Immune-Mediated Organ-Specific Reactions to COVID-19 Vaccines: A Retrospective Descriptive Study
Abstract
:1. Introduction
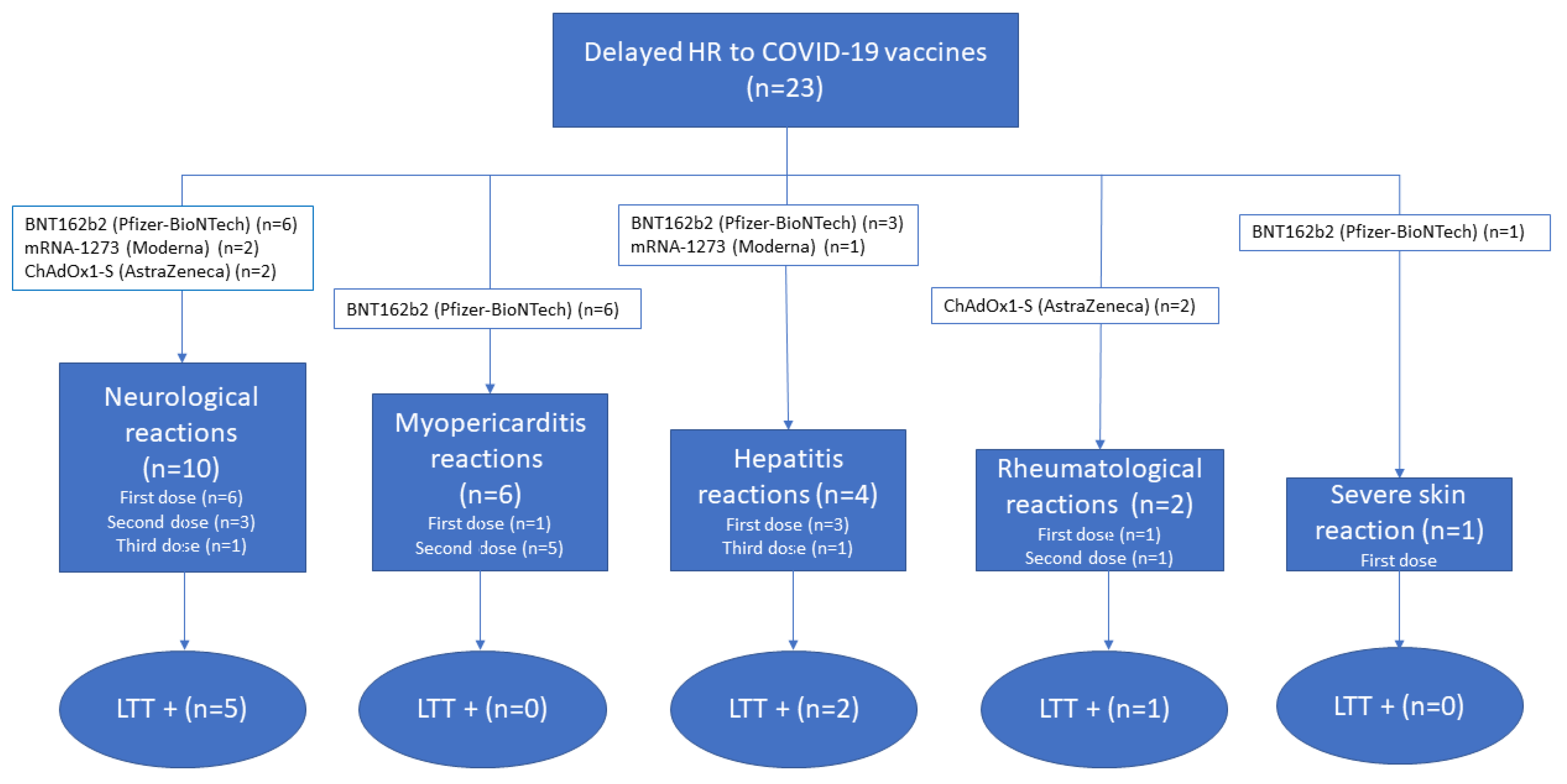
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Cellular Immune Response to COVID-19 Vaccines
4.3. Lymphocyte Transformation Test
4.4. Causality Assessment
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bok, K.; Sitar, S.; Graham, B.S.; Mascola, J.R. Accelerated COVID-19 vaccine development: Milestones, lessons, and prospects. Immunity 2021, 54, 1636–1651. [Google Scholar] [CrossRef]
- Lamprinou, M.; Sachinidis, A.; Stamoula, E.; Vavilis, T.; Papazisis, G. COVID-19 vaccines adverse events: Potential molecular mechanisms. Immunol. Res. 2023, 1–17. [Google Scholar] [CrossRef]
- Cabanillas, B.; Novak, N. Allergy to COVID-19 vaccines: A current update. Allergol. Int. 2021, 70, 313–318. [Google Scholar] [CrossRef]
- Borgsteede, S.D.; Geersing, T.H.; Tempels-Pavlica, Ž. Other excipients than PEG might cause serious hypersensitivity reactions in COVID-19 vaccines. Allergy 2021, 76, 1941–1942. [Google Scholar] [CrossRef]
- Barbaud, A.; Garvey, L.H.; Arcolaci, A.; Brockow, K.; Mori, F.; Mayorga, C.; Bonadonna, P.; Atanaskovic-Markovic, M.; Moral, L.; Zanoni, G.; et al. Allergies and COVID-19 vaccines: An ENDA/EAACI Position paper. Allergy 2022, 77, 2292–2312. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Interim Considerations: Preparing for the Potential Management of Anaphylaxis after COVID-19 Vaccination; CDC: Atlanta, GA, USA, 2020. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/anaphylaxis-management.html (accessed on 11 February 2022).
- Sokolowska, M.; Eiwegger, T.; Ollert, M.; Torres, M.J.; Barber, D.; Del Giacco, S.; Jutel, M.; Nadeau, K.C.; Palomares, O.; Rabin, R.L.; et al. EAACI statement on the diagnosis, management and prevention of severe allergic reactions to COVID-19 vaccines. Allergy 2021, 76, 1629–1639. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; de Gregorio, C.; Velissaris, D.; Petalas, K.; Brinia, A.; Assimakopoulos, S.F.; Gogos, C.; Kouni, S.N.; Kounis, G.N.; et al. Allergic Reactions to Current Available COVID-19 Vaccinations: Pathophysiology, Causality, and Therapeutic Considerations. Vaccines 2021, 9, 221. [Google Scholar] [CrossRef]
- Garvey, L.H.; Nasser, S. Anaphylaxis to the first COVID-19 vaccine: Is polyethylene glycol (PEG) the culprit? Br. J. Anaesth. 2021, 126, e106–e108. [Google Scholar] [CrossRef]
- Nilsson, L.; Csuth, Á.; Storsaeter, J.; Garvey, L.H.; Jenmalm, M.C. Vaccine allergy: Evidence to consider for COVID-19 vaccines. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 401–409. [Google Scholar] [CrossRef]
- Shelley, W.B.; Talanin, N.; Shelley, E.D. Polysorbate 80 hypersensitivity. Lancet 1995, 345, 1312–1313. [Google Scholar] [CrossRef]
- Stone, C.A., Jr.; Liu, Y.; Relling, M.V.; Krantz, M.S.; Pratt, A.L.; Abreo, A.; Hemler, J.A.; Phillips, E.J. Immediate Hypersensitivity to Polyethylene Glycols and Polysorbates: More Common Than We Have Recognized. J. Allergy Clin. Immunol. Pract. 2019, 7, 1533–1540.e8. [Google Scholar] [CrossRef] [PubMed]
- Sellaturay, P.; Nasser, S.; Ewan, P. Polyethylene Glycol-Induced Systemic Allergic Reactions (Anaphylaxis). J. Allergy Clin. Immunol. Pract. 2021, 9, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Calogiuri, G.; Foti, C.; Nettis, E.; Di Leo, E.; Macchia, L.; Vacca, A. Polyethylene glycols and polysorbates: Two still neglected ingredients causing true IgE-mediated reactions. J. Allergy Clin. Immunol. Pract. 2019, 7, 2509–2510. [Google Scholar] [CrossRef] [PubMed]
- Tramontana, M.; Bianchi, L.; Biondi, F.; Hansel, K.; Malatesta, N.; Marietti, R.; Stingeni, L. A case of delayed allergy to polyethylene glycol 2000 and polysorbate 80 confirmed by patch test: Consequences for anti-SARS-CoV2 vaccination? Contact Dermat. 2022, 87, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Sellaturay, P.; Gurugama, P.; Harper, V.; Dymond, T.; Ewan, P.; Nasser, S. The Polysorbate containing AstraZeneca COVID-19 vaccine is tolerated by polyethylene glycol (PEG) allergic patients. Clin. Exp. Allergy 2022, 52, 12–17. [Google Scholar] [CrossRef]
- Rush, C.; Faulk, K.E.; Bradley, Z.K.; Turner, A.; Krumins, M.; Greenhawt, M. The safety of SARS-CoV-2 vaccines in persons with a known history of pegaspargase allergy: A single institution experience. J. Allergy Clin. Immunol. Pract. 2022, 10, 630–632. [Google Scholar] [CrossRef]
- Copaescu, A.M.; Rosa Duque, J.S.; Phillips, E.J. What have we learned about the allergenicity and adverse reactions associated with the severe acute respiratory syndrome coronavirus 2 vaccines: One year later. Ann. Allergy Asthma Immunol. 2022, 129, 40–51. [Google Scholar] [CrossRef]
- Corey, K.B.; Koo, G.; Phillips, E.J. Adverse Events and Safety of SARS-CoV-2 Vaccines: What’s New and What’s Next. J. Allergy Clin. Immunol. Pract. 2022, 10, 2254–2266. [Google Scholar] [CrossRef]
- Juárez Guerrero, A.; Domínguez Estirado, A.; Crespo Quirós, J.; Rojas-Pérez-Ezquerra, P. Delayed cutaneous reactions after the administration of mRNA vaccines against COVID-19. J. Allergy Clin. Immunol. Pract. 2021, 9, 3811–3813. [Google Scholar] [CrossRef]
- Jover Cerdá, V.; Rodríguez Pacheco, R.; Doménech Witek, J.; Alonso Hernández, S.; Durán García, R.; Real Panisello, M.; Marco de la Calle, F.M. Allergological study in patients vaccinated against COVID-19 with suspected allergic reactions. Allergy Asthma Clin. Immunol. 2022, 18, 43. [Google Scholar] [CrossRef]
- Wolfson, A.R.; Robinson, L.B.; Li, L.; McMahon, A.E.; Cogan, A.S.; Fu, X.; Wickner, P.; Samarakoon, U.; Saff, R.R.; Blumenthal, K.G.; et al. First-Dose mRNA COVID-19 Vaccine Allergic Reactions: Limited Role for Excipient Skin Testing. J. Allergy Clin. Immunol. Pract. 2021, 9, 3308–3320.e3. [Google Scholar] [CrossRef]
- Chu, D.K.; Abrams, E.M.; Golden, D.B.K.; Blumenthal, K.G.; Wolfson, A.R.; Stone, C.A., Jr.; Krantz, M.S.; Shaker, M.; Greenhawt, M. Risk of Second Allergic Reaction to SARS-CoV-2 Vaccines: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2022, 182, 376–385. [Google Scholar] [CrossRef]
- Luxi, N.; Giovanazzi, A.; Arcolaci, A.; Bonadonna, P.; Crivellaro, M.A.; Cutroneo, P.M.; Ferrajolo, C.; Furci, F.; Guidolin, L.; Moretti, U.; et al. Allergic Reactions to COVID-19 Vaccines: Risk Factors, Frequency, Mechanisms and Management. BioDrugs 2022, 36, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Manea, M.M.; Dragoș, D.; Enache, I.; Sirbu, A.G.; Tuta, S. Multiple cranial nerve palsies following COVID-19 vaccination-Case report. Acta Neurol. Scand. 2022, 145, 257–259. [Google Scholar] [CrossRef]
- Saito, K.; Shimizu, T.; Suzuki-Inoue, K.; Ishida, T.; Wada, Y. Aseptic meningitis after vaccination of the BNT162b2 mRNA COVID-19 vaccine. Neurol. Sci. 2021, 42, 4433–4435. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Gui, H.; Sheng, Z.; Xin, H.; Xie, Q. Letter to the editor: Exacerbation of autoimmune hepatitis after COVID-19 vaccination. Hepatology 2022, 75, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Rosso, M.; Anziska, Y.; Levine, S.R. Acute Transient Encephalopathy after Moderna COVID-19 Vaccine. Case Rep. Neurol. 2022, 14, 231–236. [Google Scholar] [CrossRef]
- Karch, F.E.; Smith, C.L.; Kerzner, B.; Mazzullo, J.M.; Weintraub, M.; Lasagna, L. Adverse drug reactions-a matter of opinion. Clin. Pharmacol. Ther. 1976, 19 Pt 1, 489–492. [Google Scholar] [CrossRef]
- Blanc, S.; Leuenberger, P.; Berger, J.P.; Brooke, E.M.; Schelling, J.L. Judgments of trained observers on adverse drug reactions. Clin. Pharmacol. Ther. 1979, 25 Pt 1, 493–498. [Google Scholar] [CrossRef]
- Macedo, A.F.; Marques, F.B.; Ribeiro, C.F. Can decisional algorithms replace global introspection in the individual causality assessment of spontaneously reported ADRs? Drug. Saf. 2006, 29, 697–702. [Google Scholar] [CrossRef]
- Delgado, A.; Stewart, S.; Urroz, M.; Rodríguez, A.; Borobia, A.M.; Akatbach-Bousaid, I.; González-Muñoz, M.; Ramírez, E. Characterisation of Drug-Induced Liver Injury in Patients with COVID-19 Detected by a Proactive Pharmacovigilance Program from Laboratory Signals. J. Clin. Med. 2021, 10, 4432. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, E.; Urroz, M.; Rodríguez, A.; González-Muñoz, M.; Martín-Vega, A.; Villán, Y.; Seco, E.; Monserrat, J.; Frías, J.; Carcas, A.J.; et al. Incidence of Suspected Serious Adverse Drug Reactions in Corona Virus Disease-19 Patients Detected by a Pharmacovigilance Program by Laboratory Signals in a Tertiary Hospital in Spain: Cautionary Data. Front. Pharmacol. 2020, 11, 602841. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; García-García, I.; Martínez de Soto, L.; Gómez López De Las Huertas, A.; Borobia, A.M.; González-Torbay, A.; Akatbach-Bousaid, I.; González-Muñoz, M.; Ramírez, E. Utility of Lymphocyte Transformation Test for Assisting Updated Roussel Uclaf Causality Assessment Method in Drug-Induced Liver Injury: A Case-Control Study. Front. Pharmacol. 2022, 13, 819589. [Google Scholar] [CrossRef] [PubMed]
- Meller, S.; Gerber, P.A.; Kislat, A.; Hevezi, P.; Göbel, T.; Wiesner, U.; Kellermann, S.; Bünemann, E.; Zlotnik, A.; Häussinger, D.; et al. Allergic sensitization to pegylated interferon-α results in drug eruptions. Allergy 2015, 70, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Freeman, E.E.; Saff, R.R.; Robinson, L.B.; Wolfson, A.R.; Foreman, R.K.; Hashimoto, D.; Banerji, A.; Li, L.; Anvari, S.; et al. Delayed Large Local Reactions to mRNA-1273 Vaccine against SARS-CoV-2. N. Engl. J. Med. 2021, 384, 1273–1277. [Google Scholar] [CrossRef]
- Baeck, M.; Marot, L.; Belkhir, L. Delayed Large Local Reactions to mRNA Vaccines. N. Engl. J. Med. 2021, 384, e98. [Google Scholar] [CrossRef]
- Wenande, E.; Garvey, L.H. Immediate-type hypersensitivity to polyethylene glycols: A review. Clin. Exp. Allergy 2016, 46, 907–922. [Google Scholar] [CrossRef]
- Mohseni Afshar, Z.; Tavakoli Pirzaman, A.; Liang, J.J.; Sharma, A.; Pirzadeh, M.; Babazadeh, A.; Hashemi, E.; Deravi, N.; Abdi, S.; Allahgholipour, A.; et al. Do we miss rare adverse events induced by COVID-19 vaccination? Front. Med. 2022, 9, 933914. [Google Scholar] [CrossRef]
- Fantini, J.; Chahinian, H.; Yahi, N. Leveraging coronavirus binding to gangliosides for innovative vaccine and therapeutic strategies against COVID-19. Biochem. Biophys. Res. Commun. 2021, 538, 132–136. [Google Scholar] [CrossRef]
- Yonker, L.M.; Swank, Z.; Bartsch, Y.C.; Burns, M.D.; Kane, A.; Boribong, B.P.; Davis, J.P.; Loiselle, M.; Novak, T.; Senussi, Y.; et al. Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis. Circulation 2023, 147, 867–876. [Google Scholar] [CrossRef]
- Ling, R.R.; Ramanathan, K.; Tan, F.L.; Tai, B.C.; Somani, J.; Fisher, D.; MacLaren, G. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: A systematic review and meta-analysis. Lancet Respir. Med. 2022, 10, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Karch, F.E.; Lasagna, L. Toward the operational identification of adverse drug reactions. Clin. Pharmacol. Ther. 1977, 21, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, C.; García, M. Evaluación de la causalidad en las comunicaciones de reacciones adversas a medicamentos. Algoritmo del Sistema Español de Farmacovigilancia [Causality assessment in reports on adverse drug reactions. Algorithm of Spanish pharmacovigilance system]. Med. Clin. 2016, 147, 461–464. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Danan, G.; Teschke, R. RUCAM in Drug and Herb Induced Liver Injury: The Update. Int. J. Mol. Sci. 2016, 17, 14. [Google Scholar] [CrossRef]
- Crespo, M.; Barrilado-Jackson, A.; Padilla, E.; Eguía, J.; Echeverria-Esnal, D.; Cao, H.; Faura, A.; Folgueiras, M.; Solà-Porta, E.; Pascual, S.; et al. Negative immune responses to two-dose mRNA COVID-19 vaccines in renal allograft recipients assessed with simple antibody and interferon gamma release assay cellular monitoring. Am. J. Transplant. 2022, 22, 786–800. [Google Scholar] [CrossRef]
- Pichler, W.J.; Tilch, J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy 2004, 59, 809–820. [Google Scholar] [CrossRef]
| Patient | Sex | Age (Years) | Underlying Disease | Previous COVID-19/Previous ADR | Vaccine (Dose at Which Reaction Occurs) | Cellular Immune Response to Vaccine | Adverse Reaction | Latency | Outcome/Time until Total Recovery | Tolerance to Subsequent COVID-19 Vaccination |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | M | 30 | Allergic rhinitis and asthma | y/n | BNT162b2 (Pfizer-BioNTech) (1) | Positive | Mixed hepatitis | 7 days | Admitted to hospital ward for 4 days/98 days | Yes (mRNA-1273) |
| P2 | F | 15 | No | n/n | BNT162b2 (Pfizer-BioNTech) (1) | Positive | Hepatocellular hepatitis | 4 days | Discharged from ED/13 days | NV |
| P3 | M | 22 | No | n/n | mRNA-1273 (Moderna) (1) | Negative | Left hypoglossal nerve paresis | 6 days | Admitted to hospital ward for 8 days/63 days | NV |
| P4 | M | 15 | No | n/y (sulphonamide) | BNT162b2 (Pfizer-BioNTech) (2) | Positive | Myopericarditis | 4 days | Admitted to hospital ward for 7 days/32 days | NV |
| P5 | M | 36 | Dyslipidaemia | n/n | BNT162b2 (Pfizer-BioNTech) (1) | Positive | Myocarditis | 15 days | Admitted to hospital ward for 3 days/3 days | NV |
| P6 | M | 16 | Subclinical hypothyroidism | n/n | BNT162b2 (Pfizer-BioNTech) (2) | Positive | Myopericarditis | 4 days | Admitted to hospital ward for 3 days/3 days | NV |
| P7 | F | 67 | Hypothyroidism, dyslipidaemia | n/n | ChAdOx1-S (AstraZeneca) (1) | Positive | Cranial nerves III and IV neuropathy | 2 days | Admitted to hospital ward for 4 days/54 days | NV |
| P8 | F | 56 | Graves’ disease | n/y (acetylsalicylic acid) | mRNA-1273 (Moderna) (1) | ND | Hepatocellular hepatitis | 7 days | Admitted to hospital ward for 14 days/104 days | NV |
| P9 | M | 73 | Depression, essential tremor | n/n | BNT162b2 (Pfizer-BioNTech) (2) | Positive | Pleuropericarditis | 15 days | Admitted to hospital ward for 8 days/25 days | NV |
| P10 | F | 28 | No | n/n | BNT162b2 (Pfizer-BioNTech) (1) | Positive | Aseptic meningitis | 1 day | Admitted to hospital ward for 5 days/27 days | NV |
| P11 | F | 32 | Graves’ disease, asthma | n/n | ChAdOx1-S (AstraZeneca) (1) | Positive | Fever, arthralgia, myalgia, general discomfort | 3 days | Seen in outpatient care/No resolution yet * | NV |
| P12 | M | 38 | Multiple sclerosis | n/n | BNT162b2 (Pfizer-BioNTech) (2) | Positive | Myocarditis | 4 days | Admitted to hospital ward for 2 days/1 day | NV |
| P13 | M | 28 | No | y/n | BNT162b2 (Pfizer-BioNTech) (1) | Positive | Erythema multiforme | 10 days | Admitted to hospital ward for 6 days/24 days | NV |
| P14 | F | 76 | Dyslipidaemia, hypertension | n/n | BNT162b2 (Pfizer-BioNTech) (3) | Positive | Mixed hepatitis | 7 days | Discharged from ED after 2 days of observation/120 days | NV |
| P15 | M | 59 | Hyperlipidaemia | n/n | BNT162b2 (Pfizer-BioNTech) (2) | Positive | Guillain–Barré syndrome | 3 days | Admitted to hospital ward for 2 days/No resolution yet * | NV |
| P16 | M | 43 | Dyslipidaemia | y/n | mRNA-1273 (Moderna) (2) | Positive | Acute transient encephalopathy | 1 day | Discharged from ED after 1 day of observation/1 day | NV |
| P17 | M | 64 | Hypertension, asthma | n/n | ChAdOx1-S (AstraZeneca) (1 and 2) | Positive | Polyarthralgia (first dose) Polyserositis (second dose) | 13 days | First dose: Discharged from ED Second dose: Admitted to hospital ward for 7 days/First dose: 41 days Second dose: 17 days | No vaccination after second AR |
| P18 | F | 31 | No | n/n | BNT162b2 (Pfizer-BioNTech) (1) | Positive | Paraesthesia in right arm and foot | 4 h | Seen in outpatient care/Unknown | NV |
| P19 | M | 33 | No | n/n | BNT162b2 (Pfizer-BioNTech) (2) | Positive | Myocarditis | 1 day | Admitted to hospital ward for 3 days/3 days | NV |
| P20 | M | 51 | Urinary tract infection | n/n | BNT162b2 (Pfizer-BioNTech) (1) | Negative | Guillain–Barré syndrome | 2 days | Admitted to hospital ward for 46 days/No resolution yet | NV |
| P21 | M | 68 | Benign prostatic hyperplasia | n/n | ChAdOx1-S (AstraZeneca) (1) | Positive | Guillain–Barré syndrome | 36 days | Admitted to hospital ward for 34 days/360 days | Yes (BNT162b2) |
| P22 | M | 32 | No | n/n | BNT162b2 (Pfizer-BioNTech) (2) | ND | Guillain–Barré syndrome | 1 day | Admitted to hospital ward for 9 days/No resolution yet * | NV |
| P23 | M | 53 | Hypertension, obesity | n/n | BNT162b2 (Pfizer-BioNTech) (3) | Positive | Paraesthesia (second dose) Aggravation of paraesthesia (third dose) | 20 days from second dose | Admitted to hospital ward for 1 day/No resolution yet * | NV |
| Patient | Algorithm Score | Concomitant drugs (Algorithm Score/Stimulation Index) | Time to Analysis (Months) | Stimulation Index PEG 2000 | Stimulation Index P80 |
|---|---|---|---|---|---|
| P1 | +6 | Bilastine (+3/1.5) Terbutaline (+1/not performed) | 3 | 4.1 | 2.3 |
| P2 | +7 | No | 1.5 | 3.4 | 4.3 |
| P3 | +6 | No | 3.5 | 0.5 | 0.4 |
| P4 | +6 | No | 2 | 0.8 | 1.3 |
| P5 | +7 | No | 4 | 0.4 | 0.7 |
| P6 | +6 | No | 3 | 0.3 | 0.5 |
| P7 * | +7 | No | 7 | 12.1 | 4.6 |
| 19 | 1.6 | 1.7 | |||
| P8 | +7 | No | 6 | 1.8 | 1 |
| P9 | +7 | Tramadol (−1/not performed) | 7 | 0.7 | 0.9 |
| P10 | +6 | No | 4 | 1.4 | 0.4 |
| P11 * | +4 | No | 2.5 | 1.3 | 3.6 |
| 9 | 9.3 | 12.6 | |||
| P12 | +7 | No | 5 | 0.9 | 1.2 |
| P13 | +5 | No | 6 | 2.3 | 2.8 |
| P14 | +7 | Paracetamol (+5/0.9) | 1.5 | 0.8 | 0.8 |
| P15 * | +4 | No | 8 | 10.7 | 6.4 |
| 17 | 3.3 | 1 | |||
| P16 | +5 | No | 3 | 1.1 | 1.3 |
| P17 | +8 | No | 8 | 1.7 | 1.3 |
| P18 * | +6 | No | 7 | 11.7 | 2.8 |
| 13 | 2.7 | ND | |||
| P19 | +6 | No | 3 | 1 | 1.3 |
| P20 | +4 | No | 12 | 1.6 | 1.7 |
| P21 | +6 | Tadalafil, Silodosin (−1/not performed) | 10 | 1.1 | 1.4 |
| P22 | +4 | No | 1 | 3.8 | 10.9 |
| P23 | +7 | Enalapril/Hydrochlorothiazide (+1/not performed) | 8 | 3 | 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Fernández, C.; Cuesta, R.; Martín-López, S.; Guijarro, J.; López Gómez de las Huertas, A.; Urroz, M.; Miguel-Berenguel, L.; González-Muñoz, M.; Ramírez, E. Immune-Mediated Organ-Specific Reactions to COVID-19 Vaccines: A Retrospective Descriptive Study. Pharmaceuticals 2023, 16, 720. https://doi.org/10.3390/ph16050720
Ruiz-Fernández C, Cuesta R, Martín-López S, Guijarro J, López Gómez de las Huertas A, Urroz M, Miguel-Berenguel L, González-Muñoz M, Ramírez E. Immune-Mediated Organ-Specific Reactions to COVID-19 Vaccines: A Retrospective Descriptive Study. Pharmaceuticals. 2023; 16(5):720. https://doi.org/10.3390/ph16050720
Chicago/Turabian StyleRuiz-Fernández, Carmen, Ricardo Cuesta, Susana Martín-López, Javier Guijarro, Arturo López Gómez de las Huertas, Mikel Urroz, Laura Miguel-Berenguel, Miguel González-Muñoz, and Elena Ramírez. 2023. "Immune-Mediated Organ-Specific Reactions to COVID-19 Vaccines: A Retrospective Descriptive Study" Pharmaceuticals 16, no. 5: 720. https://doi.org/10.3390/ph16050720
APA StyleRuiz-Fernández, C., Cuesta, R., Martín-López, S., Guijarro, J., López Gómez de las Huertas, A., Urroz, M., Miguel-Berenguel, L., González-Muñoz, M., & Ramírez, E. (2023). Immune-Mediated Organ-Specific Reactions to COVID-19 Vaccines: A Retrospective Descriptive Study. Pharmaceuticals, 16(5), 720. https://doi.org/10.3390/ph16050720









