Sucrosomial® Iron: An Updated Review of Its Clinical Efficacy for the Treatment of Iron Deficiency
Abstract
1. Introduction
|
| ○ Body growth (infancy and childhood) |
| ○ Pregnancy and lactation |
| ○ Recovery from blood loss (e.g., trauma, surgery, gastrointestinal bleeding) |
| ○ Administration of erythropoiesis-stimulating agents (ESAs) |
|
| ○ Malnutrition |
| ○ Inappropriate diet: |
| ▪ Deficiency of bioavailable iron and/or ascorbic acid |
| ▪ Excess of dietary fiber, phenolic compounds from tea or coffee, and soya prod |
| ○ Malabsorption syndromes: |
| ▪ Autoimmune atrophic gastritis |
| ▪ Gastric resection |
| ▪ Bariatric surgery |
| ▪ Inflammatory bowel disease |
| ▪ Celiac disease and non-celiac gluten sensitivity |
| ▪ Helicobater pylori infection |
| ○ Medications: |
| ▪ Histamine H2 receptor antagonists, proton pump inhibitors, antacids |
| ▪ Antibiotics: tetracycline, penicillin, ciprofloxacin |
| ▪ Anticonvulsants |
| ▪ Cholestiramine |
| ○ Increased hepcidin levels: |
| ▪ Iron-refractory iron deficiency anemia (IRIDA) |
| ▪ Amenia of chronic inflammation (ACI) |
| ○ Deficiency of iron transport proteins: |
| ▪ Heme oxygenase |
| ▪ Divalent metal transporter 1 (DMT1) |
|
| ○ Major surgery and bleeding trauma |
| ○ Gastrointestinal bleeding |
| ▪ Peptic ulceration |
| ▪ Neoplasia |
| ▪ Inflammatory bowel disease |
| ▪ Vascular malformations (e.g., angiodysplasia) |
| ○ Genitourinary bleeding |
| ○ Heavy menses and multi-parity |
| ○ Multiple diagnostic phlebotomies (medical “vampirism”) |
| ○ Blood donation |
| ○ Dialysis (particularly hemodialysis) |
| ○ Medications: |
| ▪ Anti-inflammatory agents |
| ▪ Platelet anti-aggregant agents |
| ▪ Anticoagulant agents |
2. Definitions of Anemia
3. Iron Deficiency: Definitions and Diagnosis
4. Treatment Options for Iron Deficiency/Iron Deficiency Anemia
4.1. Oral Iron
4.2. Intravenous Iron
4.3. Red Blood Cell Transfusion
5. Sucrosomial® Iron for the Management of Iron Deficiency/Iron Deficiency Anemia in Different Clinical Settings
5.1. Obstetrics and Gynecology
5.2. Oncology
5.3. Nephrology
5.4. Gastroenterology
5.4.1. Inflammatory Bowel Disease
5.4.2. Celiac Disease
5.4.3. Autoimmune Atrophic Gastritis
5.4.4. Bariatric Surgery
5.5. Cardiology
5.6. Internal Medicine
5.7. Pediatrics
5.8. Patient Blood Management
| Author (Year) [Ref] Study Type | Patients | Treatment Compound (Dose) Duration | Basal Hb (g/dL) | Final Hb (g/dL) | Basal Ferritin (ng/mL) | Final Ferritin (ng/mL) | ABT Rate (%) | LOS (Days) | GI Side Effects |
|---|---|---|---|---|---|---|---|---|---|
| Orthopedic surgery | |||||||||
| Scardino et al. (2019) [128] Obs | THR ID a THR ID THR non-ID preoperative | No iron (n = 100) SI (30 mg/day, for 3–4 weeks; n = 100) No iron (n = 100) | 13.5 13.5 14.8 | 10.2 b 13.3 12.8 | 66 65 160 | --- --- --- | 7 0 0 | 6.5 4 4 | --- No --- |
| Cardiovascular surgery | |||||||||
| Pierelli et al. (2021) [130] RCT | 1000 patients preoperative | SI (60 mg/day, 30 days; n = 500) Control (standard preparation; n = 500) | --- --- | 13.9 13.3 | --- --- | 184 160 | 65 c 35 | 13 15 | 1.8% --- |
| Venturini et al. (2022) [134] Obs | 106 patients postoperative | SI (120 mg/day, 10 days + 30 mg/day, 10 days; n = 54) FCM (1000 mg IV, single dose; n = 52) | 10.1 10.1 | 12.0 12.5 | 411 386 | 220 689 | --- --- | --- --- | No No |
| Lucertini et al. (2020) [135] RCT | 51 patients AAAR postoperative | SI (30 mg/day, 30 days; starting PO10; n = 26) Control (no iron; n = 25) | 9.3 9.3 | 11.2 9.7 | --- --- | --- --- | 0 0 | --- --- | No No |
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gardner, W.; Kassebaum, N. Global, Regional, and National Prevalence of Anemia and Its Causes in 204 Countries and Territories, 1990–2019. Curr. Dev. Nutr. 2020, 4 (Suppl. S2), 830. [Google Scholar] [CrossRef]
- Safiri, S.; Kolahi, A.-A.; Noori, M.; Nejadghaderi, S.A.; Karamzad, N.; Bragazzi, N.L.; Sullman, M.J.M.; Abdollahi, M.; Collins, G.S.; Kaufman, J.S.; et al. Burden of anemia and its underlying causes in 204 countries and territories, 1990–2019: Results from the Global Burden of Disease Study 2019. J. Hematol. Oncol. 2021, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, S.-R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Peña-Rosas, J.P.; Robinson, S.; Milman, N.; Holzgreve, W.; Breymann, C.; Goffinet, F.; Nizard, J.; Christory, F.; Samama, C.-M.; et al. Patient blood management in obstetrics: Management of anaemia and haematinic deficiencies in pregnancy and in the post-partum period: NATA consensus statement. Transfus. Med. 2018, 28, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.; Beguin, Y.; Bokemeyer, C.; Dicato, M.; Gascón, P.; Glaspy, J.; Hofmann, A.; Link, H.; Littlewood, T.; Ludwig, H.; et al. Management of anaemia and iron deficiency in patients with cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29 (Suppl. S4), iv96–iv110. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Bircher, A.J.; Eckardt, K.-U.; Obrador, G.T.; Pollock, C.A.; Stenvinkel, P.; Swinkels, D.W.; Wanner, C.; Weiss, G.; Chertow, G.M.; et al. Iron management in chronic kidney disease: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2016, 89, 28–39. [Google Scholar] [CrossRef]
- Goodnough, L.T.; Comin-Colet, J.; Leal-Noval, S.; Ozawa, S.; Takere, J.; Henry, D.; Javidroozi, M.; Hohmuth, B.; Bisbe, E.; Gross, I.; et al. Management of anemia in patients with congestive heart failure. Am. J. Hematol. 2017, 92, 88–93. [Google Scholar] [CrossRef]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegård, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European Consensus on the Diagnosis and Management of Iron Deficiency and Anaemia in Inflammatory Bowel Diseases. J. Crohn’s Colitis 2015, 9, 211–222. [Google Scholar] [CrossRef]
- Bjørklund, G.; Peana, M.; Pivina, L.; Dosa, A.; Aaseth, J.; Semenova, Y.; Chirumbolo, S.; Medici, S.; Dadar, M.; Costea, D.-O. Iron Deficiency in Obesity and after Bariatric Surgery. Biomolecules 2021, 11, 613. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Comin-Colet, J.; de Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R.; et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am. J. Hematol. 2017, 92, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Judd, L.; Hof, L.; Beladdale, L.; Friederich, P.; Thoma, J.; Wittmann, M.; Zacharowski, K.; Meybohm, P.; Choorapoikayil, S. The prevalence of pre-operative anaemia in surgical patients (PANDORA) study collaborators Prevalence of pre-operative anaemia in surgical patients: A retrospective, observational, multicentre study in Germany. Anaesthesia 2022, 77, 1209–1218. [Google Scholar] [CrossRef]
- Muñoz, M.; Laso-Morales, M.J.; Gómez-Ramírez, S.; Cadellas, M.; Núñez-Matas, M.J.; García-Erce, J.A. Pre-operative haemoglobin levels and iron status in a large multicentre cohort of patients undergoing major elective surgery. Anaesthesia 2017, 72, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron-Deficiency Anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Adamson, J.W. How we diagnose and treat iron deficiency anemia. Am. J. Hematol. 2016, 91, 31–38. [Google Scholar] [CrossRef]
- De Franceschi, L.; Iolascon, A.; Taher, A.; Cappellini, M.D. Clinical management of iron deficiency anemia in adults: Systemic review on advances in diagnosis and treatment. Eur. J. Intern. Med. 2017, 42, 16–23. [Google Scholar] [CrossRef]
- Muñoz, M.; Gómez-Ramírez, S.; Besser, M.; Pavía, J.; Gomollón, F.; Liumbruno, G.M.; Bhandari, S.; Cladellas, M.; Shander, A.; Auerbach, M. Current misconceptions in diagnosis and management of iron deficiency. Blood Transfus. 2017, 15, 422–437. [Google Scholar] [CrossRef]
- Gómez-Ramírez, S.; Brilli, E.; Tarantino, G.; Muñoz, M. Sucrosomial® Iron: A New Generation Iron for Improving Oral Supplementation. Pharmaceuticals 2018, 11, 97. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; WHO/NMH/NHD/MNM/11.1. Available online: https://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf (accessed on 22 January 2023).
- Blaudszun, G.; Munting, K.E.; Butchart, A.; Gerrard, C.; Klein, A.A. The association between borderline pre-operative anaemia in women and outcomes after cardiac surgery: A cohort study. Anaesthesia 2018, 73, 572–578. [Google Scholar] [CrossRef]
- Miles, L.F.; Larsen, T.; Bailey, M.J.; Burbury, K.L.; Story, D.A.; Bellomo, R. Borderline anaemia and postoperative outcome in women undergoing major abdominal surgery: A retrospective cohort study. Anaesthesia 2020, 75, 210–217. [Google Scholar] [CrossRef]
- Muñoz, M.; Acheson, A.G.; Auerbach, M.; Besser, M.; Habler, O.; Kehlet, H.; Liumbruno, G.M.; Lasocki, S.; Meybohm, P.; Baikady, R.R.; et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2017, 72, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Shander, A.; Corwin, H.L.; Meier, J.; Auerbach, M.; Bisbe, E.; Blitz, J.; Erhard, J.; Faraoni, D.; Farmer, S.L.; Frank, S.M.; et al. Recommendations from the International Consensus Conference on Anemia Management in Surgical Patients (ICCAMS). Ann. Surg. 2023, 277, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, C.; Marques, F.; Robalo-Nunes, A.; Belo, A.; Brilhante, D.; Cortez, J. Prevalence of anaemia and iron deficiency in Portugal: The EMPIRE study. Intern. Med. J. 2016, 46, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J.J.; Khan, K.S. Non-anaemic iron deficiency—A disease looking for recognition of diagnosis: A systematic review. Eur. J. Haematol. 2016, 96, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.D. Diagnosis and management of iron-deficiency anaemia. Best Pract. Res. Clin. Haematol. 2005, 18, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Walters, G.O.; Miller, F.M.; Worwood, M. Serum ferritin concentration and iron stores in normal subjects. J. Clin. Pathol. 1973, 26, 770–772. [Google Scholar] [CrossRef]
- Urrechaga, E. Clinical utility of the new beckman-coulter parameter red blood cell size factor in the study of erithropoiesis. Int. J. Lab. Hematol. 2009, 31, 623–629. [Google Scholar] [CrossRef]
- Bisbe, E.; Basora, M.; Colomina, M.J. Spanish Best Practice in Peri-operative Anaemia Optimisation Panel. Peri-operative treatment of anaemia in major orthopaedic surgery: A practical approach from Spain. Blood Transfus. 2017, 15, 296–306. [Google Scholar] [CrossRef]
- Camaschella, C.; Girelli, D. The changing landscape of iron deficiency. Mol. Asp. Med. 2020, 75, 100861. [Google Scholar] [CrossRef]
- Barni, S.; Gascòn, P.; Petrelli, F.; García-Erce, J.A.; Pedrazzoli, P.; Rosti, G.; Giordano, G.; Mafodda, A.; Múñoz, M. Position paper on management of iron deficiency in adult cancer patients. Expert Rev. Hematol. 2017, 10, 685–695. [Google Scholar] [CrossRef]
- Moretti, D.; Goede, J.S.; Zeder, C.; Jiskra, M.; Chatzinakou, V.; Tjalsma, H.; Melse-Boonstra, A.; Brittenham, G.; Swinkels, D.W.; Zimmermann, M.B. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 2015, 126, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.; Powell, J.J. Ferrous Sulfate Supplementation Causes Significant Gastrointestinal Side-Effects in Adults: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0117383. [Google Scholar] [CrossRef]
- Cancelo-Hidalgo, M.J.; Castelo-Branco, C.; Palacios, S.; Haya-Palazuelos, J.; Ciria-Recasens, M.; Manasanch, J.; Pérez-Edo, L. Tolerability of different oral iron supplements: A systematic review. Curr. Med. Res. Opin. 2013, 29, 291–303. [Google Scholar] [CrossRef]
- Gómez-Ramírez, S.; Bisbe, E.; Shander, A.; Spahn, D.R.; Muñoz, M. Management of Perioperative Iron Deficiency Anemia. Acta Haematol. 2019, 142, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, N.U.; Cercamondi, C.I.; Brittenham, G.; Zeder, C.; Geurts-Moespot, A.J.; Swinkels, D.W.; Moretti, D.; Zimmermann, M.B. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: Two open-label, randomised controlled trials. Lancet Haematol. 2017, 4, e524–e533. [Google Scholar] [CrossRef] [PubMed]
- Snook, J.; Bhala, N.; Beales, I.L.P.; Cannings, D.; Kightley, C.; Logan, R.P.; Pritchard, D.M.; Sidhu, R.; Surgenor, S.; Thomas, W.; et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut 2021, 70, 2030–2051. [Google Scholar] [CrossRef]
- Pujol-Nicolas, A.; Morrison, R.; Casson, C.; Khan, S.; Marriott, A.; Tiplady, C.; Kotze, A.; Gray, W.; Reed, M. Preoperative screening and intervention for mild anemia with low iron stores in elective hip and knee arthroplasty. Transfusion 2017, 57, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Trentino, K.M.; Mace, H.S.; Symons, K.; Sanfilippo, F.; Leahy, M.F.; Farmer, S.L.; Hofmann, A.; Watts, R.; Wallace, M.H.; Murray, K. Screening and treating pre-operative anaemia and suboptimal iron stores in elective colorectal surgery: A cost effectiveness analysis. Anaesthesia 2021, 76, 357–365. [Google Scholar] [CrossRef]
- Muñoz, M.; Acheson, A.G.; Bisbe, E.; Butcher, A.; Gómez-Ramírez, S.; Khalafallah, A.A.; Kehlet, H.; Kietaibl, S.; Liumbruno, G.M.; Meybohm, P.; et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018, 73, 1418–1431. [Google Scholar] [CrossRef]
- Schmidt, C.; Allen, S.; Kopyt, N.; Pergola, P. Iron Replacement Therapy with Oral Ferric Maltol: Review of the Evidence and Expert Opinion. J. Clin. Med. 2021, 10, 4448. [Google Scholar] [CrossRef]
- Auerbach, M.; Gafter-Gvili, A.; Macdougall, I.C. Intravenous iron: A framework for changing the management of iron deficiency. Lancet Haematol. 2020, 7, e342–e350. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Ferric Carboxymaltose: A Review of Its Use in Iron Deficiency. Drugs 2015, 75, 101–127. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Henry, D.; DeLoughery, T.G. Intravenous ferric derisomaltose for the treatment of iron deficiency anemia. Am. J. Hematol. 2021, 96, 727–734. [Google Scholar] [CrossRef]
- Shin, H.W.; Park, J.J.; Kim, H.J.; You, H.S.; Choi, S.U.; Lee, M.J. Efficacy of perioperative intravenous iron therapy for transfusion in orthopedic surgery: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0215427. [Google Scholar] [CrossRef] [PubMed]
- Tankard, K.A.; Park, B.; Brovman, E.Y.; Bader, A.M.; Urman, R.D. The Impact of Preoperative Intravenous Iron Therapy on Perioperative Outcomes in Cardiac Surgery: A Systematic Review. J. Hematol. 2020, 9, 97–108. [Google Scholar] [CrossRef]
- Moon, T.; Smith, A.; Pak, T.; Park, B.H.; Beutler, S.S.; Brown, T.; Kaye, A.D.; Urman, R.D. Preoperative Anemia Treatment with Intravenous Iron in Patients Undergoing Abdominal Surgery: A Systematic Review. Adv. Ther. 2021, 38, 1447–1469. [Google Scholar] [CrossRef]
- Elhenawy, A.M.; Meyer, S.R.; Bagshaw, S.M.; MacArthur, R.G.; Carroll, L.J. Role of preoperative intravenous iron therapy to correct anemia before major surgery: A systematic review and meta-analysis. Syst. Rev. 2021, 10, 36. [Google Scholar] [CrossRef]
- Meyer, J.; Cirocchi, R.; Di Saverio, S.; Ris, F.; Wheeler, J.; Davies, R.J. Pre-operative iron increases haemoglobin concentration before abdominal surgery: A systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 2022, 12, 2158. [Google Scholar] [CrossRef]
- Deloughery, T.D. Safety of oral and intravenous iron. Acta Haematol. 2019, 142, 8–12. [Google Scholar] [CrossRef]
- European Medicines Agency. New Recommendations to Manage Risk of Allergic Reactions with Intravenous Iron Containing Medicines. EMA/579491/2013. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/IV_iron_31/WC500151308.pdf (accessed on 22 January 2023).
- Rampton, D.; Folkersen, J.; Fishbane, S.; Hedenus, M.; Howaldt, S.; Locatelli, F.; Patni, S.; Szebeni, J.; Weiss, G. Hypersensitivity reactions to intravenous iron: Guidance for risk minimization and management. Haematologica 2014, 99, 1671–1676. [Google Scholar] [CrossRef]
- Lim, W.; Afif, W.; Knowles, S.; Lim, G.; Lin, Y.; Mothersill, C.; Nistor, I.; Rehman, F.; Song, C.; Xenodemetropoulos, T. Canadian expert consensus: Management of hypersensitivity reactions to intravenous iron in adults. Vox Sang. 2019, 114, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Kirwan, B.-A.; Anker, S.D.; McDonagh, T.; Dorobantu, M.; Drozdz, J.; Fabien, V.; Filippatos, G.; Göhring, U.M.; Keren, A.; et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: A multicentre, double-blind, randomised, controlled trial. Lancet 2020, 396, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Kalra, P.R.; Cleland, J.G.F.; Petrie, M.C.; Thomson, E.; Kalra, P.A.; Squire, I.B.; Ahmed, F.Z.; Al-Mohammad, A.; Cowburn, P.J.; Foley, P.W.X.; et al. Intravenous ferric derisomaltose in patients with heart failure and iron deficiency in the UK (IRONMAN): An investigator-initiated, prospective, randomised, open-label, blinded-endpoint trial. Lancet 2022, 400, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Neef, V.; Baumgarten, P.; Noone, S.; Piekarski, F.; Triphaus, C.; Kleinerüschkamp, A.; Helmer, P.; Messroghli, L.; Zacharowski, K.; Choorapoikayil, S.; et al. The impact of timing of intravenous iron supplementation on preoperative haemoglobin in patients scheduled for major surgery. Blood Transfus. 2022, 20, 188–197. [Google Scholar] [CrossRef]
- Blum, L.V.; Zierentz, P.; Hof, L.; Kloka, J.A.; Messroghli, L.; Zacharowski, K.; Meybohm, P.; Choorapoikayil, S. The impact of intravenous iron supplementation in elderly patients undergoing major surgery. BMC Geriatr. 2022, 22, 293. [Google Scholar] [CrossRef]
- Muñoz, M.; García-Erce, J.A.; Díez-Lobo, A.I.; Campos, A.; Sebastianes, C.; Bisbe, E.; Anaemia Working Group España (AWGE). Usefulness of the administration of intravenous iron sucrose for the correction of preoperative anemia in major surgery patients. Med. Clin. 2009, 132, 303–306. [Google Scholar] [CrossRef]
- Murphy, M.F.; Waters, J.H.; Wood, E.M.; Yazer, M.H. Transfusing blood safely and appropriately. BMJ 2013, 347, f4303. [Google Scholar] [CrossRef]
- Carson, J.L.; Stanworth, S.J.; Dennis, J.A.; Trivella, M.; Roubinian, N.; Fergusson, D.A.; Triulzi, D.; Dorée, C.; Hébert, P.C. Transfusion thresholds for guiding red blood cell transfusion. Cochrane Database Syst. Rev. 2021, 12, CD002042. [Google Scholar] [CrossRef]
- Kietaibl, S.; Ahmed, A.; Afshari, A.; Albaladejo, P.; Aldecoa, C.; Barauskas, G.; De Robertis, E.; Faraoni, D.; Filipescu, D.C.; Fries, D.; et al. Management of severe peri-operative bleeding: Guidelines from the European Society of Anaesthesiology and Intensive Care: Second update 2022. Eur. J. Anaesthesiol. 2023, 40, 226–304. [Google Scholar] [CrossRef]
- Girelli, D.; Ugolini, S.; Busti, F.; Marchi, G.; Castagna, A. Modern iron replacement therapy: Clinical and pathophysiological insights. Int. J. Hematol. 2018, 107, 16–30. [Google Scholar] [CrossRef]
- Elli, L.; Ferretti, F.; Branchi, F.; Tomba, C.; Lombardo, V.; Scricciolo, A.; Doneda, L.; Roncoroni, L. Sucrosomial® Iron Supplementation in Anemic Patients with Celiac Disease Not Tolerating Oral Ferrous Sulfate: A Prospective Study. Nutrients 2018, 10, 330. [Google Scholar] [CrossRef]
- Ciudin, A.; Simó-Servat, O.; Balibrea, J.M.; Vilallonga, R.; Hernandez, C.; Simó, R.; Mesa, J. Response to oral sucrosomial® iron supplementation in patients undergoing bariatric surgery. The BARI-FER study. Endocrinol. Diabetes Nutr. 2018, 65, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Riccio, E.; Sabbatini, M.; Andreucci, M.; Del Rio, A.; Visciano, B. Effect of oral liposomal iron versus intravenous iron for treatment of iron deficiency anaemia in CKD patients: A randomized trial. Nephrol. Dial. Transplant. 2015, 30, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Mafodda, A.; Giuffrida, D.; Prestifilippo, A.; Azzarello, D.; Giannicola, R.; Mare, M.; Maisano, R. Oral sucrosomial® iron versus intravenous iron in anemic cancer patients without iron deficiency receiving darbepoetin alfa: A pilot study. Support. Care Cancer 2017, 25, 2779–2786. [Google Scholar] [CrossRef] [PubMed]
- Capra, A.P.; Ferro, E.; Cannavò, L.; La Rosa, M.A.; Zirilli, G. A child with severe iron-deficiency anemia and a complex TMPRSS6 genotype. Hematology 2017, 22, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Daru, J.; Zamora, J.; Fernández-Félix, B.M.; Vogel, J.; Oladapo, O.T.; Morisaki, N.; Tunçalp, Ö.; Torloni, M.R.; Mittal, S.; Jayaratne, K.; et al. Risk of maternal mortality in women with severe anaemia during pregnancy and post-partum: A multilevel analysis. Lancet Glob. Health 2018, 6, e548–e554. [Google Scholar] [CrossRef]
- World Health Organization. Who Recommendations on Antenatal Care for a Positive Pregnancy Experience. Available online: http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/anc-positive-pregnancy-experience/en/ (accessed on 27 March 2023).
- Parisi, F.; Berti, C.; Mandò, C.; Martinelli, A.; Mazzali, C.; Cetin, I. Effects of different regimens of iron prophylaxis on maternal iron status and pregnancy outcome: A randomized control trial. J. Matern. Fetal Neonatal Med. 2017, 30, 1787–1792. [Google Scholar] [CrossRef]
- Ascanio, M.; Darbá, J. Economic assessment of oral Fisiogen Ferro Forte versus intravenous iron for the management of iron deficiency in Crohn’s disease in Spain. Rev. Esp. Econ. Salud 2019, 14, 408–415. [Google Scholar]
- Farinati, F.; Maddalo, G. Iron and/or B12 deficient anemia in autoimmune gastritis. High dose sucrosomial iron supplemen-tation: Preliminary data of a single center experience. Expert Rev. Hematol. 2017, 10 (Suppl. S1), 1–40. [Google Scholar] [CrossRef]
- Karavidas, A.; Troganis, E.; Lazaros, G.; Balta, D.; Karavidas, I.-N.; Polyzogopoulou, E.; Parissis, J.; Farmakis, D. Oral sucrosomial iron improves exercise capacity and quality of life in heart failure with reduced ejection fraction and iron deficiency: A non-randomized, open-label, proof-of-concept study. Eur. J. Heart Fail. 2021, 23, 593–597. [Google Scholar] [CrossRef]
- Giordano, G.; Cutuli, M.A.; Lucchesi, A.; Magnifico, I.; Venditti, N.; Vergalito, F.; Gasperi, M.; Di Marco, R. Iron Support in Erythropoietin Treatment in Myelodysplastic Syndrome Patients Affected by Low-Risk Refractory Anaemia: Real-Life Evidence from an Italian Setting. Acta Haematol. 2020, 143, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Napolitano, M.; Di Battista, V.; Lucchesi, A. Oral high-dose sucrosomial® iron vs. intravenous iron in sideropenic anemia patients intolerant/refractory to iron sulfate: A multicentric randomized study. Ann. Hematol. 2021, 100, 2173–2176. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, G. Oral Sucrosomial® iron to prevent iron deficiency and hemoglobin drop during pregnancy: A case series in support of effectiveness and compliance. Blood Transfus. 2023, 21 (Suppl. S1), S26–S27. [Google Scholar]
- Pérez-Silvestre, L.; Quijada-Cazorla, M.A.; Gracia-Ferrón, A.A.; Jiménez-Martínez, M.D.; García-Villalba, A.; Palacios-Marqués, M.A. Study of the tolerability of Sucrosomial® iron in comparison with ferrous sulfate in pregnant women with iron deficiency anemia: A randomized controlled trial. Blood Transfus. 2023, 21 (Suppl. S1), S29. [Google Scholar] [CrossRef]
- Antoine, E.; Mehedintu, C.; Mitran, M.; Diculescu, D. Sucrosomial® iron effectiveness in recovering from mild and moderate iron-deficiency anemia in the postpartum period. BMC Pregnancy Childbirth 2023, 23, 360. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Suárez, M.J.; García-González, J.; Valdés-Lafuente, D.; Díaz de la Noval, B.; Adánez-García, J. Prospective clinical trial evaluating safety and efficacy of an original protocol of ablation of myomas by transvaginal radiofrequency developed by CUH gynecology department. Blood Transfus. 2023, 21 (Suppl. S1), S23–S24. [Google Scholar] [CrossRef]
- Busti, F.; Marchi, G.; Ugolini, S.; Castagna, A.; Girelli, D. Anemia and Iron Deficiency in Cancer Patients: Role of Iron Replacement Therapy. Pharmaceuticals 2018, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Zuccarini, A.; Cicognini, D.; Tancredi, R.; Ferrari, A.; Rizzo, G.; Lasagna, A.; Caccialanza, R.; Cavanna, L.; Orlandi, E.; Biasini, C.; et al. Randomized trial of sucrosomial iron supplementation in patients with chemotherapy-related anemia treated with ESA. Support. Care Cancer 2022, 30, 7645–7653. [Google Scholar] [CrossRef]
- Shepshelovich, D.; Rozen-Zvi, B.; Avni, T.; Gafter, U.; Gafter-Gvili, A. Intravenous Versus Oral Iron Supplementation for the Treatment of Anemia in CKD: An Updated Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2016, 68, 677–690. [Google Scholar] [CrossRef]
- Agarwal, R.; Kusek, J.W.; Pappas, M.K. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int. 2015, 88, 905–914. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Bock, A.H.; Carrera, F.; Eckardt, K.-U.; Gaillard, C.; Van Wyck, D.; Roubert, B.; Nolen, J.G.; Roger, S.D.; FIND-CKD Study Investigators. FIND-CKD: A randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol. Dial. Transplant. 2014, 29, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, I.C.; Bock, A.H.; Carrera, F.; Eckardt, K.-U.; Gaillard, C.; Van Wyck, D.; Meier, Y.; Larroque, S.; Perrin, A.; Roger, S.D.; et al. Erythropoietic response to oral iron in patients with nondialysis-dependent chronic kidney disease in the FIND-CKD trial. Clin. Nephrol. 2017, 88, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Regidor, D.L.; McAllister, C.J.; Michael, B.; Warnock, D.G. Time-Dependent Associations between Iron and Mortality in Hemodialysis Patients. J. Am. Soc. Nephrol. 2005, 16, 3070–3080. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Bhandari, S.; White, C.; Anker, S.D.; Farrington, K.; Kalra, P.A.; Mark, P.B.; McMurray, J.J.V.; Reid, C.; Robertson, M.; et al. Intravenous iron dosing and infection risk in patients on hemodialysis: A prespecified secondary analysis of the PIVOTAL trial. J. Am. Soc. Nephrol. 2020, 31, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Griveas, I. Efficacy and tolerability of oral Sucrosomial® iron in CKD patients with anemia. Expert Rev. Hematol. 2017, 10 (Suppl. S1), 8–10. [Google Scholar] [CrossRef]
- Montagud-Marrahia, E.; Arrizabalaga, P.; Abellanab, R.; Pocha, E. Liposomal iron in moderate chronic kidney disease. Ne-frología 2020, 40, 446–452. [Google Scholar] [CrossRef]
- Darbà, J.; Ascanio, M. Budget Impact Analysis of Oral Fisiogen Ferro Forte® versus Intravenous Iron for the Management of Iron Deficiency in Chronic Kidney Disease in Spain. Clin. Drug Investig. 2018, 38, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Riccio, E.; Sabbatini, M.; Capuano, I.; Pellegrino, A.M.; Petruzzelli, L.A.; Pisani, A. Oral Sucrosomial® iron versus intravenous iron for recovering iron deficiency anaemia in ND-CKD patients: A cost-minimization analysis. BMC Nephrol. 2020, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, G.; Markopoulos, K.; Albertini, R.; Di Sabatino, A.; Biagi, F.; Ciccocioppo, R.; Arbustini, E.; Corazza, G.R. Anemia of chronic disease and defective erythropoietin production in patients with celiac disease. Haematologica 2008, 93, 1785–1791. [Google Scholar] [CrossRef]
- Lee, T.; Clavel, T.; Smirnov, K.; Schmidt, A.; Lagkouvardos, I.; Walker, A.; Lucio, M.; Michalke, B.; Schmitt-Kopplin, P.; Fedorak, R.; et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut 2017, 66, 863–871. [Google Scholar] [CrossRef]
- Constante, M.; Fragoso, G.; Lupien-Meilleur, J.; Calvé, A.; Santos, M.M. Iron Supplements Modulate Colon Microbiota Composition and Potentiate the Protective Effects of Probiotics in Dextran Sodium Sulfate-induced Colitis. Inflamm. Bowel Dis. 2017, 23, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, M.; Wilkins, S.J.; Sheridan, L.; Helman, S.L.; Brilli, E.; Tarantino, G.; Gregory, J.; Anderson, G.J.; Frazer, D.M. Sup-plementation with Sucrosomial® iron leads to favourable changes in the intestinal microbiome. Blood Transfus. 2023, 21 (Suppl. S1), S6–S7. [Google Scholar] [CrossRef]
- Stein, J.; Aksan, A.; Farrag, K.; Dignass, A.; Radeke, H.H. Management of inflammatory bowel disease-related anemia and iron deficiency with specific reference to the role of intravenous iron in current practice. Expert Opin. Pharmacother. 2017, 18, 1721–1737. [Google Scholar] [CrossRef] [PubMed]
- Abbati, G.; Incerti, F.; Boarini, C.; Pileri, F.; Bocchi, D.; Ventura, P.; Buzzetti, E.; Pietrangelo, A. Safety and efficacy of sucro-somial iron in inflammatory bowel disease patients with iron deficiency anemia. Intern. Emerg. Med. 2018, 14, 423–431. [Google Scholar] [CrossRef]
- Bastida, G.; Guise, C.H.-D.; Algaba, A.; Nieto, Y.B.; Soares, J.M.; Robles, V.; Bermejo, F.; Sáez-González, E.; Gomollón, F.; Nos, P. Sucrosomial Iron Supplementation for the Treatment of Iron Deficiency Anemia in Inflammatory Bowel Disease Patients Refractory to Oral Iron Treatment. Nutrients 2021, 13, 1770. [Google Scholar] [CrossRef] [PubMed]
- Indriolo, A.; Ravelli, P. Mo1226 Comparison Between Liposomial Iron and Ferrous Sulfate in Patients with Iron Deficiency Anemia and Inflammatory Bowel Disease. A Pilot Controlled Study. Gastroenterology 2014, 146, S-591. [Google Scholar] [CrossRef]
- Bertani, L.; Tricò, D.; Zanzi, F.; Svizzero, G.B.; Coppini, F.; de Bortoli, N.; Bellini, M.; Antonioli, L.; Blandizzi, C.; Marchi, S. Oral Sucrosomial Iron Is as Effective as Intravenous Ferric Carboxy-Maltose in Treating Anemia in Patients with Ulcerative Colitis. Nutrients 2021, 13, 608. [Google Scholar] [CrossRef]
- Lindfors, K.; Ciacci, C.; Kurppa, K.; Lundin, K.E.A.; Makharia, G.K.; Mearin, M.L.; Murray, J.A.; Verdu, E.F.; Kaukinen, K. Coeliac disease. Nat. Rev. Dis. Prim. 2019, 5, 3. [Google Scholar] [CrossRef]
- Stefanelli, G.; Viscido, A.; Longo, S.; Magistroni, M.; Latella, G. Persistent Iron Deficiency Anemia in Patients with Celiac Disease Despite a Gluten-Free Diet. Nutrients 2020, 12, 2176. [Google Scholar] [CrossRef]
- Ragozzino, G.; Riccio, A.; Mattera, E. Effectiveness of oral liposomal iron (Sideral-Forte) in patients with intestinal malabsorption (Celiac disease and gluten sensitivity). Expert Rev. Hematol. 2015, 8 (Suppl. S1), 21–22. [Google Scholar]
- Lenti, M.V.; Rugge, M.; Lahner, E.; Miceli, E.; Toh, B.-H.; Genta, R.M.; De Block, C.; Hershko, C.; Di Sabatino, A. Autoimmune gastritis. Nat. Rev. Dis. Prim. 2020, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Hershko, C.; Camaschella, C. How I treat unexplained refractory iron deficiency anemia. Blood 2014, 123, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Pignata, G.; Marano, L. P5—Efficacy of food for special medical purposes with sucrosomial minerals (Sideralmed®) for correcting vitamins and mineral deficiencies after standard oral supplementation following hypoabsortive bariatric surgery. Blood Transfus. 2023, 21 (Suppl. S1), S25–S26. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; von Haehling, S.; Butler, J.; Cleland, J.G.; Ponikowski, P.; Anker, S.D. Iron deficiency and cardiovascular disease. Eur. Heart J. 2023, 44, 14–27, Erratum in Eur. Heart J. 2023, 44, 1607. [Google Scholar] [CrossRef]
- Lewis, G.D.; Malhotra, R.; Hernandez, A.F.; McNulty, S.E.; Smith, A.; Felker, G.M.; Tang, W.H.W.; LaRue, S.J.; Redfield, M.M.; Semigran, M.J.; et al. Effect of Oral Iron Repletion on Exercise Capacity in Patients with Heart Failure with Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF randomized clinical trial. JAMA 2017, 317, 1958–1966. [Google Scholar] [CrossRef]
- Ruperto, C.; Ricca, G.; Antonio, A.A.; Sfalanga, R.; Fabio Scandurra, F.; Licciardello, G. Oral Sucrosomial® iron supplementation in patients underwent percutaneous coronary intervention: Safety, efficacy and tolerability. Expert Rev. Hematol. 2017, 10 (Suppl. S1), 33–34. [Google Scholar] [CrossRef]
- Ghio, S.; Fortuni, F.; Capettini, A.C.; Scelsi, L.; Greco, A.; Vullo, E.; Raineri, C.; Guida, S.; Turco, A.; Gargiulo, C.; et al. Iron deficiency in pulmonary arterial hypertension: Prevalence and potential usefulness of oral supplementation. Acta Cardiol. 2021, 76, 162–167. [Google Scholar] [CrossRef]
- Fonseca, C.; Araujo, M.; Moniz, P.; Marques, F.; Araujo, I.; Costa, L.; Rodrigues, J.; Frade, L.; Botella, A.; Jesus, S.; et al. Prevalence and prognostic impact of anemia and iron deficiency in patients hospitalized in an internal medicine ward: The PRO-IRON study. Eur. J. Haematol. 2017, 99, 505–513. [Google Scholar] [CrossRef]
- Guerreiro, R.; Henriques, C.; Trevas, S.; Gouveia, C.; Roldão, M.; de Sousa, I.E.; Faria, C.; Pimenta, G.; Araújo, I.; Fonseca, C. Predicting Prognosis in Internal Medicine: A Short and Long-Term Mortality Comparison Analysis. Cureus 2022, 14, e21734. [Google Scholar] [CrossRef]
- Giordano, G.; Berardi, D.; Berardi, G.; Di Gregorio, G.; Carabellese, B.; Licianci, A. Sucrosomial® iron vs. different iron oral for-mulation in iron-deficiency anemia due to gastrointestinal bleeding: Multicentric randomized study. Expert Rev. Hematol. 2017, 10, 18–19. [Google Scholar]
- World Health Organization. WHO Global Anaemia Estimates, 2021 Edition. Global Anaemia Estimates in Women of Reproductive Age, by Pregnancy Status, and in Children Aged 6–59 Months; World Health Organization: Geneve, Switzerland, 2021. Available online: https://www.who.int/data/gho/data/themes/topics/anaemia_in_women_and_children (accessed on 22 January 2023).
- Cannavó, L.; De Luca, F.; Zirilli, G. Efficacy of therapy with sucosomial iton in patients with primary iron deficiency anemia in pediatric age. In Proceedings of the XLVIII National Congress AIEOP (Associazione Italiana di Ematologia e Oncologia Pediatrica), Bologne, Italy, 28–29 May 2018. Abstract N. 0064. [Google Scholar]
- Distante, M.; Verardi, S.; Tarani, F.; Musto, F.; Rossetti, D.; Aloi, M. G-P-332. Efficacy and safety of oral and parenteral iron therapy in the paediatric patient with inflammatory bowel disease. Pediatr. Gastroenterol. Nutr. 2022, 74 (Suppl. S2), 516. [Google Scholar]
- Finberg, K.E.; Heeney, M.M.; Campagna, D.R.; Aydınok, Y.; Pearson, H.A.; Hartman, K.R.; Mayo, M.M.; Samuel, S.M.; Strouse, J.J.; Markianos, K.; et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRI-DA). Nat. Genet. 2008, 40, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Asperti, M.; Brilli, E.; Denardo, A.; Gryzik, M.; Pagani, F.; Busti, F.; Tarantino, G.; Arosio, P.; Girelli, D.; Poli, M. Iron distribution in different tissues of homozygous Mask (msk/msk) mice and the effects of oral iron treatments. Am. J. Hematol. 2021, 96, 1253–1263. [Google Scholar] [CrossRef]
- Shander, A.; Hardy, J.-F.; Ozawa, S.; Farmer, S.L.; Hofmann, A.; Frank, S.M.; Kor, D.J.; Faraoni, D.; Freedman, J. A Global Definition of Patient Blood Management. Anesth. Analg. 2022, 135, 476–488. [Google Scholar] [CrossRef]
- World Health Organization. The Urgent Need to Implement Patient Blood Management: Policy Brief; World Health Organization: Geneve, Switzerland, 2021. Available online: https://apps.who.int/iris/handle/10665/346655 (accessed on 22 January 2023).
- Fowler, A.J.; Ahmad, T.; Phull, M.K.; Allard, S.; Gillies, M.A.; Pearse, R.M. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br. J. Surg. 2015, 102, 1314–1324. [Google Scholar] [CrossRef]
- Kunz, J.V.; Spies, C.D.; Bichmann, A.; Sieg, M.; Mueller, A. Postoperative anaemia might be a risk factor for postoperative delirium and prolonged hospital stay: A secondary analysis of a prospective cohort study. PLoS ONE 2020, 15, e0229325. [Google Scholar] [CrossRef]
- Piednoir, P.; Allou, N.; Driss, F.; Longrois, D.; Philip, I.; Beaumont, C.; Montravers, P.; Lasocki, S. Preoperative iron deficiency increases transfusion requirements and fatigue in cardiac surgery patients: A Prospective Observational Study. Eur. J. Anaesthesiol. 2011, 28, 796–801. [Google Scholar] [CrossRef]
- Miles, L.F.; Kunz, S.A.; Na, L.H.; Braat, S.; Burbury, K.; Story, D.A. Postoperative outcomes following cardiac surgery in non-anaemic iron-replete and iron-deficient patients—An exploratory study. Anaesthesia 2017, 73, 450–458. [Google Scholar] [CrossRef]
- Rössler, J.; Schoenrath, F.; Seifert, B.; Kaserer, A.; Spahn, G.H.; Falk, V.; Spahn, D.R. Iron deficiency is associated with higher mortality in patients undergoing cardiac surgery: A prospective study. Br. J. Anaesth. 2020, 124, 25–34. [Google Scholar] [CrossRef]
- Miles, L.F.; Soo, V.P.; Braat, S.; Bade-Boon, J.; Heritier, S.; A Klein, A.; Myles, P.S.; Richards, T.; Symons, J.; Burbury, K.L.; et al. Associations between non-anaemic iron deficiency and outcomes following elective cardiac surgery (IDOCS): A prospective cohort study. Lancet Haematol. 2022, 9, e514–e522. [Google Scholar] [CrossRef]
- Scardino, M.; Di Matteo, B.; Martorelli, F.; Tanzi, D.; Kon, E.; D’amato, T. Improved patient blood management and cost saving in hip replacement surgery through the implementation of pre-operative Sucrosomial® iron supplementation: A quality improvement assessment study. Int. Orthop. 2019, 43, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, M.; Hrelia, S.; Malaguti, M.; De Vecchi, E.; Lombardi, G.; Banfi, G.; Riso, P.; Porrini, M.; Romagnoli, S.; Pino, F.; et al. Oral Supplementation with Sucrosomial Ferric Pyrophosphate Plus L-Ascorbic Acid to Ameliorate the Martial Status: A Randomized Controlled Trial. Nutrients 2020, 12, 386. [Google Scholar] [CrossRef] [PubMed]
- Pierelli, L.; De Rosa, A.; Falco, M.; Papi, E.; Rondinelli, M.; Turani, F.; Weltert, L. Preoperative Sucrosomial Iron Supplementation Increases Haemoglobin and Reduces Transfusion Requirements in Elective Heart Surgery Patients: A Prospective Randomized Study. Surg. Technol. Int. 2021, 39, 321–328. [Google Scholar] [CrossRef]
- Klein, A.A.; Chau, M.; Yeates, J.A.; Collier, T.; Evans, C.; Agarwal, S.; Richards, T.; UK Cardiac and Vascular Surgery Interven-tional Anaemia Response (CAVIAR) study team. Preoperative intravenous iron before cardiac surgery: A prospective multicentre feasibility study. Br. J. Anaesth. 2020, 124, 243–250. [Google Scholar] [CrossRef]
- Evans, C.R.; Jones, R.; Phillips, G.; Greene, G.; Phillips, M.; Morris-Clarke, R. Observational study of pre-operative intravenous iron given to anaemic patients before elective cardiac surgery. Anaesthesia 2021, 76, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Weltert, L.P.; De Rosa, A.; Rondinelli, M.B.; Falco, M.; Turani, F.; Pierelli, L. Benefits of pre-operative oral Sucrosomial® iron supplementation in cardiac surgery: Influence of patient’s baseline hemoglobin and gender. Blood Transfus. 2022. [Google Scholar] [CrossRef]
- Venturini, E.; Iannuzzo, G.; DI Lorenzo, A.; Cuomo, G.; D’Angelo, A.; Merone, P.; Cudemo, G.; Pacileo, M.; D’Andrea, A.; Vigorito, C.; et al. Short-term treatment of iron deficiency anemia after cardiac surgery. IJC Heart Vasc. 2022, 40, 101038. [Google Scholar] [CrossRef]
- Lucertini, G.; Gazzola, V.; Boschetti, G.A.; Khourieh, T. Sucrosomial iron in the postoperative period after abdominal aortic aneurism repair. Blood Transfus 2020, 18 (Suppl. S1), s25. [Google Scholar]
- Hawkins, T.; Agarwal, S.; Evans, C.R. Centre for Perioperative Care anaemia guideline: Implications for anaesthesia. Br. J. Anaesth. 2023, 130, 115–119. [Google Scholar] [CrossRef]
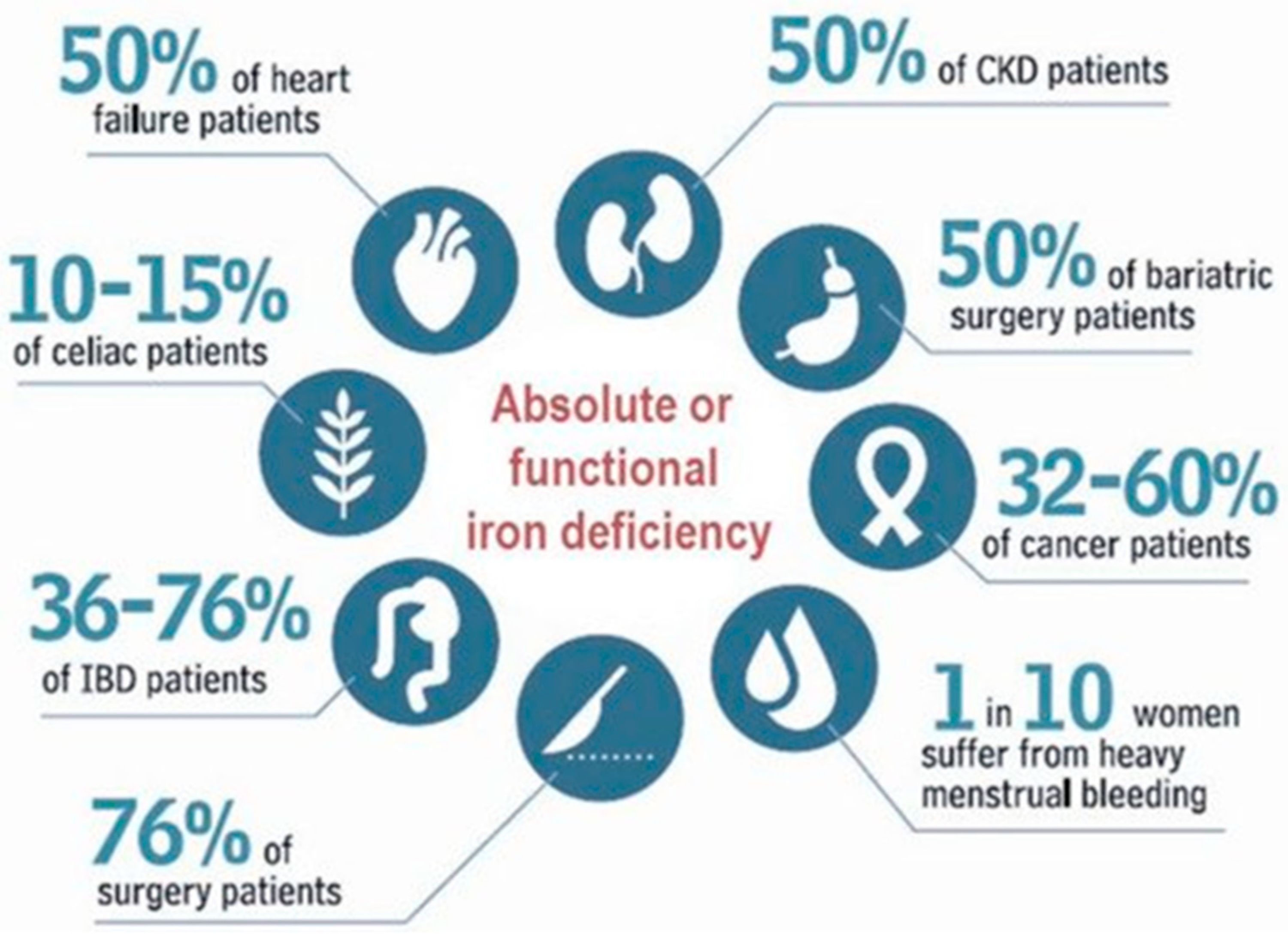
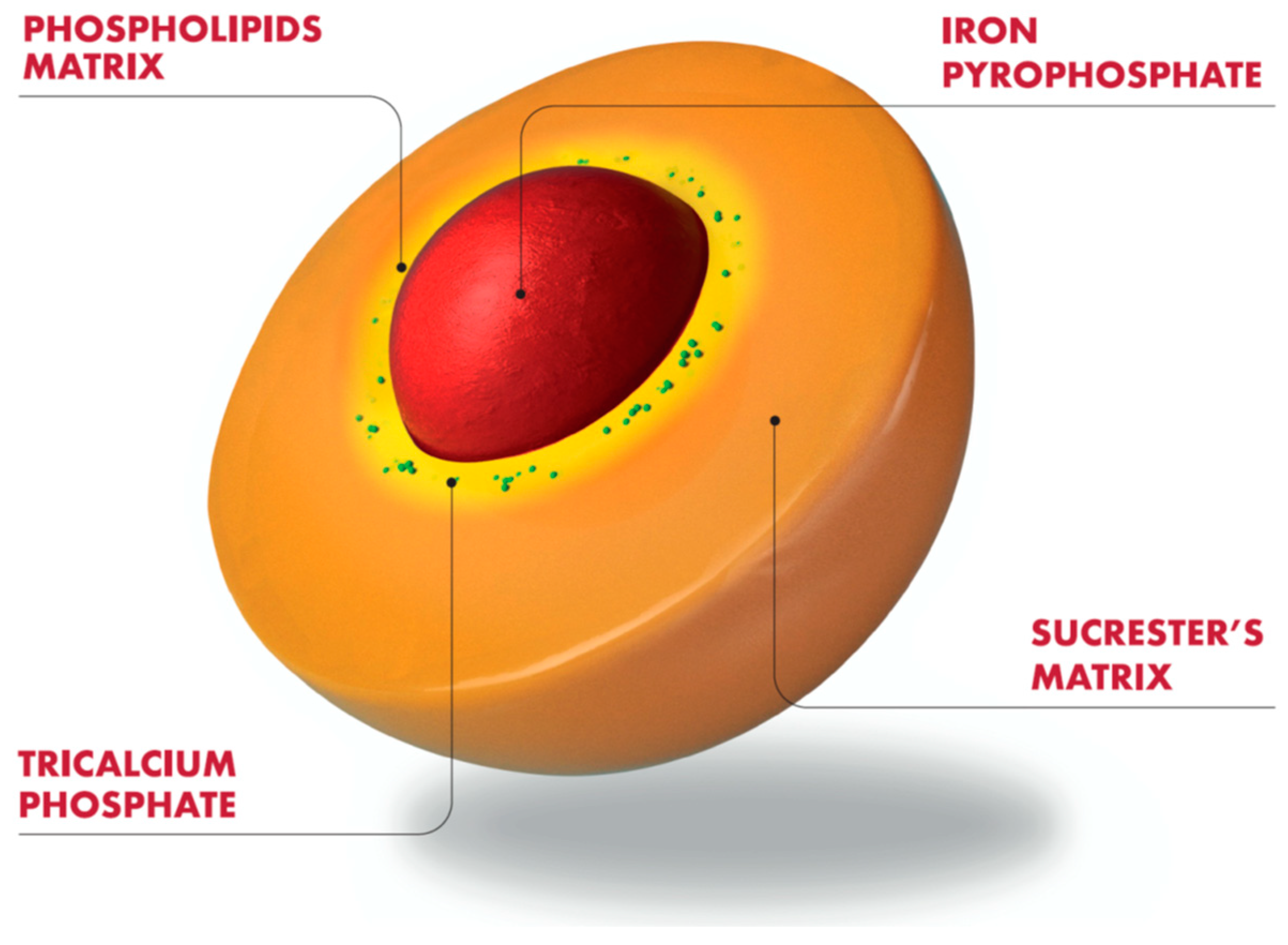
| Clinical Setting | Laboratory Data | Diagnosis |
|---|---|---|
| Anemia and/or signs and symptoms suggestive of iron deficiency | Ferritin 30–300 ng/mL +TSAT > 20% | Iron repletion |
| Ferritin < 30 ng/mL | Absolute iron deficiency | |
| Ferritin 30–100 ng/mL +TSAT < 20% or CRP > 5 mg/L | ||
| Ferritin >100 ng/mL +TSAT < 20% or CRP > 5 mg/L | Functional iron deficiency (iron sequestration) * | |
| Blood donation/pregnancy | Ferritin < 50 ng/mL +TSAT > 20% | Inadequate iron stores |
| Major surgery (Blood loss > 500 mL) | Ferritin <100 ng/mL +TSAT > 20% |
| Author (Year) [Ref] Study Type | Patients | Treatment Compound (Dose) Duration | Baseline Hb (g/dL) | Final Hb (g/dL) | Baseline Ferritin (ng/mL) | Final Ferritin (ng/mL) | Baseline TSAT (%) | Final TSAT (%) | GI Side Effects |
|---|---|---|---|---|---|---|---|---|---|
| Parisi et al. (2017) [70] RCT | 80 non-anemic Singleton pregnancy 12–14 weeks | Control (no iron) FS (30 mg/day) SI (14 mg/day) SI (28 mg/day Up to 6 weeks postpartum | 12.0 11.9 12.0 11.9 | 11.6 11.8 12.0 12.6 | 46.6 43.7 52.4 52.6 | 31.3 43.1 40.8 49.8 | 27.6 26.7 28.1 26.5 | 25.6 26.7 29.5 28.8 | 0% 0% 0% 0% |
| Mafodda et al. (2017) [66] RCT pilot | 64 patients with solid tumors | SI (30 mg/day) + DEPO 500 mcg/3 w FG (125 mg/wk) + DEPO 500 mcg/3 weeks 2 months | 9.4 9.2 | 12.7 12.9 | --- | --- | --- | --- | 3% 0% |
| Pisani et al. (2014) [65] RCT | 99 ND-CKD3-5 | SI (30 mg/day) (n = 66) FG (125 mg/week IV, up to 1000 mg) (n = 33) 3 months | 10.8 10.7 | 11.4 11.7 | 71 68 | 86 239 | 16.5 17.0 | 18.3 21.5 | 12% 18% |
| Bertani et al. (2021) [71] RCT | 42 UC Mild-to-moderate anemia | SI (60 mg/day, 2 months, plus 30 mg/day, 1 month) FCM (1000 mg IV, at baseline) 3 months | 11.1 10.3 | 12.2 11.8 | 16 10 | 26 131 | --- | --- | 5% 0% |
| Elli et al. (2018) [63] Observational | 43 celiac disease | SI (30 mg/day) intolerant to FS (n = 24) FS (105 mg/day) (n = 17) 3 months | 10.9 11.0 | 12.0 12.9 | 10.7 13.4 | 18.2 59.1 | 10.0 10.6 | 14.8 19.6 | 0% 10% |
| Farinati et al. (2018) [72] Observational | 20 women with AIAG and anemia | SI (120 mg/daily, either fasting or during meals) 8 weeks | 10.5 | 12.5 | 7 | 27 | --- | --- | 15% |
| Ciudín et al. (2017) [64] Case–control | 40 bariatric surgeries All women | SI (28 mg/day) (n = 20) IS (300 mg IV) (n = 20) 3 months | 12.4 12.5 | 12.3 12.7 | 102 98 | 89 96 | 22.9 23.6 | 24.1 26.3 | 0% 0% |
| Karavidas et al. (2021) [73] Observational | 50 patients with HFrEF (LVEF 27 ± 5) | SI (28 mg/day), 3 months (n = 25) * Matched non-treated controls (n = 25) Follow-up 3 and 6 months | 12.5 12.9 | 12.9 13.2 12.7 12.6 | 39 45 | 67 79 45 44 | --- --- | --- --- | 1 (4%) --- |
| Giordano et al. (2019) [74] RCT | 135 patients with MSD and low-risk refractory anemia | SI (28 mg/day) + EPO (n = 54) FG (62.5 mg/week) + EPO (n = 43) No iron + EPO (n = 38) 3 months | 8.9 9.3 9.7 | 12.0 12.0 12.0 | 610.2 608.8 699.5 | 607.0 # 723.7 # 730.3 # | --- --- --- | 32 # 48 # 40 # | No |
| Giordano et al. (2021) [75] RCT | 90 patients with IDA due to bleeding | SI (120 mg/day) (n = 45) FG (62.5 mg/day to cover TID) (n = 45) 4 weeks ** | 8.5 8.2 | 12.0 12.0 | 5 7 | 260 ## 18 ## | --- --- | --- --- | 36% 22% & |
| Clinical Setting | Patients Receiving Oral SI (n) | Gastrointestinal Adverse Effects (%) * |
|---|---|---|
| Obstetrics and Gynecology | 165 | 5.4 |
| Oncology | 232 | 8.9 |
| Gastroenterology | 238 | 9.2 |
| Nephrology | 301 | 4.2 |
| Cardiology | 81 | 1.2 |
| Internal Medicine | 233 | 8.2 |
| Surgery | 762 | 1.2 |
| Overall | 2012 | 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Ramírez, S.; Brilli, E.; Tarantino, G.; Girelli, D.; Muñoz, M. Sucrosomial® Iron: An Updated Review of Its Clinical Efficacy for the Treatment of Iron Deficiency. Pharmaceuticals 2023, 16, 847. https://doi.org/10.3390/ph16060847
Gómez-Ramírez S, Brilli E, Tarantino G, Girelli D, Muñoz M. Sucrosomial® Iron: An Updated Review of Its Clinical Efficacy for the Treatment of Iron Deficiency. Pharmaceuticals. 2023; 16(6):847. https://doi.org/10.3390/ph16060847
Chicago/Turabian StyleGómez-Ramírez, Susana, Elisa Brilli, Germano Tarantino, Domenico Girelli, and Manuel Muñoz. 2023. "Sucrosomial® Iron: An Updated Review of Its Clinical Efficacy for the Treatment of Iron Deficiency" Pharmaceuticals 16, no. 6: 847. https://doi.org/10.3390/ph16060847
APA StyleGómez-Ramírez, S., Brilli, E., Tarantino, G., Girelli, D., & Muñoz, M. (2023). Sucrosomial® Iron: An Updated Review of Its Clinical Efficacy for the Treatment of Iron Deficiency. Pharmaceuticals, 16(6), 847. https://doi.org/10.3390/ph16060847







