Improved Local Anesthesia at Inflamed Tissue Using the Association of Articaine and Copaiba Oil in Avocado Butter Nanostructured Lipid Carriers
Abstract
1. Introduction
2. Results and Discussion
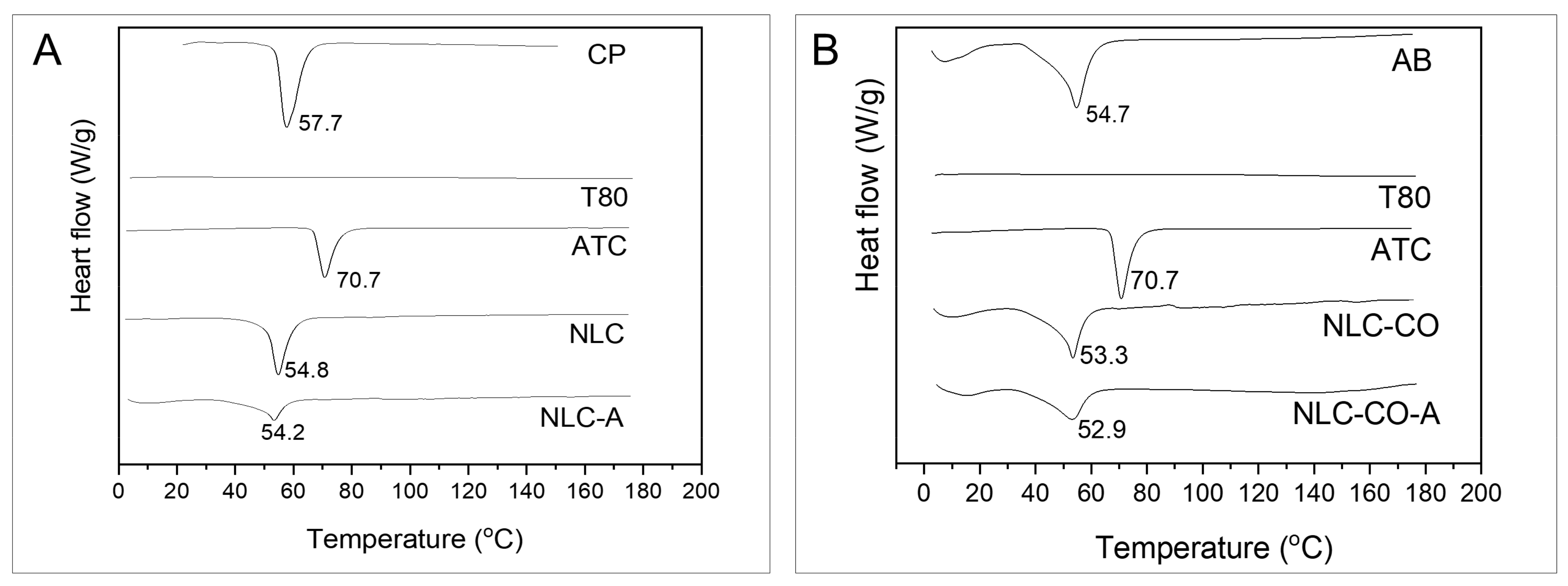
2.1. DSC and XDR Measurements
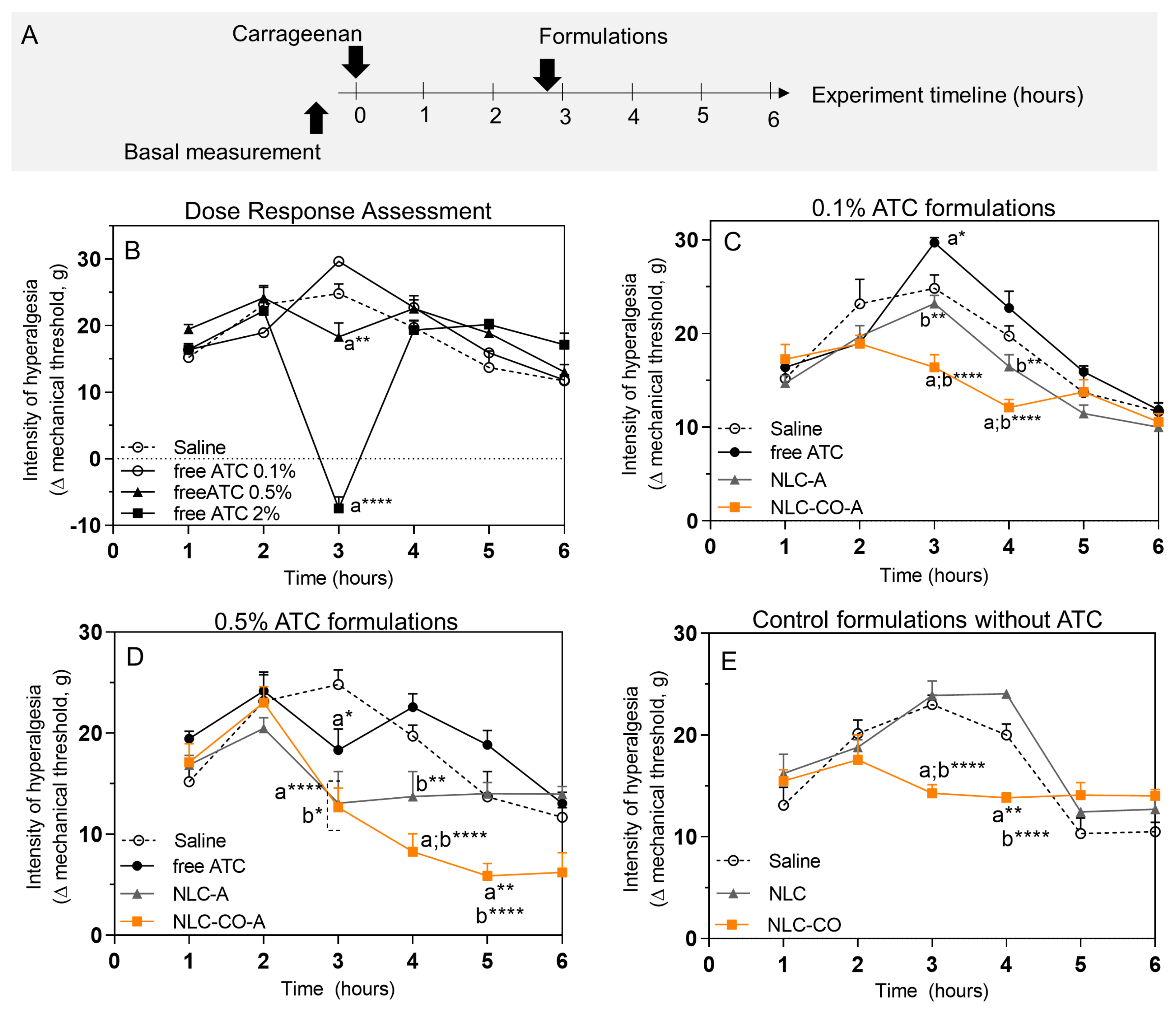
2.2. In Vivo Tests Performed through Inflammatory Pain Models
2.2.1. Subcutaneous Injection of λ-Carrageenan
2.2.2. Subcutaneous Injection of Prostaglandin E2
2.2.3. Pharmacokinetic Study (Local Microdialysis in the Inflamed Tissue)
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Articaine Quantification (by HPLC) and Assessment of the Analytical Method
4.3. NLC Preparation
4.4. Physical and Chemical Characterization of the NLC
4.4.1. Measurements of Size, Polydispersity Index, and Zeta Potential
4.4.2. Measurement of Particle Concentration
4.4.3. Differential Scanning Calorimetry and X-Ray Diffraction Measurements
4.4.4. Articaine Encapsulation Efficiency and Loading Capacity by the NLC
4.5. In Vivo Tests with Murine Models
4.5.1. Animal Maintenance Conditions
4.5.2. Carrageenan-Induced Inflammatory Hyperalgesia Model
4.5.3. Prostaglandin-E2-Induced Inflammatory Hyperalgesia Model
4.5.4. Electronic von Frey Test
4.5.5. Pharmacokinetic Study (Local Microdialysis in Inflamed Tissue)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berde, C.B.; Strichartz, G.R. Local Anesthetics. In Miller’s Anesthesia; Miller, R.D., Eriksson, L.I., Fleisher, L.A., Wiener-Kronish, J.P., Cohen, N.H., Young, W.L., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 1028–1054. [Google Scholar]
- De Araújo, D.R.; de Ribeiro, L.N.M.; de Paula, E. Lipid-Based Carriers for the Delivery of Local Anesthetics. Expert Opin. Drug Deliv. 2019, 16, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Shegokar, R.; Keck, C.M. 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.N.M.; Breitkreitz, M.C.; Guilherme, V.A.; da Silva, G.H.R.; Couto, V.M.; Castro, S.R.; de Paula, B.O.; Machado, D.; de Paula, E. Natural Lipids-Based NLC Containing Lidocaine: From Pre-Formulation to in Vivo Studies. Eur. J. Pharm. Sci. 2017, 106, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, G.; Meretski, S.; Segal, J.; Tarshis, M.; Schroeder, A.; Zanin-Zhorov, A.; Lion, G.; Ingber, A.; Hochberg, M. Polyhydroxylated Fatty Alcohols Derived from Avocado Suppress Inflammatory Response and Provide Non-Sunscreen Protection against UV-Induced Damage in Skin Cells. Arch. Dermatol. Res. 2011, 303, 239–246. [Google Scholar] [CrossRef]
- Veiga, V.F.; Rosas, E.C.; Carvalho, M.V.; Henriques, M.G.M.O.; Pinto, A.C. Chemical Composition and Anti-Inflammatory Activity of Copaiba Oils from Copaifera Cearensis Huber Ex Ducke, Copaifera Reticulata Ducke and Copaifera Multijuga Hayne? A Comparative Study. J. Ethnopharmacol. 2007, 112, 248–254. [Google Scholar] [CrossRef]
- Peters, M.C.; Botero, T.M. In Patients with Symptomatic Irreversible Pulpitis, Articaine Is 3.6 Times More Efficacious Than Lidocaine in Achieving Anesthetic Success When Used for Supplementary Infiltration After Mandibular Block Anesthesia. J. Evid. Based Dent. Pract. 2017, 17, 99–101. [Google Scholar] [CrossRef]
- Rodrigues da Silva, G.H.; Geronimo, G.; García-López, J.P.; Ribeiro, L.N.M.; de Moura, L.D.; Breitkreitz, M.C.; Feijóo, C.G.; de Paula, E. Articaine in Functional NLC Show Improved Anesthesia and Anti-Inflammatory Activity in Zebrafish. Sci. Rep. 2020, 10, 19733. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: A Review Emphasizing on Particle Structure and Drug Release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef]
- Mäder, K.; Mehnert, W. Solid Lipid Nanoparticles: Production, Characterization and Applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Rodrigues da Silva, G.H.; Ribeiro, L.N.M.; Mitsutake, H.; Guilherme, V.A.; Castro, S.R.; Poppi, R.J.; Breitkreitz, M.C.; de Paula, E. Optimised NLC: A Nanotechnological Approach to Improve the Anaesthetic Effect of Bupivacaine. Int. J. Pharm. 2017, 529, 253–263. [Google Scholar] [CrossRef]
- Rodrigues da Silva, G.H.; Geronimo, G.; Ribeiro, L.N.M.; Guilherme, V.A.; de Moura, L.D.; Bombeiro, A.L.; Oliveira, J.D.; Breitkreitz, M.C.; de Paula, E. Injectable in Situ Forming Nanogel: A Hybrid Alginate-NLC Formulation Extends Bupivacaine Anesthetic Effect. Mater. Sci. Eng. C 2020, 109, 110608. [Google Scholar] [CrossRef]
- Ribeiro, L.N.M.; Franz-Montan, M.; Breitkreitz, M.C.; Alcântara, A.C.S.; Castro, S.R.; Guilherme, V.A.; Barbosa, R.M.; de Paula, E. Nanostructured Lipid Carriers as Robust Systems for Topical Lidocaine-Prilocaine Release in Dentistry. Eur. J. Pharm. Sci. 2016, 93, 192–202. [Google Scholar] [CrossRef]
- Mooz, E.D.; Gaiano, N.M.; Shimano, M.Y.H.; Amancio, R.D.; Spoto, M.H.F. Physical and Chemical Characterization of the Pulp of Different Varieties of Avocado Targeting Oil Extraction Potential. Food Sci. Technol. 2012, 32, 274–280. [Google Scholar] [CrossRef]
- Yanty, N.A.M.; Marikkar, J.M.N.; Che Man, Y.B. Effect of Fractional Crystallization on Composition and Thermal Characteristics of Avocado (Persea Americana) Butter. J. Therm. Anal. Calorim. 2013, 111, 2203–2209. [Google Scholar] [CrossRef]
- Bunjes, H. Structural Properties of Solid Lipid Based Colloidal Drug Delivery Systems. Curr. Opin. Colloid Interface Sci. 2011, 16, 405–411. [Google Scholar] [CrossRef]
- Neupane, Y.R.; Srivastava, M.; Ahmad, N.; Kumar, N.; Bhatnagar, A.; Kohli, K. Lipid Based Nanocarrier System for the Potential Oral Delivery of Decitabine: Formulation Design, Characterization, Ex Vivo, and in Vivo Assessment. Int. J. Pharm. 2014, 477, 601–612. [Google Scholar] [CrossRef]
- Gonzalez-Mira, E.; Egea, M.A.; Souto, E.B.; Calpena, A.C.; García, M.L. Optimizing Flurbiprofen-Loaded NLC by Central Composite Factorial Design for Ocular Delivery. Nanotechnology 2011, 22, 045101. [Google Scholar] [CrossRef]
- Ruktanonchai, U.; Limpakdee, S.; Meejoo, S.; Sakulkhu, U.; Bunyapraphatsara, N.; Junyaprasert, V.; Puttipipatkhachorn, S. The Effect of Cetyl Palmitate Crystallinity on Physical Properties of Gamma-Oryzanol Encapsulated in Solid Lipid Nanoparticles. Nanotechnology 2008, 19, 095701. [Google Scholar] [CrossRef]
- Bunjes, H.; Unruh, T. Characterization of Lipid Nanoparticles by Differential Scanning Calorimetry, X-Ray and Neutron Scattering. Adv. Drug Deliv. Rev. 2007, 59, 379–402. [Google Scholar] [CrossRef]
- Jenning, V.; Gohla, S. Comparison of Wax and Glyceride Solid Lipid Nanoparticles (SLN®). Int. J. Pharm. 2000, 196, 219–222. [Google Scholar] [CrossRef]
- Napimoga, M.H.; Souza, G.R.; Cunha, T.M.; Ferrari, L.F.; Clemente-Napimoga, J.T.; Parada, C.A.; Verri, W.A.; Cunha, F.Q.; Ferreira, S.H. 15d-Prostaglandin J2 Inhibits Inflammatory Hypernociception: Involvement of Peripheral Opioid Receptor. J. Pharmacol. Exp. Ther. 2007, 324, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Nantel, F.; Denis, D.; Gordon, R.; Northey, A.; Cirino, M.; Metters, K.M.; Chan, C.C. Distribution and Regulation of Cyclooxygenase-2 in Carrageenan-Induced Inflammation. Br. J. Pharmacol. 1999, 128, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.R.; Patil, C.R. Anti-Inflammatory Activity of Bartogenic Acid Containing Fraction of Fruits of Barringtonia Racemosa Roxb. in Acute and Chronic Animal Models of Inflammation. J. Tradit. Complement. Med. 2017, 7, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Andreani, T.; Macedo, A.S.; Fangueiro, J.F.; Santana, M.H.; Silva, A.M.; Souto, E.B. Current State-of-Art and New Trends on Lipid Nanoparticles (SLN and NLC) for Oral Drug Delivery. J. Drug Deliv. 2012, 2012, 750891. [Google Scholar] [CrossRef]
- Ferreira, L.A.B.; Radaic, A.; Pugliese, G.O.; Valentini, M.B.; Oliveira, M.R.; Jesus, M.B. Endocitose e Tráfego Intracelular de Nanomateriais Endocytosis and Intracellular Trafficking of Nanomaterials. Acta Farmacêutica Portuguesa 2014, 3, 149–166. [Google Scholar]
- Scholz, A. Mechanisms of (Local) Anaesthetics on Voltage-Gated Sodium and Other Ion Channels. Br. J. Anaesth. 2002, 89, 52–61. [Google Scholar] [CrossRef]
- Lin, C.-R.; Amaya, F.; Barrett, L.; Wang, H.; Takada, J.; Samad, T.A.; Woolf, C.J. Prostaglandin E2 Receptor EP4 Contributes to Inflammatory Pain Hypersensitivity. J. Pharmacol. Exp. Ther. 2006, 319, 1096–1103. [Google Scholar] [CrossRef]
- Oertel, R.; Rahn, R.; Kirch, W. Clinical Pharmacokinetics of Articaine. Clin. Pharmacokinet. 1997, 33, 417–425. [Google Scholar] [CrossRef]
- De Ridder, N.; Politis, C. Unclarities about Articaine: Contraindications. Stomatol. Edu. J. 2020, 7, 109–116. [Google Scholar] [CrossRef]
- Virdee, S.S.; Seymour, D.; Bhakta, S. Effective Anaesthesia of the Acutely Inflamed Pulp: Part 1. The Acutely Inflamed Pulp. Br. Dent. J. 2015, 219, 385–390. [Google Scholar] [CrossRef]
- Ueno, T.; Tsuchiya, H.; Mizogami, M.; Takakura, K. Local Anesthetic Failure Associated with Inflammation: Verification of the Acidosis Mechanism and the Hypothetic Participation of Inflammatory Peroxynitrite. J. Inflamm. Res. 2008, 1, 41. [Google Scholar] [CrossRef]
- Meechan, J.G. Anaesthesia: How to Overcome Failed Local Anaesthesia. Br. Dent. J. 1999, 186, 15–20. [Google Scholar] [CrossRef]
- Becker, D.E.; Reed, K.L. Local Anesthetics: Review of Pharmacological Considerations. Anesth. Prog. 2012, 59, 90–102. [Google Scholar] [CrossRef]
- Cross, M.E.; Plunkett, E.V.E. The Meyer–Overton Hypothesis. Phys. Pharmacol. Physiol. Anaesth. 2014, 4, 146–147. [Google Scholar] [CrossRef]
- Kattan, S.; Lee, S.M.; Hersh, E.V.; Karabucak, B. Do Buffered Local Anesthetics Provide More Successful Anesthesia than Nonbuffered Solutions in Patients with Pulpally Involved Teeth Requiring Dental Therapy? A Systematic Review. J. Am. Dent. Assoc. 2019, 150, 165–177. [Google Scholar] [CrossRef]
- Burga-Sánchez, J.; Ferreira, L.E.N.; Volpato, M.C.; Cabeça, L.F.; Braga, M.; Fraceto, L.F.; de Paula, E.; Groppo, F.C. Physicochemical Characterization and Cytotoxicity of Articaine-2-Hydroxypropyl-β-Cyclodextrin Inclusion Complex. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1313–1323. [Google Scholar] [CrossRef]
- Da Silva, C.B.; Volpato, M.C.; Muniz, B.V.; dos Santos, C.P.; Serpe, L.; Nunes Ferreira, L.E.; Silva de Melo, N.F.; Fraceto, L.F.; Groppo, F.C.; Franz-Montan, M. Promising Potential of Articaine-Loaded Poly(Epsilon-Caprolactone) Nanocapules for Intraoral Topical Anesthesia. PLoS ONE 2021, 16, e0246760. [Google Scholar] [CrossRef]
- Da Silva, C.B.; Groppo, F.C.; dos Santos, C.P.; Serpe, L.; Franz-Montan, M.; de Paula, E.; Ranali, J.; Volpato, M.C. Anaesthetic Efficacy of Unilamellar and Multilamellar Liposomal Formulations of Articaine in Inflamed and Uninflamed Tissue. Br. J. Oral Maxillofac. Surg. 2016, 54, 295–300. [Google Scholar] [CrossRef]
- Hendriks, H.; Malingré, T.M.; Batterman, S.; Bos, R. Mono- and Sesqui-Terpene Hydrocarbons of the Essential Oil of Cannabis Sativa. Phytochemistry 1975, 14, 814–815. [Google Scholar] [CrossRef]
- Bahi, A.; al Mansouri, S.; al Memari, E.; al Ameri, M.; Nurulain, S.M.; Ojha, S. β-Caryophyllene, a CB2 Receptor Agonist Produces Multiple Behavioral Changes Relevant to Anxiety and Depression in Mice. Physiol. Behav. 2014, 135, 119–124. [Google Scholar] [CrossRef]
- Kuwahata, H.; Katsuyama, S.; Komatsu, T.; Nakamura, H.; Corasaniti, M.T.; Bagetta, G.; Sakurada, S.; Sakurada, T.; Takahama, K. Local Peripheral Effects of β-Caryophyllene through CB2 Receptors in Neuropathic Pain in Mice. Pharmacol. Pharm. 2012, 3, 397–403. [Google Scholar] [CrossRef]
- Aguilar-Ávila, D.S.; Flores-Soto, M.E.; Tapia-Vázquez, C.; Pastor-Zarandona, O.A.; López-Roa, R.I.; Viveros-Paredes, J.M. β-Caryophyllene, a Natural Sesquiterpene, Attenuates Neuropathic Pain and Depressive-Like Behavior in Experimental Diabetic Mice. J. Med. Food 2019, 22, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, I.; Fiorenzani, P.; Pessina, F.; Pinassi, J.; Aglianò, M.; Miragliotta, V.; Aloisi, A.M. The CB2 Agonist β-Caryophyllene in Male and Female Rats Exposed to a Model of Persistent Inflammatory Pain. Front. Neurosci. 2020, 14, 850. [Google Scholar] [CrossRef] [PubMed]
- De Melo, N.F.S.; Campos, E.V.R.; Franz-Montan, M.; de Paula, E.; da Silva, C.M.G.; Maruyama, C.R.; Stigliani, T.P.; de Lima, R.; de Araújo, D.R.; Fraceto, L.F. Characterization of Articaine-Loaded Poly(ε -Caprolactone) Nanocapsules and Solid Lipid Nanoparticles in Hydrogels for Topical Formulations. J. Nanosci. Nanotechnol. 2017, 18, 4428–4438. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahalawy, H.; Abuohashish, H.; Chathoth, S.; Al-Masoud, N.; Al-Jandan, B. Articaine Versus Lidocaine Concentration in the Palatal Tissues After Supraperiosteal Buccal Infiltration Anesthesia. J. Oral Maxillofac. Surg. 2018, 76, 315.e1–315.e7. [Google Scholar] [CrossRef]
- Schwarz, C.; Mehnert, W.; Lucks, J.S.; Müller, R.H. Solid Lipid Nanoparticles (SLN) for Controlled Drug Delivery. I. Production, Characterization and Sterilization. J. Control. Release 1994, 30, 83–96. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) in Cosmetic and Dermatological Preparations. Adv. Drug Deliv. Rev. 2002, 54, 131–155. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Zhai, Y.; Zhai, G.; Wang, Z.; Liu, J. Development of a Ropivacaine-Loaded Nanostructured Lipid Carrier Formulation for Transdermal Delivery. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 130–136. [Google Scholar] [CrossRef]
- Vivancos, G.G.; Verri, W.A.; Cunha, T.M.; Schivo, I.R.S.; Parada, C.A.; Cunha, F.Q.; Ferreira, S.H. An Electronic Pressure-Meter Nociception Paw Test for Rats. Brazilian J. Med. Biol. Res. 2004, 37, 391–399. [Google Scholar] [CrossRef]
- Joukhadar, C.; Müller, M. Microdialysis. Clin. Pharmacokinet. 2005, 44, 895–913. [Google Scholar] [CrossRef]
- Ungerstedt, U. Microdialysis—Principles and Applications for Studies in Animals and Man. J. Intern. Med. 1991, 230, 365–373. [Google Scholar] [CrossRef]



| Lipid Source | Formulation | Solid Lipid | % | Liquid Lipid | % | Surfactant | % | Drug | % |
|---|---|---|---|---|---|---|---|---|---|
| Synthetic | NLC | Cetyl palmitate | 8.75 | Dhaykol® * | 3.75 | Tween 80 | 3.75 | - | |
| NLC-A | Cetyl palmitate | 8.75 | Dhaykol® * | 3.75 | Tween 80 | 3.75 | Articaine | 2 | |
| Natural | NLC-CO | Avocado butter | 8.75 | Copaiba oil | 3.75 | Tween 80 | 3.75 | - | |
| NLC-CO-A | Avocado butter | 8.75 | Copaiba oil | 3.75 | Tween 80 | 3.75 | Articaine | 2 |
| Formulation | Size (nm) | PDI | ZP (mV) | PC (×1013 Particles/mL) | %EE | DL (%) |
|---|---|---|---|---|---|---|
| NLC | 227.1 ± 2.3 | 0.184 ± 0.034 | −26.9 ± 0.3 | 7.30 ± 0.17 | - | - |
| NLC-A | 237.6 ± 3.3 | 0.169 ± 0.015 | −42.1 ± 0.5 | 5.50 ± 0.40 | 74.0 ± 0.1 | 8.3 ± 0.1 |
| NLC-CO | 206.9 ± 1.0 | 0.161 ± 0.007 | −23.7 ± 0.3 | 6.15 ± 0.81 | - | - |
| NLC-CO-A | 217.7 ± 0.8 | 0.174 ± 0.004 | −40.2 ± 1.1 | 8.14 ± 0.71 | 78.4 ± 0.1 | 8.8 ± 0.1 |
| Sample | Melting Temperature (°C) | Enthalpy (J/g) |
|---|---|---|
| Pure cetyl palmitate | 57.7 | 222.0 |
| Pure avocado butter | 54.7 | 57.8 |
| Articaine | 70.7 | 111.6 |
| NLC | 54.8 | 121.8 |
| NLC-A | 54.2 | 107.8 |
| NLC-CO | 53.3 | 37.6 |
| NLC-CO-A | 52.9 | 35.0 |
| Formulations | |||
|---|---|---|---|
| Parameters | Free ATC | NLC-A | NLC-CO-A |
| ke (min−1) | 0.09 ± 0.01 | 0.05 ± 0.02 * | 0.04 ± 0.01 * |
| t1/2 (min) | 8.4 ± 1.1 | 15.6 ± 6.0 * | 16.8 ± 4.0 * |
| AUC (µg·min/mL) | 11,202 ± 3425 | 13,849 ± 6066 | 13,291 ± 2156 |
| C0 (µg/mL) | 1149.3 ± 704.5 | 767.1 ± 551.0 | 610.5 ± 95.0 |
| Parameter | Conditions |
|---|---|
| Column | C18 Gemini-NX 5µ 150 × 4.60 mm |
| Oven temperature | 40 °C |
| Mobile phase | Acetonitrile:KH2PO4 50 mM, 25:75 (v/v) |
| Flux | 1 mL.min−1 |
| Injection volume | 30 µL |
| Wavelength | 273 nm |
| Group | n | Treatment |
|---|---|---|
| 1 | 5 | Naïve—untreated animals (control) |
| 2 | 5 | Saline solution (positive control) |
| 3 | 5 | Free articaine 0.5% |
| 4 | 5 | NLC-A 0.5% |
| 5 | 5 | NLC-CO-A 0.5% |
| 6 | 5 | NLC (1.6 × 1013 nanoparticles/mL) |
| 7 | 5 | NLC-CO (1.6 × 1013 nanoparticles/mL) |
| 8 | 5 | Naloxone + NLC-CO (1.6 × 1013 nanoparticles/mL) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues da Silva, G.H.; Paes Lemes, J.B.; Geronimo, G.; de Carvalho, F.V.; Mendonça, T.C.; Malange, K.F.; de Lima, F.F.; Breitkreitz, M.C.; Parada, C.A.; Dalla Costa, T.; et al. Improved Local Anesthesia at Inflamed Tissue Using the Association of Articaine and Copaiba Oil in Avocado Butter Nanostructured Lipid Carriers. Pharmaceuticals 2023, 16, 546. https://doi.org/10.3390/ph16040546
Rodrigues da Silva GH, Paes Lemes JB, Geronimo G, de Carvalho FV, Mendonça TC, Malange KF, de Lima FF, Breitkreitz MC, Parada CA, Dalla Costa T, et al. Improved Local Anesthesia at Inflamed Tissue Using the Association of Articaine and Copaiba Oil in Avocado Butter Nanostructured Lipid Carriers. Pharmaceuticals. 2023; 16(4):546. https://doi.org/10.3390/ph16040546
Chicago/Turabian StyleRodrigues da Silva, Gustavo Henrique, Julia Borges Paes Lemes, Gabriela Geronimo, Fabíola Vieira de Carvalho, Talita Cesarim Mendonça, Kauê Franco Malange, Fernando Freitas de Lima, Márcia Cristina Breitkreitz, Carlos Amilcar Parada, Teresa Dalla Costa, and et al. 2023. "Improved Local Anesthesia at Inflamed Tissue Using the Association of Articaine and Copaiba Oil in Avocado Butter Nanostructured Lipid Carriers" Pharmaceuticals 16, no. 4: 546. https://doi.org/10.3390/ph16040546
APA StyleRodrigues da Silva, G. H., Paes Lemes, J. B., Geronimo, G., de Carvalho, F. V., Mendonça, T. C., Malange, K. F., de Lima, F. F., Breitkreitz, M. C., Parada, C. A., Dalla Costa, T., & de Paula, E. (2023). Improved Local Anesthesia at Inflamed Tissue Using the Association of Articaine and Copaiba Oil in Avocado Butter Nanostructured Lipid Carriers. Pharmaceuticals, 16(4), 546. https://doi.org/10.3390/ph16040546









