SIRT1 Signaling Is Involved in the Vascular Improvement Induced by Moringa Oleifera Seeds during Aging
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analysis of the Ethanolic Extract from MOI Seeds
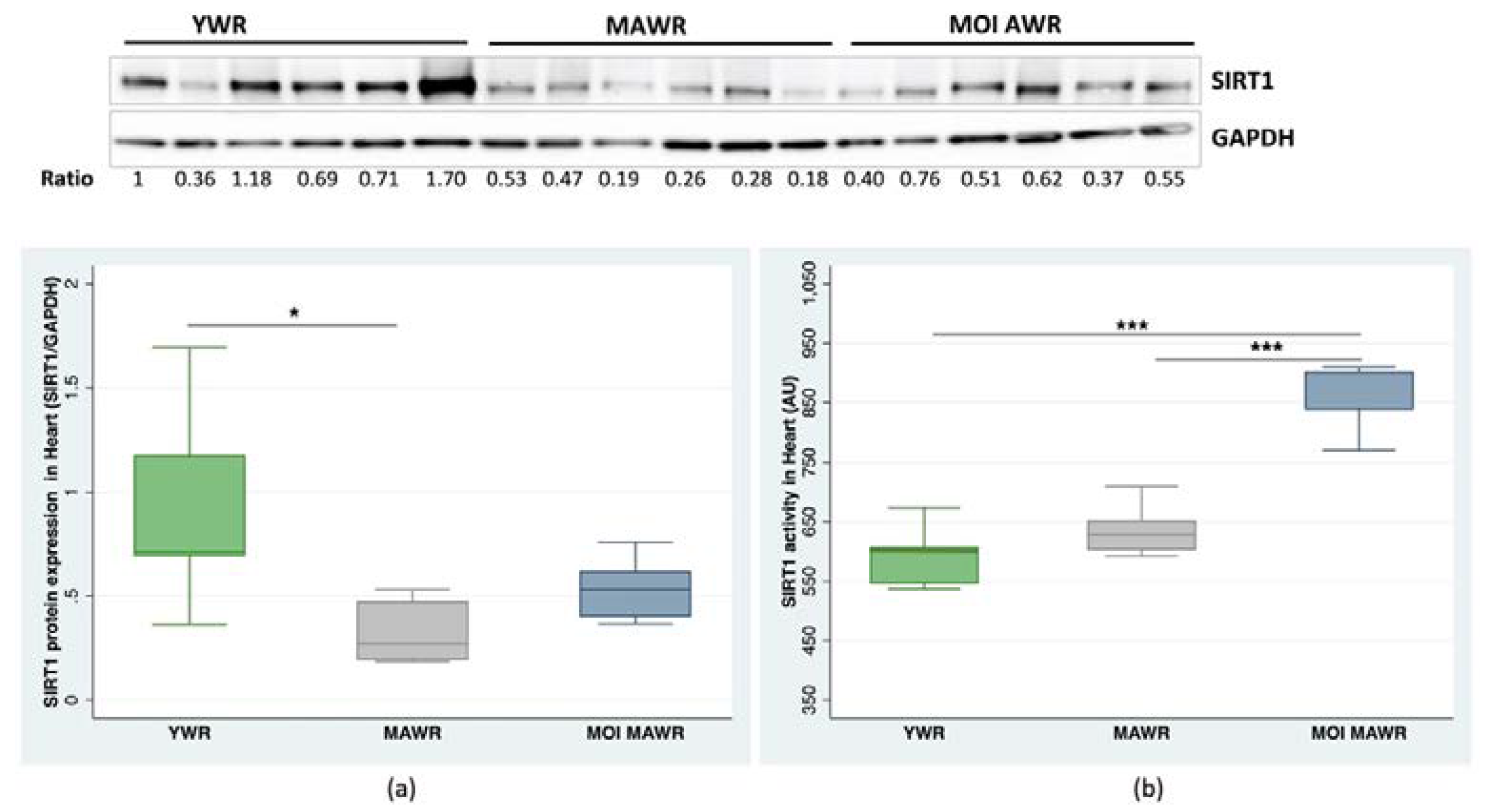
2.2. MOI Treatment Induces SIRT1 Expression and Activation in MAWR Cardiovascular Tissues
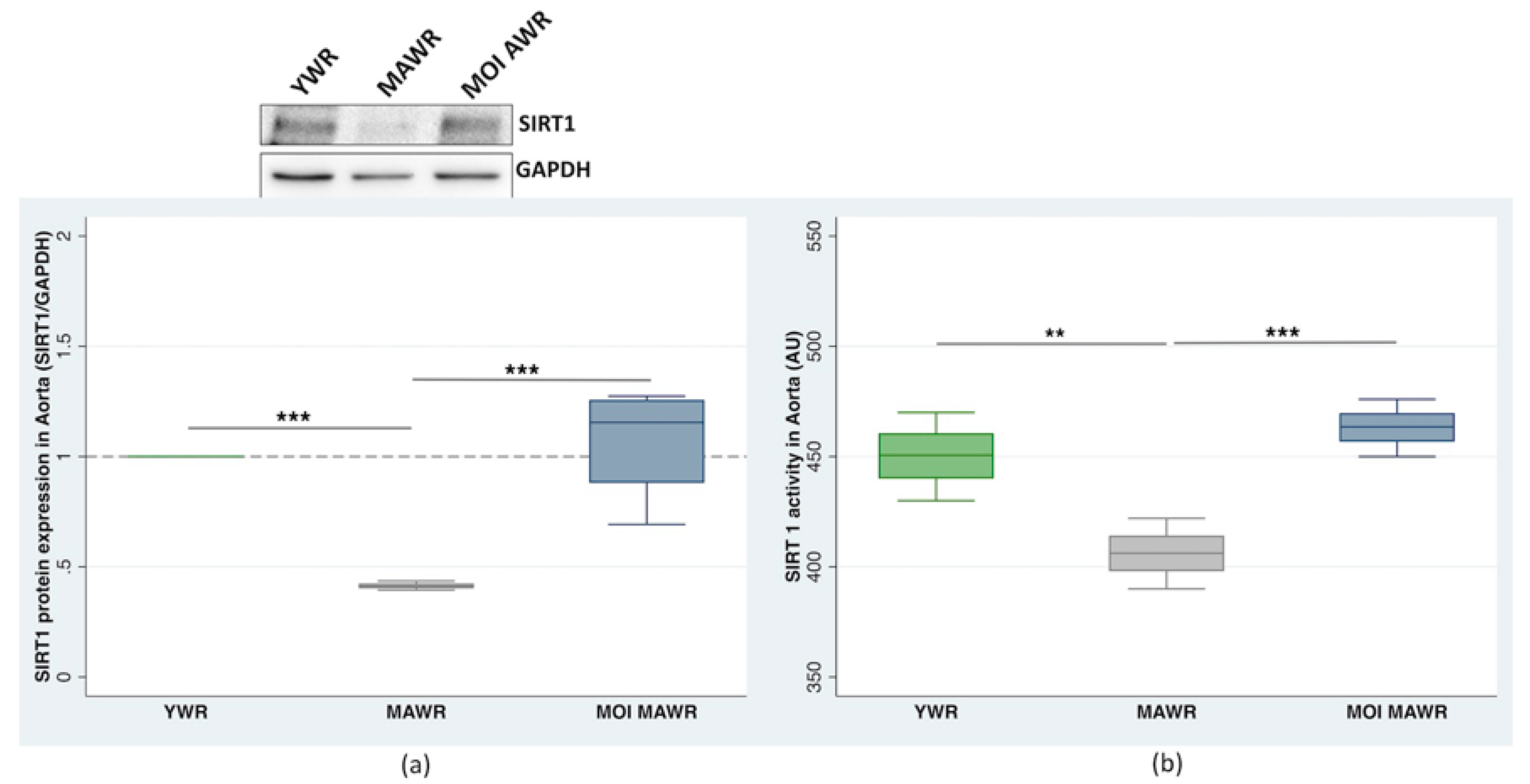
2.3. MOI Treatment Reduces FOXO1 Expression and Activation in the Aorta of MAWRs
2.4. MOI Treatment Reduces Oxidative Stress in MAWRs
3. Discussion
4. Materials and Methods
4.1. Phytochemical Screening of the MOI Seed Ethanolic Total Extract
4.2. Animal Model
4.3. Western Blot Analysis
4.4. Nucleus Extraction and SIRT1 Activity Assay
4.5. Staining and Confocal Microscopy Imaging
4.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rizki, G.; Iwata, T.N.; Li, J.; Riedel, C.G.; Picard, C.L.; Jan, M.; Murphy, C.T.; Lee, S.S. The evolutionarily conserved longevity determinants HCF-1 and SIR-2.1/SIRT1 collaborate to regulate DAF-16/FOXO. PLoS Genet. 2011, 7, e1002235. [Google Scholar] [CrossRef]
- Conti, V.; Forte, M.; Corbi, G.; Russomanno, G.; Formisano, L.; Landolfi, A.; Izzo, V.; Filippelli, A.; Vecchione, C.; Carrizzo, A. Sirtuins: Possible Clinical Implications in Cardio and Cerebrovascular Diseases. Curr. Drug Targets 2017, 18, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Kaley, G.; de Cabo, R.; Sonntag, W.E.; Csiszar, A. Mechanisms of vascular aging: New perspectives. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 1028–1041. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Oellerich, M.F.; Potente, M. FOXOs and sirtuins in vascular growth, maintenance, and aging. Circ. Res. 2012, 110, 1238–1251. [Google Scholar] [CrossRef] [PubMed]
- Sedding, D.G. FoxO transcription factors in oxidative stress response and ageing: A new fork on the way to longevity? Biol. Chem. 2008, 389, 279–283. [Google Scholar] [CrossRef]
- Hosaka, T.; Biggs, W.H., 3rd; Tieu, D.; Boyer, A.D.; Varki, N.M.; Cavenee, W.K.; Arden, K.C. Disruption of fork head transcription factor (FOXO) family members in mice reveals their functional diversification. Proc. Natl. Acad. Sci. USA 2004, 101, 2975–2980. [Google Scholar] [CrossRef]
- Potente, M.; Urbich, C.; Sasaki, K.; Hofmann, W.K.; Heeschen, C.; Aicher, A.; Kollipara, R.; DePinho, R.A.; Zeiher, A.M.; Dimmeler, S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J. Clin. Investig. 2005, 115, 2382–2392. [Google Scholar] [CrossRef]
- Corbi, G.; Conti, V.; Komici, K.; Manzo, V.; Filippelli, A.; Palazzo, M.; Vizzari, F.; Davinelli, S.; Di Costanzo, A.; Scapagnini, G.; et al. Phenolic Plant Extracts Induce Sirt1 Activity and Increase Antioxidant Levels in the Rabbit’s Heart and Liver. Oxid. Med. Cell. Longev. 2018, 2018, 2731289. [Google Scholar] [CrossRef]
- Fang, C.; Xu, H.; Yuan, L.; Zhu, Z.; Wang, X.; Liu, Y.; Zhang, A.; Shao, A.; Lou, M. Natural Compounds for SIRT1-Mediated Oxidative Stress and Neuroinflammation in Stroke: A Potential Therapeutic Target in the Future. Oxid. Med. Cell. Longev. 2022, 2022, 1949718. [Google Scholar] [CrossRef]
- Yessenkyzy, A.; Saliev, T.; Zhanaliyeva, M.; Masoud, A.R.; Umbayev, B.; Sergazy, S.; Krivykh, E.; Gulyayev, A.; Nurgozhin, T. Polyphenols as Caloric-Restriction Mimetics and Autophagy Inducers in Aging Research. Nutrients 2020, 12, 1344. [Google Scholar] [CrossRef] [PubMed]
- Corbi, G.; Conti, V.; Troisi, J.; Colucci, A.; Manzo, V.; Di Pietro, P.; Calabrese, M.C.; Carrizzo, A.; Vecchione, C.; Ferrara, N.; et al. Cardiac Rehabilitation Increases SIRT1 Activity and β-Hydroxybutyrate Levels and Decreases Oxidative Stress in Patients with HF with Preserved Ejection Fraction. Oxid. Med. Cell. Longev. 2019, 2019, 7049237. [Google Scholar] [CrossRef]
- Pearson, K.J.; Baur, J.A.; Lewis, K.N.; Peshkin, L.; Price, N.L.; Labinskyy, N.; Swindell, W.R.; Kamara, D.; Minor, R.K.; Perez, E.; et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell. Metab. 2008, 8, 157–168. [Google Scholar] [CrossRef]
- Sin, T.K.; Yu, A.P.; Yung, B.Y.; Yip, S.P.; Chan, L.W.; Wong, C.S.; Ying, M.; Rudd, J.A.; Siu, P.M. Modulating effect of SIRT1 activation induced by resveratrol on Foxo1-associated apoptotic signalling in senescent heart. J. Physiol. 2014, 592, 2535–2548. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.P. Plantes Médicinales du Nord de Madagascar: Ethnobotanique Antakarana et Informations Scientifiques; Jardins du monde. Brasparts, Antsiranana: Antsiranana, Madagascar, 2012; Volume 176–177, ISBN 978-2-9543726-0-0. [Google Scholar]
- Duranti, G.; Maldini, M.; Crognale, D.; Horner, K.; Dimauro, I.; Sabatini, S.; Ceci, R. Moringa oleifera Leaf Extract Upregulates Nrf2/HO-1 Expression and Ameliorates Redox Status in C2C12 Skeletal Muscle Cells. Molecules 2021, 26, 5041. [Google Scholar] [CrossRef]
- Randriamboavonjy, J.I.; Loirand, G.; Vaillant, N.; Lauzier, B.; Derbré, S.; Michalet, S.; Pacaud, P.; Tesse, A. Cardiac Protective Effects of Moringa oleifera Seeds in Spontaneous Hypertensive Rats. Am. J. Hypertens. 2016, 29, 873–881. [Google Scholar] [CrossRef]
- Randriamboavonjy, J.I.; Rio, M.; Pacaud, P.; Loirand, G.; Tesse, A. Moringa oleifera Seeds Attenuate Vascular Oxidative and Nitrosative Stresses in Spontaneously Hypertensive Rats. Oxid. Med. Cell. Longev. 2017, 2017, 4129459. [Google Scholar] [CrossRef] [PubMed]
- Randriamboavonjy, J.I.; Heurtebise, S.; Pacaud, P.; Loirand, G.; Tesse, A. Moringa oleifera Seeds Improve Aging-Related Endothelial Dysfunction in Wistar Rats. Oxid. Med. Cell. Longev. 2019, 2019, 2567198. [Google Scholar] [CrossRef] [PubMed]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 activation by natural phytochemicals: An overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Cheang, W.S.; Wong, W.T.; Wang, L.; Cheng, C.K.; Lau, C.W.; Ma, R.C.W.; Xu, A.; Wang, N.; Huang, Y.; Tian, X.Y. Resveratrol ameliorates endothelial dysfunction in diabetic and obese mice through sirtuin 1 and peroxisome proliferator-activated receptor delta. Pharmacol. Res. 2019, 139, 384–394. [Google Scholar] [CrossRef]
- Hung, C.H.; Chan, S.H.; Chu, P.M.; Tsai, K.L. Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Mol. Nutr. Food Res. 2015, 59, 1905–1917. [Google Scholar] [CrossRef]
- Lou, T.; Huang, Q.; Su, H.; Zhao, D.; Li, X. Targeting Sirtuin 1 signaling pathway by ginsenosides. J. Ethnopharmacol. 2021, 268, 113657. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xiong, X.; Wang, H.; Wang, J. Protective effects of panax notoginseng saponins on cardiovascular diseases: A comprehensive overview of experimental studies. Evid.-Based Complement. Altern. Med. 2014, 2014, 204840. [Google Scholar] [CrossRef] [PubMed]
- Pabón, M.F.G.; Bareño, L.L.; Puebla, P.; Feliciano, A.S. Vascular Mechanisms of triterpenoid saponins isolated from Passiflora quadrangularis L. Vitae 2020, 27, 1–11. [Google Scholar] [CrossRef]
- Ding, L.; Cheng, P.; Wang, L.; Hu, J.; Zhang, Y.X.; Cai, G.W.; Huang, G.Y.; Gao, S. The protective effects of polysaccharide extract from Xin-Ji-Er-Kang formula on Ang II-induced HUVECs injury, L-NAME-induced hypertension and cardiovascular remodeling in mice. BMC Complement. Altern. Med. 2019, 1, 127. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, Y.; Ren, D.; Yang, X. Protective Effect of Saponins-Enriched Fraction of Gynostemma pentaphyllum against High Choline-Induced Vascular Endothelial Dysfunction and Hepatic Damage in Mice. Biol. Pharm. Bull. 2020, 43, 463–473. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, J.; Sun, Z.-J.; Wu, Q.; Zhao, H.; Li, C.-W.; Cao, Y.-K. Panax notoginseng saponins alleviates advanced glycation end product-induced apoptosis by upregulating SIRT1 and antioxidant expression levels in HUVECs. Exp. Ther. Med. 2020, 20, 99. [Google Scholar] [CrossRef]
- Lee, Y.; Im, E. Regulation of miRNAs by Natural Antioxidants in Cardiovascular Diseases: Focus on SIRT1 and eNOS. Antioxidants 2021, 10, 377. [Google Scholar] [CrossRef]
- Xia, N.; Strand, S.; Schlufter, F.; Siuda, D.; Reifenberg, G.; Kleinert, H.; Förstermann, U.; Li, H. Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide 2013, 32, 29–35. [Google Scholar] [CrossRef]
- Menssen, A.; Hydbring, P.; Kapelle, K.; Vervoorts, J.; Diebold, J.; Lüscher, B.; Larsson, L.G.; Hermekin, H. The c-MYC oncoprotein, the NAMPT enzyme, the SIRT1-inhibitor DBC1, and the SIRT1 deacetylase form a positive feedback loop. Proc. Natl. Acad. Sci. USA 2012, 109, E187–E196. [Google Scholar] [CrossRef]
- Frazzi, R. SIRT1 in Secretory Organ Cancer. Front. Endocrinol. 2018, 9, 569. [Google Scholar] [CrossRef]
- Furuyama, T.; Kitayama, K.; Shimoda, Y.; Ogawa, M.; Sone, K.; Yoshida-Araki, K.; Hisatsune, H.; Nishikawa, S.; Nakayama, K.; Ikeda, K.; et al. Abnormal angiogenesis in FOXO1 (Fkhr)-deficient mice. J. Biol. Chem. 2004, 279, 34741–34749. [Google Scholar] [CrossRef]
- Potente, M.; Ghaeni, L.; Baldessari, D.; Mostoslavsky, R.; Rossig, L.; Dequiedt, F.; Haendeler, J.; Mione, M.; Dejana, E.; Alt, F.W.; et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007, 21, 2644–2658. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.T.; Johnson, T.E. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol. 2001, 11, 1975–1980. [Google Scholar] [CrossRef] [PubMed]
- Fong, H.H.S.; Tin, W.A.M.; Farnsworth, N.R. Phytochemical Screening Review; College of Pharmacy, University of Illinois: Chicago, IL, USA, 1977; pp. 275–277. [Google Scholar]
- Aiyegoro, O.A.; Okoh, A.I. Preliminary phytochemical screening and In vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement. Altern. Med. 2010, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.J., Jr.; Gutterman, D.D.; Rios, C.D.; Heistad, D.D.; Davidson, B.L. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ. Res. 1998, 82, 1298–1305. [Google Scholar] [CrossRef]



| Phytochemical Constituents | Results |
|---|---|
| Alkaloids | + |
| Flavonoids | - |
| Polysaccharides | + |
| Polyphenols | - |
| Saponins | + |
| Steroids | + |
| Tannins | - |
| Terpenoids | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, V.; Randriamboavonjy, J.I.; Rafatro, H.; Manzo, V.; Dal Col, J.; Filippelli, A.; Corbi, G.; Tesse, A. SIRT1 Signaling Is Involved in the Vascular Improvement Induced by Moringa Oleifera Seeds during Aging. Pharmaceuticals 2023, 16, 761. https://doi.org/10.3390/ph16050761
Conti V, Randriamboavonjy JI, Rafatro H, Manzo V, Dal Col J, Filippelli A, Corbi G, Tesse A. SIRT1 Signaling Is Involved in the Vascular Improvement Induced by Moringa Oleifera Seeds during Aging. Pharmaceuticals. 2023; 16(5):761. https://doi.org/10.3390/ph16050761
Chicago/Turabian StyleConti, Valeria, Joseph Iharinjaka Randriamboavonjy, Herintsoa Rafatro, Valentina Manzo, Jessica Dal Col, Amelia Filippelli, Graziamaria Corbi, and Angela Tesse. 2023. "SIRT1 Signaling Is Involved in the Vascular Improvement Induced by Moringa Oleifera Seeds during Aging" Pharmaceuticals 16, no. 5: 761. https://doi.org/10.3390/ph16050761
APA StyleConti, V., Randriamboavonjy, J. I., Rafatro, H., Manzo, V., Dal Col, J., Filippelli, A., Corbi, G., & Tesse, A. (2023). SIRT1 Signaling Is Involved in the Vascular Improvement Induced by Moringa Oleifera Seeds during Aging. Pharmaceuticals, 16(5), 761. https://doi.org/10.3390/ph16050761









