Local Delivery Strategies for Peptides and Proteins into the CNS: Status Quo, Challenges, and Future Perspectives
Abstract
1. Introduction
2. Local CNS Administration Routes and Challenges
2.1. ICV
2.2. CED
2.3. IT
2.4. Others
3. Formulation Strategies to Improve CNS Protein/Peptide Delivery
3.1. Peptide/Protein Functionalization Strategies
3.2. Long-Acting Formulations
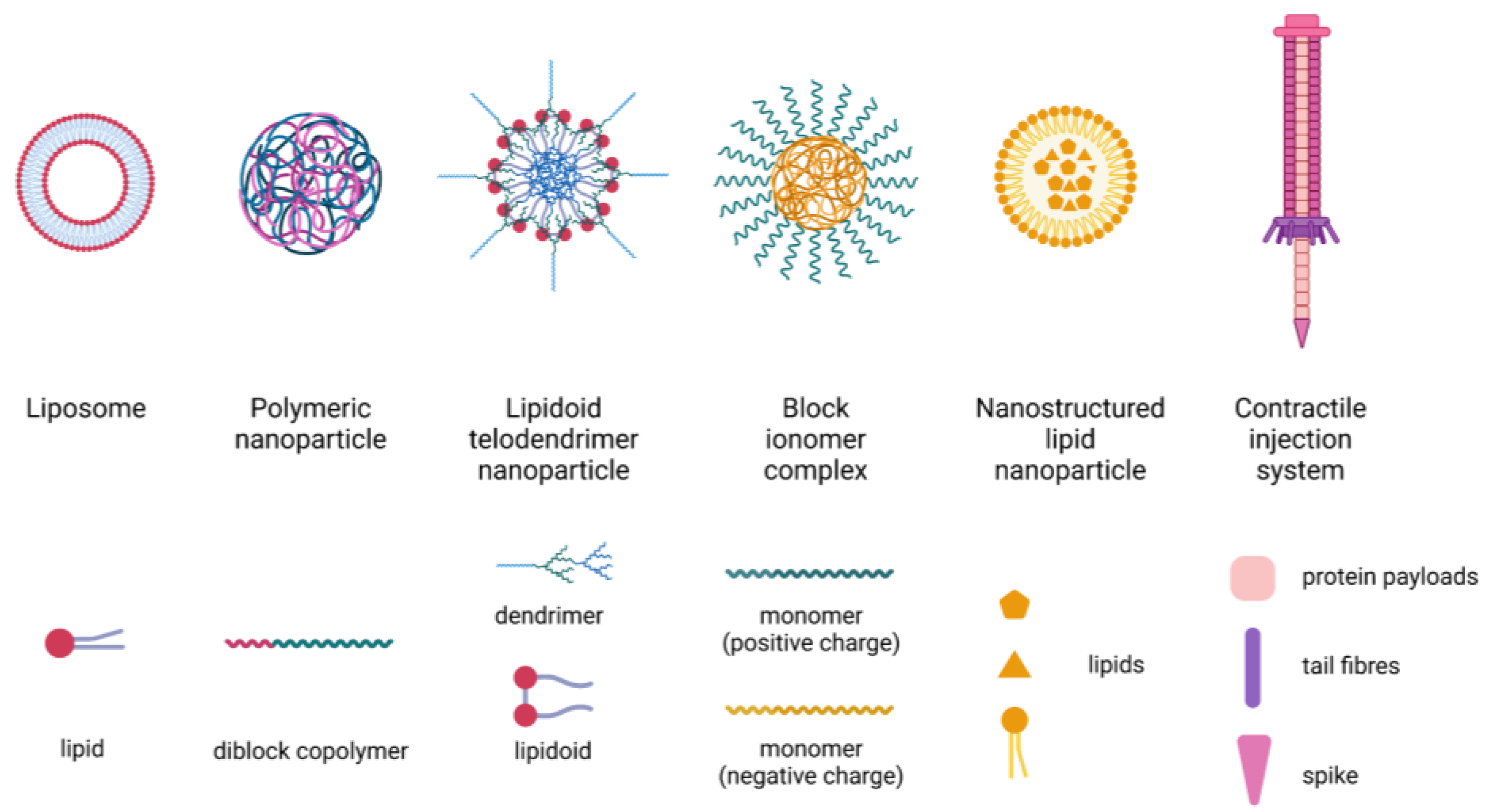
3.3. Nanotechnology-Based Delivery Systems
3.4. Extracellular Vesicles
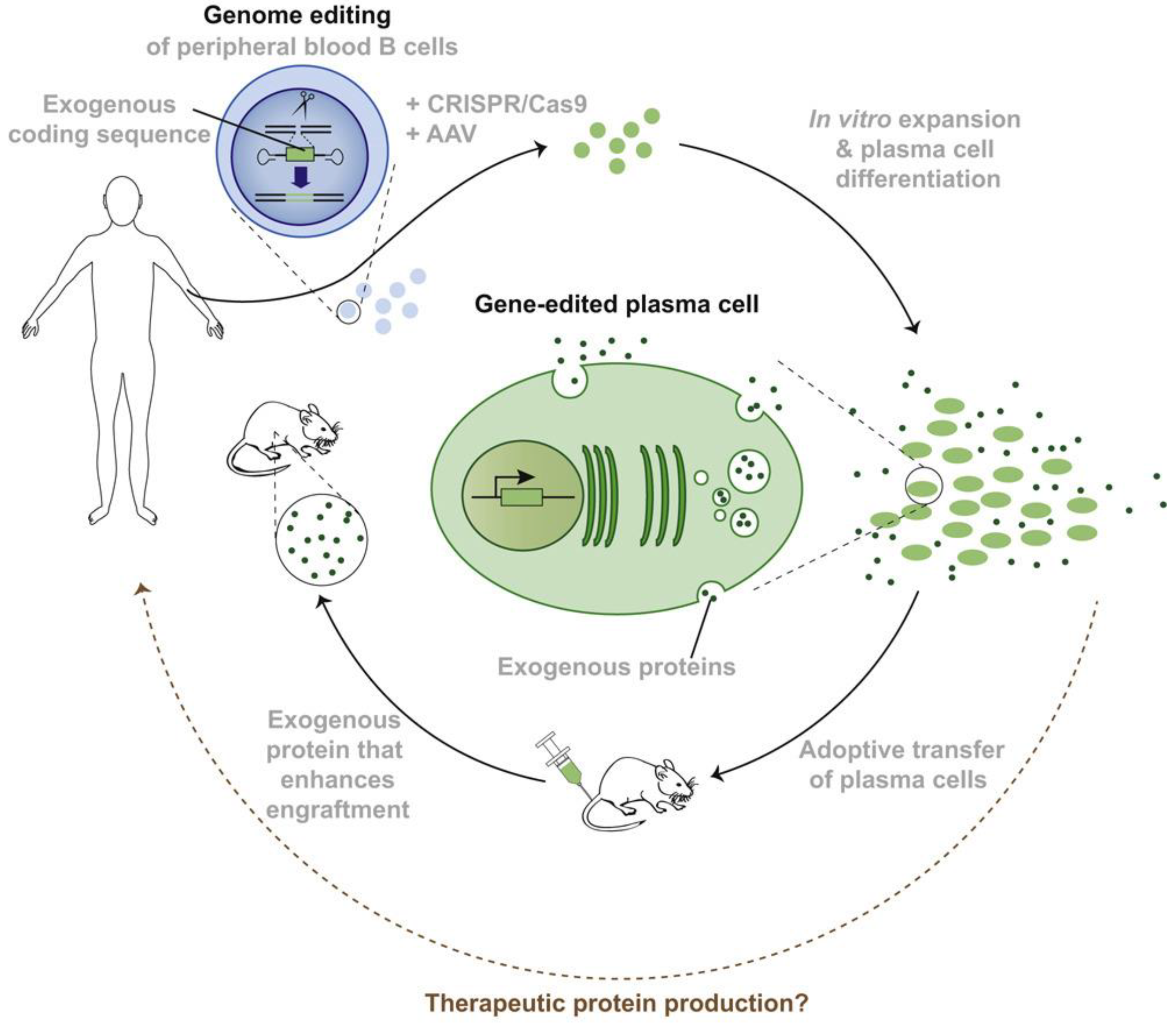
3.5. Live-Cell Therapy
4. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartley, H. Origin of the Word ‘Protein’. Nature 1951, 168, 244. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Knight, G.; Wiles, P.; Keen, H.; Ward, J.; Cauldwell, J.; Adeniyi-Jones, R.; Leiper, J.; Jones, R.; Maccuish, A.; et al. Biosynthetic Human Insulin in the Treatment of Diabetes: A Double-blind Crossover Trial in Established Diabetic Patients. Lancet 1982, 320, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Brasnjevic, I.; Steinbusch, H.W.; Schmitz, C.; Martinez-Martinez, P. Delivery of peptide and protein drugs over the blood–brain barrier. Prog. Neurobiol. 2009, 87, 212–251. [Google Scholar] [CrossRef] [PubMed]
- Hökfelt, T.; Bartfai, T.; Bloom, F. Neuropeptides: Opportunities for drug discovery. Lancet Neurol. 2003, 2, 463–472. [Google Scholar] [CrossRef]
- Oosthuyse, B.; Moons, L.; Storkebaum, E.; Beck, H.; Nuyens, D.; Brusselmans, K.; Van Dorpe, J.; Hellings, P.; Gorselink, M.; Heymans, S.; et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat. Genet. 2001, 28, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Craft, S.; Claxton, A.; Baker, L.D.; Hanson, A.J.; Cholerton, B.; Trittschuh, E.H.; Dahl, D.; Caulder, E.; Neth, B.; Montine, T.J.; et al. Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer’s Disease Biomarkers: A Pilot Clinical Trial. J. Alzheimers Dis. 2017, 57, 1325–1334. [Google Scholar] [CrossRef]
- Sayed, S.; Van Dam, N.; Horn, S.R.; Kautz, M.M.; Parides, M.; Costi, S.; Collins, K.A.; Iacoviello, B.; Iosifescu, D.V.; Mathé, A.A.; et al. A Randomized Dose-Ranging Study of Neuropeptide Y in Patients with Posttraumatic Stress Disorder. Int. J. Neuropsychopharmacol. 2018, 21, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Proulx, S.T. Cerebrospinal fluid outflow: A review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell. Mol. Life Sci. 2021, 78, 2429–2457. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. Neurorx 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Lin, J.H. Pharmacokinetics of Biotech Drugs: Peptides, Proteins and Monoclonal Antibodies. Curr. Drug Metab. 2009, 10, 661–691. [Google Scholar] [CrossRef]
- Bumbaca, B.; Li, Z.; Shah, D.K. Pharmacokinetics of protein and peptide conjugates. Drug Metab. Pharmacokinet. 2019, 34, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Filippi, C.G.; Wong, T.; Ray, A.; Fralin, S.; Tsiouris, A.J.; Praminick, B.; Demopoulos, A.; McCrea, H.J.; Bodhinayake, I.; et al. Superselective intraarterial cerebral infusion of cetuximab after osmotic blood/brain barrier disruption for recurrent malignant glioma: Phase I study. J. Neuro-Oncol. 2016, 128, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, K.L.; Pinheiro, L.; Brem, H. Delivery of local therapeutics to the brain: Working toward advancing treatment for malignant gliomas. Ther. Deliv. 2015, 6, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef]
- Yi, X.; Manickam, D.S.; Brynskikh, A.; Kabanov, A.V. Agile delivery of protein therapeutics to CNS. J. Control. Release 2014, 190, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Press Releases from BioMarin. Available online: https://investors.biomarin.com/2017-04-27-FDA-Approves-Brineura-TM-cerliponase-alfa-for-the-Treatment-of-CLN2-Disease-a-Form-of-Batten-Disease-and-Ultra-Rare-Pediatric-Brain-Disorder-in-Children (accessed on 26 April 2023).
- Schulz, A.; Ajayi, T.; Specchio, N.; Reyes, E.D.L.; Gissen, P.; Ballon, D.; Dyke, J.P.; Cahan, H.; Slasor, P.; Jacoby, D.; et al. Study of Intraventricular Cerliponase Alfa for CLN2 Disease. N. Engl. J. Med. 2018, 378, 1898–1907. [Google Scholar] [CrossRef]
- Gissen, P.; Specchio, N.; Olaye, A.; Jain, M.; Butt, T.; Ghosh, W.; Ruban-Fell, B.; Griffiths, A.; Camp, C.; Sisic, Z.; et al. Investigating health-related quality of life in rare diseases: A case study in utility value determination for patients with CLN2 disease (neuronal ceroid lipofuscinosis type 2). Orphanet J. Rare Dis. 2021, 16, 217. [Google Scholar] [CrossRef]
- Seo, J.-H.; Kosuga, M.; Hamazaki, T.; Shintaku, H.; Okuyama, T. Impact of intracerebroventricular enzyme replacement therapy in patients with neuronopathic mucopolysaccharidosis type II. Mol. Ther.-Methods Clin. Dev. 2021, 21, 67–75. [Google Scholar] [CrossRef]
- Van Damme, P.; Tilkin, P.; Mercer, K.J.; Terryn, J.; D’hondt, A.; Herne, N.; Tousseyn, T.; Claeys, K.G.; Thal, D.R.; Zachrisson, O.; et al. Intracerebroventricular delivery of vascular endothelial growth factor in patients with amyotrophic lateral sclerosis, a phase I study. Brain Commun. 2020, 2, fcaa160. [Google Scholar] [CrossRef]
- Bander, E.D.; Ramos, A.D.; Wembacher-Schroeder, E.; Ivasyk, I.; Thomson, R.; Morgenstern, P.F.; Souweidane, M.M. Repeat convection-enhanced delivery for diffuse intrinsic pontine glioma. J. Neurosurg. Pediatr. 2020, 26, 661–666. [Google Scholar] [CrossRef]
- Souweidane, M.M.; Kramer, K.; Pandit-Taskar, N.; Zhou, Z.; Haque, S.; Zanzonico, P.; Carrasquillo, J.A.; Lyashchenko, S.K.; Thakur, S.B.; Donzelli, M.; et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: A single-centre, dose-escalation, phase 1 trial. Lancet Oncol. 2018, 19, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Dali, C.; Sevin, C.; Krägeloh-Mann, I.; Giugliani, R.; Sakai, N.; Wu, J.; Wasilewski, M. Safety of intrathecal delivery of recombinant human arylsulfatase A in children with metachromatic leukodystrophy: Results from a phase 1/2 clinical trial. Mol. Genet. Metab. 2020, 131, 235–244. [Google Scholar] [CrossRef]
- Luger, T.J.; Kathrein, A.; Rieger, M.; Lorenz, I.H. Intracerebroventricular and intrathecal injectate spread in rats. Eur. J. Anaesthesiol. 2005, 22, 236–239. [Google Scholar] [CrossRef]
- Schultz, M.L.; Tecedor, L.; Chang, M.; Davidson, B.L. Clarifying lysosomal storage diseases. Trends Neurosci. 2011, 34, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Bellettato, C.M.; Scarpa, M. Pathophysiology of neuropathic lysosomal storage disorders. J. Inherit. Metab. Dis. 2010, 33, 347–362. [Google Scholar] [CrossRef]
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.E.; Boumaza, I.; Lacomis, D.; Bowser, R. Cystatin C: A Candidate Biomarker for Amyotrophic Lateral Sclerosis. PLoS ONE 2010, 5, e15133. [Google Scholar] [CrossRef]
- Okamoto, K.; Hirai, S.; Amari, M.; Watanabe, M.; Sakurai, A. Bunina bodies in amyotrophic lateral sclerosis immunostained with rabbit anti-cystatin C serum. Neurosci. Lett. 1993, 162, 125–128. [Google Scholar] [CrossRef]
- Watanabe, S.; Komine, O.; Endo, F.; Wakasugi, K.; Yamanaka, K. Intracerebroventricular administration of Cystatin C ameliorates disease in SOD1-linked amyotrophic lateral sclerosis mice. J. Neurochem. 2018, 145, 80–89. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Noguchi, Y.; Kato, M.; Ozeki, K.; Ishigai, M. Pharmacokinetics of an intracerebroventricularly administered antibody in rats. mAbs 2017, 9, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.L.; Flessner, M.F. Pharmacokinetic considerations on monoclonal antibodies. Prog. Clin. Biol. Res. 1989, 288, 429–438. [Google Scholar] [PubMed]
- Lonser, R.R.; Sarntinoranont, M.; Morrison, P.F.; Oldfield, E.H. Convection-enhanced delivery to the central nervous system. J. Neurosurg. 2015, 122, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef]
- Weber, F.W.; Floeth, F.; Asher, A.; Bucholz, R.; Berge, M.; Pradoss, M.; Chang, S.; Bruces, J.; Hall, W.; Raino, N.G.; et al. Local Convection Enhanced Delivery of IL4-Pseudomonas Exotoxin (NBI-3001) for Treatment of Patients with Recurrent Malignant Glioma; Springer: Vienna, Austria, 2003; pp. 93–103. [Google Scholar] [CrossRef]
- Kunwar, S.; Chang, S.; Westphal, M.; Vogelbaum, M.; Sampson, J.; Barnett, G.; Shaffrey, M.; Ram, Z.; Piepmeier, J.; Prados, M.; et al. Phase III randomized trial of CED of IL13-PE38QQR vs. Gliadel wafers for recurrent glioblastoma. Neuro-Oncology 2010, 12, 871–881. [Google Scholar] [CrossRef]
- Salvatore, M.F.; Ai, Y.; Fischer, B.; Zhang, A.M.; Grondin, R.C.; Zhang, Z.; Gerhardt, G.A.; Gash, D.M. Point source concentration of GDNF may explain failure of phase II clinical trial. Exp. Neurol. 2006, 202, 497–505. [Google Scholar] [CrossRef]
- Pardridge, W.M. Biopharmaceutical drug targeting to the brain. J. Drug Target. 2010, 18, 157–167. [Google Scholar] [CrossRef]
- Sampson, J.H.; Akabani, G.; Archer, G.E.; Berger, M.S.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; Greer, K.; Herndon, J.E.; Kunwar, S.; et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro-Oncology 2008, 10, 320–329. [Google Scholar] [CrossRef]
- Looseley, A. Corning and Cocaine: The Advent of Spinal Anaesthesia; Grand Rounds: Beckenham, UK, 2009; Volume 9. [Google Scholar] [CrossRef]
- Cudkowicz, M.E.; Warren, L.; Francis, J.W.; Lloyd, K.J.; Friedlander, R.M.; Borges, L.F.; Kassem, N.; Munsta, T.L.; Brown, R.H. Intrathecal administration of recombinant human superoxide dismutase 1 in amyotrophic lateral sclerosis: A preliminary safety and pharmacokinetic study. Neurology 1997, 49, 213–222. [Google Scholar] [CrossRef]
- Pizzo, M.E.; Wolak, D.J.; Kumar, N.N.; Brunette, E.; Brunnquell, C.L.; Hannocks, M.; Abbott, N.J.; Meyerand, M.E.; Sorokin, L.; Stanimirovic, D.B.; et al. Intrathecal antibody distribution in the rat brain: Surface diffusion, perivascular transport and osmotic enhancement of delivery. J. Physiol. 2018, 596, 445–475. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H., 2nd. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef] [PubMed]
- Falcone, J.A.; Salameh, T.S.; Yi, X.; Cordy, B.J.; Mortell, W.G.; Kabanov, A.V.; Banks, W.A. Intranasal Administration as a Route for Drug Delivery to the Brain: Evidence for a Unique Pathway for Albumin. Experiment 2014, 351, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Craft, S.; Baker, L.D.; Montine, T.J.; Minoshima, S.; Watson, G.S.; Claxton, A.; Arbuckle, M.; Callaghan, M.; Tsai, E.; Plymate, S.R.; et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch. Neurol. 2012, 69, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Hallschmid, M.; Schmitz, K.; Schultes, B.; Ratter, F.; Fehm, H.L.; Born, J.; Kern, W. Intranasal Insulin Improves Memory in Humans: Superiority of Insulin Aspart. Neuropsychopharmacology 2007, 32, 239–243. [Google Scholar] [CrossRef]
- Thorne, R.; Pronk, G.; Padmanabhan, V.; Frey, W. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef]
- Banks, W.A.; During, M.J.; Niehoff, M.L. Brain Uptake of the Glucagon-Like Peptide-1 Antagonist Exendin(9-39) after Intranasal Administration. Experiment 2004, 309, 469–475. [Google Scholar] [CrossRef]
- Nonaka, N.; Farr, S.A.; Kageyama, H.; Shioda, S.; Banks, W.A. Delivery of Galanin-Like Peptide to the Brain: Targeting with Intranasal Delivery and Cyclodextrins. Experiment 2008, 325, 513–519. [Google Scholar] [CrossRef]
- Ross, T.; Martinez, P.; Renner, J.; Thorne, R.; Hanson, L.; Frey, W. Intranasal administration of interferon beta bypasses the blood–brain barrier to target the central nervous system and cervical lymph nodes: A non-invasive treatment strategy for multiple sclerosis. J. Neuroimmunol. 2004, 151, 66–77. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal drug targeting of hypocretin-1 (orexin-A) to the central nervous system. J. Pharm. Sci. 2004, 98, 2501–2515. [Google Scholar] [CrossRef]
- Hellard, E.A.R.; Impastato, R.A.; Gilpin, N.W. Intra-cerebral and intra-nasal melanocortin-4 receptor antagonist blocks withdrawal hyperalgesia in alcohol-dependent rats. Addict. Biol. 2017, 22, 692–701. [Google Scholar] [CrossRef]
- Craft, S.; Raman, R.; Chow, T.W.; Rafii, M.S.; Sun, C.-K.; Rissman, R.A.; Donohue, M.C.; Brewer, J.B.; Jenkins, C.; Harless, K.; et al. Safety, Efficacy, and Feasibility of Intranasal Insulin for the Treatment of Mild Cognitive Impairment and Alzheimer Disease Dementia. JAMA Neurol. 2020, 77, 1099–1109. [Google Scholar] [CrossRef]
- Kang, C.E.; Tator, C.H.; Shoichet, M.S. Poly(ethylene glycol) modification enhances penetration of fibroblast growth factor 2 to injured spinal cord tissue from an intrathecal delivery system. J. Control. Release 2010, 144, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, D.; Thal, L. Intraparenchymal nerve growth factor improves behavioral deficits while minimizing the adverse effects of intracerebroventricular delivery. Neuroscience 2004, 124, 743–755. [Google Scholar] [CrossRef]
- Izadpanah, M.; Dargahi, L.; Ai, J.; Taei, A.A.; Barough, S.E.; Mowla, S.J.; TavoosiDana, G.; Farahmandfar, M. Extracellular Vesicles as a Neprilysin Delivery System Memory Improvement in Alzheimer’s Disease. Iran. J. Pharm. Res. 2020, 19, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Kauer, T.M.; Figueiredo, J.-L.; Hingtgen, S.; Shah, K. Encapsulated therapeutic stem cells implanted in the tumor resection cavity induce cell death in gliomas. Nat. Neurosci. 2012, 15, 197–204. [Google Scholar] [CrossRef]
- Laske, D.W.; Youle, R.J.; Oldfield, E.H. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat. Med. 1997, 3, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Frankel, A.; Liu, J.-S.; Rizzieri, D.; Hogge, D. Phase I clinical study of diphtheria toxin-interleukin 3 fusion protein in patients with acute myeloid leukemia and myelodysplasia. Leuk. Lymphoma 2008, 49, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Pastan, I.; FitzGerald, D. Recombinant Toxins for Cancer Treatment. Science 1991, 254, 1173–1177. [Google Scholar] [CrossRef]
- Tong, W.; Dwyer, C.A.; Thacker, B.E.; Glass, C.A.; Brown, J.R.; Hamill, K.; Moremen, K.W.; Sarrazin, S.; Gordts, P.L.; Dozier, L.E.; et al. Guanidinylated Neomycin Conjugation Enhances Intranasal Enzyme Replacement in the Brain. Mol. Ther. 2017, 25, 2743–2752. [Google Scholar] [CrossRef]
- Masuyer, G.; Conrad, J.; Stenmark, P. The structure of the tetanus toxin reveals pH-mediated domain dynamics. EMBO Rep. 2017, 18, 1306–1317. [Google Scholar] [CrossRef]
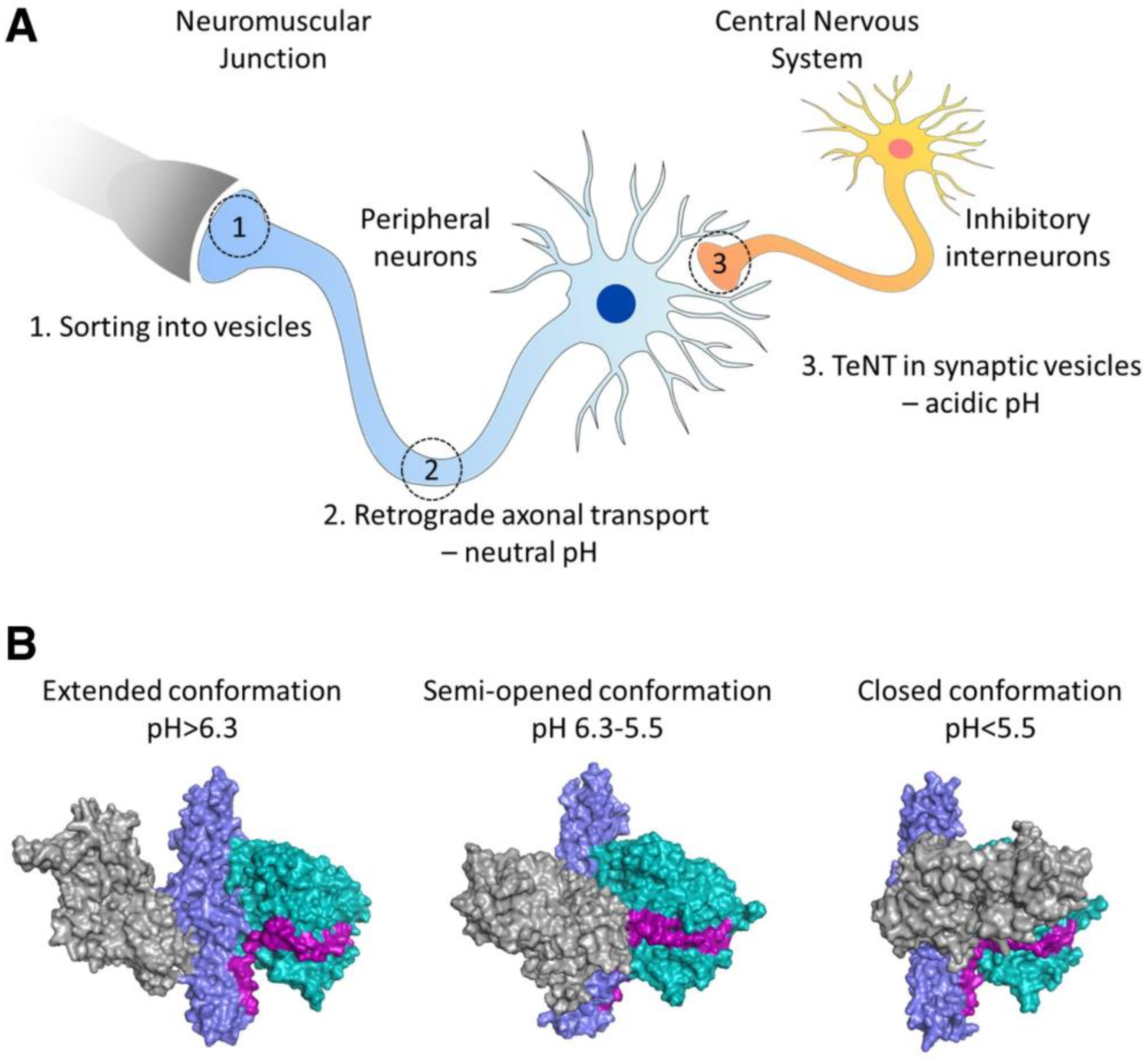
- Bizzini, B.; Stoeckel, K.; Schwab, M. An Antigenic Polypeptide Fragment Isolated from Tetanus Toxin: Chemical Characterization, Binding to Gangliosides and Retrograde Axonal Transport in Various Neuron Systems. J. Neurochem. 1977, 28, 529–542. [Google Scholar] [CrossRef]
- Fishman, P.S.; Savitt, J.M.; Farrand, D.A. Enhanced CNS uptake of systemically administered proteins through conjugation with tetanus C-fragment. J. Neurol. Sci. 1990, 98, 311–325. [Google Scholar] [CrossRef]
- Figueiredo, D.M.; Hallewell, R.A.; Chen, L.L.; Fairweather, N.F.; Dougan, G.; Savitt, J.M.; Parks, D.A.; Fishman, P.S. Delivery of Recombinant Tetanus–Superoxide Dismutase Proteins to Central Nervous System Neurons by Retrograde Axonal Transport. Exp. Neurol. 1997, 145, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Chian, R.-J.; Li, J.; Ay, I.; Celia, S.A.; Kashi, B.B.; Tamrazian, E.; Matthews, J.C.; Bronson, R.T.; Rossomando, A.; Pepinsky, R.B.; et al. IGF-1:Tetanus toxin fragment C fusion protein improves delivery of IGF-1 to spinal cord but fails to prolong survival of ALS mice. Brain Res. 2009, 1287, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, J.M.; Oliván, S.; Osta, R. Tetanus Toxin C-Fragment: The Courier and the Cure? Toxins 2010, 2, 2622–2644. [Google Scholar] [CrossRef] [PubMed]
- Bailon, P.; Won, C.-Y. PEG-modified biopharmaceuticals. Expert Opin. Drug Deliv. 2009, 6, 1–16. [Google Scholar] [CrossRef]
- Brem, H.; Piantadosi, S.; Burger, P.C.; Walker, M.; Selker, R.; Vick, N.A.; Black, K.; Sisti, M.; Brem, S.; Mohr, G.; et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. Lancet 1995, 345, 1008–1012. [Google Scholar] [CrossRef]
- Tsai, N.-M.; Chen, Y.-L.; Lee, C.-C.; Lin, P.-C.; Cheng, Y.-L.; Chang, W.-L.; Lin, S.-Z.; Harn, H.-J. The natural compound n-butylidenephthalide derived from Angelica sinensis inhibits malignant brain tumor growth in vitro and in vivo3. J. Neurochem. 2006, 99, 1251–1262. [Google Scholar] [CrossRef]
- A Phase I/IIa Study of Cerebraca Wafer Plus Adjuvant Temozolomide (TMZ) in Patients with Recurrent High Grade Glioma. Available online: https://clinicaltrials.gov/ct2/show/NCT03234595 (accessed on 22 April 2023).
- Zhang, J.; Chen, C.; Li, A.; Jing, W.; Sun, P.; Huang, X.; Liu, Y.; Zhang, S.; Du, W.; Zhang, R.; et al. Immunostimulant hydrogel for the inhibition of malignant glioma relapse post-resection. Nat. Nanotechnol. 2021, 16, 538–548. [Google Scholar] [CrossRef]
- Xu, H.-L.; Tian, F.-R.; Lu, C.-T.; Xu, J.; Fan, Z.-L.; Yang, J.-J.; Chen, P.-P.; Huang, Y.-D.; Xiao, J.; Zhao, Y.-Z. Thermo-sensitive hydrogels combined with decellularised matrix deliver bFGF for the functional recovery of rats after a spinal cord injury. Sci. Rep. 2016, 6, 38332. [Google Scholar] [CrossRef]
- Xu, H.-L.; Tian, F.-R.; Xiao, J.; Chen, P.-P.; Xu, J.; Fan, Z.-L.; Yang, J.-J.; Lu, C.-T.; Zhao, Y.-Z. Sustained-release of FGF-2 from a hybrid hydrogel of heparin-poloxamer and decellular matrix promotes the neuroprotective effects of proteins after spinal injury. Int. J. Nanomed. 2018, 13, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Donaghue, I.E.; Tator, C.H.; Shoichet, M.S. Local Delivery of Neurotrophin-3 and Anti-NogoA Promotes Repair After Spinal Cord Injury. Tissue Eng. Part A 2016, 22, 733–741. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Gao, M.; Lin, J.; Wu, W.; Wang, J.; Chew, S.Y. Three-dimensional aligned nanofibers-hydrogel scaffold for controlled non-viral drug/gene delivery to direct axon regeneration in spinal cord injury treatment. Sci. Rep. 2017, 7, 42212. [Google Scholar] [CrossRef] [PubMed]
- Pakulska, M.M.; Tator, C.H.; Shoichet, M.S. Local delivery of chondroitinase ABC with or without stromal cell-derived factor 1α promotes functional repair in the injured rat spinal cord. Biomaterials 2017, 134, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, H.; Xu, H.; Zhao, Y.; Li, Z.; Li, J.; Wang, H.; Zhuge, D.; Guo, X.; Xu, H.; et al. Novel multi-drug delivery hydrogel using scar-homing liposomes improves spinal cord injury repair. Theranostics 2018, 8, 4429–4446. [Google Scholar] [CrossRef]
- Ghosh, B.; Wang, Z.; Nong, J.; Urban, M.W.; Zhang, Z.; Trovillion, V.A.; Wright, M.C.; Zhong, Y.; Lepore, A.C. Local BDNF Delivery to the Injured Cervical Spinal Cord using an Engineered Hydrogel Enhances Diaphragmatic Respiratory Function. J. Neurosci. 2018, 38, 5982–5995. [Google Scholar] [CrossRef]
- He, Z.; Zang, H.; Zhu, L.; Huang, K.; Yi, T.; Zhang, S.; Cheng, S. An anti-inflammatory peptide and brain-derived neurotrophic factor-modified hyaluronan-methylcellulose hydrogel promotes nerve regeneration in rats with spinal cord injury. Int. J. Nanomed. 2019, 14, 721–732. [Google Scholar] [CrossRef]
- Lescure, F.; Seguin, C.; Breton, P.; Bourrinet, P.; Roy, D.; Couvreur, P. Preparation and Characterization of Novel Poly(methylidene Malonate 2.1.2.)-Made Nanoparticles. Pharm. Res. 1994, 11, 1270–1277. [Google Scholar] [CrossRef]
- Fournier, E.; Passirani, C.; Colin, N.; Sagodira, S.; Menei, P.; Benoit, J.-P.; Montero-Menei, C.N. The brain tissue response to biodegradable poly(methylidene malonate 2.1.2)-based microspheres in the rat. Biomaterials 2006, 27, 4963–4974. [Google Scholar] [CrossRef]
- Hsiao, C.-Y.; Liu, S.-J.; Ueng, S.W.-N.; Chan, E.-C. The influence of γ irradiation and ethylene oxide treatment on the release characteristics of biodegradable poly(lactide-co-glycolide) composites. Polym. Degrad. Stab. 2012, 97, 715–720. [Google Scholar] [CrossRef]
- Wang, T.; Suita, Y.; Miriyala, S.; Dean, J.; Tapinos, N.; Shen, J. Advances in Lipid-Based Nanoparticles for Cancer Chemoimmunotherapy. Pharmaceutics 2021, 13, 520. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, E.H.; Suys, E.J.; Trevaskis, N.L.; Wheatley, A.K.; Zukancic, D.; Algarni, A.; Al-Wassiti, H.; Davis, T.P.; Pouton, C.W.; Kent, S.J.; et al. From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021, 131, 16–40. [Google Scholar] [CrossRef] [PubMed]
- Pakulska, M.M.; Donaghue, I.E.; Obermeyer, J.M.; Tuladhar, A.; McLaughlin, C.K.; Shendruk, T.N.; Shoichet, M.S. Encapsulation-free controlled release: Electrostatic adsorption eliminates the need for protein encapsulation in PLGA nanoparticles. Sci. Adv. 2016, 2, e1600519. [Google Scholar] [CrossRef] [PubMed]
- Donaghue, I.E.; Tator, C.H.; Shoichet, M.S. Sustained delivery of bioactive neurotrophin-3 to the injured spinal cord. Biomater. Sci. 2015, 3, 65–72. [Google Scholar] [CrossRef]
- Wang, X.; Bodman, A.; Shi, C.; Guo, D.; Wang, L.; Luo, J.; Hall, W.A. Tunable Lipidoid-Telodendrimer Hybrid Nanoparticles for Intracellular Protein Delivery in Brain Tumor Treatment. Small 2016, 12, 4185–4192. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Feng, C.; Shao, X.; Liu, Q.; Zhang, Q.; Pang, Z.; Jiang, X. Intranasal nanoparticles of basic fibroblast growth factor for brain delivery to treat Alzheimer’s disease. Int. J. Pharm. 2014, 461, 192–202. [Google Scholar] [CrossRef]
- Zhao, Y.-Z.; Li, X.; Lu, C.-T.; Lin, M.; Chen, L.-J.; Xiang, Q.; Zhang, M.; Jin, R.-R.; Jiang, X.; Shen, X.-T.; et al. Gelatin nanostructured lipid carriers-mediated intranasal delivery of basic fibroblast growth factor enhances functional recovery in hemiparkinsonian rats. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 755–764. [Google Scholar] [CrossRef]
- Gartziandia, O.; Herran, E.; Pedraz, J.L.; Carro, E.; Igartua, M.; Hernandez, R.M. Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration. Colloids Surf. B Biointerfaces 2015, 134, 304–313. [Google Scholar] [CrossRef]
- Ribovski, L.; Hamelmann, N.M.; Paulusse, J.M.J. Polymeric Nanoparticles Properties and Brain Delivery. Pharmaceutics 2021, 13, 2045. [Google Scholar] [CrossRef]
- Khojasteh, A.; Oraee-Yazdani, S.; Dehghani, L.; Soleimani, M.; Keshel, S.H.; Saadatnia, M.; Saboori, M.; Zali, A.; Hashemi, S.M.; Soleimani, R. Safety of intraparenchymal injection of allogenic placenta mesenchymal stem cells derived exosome in patients undergoing decompressive craniectomy following malignant middle cerebral artery infarct, a pilot randomized clinical trial. Int. J. Prev. Med. 2022, 13, 7. [Google Scholar] [CrossRef]
- The Safety and the Efficacy Evaluation of Allogenic Adipose MSC-Exos in Patients with Alzheimer’s Disease. Identifier NCT04388982. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04388982 (accessed on 17 April 2023).
- Mohammed, I.; Ijaz, S.; Mokhtari, T.; Gholaminejhad, M.; Mahdavipour, M.; Jameie, B.; Akbari, M.; Hassanzadeh, G. Subventricular zone-derived extracellular vesicles promote functional recovery in rat model of spinal cord injury by inhibition of NLRP3 inflammasome complex formation. Metab. Brain Dis. 2020, 35, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.H.; Liu, Q.; Selvakumaran, S.; Haney, M.J.; Batrakova, E.V.; Allman, B.L.; Walton, P.A.; Kiser, P.; Whitehead, S.N. Brain Targeting and Toxicological Assessment of the Extracellular Vesicle-Packaged Antioxidant Catalase-SKL Following Intranasal Administration in Mice. Neurotox. Res. 2021, 39, 1418–1429. [Google Scholar] [CrossRef]
- Hung, K.; Meitlis, I.; Hale, M.; Chen, C.-Y.; Singh, S.; Jackson, S.W.; Miao, C.H.; Khan, I.F.; Rawlings, D.J.; James, R.G. Engineering Protein-Secreting Plasma Cells by Homology-Directed Repair in Primary Human B Cells. Mol. Ther. 2018, 26, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Riglar, D.; Silver, P.A. Engineering bacteria for diagnostic and therapeutic applications. Nat. Rev. Genet. 2018, 16, 214–225. [Google Scholar] [CrossRef]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.-C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and Safety of IL13Rα2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2015, 21, 4062–4072. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor–Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017, 3, 1094–1101. [Google Scholar] [CrossRef]
- Goff, S.L.; Morgan, R.A.; Yang, J.C.; Sherry, R.M.; Robbins, P.F.; Restifo, N.P.; Feldman, S.A.; Lu, Y.-C.; Lu, L.; Zheng, Z.; et al. Pilot Trial of Adoptive Transfer of Chimeric Antigen Receptor–transduced T Cells Targeting EGFRvIII in Patients with Glioblastoma. J. Immunother. 2019, 42, 126–135. [Google Scholar] [CrossRef]
- Portnow, J.; Synold, T.W.; Badie, B.; Tirughana, R.; Lacey, S.F.; D’Apuzzo, M.; Metz, M.Z.; Najbauer, J.; Bedell, V.; Vo, T.; et al. Neural Stem Cell–Based Anticancer Gene Therapy: A First-in-Human Study in Recurrent High-Grade Glioma Patients. Clin. Cancer Res. 2017, 23, 2951–2960. [Google Scholar] [CrossRef]
- Killer, K.; Le, O.; Beauséjour, C. The Intracerebroventricular Injection of Murine Mesenchymal Stromal Cells Engineered to Secrete Epidermal Growth Factor Does Not Prevent Loss of Neurogenesis in Irradiated Mice. Radiat. Res. 2021, 196, 315–322. [Google Scholar] [CrossRef]
- Aronson, J.; Katnani, H.; Pomerantseva, I.; Shapir, N.; Tse, H.; Miari, R.; Goltsman, H.; Mwizerwa, O.; Neville, C.; Neil, G.; et al. Sustained intrathecal therapeutic protein delivery using genetically transduced tissue implants in a freely moving rat model. Int. J. Pharm. 2017, 534, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.K.; Schwendeman, S.P. Characterization of Octreotide–PLGA Binding by Isothermal Titration Calorimetry. Biomacromolecules 2020, 21, 4087–4093. [Google Scholar] [CrossRef] [PubMed]
- Kreitz, J.; Friedrich, M.J.; Guru, A.; Lash, B.; Saito, M.; Macrae, R.K.; Zhang, F. Programmable protein delivery with a bacterial contractile injection system. Nature 2023, 616, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Shiue, S.-J.; Rau, R.-H.; Shiue, H.-S.; Hung, Y.-W.; Li, Z.-X.; Yang, K.D.; Cheng, J.-K. Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury–induced pain in rats. Pain 2018, 160, 210–223. [Google Scholar] [CrossRef]
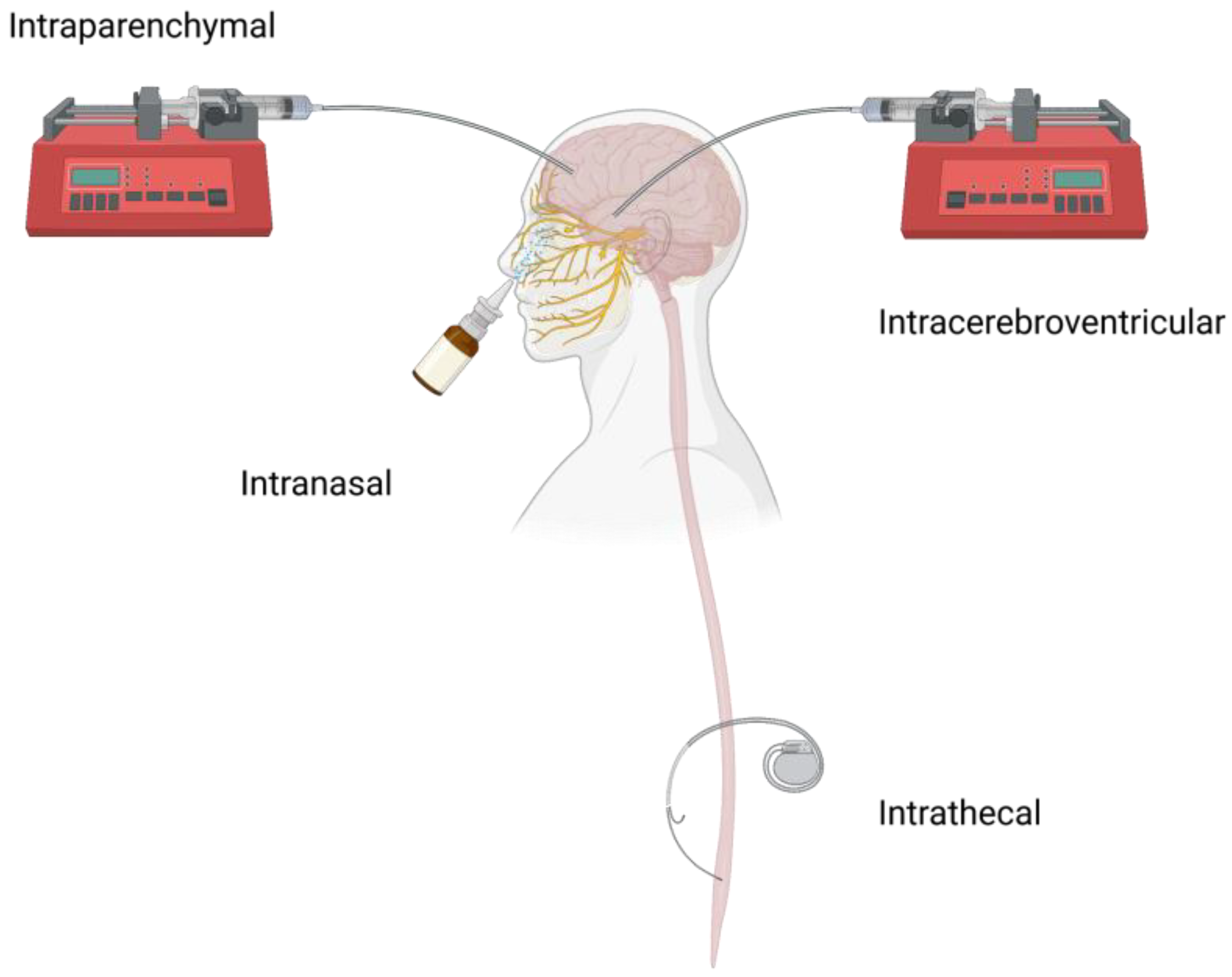
| Pros | Cons | Clinical Applications | |
|---|---|---|---|
| ICV | Direct introduction of nearly 100% therapeutics into the CSF in the lateral ventricle with minimal drug-protein bounding. | Low drug distribution in the brain parenchyma that is away from the injection site; Bulk flow of CSF may result in fast clearance and systemic exposure; Complications associated with ICV device implantation. | Brineura [16]; tripeptidyl peptidase 1 (Phase I/II) [17,18]; idursulfase-β (Phase I/II) [19]; telbermin (Phase I) [20] |
| CED | Improved drug distribution in the brain parenchyma; Topographically more restricted, thus less complications compared to ICV. | Inefficient diffusion in the parenchyma for some macromolecules; Complications from the invasiveness of the CED. | Omburtamab (Phase I) [21,22] |
| IT | Direct injection of nearly 100% therapeutics into the CSF in the spinal subarachnoid space with long half-life in the CSF due to minimal drug-protein bounding and metabolism; Less invasive compared to ICV and CED | Less macromolecule distribution in the parenchyma compared to ICV and CED; Potential complications from IT device implantation. | Recombinant human arylsulfatase A(Phase I/II) [23] |
| IN | Noninvasive | Potential systemic exposure; Device design is critical. | Insulin & insulin detemir (Phase II) [6]; neuropeptide Y (Phase I) [7] |
| Disease Model | Formulation | Cargo Therapeutic | Carrier Material | Release Duration | Ref. |
|---|---|---|---|---|---|
| GBM (GL261 mouse model) | Hydrogel | CXCL-10 | Self-assembled oligopeptide | >12 days | [73] |
| Spinal cord injury (Rat model) | Hydrogel | basic fibroblast growth factor (bFGF) or FGF2 | Heparin-modified poloxamer and lyophilized acellular spinal cord | >7 days | [74,75] |
| Nanoparticle (NP)-hydrogel composite | neurotrophin-3 (NT-3) + antibody 11c7 (anti-NogoA) | Hyaluronan-methylcellulose (HAMC) and poly(lactic-co-glycolic acid) (PLGA) | NT-3: >58 days; anti-NogoA: >10 days | [76] | |
| Hydrogel | NT-3 | Heparin-contained collagen | >90 days | [77] | |
| NP-hydrogel composite | chondroitinase ABC (ChABC) + stromal cell-derived factor 1α (SDF) | SH3 binding peptide-modified methylcellulose and PLGA | ChABC: >7 days; SDF: >14 days | [78] | |
| Liposome-hydrogel composite | brain-derived neurotrophic factor (BDNF) + acidic FGF (aFGF) | Heparin-modified poloxamer and scar-targeted tetrapeptide-modified liposomes | BDNF/aFGF: >21 days | [79] | |
| NP-hydrogel composite | BDNF | Agarose + polysaccharide polyelectrolyte complexes | >17 days | [80] | |
| Hydrogel | Anti-inflammatory peptide KAFAK + BDNF | HAMC | KAFAK/BDNF: >4 days (only ~50% of payloads released) | [81] |
| Disease Model | Formulation | Cargo Therapeutic | Administration | Ref. |
|---|---|---|---|---|
| Spinal cord injury (Rat model) | PLGA NP | NT-3 | IT | [88] |
| Brain tumor (Mouse model) | Lipidoid-telodendrimer binary hybrid NP | DT390 | CED | [89] |
| AD (Rat model) | Lectin-modified PEG-PLGA NP | bFGF | IN | [90] |
| PD (Rat model) | Phospholipid-based gelatin NP | bFGF | IN | [91] |
| Mouse model | Chitosan coated nanostructured lipid carriers | human IGF-I (hIGF-I) | IN | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, W.; Shen, J. Local Delivery Strategies for Peptides and Proteins into the CNS: Status Quo, Challenges, and Future Perspectives. Pharmaceuticals 2023, 16, 810. https://doi.org/10.3390/ph16060810
Yue W, Shen J. Local Delivery Strategies for Peptides and Proteins into the CNS: Status Quo, Challenges, and Future Perspectives. Pharmaceuticals. 2023; 16(6):810. https://doi.org/10.3390/ph16060810
Chicago/Turabian StyleYue, Weizhou, and Jie Shen. 2023. "Local Delivery Strategies for Peptides and Proteins into the CNS: Status Quo, Challenges, and Future Perspectives" Pharmaceuticals 16, no. 6: 810. https://doi.org/10.3390/ph16060810
APA StyleYue, W., & Shen, J. (2023). Local Delivery Strategies for Peptides and Proteins into the CNS: Status Quo, Challenges, and Future Perspectives. Pharmaceuticals, 16(6), 810. https://doi.org/10.3390/ph16060810







