Advanced Therapy Medicinal Products for Age-Related Macular Degeneration; Scaffold Fabrication and Delivery Methods
Abstract
1. Introduction
2. Pathogenesis of AMD and Treatment Options
3. Advanced Therapy Medicines for Treatment of AMD
| Methods | Product Name | Study Phase | Therapeutic Agent (s) | Method of Delivery | |
|---|---|---|---|---|---|
| Cell-Based Therapy | Scaffold-free | OpRegen [65] | Phase IIa | Human embryonic stem cell (hESC)-derived RPE cells | Subretinal administration as a cell suspension either in ophthalmic Balanced Salt Solution Plus (BSS Plus) or in CryoStor® 5 (Thaw-and-Inject, TAI) |
| RPESC-RPE-4W [79] | Phase I/IIa | Allogeneic RPE stem cell (RPESC)-derived RPE cells | Subretinal administration; RPESC-RPE cell obtained after 4 weeks of differentiation (RPESC-RPE-4W). The RPESC-RPE-4W progenitor stage cell has shown increased engraftment and vision rescue compared to more mature RPE cell products | ||
| AlloRx [80] | Phase I | Cultured allogeneic adult umbilical cord derived mesenchymal stem cells | Intravenous and sub-tenon administration; It has the potential to reduce inflammation through activation of anti-inflammatory biochemical and cellular pathways | ||
| Scaffold-based | iPSC-derived RPE/PLGA transplantation [66] | Phase I/IIa | iPSC-derived RPE | Subretinal administration; iPSCs are differentiated into RPE, which is grown as a monolayer on a thin poly lactic-co-glycolic acid (PLGA) scaffold | |
| CPCB-RPE1 [81] | Phase I/II | Human embryonic stem cell (hESC)-derived RPE cells | Subretinal administration; Implant is designed to replace the RPE and Bruch’s membrane in the eye that degenerate in AMD | ||
| PF-05206388 [82] | Phase I | Human embryonic stem cell derived retinal pigment epithelium | Subretinal administration; Monolayer of RPE cells immobilized on a polyester membrane It is a living tissue equivalent, which is designed to remain in situ life-long | ||
| Gene-Based Therapy | ADVM-022 [83] | Phase I | AAV.7m8 gene vector carrying a coding sequence for aflibercept | Intravitreal administration: One-time IVT administration of ADVM-022 provides durable expression of therapeutic levels of intraocular anti-VEGF protein (aflibercept) | |
| FT-003 [84] | Phase I | AAV vector | Intravitreal administration FT-003 has the potential to treat AMD by providing durable expression of therapeutic levels of intraocular protein | ||
| 4D-150 IVT [85] | Phase I/II | AAV-based gene therapy carrying miRNA targeting VEGF-C and codon-optimized sequence encoding aflibercept | Intravitreal administration: Dual-transgene gene therapy designed to inhibit four distinct angiogenic factors to prevent angiogenesis and reduce vascular permeability. | ||
| BD311 [86] | Phase I | Integration-deficient lentiviral vector (IDLV) expressing VEGFA antibody | Suprachoroidal administration: Gene is delivered to the RPE cells to express the VEGFA antibody to neutralizes the VEGFA activity in the posterior segment | ||
| RGX-314 [87] | Phase II | AAV8 vector that contains a gene to encode for a monoclonal antibody fragment to neutralizes VEGF | Subretinal administration: RGX-314 is being developed as a potential one-time treatment for wet AMD | ||
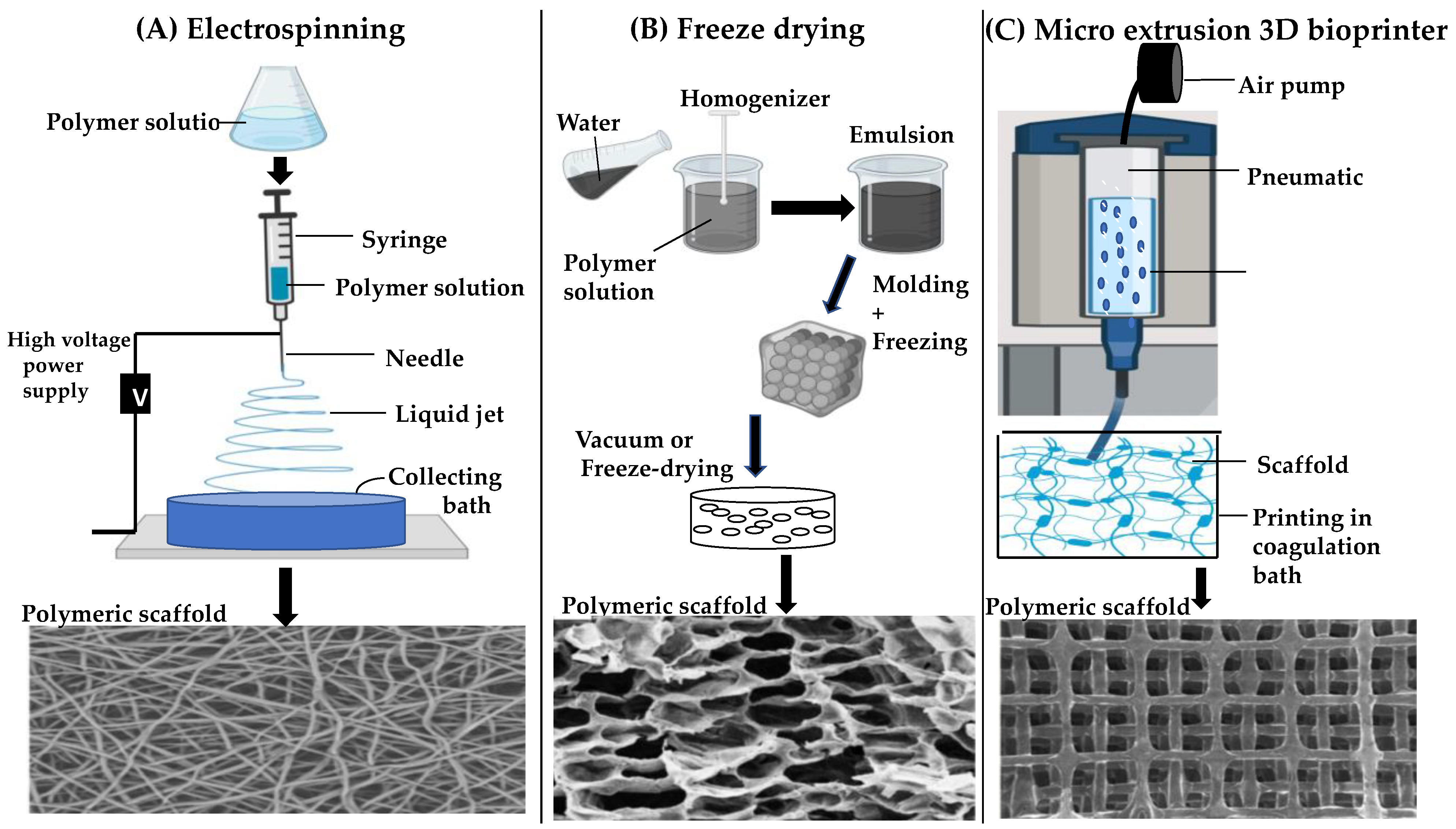
4. Fabrication Strategies for Scaffold-Based Retinal Implants
5. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vyawahare, H.; Shinde, P. Age-related macular degeneration: Epidemiology, pathophysiology, diagnosis, and treatment. Cureus 2022, 14, e29583. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Stahl, A. The diagnosis and treatment of age-related macular degeneration. Dtsch. Arztebl. Int. 2020, 117, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.; Menten, M.J.; Riedl, S.; Bogunović, H.; Leingang, O.; Anders, P.; Hagag, A.M.; Waldstein, S.; Wilson, A.; Cree, A.J.; et al. Developing and validating a multivariable prediction model which predicts progression of intermediate to late age-related macular degeneration-the PINNACLE trial protocol. Eye 2023, 37, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Asp. Med. 2012, 33, 295–317. [Google Scholar] [CrossRef]
- Galindo-Camacho, R.M.; Blanco-Llamero, C.; da Ana, R.; Fuertes, M.A.; Señoráns, F.J.; Silva, A.M.; García, M.L.; Souto, E.B. Therapeutic approaches for age-related macular degeneration. Int. J. Mol. Sci. 2022, 23, 11769. [Google Scholar] [CrossRef]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The effect of tortuosity on permeability of porous scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Cho, E.; Hung, S.; Willett, W.C.; Spiegelman, D.; Rimm, E.B.; Seddon, J.M.; Colditz, G.A.; Hankinson, S.E. Prospective study of dietary fat and the risk of age-related macular degeneration. Am. J. Clin. Nutr. 2001, 73, 209–218. [Google Scholar] [CrossRef]
- Feigl, B. Age-related maculopathy—Linking aetiology and pathophysiological changes to the ischaemia hypothesis. Prog. Retin. Eye Res. 2009, 28, 63–86. [Google Scholar] [CrossRef]
- Dehghan, S.; Mirshahi, R.; Shoae-Hassani, A.; Naseripour, M. Human-induced pluripotent stem cells-derived retinal pigmented epithelium, a new horizon for cells-based therapies for age-related macular degeneration. Stem Cell Res. Ther. 2022, 13, 217. [Google Scholar] [CrossRef] [PubMed]
- van Lookeren Campagne, M.; LeCouter, J.; Yaspan, B.L.; Ye, W. Mechanisms of age-related macular degeneration and therapeutic opportunities: Pathology, genetics, animal models, and therapeutic rationale of AMD. J. Pathol. 2014, 232, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Jiang, S.; Gericke, A. Age-related macular degeneration: Role of oxidative stress and blood vessels. Int. J. Mol. Sci. 2021, 22, 1296. [Google Scholar] [CrossRef]
- Fabre, M.; Mateo, L.; Lamaa, D.; Baillif, S.; Pagès, G.; Demange, L.; Ronco, C.; Benhida, R. Recent advances in age-related macular degeneration therapies. Molecules 2022, 27, 5089. [Google Scholar] [CrossRef] [PubMed]
- Tarau, I.-S.; Berlin, A.; Curcio, C.A.; Ach, T. The cytoskeleton of the retinal pigment epithelium: From normal aging to age-related macular degeneration. Int. J. Mol. Sci. 2019, 20, 3578. [Google Scholar] [CrossRef]
- Choi, E.H.; Suh, S.; Einstein, D.E.; Leinonen, H.; Dong, Z.; Rao, S.R.; Fliesler, S.J.; Blackshaw, S.; Yu, M.; Peachey, N.S.; et al. An inducible Cre mouse for studying roles of the RPE in retinal physiology and disease. JCI Insight 2021, 6, e146604. [Google Scholar] [CrossRef] [PubMed]
- Chirco, K.R.; Sohn, E.H.; Stone, E.M.; Tucker, B.A.; Mullins, R.F. Structural and molecular changes in the aging choroid: Implications for age-related macular degeneration. Eye 2017, 31, 10–25. [Google Scholar] [CrossRef]
- Stern, J.H.; Tian, Y.; Funderburgh, J.; Pellegrini, G.; Zhang, K.; Goldberg, J.L.; Ali, R.R.; Young, M.; Xie, Y.; Temple, S. Regenerating eye tissues to preserve and restore vision. Cell Stem Cell 2018, 22, 834–849. [Google Scholar] [CrossRef]
- Tan, W.; Zou, J.; Yoshida, S.; Jiang, B.; Zhou, Y. The role of inflammation in age-related macular degeneration. Int. J. Biol. Sci. 2020, 16, 2989–3001. [Google Scholar] [CrossRef]
- Armento, A.; Ueffing, M.; Clark, S.J. The complement system in age-related macular degeneration. Cell. Mol. Life Sci. 2021, 78, 4487–4505. [Google Scholar] [CrossRef]
- Somasundaran, S.; Constable, I.J.; Mellough, C.B.; Carvalho, L.S. Retinal pigment epithelium and age-related macular degeneration: A review of major disease mechanisms. Clin. Exp. Ophthalmol. 2020, 48, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I. Oxidative stress in age-related macular degeneration: Nrf2 as therapeutic target. Front. Pharmacol. 2018, 9, 1280. [Google Scholar] [CrossRef] [PubMed]
- Domènech, E.B.; Marfany, G. The relevance of oxidative stress in the pathogenesis and therapy of retinal dystrophies. Antioxidants 2020, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Rohowetz, L.J.; Kraus, J.G.; Koulen, P. Reactive oxygen species-mediated damage of retinal neurons: Drug development targets for therapies of chronic neurodegeneration of the retina. Int. J. Mol. Sci. 2018, 19, 3362. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, S.; Musayeva, A.; Gericke, A. Oxidative stress and vascular dysfunction in the retina: Therapeutic strategies. Antioxidants 2020, 9, 761. [Google Scholar] [CrossRef]
- Cho, Y.-K.; Park, D.-H.; Jeon, I.-C. Medication trends for age-related macular degeneration. Int. J. Mol. Sci. 2021, 22, 11837. [Google Scholar] [CrossRef]
- Hanneken, A.; Lin, F.-F.; Johnson, J.; Maher, P. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress–induced death. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3164. [Google Scholar] [CrossRef]
- Csader, S.; Korhonen, S.; Kaarniranta, K.; Schwab, U. The effect of dietary supplementations on delaying the progression of age-related macular degeneration: A systematic review and meta-analysis. Nutrients 2022, 14, 4273. [Google Scholar] [CrossRef]
- Eisenhauer, B.; Natoli, S.; Liew, G.; Flood, V.M. Lutein and zeaxanthin-food sources, bioavailability and dietary variety in age-related macular degeneration protection. Nutrients 2017, 9, 120. [Google Scholar] [CrossRef]
- Bianchetti, G.; Clementi, M.E.; Sampaolese, B.; Serantoni, C.; Abeltino, A.; De Spirito, M.; Sasson, S.; Maulucci, G. Investigation of DHA-induced regulation of redox homeostasis in retinal pigment epithelium cells through the combination of metabolic imaging and molecular biology. Antioxidants 2022, 11, 1072. [Google Scholar] [CrossRef]
- Bianchetti, G.; Clementi, M.E.; Sampaolese, B.; Serantoni, C.; Abeltino, A.; De Spirito, M.; Sasson, S.; Maulucci, G. Metabolic imaging and molecular biology reveal the interplay between lipid metabolism and DHA-induced modulation of redox homeostasis in RPE cells. Antioxidants 2023, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Group A-REDSR. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Van Asten, C.Y.; Chiu, E.; Agrón, T.E.; Clemons, R.; Ratnapriya, A.; Swaroop, M.L.; Klein, M.L.; Fan, R.; Chew, E.Y.; Age-Related Eye Disease Study 2 Research Group. No CFH or ARMS2 interaction with omega-3 fatty acids, low versus high zinc, or β-carotene versus lutein and zeaxanthin on progression of age-related macular degeneration in the age-related eye disease study 2: Age. Ophthalmology 2019, 126, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Li, F.; Anderson, R.E. Protection of retinal pigment epithelium by OT-551 and its metabolite TEMPOL-H against light-induced damage in rats. Exp. Eye Res. 2010, 91, 111–114. [Google Scholar] [CrossRef]
- Clementi, M.E.; Pizzoferrato, M.; Bianchetti, G.; Brancato, A.; Sampaolese, B.; Maulucci, G.; Tringali, G. Cytoprotective effect of idebenone through modulation of the intrinsic mitochondrial pathway of apoptosis in human retinal pigment epithelial cells exposed to oxidative stress induced by hydrogen peroxide. Biomedicines 2022, 10, 503. [Google Scholar] [CrossRef]
- Chen, E.; Kaiser, P. Therapeutic Potential of the Ranibizumab Port Delivery System in the Treatment of AMD: Evidence to Date. Clin. Ophthalmol. 2020, 14, 1349–1355. [Google Scholar] [CrossRef]
- Xu, M.; Fan, R.; Fan, X.; Shao, Y.; Li, X. Progress and challenges of anti-VEGF agents and their sustained-release strategies for retinal angiogenesis. Drug Des. Dev. Ther. 2022, 16, 3241–3262. [Google Scholar] [CrossRef]
- Rubido, M.; Sadikhov, S.; Szczesny, P.; Schwab, D.; Nogoceke, E.; Weikert, R. Safety and Efficacy of Different Doses and Regimens of Faricimab vs Ranibizumab in Neovascular Age-Related macular degeneration: The AVENUE phase 2 randomized clinical trial. JAMA Ophthalmol. 2020, 138, 955–963. [Google Scholar]
- Wu, A.; Lu, R.; Lee, E. Tissue engineering in age-related macular degeneration: A mini-review. J. Biol. Eng. 2022, 16, 11. [Google Scholar] [CrossRef]
- Cabral de Guimaraes, T.A.; Daich Varela, M.; Georgiou, M.; Michaelides, M. Treatments for dry age-related macular degeneration: Therapeutic avenues, clinical trials and future directions. Br. J. Ophthalmol. 2022, 106, 297–304. [Google Scholar] [CrossRef]
- Lin, J.B.; Murakami, Y.; Miller, J.W.; Vavvas, D.G. Neuroprotection for age-related macular degeneration. Ophthalmol. Sci. 2022, 2, 100192. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Dugel, P.U.; Holz, F.G.; Heier, J.S.; Pearlman, J.A.; Novack, R.L.; Csaky, K.G.; Koester, J.M.; Gregory, J.K.; Kubota, R. Emixustat hydrochloride for geographic atrophy secondary to age-related macular degeneration. Ophthalmology 2018, 125, 1556–1567. [Google Scholar] [CrossRef] [PubMed]
- Racz, B.; Varadi, A.; Kong, J.; Allikmets, R.; Pearson, P.G.; Johnson, G.; Cioffi, C.L.; Petrukhin, K. A non-retinoid antagonist of retinol-binding protein 4 rescues phenotype in a model of Stargardt disease without inhibiting the visual cycle. J. Biol. Chem. 2018, 293, 11574–11588. [Google Scholar] [CrossRef] [PubMed]
- Saad, L.; Washington, I. Can vitamin A be improved to prevent blindness due to age-related macular degeneration, stargardt disease and other retinal dystrophies? In Retinal Degenerative Diseases; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Zelek, W.M.; Morgan, B.P. Monoclonal antibodies capable of inhibiting complement downstream of C5 in multiple species. Front. Immunol. 2020, 11, 612402. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Sadda, S.R.; Busbee, B.; Chew, E.Y.; Mitchell, P.; Tufail, A.; Brittain, C.; Ferrara, D.; Gray, S.; Honigberg, L.; et al. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: Chroma and spectri phase 3 randomized clinical trials. JAMA Ophthalmol. 2018, 136, 666. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Westby, K.; Csaky, K.G.; Monés, J.; Pearlman, J.A.; Patel, S.S.; Joondeph, B.C.; Randolph, J.; Masonson, H.; Rezaei, K.A. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: A randomized pivotal phase 2/3 trial. Ophthalmology 2021, 128, 576–586. [Google Scholar] [CrossRef]
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: A randomized phase 2 trial. Ophthalmology 2020, 127, 186–195. [Google Scholar] [CrossRef]
- Halawa, O.A.; Lin, J.B.; Miller, J.W.; Vavvas, D.G. A review of completed and ongoing complement inhibitor trials for geographic atrophy secondary to age-related macular degeneration. J. Clin. Med. 2021, 10, 2580. [Google Scholar] [CrossRef]
- Shaw, L.T.; Mackin, A.; Shah, R.; Jain, S.; Jain, P.; Nayak, R.; Hariprasad, S.M. Risuteganib-a novel integrin inhibitor for the treatment of non-exudative (dry) age-related macular degeneration and diabetic macular edema. Expert Opin. Investig. Drugs 2020, 29, 547–554. [Google Scholar] [CrossRef]
- Nhu, N.T.; Xiao, S.-Y.; Liu, Y.; Kumar, V.B.; Cui, Z.-Y.; Lee, S.-D. Neuroprotective Effects of a Small Mitochondrially-Targeted Tetrapeptide Elamipretide in Neurodegeneration. Front. Integr. Neurosci. 2022, 15, 747901. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Berger, B.; Reichel, E. A randomized phase 2 study of an anti- amyloid β monoclonal antibody in geographic atrophy secondary to age-related macular degeneration. Ophthalmol. Retin. 2018, 2, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Hanus, J.; Zhao, F.; Wang, S. Current therapeutic developments in atrophic age-related macular degeneration. Br. J. Ophthalmol. 2016, 100, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sato, K.; Gordon, W.C.; Sendtner, M.; Bazan, N.G.; Jin, M. Ciliary neurotrophic factor (CNTF) protects retinal cone and rod photoreceptors by suppressing excessive formation of the visual pigments. J. Biol. Chem. 2018, 293, 15256–15268. [Google Scholar] [CrossRef] [PubMed]
- Tamhane, M.; Luu, K.T.; Attar, M. Ocular pharmacokinetics of brimonidine drug delivery system in monkeys and translational modeling for selection of dose and frequency in clinical trials. J. Pharmacol. Exp. Ther. 2021, 378, 207–214. [Google Scholar] [CrossRef]
- López-Paniagua, M.; de la Mata, A.; Galindo, S.; Blázquez, F.; Calonge, M.; Nieto-Miguel, T. Advanced therapy medicinal products for the eye: Definitions and regulatory framework. Pharmaceutics 2021, 13, 347. [Google Scholar] [CrossRef] [PubMed]
- Rubner, R.; Li, K.V.; Canto-Soler, M.V. Progress of clinical therapies for dry age-related macular degeneration. Int. J. Ophthalmol. 2022, 15, 157–166. [Google Scholar] [CrossRef]
- Rizzolo, L.J.; Nasonkin, I.O.; Adelman, R.A. Retinal cell transplantation, biomaterials, and in vitro models for developing next-generation therapies of age-related macular degeneration. Stem Cells Transl. Med. 2022, 11, 269–281. [Google Scholar] [CrossRef]
- Jemni-Damer, N.; Guedan-Duran, A.; Fuentes-Andion, M.; Serrano-Bengoechea, N.; Alfageme-Lopez, N.; Armada-Maresca, F.; Guinea, G.V.; Perez-Rigueiro, J.; Rojo, F.; Gonzalez-Nieto, D.; et al. Biotechnology and biomaterial-based therapeutic strategies for age-related Macular Degeneration. Part II: Cell and tissue engineering therapies. Front. Bioeng. Biotechnol. 2020, 8, 588014. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, F.; Zhang, M.; Zheng, Y.; Zhang, F.; Xu, L.; Cao, L.; He, W. Intravitreal injection of human retinal progenitor cells for treatment of retinal degeneration. Med. Sci. Monit. 2020, 26, e921184. [Google Scholar] [CrossRef]
- Nair, D.S.R.; Seiler, M.J.; Patel, K.H.; Thomas, V.; Camarillo, J.C.M.; Humayun, M.S.; Thomas, B.B. Tissue engineering strategies for retina regeneration. Appl. Sci. 2021, 11, 2154. [Google Scholar] [CrossRef]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Dang, W.; Lin, C.-M.; Mitra, D.; Zhu, D.; Thomas, B.B.; et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci. Transl. Med. 2018, 10, eaao4097. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.A.B.; Stefanini, F.R.; Falabella, P.; Koss, M.J.; Wells, T.; Diniz, B.; Ribeiro, R.; Schor, P.; Maia, M.; Penha, F.M.; et al. Development of a new tissue injector for subretinal transplantation of human embryonic stem cell derived retinal pigmented epithelium. Int. J. Retin. Vitr. 2017, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Gullapalli, V.K.; Zarbin, M.A. New prospects for retinal pigment epithelium transplantation. Asia Pac. J. Ophthalmol. 2022, 11, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Safety and Efficacy Study of OpRegen for Treatment of Advanced Dry-Form Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT02286089 (accessed on 24 March 2023).
- Autologous Transplantation of Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium for Geographic Atrophy Associated with Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT04339764 (accessed on 24 March 2023).
- Hinkle, J.W.; Mahmoudzadeh, R.; Kuriyan, A.E. Cell-based therapies for retinal diseases: A review of clinical trials and direct to consumer “cell therapy” clinics. Stem Cell Res. Ther. 2021, 12, 538. [Google Scholar] [CrossRef]
- Ru, L.; Wu, N.; Wei, K.; Zeng, Y.; Li, Q.; Weng, C.; Ren, C.; Ren, B.; Huo, D.; Li, Y.; et al. Improving cell survival and engraftment in vivo via layer-by-layer nanocoating of hESC-derived RPE cells. Stem Cell Res. Ther. 2020, 11, 495. [Google Scholar] [CrossRef]
- Arabi, A.; Shahraki, T. Novel treatments and genetics of age-related macular degeneration-a narrative review. Ann. Eye Sci. 2021, 6, 38. [Google Scholar] [CrossRef]
- Guimaraes, T.A.C.; de Georgiou, M.; Bainbridge, J.W.B.; Michaelides, M. Gene therapy for neovascular age-related macular degeneration: Rationale, clinical trials and future directions. Br. J. Ophthalmol. 2021, 105, 151–157. [Google Scholar] [CrossRef]
- Hill, A.B.; Chen, M.; Chen, C.-K.; Pfeifer, B.A.; Jones, C.H. Overcoming gene-delivery hurdles: Physiological considerations for nonviral vectors. Trends Biotechnol. 2016, 34, 91–105. [Google Scholar] [CrossRef]
- Crane, R.; Conley, S.M.; Al-Ubaidi, M.R.; Naash, M.I. Gene therapy to the retina and the cochlea. Front. Neurosci. 2021, 15, 652215. [Google Scholar] [CrossRef]
- Muhuri, M.; Levy, D.I.; Schulz, M.; McCarty, D.; Gao, G. Durability of transgene expression after rAAV gene therapy. Mol. Ther. 2022, 30, 1364–1380. [Google Scholar] [CrossRef]
- SparingVision’s Lead Product, SPVN06, Is a Breakthrough Gene Therapy Approach Targeting Inherited Retinal Diseases; SpringVision: Paris, France, 2022.
- AAVCAGsCD59 for the Treatment of Wet AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT03585556 (accessed on 24 March 2023).
- FOCUS: First in Human Study to Evaluate the Safety and Efficacy of GT005 Administered in Subjects with Dry AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT03846193 (accessed on 24 March 2023).
- Becker, J.; Fakhiri, J.; Grimm, D. Fantastic AAV gene therapy vectors and how to find them-random diversification, rational design and machine learning. Pathogens 2022, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 1–24. [Google Scholar] [CrossRef]
- Safety and Tolerability of RPE Stem Cell-Derived RPE(RPESC-RPE) Transplantation in Patients with Dry Age-Related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT04627428 (accessed on 24 March 2023).
- Safety of Cultured Allogeneic Adult Umbilical Cord Derived Mesenchymal Stem Cells for Eye Diseases. Available online: https://clinicaltrials.gov/ct2/show/NCT05147701 (accessed on 24 March 2023).
- Regenerative Patch Technologies LLC. Study of Subretinal Implantation of Human Embryonic Stem Cell-Derived RPE Cells in Advanced Dry AMD. ClinicalTrials.gov Identifier: NCT02590692 Updated. 2020, 29. Available online: https://clinicaltrials.gov/ct2/show/NCT02590692 (accessed on 24 March 2023).
- A Study of Implantation of Retinal Pigment Epithelium in Subjects with Acute Wet Age Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01691261 (accessed on 24 March 2023).
- Safety and Efficacy of ADVM-022 in Treatment-Experienced Patients with Neovascular Age-Related Macular Degeneration [LUNA]. Available online: https://clinicaltrials.gov/ct2/show/NCT05536973 (accessed on 24 March 2023).
- Gene Therapy for Wet AMD. Available online: https://clinicaltrials.gov/ct2/show/NCT05611424 (accessed on 24 March 2023).
- 4D-150 in Patients With Neovascular (Wet) Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT05197270 (accessed on 24 March 2023).
- VEGFA-Targeting Gene Therapy to Treat Retinal and Choroidal Neovascularization Diseases. Available online: https://clinicaltrials.gov/ct2/show/NCT05099094 (accessed on 24 March 2023).
- RGX-314 Gene Therapy Pharmacodynamic Study for Neovascular Age-related Macular Degeneration (nAMD). Available online: https://clinicaltrials.gov/ct2/show/NCT04832724 (accessed on 24 March 2023).
- Maddalena, A.; Tornabene, P.; Tiberi, P.; Minopoli, R.; Manfredi, A.; Mutarelli, M.; Rossi, S.; Simonelli, F.; Naggert, J.K.; Cacchiarelli, D.; et al. Triple vectors expand AAV transfer capacity in the retina. Mol. Ther. 2018, 26, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Abbott, R.D.; Kaplan, D.L. Engineering biomaterials for enhanced tissue regeneration. Curr. Stem Cell Rep. 2016, 2, 140–146. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Bitar, K.N.; Zakhem, E. Design strategies of biodegradable scaffolds for tissue regeneration. Biomed. Eng. Comput. Biol. 2014, 6, BECB.S10961. [Google Scholar] [CrossRef]
- Wintermantel, E.; Mayer, J.; Blum, J.; Eckert, K.L.; Lüscher, P.; Mathey, M. Tissue engineering scaffolds using superstructures. Biomaterials 1996, 17, 83–91. [Google Scholar] [CrossRef]
- Mikos, A.G.; McIntire, L.V.; Anderson, J.M.; Babensee, J.E. Host response to tissue engineered devices. Adv. Drug Deliv. Rev. 1998, 33, 111–139. [Google Scholar] [CrossRef]
- Brown, B.N.; Valentin, J.E.; Stewart-Akers, A.M.; McCabe, G.P.; Badylak, S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 2009, 30, 1482–1491. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Caramella, C.; Catenacci, L.; Conti, B.; Dorati, R.; Ferrari, F.; Genta, I.; Modena, T.; Perteghella, S.; Rossi, S.; et al. Biomaterials for soft tissue repair and regeneration: A focus on Italian research in the field. Pharmaceutics 2021, 13, 1341. [Google Scholar] [CrossRef]
- Mastrullo, V.; Cathery, W.; Velliou, E.; Madeddu, P.; Campagnolo, P. Angiogenesis in Tissue Engineering: As Nature Intended? Front. Bioeng. Biotechnol. 2020, 8, 188. [Google Scholar] [CrossRef] [PubMed]
- White, C.E.; Olabisi, R.M. Scaffolds for retinal pigment epithelial cell transplantation in age-related macular degeneration. J. Tissue Eng. 2017, 8, 2041731417720841. [Google Scholar] [CrossRef] [PubMed]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes-different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.; McLaughlin, C.; Koduru, S.V.; Ravnic, D.J. Regenerative engineering: Current applications and future perspectives. Front. Surg. 2021, 8, 731031. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Lin, K.T.; Wang, A.; Nguyen, A.B.; Iyer, J.; Tran, S.D. Recent advances in hydrogels: Ophthalmic applications in cell delivery, vitreous substitutes, and ocular adhesives. Biomedicines 2021, 9, 1203. [Google Scholar] [CrossRef]
- Parisi, L.; Toffoli, A.; Ghiacci, G.; Macaluso, G.M. Tailoring the interface of biomaterials to design effective scaffolds. J. Funct. Biomater. 2018, 9, 50. [Google Scholar] [CrossRef]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of scaffolds from bio-based natural materials for tissue regeneration applications: A review. Gels 2023, 9, 100. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Hong, J.; Yeo, M.; Yang, G.H.; Kim, G. Cell-electrospinning and its application for tissue engineering. Int. J. Mol. Sci. 2019, 20, 6208. [Google Scholar] [CrossRef] [PubMed]
- Beachley, V.; Wen, X. Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Prog. Polym. Sci. 2010, 35, 868–892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Leong, K.; Fisher, J. 3D Bioprinting and Nanotechnology in Tissue Engineering and Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Wang, Z.; Wang, Y.; Yan, J.; Zhang, K.; Lin, F.; Xiang, L.; Deng, L.; Guan, Z.; Cui, W.; Zhang, H. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv. Drug Deliv. Rev. 2021, 174, 504–534. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R. Scaffold: A novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2012, 29, 1–63. [Google Scholar] [CrossRef]
- Capuana, E.; Lopresti, F.; Carfì Pavia, F.; Brucato, V.; La Carrubba, V. Solution-based processing for scaffold fabrication in tissue engineering applications: A brief review. Polymers 2021, 13, 2041. [Google Scholar] [CrossRef]
- Yang, P.; Ju, Y.; Hu, Y.; Xie, X.; Fang, B.; Lei, L. Emerging 3D bioprinting applications in plastic surgery. Biomater. Res. 2023, 27, 1. [Google Scholar] [CrossRef]
- Yazdanpanah, Z.; Johnston, J.D.; Cooper, D.M.L.; Chen, X. 3D bioprinted scaffolds for bone tissue engineering: State-of-the-art and emerging technologies. Front. Bioeng. Biotechnol. 2022, 10, 824156. [Google Scholar] [CrossRef]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J. Pharm. Sci. 2020, 15, 529–557. [Google Scholar] [CrossRef]
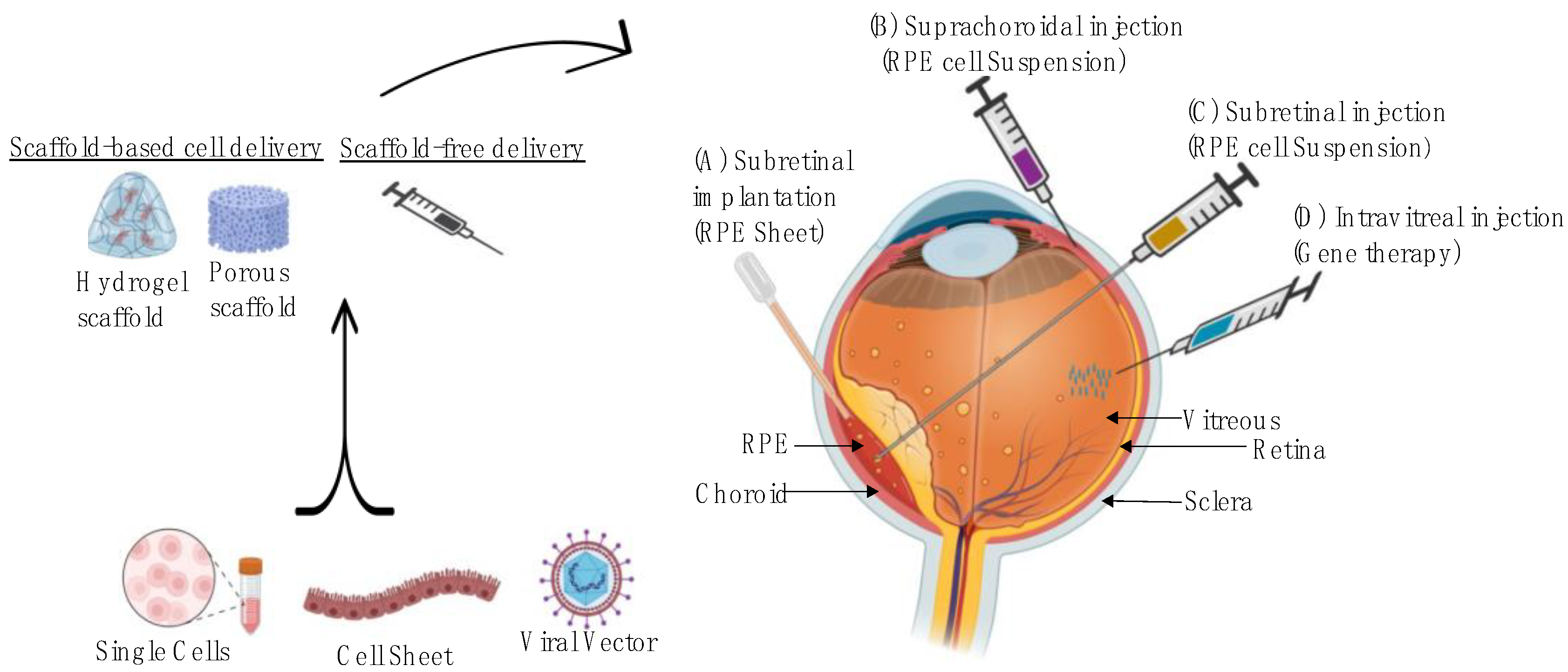

| Disease Prevention | Product Category | Product Name | Study Phase | Mechanism of Action | Method of Delivery |
| Antioxidant | AREDS [28] | Phase III | vitamin supplement | Oral | |
| OT-551 [40,41] | Phase II | vitamin supplement | Eye drop | ||
| Visual cycle modulators (Prevent drusen formation) | ACU-4429 [42] | Phase II/III | Inhibits the formation of 11-cis-retinal to slow the rate of retinoid metabolism and A2E generation | Oral | |
| Fenretinide [43] | Phase II | Synthetic retinoid (vitamin A); reduce accumulation of lipofuscin through binding to its carrier protein | Oral | ||
| C20-D3-vitamin A (ALK-001) [44] | Phase III | A modified form of Vitamin A to decrease toxic by-product formation through reducing A2E biosynthesis | Oral | ||
| Halting Disease Progression | Anti-inflammatory drugs (anti-complement pathways) | Eculizumab [45] | Phase III | A monoclonal antibody to inhibit the complement protein C5, preventing MAC formation | IV |
| Lampalizumab [46] | Phase III | A monoclonal antibody to inhibit complement factor D | Intravitreal | ||
| Avacincaptad pegol (Zimura) [47] | Phase II/III | Anti-complement factor 5, preventing MAC formation | Intravitreal | ||
| Pegcetacoplan (APL-2) [48] | Phase II | Complement C3 inhibitor and prevents downstream activation of C3b | Intravitreal | ||
| LFG316 [49] | Phase II | A monoclonal antibody to inhibit the complement protein C5 | Intravitreal | ||
| Oxidative stress | Risuteganib [50] | Phase II | An integrin inhibitor of αVβ3/αVβ5 and α5β1 to target multiple oxidative stress factors | Intravitreal | |
| Mitochondrial enhancer | Elamipretide [51] | Phase III | A small mitochondrially targeted tetrapeptide to reduce the production of toxic ROS and stabilize cardiolipin levels | Subcutaneous | |
| β-amyloid inhibitors | GSK933776 [52] | Phase II | An anti-amyloid β monoclonal antibody | IV | |
| RN6G [53] | Phase II | A humanized antibody to inhibit accumulation of amyloid β-40 and β-42 | IV | ||
| Neuroprotection | Ciliary nerve trophic factor [54] | Phase II | Protects rod photoreceptors and retinal cones by improving morphology of photoreceptor mitochondria and reduingoxygen consumption | Intravitreal | |
| Brimonidine tartrate [55] | Phase II | An alpha2-adrenergic receptor agonist | Intravitreal |
| Scaffold Property | Biological Significance | Refs |
| Biocompatibility |
| [93,94] |
| Biodegradability |
| [95,96,97] |
| Mechanical properties |
| [96,97] |
| Scaffold architecture |
| [98,99,100] |
| Manufacturing technology |
| [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalili, H.; Kashkoli, H.H.; Weyland, D.E.; Pirkalkhoran, S.; Grabowska, W.R. Advanced Therapy Medicinal Products for Age-Related Macular Degeneration; Scaffold Fabrication and Delivery Methods. Pharmaceuticals 2023, 16, 620. https://doi.org/10.3390/ph16040620
Khalili H, Kashkoli HH, Weyland DE, Pirkalkhoran S, Grabowska WR. Advanced Therapy Medicinal Products for Age-Related Macular Degeneration; Scaffold Fabrication and Delivery Methods. Pharmaceuticals. 2023; 16(4):620. https://doi.org/10.3390/ph16040620
Chicago/Turabian StyleKhalili, Hanieh, Hamid Heidari Kashkoli, David Edward Weyland, Sama Pirkalkhoran, and Wiktoria Roksana Grabowska. 2023. "Advanced Therapy Medicinal Products for Age-Related Macular Degeneration; Scaffold Fabrication and Delivery Methods" Pharmaceuticals 16, no. 4: 620. https://doi.org/10.3390/ph16040620
APA StyleKhalili, H., Kashkoli, H. H., Weyland, D. E., Pirkalkhoran, S., & Grabowska, W. R. (2023). Advanced Therapy Medicinal Products for Age-Related Macular Degeneration; Scaffold Fabrication and Delivery Methods. Pharmaceuticals, 16(4), 620. https://doi.org/10.3390/ph16040620








