1. Introduction
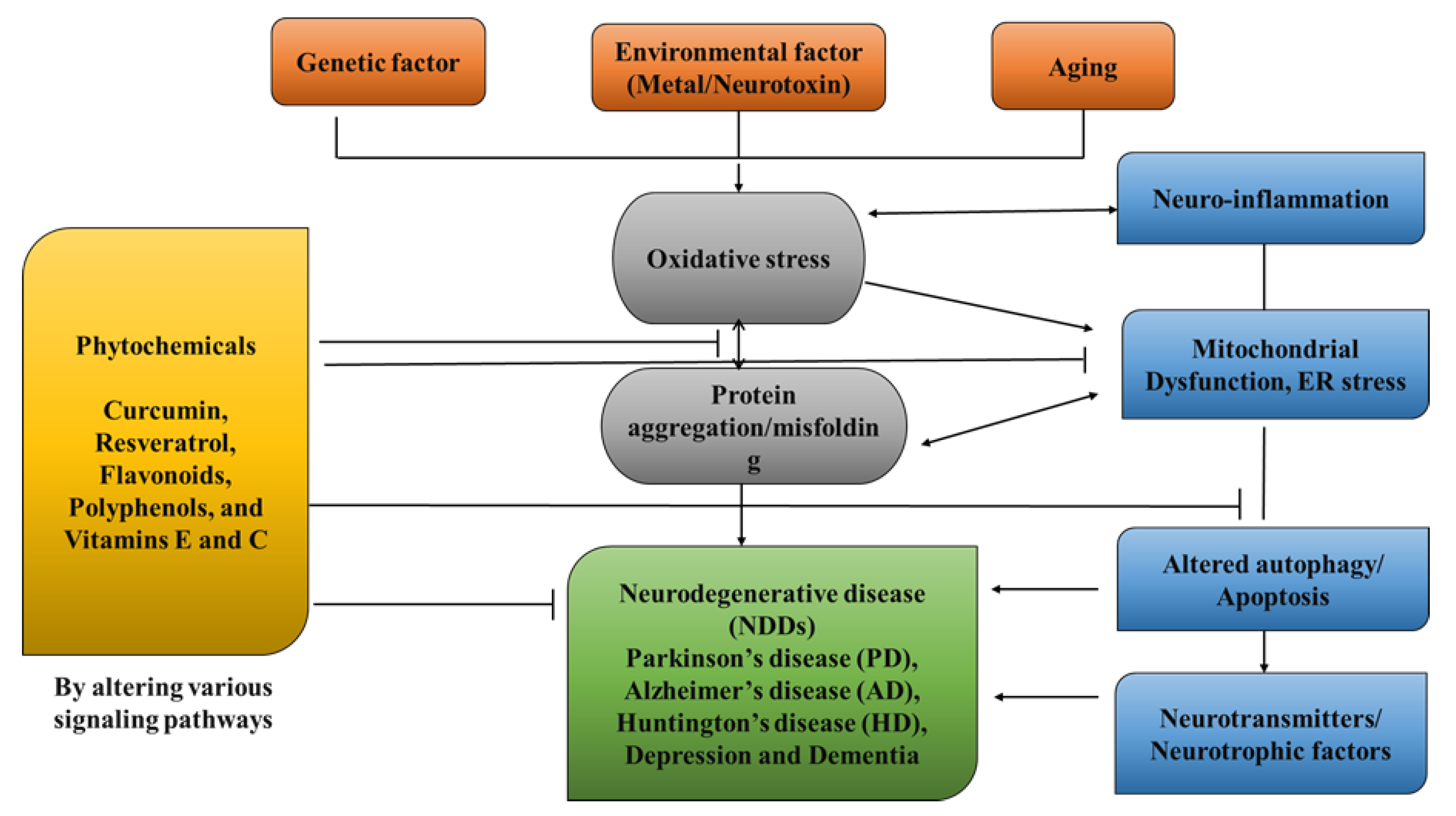
NDDs (neurodegenerative disorders) are a collection of disorders with various clinical implications and etiology. ALS (amyotrophic lateral sclerosis), cerebellar illness, Huntington’s chorea, Parkinson’s disease, Alzheimer’s, dementia, and schizophrenia are all examples of NDDs [
1,
2,
3,
4]. Age groups, hereditary diseases, non-enzymatic antioxidants, excitotoxicity, cytoskeletal abnormalities, autoimmunity, asymmetry, toxicity, elevated blood pressure, oxidative stress, and peripheral vascular diseases are subject to both experimental and epidemiological research. Some of the risk variables that have been discovered through the clinical manifestations of NDDs are associated with free radical toxicity, radical-mediated alteration, oxidase dysfunction, and endoplasmic reticulum stress caused by perinatal genetic abnormalities. The most important common symptoms are disturbances in balance, breathing, movement, reflexes, motor skills, or cardiac activity. Antioxidants such as flavonoids, polyphenols, and vitamins E and C can help to prevent these symptoms [
5,
6,
7]. Antioxidants have a great impact on human health, as they can fight free radicals and, thus, halt the aging process and decrease the effects of oxidative damage caused by, for example, an unbalanced diet [
8,
9]. As a result, the long-term risk of neurodegenerative diseases is reduced (
Figure 1). Although they can be treated, there is currently no cure for neurodegenerative diseases. Treatment of this disease relieves the symptoms in order to preserve the quality of life. Natural antioxidants, such as polyphenols, are becoming more popular because they can be obtained from foods and supplements and offer a range of health benefits [
10]. Although it affects both males and females with increase in their age but males tend to be affected more frequently [
11]. It is considered an international disease that does not distinguish between social class or race. The disease is estimated to affect 1% of people over the age of 65 worldwide, accounting for up to two-thirds of all people with movement disorders [
12].
As the population ages, PD has become more prevalent, with those over 85 years old reaching 2.6%. The non-motor symptoms that patients with PD encounter include pain, exhaustion, autonomic dysfunction, changed mood, sleep disturbance, and cognitive abnormalities [
13]. PD is referred to asa synucleinopathy because Lewy bodies, a crucial clinical characteristic of the disease, accumulate due to the misfolding of β-synuclein as a major feature. Furthermore, β-synuclein appears to be connected to both idiopathic and inherited forms of Parkinson’s disease and has a special role in the disease’s pathogenesis. It is worth noting that β-synuclein buildup has been closely linked to posttranslational modifications, systemic inflammation, oxidative stress, mitochondrial biogenesis, changed mitochondrial physical properties, synapse dysfunction, glycolipids, ER stress, and metal complexes. The overproduction of ROS and years of age breakdown the antioxidant defense system and increase oxidative stress in certainbrain areas, which can contribute to the misfolding of β-synuclein being the catalyst for the aging process in PD [
14,
15]. Although it is not a cure, levodopa has emerged as an efficacious drug for PD’s first motor symptoms. Despite the fact that stiffness and bradykinesia respond the best, tremors may only be somewhat reduced by levodopa. Other symptoms, such as balance issues, may worsen. L-dopa, however, cannot be used to treat Lewy disease, non-motor symptoms, or neuronal loss [
16]. Patients require greater L-dopa doses over time, which is accompanied by a rise in side effects such as dyskinesias. Amantadine, an antiviral medication, appears to alleviate motor problems as well. Medicines based on dopamine agonists are also administered to address a range of neuromotor symptoms that are associated with disease progression. The long-term use of conventional PD medications can lead to adverse effects, such as dyskinesias and motor fluctuations (
Table 1). As a result, novel therapeutic techniques used to prevent neurodegeneration, non-motor symptoms, Lewy disease accumulation, or synuclein aggregation in the brain are required [
17,
18].
Complementary Therapies for PD
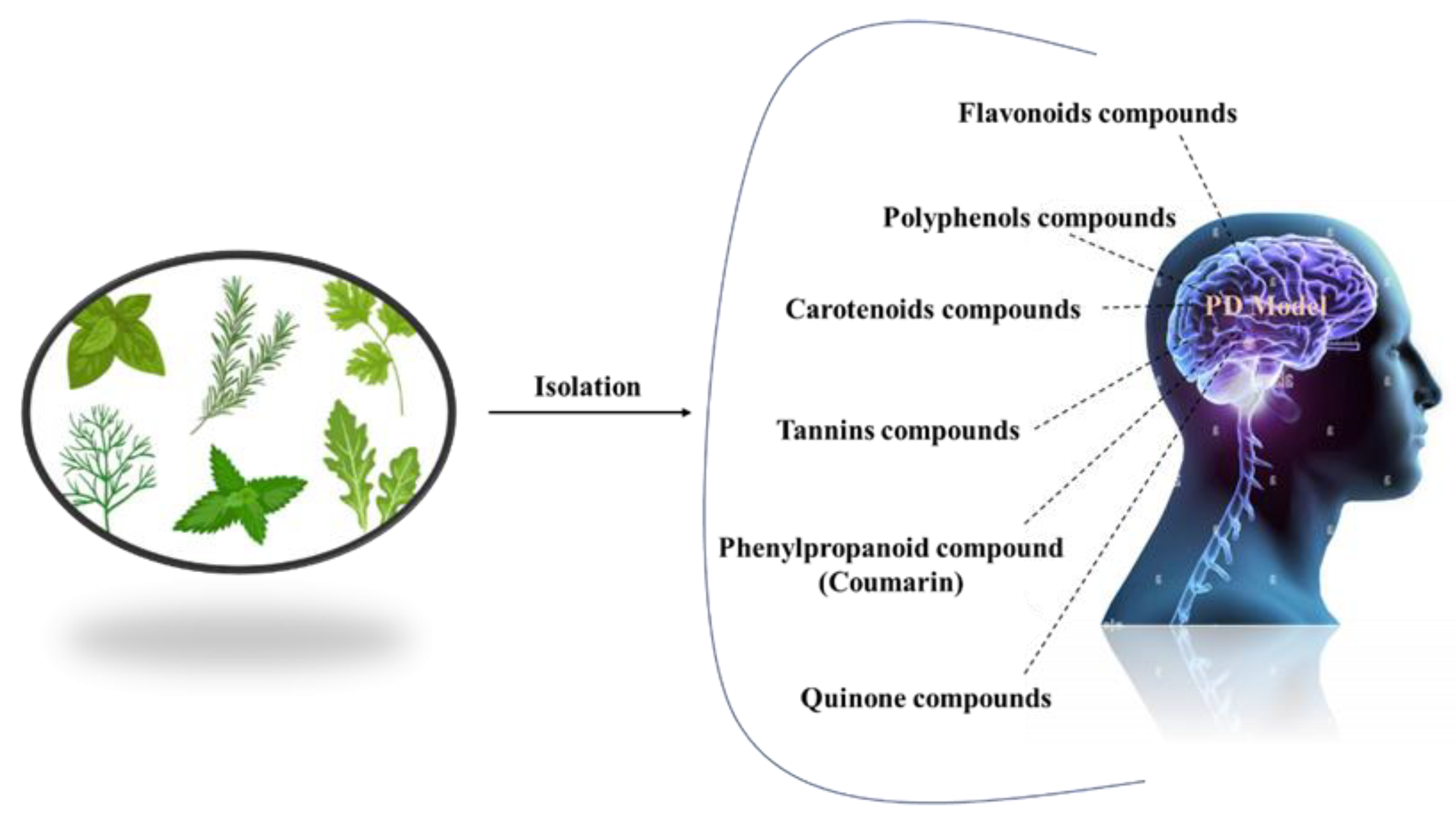
Complementary therapies and phytonutrients derived from plant sources have been proposed as treatments for Parkinson’s disease [
24]. Numerous natural phytochemicals have emerged as therapeutically interesting compounds, drug entities, and phytochemicals for the treatment of inflammatory disorders [
25]. Additionally, numerous pharmacological studies have shown that phytochemicals are useful in treating neurodegenerative diseases (NDDs), depression, and dementia [
26,
27,
28,
29,
30]. Physiologically active phytochemicals are important therapeutically because they serve as the antioxidant defense system’s primary and secondary metabolites, which protect against a variety of stress-related disorders and clinical symptoms [
31]. These phytochemicals’ positive and therapeutic effects include antioxidant capacity, pathogen prevention, immune system activation, and nutritional support for healthy living cells [
32]. Botanical substances, with their active phytonutrients, efficiently salvage oxygen-based free radicals, influencing the antioxidative defense system and contributing to major cognitive impairments such as dyskinesias, residual tremors, muscle stiffness, and instability [
33]. This review holds prime importance, as it addresses the treatment gaps in PD and explores the potential of natural antioxidants as a therapeutic approach. By summarizing the current literature on natural antioxidants and their effects on PD, this review aims to provide a comprehensive overview that can guide further research and clinical trials. Understanding the role of natural antioxidants in PD treatment may lead to the development of novel therapeutic strategies that can modify the disease course, mitigate the symptoms, and improve the quality of life for individuals living with PD. In this review, we address the natural antioxidants that can be used to treat Parkinson’s disease, as well as their mode of action (
Figure 2).
Pathogenesis
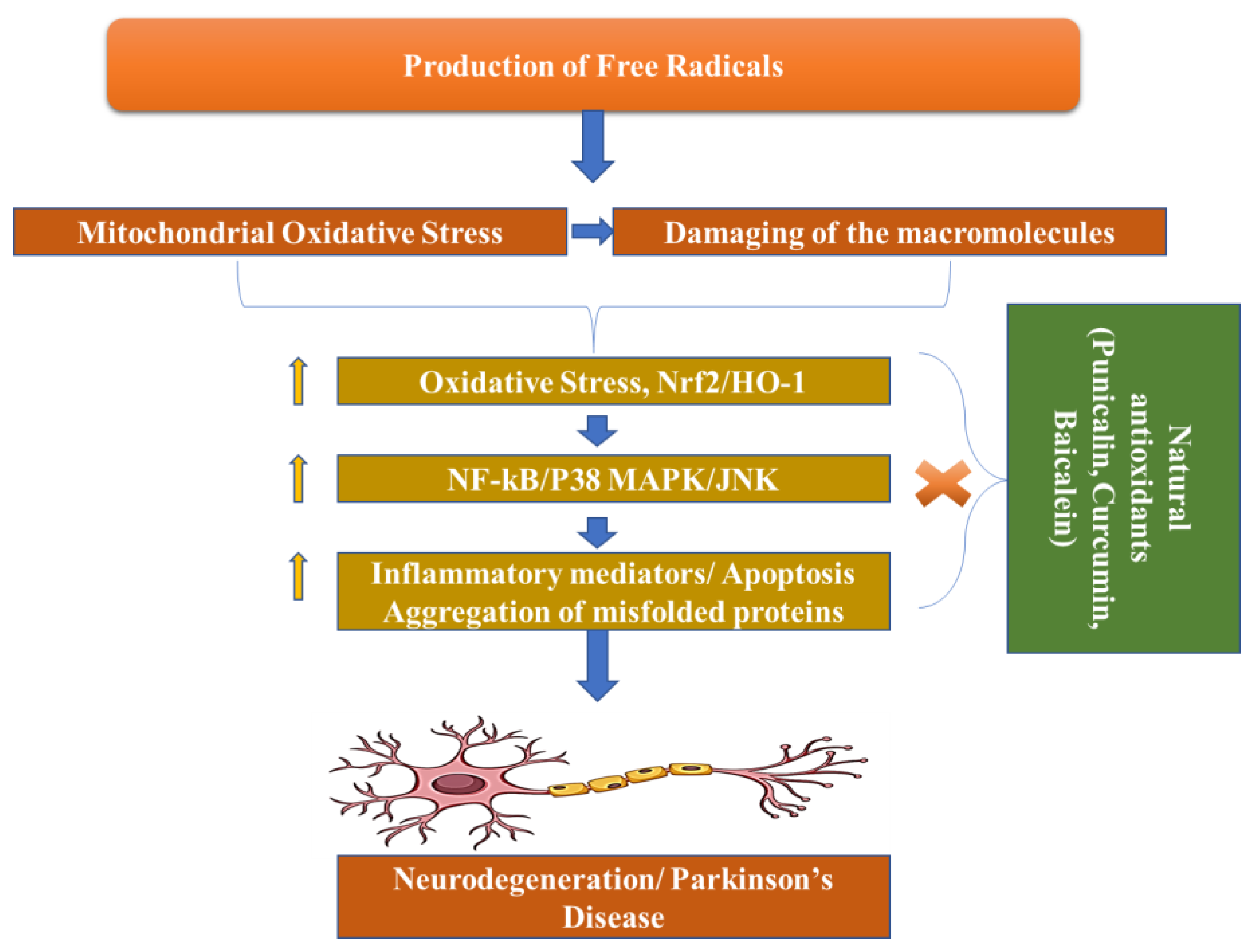
Dopamine levels in the caudal nucleus, striatum, and expression medium fall as a result of the death of dopaminergic neurons in the substantia nigra pars compacta, which is associated with Parkinson’s disease. The progressive loss of cholinergic neurons is the most significant pathogenic discovery in Parkinson’s disease sufferers’ brains. For this reason, with the loss of these neurons, the dopamine levels fall. When 50–60% of the dopamine pathway is shut down and striatal dopamine levels are lowered by 80–85%, symptoms of the disease arise. However, Parkinson’s disease may have multiple etiological factors, including mitochondrial dysfunction, neuroinflammation, oxidative stress, and the formation of protein kinases called Lewy bodies in the cytosol (
Figure 3) [
34].
Phytochemicals and Parkinson’s Disease
Neurodegenerative diseases, including Parkinson’s disease, contributes to N increase in nicotinamide adenine dinucleotide phosphate oxidase and intracellular RNS, and, thus, result in cell damage due to oxidative stress [
35].
Free radicals also damage cell membranes by oxidizing proteins, lipids, and nucleic acids, causing peptide cross-links [
36]. The antioxidant and chelating effects of flavonoids and terpenoids are believed to be accountable for their therapeutic effects. Terpenoids, phenols, and flavonoids show important neuroprotective effects, due to their oxidative capacity [
37]. By releasing extra protons, flavonoids scavenge superoxide, hydroxyl, and peroxyl radicals by donating an extra proton. This prevents ROS generation by the formation of complex structures containing dihydroxy groups, copper, multiple ions of transition metals, and iron [
38].
Glutathione reductase, septo-optic dysplasia, and glutathione-S-transferase (GST) are the polyphenols that activate antioxidant enzymes. Polyphenols can raise the antioxidant levels by enhancing cell-signaling pathways through these enzymes. By generating unsaturated carbon–carbon double bonds with lipid bilayers, these phenolic acids inhibit lipid peroxidation and, thus, work as a powerful therapeutic and preventative agent [
39].
The lack of oxygenation of the rings is assumed to be responsible for chrysin’s chemical capabilities, which include anti-inflammatory and antioxidant potential [
40]. Diverse structures of flavones have been discovered to increase the capacity of antioxidant enzymes and also work in inhibiting COX-2, which is a pro-inflammatory mediator. Experiments with xanthine oxidase demonstrate that chrysin dramatically reduces ROS production. It is worth noting that rodent models require polyphenol dosages ranging from 10 µM to 100 µM to exercise their putative antioxidant and anti-inflammatory properties [
41].
Neuroprotective Dietary Antioxidants
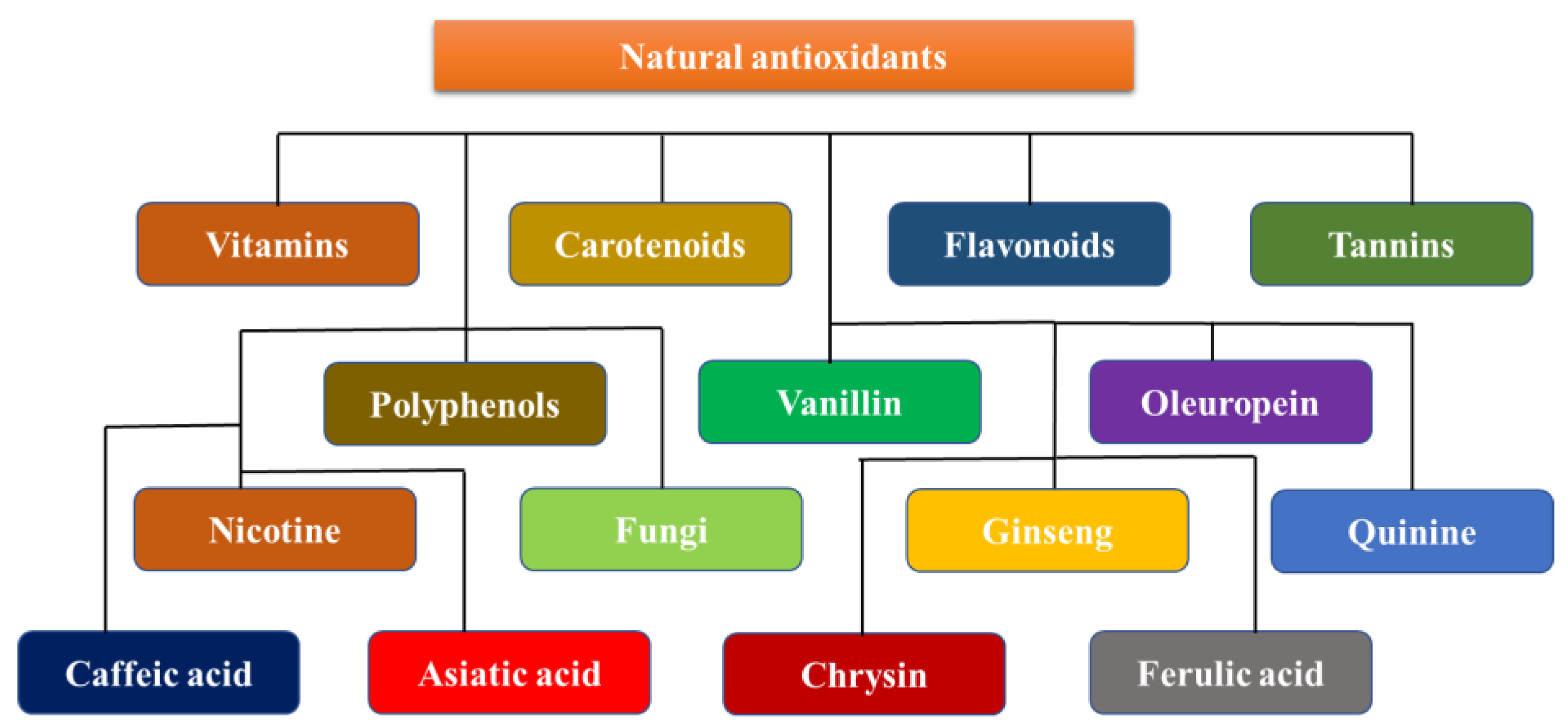
Antioxidants are neuroprotective compounds that protect or repair cellular components from oxidative damage. They efficiently halt neuronal lipid, protein, and DNA breakdown. Vitamins, flavonoids, carotenoids, tannins, and polyphenols are all antioxidants [
42]. Oxidative stress damage is a prominent manifestation of neurodegenerative disorders. However, few efficacious agents have been found for clinical use, and even fewer have been successful because of their toxicity and cancer risks [
43]. Evidence suggests that neuroprotection is a potential pharmaceutical target for neurodegenerative diseases, suggesting that it is possible. The use of relatively harmless antioxidant molecules found in food as remedies for many ailments, on the other hand, is appealing; however, it is limited by the difficulty of creating positive feedback in the brain. Polyphenols, a class of antioxidants, have been shown to have neuroprotective properties. Many biological processes, such as interactions with metal complexes, the scavenging of free radicals, changes in the activity of different enzymes, and impacts on intracellular signaling pathways and gene expression are all biological processes that contribute to this protection. A diet that is high in antioxidants is essential to prevent many diseases [
44]. A reduction in the incidence of stroke, neurological disease, heart disease, cancer, and high blood pressure has been associated with these chemicals, which are primarily present in vegetables and fruit. The body’s natural antioxidant system needs help from the exogenous antioxidants that are found in fruits, nuts, vegetables, and other foods. Exogenous antioxidants are often classified as vitamins and non-vitamins. This section reviews the specific properties and roles of the key exogenous antioxidants in neuroprotection (
Figure 4) [
45].
Vitamin E
Four tocopherols, trocochromanols or four tocotrienols, and major fat-soluble antioxidants are collectively referred to as vitamin E [
46]. The human body cannot produce this vitamin itself, therefore, it must be obtained from food (
Table 2).
Each vitamin E isoform has complex antioxidant properties, and it is currently unknown how these effects work. It is suspected that they work by stopping the chain process of lipid peroxidation, therefore, important cellular components can be protected [
47]. Vitamin E bioavailability varies with many factors, including the dietary matrix, genetics, and metabolic fat, and ranges from 10 to 33%. Preclinical studies in Parkinson’s disease have shown conflicting results regarding the efficacy of vitamin E as a disease-modifying agent [
48]. Vitamin E deficiency increased substantia nigra MPTP toxicity, while partially protecting against neurotransmitter and metabolite deficiencies caused by striatal MPTP. Dopamine degradation could not be stopped, even by pre-MPTP injection therapy with β-tocopherol. However, substantial amounts of vitamin E may partially protect dopaminergic neurons from MPTP-mediated toxicity [
37]. During the Deprenyl and Tocopherol antioxidative treatment of Parkinsonism study, patients with early or untreated Parkinson’s disease were given deprenyl (10 micrograms daily) and tocopherol as a treatment. The results of the study demonstrated that the monoamine oxidase inhibitor (deprenyl) slowed the progression of the disorders.However, the modest improvement in motor function gradually diminished when deprenyl was discontinued [
49]. In individuals with Parkinson’s disease with a recent diagnosis, vitamin C and alpha-tocopherol supplementation delayed the initiation of levodopa therapy by 25 years.
Table 2.
Some other potent activities of Vitamin E.
Table 2.
Some other potent activities of Vitamin E.
| Compound Name | Disease | Impact | Ref. |
|---|
| Vitamin E | Alzheimer’s disease (AD) | Effective against AD when consumed with vitamin C in the earlier phase of AD. | [50] |
| Cell membrane dysfunction | Effective in the protection of the cell membrane | [51] |
| Cardiovascular disorder | Prevents the clot formation and buildup of bad cholesterol | [52] |
Ascorbic Acid
Ascorbate, or ascorbic acid, is a vitamin that is soluble in water that is necessary for catecholamine production and other enzyme processes. Ascorbic acid shows its antioxidant capabilities by reduction inO2, alkoxyl (RO), hydroxyl (HO), peroxyl (ROO), and other free radicals in body. When vitamin E is oxidized while scavenging free radicals from the lipid membranes, the radical tocopheroxyl is formed, which interacts with vitamin C in order to replenish vitamin E. Vitamin C absorption, distribution, and metabolism are more complex than those of most lighter weight molecular compounds [
53]. A family of sodium-dependent vitamin C transporters contains the key proteins that are involved in substance distribution and tissue absorption. The brain contains one of the highest concentrations of vitamin C in the body. Concentrations vary per brain region, with the motor cortex having the lowest concentration [
54]. Different transporters are present in the brain for reduced and oxidized forms of vitamin C (SVCT2 or GLUT family). Individuals whosuffer from neurodegenerative conditions such as Parkinson’s disease (PD) exhibit low plasma exogenous antioxidant concentrations (including vitamin C). A lack of vitamin C has also been related to a higher incidence of Parkinson’s disease. It has been proposed that the level of vitamin C in lymphocytes could serve as a diagnostic indicator of Parkinson’s disease progression. Vitamin C supplementation is one of the medicinal methods used to cure Parkinson’s disease [
55] (
Table 3).
Flavonoids
It is the largest polyphenol class and has a backbone that is made up of one heterocyclic ring and two phenyl rings. The side chain configuration and quantity of hydroxyl groups influence the biological features, such as antioxidant activity and the modification of enzyme activity [
40].
By reacting with free radicals and serving as metal catalysts, flavonoids act as radical chain terminators. In order to inhibit oxidation, phenolic antioxidants (PhOH) provide hydrogen atoms to the free radicals. There are several different types of flavonoids present in legumes, plants, and fruits (obtained from celery, citrus peels, chamomile, paprika, ginkgo biloba, parsley leaves, and mint). Crops, flowers, and leaves contain flavones as glucosides [
59].
Kaempferol, quercetin, ferulic acid, and myricetin are examples of flavonols. In 6-OHDA-treated PC12 cells, 20 µM of quercetin boosted the mitochondrial activity, decreased the oxidative stress, and decreased β-synuclein protein synthesis [
60]. Furthermore, oral quercetin administration improved the motor performance in arat model by lowering the mitochondrial oxidative stress, β-synuclein aggregation formation, and neuronal cell death [
46].
Chalcone, isoflavonoids (such as daidzein and genistein), water-soluble anthocyanidin pigments (such as delphinidin, pelargonidin, peonidin, and cyanidin), and aromatic colorless flavanones (such as hesperetin, naringin, and hesperidin) are the other class of flavonoids that can be useful in the treatment of PD (
Table 4) [
61].
Green Tea Polyphenols
Green tea polyphenols, notably EGCG, exhibit a broad range of pharmacological properties, involving antimutagenic and anticarcinogenic properties. Green tea polyphenols’ strong antioxidant capacity is responsible for these positive effects. They are potent antioxidants that combat free radicals such as lipid radicals, phenolic radicals, superoxide anion radicals, and hydroxide ions [
65]. When they are taken orally, the flavonoids and green tea’s polyphenols have been demonstrated to prevent aging. Tea catechins (TC) are commonly thought to be radical scavengers, although other components of tea have numerous applications, possibly protecting against 6-OHDP-induced apoptosis. In MTT assays, 6-OHDA treatment decreased the cell viability in a concentration-dependent manner, whereas flow cytometry, fluorescence microscopy, and DNA fragmentation confirmed apoptosis in PC12 cells. TC substantially reduces PC12 cell death. EGC and EC had lower efficacy, but EGCG and ECG were more efficacious compared to TC. The protective effect increased from 50 µM to 400 µM, and, at these concentrations, EGCG outperformed the green tea polyphenols. They inhibit at 83.2% and 84.9%, respectively, while EGCG inhibitory concentration is at 88.4% and 90.2% [
54]. According to flow cytometry data, 200–400 µM of green tea polyphenols and EGCG dramatically decrease the number of apoptotic cells. Notably, EGCG-protected PC12 cells abolished nuclear changes indicative of apoptosis [
66] (
Table 5).
Isoflavones
Genistein, the most active soy isoflavone, has estrogen receptor affinity, antioxidant characteristics, increased cytoplasmic glutathione peroxidase, PTK inhibition, and other physiological effects. Soy isoflavones have protective efficacy against a variety of illnesses, including atherosclerosis, the consequences of estrogen deficiency in menopause, and hormone-dependent breast and prostate cancer (
Table 6) [
70]. It has been found that it exhibits neuroprotection by antagonizing the toxic effect of the critical Aβ protein [
71]. As a result, it can be used for PD management.
Nicotine
In hippocampus cultures, nicotine significantly inhibits apoptosis induced by Ab and increases caspase activity. Nicotine reduced the number of free radicals accumulated by the Ab. The cholinergic antagonist mecamylamine decreased nicotine’s capacity to protect cells from Ab-induced caspase-3 activation and ROS buildup [
75]. According to research, nicotine receptors play a major role in nicotine’s protective potential. The findings imply that nicotine may assist in averting the emergence of neurodegenerative diseases such as PD and Alzheimer’s. Although smoking has been linked to a lower incidence of Parkinson’s disease, the specific mechanism for this is unknown. Nicotine has been discovered to suppress cytochrome C release from intact mitochondria, as well as the high-amplitude mitochondrial swelling that is caused by calcium and
N-methyl-4-phenylpyridine (MPPt). The redox state within mitochondria was also maintained by nicotine, which was likelyas a result of decreased mitochondrial permeability. Nicotine did not prevent the reduction in mitochondrial membrane potential by MPPt or calcium, but decreased electron leakage at sites of respiratory chain complex I and 6-OHDA, causing cytochrome C release and mitochondrial enlargement in SH-SY5Y cells [
66].
This suggests that nicotine has receptor-independent neuroprotective effects. These findings imply that, when evaluating nicotine’s neuroprotective benefits, it is critical to consider both its interaction with the mitochondrial respiratory chain and its antioxidant activity. It is not entirely clear how nicotine attenuates β-amyloidosis and prevents PD.
In one study, after nicotine treatment, pure zincand copper metal concentrations in amyloid plaques and neuropils dropped considerably. The density of zinc and copper distribution in the hippocampus CA1 area is likewise lowered after nicotine administration. Researchers examined how nicotine affected the homeostasis of metals in SH-SY5Y cells that were overexpressing the Swedish variant of human APP (APPsw). These effects are not dependent on nicotinic acetylcholine receptor activation and are mediated by a decrease in intracellular copper concentrations with nicotine therapy, as well as a reduction in Ab-induced neurotoxicity, helped by copper addition [
76] (
Table 7).
Carotenoids
There are about 850 fat-soluble tetraterpenes in the class of carotenoids that are produced by photosynthetic plants, algae, and bacteria. They support plants in both photosynthesis and photo-defense. In addition to fruits and vegetables, such as beets, cherries, melons, and pumpkins, foods such as microalgae, fish, and shrimp also contain yellow, orange, and red pigments called carotenoids [
80]. Serum lycopene, β, and α-carotene levels are considerably lower in Parkinson’s disease patients, and lower serum carotenoid levels are also linked to motor impairment. Carotenoids act as antioxidants by eliminating ROS via chemical and physical reactions; for example, β-carotene absorbs thermal energy from singlet oxygen and/or peroxynitrite, therefore, inhibiting tyrosine nitration. Carotenoids protect neurons against neurodegenerative diseases in multiple ways, including ROS quenching, the activation of antioxidant enzyme systems, and anti-neuroinflammatory effects [
81] (
Table 8).
Resveratrol
Resveratrol is a phenolic compound chemical that is accessible in several plants, including berries and grapes. In animal studies of Parkinson’s disease, they help with movement deficits, oxidative stress, and the elimination of TH neurons [
85]. Resveratrol inhibits chromatin condensation and mitochondrial expansion, while decreasing COX-2 and TNF gene output [
86]. This phenol has a short oral survival period due to its low water solubility and short half-life (10 min). Furthermore, resveratrol derivatives triethylsilyl and ditriisopropylsilyl show superior action in invitro anti-inflammatory and neuroprotective properties when compared to pure resveratrol. Resveratrol is employed because it has shown increased biocompatibility and neuroprotective properties in zebrafish embryos. Also selected from the prodrugs, this chemical reduced clinical scores and the severity of motor impairment in a 3-nitropropionic acid PD mousemodel of experimental autoimmune encephalomyelitis [
87] (
Table 9).
Tannins
These phenolic compounds have enough hydroxyl and carboxyl groups and high molecular weight to form a complex with biomolecules (0.5–20 kDa). They are also water-soluble. Tannins are unique in their ability to precipitate and link to alkaloids, proteins, and amino acids.There are several methods for removing tannins, however, acetone solvent extraction is the most effective for reducing tannin–protein complexes [
91]. Tannic acid was chosen as the most optimistic molecule to minimize α-synuclein fibrosis without significantly raising toxicity levels [
92] (
Table 10).
Curcumin Polyphenol
Curcumin (10 µM) therapy also lowers oxidation-related protein changes such as carbonylation and nitrotyrosine production, which help to sustain dopaminergic cells. Curcumin, an antioxidant, effectively protects against oxidative-stress-induced mitochondrial damage [
96,
97]. In MPTP-induced mouse striatum, curcumin inhibits TH-positive cell death and DA depletion. In addition, it reduces inflammatory markers such as total nitrite, cytokines, and inducible nitric oxide synthase [
98] (
Table 11).
Herbs’ most prevalent chemical ingredients are ginkgolic acids, terpenoids, and flavonoids. Quercetin, isorhamnetin, and kaempferol are the most prevalent flavonoids in a registered standardized extract of ginkgo biloba (EGb 761) produced by solid–liquid extraction using aqueous acetone. Terpene lactones account for 6% of the extract (3.1% ginkgolides and 2.9% bilobalides) and other components (e.g., glucose and organic acids).
The long-term injection of EGb761 reduced dopaminergic nerve terminal degeneration caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in a Parkinson’s disease rat model (MPTP) [
102]. Before and after treatment, EGb761 has been shown to guard against MPTP-induced dopaminergic neurotoxicity. Furthermore, EGb761 reduced the neurotoxicity of levodopa in the Parkinson’s disease 6-hydroxydopamine (6-OHDA) model (PD). Monoamine oxidase, an enzyme that is involved in dopamine metabolism and the production of free radicals that damage nigrostriatal neurons, was either inhibited or lowered by EGb761 [
103] (
Table 12).
Pomegranate
Punica granatum, belonging to the Punica family, and the earliest known edible fruit, is endemic to Iran. It is high in polyphenolic chemicals. Pomegranate juice contains soluble polyphenolic components such as catechins, anthocyanins, ellagitannins, gallic acid, and ellagic acid. The anti-inflammatory, antiapoptotic, and antioxidant efficacy of the juice are supported by ellagic acid, punicalin, punicalagin, pedunculagin, gallic acid, glucose esters of ellagic acid, and their metabolites. Ellagitannins are primarily responsible for the neuroprotective properties [
107]. In a dose-dependent way, the oral pre-administration of pomegranate extract standardized to 40% ellagic acid shields adult rats against the neuronal damage that is brought on by cerebral ischemia-reperfusion brain injury. The neuroprotective antiapoptotic effect of the extract was in addition to oxidative stress-induced apoptosis, reduce levels of TNF and caspase-3-attributed nuclear factor NF-B p65, and increased interleukin-10 and brain ATP [
108] (
Table 13).
Baicalein
A substance called baicalein (Lamiaceae) is created from the dehydrated roots of
Scutellaria baicalensis. When it was tested for rotenone-induced neurotoxicity, baicalein decreased ROS generation, apoptosis, ATP deficit, and mitochondrial transmembrane breach PC12 cells [
112]. Baicalein treatment raises and maintains dopamine and 5-hydroxytryptamine levels in the basal ganglia. In Hela and SH-SY5Y cells, baicalein reduced α-synuclein oligomerization and aggregation [
113] (
Table 14).
Peganum harmara
Peganum harmara (Nitralaceae) reduced muscle stiffness, prevented the oxidation of brain proteins and lipids, and stopped the deterioration of dopaminergic neurons. The herb’s ability to reduce angiotensin II activity is thought to provide neuroprotective effects. The opaminergic neurons are protected and oxidative damage is reduced [
116] (
Table 15).
Carthamus tinctorius L. (Safflower)
Safflower (Asteraceae), which contains flavonoids, is widely used in China as a traditional remedy for diseases of the cerebrovascular system [
112]. In addition to increasing DA levels, DJ-1 and DA transporter expression were also improved. Safflower can reduce the reactive astrogliosis, overexpression, or aggregation of β-synuclein [
120] (
Table 16).
Pueraria lobata
Puerarin (legume family) has been found to inhibit ubiquitin-binding protein accumulation, proteasome malfunction, and the creation of other potentially hazardous proteins. Contrarily, puerarin lowers the ratio of caspase-3 activity to that of bcl-2/bax [
124]. Puerarin, is a drug that protects DA, and also safeguards tyrosine hydroxylase (TH)-positive neurons from the damage that is caused by 6-OHDA [
125] (
Table 17).
Ginseng
Rb1 and Rg1 ginsenosides are most likely the primary ginseng mediators (Araliaceae). In SNK-SH cells, MPTP-induced cell death was prevented by ginsenosides Rb1 and Rg1 (neuroblastoma cell line). By the upregulation of Bcl-2 and Bcl-xl, the downregulation of Bax and iNOS, and the inhibition of caspase-3 activation, Rg1 protects the cells from MPTP-induced apoptosis. Ginsenosides protect cells by increasing the antioxidant activity, lowering intracellular reactive oxygen species (ROS), maintaining complex I activity, and increasing intracellular adenosine triphosphate (ATP) [
129].
In mice that were given MPTP, Rg1 enhanced their movement and boosted dopaminergic neurons in the striatum and substantia nigra (SN). Increased ginsenoside Rb1 levels can also prevent α-synuclein polymerization and disintegrate fibrils [
130] (
Table 18).
Passionflower
Passionflower, or
Passiflora incarnata, contains glycosides, flavonoids, alkaloids, and phenolic chemicals. It has the potential to treat a variety of conditions such as anxiety, epilepsy, restlessness, and muscle spasms [
134]. Tacrine, a well-known animal model for Parkinson’s tremors, induces jaw movements that can be reduced by passionflower extract. The duration of haloperidol-induced catalepsy in animals was significantly shortened, and cognitive performance improved. The antioxidant activity of this herb is responsible for its PD effects [
135] (
Table 19).
St. John’s Wort
The active ingredients in this plant include naphthodianthrone, phloroglucinol, flavonoids, and essential oils. St. John’s wort (Hypericaceae) also has antioxidant and neuroprotective properties [
138]. St. John’s wort extract decreased the neurotoxicity that was produced by long-term rotenone therapy in rats. St. John’s wort reduces the damage caused to neurons and also prevents apoptosis by lowering the Bax levels. After dopaminergic neuron lesioning in rats, it lowered striatal malondialdehyde levels, catalase activity, glutathione (GSH) content, tumor necrosis factor-alpha (TNF-) expression, and DNA fragmentation [
139] (
Table 20).
Bacopa monnieri
The efficacy of
B. monnieri against Parkinson’s disease was studied in vitro, as well as in animal models of the neurodegenerative disease.
B. monnieri’s antioxidant and neuroprotective characteristics result in anti-parkinsonian effects linked toreduced synuclein protein aggregation and selective dopaminergic neurodegeneration. In Ayurvedic medicine, this herb is also often used as a brain stimulant. Because of its antioxidant capacity and restoration of mitochondrial ETC complex activity,
B. monnieri extract has been demonstrated to greatly diminish paraquat-induced Parkinson’s disease in both Drosophila and mice models [
143] (
Table 21).
Mushrooms
Metabolites from sea
Mushrooms may have anti-PD neuroprotective effects. Neoechinulin A is an isoprene quinone alkaloid that can be isolated from two Aspergillus sp. red algal-based fungi, as well as Microsporum [
148]. By addressing mitochondrial complex I malfunction, it can protect PC12 cells from neuronal cell death caused by MPP+ and peroxynitrite [
149].
Secalonic acid A is a natural substance derived from two species of marine fungi, Aspergillus ochraceous and Paecilomyces [
150]. By blocking p38 phosphorylation, JNK, calcium entry, and caspase-3 activation in colchicine-induced mortality of cortical neurons were significantly reduced at concentrations of 3–10 µM [
151] (
Table 22).
Sea Cucumbers
Nutrient-dense foods include
sea cucumbers and sea mollusks. In many oriental countries,
sea cucumbers are considered a restorative and conventional therapy for neurological diseases. These compounds dramatically reduce the 6-OHDA-induced loss of DA neurons in Caenorhabditis elegans [
155]. These extracts may also increase lipid levels and inhibit synuclein aggregation.
Cucumaria frondosa sea cucumber extract has high levels of sphingolipids SCG-1, SCG-2, and SCG-3, which raises TrkA phosphorylation and BDNF expression [
156], thus, promoting their potent anti-PD action (
Table 23).
Oleuropein
Oleuropein is made up of the following three components: elenolic acid, glucose, and hydroxytyrosol. It is a well-known phenolic component that is present in olive oil [
160]. Although oleuropein is the most abundant, olive oil also contains aglycones and oleurosides, as well as other oleuropein derivatives, such as beta-6-sulfonic acid, and a radical scavenger of 2,2-diphenyl-1-picrylhydrazyl radicals. The use of oleuropein and its derivatives has been found to minimize ROS accumulation [
161,
162] (
Table 24).
Theaflavin
Polyphenol theaflavin (TF) improves the flavor and color of black tea [
166]. Because of its antioxidant capabilities, which include the capacity to minimize excessive free radical formation and metal chelation, it is widely known for having a wide range of positive effects against many disorders [
167]. According to recently released scholarly research, it may have neuroprotective properties. Theaflavin has been shown to be similarly as efficient as EGCG in reducingamyloid- and α-synuclein-induced neurotoxicity because of its potential antioxidant properties [
168] (
Table 25).
Caffeic Acid
Caffeic acid (CA), a phenol molecule that is present in a variety of vegetation types, is prevalent in caffeine and wines, as well as brews. It contains therapeutic qualities that are similar to propolis, whose remarkable antioxidant potential has been well studied [
173]. It also contains pharmacological potential properties, such as anti-cancer, anti-inflammatory, and neuroprotective qualities [
174]. It has been discovered that it can interact with the peroxy radicals that are responsible for lipid peroxidation, allowing it to successfully cure a variety of disorders. It has been demonstrated that caffeine increases the activity of antioxidant enzymes such SOD, GPx, GSH, and CAT, and also reduces the overproduction of ROS and RNS [
175]. Furthermore, after delivery, striatal DA levels increased significantly, while inflammatory mediators dropped [
176] (
Table 26).
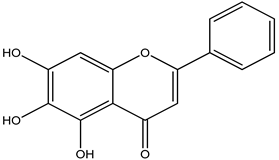
Chrysin
Chrysin is a flavonoid, a naturally produced polyphenolic substance. A wide range of foods, including fruits, honey, vegetables, mushrooms, plants, and blue
passionflowers contain chrysin [
180].
Chrysin’s anti-inflammatory and antioxidant abilities, and variety of pharmacological characteristics, have been researched for their neuroprotective effects. The bioavailability and amount that can be attained in rat cells and target tissues influence its adaptability of therapeutic efficacy. Increased DA levels are inversely linked with dopaminergic neuron mortality in both in vivo and in vitro research, supporting chrysin’s neuroprotective action. It also boosted DA levels in PD mousemodels and CGN cells treated with MPP+ and MPTP by inhibiting monoamine oxidase B [
181].
Furthermore, in Barnes maze, rotational behavior models, chrysin greatly corrected motor and cognitive deficiencies in such animals [
182]. Chrysin is thus a potential disease modulator that can also assist in the decrease of Parkinson’s disease symptoms (
Table 27).
Vanillin
Vanillin, a phenolic aldehyde molecule, is a popular flavoring component that is found all over the world. It is employed by various plant species and is frequently used in food, drinks, pharmaceuticals, aromatherapy, and personal care items. Vanillin’s structure and key biological functions, such as vanillyl alcohol and vanillic acid, are well known [
186]. It can simply pass the blood–brain barrier, preventing oxidative damage to the brain [
187] (
Table 28).
Asiatic Acid
As a neuroprotective drug, asiatic acid, a naturally occurring pentacyclic triterpenoid, has several pharmacological effects. Many biological roles of asiatic acid have been demonstrated, including antioxidative, anticarcinogenic, and neuroprotective characteristics. It protects against Parkinson’s disease by reducing mitochondrial oxidative stress [
191] (
Table 29).
Thymoquinone
Black cumin, as well as other Labiatae plants’ seeds, contains the pharmacologically active compound thymoquinone (TQ) [
197]. Thymoquinone therapy reduced the mortality of primary dopaminergic neurons in a rotenone-induced Parkinson’s disease model, confirming the compound’s neuroprotective effect [
198].
Thymoquinone dramatically increased the expression of neuroprotective proteins, decreased NF-B activation, and significantly reduced the expression of pro-inflammatory cytokines in LPS/IFN-activated BV-2 microglial cells. In addition, it reduces oxidative stress via the Nrf2/ARE signaling pathway, which delays dopaminergic neurodegeneration [
199] (
Table 30).
Ferulic Acid
Ferulic acid (FA) is a naturally occurring phenolic phytochemical that is present in a number of foods. Eating vegetables, fruits, and wholegrains has been shown to help prevent illnesses such as malignancy, obesity, coronary heart disease, and Parkinson’s disease [
203]. It contains immunomodulatory, oxidative, chemotherapeutic, and therapeutic properties [
204]. It also has a low toxicity level. Through decreasing lipid peroxidation and ROS production through the action of its phenolic hydroxyl group, ferulic acid is able to carry out these functions. It also suppresses the expression of enzymes that promote inflammation [
203] (
Table 31 and
Table 32).
Recent Clinical Trials
Below are some of the phytoconstituents that have been used in various disorders that shown their effect in different clinical trials (
Table 33).
Phytochemical-Based Formulations
Below are some phytochemical-based market formulations for the treatment of neurodegenerative disorders (
Table 34).
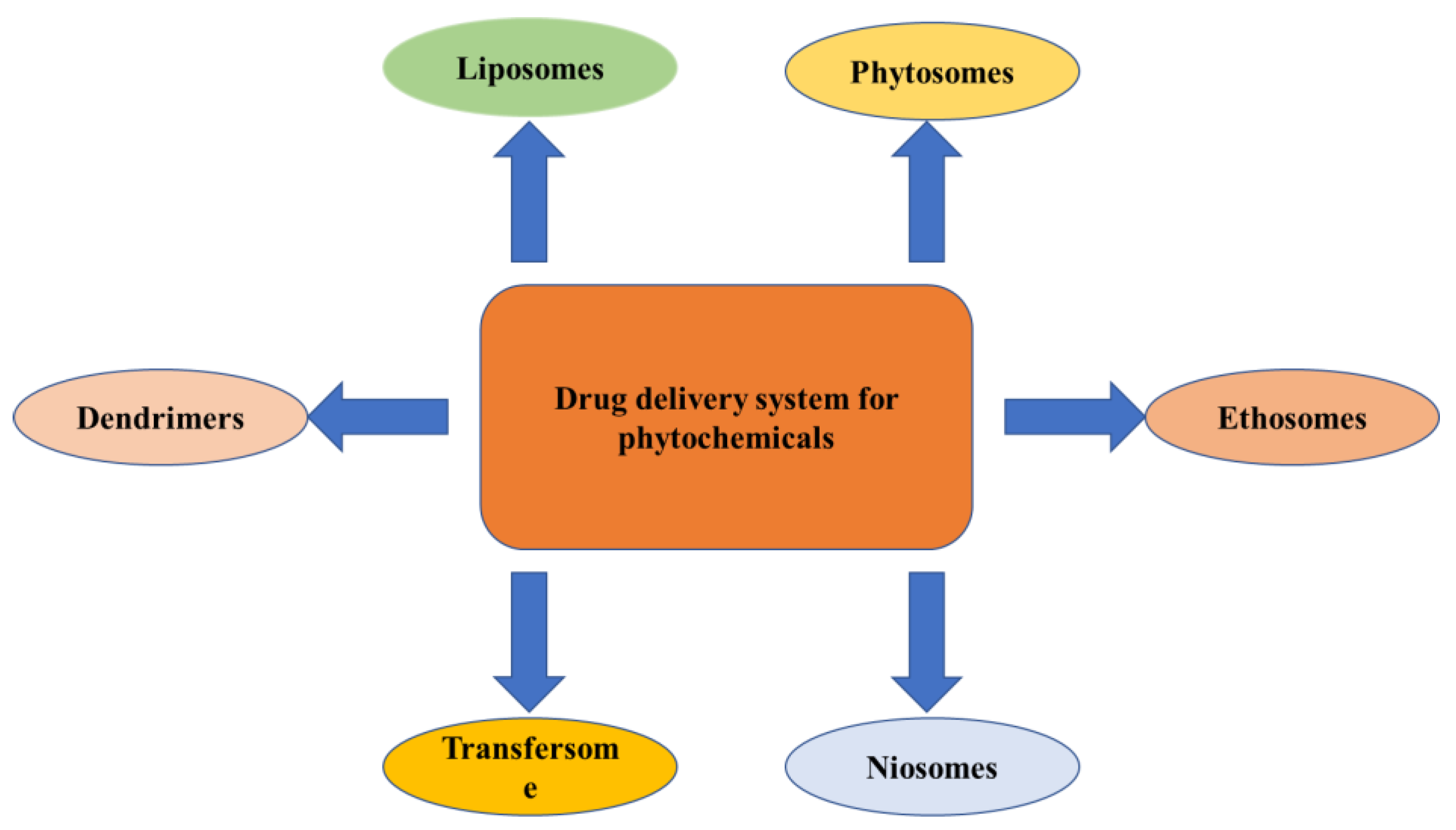
Novel Drug Delivery Approaches for Phytoconstituents
The novel techniques for the drug delivery of phytochemicals use liposomes, phytosomes, niosomes, transferosomes, ethosomes, etc., which are discussed below (
Figure 5).
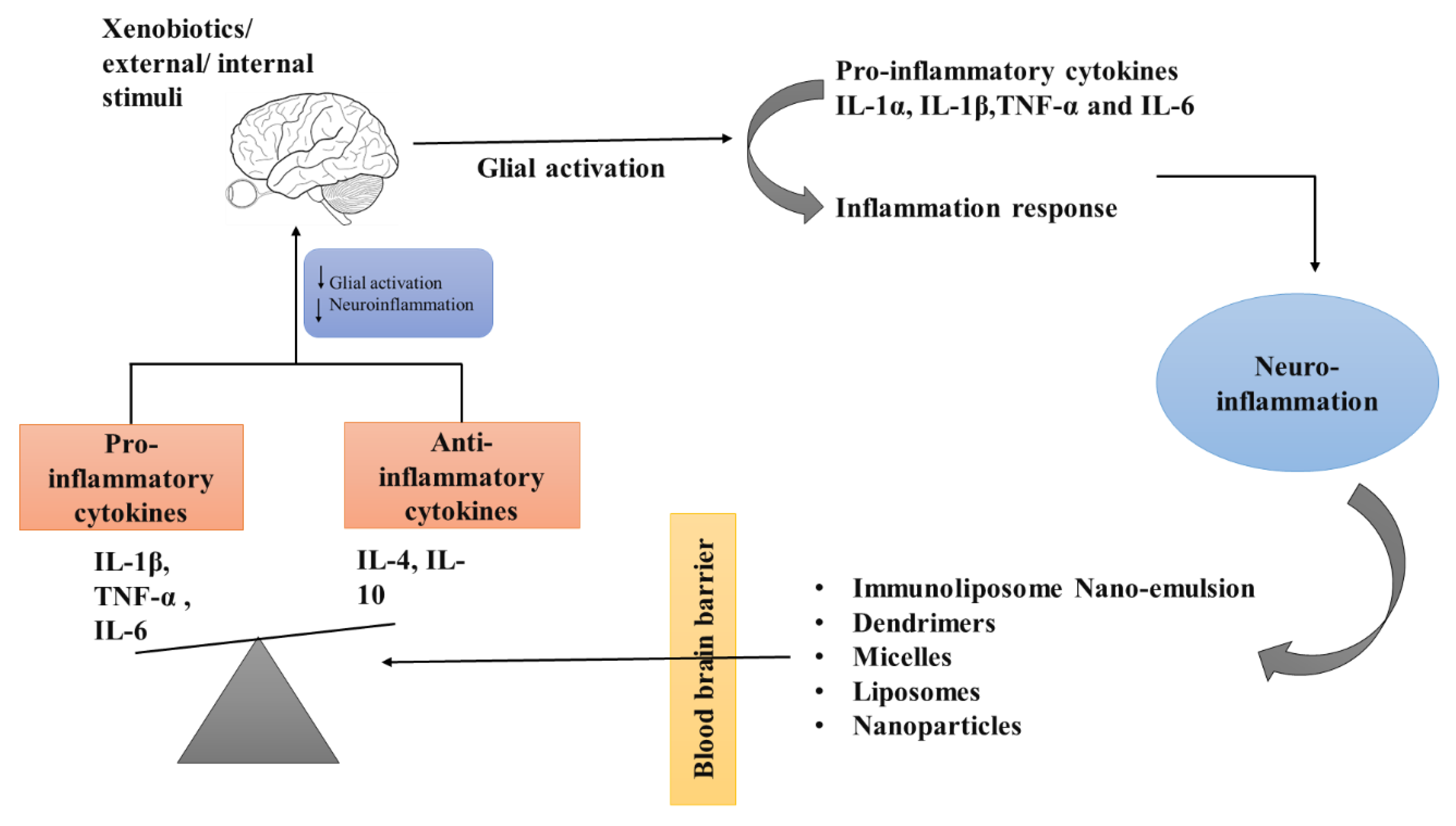
Role of Nanotechnology in Various Neurodegenerative Disorders
The therapy of neurodegenerative disorders such as AD, PD, stroke, epilepsy, and Huntington’s disease has been improved by the development of newer medication delivery strategies such as nanoparticles. The nanoparticles that are given have the ability to cross the blood–brain barrier and then target a particular cell or signaling pathway (
Figure 6).
Nanotechnology-Based Brain Targeting via Alteration of Blood–Brain Barrier
The BBB is a selective permeability barrier that works as a local gateway for circulating antigens and foreign particles. This is the most complex gateway to be crossed and acts as a major challenge for the drug delivery vehicles to target the drug molecules to the brain. Emerging nanotechnologies, involve a number of fascinating concepts that have the potential to serve as the answer to this persistent challenge.
The advancement of nanotechnology through interdisciplinary collaboration will lead to new understandings of how neural circuits function, as well as the methods for the detection and treatment of brain disorders. A new drug delivery platform that makes it simple to transfer therapeutic compounds to the brain can be developed by utilizing the unique qualities of nanomaterials, such as their smaller size, biocompatibility, longer blood circulation, and lack of toxicity. Both specialized and general targeting strategies for brain locations are used in nanotechnology-mediated medication delivery systems. The formulation of nano-vehicles for the delivery of drugs, such as nanoparticles, liposomes, dendrimers, micelles, and carbon nanotubes, has received a lot of attention recently. These vehicles can deliver pharmaceuticals, peptides, proteins, vaccines, or nucleic acids [
220].
Alzheimer’s Disease—Researchers have developed a wide variety of nano-formulations in the hopes that they will have a positive effect on AD patients. The studies that were conducted in vitro on the SHSY-5Y human neuroblastoma cell line showed that phospholipid-based PEG-stabilized nano-micelles inhibit Aβaggregation and reduce Aβ-induced neurotoxicity [
230]. The phytochemical curcumin was shown to have the power to decrease Aβ oligomerization and cell proliferation in the in vitro study; however, when it was injected into mice, it showed poor bioavailability [
231]. Curcumin was formulated by using nanoliposomes, which improved the bioavailability without compromising the drug’s ability to prevent Aβ aggregation [
232].
Parkinson’s Disease—According to one study, L-Dopa and nerve-growth-factor-bound PBCA nanoparticles can cross the BBB and treat the primary signs and symptoms of Parkinson’s disease (PD) [
233].
In addition, in another study, SA (Schisantherin A), which is used for the treatment of PD, was encapsulated in nanoparticles, which increasedits circulation in the blood stream and also its uptake in the brain. This showed that delivery with nanoparticles increases the efficacy of SA and makesit a potent compound for the treatment of PD [
234].
Huntington’s Disease—Nitrendipine, a calcium channel blocker, has been demonstrated to significantly lower the incidence of dementia in HD patients over the duration of two years. However, this drug has permeability issues, due to its hydrophilic nature, and, as a result, it crosses the BBB very poorly. A study was conducted in which SLNs of nitrendipine were prepared, and a comparison of the uptake of nano-formulation and the bulk drug was carried out. According to the findings, the drug was taken in at a higher rate when it was encapsulated in SLNs [
235].
Epilepsy—It has been discovered that solid lipid nanoparticles packed with carbamazepine and PLGA nanoparticles filled with β-carotene have the ability to have a greater anticonvulsant impact compared to polysorbate-80 and carbamazepine-packed, nanoemulged-covered nanoparticles [
236].
In a rat model, subcutaneous injection of ethosuximide-encapsulated chitosan nano-capsules was found to reduce the spike-wave discharge. This finding was made by researchers using the rat as a model. Because they can provide a consistent release of the medication, these nanoparticles can be developed as depot drug delivery systems, enabling the long-term usage of antiepileptic drugs [
237].
Advantages of Various Novel Drug Delivery Methods
There are various advantages of novel approaches for drug delivery using nanoparticles (
Table 35).