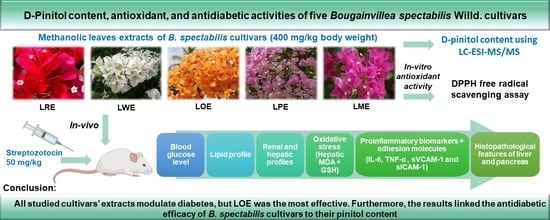
D-Pinitol Content and Antioxidant and Antidiabetic Activities of Five Bougainvillea spectabilis Willd. Cultivars
Abstract
1. Introduction
2. Results
2.1. Yields of Extracts
2.2. D-Pinitol Content
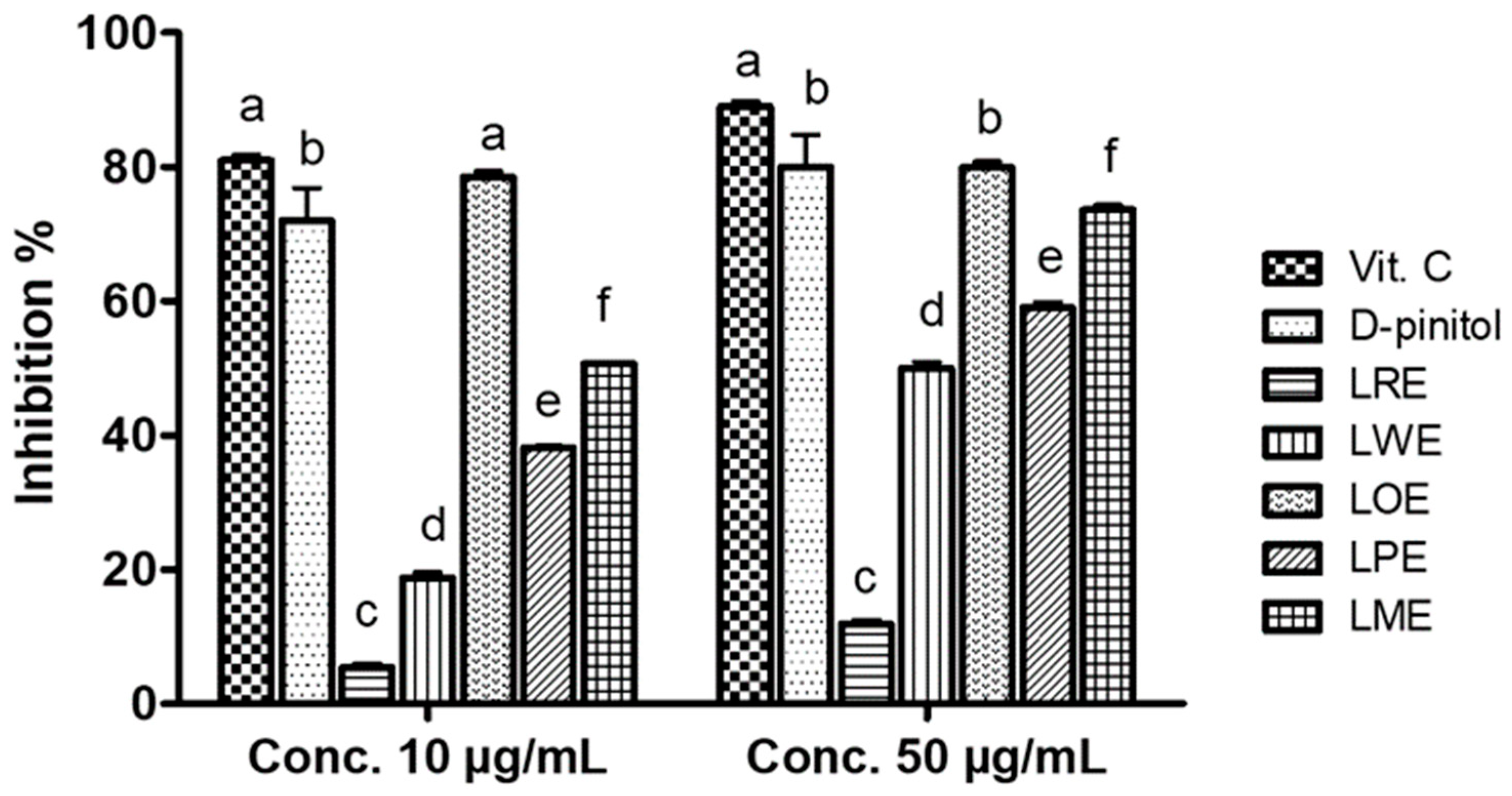
2.3. DPPH• Scavenging Activity
2.4. Antidiabetic Activity
2.4.1. Acute Toxicity Study
2.4.2. Fasting Blood Glucose Levels
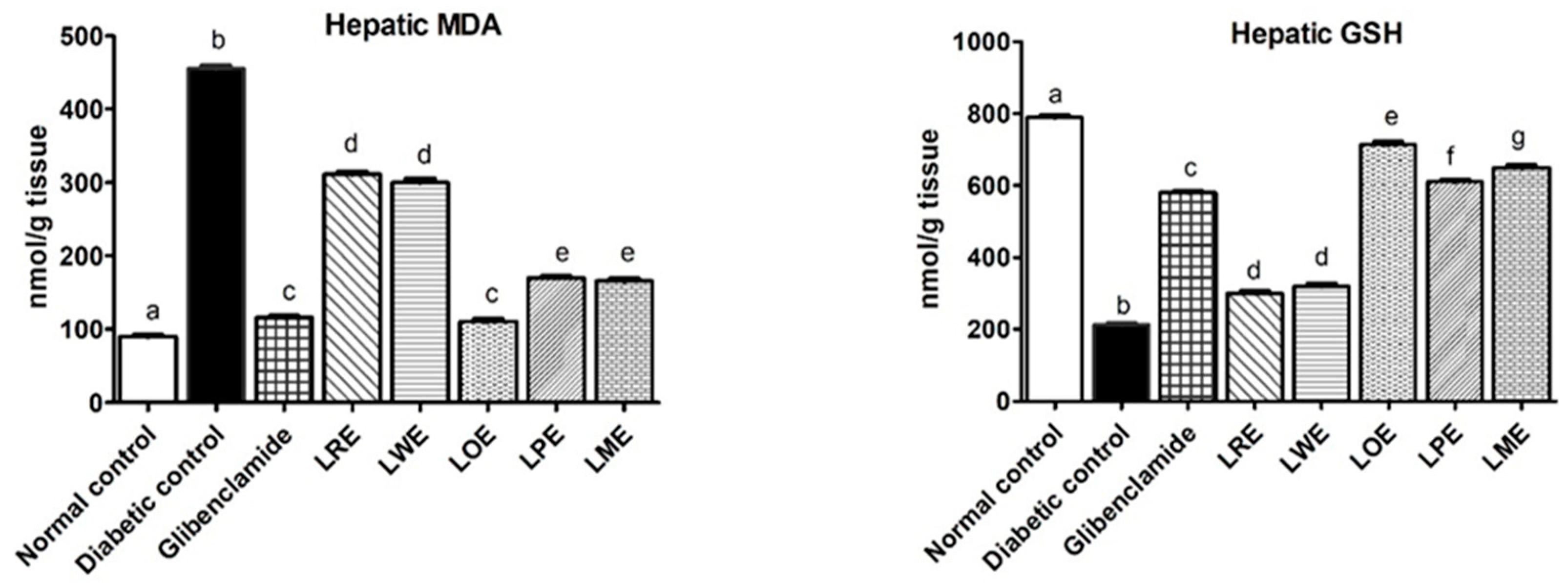
2.4.3. Oxidative Stress Markers
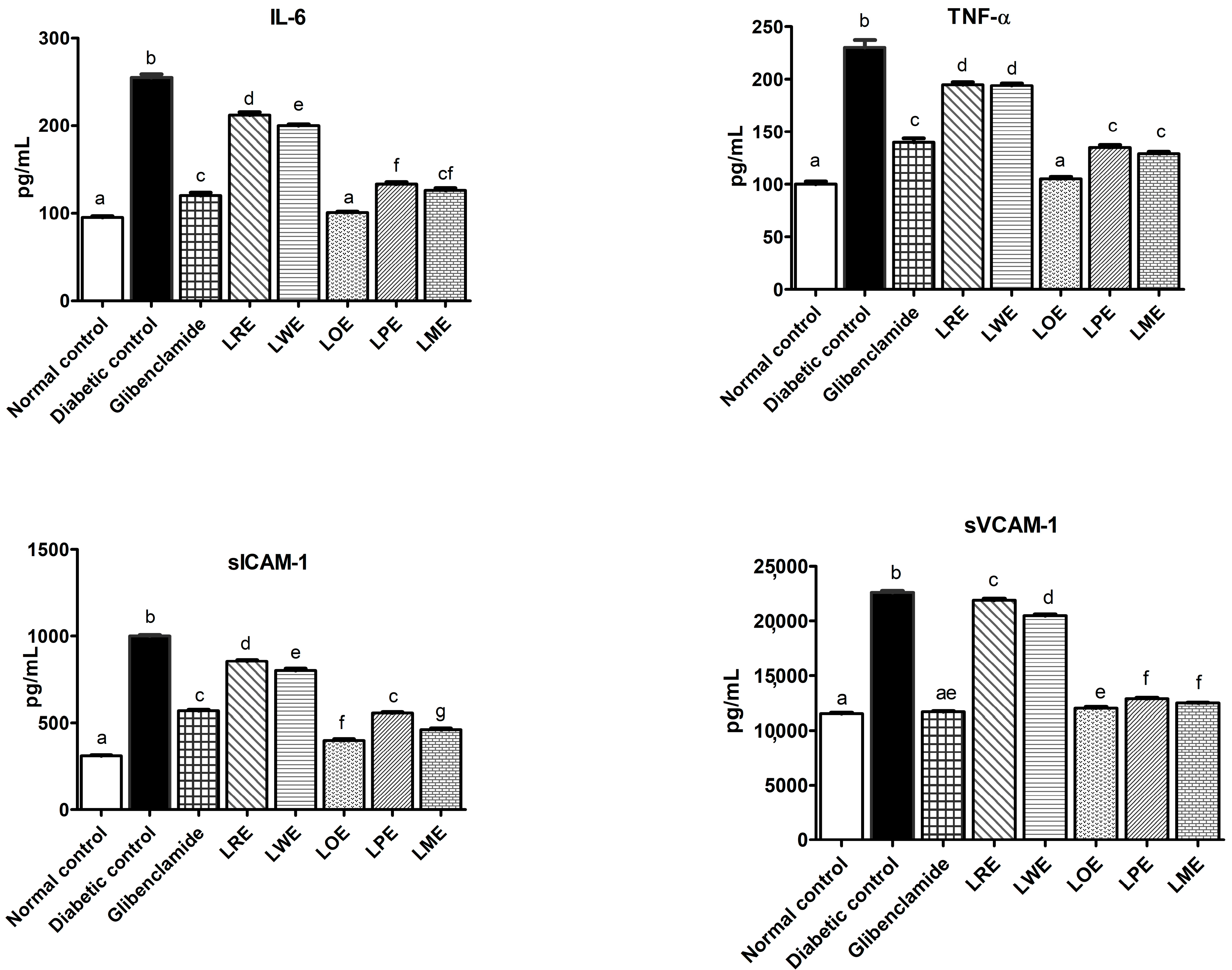
2.4.4. Pro-Inflammatory Markers and Adhesion Molecules
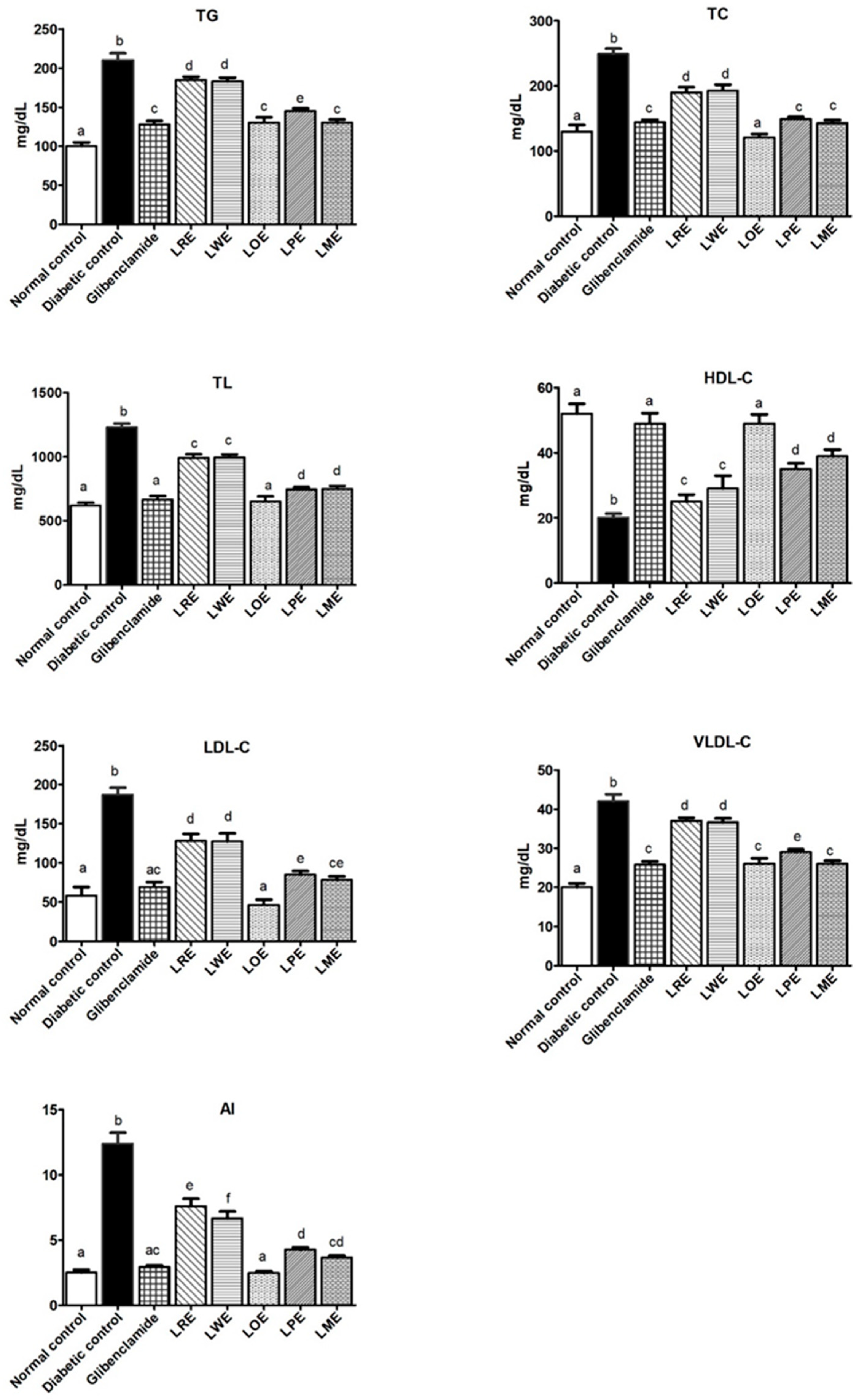
2.4.5. Lipid Profiles
2.4.6. Renal Function Markers
2.4.7. Liver Function Parameters
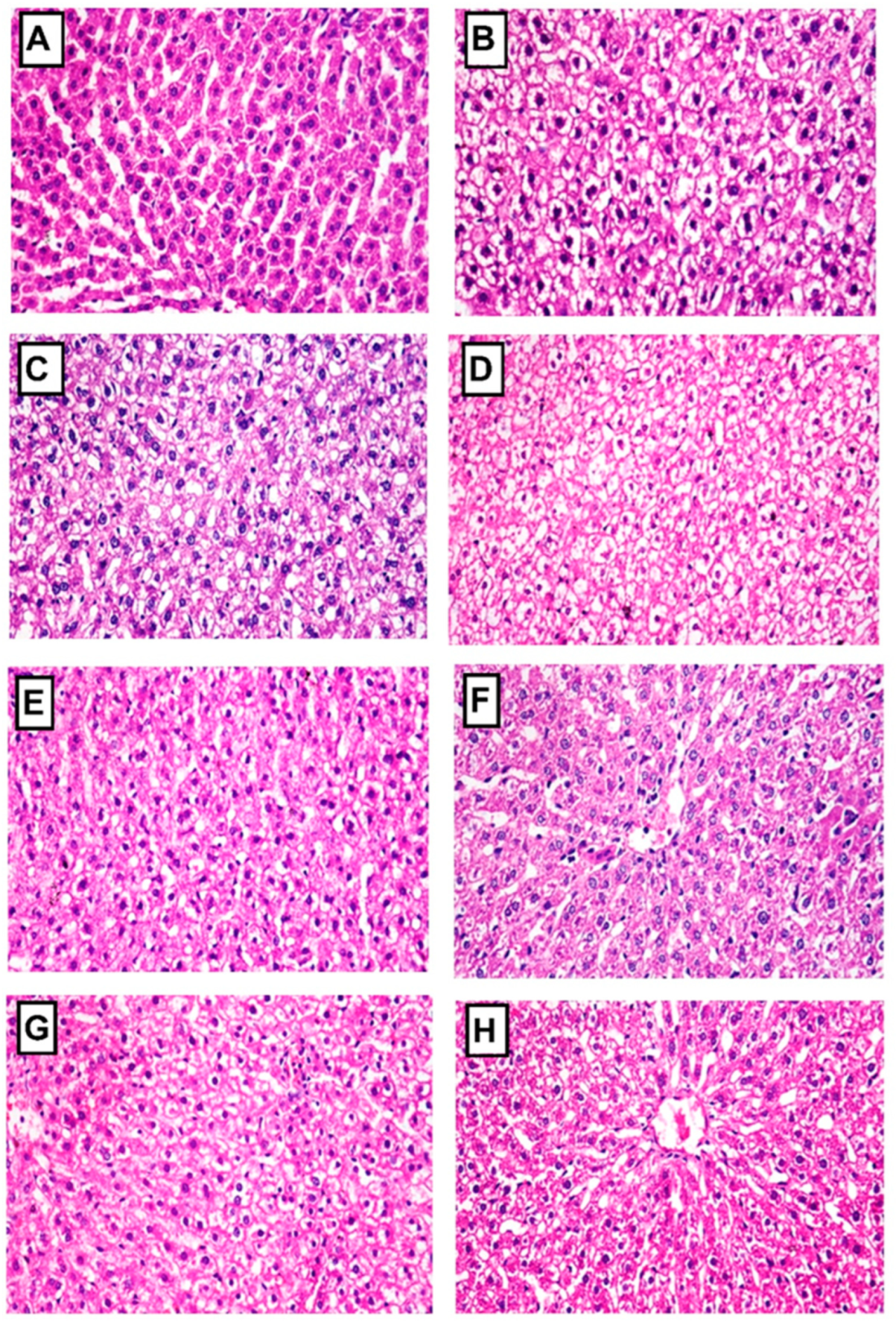
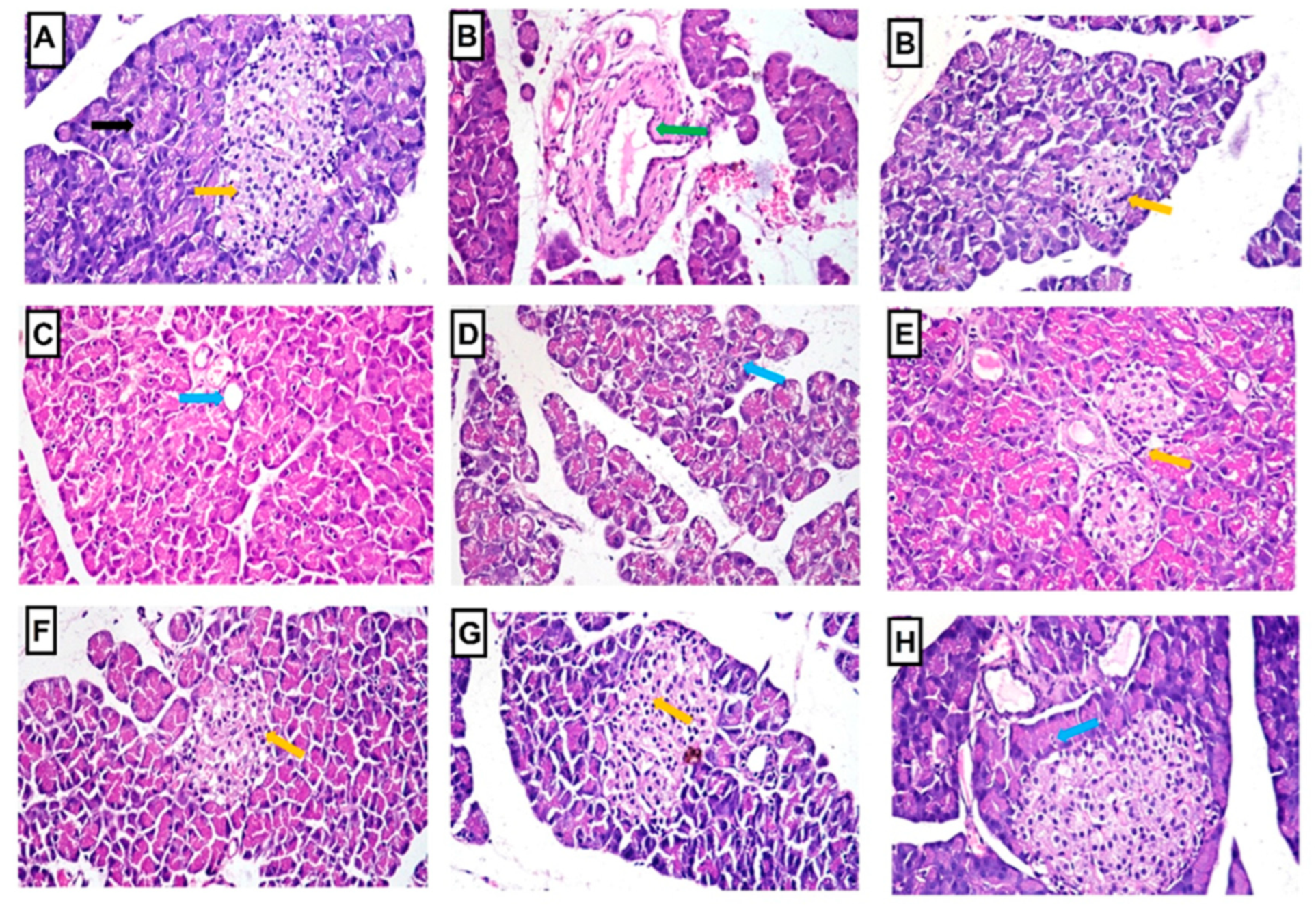
2.4.8. Histopathology of Liver and Pancreas
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Extraction
4.4. LC-ESI-MS/MS Study
4.5. In Vitro Antioxidant Activity (DPPH• Scavenging Assay)
4.6. In Vivo Antidiabetic Activity
4.6.1. Animals
4.6.2. Acute Toxicity Study
4.6.3. Experimental Design
4.6.4. Induction of Diabetes
4.6.5. Sample Preparation
4.6.6. Biochemical Investigations
Estimation of Blood Glucose
Estimation of Oxidative Stress Markers
Estimation of Pro-Inflammatory Markers
Estimation of Lipid Profiles
Estimation of Renal Function
Estimation of Liver Function
4.6.7. Histopathological Investigation
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDF. IDF Diabetes Atlas. 2021. Available online: www.diabetesatlas.org (accessed on 22 December 2022).
- Goyal, R.; Jialal, I. Diabetes Mellitus Type 2; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Li, W.L.; Zheng, H.C.; Bukuru, J.; De Kimpe, N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef]
- Singh, N.; Gautam, G.K.; Ved, A.; Shukla, K.S. Anti Diabetic Evaluation of Methanolic Extract of Psoralea corylifolia L. & Psoralea esculenta L. Seeds in Streptozotocin Induced Diabetic Rats and Histopathological Changes in Diabetic Rats Pancreas: A Comparative Study. J. Nat. Prod. 2022, 12, 74–79. [Google Scholar]
- Abarca-Vargas, R.; Petricevich, V.L. Bougainvillea genus: A review on phytochemistry, pharmacology, and toxicology. Evid. Based Complement. Alternat. Med. 2018, 2018, 9070927. [Google Scholar] [CrossRef]
- Srivastava, R.; Shukla, S.; Soni, A.; Kumar, A. RAPD-based genetic relationships in different Bougainvillea cultivars. Crop Breed. Appl. Biotechnol. 2009, 9, 154–163. [Google Scholar] [CrossRef]
- Wang, N.; Qiu, M.-Y.; Yang, Y.; Li, J.-W.; Zou, X.-X. Complete chloroplast genome sequence of Bougainvillea spectabilis (Nyctaginaceae). Mitochondrial DNA Part B 2019, 4, 4010–4011. [Google Scholar] [CrossRef] [PubMed]
- Gobato, R.; Gobato, A.; Fedrigo, D.F.G. Study of the molecular electrostatic potential of D-Pinitol an active hypoglycemic principle found in Spring flower Three Marys (Bougainvillea species) in the Mm+ method. Parana J. Sci. Educ. 2016, 2, 1–9. [Google Scholar]
- Salam, P.; Bhargav, V.; Gupta, Y.C.; Nimbolkar, P.K. Evolution in bougainvillea (Bougainvillea Commers.)—A review. J. Appl. Nat. Sci. 2017, 9, 1489–1494. [Google Scholar] [CrossRef]
- Chatterjee, J.; Mandal, A.K.A.; Chakrabarty, D.; Datta, S.K. Use of RAPD Analysis to Determine Genetic Diversity and Relationships among Bougainvillea Cultivars at Intra-and Inter-Specific Levels. Hortic. Environ. Biotechnol. 2007, 48, 43. [Google Scholar]
- Kumar, P.P.; Janakiram, T.; Bhatt, K.V.; Prasad, K.V.; Jain, R. Genetic divergence analysis of bougainvillea (Bougainvillea spp) cultivars using morphological markers. Indian J. Agric. Sci. 2015, 85, 661–665. [Google Scholar]
- Datta, S.K.; Jayanthi, R.; Janakiram, T. Bougainvillea. In Floriculture and Ornamental Plants; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–34. [Google Scholar]
- Kaushik, D.; Kumar, M.; Proestos, C.; Oz, F.; Gupta, P.; Kumar, A.; Kundu, P.; Kaur, J.; Kumar, V.; Anjali, A.; et al. A narrative review on the anti-inflammatory efficacy of Bougainvillea spectabilis Willd. and its various applications. J. Agric. Food Res. 2023, 12, 100570. [Google Scholar] [CrossRef]
- Soumyanath, A. Traditional medicines for modern times. In Antidiabetic Plants; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Bhat, M.; Zinjarde, S.S.; Bhargava, S.Y.; Kumar, A.R.; Joshi, B.N. Antidiabetic Indian Plants: A Good Source of Potent Amylase Inhibitors. Evid. Based Complement. Alternat. Med. 2011, 2011, 810207. [Google Scholar] [CrossRef] [PubMed]
- Jawla, S.; Kumar, Y.; Khan, M.S.Y. Isolation of antidiabetic principle from Bougainvillea spectabilis Willd (Nyctaginaceae) stem bark. Trop. J. Pharm. Res. 2013, 12, 761–765. [Google Scholar] [CrossRef]
- Adebayo, G.I.; Alabi, O.T.; Owoyele, B.V.; Soladoye, A.O. Anti-diabetic properties of the aqueous leaf extract of Bougainvillea glabra (Glory of the Garden) on alloxan-induced diabetic rats. Rec. Nat. Prod. 2009, 3, 187. [Google Scholar]
- Jawla, S.; Kumar, Y.; Khan, M.S.Y. Hypoglycemic potential of Bougainvillea spectabilis root bark in normal and Alloxaninduced diabetic rats. Pharmacologyonline 2011, 3, 73–87. [Google Scholar]
- Jawla, S.; Kumar, Y.; Khan, M.S.Y. Hypoglycemic activity of Bougainvillea spectabilis stem bark in normal and alloxan-induced diabetic rats. Asian Pac. J. Trop. Biomed. 2012, 2, S919–S923. [Google Scholar] [CrossRef]
- Mandal, G.; Chatterjee, C.; Chatterjee, M. Evaluation of anti-inflammatory activity of methanolic extract of leaves of Bougainvillea spectabilis in experimental animal models. Pharmacogn. Res. 2015, 7, 18–22. [Google Scholar]
- Ferdous, A.; Janta, R.A.; Arpa, R.N.; Afroze, M.; Khan, M.; Moniruzzaman, M. The leaves of Bougainvillea spectabilis suppressed inflammation and nociception in vivo through the modulation of glutamatergic, cGMP, and ATP-sensitive K+ channel pathways. J. Ethnopharmacol. 2020, 261, 113148. [Google Scholar] [CrossRef]
- Devi, M.R.; Ramesh, B. Hypoglycemic activity of Leaves of Bougainvillea spectabilis extract in streptozotocin-induced diabetic rats. AJPRes 2018, 8, 99–103. [Google Scholar] [CrossRef]
- Malviya, N.; Jain, S.; Malviya, S. Antidiabetic potential of medicinal plants. Acta Pol. Pharm. 2010, 67, 113–118. [Google Scholar]
- Ghogar, A.; Jiraungkoorskul, W. Antifertility Effect of Bougainvillea spectabilis or Paper Flower. Pharmacogn. Rev. 2017, 11, 19–22. [Google Scholar]
- Anandakumar, P.; Vanitha, M.K.; Gizaw, M.; Dereje, G. A review on the diverse effects of D-Pinitol. Adv. J. Pharm. Life Sci. Res. 2018, 6, 1–7. [Google Scholar]
- Shaiq Ali, M.; Amir Ibrahim, S.; Ahmed, F.; Kashif Pervez, M. Color versus bioactivity in the flowers of Bougainvillea spectabilis (Nyctaginaceae). Nat. Prod. Res. 2005, 19, 1–5. [Google Scholar] [CrossRef]
- Gundala, N.K.V.; Naidu, V.G.M.; Das, U.N. Amelioration of streptozotocin-induced type 2 diabetes mellitus in Wistar rats by arachidonic acid. Biochem. Biophys. Res. Commun. 2018, 496, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Jamali-Raeufy, N.; Baluchnejadmojarad, T.; Roghani, M. Isorhamnetin exerts neuroprotective effects in STZ-induced diabetic rats via attenuation of oxidative stress, inflammation and apoptosis. J. Chem. Neuroanat. 2019, 102, 101709. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Mahajan, S.; Kulshrestha, A.; Shrivastava, S.; Sharma, B.; Goswamy, H.M.; Prasad, G.B.K.S. Bougainvillea spectabilis exhibits antihyperglycemic and antioxidant activities in experimental diabetes. J. Evid. Based Complement. Altern. Med. 2016, 21, 177–185. [Google Scholar] [CrossRef]
- Sivakumar, S.; Palsamy, P.; Subramanian, S.P. Impact of D-pinitol on the attenuation of proinflammatory cytokines, hyperglycemia-mediated oxidative stress and protection of kidney tissue ultrastructure in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2010, 188, 237–245. [Google Scholar] [CrossRef]
- Al-Brakati, A.; Albarakati, A.J.A.; Daabo, H.M.A.; Baty, R.S.; Salem, F.E.H.; Habotta, O.A.; Elmahallawy, E.K.; Abdel-Mohsen, D.M.; Taha, H.; Akabawy, A.M.A.; et al. Neuromodulatory effects of green coffee bean extract against brain damage in male albino rats with experimentally induced diabetes. Metab. Brain Dis. 2020, 35, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Zhang, P.; Zhang, L.; Wang, H.; Ho, C.T.; Li, S.; Shahidi, F.; Zhao, H. Detection of cellular redox reactions and antioxidant activity assays. J. Funct. Foods 2017, 37, 467–479. [Google Scholar] [CrossRef]
- Meng, D.; Zhang, P.; Zhang, L.; Wang, H.; Ho, C.-T.; Li, S.; Shahidi, F.; Zhao, H. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar]
- Arcambal, A.; Taïlé, J.; Rondeau, P.; Viranaïcken, W.; Meilhac, O.; Gonthier, M.-P. Hyperglycemia modulates redox, inflammatory and vasoactive markers through specific signaling pathways in cerebral endothelial cells: Insights on insulin protective action. Free Radic. Biol. Med. 2019, 130, 59–70. [Google Scholar] [CrossRef]
- Samarghandian, S.; Borji, A.; Farkhondeh, T. Attenuation of oxidative stress and inflammation by Portulaca oleracea in streptozotocin-induced diabetic rats. J. Evid. Based Complement. Altern. Med. 2017, 22, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef]
- Hadi, H.A.; Suwaidi, J.A. Endothelial dysfunction in diabetes mellitus. Vasc. Health Risk Manag. 2007, 3, 853–876. [Google Scholar]
- Rashid, M.; Verhoeven, A.J.M.; Mulder, M.T.; Timman, R.; Ozcan, B.; van Beek-Nieuwland, Y.; Chow, L.M.; van de Laar, R.J.J.M.; Dik, W.A.; Sijbrands, E.J.G.; et al. The effect of monomeric and oligomeric FLAVAnols in patients with type 2 diabetes and microalbuminuria (FLAVA-trial): A double-blind randomized controlled trial. Clin. Nutr. 2021, 40, 5587–5594. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D. Dyslipidemia in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 150–159. [Google Scholar] [CrossRef]
- Goldberg, I.J. Diabetic Dyslipidemia: Causes and Consequences. J. Clin. Endocrinol. Metab. 2001, 86, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Meydani, M. Vitamin E and Atherosclerosis: Beyond Prevention of LDL Oxidation. J. Nutr. 2001, 131, 366S–368S. [Google Scholar] [CrossRef]
- Li, Y.-W.; Kao, T.-W.; Chang, P.-K.; Chen, W.-L.; Wu, L.-W. Atherogenic index of plasma as predictors for metabolic syndrome, hypertension and diabetes mellitus in Taiwan citizens: A 9-year longitudinal study. Sci. Rep. 2021, 11, 9900. [Google Scholar] [CrossRef] [PubMed]
- Madhuri, K.; Naik, P.R. Ameliorative effect of borneol, a natural bicyclic monoterpene against hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic Wistar rats. Biomed. Pharmacother. 2017, 96, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Saikia, H.; Lama, A. Effect of Bougainvillea spectabilis leaves on serum lipids in albino rats fed with high fat diet. Int. J. Pharm. Sci. Drug Res. 2011, 3, 141–145. [Google Scholar]
- Geethan, P.K.M.A.; Prince, P.S.M. Antihyperlipidemic effect of D-pinitol on streptozotocin-induced diabetic wistar rats. J. Biochem. Mol. Toxicol. 2008, 22, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Giunti, S.; Barit, D.; Cooper, M.E. Mechanisms of diabetic nephropathy: Role of hypertension. Hypertension. 2006, 48, 519–526. [Google Scholar] [CrossRef]
- Samadi, M.; Aziz, S.G.; Naderi, R. The effect of tropisetron on oxidative stress, SIRT1, FOXO3a, and claudin-1 in the renal tissue of STZ-induced diabetic rats. Cell Stress Chaperones. 2021, 26, 217–227. [Google Scholar] [CrossRef]
- Dango, D.; Umeta, M.; Genet, S.; Menon, M.; Kebede, T.; Beker, J. Profiles of liver function tests among type 2 diabetic patients who are receiving different anti-diabetic drugs attending tikur anbessa specialized hospitals. J. Pharm. Pharm. 2016, 7, 2153-0645. [Google Scholar] [CrossRef]
- Eluehike, N.; Innih, S.O.; Ukwuonwo-Ediale, A.C.; Onoagbe, I.O. Liver function status in Streptozotocin Induced Diabetic Rats Treated with Extracts of some Anti-diabetic Medicinal plants. J. Appl. SCI Environ. Manag. 2022, 26, 399–405. [Google Scholar] [CrossRef]
- Agrawal, S.; Dhiman, R.K.; Limdi, J.K. Evaluation of abnormal liver function tests. Postgrad. Med. J. 2016, 92, 223. [Google Scholar] [CrossRef] [PubMed]
- Beyer, J.; Peters, F.T.; Kraemer, T.; Maurer, H.H. Detection and validated quantification of toxic alkaloids in human blood plasma—Comparison of LC-APCI-MS with LC-ESI-MS/MS. J. Mass. Spectrom. 2007, 42, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hidalgo, M.; León-González, A.J.; Gálvez-Peralta, M.; González-Mauraza, N.H.; Martin-Cordero, C. d-Pinitol: A cyclitol with versatile biological and pharmacological activities. Phytochem. Rev. 2021, 20, 211–224. [Google Scholar] [CrossRef]
- Azab, A. D-Pinitol—Active Natural Product from Carob with Notable Insulin Regulation. Nutrients 2022, 14, 1453. [Google Scholar] [CrossRef]
- Abo-Elghiet, F.; Abd-elsttara, A.; Metwaly, A.M.; Mohammad, A.-E.I. Antioxidant, anti-inflammatory, and antimicrobial evaluation of Terminalia arjuna leaves, fruits, and bark. Azhar Int. J. Pharm. Med Sci. 2022, 2, 148–158. [Google Scholar] [CrossRef]
- Clark, J.D.; Gebhart, G.F.; Gonder, J.C.; Keeling, M.E.; Kohn, D.F. The 1996 guide for the care and use of laboratory animals. ILAR J. 1997, 38, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Jaishree, V.; Narsimha, S. Swertiamarin and quercetin combination ameliorates hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced type 2 diabetes mellitus in wistar rats. Biomed. Pharmacother. 2020, 130, 110561. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Subramanya, S.; Kim, S.-S.; Abraham, S.; Yao, J.; Kumar, M.; Kumar, P.; Haridas, V.; Lee, S.-K.; Shultz, L.D.; Greiner, D.; et al. Targeted delivery of small interfering RNA to human dendritic cells to suppress dengue virus infection and associated proinflammatory cytokine production. J. Virol. 2010, 84, 2490–2501. [Google Scholar] [CrossRef]
- Samuvel, D.J.; Sundararaj, K.P.; Li, Y.; Lopes-Virella, M.F.; Huang, Y. Adipocyte-mononuclear cell interaction, Toll-like receptor 4 activation, and high glucose synergistically up-regulate osteopontin expression via an interleukin 6-mediated mechanism. J. Biol. Chem. 2010, 285, 3916–3927. [Google Scholar] [CrossRef]
- Lynch, D.F., Jr.; Hassen, W.; Clements, M.A.; Schellhammer, P.F.; Wright, G.L., Jr. Serum levels of endothelial and neural cell adhesion molecules in prostate cancer. Prostate 1997, 32, 214–220. [Google Scholar] [CrossRef]
- Ben Khaled, H.; Ghlissi, Z.; Chtourou, Y.; Hakim, A.; Ktari, N.; Fatma, M.A.; Barkia, A.; Sahnoun, Z.; Nasri, M. Effect of protein hydrolysates from sardinelle (Sardinella aurita) on the oxidative status and blood lipid profile of cholesterol-fed rats. Food Res. Int. 2012, 45, 60–68. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Malaspina, J.P.; Bussiére, H.; Le Calve, G. The total cholesterol/hdl cholesterol ratio: A suitable atherogenesis index. Atherosclerosis 1981, 40, 373–375. [Google Scholar] [CrossRef]
- Salama, S.A.; Arab, H.H.; Maghrabi, I.A.; Hassan, M.H.; AlSaeed, M.S. Gamma-Glutamyl Cysteine Attenuates Tissue Damage and Enhances Tissue Regeneration in a rat Model of Lead-Induced Nephrotoxicity. Biol. Trace Elem. Res. 2016, 173, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Belfield, A.; Goldberg, D. Colorimetric determination of alkaline phosphatase activity. Enzyme 1971, 12, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Walters, M.I.; Gerarde, H.W. An ultramicromethod for the determination of conjugated and total bilirubin in serum or plasma. Microchem. J. 1970, 15, 231–243. [Google Scholar] [CrossRef]
- Hoefs, J.C. Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J. Lab. Clin. Med. 1983, 102, 250–259. [Google Scholar]
- Carleton, H.M.; Drury, R.A.B.; Wallington, E.A. Carleton’s Histological Technique; Oxford University Press: New York, NY, USA, 1980. [Google Scholar]
- Korany, R.M.; Ahmed, K.S.; Halawany, H.A.; Ahmed, K.A. Effect of long-term arsenic exposure on female Albino rats with special reference to the protective role of Spirulina platensis. Explor. Anim. Med. Res. 2019, 9, 125–136. [Google Scholar]
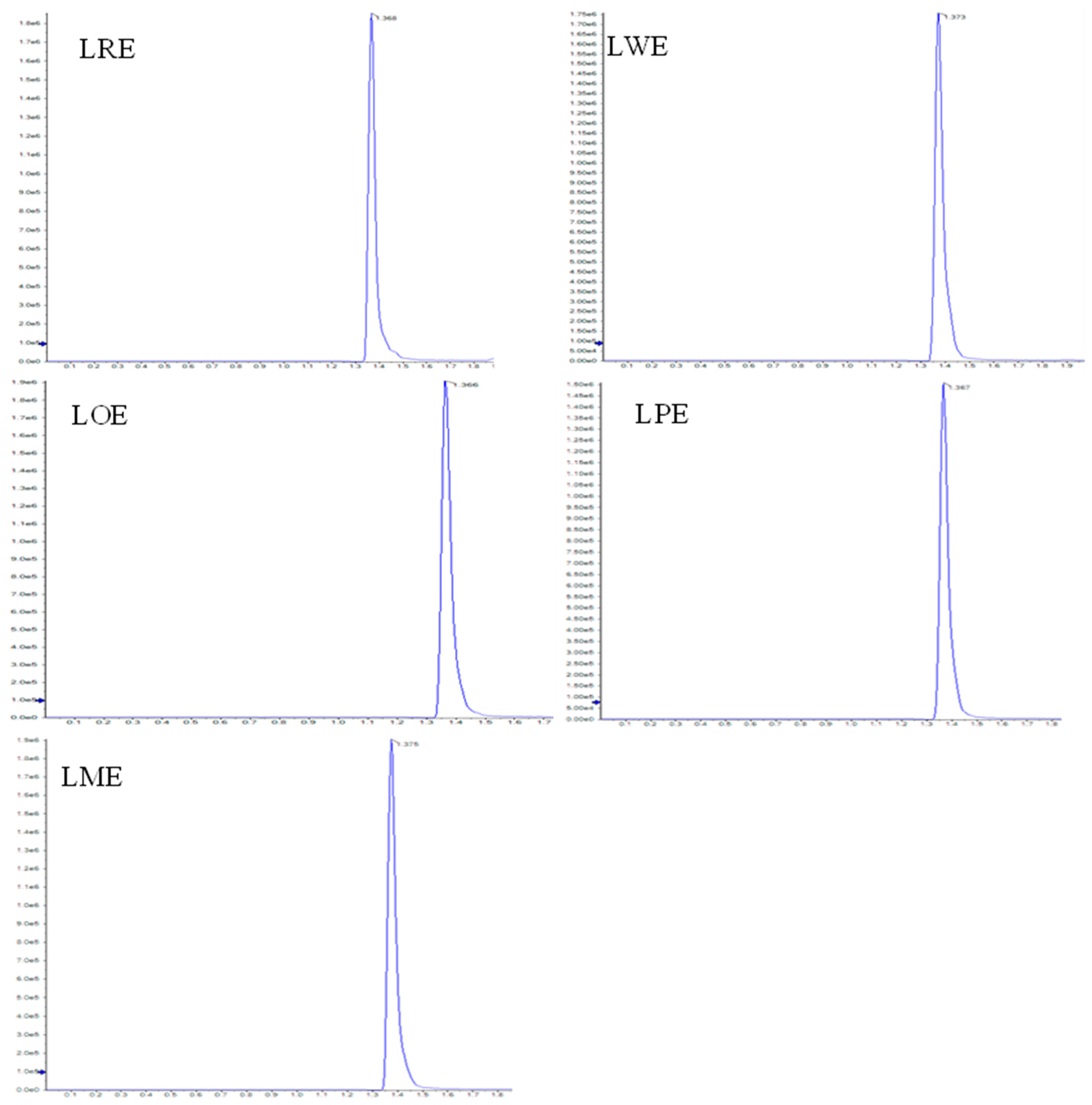



| Leaves Methanolic Extracts of B. spectabilis Cultivars | D-Pinitol Conc. (mg/g Extract) |
|---|---|
| LRE | 5.08 |
| LWE | 5.25 |
| LOE | 6.95 |
| LPE | 6.17 |
| LME | 6.66 |
| Groups | Liver Lesions | Pancreas Lesions | |||
|---|---|---|---|---|---|
| Vacuolar Degeneration of Hepatocytes | Distortion and Atrophy of Islets of Langerhans | Interstitial Congestion, Edema, and Hemorrhage | Periductal Fibrosis | Degeneration of the Exocrine Pancreatic Tissue | |
| Normal control | 0 | 0 | 0 | 0 | 0 |
| STZ-diabetic control | 3 | 3 | 3 | 3 | 3 |
| Glibenclamide | 1 | 0 | 0 | 0 | 1 |
| LRE | 2 | 1 | 1 | 1 | 2 |
| LWE | 2 | 1 | 1 | 0 | 1 |
| LOE | 1 | 1 | 0 | 0 | 1 |
| LPE | 1 | 1 | 1 | 0 | 1 |
| LME | 1 | 1 | 0 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abo-Elghiet, F.; Ahmed, A.H.; Aly, H.F.; Younis, E.A.; Rabeh, M.A.; Alshehri, S.A.; Alshahrani, K.S.A.; Mohamed, S.A. D-Pinitol Content and Antioxidant and Antidiabetic Activities of Five Bougainvillea spectabilis Willd. Cultivars. Pharmaceuticals 2023, 16, 1008. https://doi.org/10.3390/ph16071008
Abo-Elghiet F, Ahmed AH, Aly HF, Younis EA, Rabeh MA, Alshehri SA, Alshahrani KSA, Mohamed SA. D-Pinitol Content and Antioxidant and Antidiabetic Activities of Five Bougainvillea spectabilis Willd. Cultivars. Pharmaceuticals. 2023; 16(7):1008. https://doi.org/10.3390/ph16071008
Chicago/Turabian StyleAbo-Elghiet, Fatma, Amal H. Ahmed, Hanan F. Aly, Eman A. Younis, Mohamed A. Rabeh, Saad Ali Alshehri, Khalid S. A. Alshahrani, and Shaza A. Mohamed. 2023. "D-Pinitol Content and Antioxidant and Antidiabetic Activities of Five Bougainvillea spectabilis Willd. Cultivars" Pharmaceuticals 16, no. 7: 1008. https://doi.org/10.3390/ph16071008
APA StyleAbo-Elghiet, F., Ahmed, A. H., Aly, H. F., Younis, E. A., Rabeh, M. A., Alshehri, S. A., Alshahrani, K. S. A., & Mohamed, S. A. (2023). D-Pinitol Content and Antioxidant and Antidiabetic Activities of Five Bougainvillea spectabilis Willd. Cultivars. Pharmaceuticals, 16(7), 1008. https://doi.org/10.3390/ph16071008