Honey-Related Treatment Strategies in Dry Eye Disease
Abstract
1. Introduction
2. Results
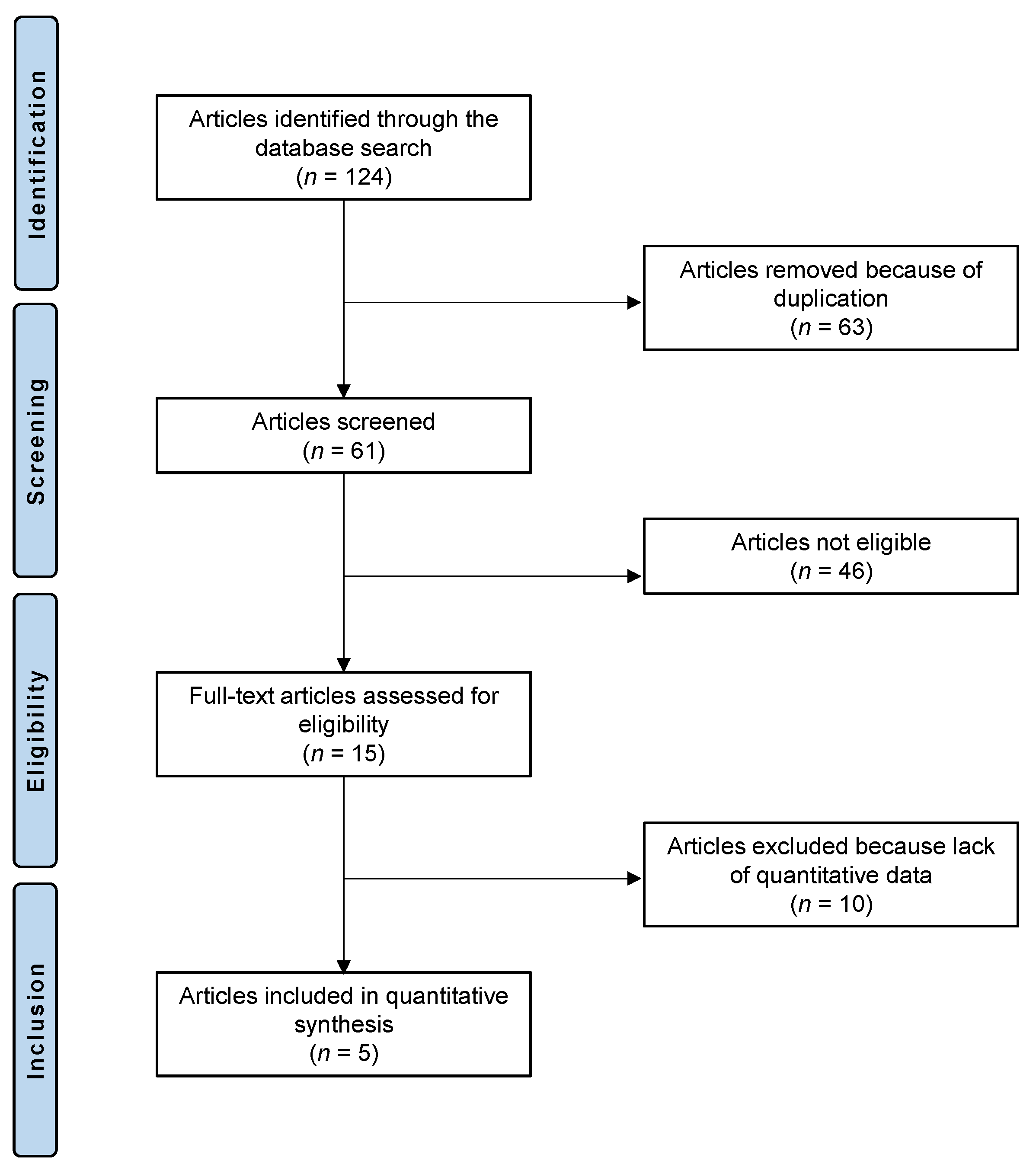
2.1. Study Selection
2.2. Study Risk of Bias Assessment
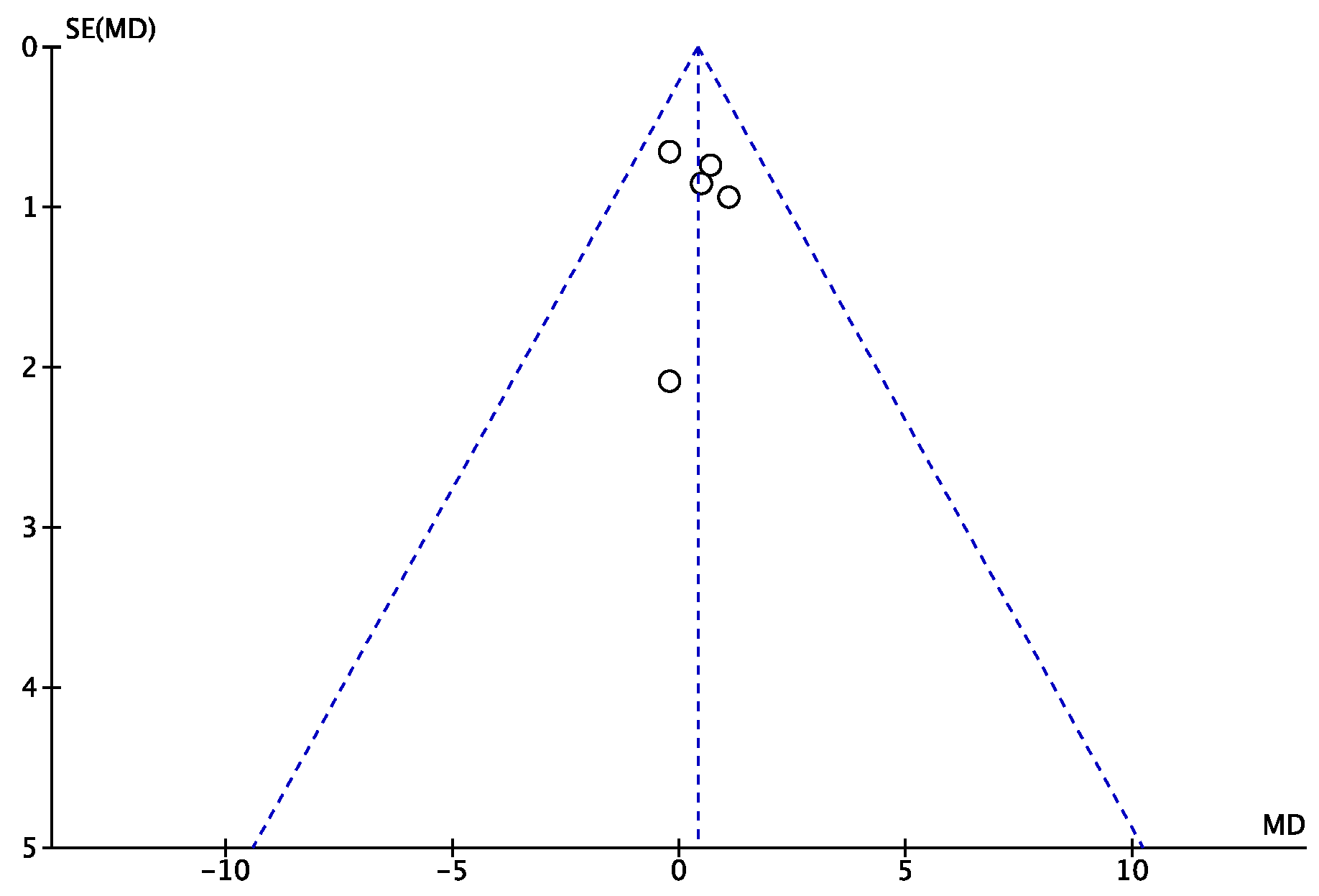
2.3. Risk of Publication Bias
2.4. Study Characteristics and Results of Individual Studies
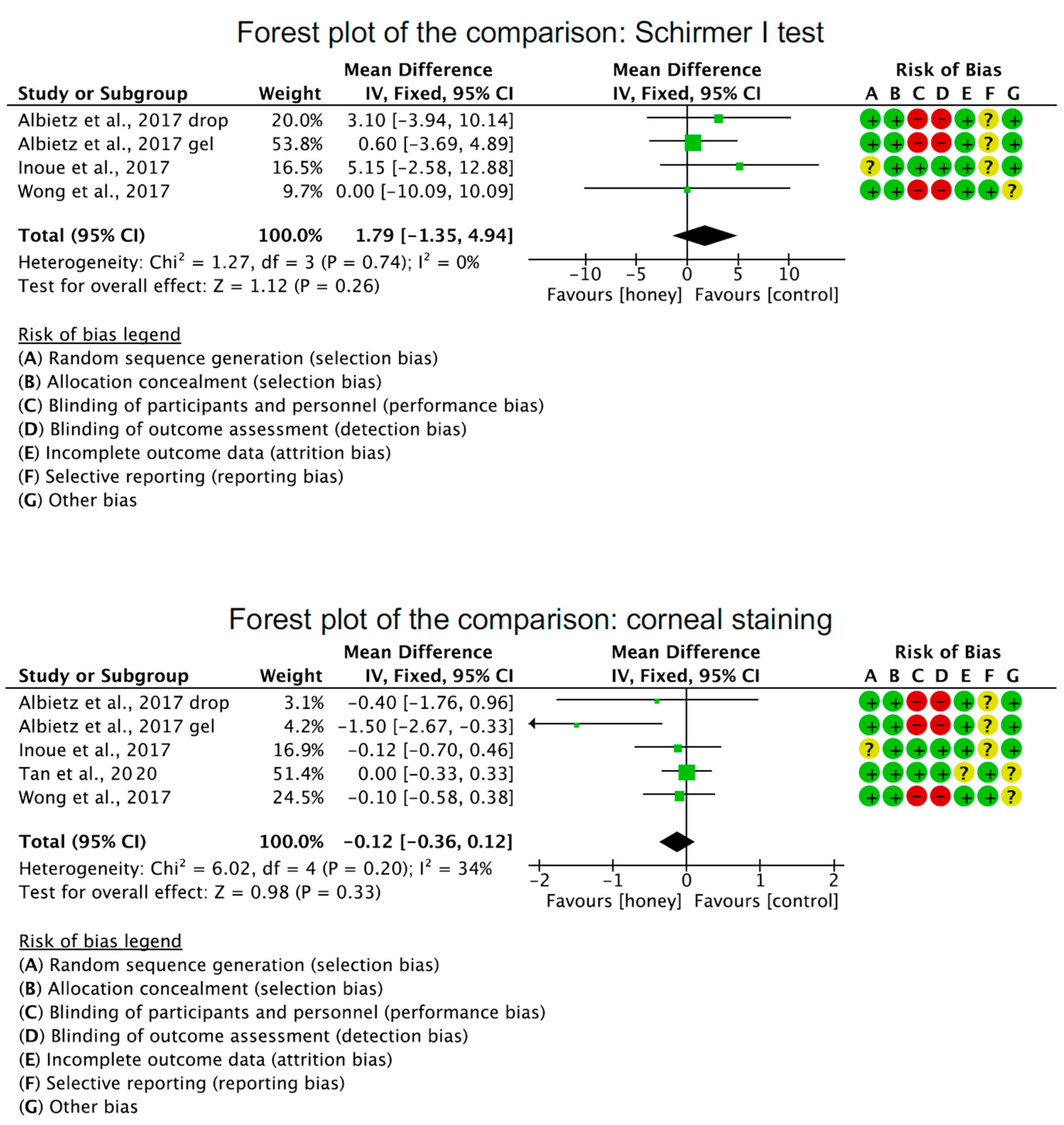
2.5. Efficacy of Honey-Related Treatment Strategies
2.6. Honey-Related Treatment Strategies Compared to Other Treatments
3. Discussion
4. Materials and Methods
4.1. Eligibility Criteria
4.2. Search Strategy
- P (Population): patients with DED;
- I (Intervention): Honey-related treatment strategies, including Manuka honey, Royal Jelly;
- C (Comparison): improvement at the last follow-up and compared with placebo or control group;
- O (Outcomes): Ocular Surface Disease Index; Tear breakup time test; Schirmer I test, corneal staining, adverse events.
4.3. Selection and Data Collection
4.4. Data Items
4.5. Study Risk of Bias Assessment
4.6. Synthesis Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Dogru, M.; Yagi, Y.; Goto, E.; Tomita, M.; Kon, T.; Saiki, M.; Matsumoto, Y.; Uchino, Y.; Yokoi, N.; et al. The features of dry eye disease in a Japanese elderly population. Optom. Vis. Sci. 2006, 83, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Nagino, K.; Sung, J.; Oyama, G.; Hayano, M.; Hattori, N.; Okumura, Y.; Fujio, K.; Akasaki, Y.; Huang, T.; Midorikawa-Inomata, A.; et al. Prevalence and characteristics of dry eye disease in Parkinson’s disease: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 18348. [Google Scholar] [CrossRef] [PubMed]
- Hazra, D.; Yotsukura, E.; Torii, H.; Mori, K.; Maruyama, T.; Ogawa, M.; Hanyuda, A.; Tsubota, K.; Kurihara, T.; Negishi, K. Relation between dry eye and myopia based on tear film breakup time, higher order aberration, choroidal thickness, and axial length. Sci. Rep. 2022, 12, 10891. [Google Scholar] [CrossRef]
- Javadi, M.A.; Feizi, S. Dry eye syndrome. J. Ophthalmic Vis. Res. 2011, 6, 192–198. [Google Scholar]
- Alves, M.; Novaes, P.; Morraye, M.D.A.; Reinach, P.S.; Rocha, E.M. Is dry eye an environmental disease? Arq. Bras. Oftalmol. 2014, 77, 193–200. [Google Scholar] [CrossRef]
- Seen, S.; Tong, L. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018, 96, e412–e420. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Martin, L.M.; Jeyabalan, N.; Tripathi, R.; Panigrahi, T.; Johnson, P.J.; Ghosh, A.; Mohan, R.R. Autophagy in corneal health and disease: A concise review. Ocul. Surf. 2019, 17, 186–197. [Google Scholar] [CrossRef]
- Ohashi, Y.; Ishida, R.; Kojima, T.; Goto, E.; Matsumoto, Y.; Watanabe, K.; Ishida, N.; Nakata, K.; Takeuchi, T.; Tsubota, K. Abnormal protein profiles in tears with dry eye syndrome. Am. J. Ophthalmol. 2003, 136, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Downie, L.E.; Ng, S.M.; Lindsley, K.B.; Akpek, E.K. Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease. Cochrane Database Syst. Rev. 2019, 12, CD011016. [Google Scholar] [CrossRef] [PubMed]
- Ervin, A.M.; Law, A.; Pucker, A.D. Punctal occlusion for dry eye syndrome: Summary of a Cochrane systematic review. Br. J. Ophthalmol. 2019, 103, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Q.; Wei, R.L. Topical cyclosporine A in the treatment of dry eye: A systematic review and meta-analysis. Cornea 2014, 33, 760–767. [Google Scholar] [CrossRef]
- Lee, H.K.; Ryu, I.H.; Seo, K.Y.; Hong, S.; Kim, H.C.; Kim, E.K. Topical 0.1% prednisolone lowers nerve growth factor expression in keratoconjunctivitis sicca patients. Ophthalmology 2006, 113, 198–205. [Google Scholar] [CrossRef]
- Prinz, J.; Maffulli, N.; Fuest, M.; Walter, P.; Bell, A.; Migliorini, F. Efficacy of Topical Administration of Corticosteroids for the Management of Dry Eye Disease: Systematic Review and Meta-Analysis. Life 2022, 12, 1932. [Google Scholar] [CrossRef]
- Lemp, M.A. Management of dry eye disease. Am. J. Manag. Care 2008, 14, S88–S101. [Google Scholar]
- McGhee, C.N.; Dean, S.; Danesh-Meyer, H. Locally administered ocular corticosteroids: Benefits and risks. Drug Saf. 2002, 25, 33–55. [Google Scholar] [CrossRef]
- Majtanova, N.; Cernak, M.; Majtan, J. Honey: A Natural Remedy for Eye Diseases. Res. Complement. Med. 2016, 23, 364–369. [Google Scholar] [CrossRef]
- Carnahan, M.C.; Goldstein, D.A. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr. Opin. Ophthalmol. 2000, 11, 478–483. [Google Scholar] [CrossRef]
- Mittal, R.; Patel, S.; Galor, A. Alternative therapies for dry eye disease. Curr. Opin. Ophthalmol. 2021, 32, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Jabarzare, S.; Neurmohamadi, M.; Kheiri, S.; Rafieian-Kopaei, M. A double blind clinical trial on the efficacy of honey drop in vernal keratoconjunctivitis. Evid. Based Complement. Altern. Med. 2014, 2014, 287540. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Coyle, S.; Warnock, M.; Gow, I.; Fyfe, L. Anti-microbial activity and composition of manuka and portobello honey. Phytother. Res. 2013, 27, 1162–1168. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Hills, S.P.; Mitchell, P.; Wells, C.; Russell, M. Honey Supplementation and Exercise: A Systematic Review. Nutrients 2019, 11, 1586. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Albietz, J.M.; Tran, H.; Du Toit, C.; Li, A.H.; Yun, T.; Han, J.; Schmid, K.L. Treatment of contact lens related dry eye with antibacterial honey. Contact Lens Anterior Eye 2017, 40, 389–393. [Google Scholar] [CrossRef]
- Chong, K.K.; Lai, F.H.; Ho, M.; Luk, A.; Wong, B.W.; Young, A. Randomized trial on silicone intubation in endoscopic mechanical dacryocystorhinostomy (SEND) for primary nasolacrimal duct obstruction. Ophthalmology 2013, 120, 2139–2145. [Google Scholar] [CrossRef]
- Inoue, S.; Kawashima, M.; Hisamura, R.; Imada, T.; Izuta, Y.; Nakamura, S.; Ito, M.; Tsubota, K. Clinical Evaluation of a Royal Jelly Supplementation for the Restoration of Dry Eye: A Prospective Randomized Double Blind Placebo Controlled Study and an Experimental Mouse Model. PLoS ONE 2017, 12, e0169069. [Google Scholar] [CrossRef]
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.A.D.T.G.; Cremers, N.A.J. Defining the standards for medical grade honey. J. Apic. Res. 2020, 59, 125–135. [Google Scholar] [CrossRef]
- Bashkaran, K.; Zunaina, E.; Bakiah, S.; Sulaiman, S.A.; Sirajudeen, K.; Naik, V. Anti-inflammatory and antioxidant effects of Tualang honey in alkali injury on the eyes of rabbits: Experimental animal study. BMC Complement. Altern. Med. 2011, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Albietz, J.M.; Lenton, L.M. Standardised antibacterial Manuka honey in the management of persistent post-operative corneal oedema: A case series. Clin. Exp. Optom. 2015, 98, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.M.; Zein, W.; Haddad, R.; Khoury, J. Bullous keratopathy treated with honey. Acta Ophthalmol. Scand. 2004, 82, 312–313. [Google Scholar] [CrossRef] [PubMed]
- Albietz, J.M.; Lenton, L.M. Late reactivation of herpes zoster keratitis results in band keratopathy. Optom. Vis. Sci. 2014, 91, e149–e155. [Google Scholar] [CrossRef]
- Tonks, A.J.; Cooper, R.A.; Jones, K.P.; Blair, S.; Parton, J.; Tonks, A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine 2003, 21, 242–247. [Google Scholar] [CrossRef]
- Albietz, J.M.; Schmid, K.L. Randomised controlled trial of topical antibacterial Manuka (Leptospermum species) honey for evaporative dry eye due to meibomian gland dysfunction. Clin. Exp. Optom. 2017, 100, 603–615. [Google Scholar] [CrossRef]
- Craig, J.P.; Cruzat, A.; Cheung, I.M.Y.; Watters, G.A.; Wang, M.T.M. Randomized masked trial of the clinical efficacy of MGO Manuka Honey microemulsion eye cream for the treatment of blepharitis. Ocul. Surf. 2020, 18, 170–177. [Google Scholar] [CrossRef]
- Tan, J.; Jia, T.; Liao, R.; Stapleton, F. Effect of a formulated eye drop with Leptospermum spp honey on tear film properties. Br. J. Ophthalmol. 2020, 104, 1373–1377. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Betiku, A.O.; Oduyoye, O.O.; Jagun, O.O.; Olajide, O.S.; Adebusoye, S.O.; Aham-Onyebuchi, U.O. Prevalence and risk factors associated with dry eye disease among adults in a population-based setting in South-West Nigeria. Niger. J. Clin. Pract. 2022, 25, 354–360. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Giampieri, F. The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Abd Rashid, N.; Mohammed, S.N.F.; Syed Abd Halim, S.A.; Ghafar, N.A.; Abdul Jalil, N.A. Therapeutic Potential of Honey and Propolis on Ocular Disease. Pharmaceuticals 2022, 15, 1419. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef] [PubMed]
- Uchino, Y.; Kawakita, T.; Miyazawa, M.; Ishii, T.; Onouchi, H.; Yasuda, K.; Ogawa, Y.; Shimmura, S.; Ishii, N.; Tsubota, K. Oxidative stress induced inflammation initiates functional decline of tear production. PLoS ONE 2012, 7, e45805. [Google Scholar] [CrossRef]
- Navel, V.; Sapin, V.; Henrioux, F.; Blanchon, L.; Labbé, A.; Chiambaretta, F.; Baudouin, C.; Dutheil, F. Oxidative and antioxidative stress markers in dry eye disease: A systematic review and meta-analysis. Acta Ophthalmol. 2022, 100, 45–57. [Google Scholar] [CrossRef]
- Dogru, M.; Kojima, T.; Simsek, C.; Tsubota, K. Potential Role of Oxidative Stress in Ocular Surface Inflammation and Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES163–DES168. [Google Scholar] [CrossRef]
- Imada, T.; Nakamura, S.; Kitamura, N.; Shibuya, I.; Tsubota, K. Oral administration of royal jelly restores tear secretion capacity in rat blink-suppressed dry eye model by modulating lacrimal gland function. PLoS ONE 2014, 9, e106338. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxid. Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, Y.; Cao, W. The Protective Effect of Whole Honey and Phenolic Extract on Oxidative DNA Damage in Mice Lymphocytes Using Comet Assay. Plant. Foods Hum. Nutr. 2017, 72, 388–395. [Google Scholar] [CrossRef]
- Asgary, S.; Sahebkar, A.; Afshani, M.R.; Keshvari, M.; Haghjooyjavanmard, S.; Rafieian-Kopaei, M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother. Res. 2014, 28, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Kelishadi, R.; Rafieian-Kopaei, M.; Najafi, S.; Najafi, M.; Sahebkar, A. Investigation of the lipid-modifying and antiinflammatory effects of Cornus mas L. supplementation on dyslipidemic children and adolescents. Pediatr. Cardiol. 2013, 34, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.P. The role of bacteria in blepharitis. Ocul. Surf. 2009, 7, S21–S22. [Google Scholar] [CrossRef] [PubMed]
- Albietz, J.M.; Lenton, L.M. Effect of antibacterial honey on the ocular flora in tear deficiency and meibomian gland disease. Cornea 2006, 25, 1012–1019. [Google Scholar] [CrossRef]
- McCulley, J.P.; Dougherty, J.M. Bacterial aspects of chronic blepharitis. Trans. Ophthalmol. Soc. U. K. 1986, 105 Pt 3, 314–318. [Google Scholar] [PubMed]
- Doughty, M.J. Contact lens wear and the goblet cells of the human conjunctiva-A review. Contact Lens Anterior Eye 2011, 34, 157–163. [Google Scholar] [CrossRef]
- Terry, R.L.; Schnider, C.M.; Holden, B.A.; Cornish, R.; Grant, T.; Sweeney, D.; La Hood, D.; Back, A. CCLRU standards for success of daily and extended wear contact lenses. Optom. Vis. Sci. 1993, 70, 234–243. [Google Scholar] [CrossRef]
- Bron, A.J.; Evans, V.E.; Smith, J.A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003, 22, 640–650. [Google Scholar] [CrossRef]
- Bergman, A.; Yanai, J.; Weiss, J.; Bell, D.; David, M.P. Acceleration of wound healing by topical application of honey. An animal model. Am. J. Surg. 1983, 145, 374–376. [Google Scholar] [CrossRef]
- Tonks, A.J.; Dudley, E.; Porter, N.G.; Parton, J.; Brazier, J.; Smith, E.L.; Tonks, A. A 5.8-kDa component of manuka honey stimulates immune cells via TLR4. J. Leukoc. Biol. 2007, 82, 1147–1155. [Google Scholar] [CrossRef]
- Snowdon, J.A.; Cliver, D.O. Microorganisms in honey. Int. J. Food Microbiol. 1996, 31, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Kawaji, T.; Inatani, M.; Kameda, T.; Yoshimura, N.; Tanihara, H. Simultaneous increases in multiple proinflammatory cytokines in the aqueous humor in pseudophakic glaucomatous eyes. J. Cataract. Refract. Surg. 2012, 38, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Irish, J.; Blair, S.; Carter, D.A. The antibacterial activity of honey derived from Australian flora. PLoS ONE 2011, 6, e18229. [Google Scholar] [CrossRef] [PubMed]
- Howick, J.; Chalmers, I.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H.; Goddard, O.; et al. The 2011 Oxford CEBM Levels of Evidence. Oxf. Cent. Evid. Based Med. 2011, 1, 1–3. Available online: https://www.cebm.net/index.aspx?o=5653 (accessed on 14 April 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Walt, J.G.; Rowe, M.M.; Stern, K.L. Evaluating the functional impact of dry eye: The Ocular Surface Disease Index. Drug Inf. J. 1997, 31, b5. [Google Scholar]
- Cho, P.; Leung, L.; Lam, A.; Choi, A. Tear break-up time: Clinical procedures and their effects. Ophthalmic Physiol. Opt. 1998, 18, 319–324. [Google Scholar] [CrossRef]
- Cho, P.; Yap, M. Schirmer test. I. A review. Optom. Vis. Sci. 1993, 70, 152–156. [Google Scholar] [CrossRef]

| Author, Year | Journal | Follow-Up (Weeks) | Treatment | Patients (n) | Mean Age | Women (%) |
|---|---|---|---|---|---|---|
| Albietz et al., 2017 [36] | Clin. Exp. Optom. | 8 | Honey (Optimel Manuka gel) plus conventional therapy | 37 | 58.9 | 42.9 |
| Honey (Optimel Manuka drops) plus conventional therapy | 37 | 62.2 | 42.4 | |||
| Conventional therapy | 40 | 61.4 | 41.2 | |||
| Craig et al., 2020 [37] | Ocul. Surf. | 13 | Honey (Manuka microemulsion) | 53 | 60.0 | 60.0 |
| No treatment | 53 | 60.0 | 60.0 | |||
| Inoue et al., 2017 [29] | PLoS ONE | 8 | Honey (Royal Jelly) | 22 | 29.6 | 28.6 |
| Placebo | 19 | 37.0 | 54.5 | |||
| Tan et al., 2020 [38] | Br. J. Ophthalmol. | 4 | Honey (Optimel Manuka+ honey eye drops) | 21 | 22.2 | 57.1 |
| Artificial tears | 21 | 20.6 | 76.2 | |||
| Wong et al., 2017 [27] | Cont. Lens Anterior Eye | 2 | Honey (Optimel Manuka drops) | 10 | 25.7 | 55.0 |
| Artificial tears | 10 | 25.7 | 55.0 |
| Endpoint | Baseline | Last FU | MD | SE | 95% CI | T-Value | p |
|---|---|---|---|---|---|---|---|
| Tear breakup time | 5.0 ± 3.3 | 6.1 ± 2.7 | 1.1 | 0.426 | 0.25 to 1.94 | 2.58 | 0.01 |
| Ocular Surface Disease Index | 32.9 ± 9.9 | 20.1 ± 6.5 | −12.8 | 1.184 | −15.13 to −10.46 | −10.808 | <0.0001 |
| Schirmer I test | 16.7 ± 4.3 | 18.5 ± 1.7 | 1.8 | 0.462 | 0.88 to 2.71 | 3.893 | 0.0001 |
| Corneal Staining | 2.3 ± 2.6 | 1.1 ± 0.9 | −1.2 | 0.275 | −1.74 to −0.65 | −4.361 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prinz, J.; Maffulli, N.; Fuest, M.; Walter, P.; Hildebrand, F.; Migliorini, F. Honey-Related Treatment Strategies in Dry Eye Disease. Pharmaceuticals 2023, 16, 762. https://doi.org/10.3390/ph16050762
Prinz J, Maffulli N, Fuest M, Walter P, Hildebrand F, Migliorini F. Honey-Related Treatment Strategies in Dry Eye Disease. Pharmaceuticals. 2023; 16(5):762. https://doi.org/10.3390/ph16050762
Chicago/Turabian StylePrinz, Julia, Nicola Maffulli, Matthias Fuest, Peter Walter, Frank Hildebrand, and Filippo Migliorini. 2023. "Honey-Related Treatment Strategies in Dry Eye Disease" Pharmaceuticals 16, no. 5: 762. https://doi.org/10.3390/ph16050762
APA StylePrinz, J., Maffulli, N., Fuest, M., Walter, P., Hildebrand, F., & Migliorini, F. (2023). Honey-Related Treatment Strategies in Dry Eye Disease. Pharmaceuticals, 16(5), 762. https://doi.org/10.3390/ph16050762