Pectin Nanoparticle-Loaded Soft Coral Nephthea sp. Extract as In Situ Gel Enhances Chronic Wound Healing: In Vitro, In Vivo, and In Silico Studies
Abstract
1. Introduction
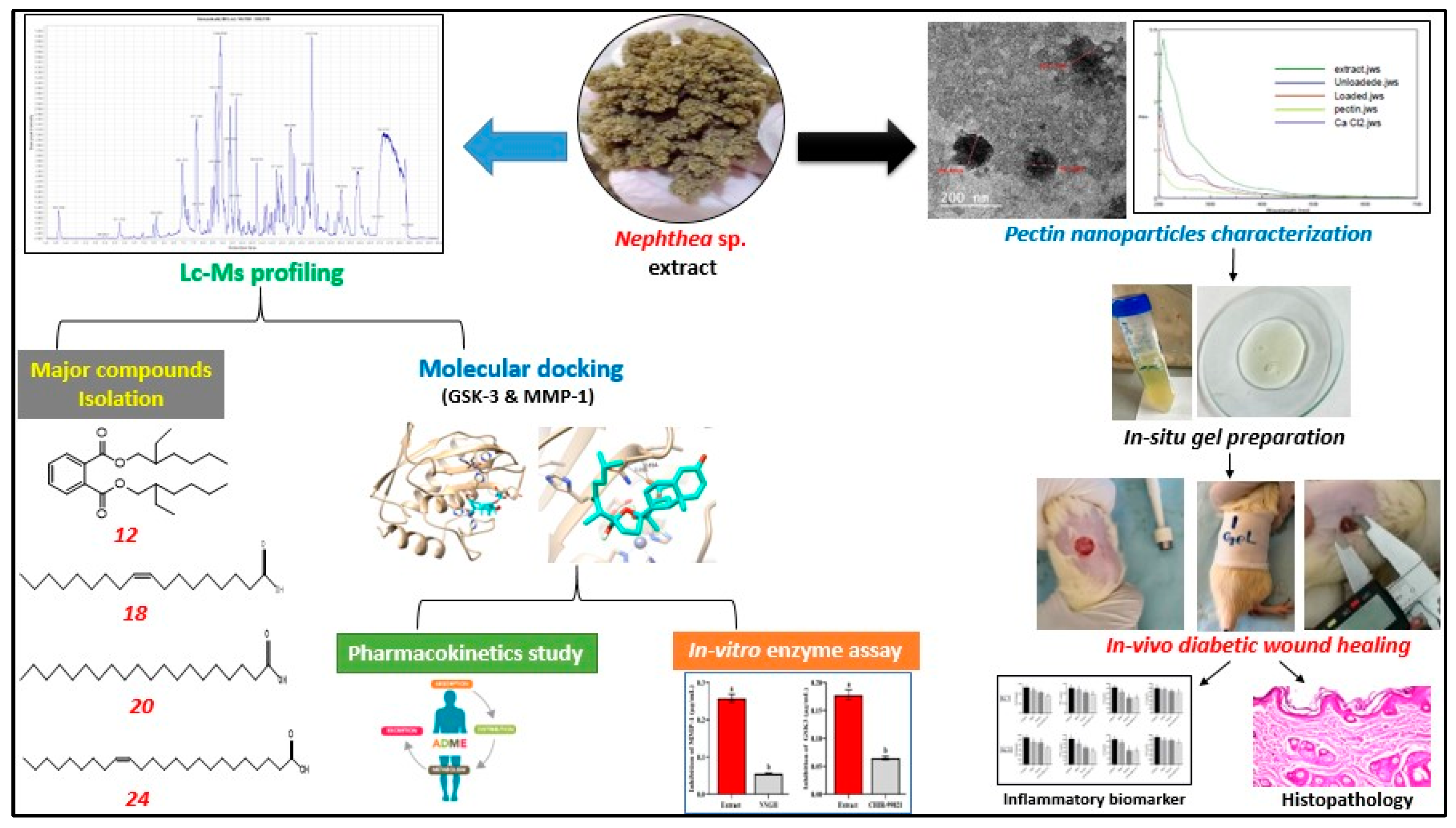
2. Results and Discussion
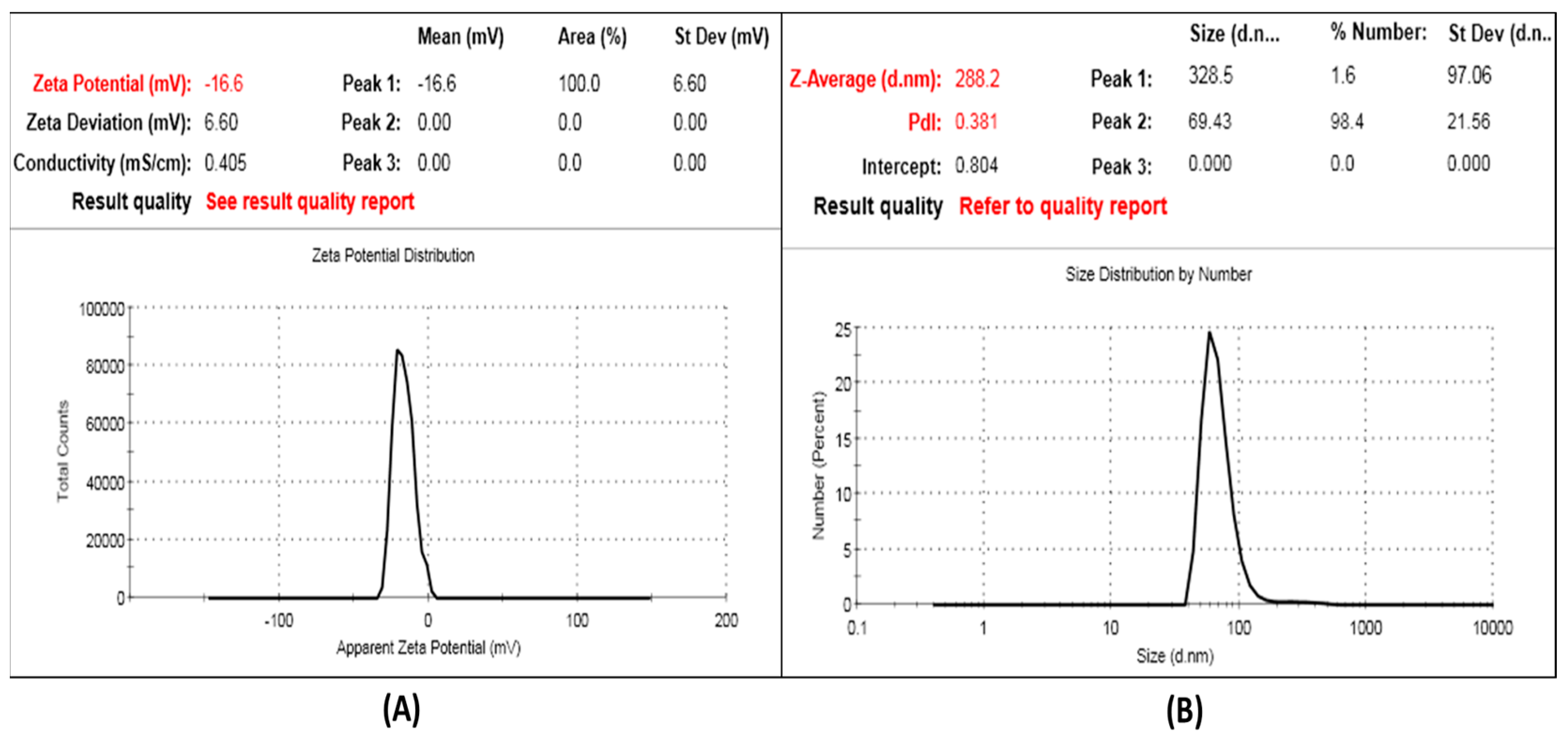
2.1. Preparation and Characterization of SCN-LPN
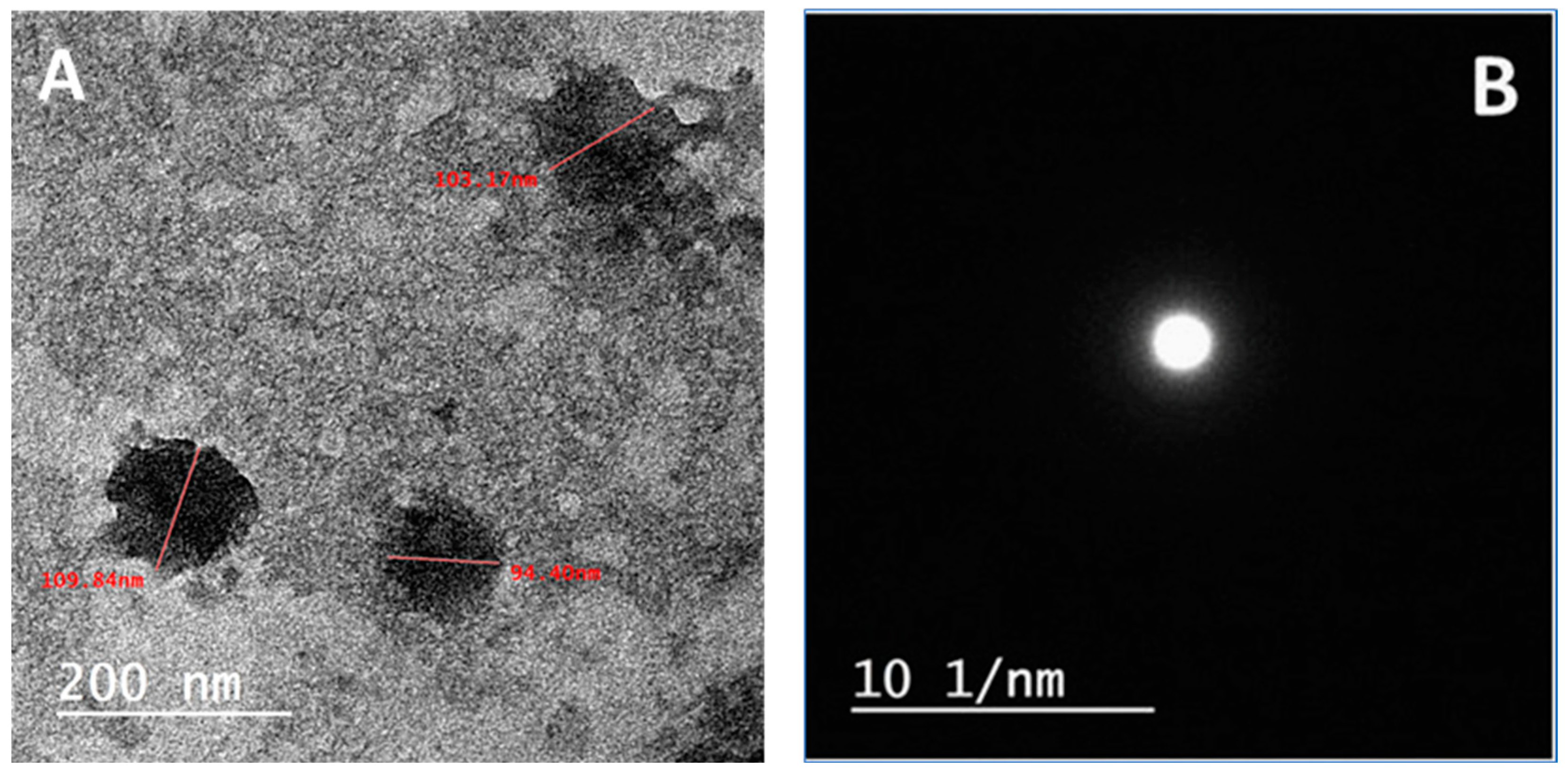
2.1.1. HRTEM Characterization of the Selected SCN-LPN
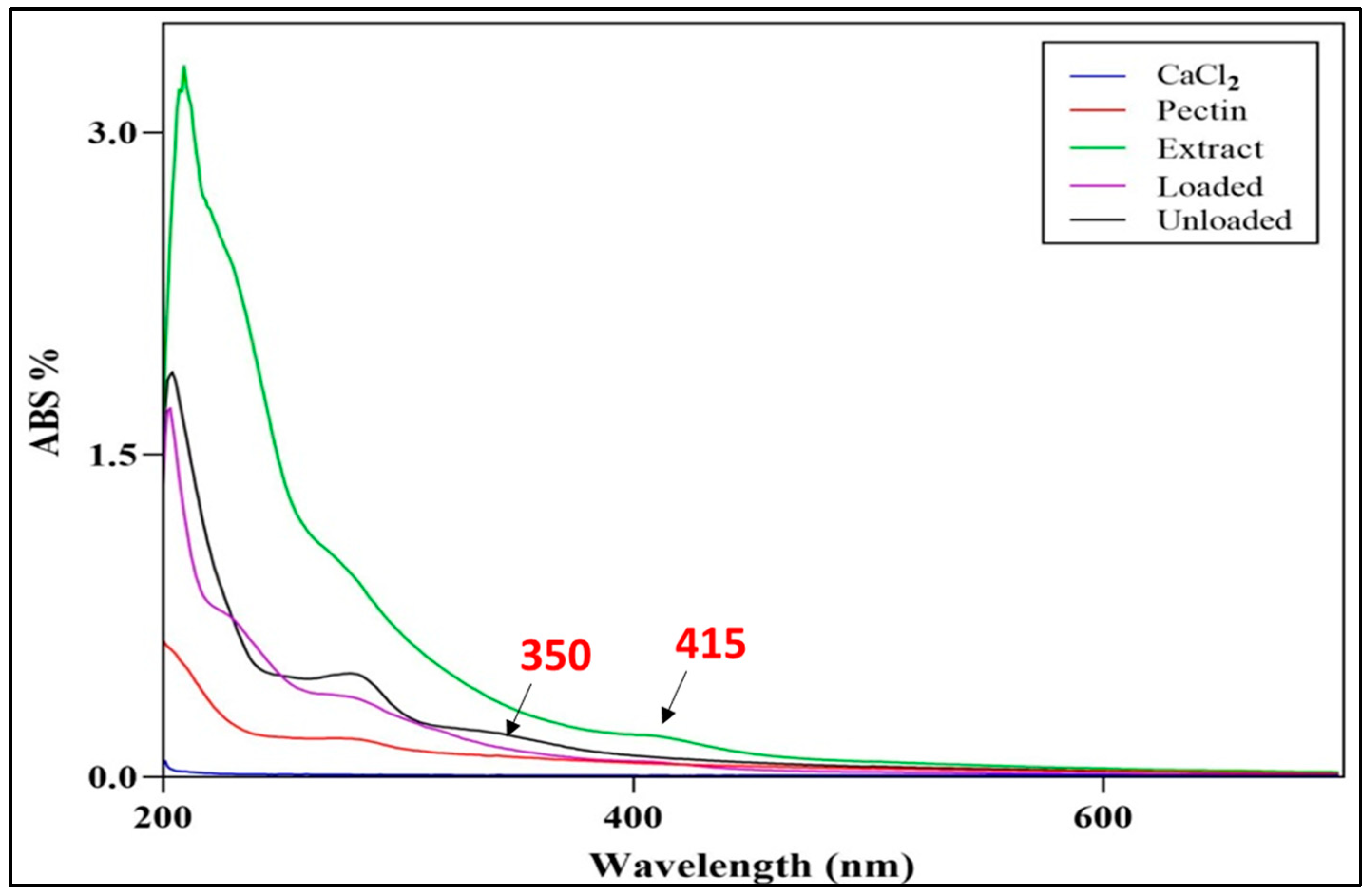
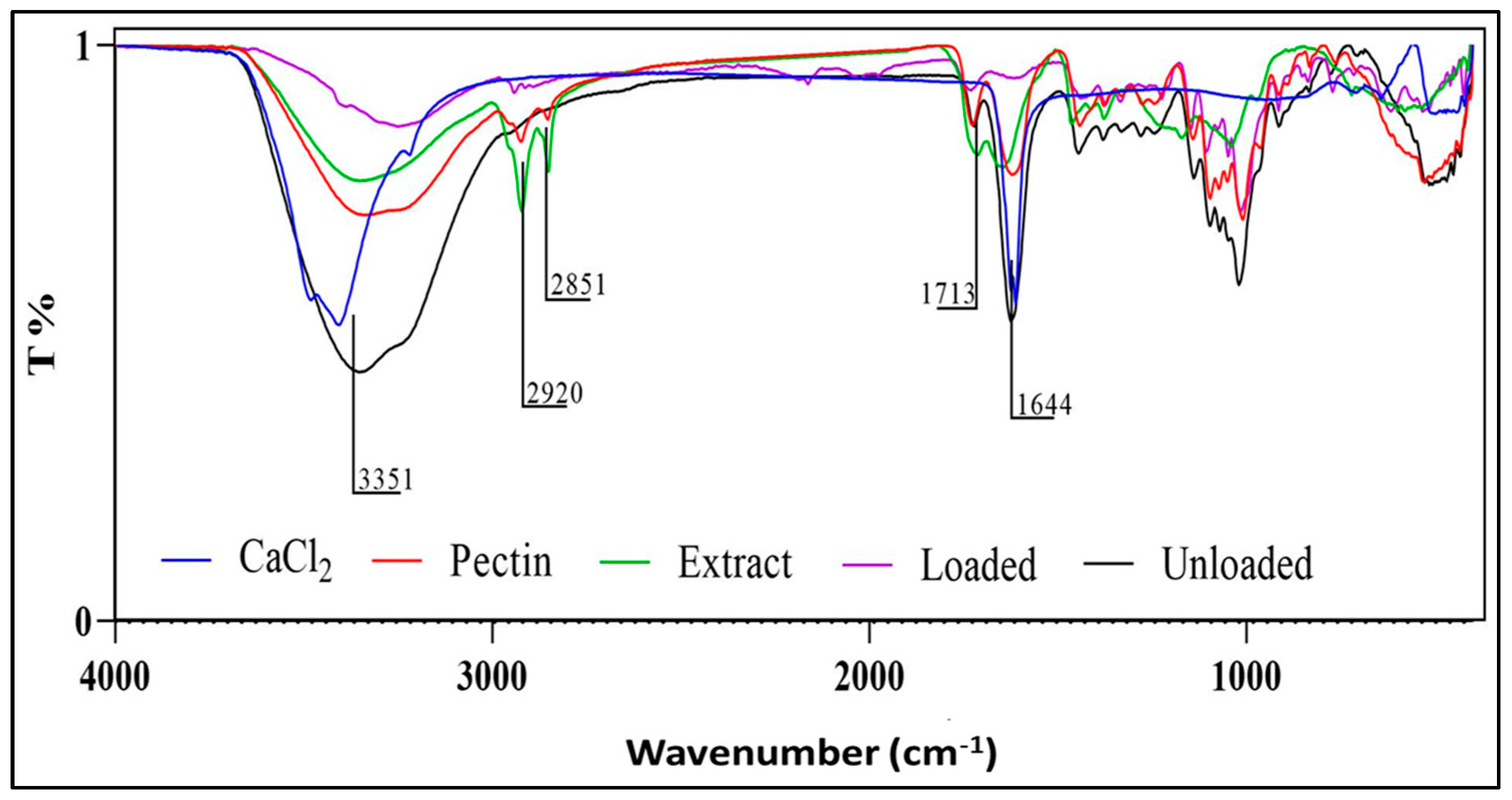
2.1.2. UV-Visible and FTIR Characterization of the Selected SCN-LPN Formulation
2.1.3. Characterization of SCN-LPN-ISG
2.2. Metabolomic Profiling Study
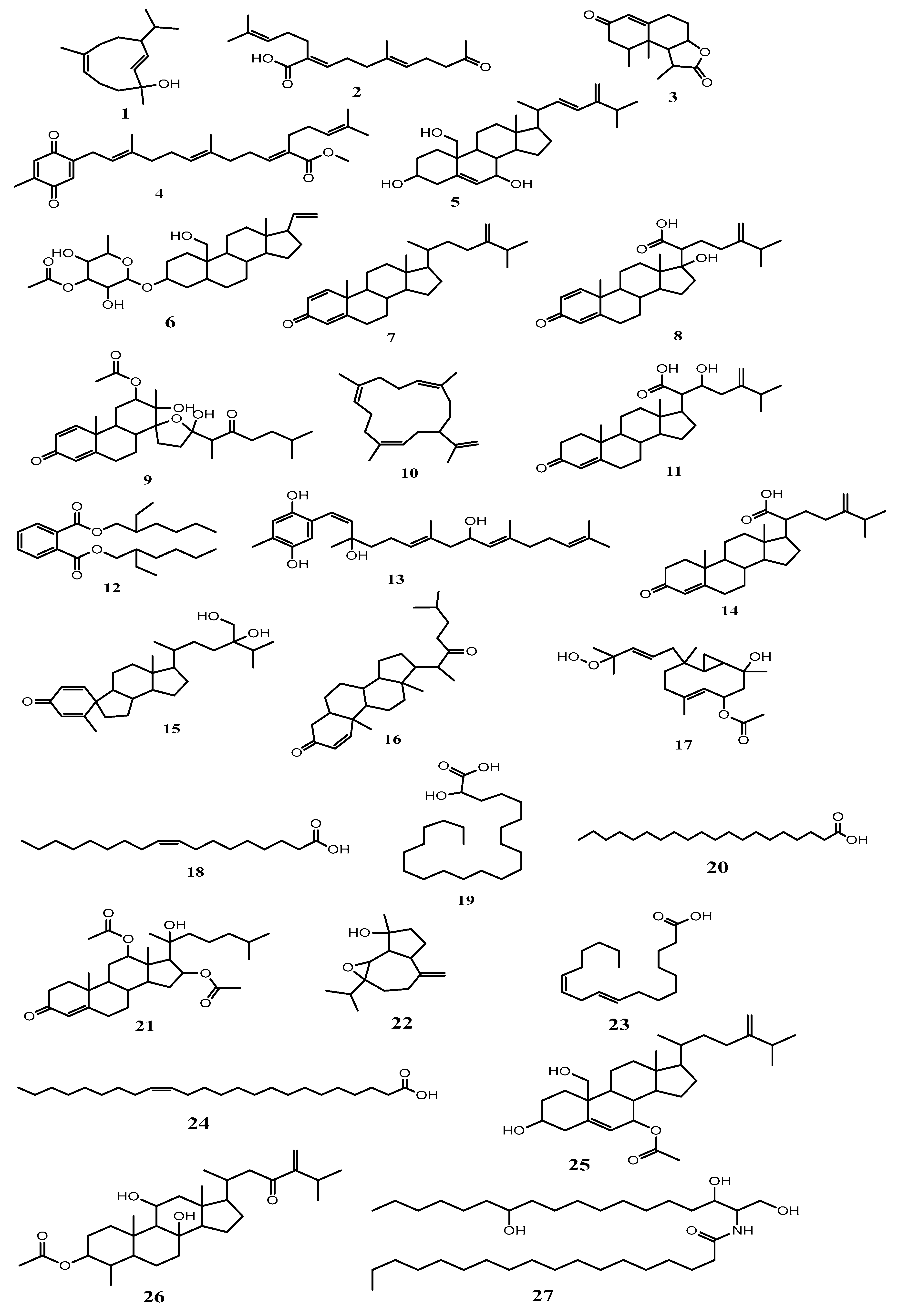
Isolation of the Major Metabolites
| No. | Identified Metabolites | Molecular Formula | Rt. (min) | Ionization Mode | m/z | Molecular Weight | ∆Mass (ppm) | Chemical Class | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Nephthenol | C15H26O | 5.56 | Negative | 221.376520 | 222.198365 | −2.3185972 | Cembrane diterpene | [33] |
| 2 | Ketochabrolic acid | C18H28O3 | 5.75 | Positive | 293.227486 | 292.203803 | −1.9893311 | Terpenoid-related carboxylic acids | [34] |
| 3 | Armatin F | C15H20O3 | 6.48 | Positive | 249.149275 | 248.141930 | 2.7641515 | Sesquiterpene | [26] |
| 4 | Chabrolobenzoquinone B | C28H38O4 | 7.79 | Positive | 439.283800 | 438.276500 | −1.1620504 | Diterpene | [34] |
| 5 | Erectasteroid H | C28H44O3 | 8.31 | Positive | 429.336642 | 428.328765 | −0.6532361 | Steroid | [26] |
| 6 | Pregn-20-ene-3,19-diol; (3β,5α)-form, 3-O-(3-O-acetyl-α-L-fucopyranoside) | C29H46O7 | 9.11 | Negative | 505.315812 | 506.323088 | −2.5011635 | Steroid | [14] |
| 7 | Ergosta-1,4,24(28)-trien-3-one | C28H42O | 9.26 | Positive | 395.653219 | 394.323565 | −1.9664193 | Steroid | [35] |
| 8 | Chabrolosteroid H | C28H40O4 | 9.43 | Positive | 441.299122 | 440.291794 | −1.9664193 | Steroid | [34], [36] |
| 9 | Isogosterone B | C29H42O7 | 9.87 | Negative | 501.284708 | 502.291984 | −2.1308277 | ||
| 10 | Cembrene A | C20H32 | 10.35 | Negative | 271.346700 | 272.250976 | −2.8617931 | Cembrane diterpene | [37] |
| 11 | Chabrolosteroid G | C28H42O4 | 10.41 | Positive | 443.314955 | 442.307657 | −1.4758935 | Steroid | [34] |
| 12 | Bis(2-ethylhexyl)phthalate * | C24H38O4 | 10.74 | Positive | 391.539732 | 390.426789 | −2.9895325 | Phthalates | [27] |
| 13 | Chabrolohydroxybenzoquinone F | C27H40O4 | 11.45 | Negative | 427.284428 | 428.291704 | −2.2307176 | Diterpene | [34] |
| 14 | Chabrolosteroid E | C28H42O3 | 11.56 | Positive | 427.319666 | 426.312288 | −2.5948047 | Steroid | [34] |
| 15 | Chabrolosteroid C | C28H44O3 | 12.04 | Positive | 429.336311 | 428.329034 | −0.0238134 | ||
| 16 | Cholest-1-ene-3,22-dione | C27H42O2 | 12.35 | Positive | 399.420384 | 398.318485 | 0.4476480 | Steroid | [14] |
| 17 | Pacificin G | C22H36O5 | 12.65 | Negative | 379.896542 | 380.256275 | −1.3900752 | Diterpene | [26] |
| 18 | Oleic acid * | C18H34O2 | 12.95 | Negative | 281.754231 | 282.586432 | −2.0964387 | Fatty acid | [15] |
| 19 | 2-Hydroxydocosanoic acid | C22H44O3 | 13.26 | Negative | 355.320800 | 356.328077 | −2.7163095 | Fatty acid | [38] |
| 20 | Arachidic acid * | C20H40O2 | 13.58 | Negative | 311.054217 | 312.502653 | −1.9700365 | Fatty acid | [15] |
| 21 | Nanjiol B | C31H48O6 | 13.42 | Positive | 517.351338 | 516.343721 | −2.6503592 | Steroid | [26] |
| 22 | Orientalol C | C15H24O2 | 14.43 | Positive | 237.542987 | 236.177638 | −2.5296721 | Sesquiterpene | [39] |
| 23 | Linoleic acid | C18H32O2 | 14.86 | Positive | 281.245678 | 280.240232 | 1.5898976 | Fatty acid | [15] |
| 24 | Nervonic acid * | C24H46O2 | 14.32 | Negative | 365.905392 | 366.219853 | −2.9065342 | Fatty acid | [15] |
| 25 | Nephalsterol C | C30H48O4 | 15.39 | Negative | 471.875324 | 472.355263 | −2.9850569 | Steroid | [40] |
| 26 | Nebrosteroid B | C31H50O5 | 15.53 | Positive | 503.254835 | 502.365825 | −0.2345662 | Steroid | [14] |
| 27 | Nephtixamide B | C36H73NO4 | 16.46 | Negative | 582.763198 | 583.553959 | −1.9956883 | Ceramide | [18] |
2.3. Wound Healing Activity
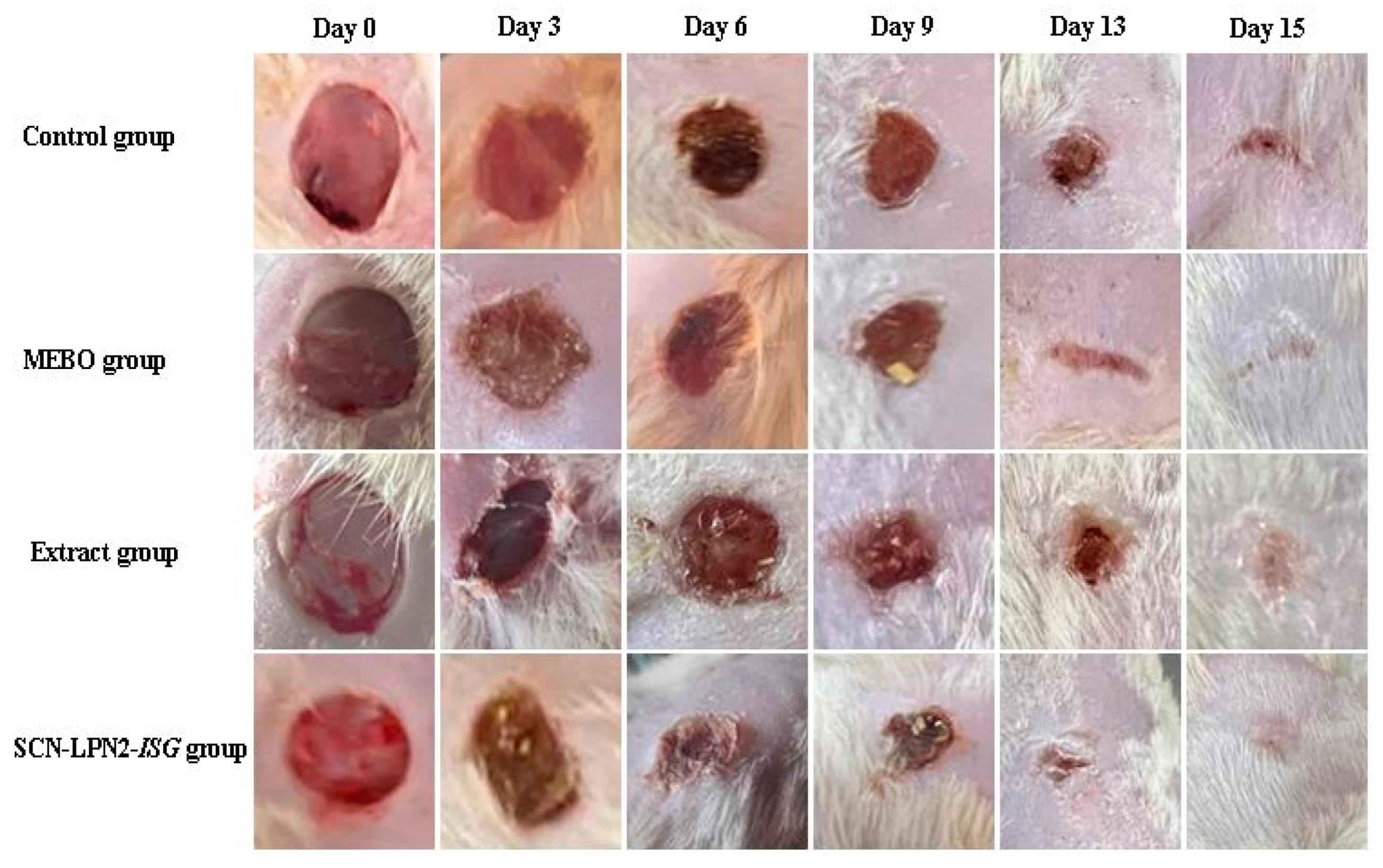
2.3.1. Histopathological Examination of Skin Tissue
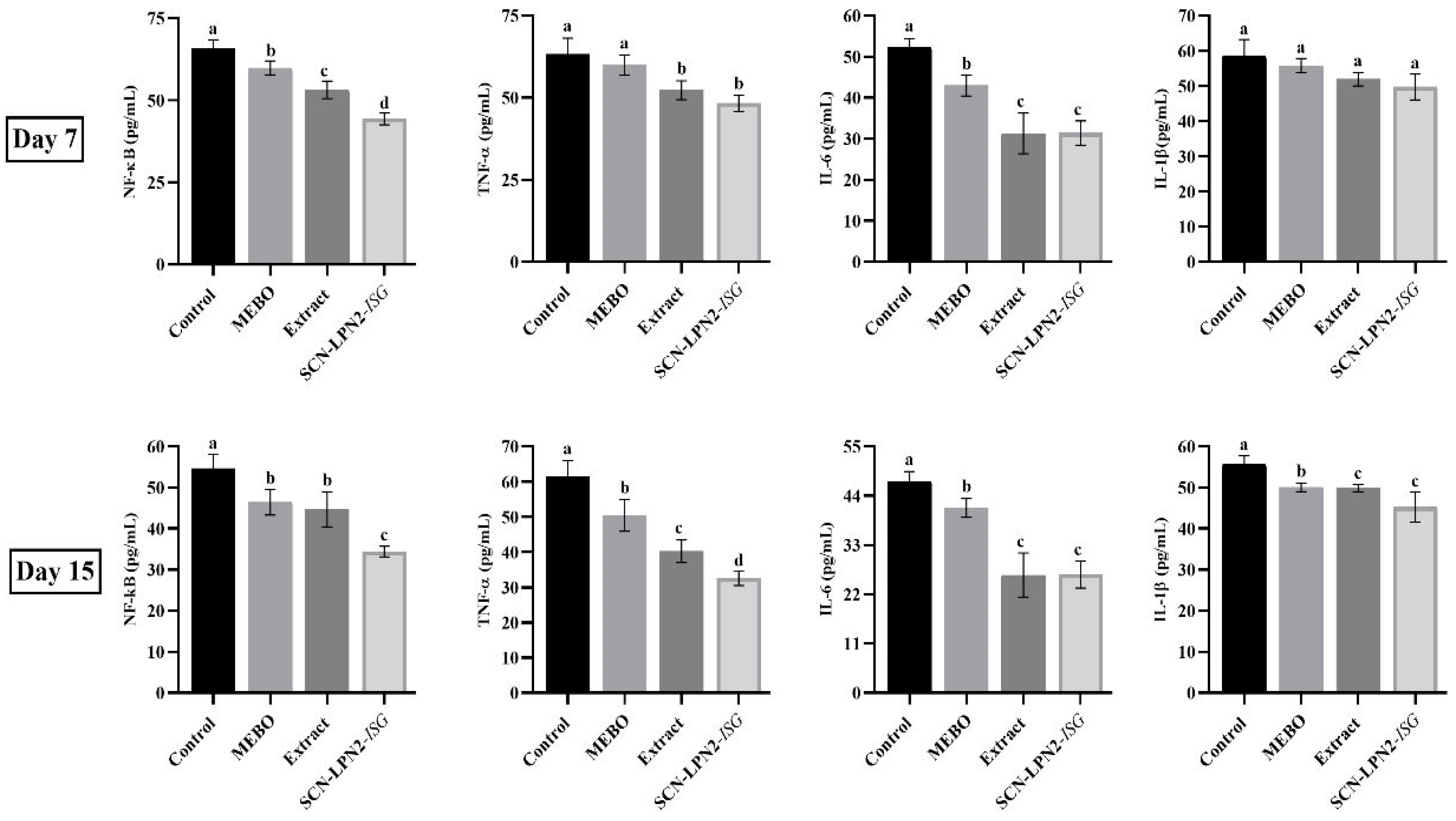
2.3.2. Inflammation Markers
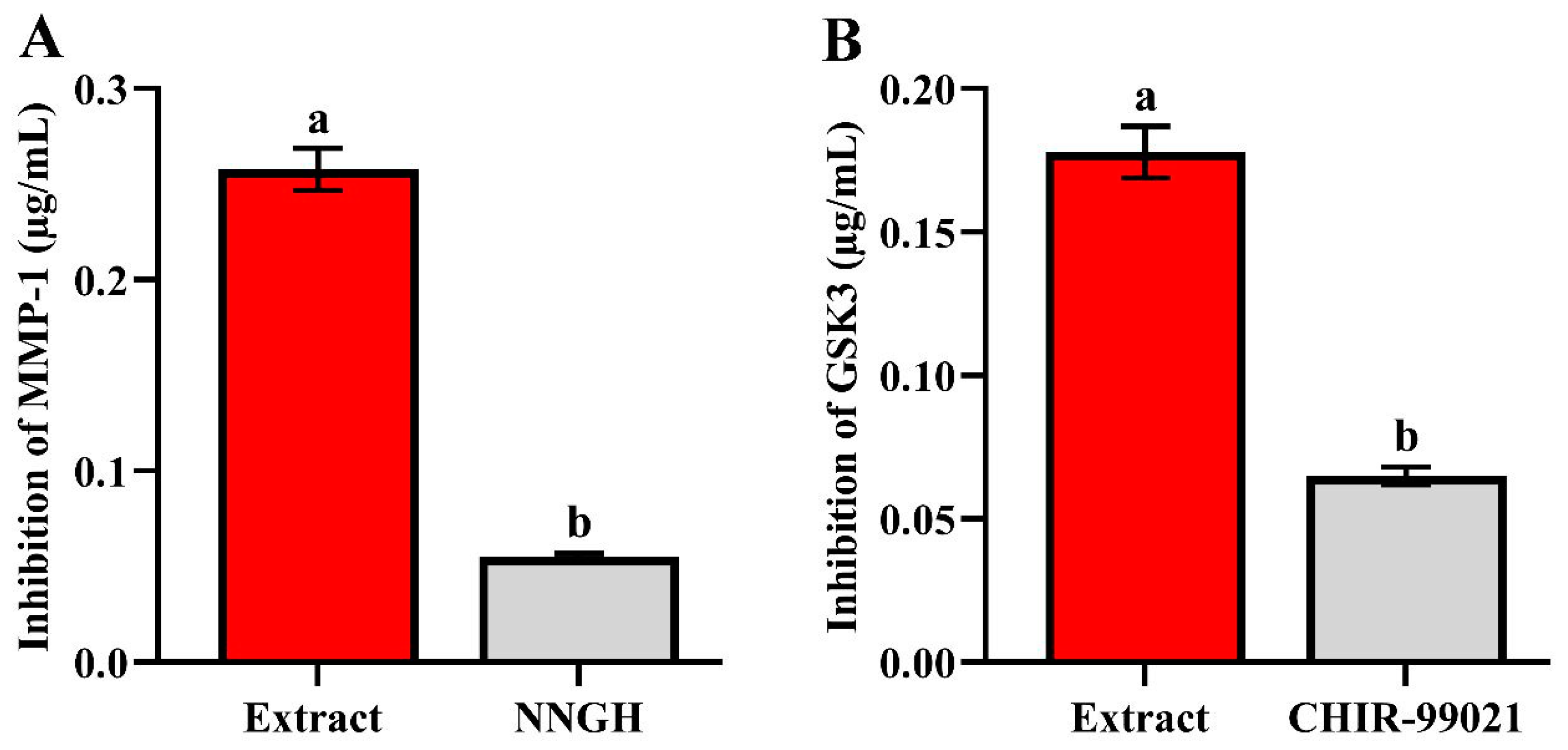
2.4. In Vitro Inhibition Assay of GSK-3 and MMP-1 Enzymes
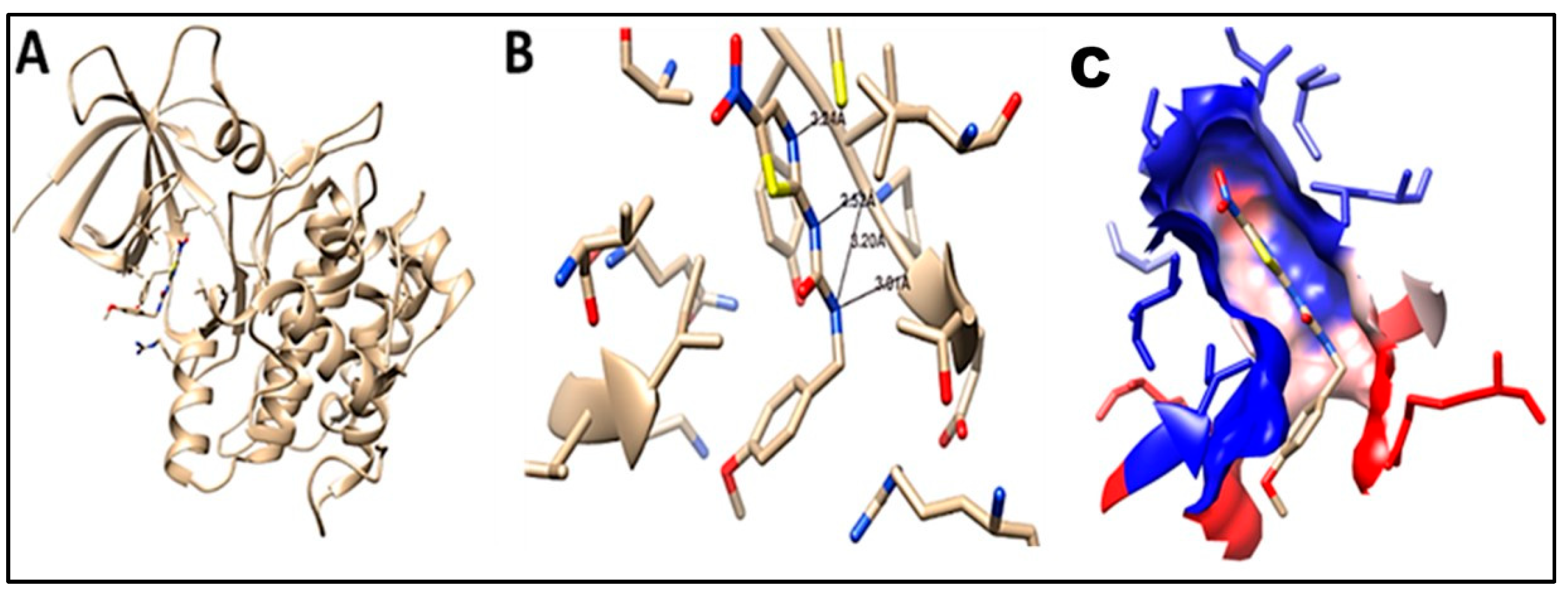
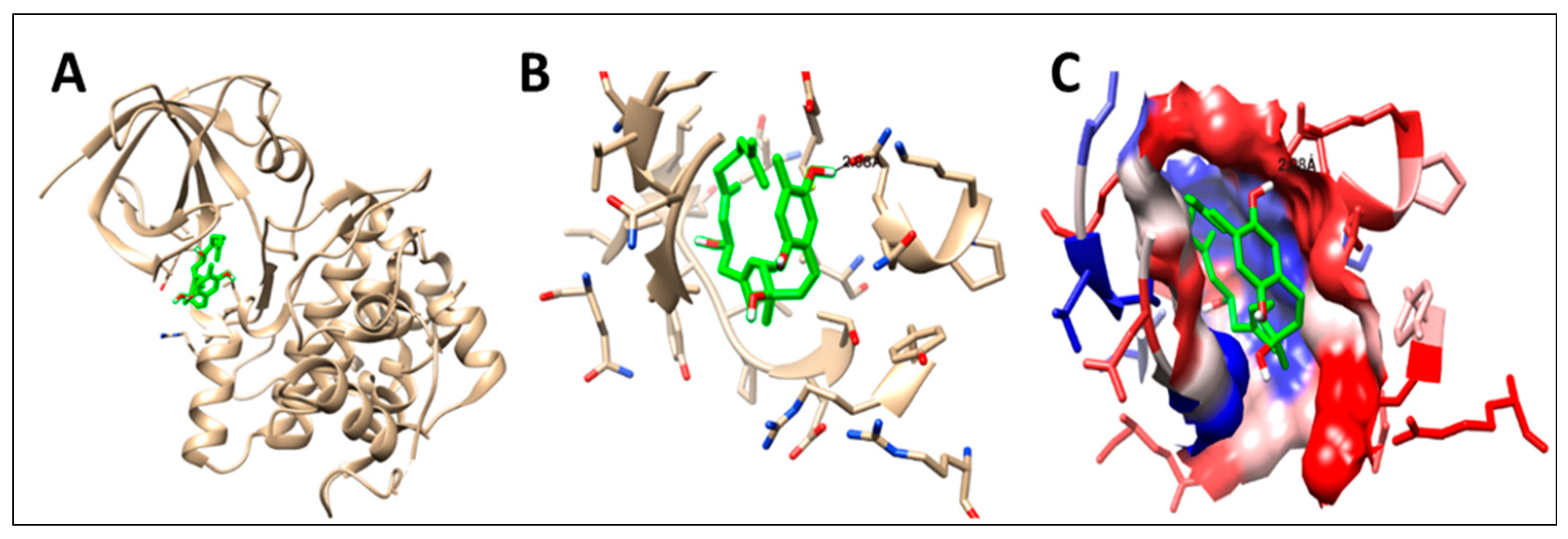
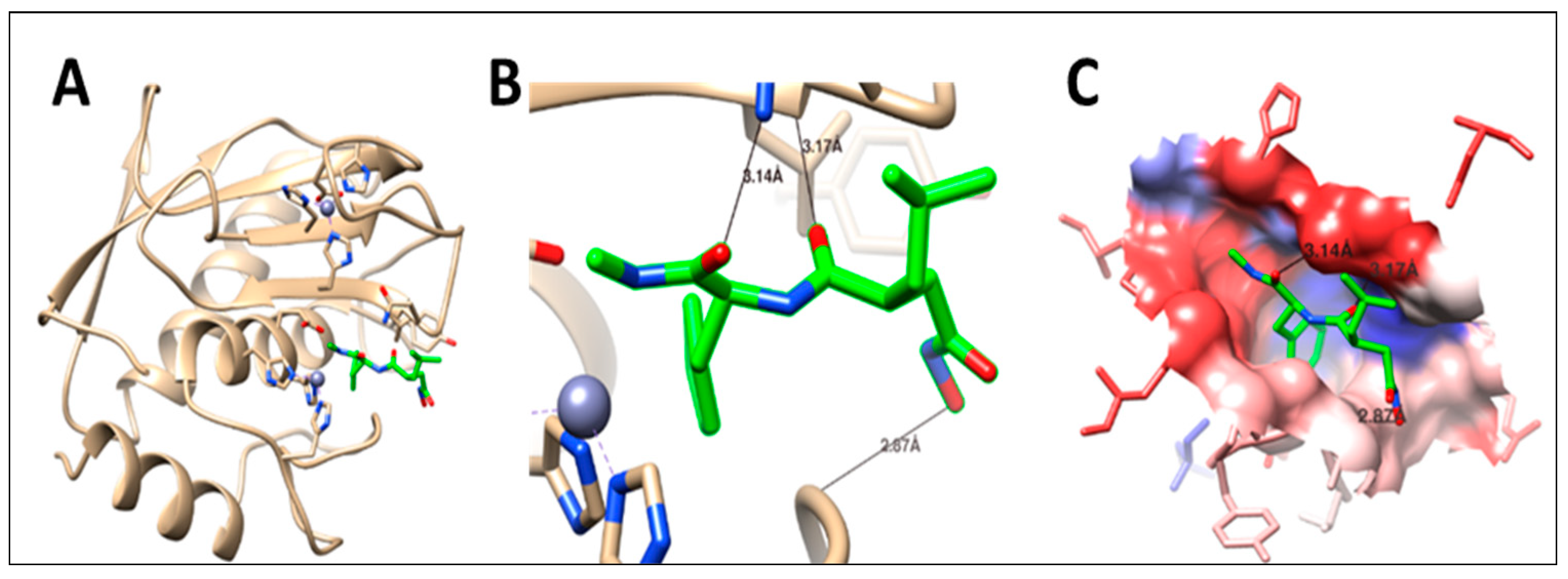
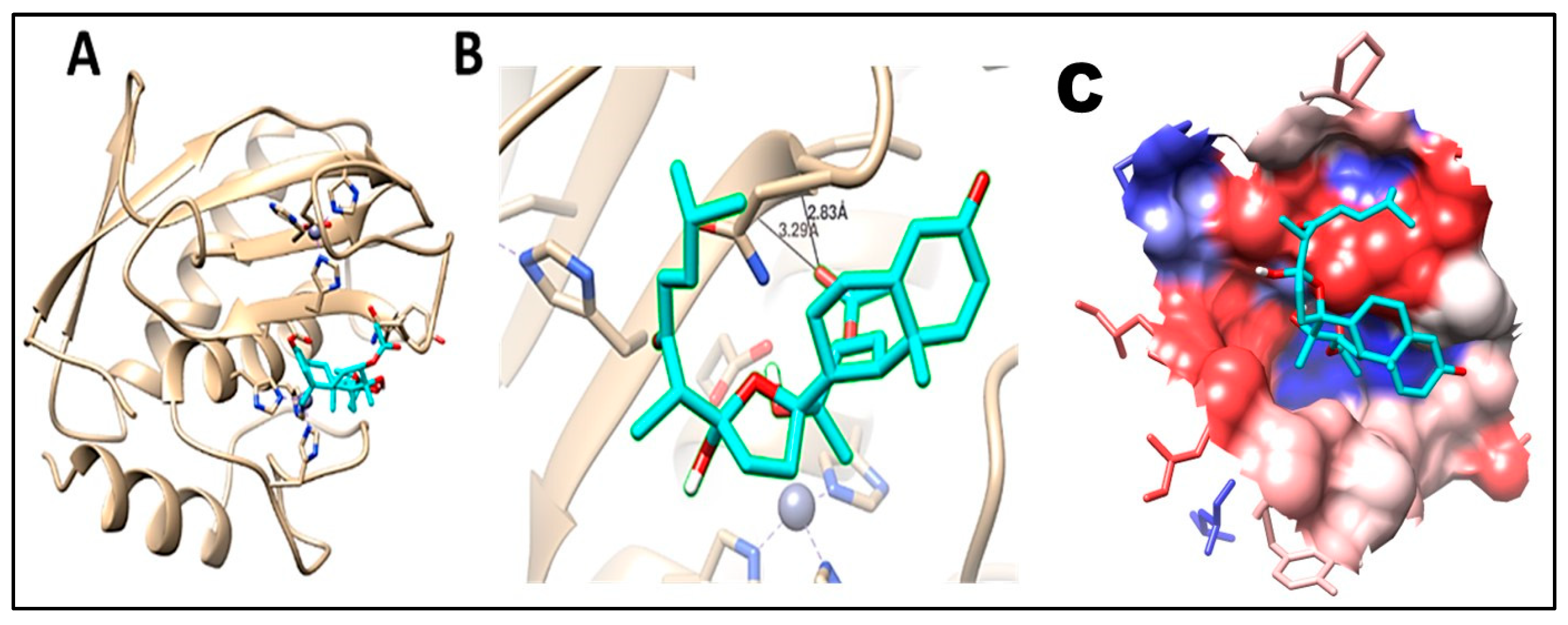
2.5. Molecular Docking
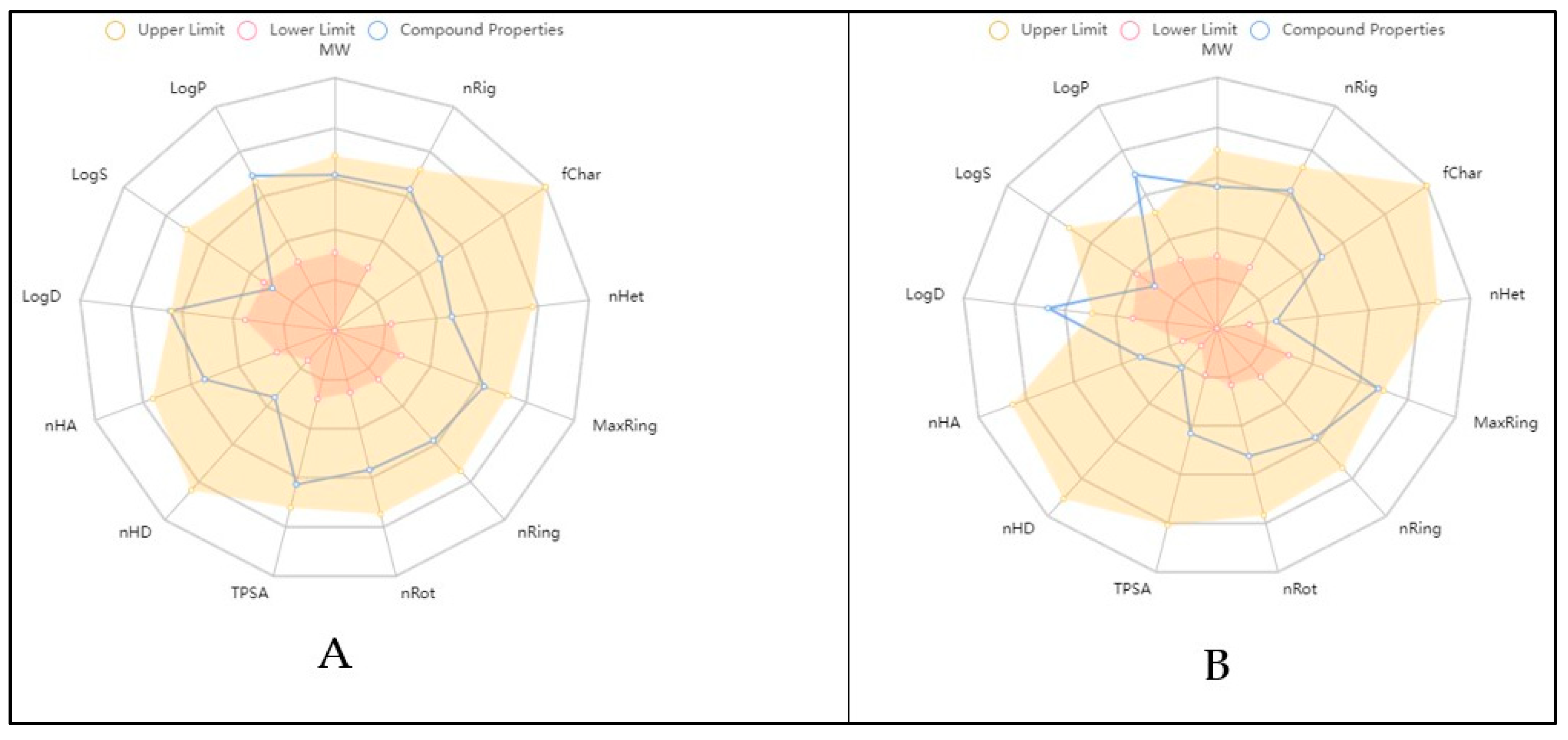
2.6. Physicochemical and ADME In Silico Study
3. Materials and Methods
3.1. Soft Coral Material and Chemicals
Extraction and Preparation
3.2. Metabolomic Profiling Study
3.3. Isolation of the Major Metabolites
3.4. Pharmaceutical Preparation
3.4.1. Preparation and Characterization of Soft Coral Nephthea sp. Loaded with Pectin Nanoparticles (SCN-LPN)
Preparation of SCN-LPN
3.4.2. Characterization of SCN-LPN
Determination of % SCN Entrapment Efficiency (% E.E.)
Determination of Particle Size (PS), Polydispersity Index (PDI), and Zeta Potential (ZP)
Transmission Electron Microscopy (TEM)
Fourier Transform Infrared Spectroscopy (FTIR)
3.4.3. Preparation and Characterization of Soft Coral Nephthea sp. Loaded with Pectin Nanoparticles In-Situ Gel (SCN-LPN-ISG)
Preparation of SCN-LPN-ISG
3.4.4. Characterization of SCN-LPN-ISG
Visual Characterization, pH, and Drug Content (%) Analysis
Sol–Gel Transformation Temperature
Rheology Properties
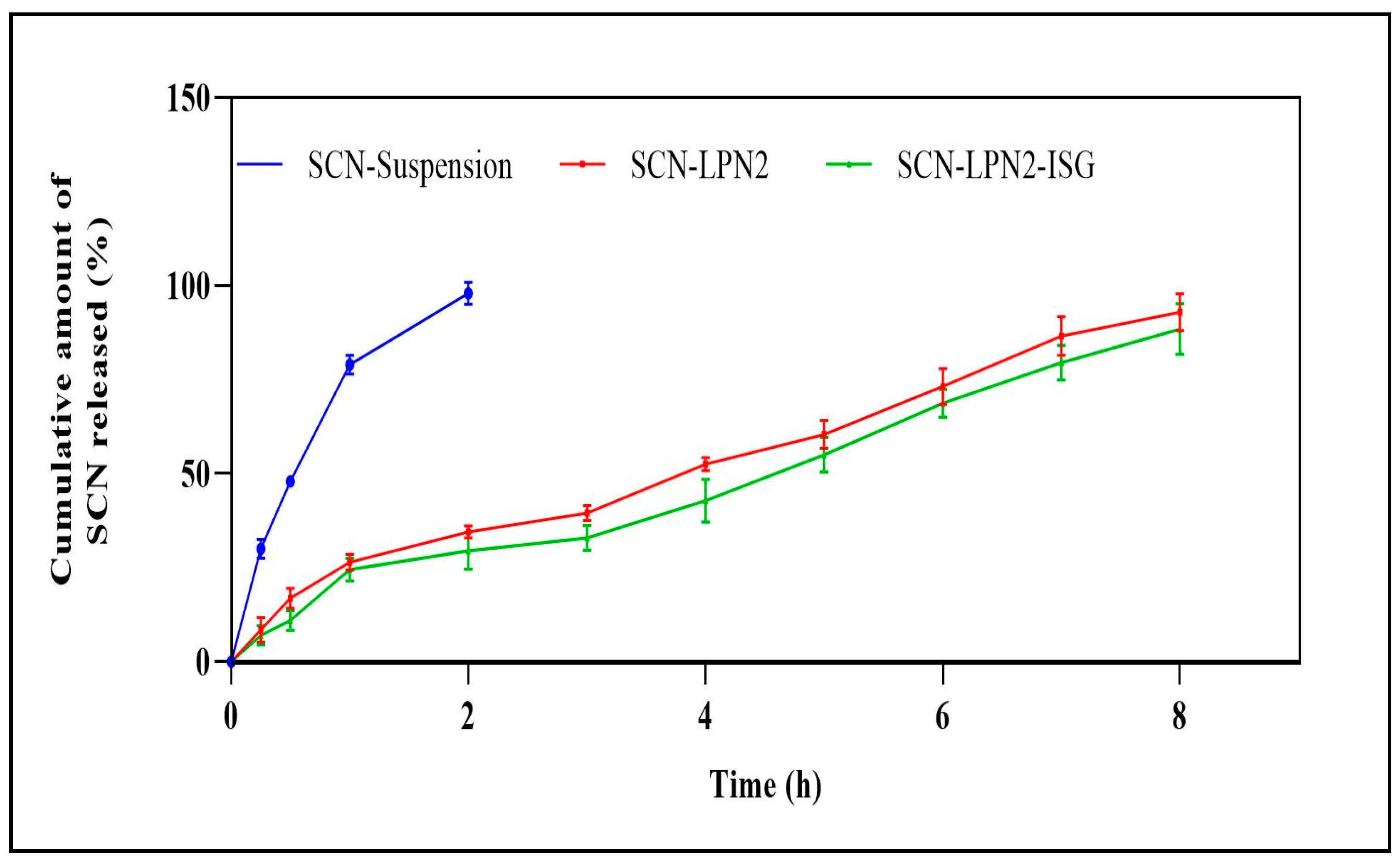
3.5. In Vitro Release Studies
3.6. In Vivo Diabetic Wound Healing Study
3.6.1. Experimental Animals and Ethical Statement
3.6.2. Diabetic Model Induced
3.6.3. Circular Excision Wound Model
- The first group was considered as a control (untreated).
- In the second group, the rats received a prepared extract of Nephthea sp. (applied as small pieces of sterile gauze macerated in 1.5 gm of the extract) and two pieces (30 mg) per wound dressing day-after-day for 15 d.
- The third group received 1 cm from SCN-LPN-ISG (Conc.: 2 mg/mL), changed day-after-day for 15 d.
- The fourth group served as a positive control, receiving MEBO ointment (as the market reference drug, 100 mg/wound, daily, Gulf Pharmaceutical Industries Company, Ras Al Khaimah, United Arab Emirates) day-after-day throughout the experiment period.
3.6.4. Wound Concentration Analysis
3.7. Histological and Histochemical Assessment Studies
3.8. Inflammatory Biomarker Assays
3.9. Inhibition Activity of Nephthea sp. Extract against GSK-3 and MMP-1 Enzymes (In Vitro)
3.10. In Silico Studies
Molecular Docking and Pharmacokinetics, “ADME” Activity
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonilla-Escobar, F.J.; Gutiérrez, M.I. Injuries are not accidents: Towards a culture of prevention. Colomb. Med. 2014, 45, 132–135. [Google Scholar] [CrossRef]
- Al-Warhi, T.; Zahran, E.M.; Selim, S.; Al-Sanea, M.M.; Ghoneim, M.M.; Maher, S.A.; Mostafa, Y.A.; Alsenani, F.; Elrehany, M.A.; Almuhayawi, M.S. Antioxidant and wound healing potential of vitis vinifera seeds supported by phytochemical characterization and docking studies. Antioxidants 2022, 11, 881. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.a.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Tenci, M.; Rossi, S.; Bonferoni, M.C.; Sandri, G.; Boselli, C.; Di Lorenzo, A.; Daglia, M.; Cornaglia, A.I.; Gioglio, L.; Perotti, C. Particulate systems based on pectin/chitosan association for the delivery of manuka honey components and platelet lysate in chronic skin ulcers. Int. J. Pharm. 2016, 509, 59–70. [Google Scholar] [CrossRef]
- Soubhagya, A.; Moorthi, A.; Prabaharan, M. Preparation and characterization of chitosan/pectin/zno porous films for wound healing. Int. J. Biol. Macromol. 2020, 157, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Emam, M.; El Raey, M.A.; Eisa, W.H.; El-Haddad, A.E.; Osman, S.M.; El-Ansari, M.A.; Rabie, A.-G.M. Green synthesis of silver nanoparticles from caesalpinia gilliesii (hook) leaves: Antimicrobial activity and in vitro cytotoxic effect against bj-1 and mcf-7 cells. J. Appl. Pharm. Sci. 2017, 7, 226–233. [Google Scholar]
- Marimuthu, S.; Rahuman, A.A.; Kirthi, A.V.; Santhoshkumar, T.; Jayaseelan, C.; Rajakumar, G. Eco-friendly microbial route to synthesize cobalt nanoparticles using bacillus thuringiensis against malaria and dengue vectors. Parasitol. Res. 2013, 112, 4105–4112. [Google Scholar] [CrossRef]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef]
- Bera, S.; Mitra, R.; Singh, J. Recent advancement in protected delivery methods for carotenoid: A smart choice in modern nutraceutical formulation concept. Biotechnol. Genet. Eng. Rev. 2023, 1–57. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, P.; Liu, Y.; Wu, Z.; Wang, L.; Liu, L. Citrus pectin research advances: Derived as a biomaterial in the construction and applications of micro/nano-delivery systems. Food Hydrocoll. 2022, 133, 107910. [Google Scholar] [CrossRef]
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Sato, Y.; Hattori, H. Polyelectrolyte complexes of natural polymers and their biomedical applications. Polymers 2019, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, V.; Kumar, R.; Pruncu, C.I. Fabrication and characterization of ceftizoxime-loaded pectin nanocarriers. Nanomaterials 2020, 10, 1452. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Saraf, S.; Saraf, S. Understanding the role of poloxamer 407 based thermoreversible in situ gelling hydrogel for delivery of pegylated melphalan conjugate. Curr. Drug Deliv. 2016, 13, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafez, O.H.; Fahim, J.R.; Desoukey, S.Y.; Kamel, M.S.; Abdelmohsen, U.R. Recent updates on corals from nephtheidae. Chem. Biodivers. 2019, 16, e1800692. [Google Scholar] [CrossRef]
- Hassan, N.H.; El-Hawary, S.S.; Emam, M.; Rabeh, M.A.; Abdelmohsen, U.R.; Selim, N.M. Potential inhibitors of cyp51 enzyme in dermatophytes by red sea soft coral Nephthea sp.: In silico and molecular networking studies. ACS Omega 2022, 7, 13808–13817. [Google Scholar] [CrossRef]
- Hassan, N.H.; Elhawary, S.; Emam, M.; Rabeh, M.A.; Muhsinah, A.B.; Asiri, Y.I.; Hamed, E.A.; AbdelMohsen, U.R.; Selim, N.M. Bioactive constituents of marine soft coral Nephthea sp. Against herpes simplex type i (hsv-1) and coxsackie b4 (coxb4) viruses; in-vitro and in silico studies. Egypt. J. Chem. 2022. [Google Scholar] [CrossRef]
- Hassan, N.H.; El-Hawary, S.S.; Emam, M.; Safwat, N.A.; Rabeh, M.A.; Abdelmohsen, U.R.; Selim, N.M. Nephthea sp. Inhibits biofilm, DNA gyrase, hsp90, and dhfr: In vitro, in silico, and pharmacokinetics studies. Nat. Prod. Res. 2022, 1–6. [Google Scholar] [CrossRef]
- Abdelhafez, O.H.; Fahim, J.R.; Mustafa, M.; AboulMagd, A.M.; Desoukey, S.Y.; Hayallah, A.M.; Kamel, M.S.; Abdelmohsen, U.R. Natural metabolites from the soft coral Nephthea sp. As potential SARS-COV-2 main protease inhibitors. Nat. Prod. Res. 2022, 36, 2893–2896. [Google Scholar] [CrossRef]
- Hamed, A.; Abdel-Razek, A.S.; Araby, M.; Abu-Elghait, M.; El-Hosari, D.G.; Frese, M.; Soliman, H.S.; Stammler, H.G.; Sewald, N.; Shaaban, M. Meleagrin from marine fungus emericella dentata nq45: Crystal structure and diverse biological activity studies. Nat. Prod. Res. 2021, 35, 3830–3838. [Google Scholar] [CrossRef]
- Yener, S.; Akbulut, K.G.; Karakuş, R.; Erdoğan, D.; Acartürk, F. Development of melatonin loaded pectin nanoparticles for the treatment of inflammatory bowel disease: In vitro and in vivo studies. J. Drug Deliv. Sci. Technol. 2022, 67, 102861. [Google Scholar] [CrossRef]
- Oveissi, F.; Tavakoli, N.; Minaiyan, M.; Mofid, M.R.; Taheri, A. Alginate hydrogel enriched with ambystoma mexicanum epidermal lipoxygenase-loaded pectin nanoparticles for enhanced wound healing. J. Biomater. Appl. 2020, 34, 1171–1187. [Google Scholar] [CrossRef]
- Abd-Elal, R.M.; Shamma, R.N.; Rashed, H.M.; Bendas, E.R. Trans-nasal zolmitriptan novasomes: In-vitro preparation, optimization and in-vivo evaluation of brain targeting efficiency. Drug Deliv. 2016, 23, 3374–3386. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elal, R.M.; Elosaily, G.H.; Gad, S.; Khafagy, E.-S.; Mostafa, Y. Full factorial design, optimization, in vitro and ex vivo studies of ocular timolol-loaded microsponges. J. Pharm. Innov. 2020, 15, 651–663. [Google Scholar] [CrossRef]
- Ghasemi, S.; Jafari, S.M.; Assadpour, E.; Khomeiri, M. Production of pectin-whey protein nano-complexes as carriers of orange peel oil. Carbohydr. Polym. 2017, 177, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Chinnaiyan, S.K.; Karthikeyan, D.; Gadela, V.R. Development and characterization of metformin loaded pectin nanoparticles for t2 diabetes mellitus. Pharm. Nanotechnol. 2018, 6, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Amir, F.; Koay, Y.C.; Yam, W.S. Chemical constituents and biological properties of the marine soft coral nephthea: A review (part 1). Trop. J. Pharm. Res. 2012, 11, 485–498. [Google Scholar]
- Priya, A.M.; Jayachandran, S. Induction of apoptosis and cell cycle arrest by bis (2-ethylhexyl) phthalate produced by marine bacillus pumilus mb 40. Chem.-Biol. Interact. 2012, 195, 133–143. [Google Scholar] [CrossRef]
- Elhagali, G.A.; Abozeed, A.E.; Youssif, Y.M. Investigation of bioactive constituents and biological activities of different fractions from herniaria hemistemon J. Gay. Al-Azhar Bull. Sci. 2019, 30, 67–80. [Google Scholar] [CrossRef]
- Abbas, A.S.; Abbas, S.M. Kinetic study and simulation of oleic acid esterification in different type of reactors. Iraqi J. Chem. Pet. Eng. 2013, 14, 13–20. [Google Scholar]
- Termsarasab, U.; Cho, H.-J.; Kim, D.H.; Chong, S.; Chung, S.-J.; Shim, C.-K.; Moon, H.T.; Kim, D.-D. Chitosan oligosaccharide–arachidic acid-based nanoparticles for anti-cancer drug delivery. Int. J. Pharm. 2013, 441, 373–380. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Yu, X.; Gao, J.-M. A mini review of nervonic acid: Source, production, and biological functions. Food Chem. 2019, 301, 125286. [Google Scholar] [CrossRef] [PubMed]
- Giwa, A.S.; Ali, N. Perspectives of nervonic acid production by yarrowia lipolytica. Biotechnol. Lett. 2022, 44, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Kamada, T.; Vairappan, C.S. Three new cembranoids from the bornean soft coral Nephthea sp. J. Asian Nat. Prod. Res. 2016, 18, 415–422. [Google Scholar] [CrossRef]
- Amir, F.; Koay, Y.C.; Yam, W.S. Chemical constituents and biological properties of the marine soft coral nephthea: A review (part 2). Trop. J. Pharm. Res. 2012, 11, 499–517. [Google Scholar]
- Yan, X.-H.; Liu, H.-L.; Huang, H.; Li, X.-B.; Guo, Y.-W. Steroids with aromatic a-rings from the hainan soft coral dendronephthya studeri ridley. J. Nat. Prod. 2011, 74, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Sarma, N.S.; Krishna, M.S.; Pasha, S.G.; Rao, T.S.P.; Venkateswarlu, Y.; Parameswaran, P. Marine metabolites: The sterols of soft coral. Chem. Rev. 2009, 109, 2803–2828. [Google Scholar] [CrossRef]
- Ishii, T.; Zhaoqi, Z.; Vairappan, C.S. A new cembrane diterpene from the bornean soft coral Nephthea sp. Molecules 2010, 15, 3857–3862. [Google Scholar] [CrossRef]
- Farokhi, F.; Wielgosz-Collin, G.; Clement, M.; Kornprobst, J.-M.; Barnathan, G. Cytotoxicity on human cancer cells of ophidiacerebrosides isolated from the african starfish narcissia canariensis. Mar. Drugs 2010, 8, 2988–2998. [Google Scholar] [CrossRef]
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2010, 27, 1681–1708. [Google Scholar] [CrossRef]
- Hsiao, T.-H.; Cheng, C.-H.; Wu, T.-Y.; Lu, M.-C.; Chen, W.-F.; Wen, Z.-H.; Dai, C.-F.; Wu, Y.-C.; Sung, P.-J. New cembranoid diterpenes from the cultured octocoral nephthea columnaris. Molecules 2015, 20, 13205–13215. [Google Scholar] [CrossRef]
- Serra, M.B.; Barroso, W.A.; Silva, N.N.d.; Silva, S.d.N.; Borges, A.C.R.; Abreu, I.C.; Borges, M.O.d.R. From inflammation to current and alternative therapies involved in wound healing. Int. J. Inflamm. 2017, 2017, 3406215. [Google Scholar] [CrossRef]
- Akita, S. Wound repair and regeneration: Mechanisms, signaling. Int. J. Mol. Sci. 2019, 20, 6328. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Tian, R.; Zhang, Y.; Drutskaya, M.S.; Wang, C.; Ge, J.; Fan, Z.; Kong, D.; Wang, X. Macrophages induce akt/β-catenin-dependent lgr5+ stem cell activation and hair follicle regeneration through tnf. Nat. Commun. 2017, 8, 14091. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Jeong, M.J.; Ashworth, J.J.; Hardman, M.; Jin, W.; Moutsopoulos, N.; Wild, T.; McCartney-Francis, N.; Sim, D.; McGrady, G. Tumor necrosis factor-alpha (tnf-α) is a therapeutic target for impaired cutaneous wound healing. Wound Repair Regen. 2012, 20, 38–49. [Google Scholar] [CrossRef]
- Rai, N.K.; Tripathi, K.; Sharma, D.; Shukla, V.K. Apoptosis: A basic physiologic process in wound healing. Int. J. Low Extrem. Wounds 2005, 4, 138–144. [Google Scholar] [CrossRef]
- Ahamed, K.B.; Gowdru, H.B.; Rajashekarappa, S.; Malleshappa, K.S.H.; Krishna, V. Molecular docking of glycogen synthase kinase3-β inhibitor oleanolic acid and its wound-healing activity in rats. Med. Chem. Res. 2013, 22, 156–164. [Google Scholar] [CrossRef]
- Tantawy, M.A.; Shalby, A.B.; Barnawi, I.O.; Kattan, S.W.; Abd-Rabou, A.A.; Elmegeed, G.A. Anti-cancer activity, and molecular docking of novel hybrid heterocyclic steroids revealed promising anti-hepatocellular carcinoma agent: Implication of cyclin dependent kinase-2 pathway. Steroids 2023, 193, 109187. [Google Scholar] [CrossRef]
- Qin, C.; Li, Y.; Zhang, Y.; Liu, L.; Wu, Z.; Weng, P. Insights into oat polyphenols constituent against advanced glycation end products mechanism by spectroscopy and molecular interaction. Food Biosci. 2021, 43, 101313. [Google Scholar] [CrossRef]
- Abdelshaheed, M.M.; El Subbagh, H.I.; Tantawy, M.A.; Attia, R.T.; Youssef, K.M.; Fawzy, I.M. Discovery of new pyridine heterocyclic hybrids; design, synthesis, dynamic simulations, and in vitro and in vivo breast cancer biological assays. RSC Adv. 2023, 13, 15689–15703. [Google Scholar] [CrossRef]
- Gupta, S.; Shende, P. L-proline adsorbed oxygen-loaded nanobubbles in-situ gel for wound healing. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129028. [Google Scholar] [CrossRef]
- Kurniawansyah, I.S.; Rusdiana, T.; Sopyan, I.; Ramoko, H.; Wahab, H.A.; Subarnas, A. In situ ophthalmic gel forming systems of poloxamer 407 and hydroxypropyl methyl cellulose mixtures for sustained ocular delivery of chloramphenicole: Optimization study by factorial design. Heliyon 2020, 6, e05365. [Google Scholar] [CrossRef]
- Lu, C.; Liu, M.; Fu, H.; Zhang, W.; Peng, G.; Zhang, Y.; Cao, H.; Luo, L. Novel thermosensitive in situ gel based on poloxamer for uterus delivery. Eur. J. Pharm. Sci. 2015, 77, 24–28. [Google Scholar] [CrossRef]
- Rarokar, N.R.; Saoji, S.D.; Raut, N.A.; Taksande, J.B.; Khedekar, P.B.; Dave, V.S. Nanostructured cubosomes in a thermoresponsive depot system: An alternative approach for the controlled delivery of docetaxel. AAPS Pharmscitech 2016, 17, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Kesarla, R.; Tank, T.; Vora, P.A.; Shah, T.; Parmar, S.; Omri, A. Preparation and evaluation of nanoparticles loaded ophthalmic in situ gel. Drug Deliv. 2016, 23, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Mansor, L.S.; Gonzalez, E.R.; Cole, M.A.; Tyler, D.J.; Beeson, J.H.; Clarke, K.; Carr, C.A.; Heather, L.C. Cardiac metabolism in a new rat model of type 2 diabetes using high-fat diet with low dose streptozotocin. Cardiovasc. Diabetol. 2013, 12, 136. [Google Scholar] [CrossRef]
- Gopal, A.; Kant, V.; Gopalakrishnan, A.; Tandan, S.K.; Kumar, D. Chitosan-based copper nanocomposite accelerates healing in excision wound model in rats. Eur. J. Pharmacol. 2014, 731, 8–19. [Google Scholar] [CrossRef]
- Gushiken, L.F.S.; Beserra, F.P.; Hussni, M.F.; Gonzaga, M.T.; Ribeiro, V.P.; De Souza, P.F.; Campos, J.C.L.; Massaro, T.N.C.; Hussni, C.A.; Takahira, R.K. Beta-caryophyllene as an antioxidant, anti-inflammatory and re-epithelialization activities in a rat skin wound excision model. Oxidative Med. Cell. Longev. 2022, 9004014. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D. Histochemical Techniques; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Seif, M.; Deabes, M.; El-Askary, A.; El-Kott, A.F.; Albadrani, G.M.; Seif, A.; Wang, Z. Ephedra sinica mitigates hepatic oxidative stress and inflammation via suppressing the tlr4/myd88/nf-κb pathway in fipronil-treated rats. Environ. Sci. Pollut. Res. 2021, 28, 62943–62958. [Google Scholar] [CrossRef]
- Asawa, Y.; Yoshimori, A.; Bajorath, J.; Nakamura, H. Prediction of an mmp-1 inhibitor activity cliff using the sar matrix approach and its experimental validation. Sci. Rep. 2020, 10, 14710. [Google Scholar] [CrossRef]
- Pedretti, A.; Villa, L.; Vistoli, G. Vega–an open platform to develop chemo-bio-informatics applications, using plug-in architecture and script programming. J. Comput.-Aided Mol. Des. 2004, 18, 167–173. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A. Software news and update autodock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar]
- Petrescu, I.; Lamotte-Brasseur, J.; Chessa, J.-P.; Ntarima, P.; Claeyssens, M.; Devreese, B.; Marino, G.; Gerday, C. Xylanase from the psychrophilic yeast cryptococcus adeliae. Extremophiles 2000, 4, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, M.A.; Sroor, F.M.; Mohamed, M.F.; El-Naggar, M.E.; Saleh, F.M.; Hassaneen, H.M.; Abdelhamid, I.A. Molecular docking study, cytotoxicity, cell cycle arrest and apoptotic induction of novel chalcones incorporating thiadiazolyl isoquinoline in cervical cancer. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2020, 20, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. Swissadme: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A. Admetlab 2.0: An integrated online platform for accurate and comprehensive predictions of admet properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]
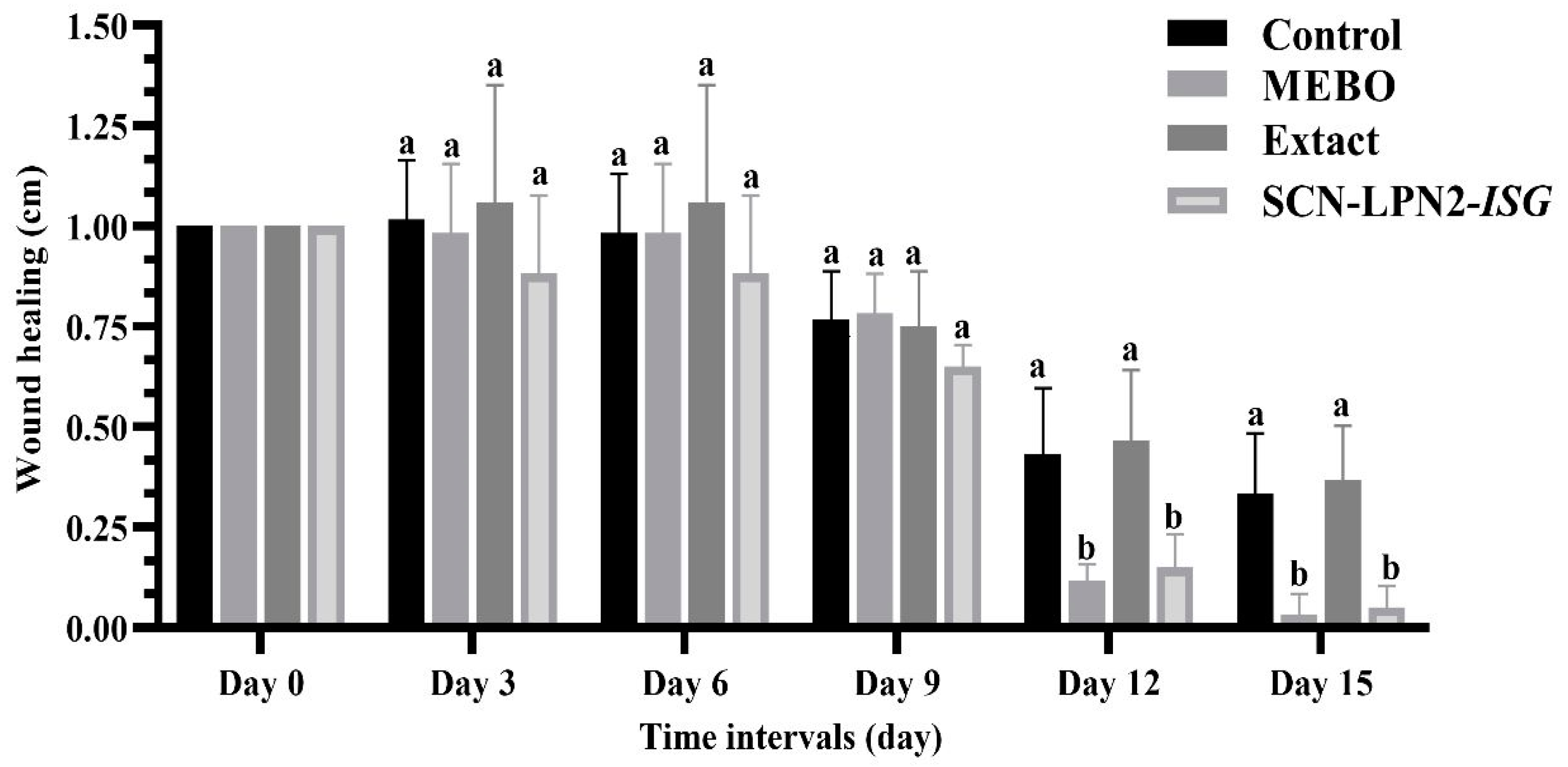

| Experimental Group Wounds | Wound Healing (%) | ||||
|---|---|---|---|---|---|
| Day 3 | Day 6 | Day 9 | Day 12 | Day 15 | |
| Control (untreated) | −1.666 | 1.666 | 13.333 | 56.666 | 66.666 |
| MEBO-treated group | 1.666 | 1.666 | 21.666 | 88 | 98 |
| Extract-treated group | −5.833 | −5.833 | 25 | 48 | 60 |
| SCN-LPN2-ISG group | 11.666 | 11.666 | 35 | 75 | 90 |
| Compound | GSK-3 Protein (PDB: 1Q5K) | MMP-1 Protein (PDB: 1HFC) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Free Binding of Energy (Kcal/mol) | H-Bond | Free Binding of Energy (Kcal/mol) | H-Bond | ||||||
| No. of H-Bonds | Amino Acid | Length Å | No. of H-Bonds | Amino Acid | Length Å | ||||
| Co-crystallized ligand | N-(4-Methoxybenzyl)-N’-(5-nitro-1,3-thiazol-2-yl)urea (TMU) | −7.1 | 4 | PRO136 | 3.01 | ----------------------------------------- | |||
| VAL135 | 3.20 | ||||||||
| VAL135 | 2.52 | ||||||||
| VAL135 | 3.25 | ||||||||
| Methylamino-phenylalanyl-leucyl-hydroxamic acid (PLH) | ------------------------------------- | −7.2 | 3 | LEU181 | 3.17 | ||||
| ASN180 | 3.14 | ||||||||
| TYR240 | 2.87 | ||||||||
| Nephthea sp. identified metabolites | (1) 2-Hydroxydocosanoic acid | −5.5 | − | − | − | −4.5 | 1 | ASN180 | 2.94 |
| (2) Armatin F | −6.9 | 1 | GLN185 | 3.05 | −6.0 | 1 | SER172 | 2.97 | |
| (3) Chabrolobenzoquinone B | −7.6 | 2 | ARG144 | 3.11 | −6.4 | 3 | TYR240 | 2.84 | |
| ARG144 | 3.17 | LEU181 | 2.97 | ||||||
| LEU182 | 3.05 | ||||||||
| (4) Chabrolohydroxybenzoquinone F (13) | −8.2 | 1 | ASN186 | 2.08 | −7.4 | 3 | GLY179 | 3.04 | |
| GLY179 | 2.39 | ||||||||
| LEU181 | 2.86 | ||||||||
| (5) Chabrolosteroid C | −6.9 | 2 | VAL135 | 3.37 | −7.1 | 3 | GLY179 | 3.27 | |
| ARG144 | 3.15 | LEU181 | 3.07 | ||||||
| ALA182 | 3.08 | ||||||||
| (6) Chabrolosteroid E | −9.6 | 1 | GLN185 | 2.52 | −5.7 | 2 | SER172 | 3.20 | |
| LEU181 | 3.35 | ||||||||
| (7) Chabrolosteroid G | −7.5 | 1 | CYS199 | 3.47 | −7.1 | 5 | GLY179 | 2.44 | |
| ELU181 | 3.07 | ||||||||
| LEU181 | 2.89 | ||||||||
| ALA182 | 2.81 | ||||||||
| ALA182 | 2.03 | ||||||||
| (8) Chabrolosteroid H | −7.6 | 1 | ILE62 | 2.00 | −6.6 | 1 | ASN180 | 2.88 | |
| (9) Cholest-1-ene-3,22-dione | −7.0 | - | - | - | −7.0 | - | |||
| (10) Erectasteroid H | −7.2 | 1 | ARG141 | 3.39 | −6.8 | - | |||
| (11) Ergosta-1,4,24(28)-trien-3-one | −7.2 | 1 | TYR134 | 3.22 | −7.1 | 1 | SER172 | 2.95 | |
| (12) Isogosterone B (9) | −6.0 | 1 | ARG141 | 3.27 | −7.8 | 2 | ALA182 | 3.29 | |
| LEU181 | 2.83 | ||||||||
| (13) Ketochabrolic acid | −6.8 | 1 | VAL135 | 3.09 | −6.7 | 2 | ALA187 | 2.32 | |
| TYR120 | 2.90 | ||||||||
| (14) Linoleic acid | −5.6 | 1 | CYS199 | 3.69 | −5.8 | 1 | ARG214 | 3.07 | |
| (15) Nanjiol B | −7.4 | 1 | LYS183 | 3.19 | −6.5 | 1 | TYR240 | 3.19 | |
| (16) Nebrosteroid B | −8.0 | 3 | VAL135 | 2.83 | −5.7 | 2 | ASN180 | 2.86 | |
| GLN185 | 1.72 | ASN180 | 2.94 | ||||||
| GLN185 | 2.69 | ||||||||
| (17) Nephalsterol C | −7.0 | 1 | LYS85 | 2.86 | −6.7 | 1 | ALA184 | 2.40 | |
| (18) Nephthenol | −7.4 | 1 | CYS199 | 3.68 | −6.2 | 1 | TYR240 | 3.21 | |
| (19) Nephtixamide B | −5.8 | 1 | VAL135 | 3.16 | −4.9 | 2 | SER172 | 3.30 | |
| SER172 | 3.01 | ||||||||
| (20) Orientalol C | −7.3 | - | - | - | −5.6 | 1 | TYR240 | 2.87 | |
| (21) Pacificin G | −7.9 | 3 | VAL135 | 2.98 | −6.1 | 3 | HIS183 | 3.04 | |
| GLN185 | 3.03 | LEU181 | 2.95 | ||||||
| GLN185 | 2.91 | ALA182 | 3.19 | ||||||
| (22) Arachidic acid | −5.5 | 1 | TYR134 | 3.02 | −5.1 | 2 | ARG214 | 3.11 | |
| ARG214 | 3.00 | ||||||||
| (23) Nervonic A | −6.0 | - | - | - | −5.4 | 2 | ARG214 | 3.01 | |
| ARG214 | 2.93 | ||||||||
| (24) Bis(2-ethylhexyl)phthalate | −6.4 | 1 | CYS199 | 3.80 | −5.9 | 1 | ASN180 | 2.91 | |
| (25) Cembrene A | −9.4 | - | - | - | −7.0 | - | - | - | |
| (26) Oleic acid | −5.8 | 1 | GLY68 | 2.12 | −5.7 | - | - | - | |
| (27) Pregn-20-ene-3,19-diol; (3ß,5a)-form; 3-O-(3-O-acetyl-α-L-fucopyranoside) | −7.3 | −3 | TYR134 | 2.99 | −7.6 | - | - | - | |
| ARG141 | 3.18 | ||||||||
| THR138 | 2.93 | ||||||||
| Parameter | Compounds | Comment | |
|---|---|---|---|
| Chabrolohydroxybenzoquinone F (13) | Isogosterone B (9) | ||
| MW | 428.290 | 502.290 | No. of hydrogen atoms. Optimal: 100–600 |
| nHA | 4 | 7 | No. of hydrogen bond acceptors. Optimal: 0–12 |
| nHD | 4 | 2 | No. of hydrogen bond donors. Optimal: 0–7 |
| TPSA | 80.920 | 110.130 | Topological polar surface area. Optimal: 0–140 |
| nRot | 11 | 7 | No. of rotatable bonds. Optimal: 0–11 |
| nRing | 1 | 4 | No. of rings. Optimal: 0–6 |
| MaxRing | 6 | 14 | No. of atoms in the biggest ring. Optimal: 0–18 |
| nHet | 4 | 7 | No. of heteroatoms. Optimal: 1–15 |
| fChar | 0 | 0 | Formal charge. Optimal: −4 |
| nRig | 10 | 24 | No. of rigid bonds. Optimal: 0–30 |
| Flex | 1.100 | 0.292 | Flexibility = nRot/nRig |
| nStereo | 2 | 8 | No. of stereocenters. Optimal: ≤2 |
| LogS | −3.650 | −4.500 | Log of aqueous solubility. Optimal: −4–0.5 log mol/L |
| LogD | 4.452 | 3.036 | Log of octanol/water partition coefficient. Optimal: 0–3 |
| LogP | 5.634 | 3.217 | LogP at physiological pH 7.4. Optimal: 1–3 |
| Formulation | Organic Phase | Aqueous Phases | Evaluation Parameters | |||||
|---|---|---|---|---|---|---|---|---|
| SCN | Methanol | Pectin Concentration | Pectin: CaCl2 Ratio | E.E. * | PS * | PDI * | ZP * | |
| (mg) | (mL) | (% w/v) | (%) | (nm) | (mV) | |||
| SCN-LPN1 | 20 | 3 | 0.5 | 1:1 | 92.84 ± 2.20 | 241.5 ± 17.5 | 0.290 ± 0.22 | (−5.34) ± 1.20 |
| SCN-LPN2 | 20 | 3 | 0.5 | 1:2 | 98.55 ± 2.20 | 288.2 ± 25.66 | 0.381 ± 0.12 | (−16.6) ± 2.4 |
| SCN-LPN3 | 20 | 3 | 0.5 | 1:3 | 97.55 ± 1.57 | 269.5 ± 34.7 | 0.523 ± 0.12 | (−5.54) ± 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, N.H.; El-Hawary, S.S.; Emam, M.; Rabeh, M.A.; Tantawy, M.A.; Seif, M.; Abd-Elal, R.M.A.; Bringmann, G.; Abdelmohsen, U.R.; Selim, N.M. Pectin Nanoparticle-Loaded Soft Coral Nephthea sp. Extract as In Situ Gel Enhances Chronic Wound Healing: In Vitro, In Vivo, and In Silico Studies. Pharmaceuticals 2023, 16, 957. https://doi.org/10.3390/ph16070957
Hassan NH, El-Hawary SS, Emam M, Rabeh MA, Tantawy MA, Seif M, Abd-Elal RMA, Bringmann G, Abdelmohsen UR, Selim NM. Pectin Nanoparticle-Loaded Soft Coral Nephthea sp. Extract as In Situ Gel Enhances Chronic Wound Healing: In Vitro, In Vivo, and In Silico Studies. Pharmaceuticals. 2023; 16(7):957. https://doi.org/10.3390/ph16070957
Chicago/Turabian StyleHassan, Nevine H., Seham S. El-Hawary, Mahmoud Emam, Mohamed A. Rabeh, Mohamed A. Tantawy, Mohamed Seif, Radwa M. A. Abd-Elal, Gerhard Bringmann, Usama Ramadan Abdelmohsen, and Nabil M. Selim. 2023. "Pectin Nanoparticle-Loaded Soft Coral Nephthea sp. Extract as In Situ Gel Enhances Chronic Wound Healing: In Vitro, In Vivo, and In Silico Studies" Pharmaceuticals 16, no. 7: 957. https://doi.org/10.3390/ph16070957
APA StyleHassan, N. H., El-Hawary, S. S., Emam, M., Rabeh, M. A., Tantawy, M. A., Seif, M., Abd-Elal, R. M. A., Bringmann, G., Abdelmohsen, U. R., & Selim, N. M. (2023). Pectin Nanoparticle-Loaded Soft Coral Nephthea sp. Extract as In Situ Gel Enhances Chronic Wound Healing: In Vitro, In Vivo, and In Silico Studies. Pharmaceuticals, 16(7), 957. https://doi.org/10.3390/ph16070957







