Abstract
Angelica keiskei is a perennial plant, belonging to the Apiaceae family and originating from Japan. This plant has been reported to act as a diuretic, analeptic, antidiabetic, hypertensive, tumor, galactagogue, and laxative. The mechanism of action of A. keiskei is not known, but previous studies have suggested that it may act as an antioxidant. In this work, we used Drosophila melanogaster to evaluate the impact of A. keiskei on lifespan and healthspan and its potential anti-aging mechanism by conducting multiple assays on three fly strains: w1118, chico, and JIV. We observed that the extract extended lifespan and improved healthspan in a sex- and strain-dependent manner. A. keiskei extended lifespan and improved reproductive fitness in female flies and either had no effect or decreased survival and physical performance in males. The extract protected against the superoxide generator paraquat in both sexes. These sex-specific effects suggest that A. keiskei may act through age-specific pathways such as the insulin and insulin-like growth factor signaling (IIS) pathways. Upon examination, we found that the increased survival of A. keiskei-fed females was dependent on the presence of the insulin receptor substrate chico, supporting the role of IIS in the action of A. keiskei.
1. Introduction
Angelica keiskei is a hardy perennial plant, native to the pacific coast of Japan, commonly known as ashitaba or “tomorrow’s leaf” [1]. The notoriety of A. keiskei as a medicinal herb has increased due to its reported range of health benefits, including its potential antioxidative, anti-inflammatory, diabetic, hypotensive, microbial, tumor, anti-fibrotic, laxative, stimulant, and galactagogue properties [1,2,3,4,5]. The beneficial properties of A. keiskei extend throughout the plant, with the stems and leaves typically consumed as a health food, while the roots are utilized for their medicinal properties, including analgesic and glucose-lowering in patients with Type II Diabetes [6,7,8]. The precise active compounds in A. keiskei are not known. However, phytochemical analysis has led to the identification of over 100 compounds, including chalcones, coumarins, and flavanones [5].
Chalcones, arguably the most abundant bioactive components of the plant, are a class of unsaturated aromatic ketones, with over 20 forms identified, the most potent and abundant being 4-hydroxyderricin and xanthoangelol [9]. Chalcones are recognized for their antibacterial, antifungal, antiviral, antitumor, antidiabetic, and anti-inflammatory properties, mediated by their action on nitric oxide regulation and macrophage stimulation [2,10,11,12,13,14]. They have also been identified as potent antioxidants, due to their superoxide-scavenging activity [15,16].
Prior work on the antioxidant and oxidative-protective roles of A. keiskei has predominantly been explored in cell culture models [1,2,17]. To the best of our knowledge, this is the first study that evaluated the impact of A. keiskei supplementation on the lifespan and healthspan of an animal. We chose the model organism Drosophila melanogaster as 75% of its genes have functional homology to humans and its short lifecycle and lifespan of about 60 days makes it ideal for anti-aging studies [18]. Furthermore, previous experiments have successfully used D. melanogaster to study the impact of pharmacological interventions such as Rhodiola rosea and resveratrol on the lifespan and healthspan of flies [19,20,21,22,23]. In this work, we first evaluated the impact of A. keiskei on the lifespan and healthspan of D. melanogaster and then we aimed to understand its mechanism of action.
2. Results
Upon extraction and subsequent HPLC analysis of A. keiskei, 4-hydroxyderricin and xanthoangelol were identified as major constituents in chromatograms at 365 and 254 nm (available as Supplementary File, Figure S1). Following initial dose-finding studies at 0, 0.1, 0.2, and 0.4 mg/mL, we observed that only the 0.4 mg/mL of A. keiskei resulted in a significant increase in lifespan in a sex and genetic background-dependent manner. At doses higher than 0.4 mg/mL, the plant extract appeared to be toxic and increased mortality (unpublished data), while a lower dose of 0.2 mg/mL was found to be detrimental to the lifespan of w1118 male flies compared to those fed with standard diet (p < 0.05, hazard ratio = 1.251, Mantel–Haenszel test was 1.251). Lifespan experiments were carried out using two fly strains, w1118 and JIV, at different concentrations of A. keiskei (0, 0.1, 0.2, 0.4 mg/mL). We found that A. keiskei was, in general, detrimental to w1118 male flies, as evidenced by a shortened lifespan (Figure 1A), whereas it had no effect on the lifespan of JIV male flies (Figure 1C). Conversely, 0.4 mg/mL A. keiskei increased the lifespan of female w1118 and JIV flies (Figure 1 B-D). Other doses (0.1 and 0.2 mg/mL) of A. keiskei had no significant effect on female lifespan.
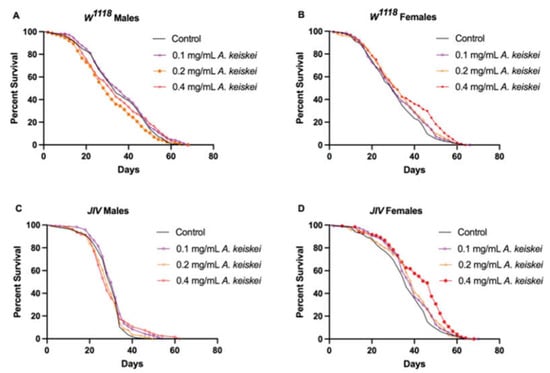
Figure 1.
The impact of Angelica keiskei on lifespan of Drosophila melanogaster (A) w1118 males, (B) w1118 females, (C) JIV males, and (D) JIV females. Angelica keiskei was found to be detrimental to w1118 male flies, as evidenced by a shortened lifespan (A), while it had no effect on the lifespan of JIV male flies (C). Both female w1118 and JIV strains were found to have an increased lifespan with 0.4 mg/mL of A. keiskei supplementation (B,D). p-values were calculated using the Mantel–Cox log-rank test (n = 190–210 for w1118 flies and 120 for JIV flies). Survival curves with symbols filled with color denote statistical significance of the dose compared to control (males, p < 0.05; females p < 0.001); clear symbols denote lack of statistical significance compared to control.
Next, we wished to determine whether A. keiskei could improve healthspan along with lifespan. Since we only observed lifespan extension with 0.4 mg/mL of A. keiskei, this dose was used in subsequent healthspan assays. We examined the effect of the extract on physical performance, a fundamental measurement of animal health. To do this, we utilized the Rapid Iterative Negative Geotaxis (RING) assay to determine the animal’s ability to complete a strenuous activity (i.e., vertical climbing) [24]. In this context, we take advantage of the animal’s innate ability to work against gravity and climb up the vial, providing insight into their physical robustness. By week 3, A. keiskei-fed males displayed significantly reduced climbing abilities compared to their control-fed counterparts (Figure 2A). In contrast, control or A. keiskei-fed female flies showed no significant difference in climbing performance throughout all five weeks of treatment (Figure 2B). As the male flies were shorter-lived than the female flies, no data was available for male flies by week 5. These findings demonstrate a detrimental impact by A. keiskei on physical performance in male flies.
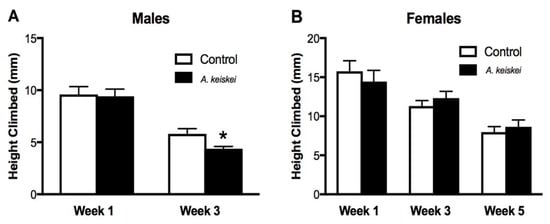
Figure 2.
The impact of Angelica keiskei on locomotion. (A) By week 3, A. keiskei-fed male flies showed a significant decrease in locomotion compared to controls (* p < 0.05, Mann–Whitney test). (B) Control or A. keiskei-fed female flies showed no significant difference in locomotion throughout 5 weeks of treatment (Mann–Whitney test). For both males and females, n = 9 groups of 20 flies.
Reproductive rates and lifespan are typically inversely related [25]. Interventions that increase lifespan in female flies often do so by decreasing fecundity, a physiological trade-off, and blocking female reproduction has typically led to increased lifespan [25]. To determine if the observed lifespan extension in females was a direct effect of A. keiskei treatment or a secondary physiological trade-off, we assessed female fecundity following A. keiskei supplementation. We observed that A. keiskei supplementation at 0.4 mg/mL increased fecundity in w1118 females (Figure 3A), despite increasing w1118 female lifespan (Figure 1B). Female JIV flies also showed lifespan extension (Figure 1D), but their fecundity was not affected by A. keiskei supplementation (Figure 3B). Together, these results demonstrate that A. keiskei’s positive impact on female w1118 and JIV lifespan is not caused by a decrease in fecundity.
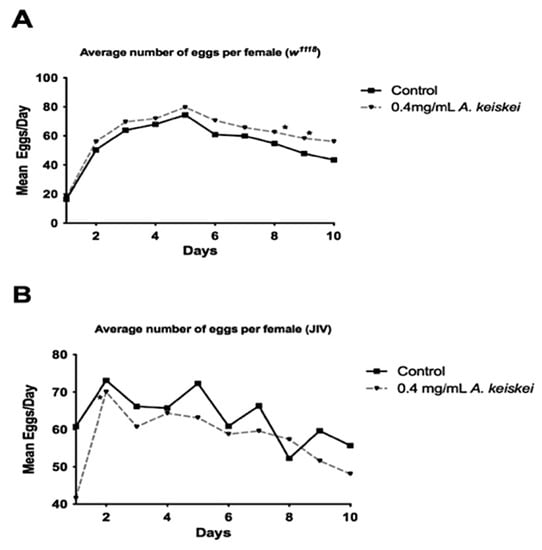
Figure 3.
The impact of Angelica keiskei on fecundity. (A) w1118 females fed A. keiskei showed a significant improvement in fecundity (*p < 0.0001) compared to controls. (B) JIV females, irrespective of treatment, showed no difference in average progeny number per female. Statistical significance was calculated using 2-Way ANOVA for each time point, n = 20 individually housed females for each group.
After completing the lifespan and healthspan assays, we attempted to understand the mechanism of action of A. keiskei. Many long-lived organisms demonstrate high levels of resistance to environmental stressors, including oxidative stress and starvation [26,27]. We chose to challenge flies to paraquat, iron, heat, starvation, and desiccation as they are commonly used markers to evaluate tolerance to oxidative and environmental stress [28,29]. Following a 10-day feeding period either in the absence or presence of A. keiskei, flies were subjected to a semi-lethal amount of paraquat (redox cycler of superoxide). Since we observed lifespan extension only with 0.4 mg/mL A. keiskei, we used this dose in our healthspan and stress-resistance assays. Both males and females pretreated with A. keiskei at 0.4 mg/mL showed increased survival against the superoxide generator paraquat (Figure 4A). In contrast, flies pretreated with A. keiskei showed no protection against oxidative stress induced by iron (Figure 4B). In addition, A. keiskei had no protective effect against heat (Figure 4C), starvation (Figure 4D), and desiccation (Figure 4E). These outcomes suggest the specific role of A. keiskei as a superoxide scavenger, as it protected against paraquat toxicity, a known redox generator of superoxide [16].
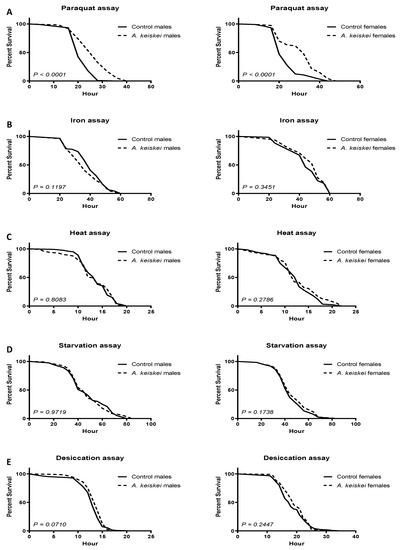
Figure 4.
The protective effect of Angelica keiskei against oxidative and environmental stress in JIV flies. (A) Both male and female flies pretreated with A. keiskei showed protection against paraquat toxicity; neither male nor female flies showed protection against oxidative stress induced by (B) iron, (C) heat, (D) starvation and (E) desiccation. Statistically significant differences were calculated using the Mantel–Cox log-rank test with n = 100–220 flies per group.
Previous studies of insulin and insulin-like growth factor signaling (IIS) in flies showed a sex- and dose-dependent effect on lifespan. Females heterozygous or homozygous for the insulin receptor substrate chico have an increased lifespan. While heterozygous males live longer, those lacking chico have shortened lifespans [30]. This finding led us to hypothesize that A. keiskei exerts its positive impact on female lifespan through the IIS pathway, as a similar female-benefit/male-detriment pattern in lifespan was observed following A. keiskei supplementation. Therefore, we evaluated the effect of A. keiskei on the lifespan of homozygous chico flies. We found that homozygous chico mutant females no longer showed lifespan extension when fed A. keiskei (Figure 5B). These findings suggest that A. keiskei may exert its effects through the IIS pathway. Similar to our previous results in w1118 flies, A. keiskei did not extend lifespan in homozygous chico mutant males (Figure 5A).
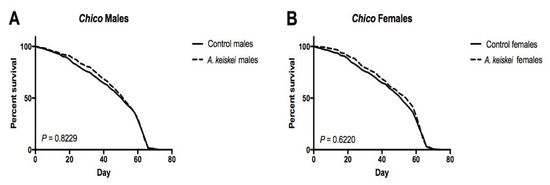
Figure 5.
Angelica keiskei requires the insulin receptor substrate (chico) to extend the lifespan of female flies. (A) Male and (B) female homozygous mutant chico flies showed no significant effect on longevity, when fed A. keiskei. Mantel–Cox log-rank test with n = 100 flies per group.
3. Discussion
Angelica keiskei is a highly cultivated and popular plant within many Asian countries (including Japan and Korea) due to its reported medicinal properties. It has been reported to act as a diuretic, analeptic, antidiabetic, galactagogue, and a laxative [5]. Over 100 biologically active compounds have been identified in A. keiskei [7,31], with the two most notable and abundant groups of compounds being coumarins and chalcones, both of which appear to have antitumor and antioxidant properties as demonstrated in cell culture models [2,32]. Cytoprotective effects of these compounds against oxidative stress have also been demonstrated in a previous study [33]. To our knowledge, this is the first study that evaluated the impact of the plant on D. melanogaster lifespan and healthspan.
The main aim of our study was to assess the impact of A. keiskei on the lifespan and healthspan of the model organism, D. melanogaster. Initial dose-finding studies indicated that A. keiskei at 0.4 mg/mL modulated the lifespan and healthspan of D. melanogaster in a sex- and strain-dependent manner. A. keiskei increased the lifespan of female flies and either had no effect or reduced survival in males. The impact of A. keiskei on the health of D. melanogaster was also evaluated using two known healthspan assays: locomotion and fecundity [34,35,36]. We found that A. keiskei improved the reproductive fitness in female flies and reduced physical performance in males. Because of the positive effect of the plant extract in females and negative effect in males, we suspected that A. keiskei may be working through the insulin and insulin-like growth factor signaling (IIS) pathway. Upon examining the effect of A. keiskei in flies lacking chico, the insulin-receptor substrate, we found that A. keiskei did not extend their lifespan, supporting the involvement of IIS in the action of A. keiskei. Moreover, A. keiskei has been hypothesized to function as an antioxidant [17]. This was supported by its ability to protect flies against the superoxide generator paraquat.
One of the possible explanations for an increased lifespan is through an inhibition of reproductive fitness. However, we found that reproduction was unaffected in JIV flies, and actually increased in w1118 flies (Figure 3). While unusual, this finding is not a unique phenomenon. For example, proanthocyanidins-enriched cranberry extract was found to increase egg-laying in flies while also increasing their lifespan [37]. In this case, cranberry extract also increased lifespan, but this effect was dependent on the dietary ratio of carbohydrate: protein or sugar: yeast. Flies fed with high or equal ratio of sugar:yeast (9:1) were found to have an increased lifespan when supplemented with cranberry, while flies fed with lower sugar:yeast ratio (1:9) had little to no effect on their lifespans. One way to remedy the lack of lifespan extension in the case of flies fed with lower dietary carbohydrate: protein ratio is to provide cholesterol supplementation, which then supports reproductive fitness, and indirectly results in lifespan extension [25]. Therefore, although lifespan extension in females often occurs at the expense of reproductive fitness, manipulating dietary macronutrients may remedy the negative correlation. Further nutrition-based studies are needed to confirm this analysis.
A common characteristic of many long-lived species is being highly stress-resistant [38]. In turn, the ability to mitigate cellular damage enables organisms to better combat chronic oxidative stress [39,40]. Supplements that can promote stress resistance offer a viable option for improved healthspan. One of the active compounds, flavonoid 4,4′-dimethoxychalcone chalcones, isolated from A. keiskei, has been shown to extend D. melanogaster lifespan, owing to its autophagy-inducing properties [41]. Angelica keiskei has also been previously implicated as a superoxide scavenger [16], and indeed, we showed that flies supplemented with the plant extract were protected against paraquat toxicity, a known superoxide generator. Surprisingly, however, the plant was unable to protect against other forms of oxidative and environmental stressors. These findings suggest that A. keiskei confers little or no general protective effect against environmental stressors, however, it may act as an antioxidant against specific targets. It is likely that one or more of the constituent compounds, such as one of the coumarins or chalcones, are directly able to protect against superoxide [5]. This hypothesis is further complicated by the findings that A. keiskei did not afford protection against iron, which should generate toxicity in an oxidative stress-dependent manner. Although, this variable protection against oxidative stress may not be that surprising as melatonin also protected against paraquat, but not iron [32]. We also found the opposite in green tea, where it protected against iron, but not against paraquat [28]. These findings suggest that, although both iron and paraquat generate oxidative stress, the mechanisms by which a pharmacological agent resists the two insults differs.
The differences in survival highlight the genetic fitness between strains. Earlier work showed that variation in D. melanogaster lifespan is highly strain-dependent [42,43]. Moreover, stress tolerance is also largely determined by genetic background [44,45]. Therefore, it should be of little surprise that A. keiskei may have a strain-specific benefit on lifespan. As mentioned above, one surprising finding here is that A. keiskei could increase lifespan in females, while compromising lifespan in males. This is reminiscent of the action of the IIS, in which females with either a heterozygous or homozygous deficiency in the insulin receptor substrate chico lived longer, while only the heterozygous males were longer-lived [30]. Males lacking chico were actually shorter-lived [30]. This reveals that while impairment of insulin signaling can extend lifespan in flies, doing so “too much” is detrimental. It also suggests that females may use alternative pathways for insulin signaling that are not present in males. Aligning with previous research, the results of our study illustrated that A. keiskei may have similar functional characteristics to a knockout of the chico allele. Interestingly, Bai et al. found that chico heterozygous or homozygous flies were protected against paraquat, however, our study found this protection was only conferred to homozygous deficient males [46]. This shows a complex interplay between the sex of the fly, IIS, and the resistance to oxidative stress, particularly against paraquat. Therefore, it should not actually be unexpected that the action of A. keiskei with respect to lifespan differs between the sexes while conferring a protective effect against paraquat in both sexes. Future studies will need to be undertaken to clarify the interaction between sex, IIS, and paraquat tolerance.
4. Materials and Methods
4.1. Plant Material
The aerial parts (stems and leaves) of A. keiskei Koidz. (Apiaceae) were collected in Icheon, Gyeonggi-do, South Korea, in September 2011 and identified by Professor Je-Hyun Lee of Oriental Medicine at Dongguk University. A voucher specimen (no. EA327) was deposited at the Natural Product Chemistry Laboratory, College of Pharmacy, Ewha Womans University.
4.2. Preparation of Ethanol Extract of A. keiskei
10 g of raw A. keiskei were extracted 3 times by sonication with 200 mL of 80% ethanol at 25 °C for 30 min each time. The collected extracts were filtered through filter paper, concentrated in vacuo, and freeze-dried to obtain a dried extract (3.5 g, approximately 35% yield). HPLC analysis was performed as previously reported, using a Waters system composed of a 996 PDA detector and 1525 binary HPLC pump (Waters Co., Milford, MA, USA) with a Phenomenex Luna 5 μm C18 column (250 × 4.6 mm) and a gradient solvent system of 60–100% methanol in deionized water (flow rate, 1 mL/min) [47].
4.3. Drosophila melanogaster Strains and Culture
Three fly strains were used: w1118, chico, and JIV. The w1118 and chico flies were obtained from the Bloomington Drosophila Stock Center (BDSC) at Indiana University, USA (w1118: BDSC #3605 and chico BDSC #10738). The JIV flies are an outbred population derived from flies originally collected in 1978, derived from an Amherst, Massachusetts population established by P.T. Ives in 1975 (#149) [48,49]. This population has been cultured for more than 700 generations with controlled densities of 50–80 eggs per vial. Flies were maintained in vials incubated at 23 °C under 12-hour light and 12-hour dark cycles.
The Drosophila banana–molasses food, a standard lab diet for fruit flies, was used for fly maintenance as well as in all feeding assays. It consists of 5 mL of banana–molasses food coated with a yeast solution overlaid on food. Control diets included a 75 μL yeast solution (3% yeast, 1% acetic acid) overlay on food, which was allowed to dry and was refrigerated for at least 24 h before use. Treatment diets were made by dissolving various concentrations of A. keiskei extract into the yeast overlay solution.
4.4. Lifespan Assays
Lifespan assays were performed as previously described [50]. Forty vials per group (A. keiskei supplemented or control), with 6 males and 6 females per vial, were set up with both w1118 and JIV fly strains. Flies were fed standard banana–molasses food coated with yeast solution that contained 0 mg/mL for control groups and 0.1, 0.2, or 0.4 mg/mL of A. keiskei for treatment groups. Flies were transferred to fresh food vials every other day, with the number of dead flies recorded. Due to a significant increase in lifespan with the 0.4 mg/mL of A. keiskei female flies, this dose was subsequently used in all healthspan (climbing and fecundity) and stress resistance assays.
Lifespan assays were also performed with chico flies. Balancer chromosome CyO “Curly O” allowed for the identification of homozygous mutant chico flies as those displaying a straight-winged phenotype. Each group, control-fed and 0.4 mg/mL-A. keiskei-fed, consisted of 20 vials with 6 females and 6 males per vial. Flies were transferred to fresh food vials every other day, and the number of dead flies was recorded.
4.5. Stress Resistance Assays
Following a 10-day feeding period in which w1118 flies were fed 0.4 mg/mL of A. keiskei, stress resistance assays were performed as previously described [50]. Death counts were recorded differently depending on the stress condition: every hour under heat stress, every 2 h under desiccation and starvation, and every 4 h for the paraquat and iron conditions.
For the desiccation assay, flies were housed in empty vials, and deaths were recorded every 2 h. For the starvation assay, flies were housed in vials containing 2% agarose to provide moisture with no nutritional value. Deaths were recorded every 4 h. Survival for both assays was determined by the log-rank Mantel–Cox test.
4.6. Climbing Assay
JIV flies were split into 4 groups: control males, control females, 0.4 mg/mL A. keiskei-fed males, and 0.4 mg/mL A. keiskei-fed females. Flies were transferred into empty vials (20 flies per vial) and placed into the RING apparatus [24], with the apparatus tapped to dislodge all flies to the bottom, and then recording the vertical distance the flies travel in 4 s. The procedure was performed 6 times for each of the 4 groups. The assay was performed every other week (week 1, week 3, week 5). Climbing heights were measured by pausing the video recording at 4 s and using ImageJ to assess the distance travelled.
4.7. Fecundity Assay
Fecundity assays were performed as previously described [51]. Briefly, within the first 24 h of emergence, adult flies were collected, and each vial was set up with 1 female and 1 male. Each female was allotted exactly 24 h to lay eggs. Flies were transferred to new vials daily, and fecundity (number of eggs laid by each female) was evaluated per day for 10 days.
4.8. Statistical Analysis
Statistical analyses were performed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA). For all survival and lifespans assays, p-values were calculated using the Mantel–Cox log-rank test, with n = 190–210 for w1118 flies, n = 120 for JIV flies, and n = 100 for chico flies. Fecundity experiments were analyzed by two-way ANOVA and data were presented as means ± SEM, with n = 20 individually housed females for each group. Climbing experiments were analyzed with the Mann–Whitney nonparametric test with n = 9 of independent trials with 20 flies per trial.
5. Conclusions
The results of this study shed light on the action of A. keiskei on the lifespan and healthspan of D. melanogaster. We found that this plant exhibited significant strain and sex-dependent effects with respect to lifespan, reproductive fitness, and physical performance. In general, the A. keiskei has positive effects in females and negative effects in males. This led us to identify a potential mechanism for lifespan extension of A. keiskei through the IIS pathway, a pathway conserved in both D. melanogaster and humans. The one commonality between the sexes is that A. keiskei did protect both males and females against paraquat, consistent with its presumed mode of action as an antioxidant. In summary, this study supports the action of A. keiskei as an antioxidant, that it requires IIS to extend lifespan in females, and a complex interaction between the plant and sex and genetic background of the flies being investigated.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph16050738/s1, Figure S1: HPLC chromatograms of Angelica keiskei extract.
Author Contributions
Conceptualization, M.J. and E.K.S.; methodology, M.J., S.E.S., S.T.P. and E.K.S.; formal analysis, M.J., Y.-S.K., E.K.S. and S.E.S.; plant sources and quality control, Y.-S.K. and E.K.S.; investigation, M.J., S.T.P. and S.E.S.; data curation, M.J. and S.E.S.; writing—original draft preparation, M.J.; writing—review and editing, M.J., S.E.S., Y.-S.K., S.T.P. and E.K.S.; supervision, M.J. and S.E.S.; project administration, M.J.; funding acquisition, M.J. and S.T.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no funding from an external funding agency. The work was partially supported by a grant from the Undergraduate Research Opportunities Program (UROP) at the University of California, Irvine that provides small grants to students’ research projects.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in a FigShare link at: https://figshare.com/s/4fcdf73e8ca419b2c519 accessed on 5 May 2023.
Acknowledgments
The authors wish to thank Farheen Nafees for her assistance in reference management and formatting the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amalia, R.; Aulifa, D.L.; Zain, D.N.; Pebiansyah, A.; Levita, J. The Cytotoxicity and Nephroprotective Activity of the Ethanol Extracts of Angelica keiskei Koidzumi Stems and Leaves against the NAPQI-Induced Human Embryonic Kidney (HEK293) Cell Line. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–6. [Google Scholar] [CrossRef]
- Bae, U.-J.; Ryu, J.-H.; Park, B.-H.; Bae, E.J. Angelica keiskei Root Extract Attenuates Bile Duct Ligation-Induced Liver Injury in Mice. J. Med. Food 2022, 25, 435–442. [Google Scholar] [CrossRef]
- Noh, S.; Go, A.; Kim, D.B.; Park, M.; Jeon, H.W.; Kim, B. Role of Antioxidant Natural Products in Management of Infertility: A Review of Their Medicinal Potential. Antioxidants 2020, 9, 957. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Samukawa, Y.; Yamashita, Y.; Ashida, H. 4-Hydroxyderricin and Xanthoangelol Isolated from Angelica keiskei Prevent Dexamethasone-Induced Muscle Loss. Food Funct. 2020, 11, 5498–5512. [Google Scholar] [CrossRef]
- Kil, Y.-S.; Pham, S.T.; Seo, E.K.; Jafari, M. Angelica keiskei, an Emerging Medicinal Herb with Various Bioactive Constituents and Biological Activities. Arch. Pharm. Res. 2017, 40, 655–675. [Google Scholar] [CrossRef]
- Kweon, M.; Lee, H.; Park, C.; Choi, Y.H.; Ryu, J.-H. A Chalcone from Ashitaba (Angelica keiskei) Stimulates Myoblast Differentiation and Inhibits Dexamethasone-Induced Muscle Atrophy. Nutrients 2019, 11, 2419. [Google Scholar] [CrossRef]
- Tu, L.; Wang, R.; Fang, Z.; Sun, M.; Sun, X.; Wu, J.; Dang, Y.; Liu, J. Assessment of the Hypoglycemic and Hypolipidemic Activity of Flavonoid-Rich Extract from Angelica keiskei. Molecules 2022, 27, 6625. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, Q.; Luo, J.; Wu, J.; Wang, Z. Phytonutrient and Anti-Diabetic Functional Properties of Flavonoid-Rich Ethanol Extract from Angelica keiskei Leaves. J. Food Sci. Technol. 2018, 55, 4406–4412. [Google Scholar] [CrossRef]
- Park, C.; Kim, D.H.; Kim, T.H.; Jeong, S.U.; Yoon, J.H.; Moon, S.-K.; Kwon, C.-Y.; Park, S.-H.; Hong, S.H.; Shim, J.-H.; et al. Improvement of Oxidative Stress-Induced Cytotoxicity of Angelica keiskei (Miq.) Koidz. Leaves Extract through Activation of Heme Oxygenase-1 in C2C12 Murine Myoblasts. Biotechnol. Bioproc. E 2023, 28, 51–62. [Google Scholar] [CrossRef]
- Aulifa, D.L.; Adnyana, I.K.; Sukrasno, S.; Levita, J. Inhibitory Activity of Xanthoangelol Isolated from Ashitaba (Angelica keiskei Koidzumi) towards α-Glucosidase and Dipeptidyl Peptidase-IV: In Silico and In Vitro Studies. Heliyon 2022, 8, e09501. [Google Scholar] [CrossRef]
- Mottin, M.; Caesar, L.K.; Brodsky, D.; Mesquita, N.C.M.R.; de Oliveira, K.Z.; Noske, G.D.; Sousa, B.K.P.; Ramos, P.R.P.S.; Jarmer, H.; Loh, B.; et al. Chalcones from Angelica keiskei (Ashitaba) Inhibit Key Zika Virus Replication Proteins. Bioorganic Chem. 2022, 120, 105649. [Google Scholar] [CrossRef]
- Caesar, L.K.; Kellogg, J.J.; Kvalheim, O.M.; Cech, R.A.; Cech, N.B. Integration of Biochemometrics and Molecular Networking to Identify Antimicrobials in Angelica keiskei. Planta Med. 2018, 84, 721–728. [Google Scholar] [CrossRef]
- Karimi-Sales, E.; Mohaddes, G.; Alipour, M.R. Chalcones as Putative Hepatoprotective Agents: Preclinical Evidence and Molecular Mechanisms. Pharmacol. Res. 2018, 129, 177–187. [Google Scholar] [CrossRef]
- Rocha, S.; Ribeiro, D.; Fernandes, E.; Freitas, M. A Systematic Review on Anti-Diabetic Properties of Chalcones. CMC 2020, 27, 2257–2321. [Google Scholar] [CrossRef]
- Martins, T.; Fonseca, B.M.; Rebelo, I. Antioxidant Effects of Chalcones during the Inflammatory Response: An Overall Review. Curr. Med. Chem. 2021, 28, 7658–7713. [Google Scholar] [CrossRef]
- Aoki, N.; Muko, M.; Ohta, E.; Ohta, S. C-Geranylated Chalcones from the Stems of Angelica keiskei with Superoxide-Scavenging Activity. J. Nat. Prod. 2008, 71, 1308–1310. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Q.; Fredimoses, M.; Gao, G.; Wang, K.; Chen, H.; Wang, T.; Oi, N.; Zykova, T.A.; Reddy, K.; et al. The Ashitaba (Angelica keiskei) Chalcones 4-Hydroxyderricin and Xanthoangelol Suppress Melanomagenesis by Targeting BRAF and PI3K. Cancer Prev. Res. 2018, 11, 607–620. [Google Scholar] [CrossRef]
- Ogienko, A.A.; Omelina, E.S.; Bylino, O.V.; Batin, M.A.; Georgiev, P.G.; Pindyurin, A.V. Drosophila as a Model Organism to Study Basic Mechanisms of Longevity. Int. J. Mol. Sci. 2022, 23, 11244. [Google Scholar] [CrossRef]
- Bass, T.M.; Weinkove, D.; Houthoofd, K.; Gems, D.; Partridge, L. Effects of Resveratrol on Lifespan in Drosophila melanogaster and Caenorhabditis Elegans. Mech. Ageing Dev. 2007, 128, 546–552. [Google Scholar] [CrossRef]
- Islam, M.S.; Jin, Y.Y.; Chung, H.-J.; Kim, H.-J.; Baek, S.-H.; Hong, S.-T. Effect of the Resveratrol Rice DJ526 on Longevity. Nutrients 2019, 11, 1804. [Google Scholar] [CrossRef]
- Abolaji, A.O.; Adedara, A.O.; Adie, M.A.; Vicente-Crespo, M.; Farombi, E.O. Resveratrol Prolongs Lifespan and Improves 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Oxidative Damage and Behavioural Deficits in Drosophila melanogaster. Biochem. Biophys Res. Commun. 2018, 503, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Staats, S.; Wagner, A.E.; Kowalewski, B.; Rieck, F.T.; Soukup, S.T.; Kulling, S.E.; Rimbach, G. Dietary Resveratrol Does Not Affect Life Span, Body Composition, Stress Response, and Longevity-Related Gene Expression in Drosophila melanogaster. Int. J. Mol. Sci. 2018, 19, 223. [Google Scholar] [CrossRef] [PubMed]
- Arabit, J.; Elhaj, R.; Schriner, S.E.; Sevrioukov, E.A.; Jafari, M. Rhodiola rosea improves lifespan, locomotion, and neurodegeneration in a Drosophila melanogaster model of Huntington’s disease. Biomed. Res. Int. 2019, 85, 6726874. [Google Scholar]
- Kil, Y.-S.; Nam, J.-W.; Lee, J.; Seo, E.K. Separation of Two Major Chalcones from Angelica keiskei by High-Speed Counter-Current Chromatography. Arch. Pharm. Res. 2015, 38, 1506–1511. [Google Scholar] [CrossRef]
- Rose, M.R.; Charlesworth, B. Genetics of Life History in Drosophila melanogaster. I. Sib analysis of adult females. Genetics 1981, 97, 173–186. [Google Scholar] [CrossRef]
- Rose, M.R.; Drapeau, M.D.; Yazdi, P.G.; Shah, K.H.; Moise, D.B.; Thakar, R.R.; Rauser, C.L.; Mueller, L.D. Evolution of Late-Life Mortality in Drosophila melanogaster. Evolution 2002, 56, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- Schriner, S.E.; Katoozi, N.S.; Pham, K.Q.; Gazarian, M.; Zarban, A.; Jafari, M. Extension of Drosophila Lifespan by Rosa Damascena Associated with an Increased Sensitivity to Heat. Biogerontology 2012, 13, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Gargano, J.W.; Martin, I.; Bhandari, P.; Grotewiel, M.S. Rapid Iterative Negative Geotaxis (RING): A New Method for Assessing Age-Related Locomotor Decline in Drosophila. Exp. Gerontol. 2005, 40, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Lee, B.-S.; Semnani, S.; Avanesian, A.; Um, C.-Y.; Jeon, H.-J.; Seong, K.-M.; Yu, K.; Min, K.-J.; Jafari, M. Curcumin Extends Life Span, Improves Health Span, and Modulates the Expression of Age-Associated Aging Genes in Drosophila Melanogaster. Rejuvenation Res. 2010, 13, 561–570. [Google Scholar] [CrossRef]
- Zanco, B.; Mirth, C.K.; Sgrò, C.M.; Piper, M.D. A Dietary Sterol trade-off Determines Lifespan Responses to Dietary Restriction in Drosophila melanogaster Females. eLife 2021, 10, e62335. [Google Scholar] [CrossRef]
- Yang, F.; Xiu, M.; Yang, S.; Li, X.; Tuo, W.; Su, Y.; He, J.; Liu, Y. Extension of Drosophila Lifespan by Astragalus Polysaccharide through a Mechanism Dependent on Antioxidant and Insulin/IGF-1 Signaling. Evid. Based Complement. Altern. Med. 2021, 2021, e6686748. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Xie, M.; Luo, L.; Tian, Y.; Yu, G.; Wu, Q.; Fan, X.; Yang, D.; Mao, X.; Gaur, U.; et al. Inhibitor GSK690693 Extends Drosophila Lifespan via Reduce AKT Signaling Pathway. Mech. Ageing Dev. 2022, 202, 111633. [Google Scholar] [CrossRef] [PubMed]
- Lopez, T.; Schriner, S.E.; Okoro, M.; Lu, D.; Chiang, B.T.; Huey, J.; Jafari, M. Green Tea Polyphenols Extend the Lifespan of Male Drosophila melanogaster while Impairing Reproductive Fitness. J. Med. Food 2014, 17, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-N.; Shim, Y.-J.; Kang, B.-H.; Park, J.-J.; Min, B.-H. Over-Expression of Human Clusterin Increases Stress Resistance and Extends Lifespan in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2012, 420, 851–856. [Google Scholar] [CrossRef]
- Clancy, D.J.; Gems, D.; Harshman, L.G.; Oldham, S.; Stocker, H.; Hafen, E.; Leevers, S.J.; Partridge, L. Extension of Life-Span by Loss of CHICO, a Drosophila Insulin Receptor Substrate Protein. Science 2001, 292, 104–106. [Google Scholar] [CrossRef]
- Kim, D.W.; Curtis-Long, M.J.; Yuk, H.J.; Wang, Y.; Song, Y.H.; Jeong, S.H.; Park, K.H. Quantitative Analysis of Phenolic Metabolites from Different Parts of Angelica keiskei by HPLC–ESI MS/MS and Their Xanthine Oxidase Inhibition. Food Chem. 2014, 153, 20–27. [Google Scholar] [CrossRef]
- Li, L.; Aldini, G.; Carini, M.; Chen, C.-Y.O.; Chun, H.-K.; Cho, S.-M.; Park, K.-M.; Correa, C.R.; Russell, R.M.; Blumberg, J.B.; et al. Characterisation, Extraction Efficiency, Stability and Antioxidant Activity of Phytonutrients in Angelica keiskei. Food Chem. 2009, 115, 227–232. [Google Scholar] [CrossRef]
- Yan, R.; Yan, J.; Chen, X.; Yu, Y.; Sun, T. Xanthoangelol Prevents Ox-LDL–Induced Endothelial Cell Injury by Activating Nrf2/ARE Signaling. J. Cardiovasc. Pharmacol. 2019, 74, 162. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Zhai, L.; Sun, L.; Zhao, D.; Wang, Z.; Li, X. Ginsenoside Extract from Ginseng Extends Lifespan and Health Span in Caenorhabditis Elegans. Food Funct. 2021, 12, 6793–6808. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, X.; Zhuang, C.; Lin, Y.; Cao, Y.; Chen, Y. Healthspan Improvements in Caenorhabditis Elegans with Traditional Chinese Herbal Tea. Oxidative Med. Cell. Longev. 2020, 2020, e4057841. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Zhu, L.J.; Yen, K.; Tissenbaum, H.A. Uncoupling Lifespan and Healthspan in Caenorhabditis Elegans Longevity Mutants. Proc. Natl. Acad. Sci. USA 2015, 112, E277–E286. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yolitz, J.; Alberico, T.; Laslo, M.; Sun, Y.; Wheeler, C.T.; Sun, X.; Zou, S. Cranberry Interacts with Dietary Macronutrients to Promote Healthy Aging in Drosophila. J. Gerontol. Ser. A 2014, 69, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C. The Plasticity of Aging: Insights from Long-Lived Mutants. Cell 2005, 120, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Leiser, S.F.; Miller, R.A. Nrf2 Signaling, a Mechanism for Cellular Stress Resistance in Long-Lived Mice. Mol. Cell Biol. 2010, 30, 871–884. [Google Scholar] [CrossRef]
- Harper, J.M.; Salmon, A.B.; Leiser, S.F.; Galecki, A.T.; Miller, R.A. Skin-Derived Fibroblasts from Long-Lived Species Are Resistant to Some, but Not All, Lethal Stresses and to the Mitochondrial Inhibitor Rotenone. Aging Cell 2007, 6, 1–13. [Google Scholar] [CrossRef]
- Carmona-Gutierrez, D.; Zimmermann, A.; Kainz, K.; Pietrocola, F.; Chen, G.; Maglioni, S.; Schiavi, A.; Nah, J.; Mertel, S.; Beuschel, C.B.; et al. The Flavonoid 4,4′-Dimethoxychalcone Promotes Autophagy-Dependent Longevity across Species. Nat. Commun. 2019, 10, 651. [Google Scholar] [CrossRef]
- Niraula, P.; Kim, M.S. N-Acetylcysteine Extends Lifespan of Drosophila via Modulating ROS Scavenger Gene Expression. Biogerontology 2019, 20, 533–543. [Google Scholar] [CrossRef]
- Cross, S.T.; Brehm, A.L.; Dunham, T.J.; Rodgers, C.P.; Keene, A.H.; Borlee, G.I.; Stenglein, M.D. Galbut Virus Infection Minimally Influences Drosophila melanogaster Fitness Traits in a Strain and Sex-Dependent Manner. Viruses 2023, 15, 539. [Google Scholar] [CrossRef]
- Ramnarine, T.J.S.; Grath, S.; Parsch, J. Natural Variation in the Transcriptional Response of Drosophila melanogaster to Oxidative Stress. G3 Bethesda 2021, 12, jkab366. [Google Scholar] [CrossRef]
- Malacrida, S.; De Lazzari, F.; Mrakic-Sposta, S.; Vezzoli, A.; Zordan, M.A.; Bisaglia, M.; Menti, G.M.; Meda, N.; Frighetto, G.; Bosco, G.; et al. Lifespan and ROS Levels in Different Drosophila melanogaster Strains after 24 h Hypoxia Exposure. Biol. Open 2022, 11, bio059386. [Google Scholar] [CrossRef]
- Bai, H.; Post, S.; Kang, P.; Tatar, M. Drosophila Longevity Assurance Conferred by Reduced Insulin Receptor Substrate Chico Partially Requires D4eBP. PLoS ONE 2015, 10, e0134415. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).