Binary Polymeric Surfactant Mixtures for the Development of Novel Loteprednol Etabonate Nanomicellar Eyedrops
Abstract
1. Introduction
2. Results and Discussion
2.1. Development of LE-Loaded Mixed Nanomicelles (LE-MixNano)
2.2. CMC Determinations and Synergistic Effect of Surfactant Mixtures
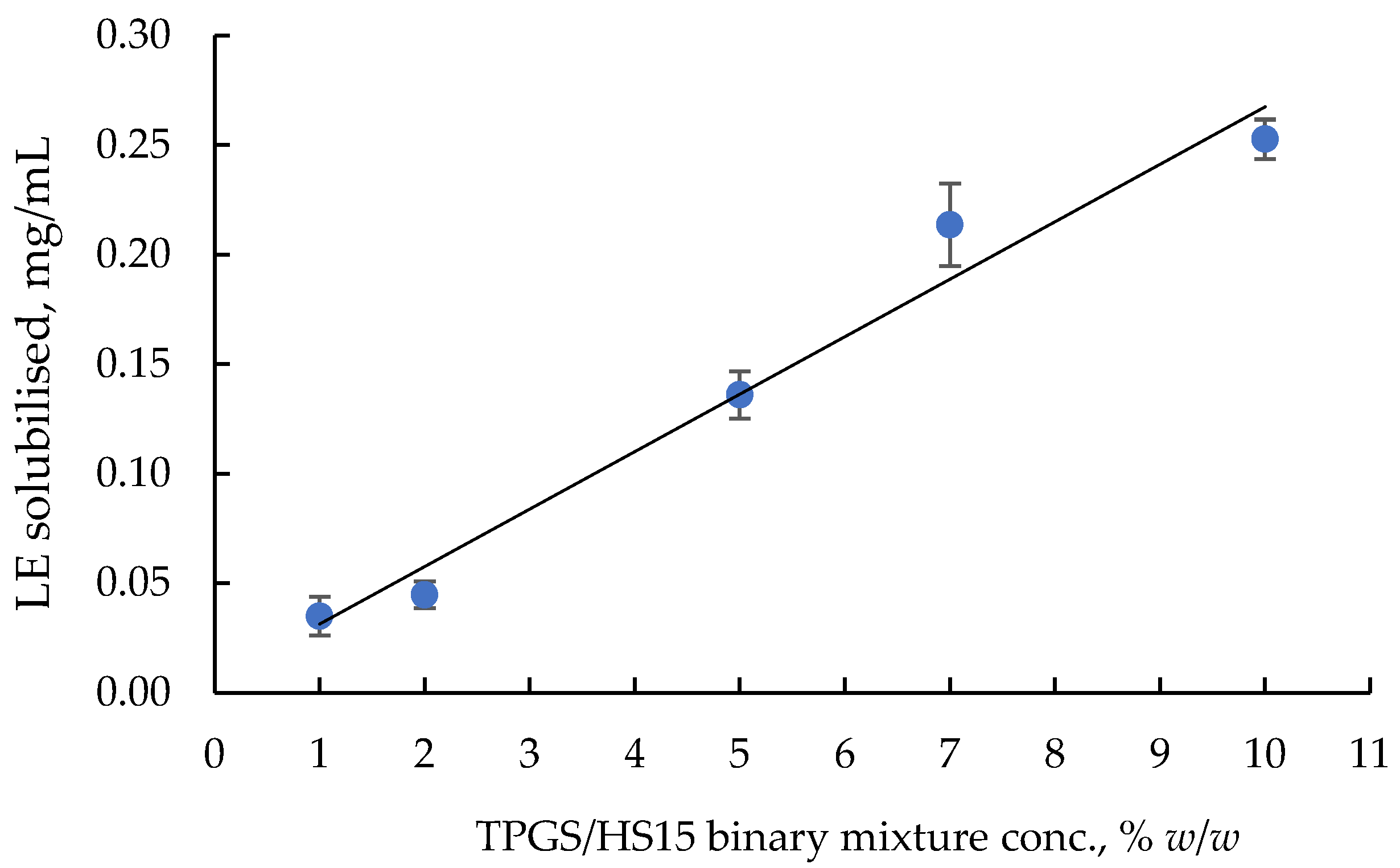
2.3. Optimisation of LE-TPGS/HS Nanomicelles
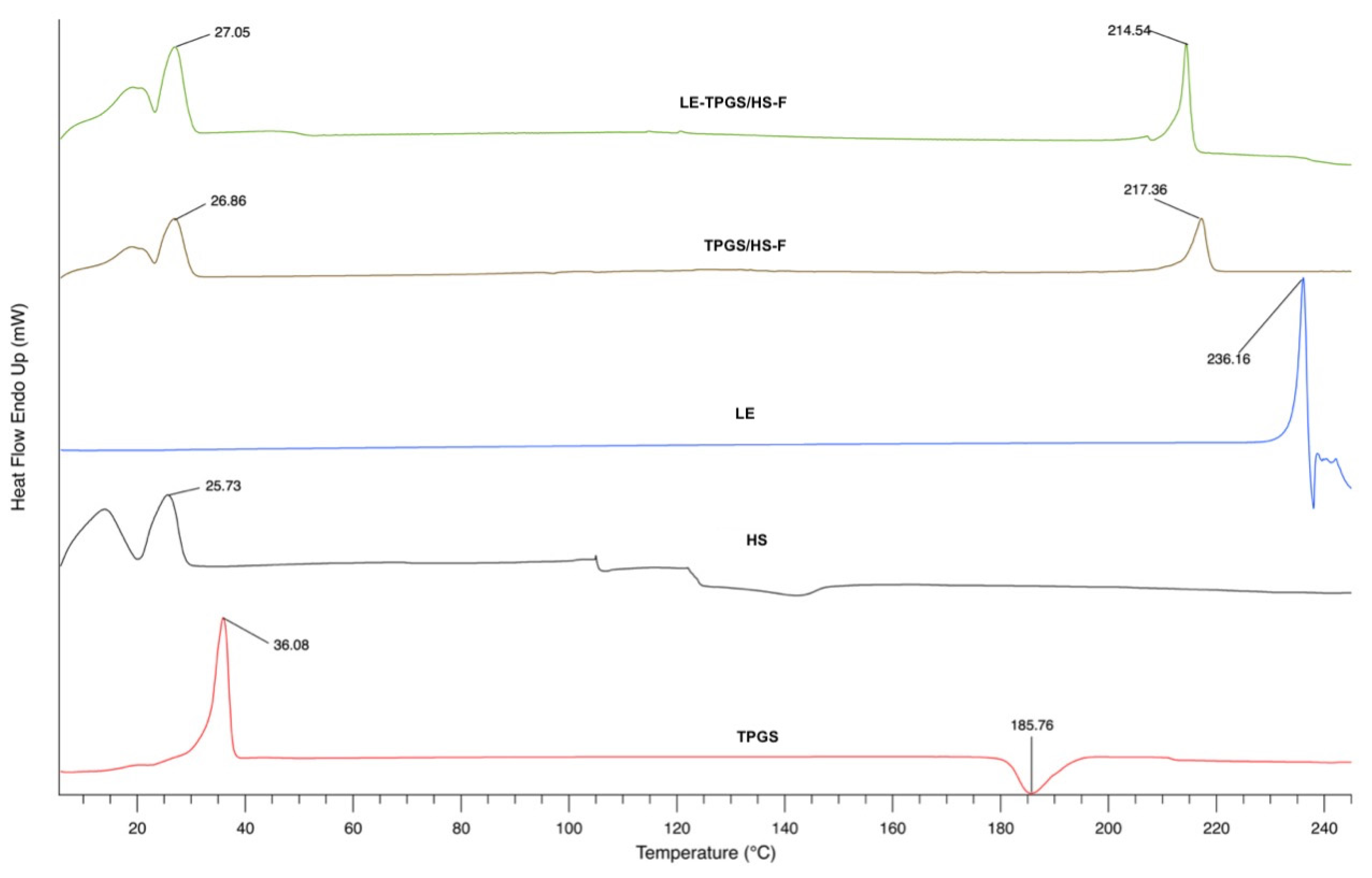
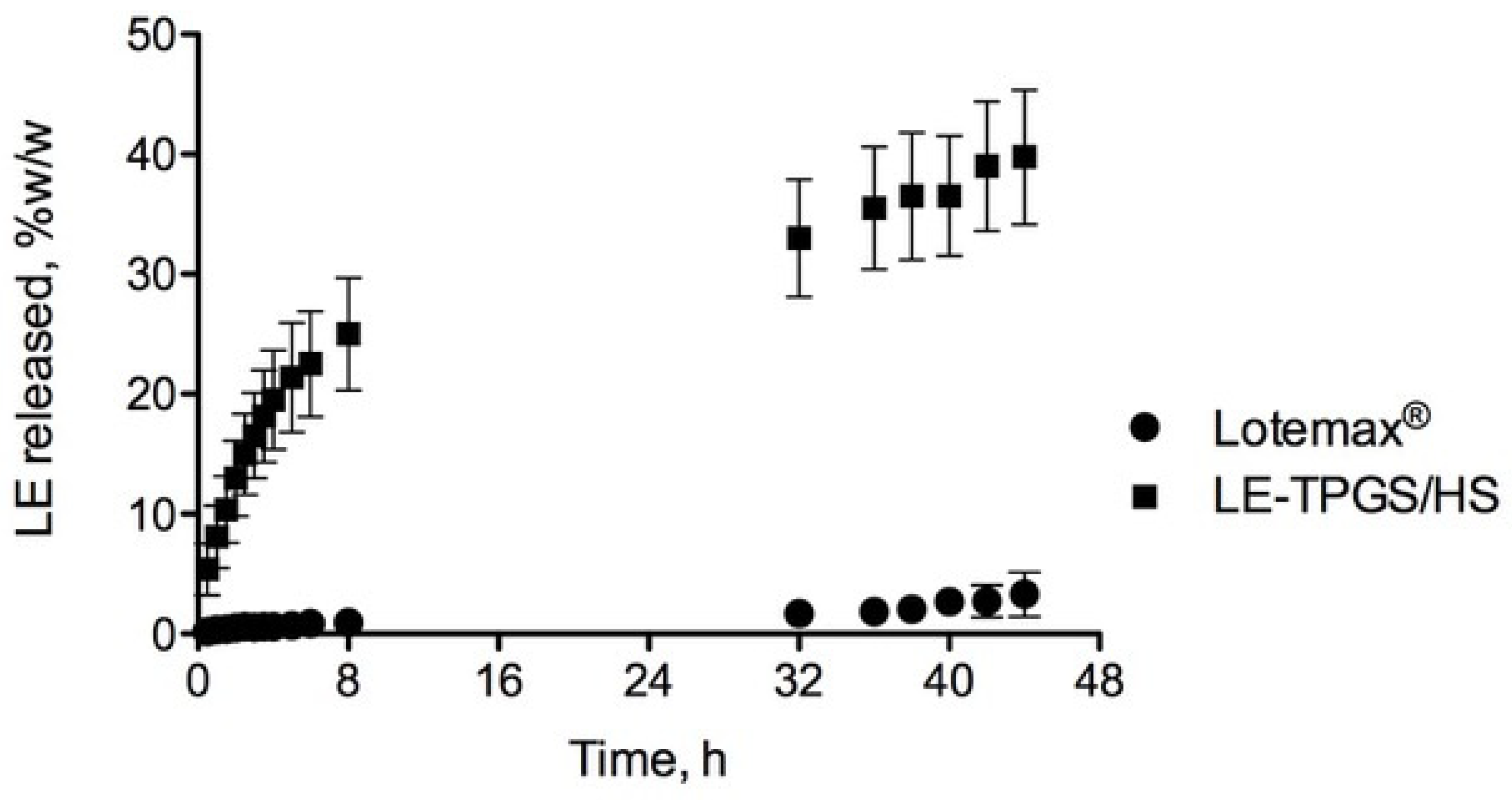
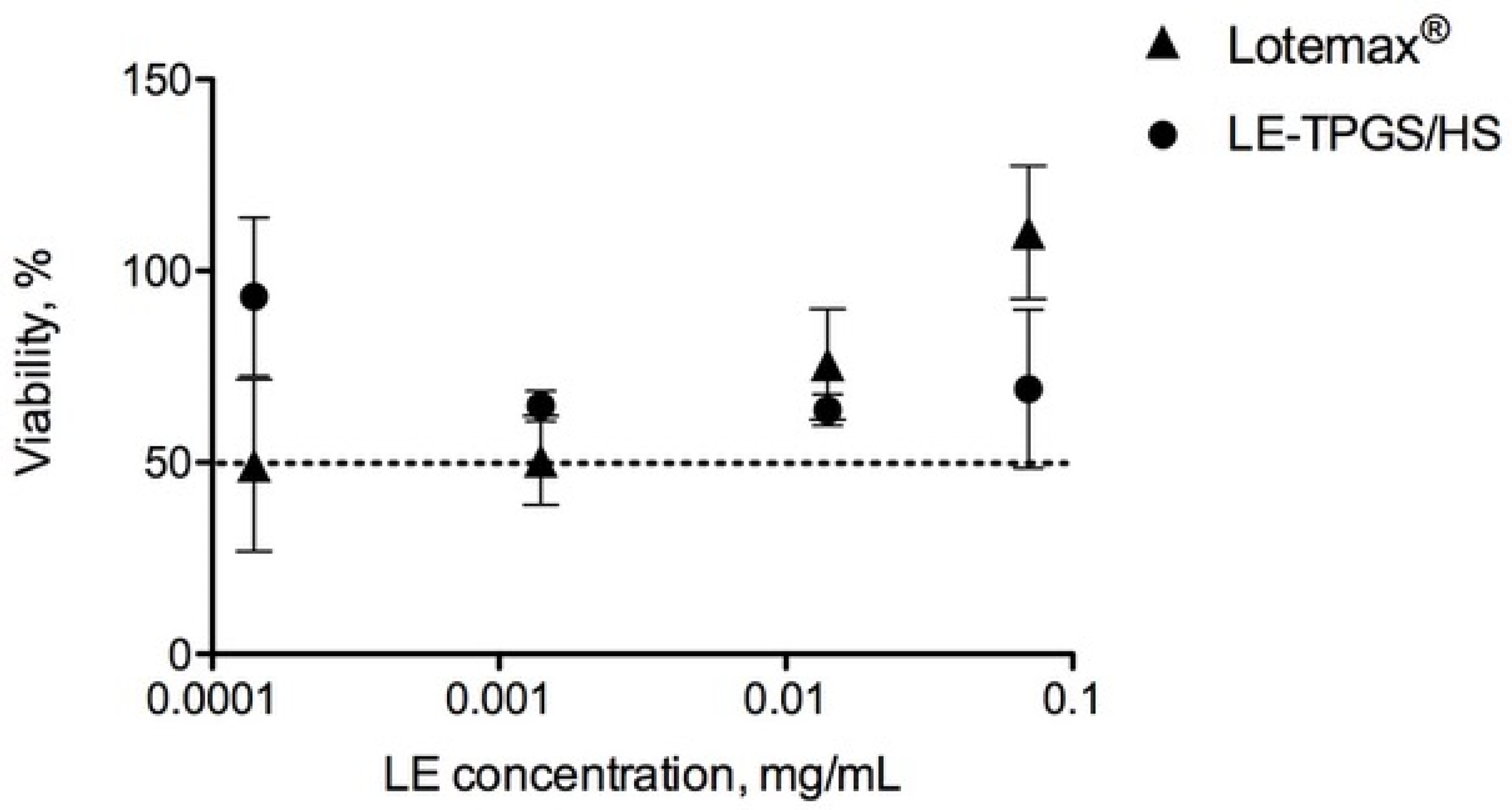
2.4. Physico-Chemical and Biological Characterisation of LE-Loaded Mixed Nanomicelles (LE-TPGS/HS)
3. Materials and Methods
3.1. Chemicals
3.2. Cell Cultures
3.3. HPLC Analytical Method
3.4. Solubility Study of Loteprednol Etabonate
3.5. Preparation of LE-Loaded Mixed Nanomicelles (LE-MixNano)
3.6. Characterisation of LE-Loaded Mixed Nanomicelles (LE-MixNano)
3.6.1. Size Distribution and Polydispersity Index analysis
3.6.2. Determination of the Amount of Solubilised LE (LE-In) and of LE Encapsulation Efficiency (LE-EE) in MixNano Formulations
3.6.3. Evaluation of the Clarity and Filterability of LE-MixNano Formulations
3.6.4. Investigation on the Synergism between the Different Surfactant Mixtures
3.6.5. Influence of Concentration of TPGS/HS Mixture on LE Solubilisation
3.6.6. Determination of Experimental Critical Micellar Concentration (CMCexp)
3.7. Freeze-Drying of the Selected LE-MixNano Formulation
3.8. Thermal Analysis of the Freeze-Dried Formulations by Differential Scanning Calorimetry (DSC)
3.9. Cytotoxicity Assay
3.10. Physico-Chemical Stability of the Selected Nanomicellar Formulation
3.11. In Vitro LE Release Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazet, R.; Yaméogo, J.B.G.; Wouessidjewe, D.; Choisnard, L.; Gèze, A. Recent advances in the design of topical ophthalmic delivery systems in the treatment of ocular surface inflammation and their biopharmaceutical evaluation. Pharmaceutics 2020, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- Gaballa, S.A.; Kompella, U.B.; Elgarhy, O.; Alqahtani, A.M.; Pierscionek, B.; Alany, R.G.; Abdelkader, H. Corticosteroids in ophthalmology: Drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Deliv. Transl. Res. 2021, 11, 866–893. [Google Scholar] [CrossRef] [PubMed]
- McGhee, C.N.J.; Dean, S.; Danesh-Meyer, H. Locally administered ocular corticosteroids: Benefits and risks. Drug Saf. 2002, 25, 33–55. [Google Scholar] [CrossRef]
- Shen, L.; Fang, G.; Tang, B.; Zhu, Q. Enhanced topical corticosteroids delivery to the eye: A trade-off in strategy choice. J. Control. Release 2021, 339, 91–113. [Google Scholar] [CrossRef]
- Patton, T.F.; Robinson, J.R. Quantitative precorneal disposition of topically applied pilocarpine nitrate in rabbit eyes. J. Pharm. Sci. 1976, 65, 1295–1301. [Google Scholar] [CrossRef]
- Sieg, J.W.; Robinson, J.R. Vehicle effects on ocular drug bioavailability II: Evaluation of pilocarpine. J. Pharm. Sci. 1977, 66, 1222–1228. [Google Scholar] [CrossRef]
- Hui, H.-W.; Robinson, J.R. Effect of particle dissolution rate on ocular drug bioavailability. J. Pharm. Sci. 1986, 75, 280–287. [Google Scholar] [CrossRef]
- Ali, Y.; Lehmussaari, K. Industrial perspective in ocular drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.S.M.; York, P.; Ali, A.M.A.; Blagden, N. Hydrocortisone nanosuspensions for ophthalmic delivery: A comparative study between microfluidic nanoprecipitation and wet milling. J. Control. Release 2011, 149, 175–181. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Golovkin, A.S.; Kudryavtsev, I.V.; Prikhodko, S.S.; Trulioff, A.S.; Bokatyi, A.N.; Poshina, D.N.; Raik, S.V.; Skorik, Y.A. Mucoadhesive cholesterol-chitosan self-assembled particles for topical ocular delivery of dexamethasone. Int. J. Biol. Macromol. 2020, 158, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Balla, A.; Ruponen, M.; Valtari, A.; Toropainen, E.; Tuomainen, M.; Alvarez-Lorenzo, C.; Del Amo, D.M.; Urtti, A.; Vellonen, K.-S. Understanding dexamethasone kinetics in the rabbit tear fluid: Drug release and clearance from solution, suspension and hydrogel formulations. Eur. J. Pharm. Biopharm. 2022, 172, 53–60. [Google Scholar] [CrossRef]
- Mathiowitz, E.; Chickering, D.; Lehr, C. Bioadhesive Drug Delivery Systems; Marcel Dekker: New York, NY, USA, 1999; pp. 179–202. [Google Scholar]
- Mandal, A.; Patel, P.; Pal, D.; Mitra, A.K. Multi-Layered nanomicelles as self-assembled nanocarrier systems for ocular peptide delivery. AAPS PharmSciTech 2019, 20, 66. [Google Scholar] [CrossRef] [PubMed]
- Kashapov, R.; Gaynanova, G.; Gabdrakhmanov, D.; Kuznetsov, D.; Pavlov, R.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-assembly of amphiphilic compounds as a versatile tool for construction of nanoscale drug carriers. Int. J. Mol. Sci. 2020, 21, 6961. [Google Scholar] [CrossRef] [PubMed]
- Terreni, E.; Chetoni, P.; Tampucci, S.; Burgalassi, S.; Al-Kinani, A.A.; Alany, R.G.; Monti, D. Assembling surfactants-mucoadhesive polymer nanomicelles (ASMP-Nano) for ocular delivery of cyclosporine-A. Pharmaceutics 2020, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Uchegbu, I.F.; Breznikar, J.; Zaffalon, A.; Odunze, U.; Schätzlein, A.G. Polymeric micelles for the enhanced deposition of hydrophobic drugs into ocular tissues, without plasma exposure. Pharmaceutics 2021, 13, 744. [Google Scholar] [CrossRef]
- Comstock, T.L.; Sheppard, J.D. Loteprednol etabonate for inflammatory conditions of the anterior segment of the eye: Twenty years of clinical experience with a retrometabolically designed corticosteroid. Expert Opin. Pharmacother. 2018, 19, 337–353. [Google Scholar] [CrossRef]
- Coffey, M.J.; DeCory, H.H.; Lane, S.S. Development of a non-settling gel formulation of 0.5% loteprednol etabonate for anti-inflammatory use as an ophthalmic drop. Clin. Ophthalmol. 2013, 7, 299–312. [Google Scholar] [CrossRef][Green Version]
- Kang, C.; Keam, J.S.; Shirley, M.; Syed, Y.Y. Loteprednol etabonate (submicron) ophthalmic gel 0.38%: A review in post-operative inflammation and pain following ocular surgery. Clin. Drug Investig. 2020, 40, 387–394. [Google Scholar] [CrossRef]
- Popov, A. mucus-penetrating particles and the role of ocular mucus as a barrier to micro- and nanosuspensions. J. Ocul. Pharmacol. Ther. 2020, 36, 366–375. [Google Scholar] [CrossRef]
- Beckman, K.; Katz, J.; Majmudar, P.; Rostov, A. Loteprednol etabonate for the treatment of dry eye disease. J. Ocul. Pharmacol. Ther. 2020, 36, 497–511. [Google Scholar] [CrossRef]
- Patel, N.; Nakrani, H.; Raval, M.; Sheth, N. Development of loteprednol etabonate-loaded cationic nanoemulsified in-situ ophthalmic gel for sustained delivery and enhanced ocular bioavailability. Drug Deliv. 2016, 23, 3712–3723. [Google Scholar] [CrossRef]
- El-Aazeem Soliman, O.A.; Mohamed, E.A.M.; El-Dahan, M.S.; Khatera, N.A.A. Potential use of cyclodextrin complexes for enhanced stability, anti-inflammatory efficacy, and ocular bioavailability of loteprednol etabonate. AAPS PharmSciTech 2016, 18, 1228–1241. [Google Scholar] [CrossRef]
- Sah, A.K.; Suresh, P.K.; Verma, V.K. PLGA nanoparticles for ocular delivery of loteprednol etabonate: A corneal penetration study. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1156–1164. [Google Scholar] [CrossRef]
- Nasr, F.H.; Khoee, S. Design, characterization and in vitro evaluation of novel shell crosslinked poly(butylene adipate)-co-N-succinyl chitosan nanogels containing loteprednol etabonate: A new system for therapeutic effect enhancement via controlled drug delivery. Eur. J. Med. Chem. 2015, 102, 132–142. [Google Scholar] [CrossRef]
- Sharma, P.K.; Sharma, H.P.; Chakole, C.M.; Pandey, J.; Chauhan, M.K. Application of Vitamin E TPGS in ocular therapeutics—Attributes beyond excipient. J. Indian Chem. Soc. 2022, 99, 100387. [Google Scholar] [CrossRef]
- Kang, H.; Cha, K.-H.; Cho, W.; Park, J.; Park, H.J.; Sun, B.K.; Hyun, S.-M.; Hwang, S.-J. Cyclosporine amicellar delivery system for dry eyes. Int. J. Nanomed. 2016, 11, 2921–2933. [Google Scholar] [CrossRef]
- Zhoua, T.; Zhub, L.; Xiaa, H.; Hea, J.; Liua, S.; Hea, S.; Wanga, L.; Zhanga, J. Micelle carriers based on macrogol 15 hydroxystearate for ocular delivery of terbinafine hydrochloride: In vitro characterization and in vivo permeation. Eur. J. Pharm. Sci. 2017, 109, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Soluplus micelles for acyclovir ocular delivery: Formulation and cornea and sclera permeability. Int. J. Pharm. 2018, 552, 39–47. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, F.; Lan, J.; Sun, F.; Li, J.; Li, M.; Song, K.; Wua, X. Ultra-small micelles based on polyoxyl 15 hydroxystearate for ocular delivery of myricetin: Optimization, in vitro, and in vivo evaluation. Drug Deliv. 2019, 26, 158–167. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, X.; Voo, Z.X.; Yap, S.S.L.; Yang, C.; Gao, S.; Liu, S.; Venkataraman, S.; Obuobi, S.A.O.; Khara, J.S.; et al. Biodegradable functional polycarbonate micelles for controlled release of amphotericin B. Acta Biomater. 2016, 46, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Miyagishi, S.; Okada, K.; Asakawa, T. Salt effect on critical micelle concentrations of nonionic surfactants, N-Acyl-N-methylglucamides (MEGA-n). J. Colloid Interface Sci. 2001, 238, 91–95. [Google Scholar] [CrossRef]
- Clint, J.H. Micellization of mixed nonionic surface active agents. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1975, 71, 1327–1334. [Google Scholar] [CrossRef]
- Bernabeu, E.; Gonzalez, L.; Cagel, M.; Gergic, E.P.; Moretton, M.A.; Chiappetta, D.A. Novel Soluplus®-TPGS mixed micelles for encapsulation of paclitaxel with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines. Colloids Surf. B Biointerfaces 2016, 140, 403–411. [Google Scholar] [CrossRef]
- Terreni, E.; Burgalassi, S.; Chetoni, P.; Tampucci, S.; Zucchetti, E.; Fais, R.; Ghelardi, D.E.; Lupetti, A.; Monti, D. Development and characterization of a novel peptide-loaded antimicrobial ocular insert. Biomolecules 2020, 10, 664. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, C.; Liu, W.; Yang, M.; Xu, B.; Chen, Y. Fast in vitro release and in vivo absorption of an anti-schizophrenic drug paliperidone from Its Soluplus®/TPGS mixed micelles. Pharmaceutics 2022, 14, 889. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, T.; Qi, Y.; Zhang, Z. Recent advances in the application of Vitamin E TPGS for drug delivery. Theranostics 2018, 8, 464–485. [Google Scholar] [CrossRef]
- Younes, N.F.; Abdel-Halim, S.A.; Elassasy, A.I. Solutol HS15 based binary mixed micelles with penetration enhancers for augmented corneal delivery of sertaconazole nitrate: Optimization, in vitro, ex vivo and in vivo characterization. Drug Deliv. 2018, 25, 1706–1717. [Google Scholar] [CrossRef]
- Bergonzi, M.C.; Vasarri, M.; Marroncini, G.; Barletta, E.; Degl’Innocenti, D. Thymoquinone-loaded Soluplus®-Solutol® HS15 mixed micelles: Preparation, in vitro characterization, and effect on the SH-SY5Y cell migration. Molecules 2020, 25, 4707. [Google Scholar] [CrossRef] [PubMed]
- Malkawi, A.; Jalil, A.; Nazir, I.; Matuszczak, B.; Kennedy, R.; Bernkop-Schnürch, A. Self-emulsifying drug delivery systems: Hydrophobic drug polymer. Complexes provide a sustained release in vitro. Mol. Pharm. 2020, 17, 3709–3719. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, P.S.; Nadaf, S.; Manjappa, A.S.; Jha, N.K.; Shinde, S.S.; Chopade, S.S.; Shete, A.S.; Disouza, J.; Sambamoorthy, U.; Kum, S.A. D-α-tocopheryl polyethylene glycol succinate: A review of multifarious applications in nanomedicines. OpenNano 2022, 6, 100036. [Google Scholar] [CrossRef]
- Chetoni, P.; Burgalassi, S.; Zucchetti, E.; Granchi, C.; Minutolo, F.; Tampucci, S.; Monti, D. MAGL inhibitor NanoMicellar formulation (MAGL-NanoMicellar) for the development of an antiglaucoma eye drop. Int. J. Pharm. 2022, 625, 122078. [Google Scholar] [CrossRef]
- Makwana, S.B.; Patel, V.A.; Parmar, S.J. Development and characterization of in-situ gel for ophthalmic formulation containing ciprofloxacin hydrochloride. Results Pharma Sci. 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rubingh, D.N. Mixed Micelle Solutions; Springer: Berlin/Heidelberg, Germany, 1979; pp. 337–354. [Google Scholar]
- Zhang, Y.; Lam, Y.M. Study of mixed micelles and interaction parameters for polymeric nonionic and normal surfactants. J. Nanosci. Nanotechnol. 2006, 6, 3877–3881. [Google Scholar] [CrossRef] [PubMed]
- Burgalassi, S.; Zucchetti, E.; Ling, L.; Chetoni, P.; Tampucci, S.; Monti, D. Hydrogels as corneal stroma substitutes for in vitro evaluation of drug ocular permeation. Pharmaceutics 2022, 14, 850. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.D.; Neeson, M.J.; Dagastine, R.R.; Chan, D.Y.C.; Tabor, R.F. Measurement of surface and interfacial tension using pendant drop tensiometry. J. Colloid Interface Sci. 2015, 454, 226–237. [Google Scholar] [CrossRef]
- Burgalassi, S.; Monti, D.; Chetoni, P.; Saettone, M.F. Cytotoxicity of potential ocular permeation enhancers evaluated on rabbit and human corneal epithelial cell lines. Toxicol. Lett. 2001, 122, 1–8. [Google Scholar] [CrossRef]
- Chetoni, P.; Monti, D.; Tampucci, S.; Matteoli, B.; Ceccherini-Nelli, L.; Subissi, A.; Burgalassi, S. Liposomes as a potential ocular delivery system of distamycin A. Int. J. Pharm. 2015, 492, 120–126. [Google Scholar] [CrossRef]
- Cavet, M.E.; Glogowski, S.; Lowe, E.R.; Phillips, E. Rheological Properties, Dissolution kinetics, and ocular pharmacokinetics of loteprednol etabonate (submicron) ophthalmic gel 0.38%. J. Ocul. Pharmacol. Ther. 2019, 35, 291–300. [Google Scholar] [CrossRef]
- Toropainen, E.; Fraser-Miller, S.J.; Novakovic, D.; Del Amo, E.M.; Vellonen, K.-S.; Ruponen, M.; Viitala, T.; Korhonen, O.; Auriola, S.; Hellinen, L.; et al. Biopharmaceutics of topical ophthalmic suspensions: Importance of viscosity and particle size in ocular absorption of indomethacin. Pharmaceutics 2021, 13, 452. [Google Scholar] [CrossRef]
- Sheppard, J.D.; Cockrum, P.C.; Justice, A.; Jasek, M.C. In vivo pharmacokinetics of bromfenac ophthalmic solution 0.075%, bromfenac ophthalmic solution 0.07%, and nepafenac/amfenac ophthalmic suspension 0.3% in rabbits. Ophthalmol. Ther. 2018, 7, 157–165. [Google Scholar] [CrossRef]

| Types of LE-MixNano | LE-In (mg/mL) | LE-EE (% w/w) | Dh (nm) | PI | Clarity | Filterability |
|---|---|---|---|---|---|---|
| LE-TPGS/HS | 0.253 (±0.007) | 25.3 (±0.7) | 13.57 (±0.49) | 0.271 (±0.110) | +++ | ++ |
| LE-TPGS/SolP | 0.192 (±0.003) | 19.2 (±0.3) | 17.93 (±0.91) | 0.343 (±0.150) | + | + |
| LE-TPGS/EL | 0.200 (±0.010) | 20.1 (±1.0) | 12.03 (±0.25) | 0.303 (±0.091) | ++ | ++ |
| LE-HS/EL | 0.129 (±0.002) | 12.9 (±0.2) | 13.47 (±0.31) | 0.145 (±0.115) | ++ | ++ |
| LE-HS/SolP | 0.176 (±0.007) | 17.6 (±0.7) | 85.13 (±0.81) | 0.507 (±0.020) | + | + |
| LE-EL/SolP | 0.121 (±0.004) | 12.1 (±0.4) | 68.27 (±1.63) | 0.471 (±0.034) | + | + |
| Polymeric Surfactant | CMCexp (mM) | CMCexp (% w/w) | CMCtheor (mM) | CMCtheor (% w/w) |
|---|---|---|---|---|
| TPGS | 15.33 × 10−2 | 2.32 × 10−2 | 13.22 × 10−2 | 2.00 × 10−2 |
| SolP | 7.12 × 10−5 | 0.84 × 10−3 | 6.44 × 10−5 | 7.60 × 10−4 |
| EL | 163.06 × 10−2 | 2.22 × 10−2 | 146.90 × 10−2 | 2.00 × 10−2 |
| HS | 7.83 × 10−2 | 1.22 × 10−2 | 8.74 × 10−2 # | 1.25 × 10−2 # |
| Binary Mixtures | α1 | (mM) | |
|---|---|---|---|
| TPGS/HS | 0.4859 | 0.1027 | 32.56 × 10−2 |
| TPGS/SolP | 0.9873 | 0.0054 | 2.31 × 10−4 |
| TPGS/EL | 0.0825 | 0.9082 | 44.78 × 10−2 |
| HS/EL | 0.0869 | 0.5988 | 62.47 × 10−2 |
| HS/SolP | 0.9880 | 0.0055 | 4.51 × 10−4 |
| EL/SolP | 0.9989 | 0.0595 | 2.18 × 10−5 |
| Type of Nanomicelles | CMCTPGS (mM) | CMCHS (mM) | CMC*TPGS/HS (mM) | CMCTPGS/HS (mM) | X1TPGS | TPGS | βTPGS/HS |
|---|---|---|---|---|---|---|---|
| LE-TPGS/HS | 0.1533 | 0.0783 | 0.1027 | 0.0983 | 0.3398 | 0.3256 | −0.1322 |
| Time Days | Dh nm | PI | LE Recovered % w/w | Clarity |
|---|---|---|---|---|
| 0 | 12.93 (±0.61) | 0.199 (±0.020) | 100.00 (±0.95) | +++ |
| 30 | 12.97 (±0.42) | 0.220 (±0.081) | 96.32 (±3.77) | +++ |
| 90 | 13.87 (±3.39) | 0.869 (±0.592) | 94.82 (±0.77) | +++ |
| Type of Polymer | HLB | CMCtheor % w/w |
|---|---|---|
| TPGS | 13 | 0.02 & |
| HS | 14–16 | 0.005–0.02 § |
| SolP | 14 | 0.00076 § |
| EL | 12–14 | 0.02 § |
| Clarity | Grade |
|---|---|
| Cloudy: the formulation is a milky-white solution. | + |
| Opaque: the formulation is colourless and slightly opalescent with suspended particles. | ++ |
| Transparent: the formulation is completely clear. | +++ |
| Filterability | Grade |
|---|---|
| Hard to filter: the formulation presents strong resistance to filtration, clogging the filter. | + |
| Filterable: the formulation is easily filtrable without any impediment. | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tampucci, S.; Monti, D.; Burgalassi, S.; Terreni, E.; Paganini, V.; Di Gangi, M.; Chetoni, P. Binary Polymeric Surfactant Mixtures for the Development of Novel Loteprednol Etabonate Nanomicellar Eyedrops. Pharmaceuticals 2023, 16, 864. https://doi.org/10.3390/ph16060864
Tampucci S, Monti D, Burgalassi S, Terreni E, Paganini V, Di Gangi M, Chetoni P. Binary Polymeric Surfactant Mixtures for the Development of Novel Loteprednol Etabonate Nanomicellar Eyedrops. Pharmaceuticals. 2023; 16(6):864. https://doi.org/10.3390/ph16060864
Chicago/Turabian StyleTampucci, Silvia, Daniela Monti, Susi Burgalassi, Eleonora Terreni, Valentina Paganini, Mariacristina Di Gangi, and Patrizia Chetoni. 2023. "Binary Polymeric Surfactant Mixtures for the Development of Novel Loteprednol Etabonate Nanomicellar Eyedrops" Pharmaceuticals 16, no. 6: 864. https://doi.org/10.3390/ph16060864
APA StyleTampucci, S., Monti, D., Burgalassi, S., Terreni, E., Paganini, V., Di Gangi, M., & Chetoni, P. (2023). Binary Polymeric Surfactant Mixtures for the Development of Novel Loteprednol Etabonate Nanomicellar Eyedrops. Pharmaceuticals, 16(6), 864. https://doi.org/10.3390/ph16060864











