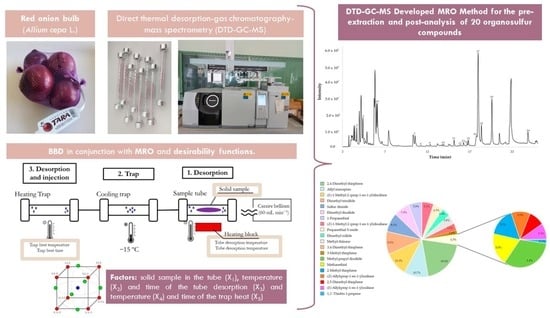
Application of Direct Thermal Desorption–Gas Chromatography–Mass Spectrometry for Determination of Volatile and Semi-Volatile Organosulfur Compounds in Onions: A Novel Analytical Approach
Abstract
1. Introduction
2. Results and Discussion
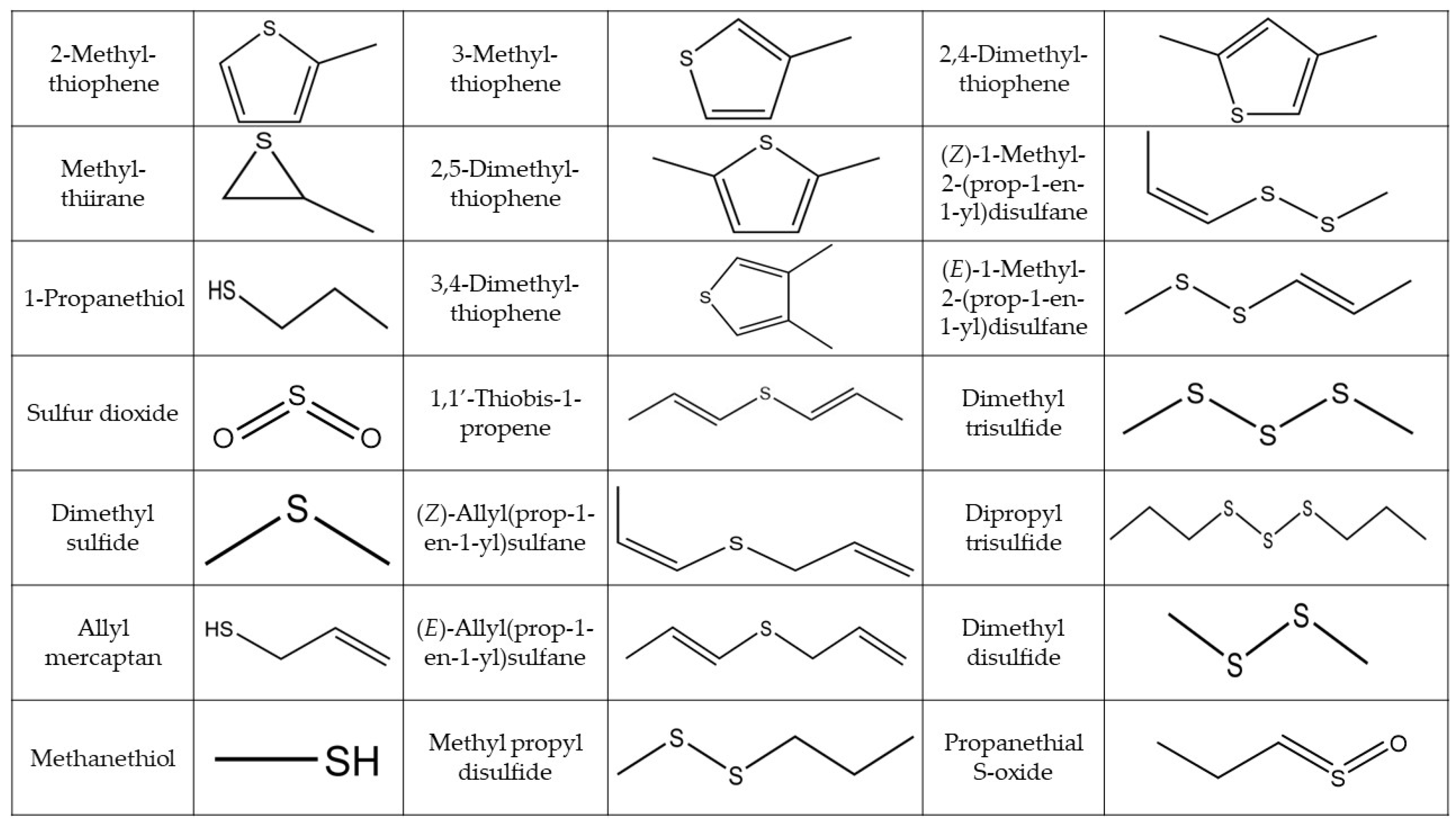
2.1. Qualitative Analyses
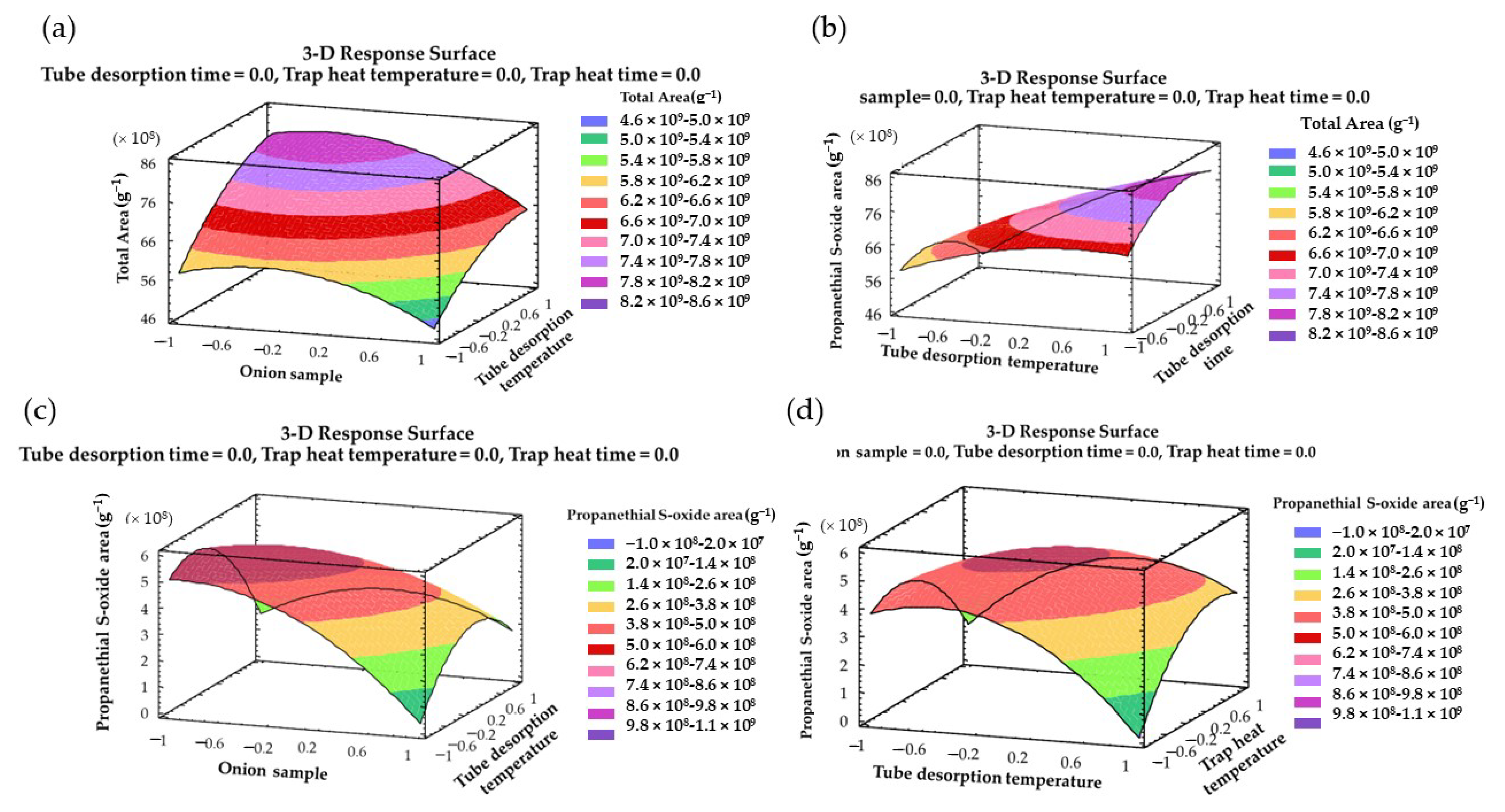
2.2. Individual Box–Behnken Designs and Analysis of Variances
2.3. Multi-Response Optimization
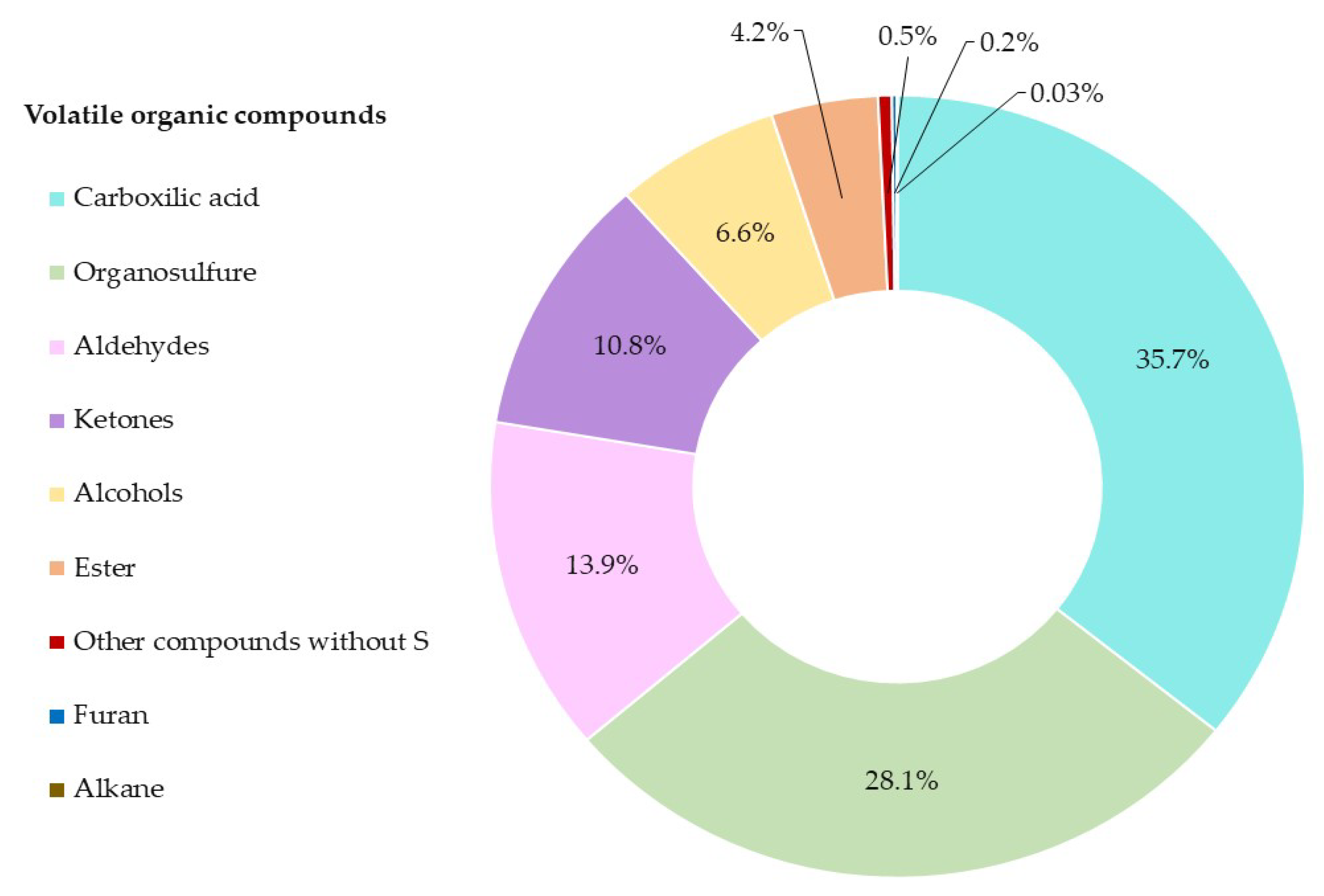
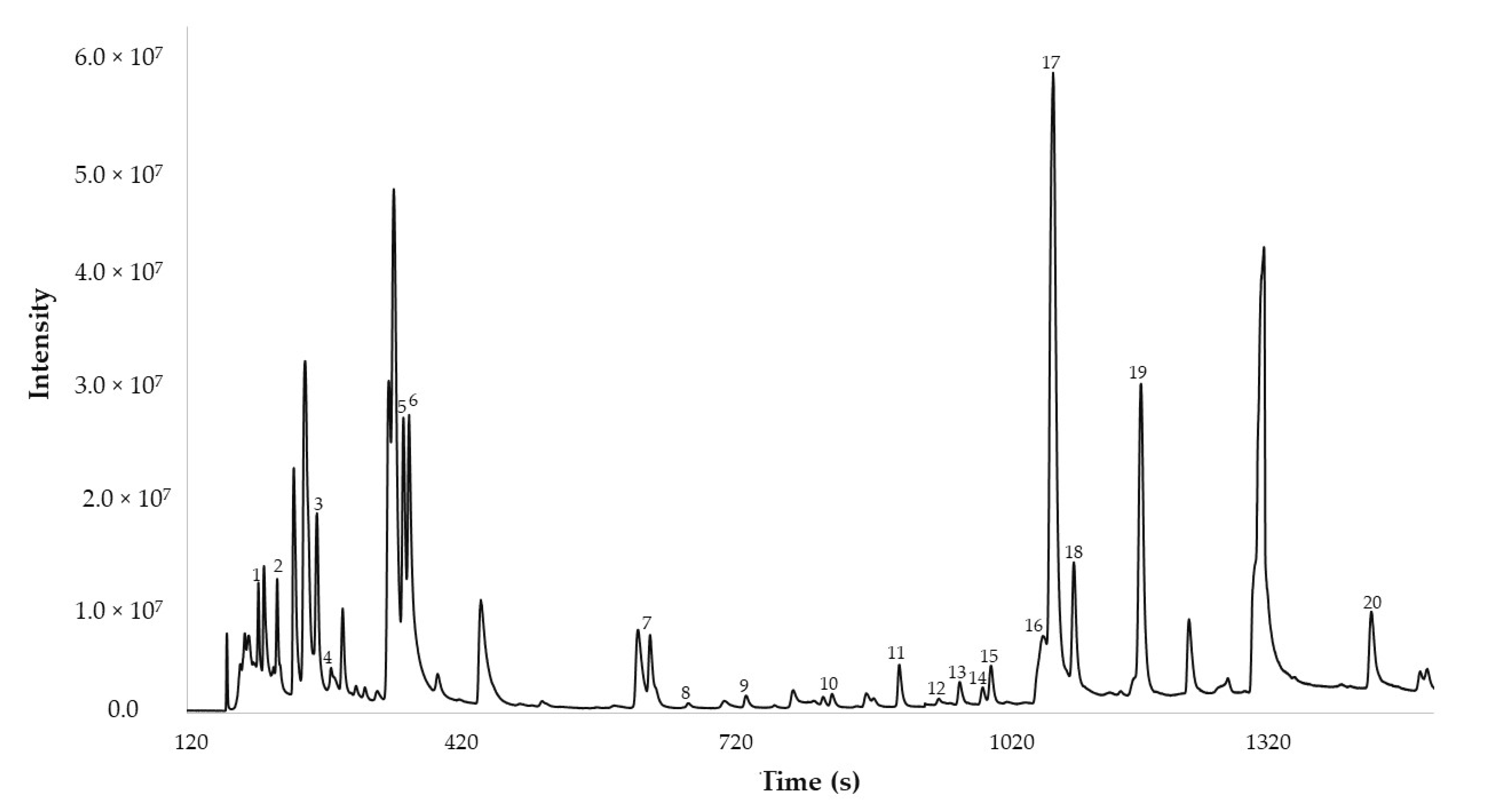
2.4. Distribution of the Organosulfur Compound in Red Onion by MRO DTD-GC-MS Method
2.5. Comparison of DTD Methodology with Another Extraction Techniques
3. Materials and Methods
3.1. Onion Samples
3.2. DTD-GC-MS Procedure and Conditions
3.3. Box–Behnken Design
3.4. Response Surface Methodology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Böttcher, C.; Krähmer, A.; Stürtz, M.; Widder, S.; Schulz, H. Comprehensive Metabolite Profiling of Onion Bulbs (Allium cepa) Using Liquid Chromatography Coupled with Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry. Metabolomics 2017, 13, 35. [Google Scholar] [CrossRef]
- Crowther, T.; Collin, H.A.; Smith, B.; Tomsett, A.B.; O’Connor, D.; Jones, M.G. Assessment of the flavour of fresh uncooked onions by taste-panels and analysis of flavour precursors, pyruvate and sugars. J. Sci. Food Agric. 2005, 85, 112–120. [Google Scholar] [CrossRef]
- Goncharov, N.V.; Belinskaia, D.A.; Ukolov, A.I.; Jenkins, R.O.; Avdonin, P.V. Chapter 41—Organosulfur Compounds as Nutraceuticals. In Nutraceuticals: Efficacy, Safety and Toxicity; Ramesh, C.G., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 555–568. [Google Scholar] [CrossRef]
- Arshad, M.S.; Sohaib, M.; Nadeem, M.; Saeed, F.; Imran, A.; Javed, A.; Amjad, A.; Batool, S.M.; Yildiz, F. Status and trends of nutraceuticals from onion and onion by-products: A critical review. Cogent Food Agric. 2017, 3, 1280254. [Google Scholar] [CrossRef]
- Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and Onions: Their Cancer Prevention Properties. Cancer Prev. Res. 2015, 8, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Abouzed, T.K.; del Mar Contreras, M.; Sadek, K.M.; Shukry, M.; Abdelhady, H.D.; Gouda, W.M.; Abdo, W.; Nasr, N.E.; Mekky, R.H.; Segura-Carretero, A.; et al. Red Onion Scales Ameliorated Streptozotocin-Induced Diabetes and Diabetic Nephropathy in Wistar Rats in Relation to Their Metabolite Fingerprint. Diabetes Res. Clin. Pract. 2018, 140, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Ferioli, F.; D’Antuono, L.F. Evaluation of Phenolics and Cysteine Sulfoxides in Local Onion and Shallot Germplasm from Italy and Ukraine. Genet. Resour. Crop Evol. 2016, 63, 601–614. [Google Scholar] [CrossRef]
- Fredotović, Ž.; Soldo, B.; Šprung, M.; Marijanović, Z.; Jerković, I.; Puizina, J. Comparison of Organosulfur and Amino Acid Composition between Triploid Onion Allium cornutum clementi Ex Visiani, 1842, and Common Onion Allium cepa L., and Evidences for Antiproliferative Activity of Their Extracts. Plants 2020, 9, 98. [Google Scholar] [CrossRef]
- González-de-peredo, A.V.; Vázquez-espinosa, M.; Espada-bellido, E.; Carrera, C.; Ferreiro-gonzález, M.; Barbero, G.F.; Palma, M. Flavonol Composition and Antioxidant Activity of Onions (Allium cepa L.) Based on the Development of New Analytical Ultrasound-assisted Extraction Methods. Antioxidants 2021, 10, 273. [Google Scholar] [CrossRef]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Barbero, G.F.; Palma, M. Extraction of Antioxidant Compounds from Onion Bulb (Allium cepa L.) Using Individual and Simultaneous Microwave-Assisted Extraction Methods. Antioxidants 2022, 11, 846. [Google Scholar] [CrossRef]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Barbero, G.F.; Palma, M. Development of Optimized Ultrasound-Assisted Extraction Methods for the Recovery of Total Phenolic Compounds and Anthocyanins from Onion Bulbs. Antioxidants 2021, 10, 1755. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.B.; Jin, W.; Park, J.; Yoon, W.; Lee, Y.; Kim, S.; Lee, S.; Kim, S.; Lee, O.H.; et al. Comparative Studies of Bioactive Organosulphur Compounds and Antioxidant Activities in Garlic (Allium sativum L.), Elephant Garlic (Allium ampeloprasum L.) and Onion (Allium cepa L.). Nat. Prod. Res. 2017, 32, 1193–1197. [Google Scholar] [CrossRef]
- Cardozo, L.F.M.F.; Pedruzzi, L.M.; Stevinkel, P.; Stockler-Pinto, M.B.; Daleprane, J.B.; Leire, M., Jr.; Mafra, D. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie 2013, 95, 1525–1533. [Google Scholar] [CrossRef]
- Wu, S.; Liao, X.; Zhu, Z.; Huang, R.; Chen, M.; Huang, A.; Zhang, J.; Wu, Q.; Wang, J.; Ding, Y. Antioxidant and anti-inflammation effects of dietary phytochemicals: The Nrf2/NF-κB signalling pathway and upstream factors of Nrf2. Phytochemistry 2022, 204, 113429. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Yarjanli, Z.; Pakniya, F.; Bridam, E.; Los, M.J.; Eshraghi, M.; Klionsky, D.J.; Ghavami, S.; Zarrabi, A. Targeting autophagy, oxidative stress, and ER stress for neurodegenerative disease treatment. J. Control. Release 2022, 345, 147–175. [Google Scholar] [CrossRef]
- Kato, M.; Masamura, N.; Shono, J.; Okamoto, D.; Abe, T.; Imai, S. Production and Characterization of Tearless and Non-Pungent Onion. Sci. Rep. 2016, 6, 23779. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Saito, K. S-Alk(En)Ylcysteine Sulfoxides in the Genus Allium: Proposed Biosynthesis, Chemical Conversion, and Bioactivities. J. Exp. Bot. 2019, 70, 4123–4137. [Google Scholar] [CrossRef]
- Ramirez, D.A.; Locatelli, D.A.; González, R.E.; Cavagnaro, P.F.; Camargo, A.B. Analytical Methods for Bioactive Sulfur Compounds in Allium: An Integrated Review and Future Directions. J. Food Compos. Anal. 2017, 61, 4–19. [Google Scholar] [CrossRef]
- Tocmo, R.; Liang, D.; Lin, Y.; Huang, D. Chemical and Biochemical Mechanisms Underlying the Cardioprotective Roles of Dietary Organopolysulfides. Front. Nutr. 2015, 2, 1. [Google Scholar] [CrossRef]
- Bahram-Parvar, M.; Lim, L.T. Fresh-Cut Onion: A Review on Processing, Health Benefits, and Shelf-Life. Compr. Rev. Food Sci. Food Saf. 2018, 17, 290–308. [Google Scholar] [CrossRef]
- Nandakumar, R.; Eyres, G.T.; Burritt, D.J.; Kebede, B.; Leus, M.; Oey, I. Impact of Pulsed Electric Fields on the Volatile Compounds Produced in Whole Onions (Allium cepa and Allium fistulosum). Foods 2018, 7, 183. [Google Scholar] [CrossRef]
- Cazzola, R.; Cestaro, B. Chapter 9 - Antioxidant Spices and Herbs Used in Diabetes. In Diabetes: Oxidative Stress Diet Antioxidants; Preedy, V.R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 89–97. [Google Scholar] [CrossRef]
- Rektorisova, M.; Hrbek, V.; Jiru, M.; Ovesna, J.; Hajslova, J. Variability in S-Alk(En)Yl-l-Cysteine Sulfoxides in Garlic within a Seven-Month Period Determined by a Liquid Chromatography—Tandem Mass Spectrometry Method. Plant Foods Hum. Nutr. 2020, 75, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, L.; Ieri, F.; Vignolini, P.; Mulinacci, N.; Romani, A. Characterization of Volatile and Flavonoid Composition of Different Cuts of Dried Onion (Allium cepa L.) by HS-SPME-GC-MS, HS-SPME-GC×GC-TOF and HPLC-DAD. Molecules 2020, 25, 408. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-h.; Zhong, Y.; Zhou, Y.; Zhang, Y.; Feng, X.-s. Organosulfur in Food Samples: Recent Updates on Sampling, Pretreatment and Determination Technologies. J. Chromatogr. A 2023, 1689, 463769. [Google Scholar] [CrossRef]
- Gîtin, L.; Dinică, R.; Neagu, C.; Dumitrascu, L. Sulfur Compounds Identification and Quantification from Allium Spp. Fresh Leaves. J. Food Drug Anal. 2014, 22, 425. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, J.; Gonçalves, J.L.; Alves, V.L.; Câmara, J.S. Relationship between Volatile Composition and Bioactive Potential of Vegetables and Fruits of Regular Consumption—An Integrative Approach. Molecules 2021, 26, 3653. [Google Scholar] [CrossRef]
- Colina-Coca, C.; González-Peña, D.; Vega, E.; De Ancos, B.; Sánchez-Moreno, C. Novel Approach for the Determination of Volatile Compounds in Processed Onion by Headspace Gas Chromatography-Mass Spectrometry (HS GC-MS). Talanta 2013, 103, 137–144. [Google Scholar] [CrossRef]
- Flores, R.M.; Mertoglu, E.; Durenne, B.; Blondel, A.; Druart, P.; Fauconnier, M.L.; Duan, Z.; Kjeldsen, P.; Scheutz, C. Optimization of a Thermal Desorption-Gas Chromatography/Mass Spectrometry Method for Characterization of Semi-Volatile Organic Compounds in High Time Resolved PM2.5. Atmos. Pollut. Res. 2020, 11, 619–629. [Google Scholar] [CrossRef]
- Gouda, M.; Ma, M.; Sheng, L.; Xiang, X. SPME-GC-MS & Metal Oxide E-Nose 18 Sensors to Validate the Possible Interactions between Bio-Active Terpenes and Egg Yolk Volatiles. Food Res. Int. 2019, 125, 108611. [Google Scholar] [CrossRef]
- Cheng, Z.; Mannion, D.T.; O’Sullivan, M.G.; Miao, S.; Kerry, J.P.; Kilcawley, K.N. Comparison of Automated Extraction Techniques for Volatile Analysis of Whole Milk Powder. Foods 2021, 10, 2061. [Google Scholar] [CrossRef]
- Shiea, C.; Huang, Y.L.; Liu, D.L.; Chou, C.C.; Chou, J.H.; Chen, P.Y.; Shiea, J.; Huang, M.Z. Rapid Screening of Residual Pesticides on Fruits and Vegetables Using Thermal Desorption Electrospray Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 163–170. [Google Scholar] [CrossRef]
- Humbert, K.; Debret, M.; Morin, C.; Cosme, J.; Portet-Koltalo, F. Direct Thermal Desorption-Gas Chromatography-Tandem Mass Spectrometry versus Microwave Assisted Extraction and GC-MS for the Simultaneous Analysis of Polyaromatic Hydrocarbons (PAHs, PCBs) from Sediments. Talanta 2022, 250, 123735. [Google Scholar] [CrossRef]
- Elorduy, I.; Elcoroaristizabal, S.; Durana, N.; García, J.A.; Alonso, L. Diurnal Variation of Particle-Bound PAHs in an Urban Area of Spain Using TD-GC/MS: Influence of Meteorological Parameters and Emission Sources. Atmos. Environ. 2021, 138, 87–98. [Google Scholar] [CrossRef]
- Vera Candioti, L.; De Zan, M.M.; Cámara, M.S.; Goicoechea, H.C. Experimental Design and Multiple Response Optimization. Using the Desirability Function in Analytical Methods Development. Talanta 2014, 124, 123–138. [Google Scholar] [CrossRef]
- Villière, A.; Le Roy, S.; Fillonneau, C.; Guillet, F.; Falquerho, H.; Boussely, S.; Prost, C. Evaluation of Aroma Profile Differences between Sué, Sautéed, and Pan-Fried Onions Using an Innovative Olfactometric Approach. Flavour 2015, 4, 24. [Google Scholar] [CrossRef]
- Elorduy, I.; Durana, N.; García, J.A.; Gómez, M.C.; Alonso, L. Optimization and Validation of Thermal Desorption Gas Chromatography-Mass Spectrometry for the Determination of Polycyclic Aromatic Hydrocarbons in Ambient Air. J. Anal. Methods Chem. 2018, 2018, 8734013. [Google Scholar] [CrossRef]
- Gao, S.; Zuom, G.; Chen, J.; Zhou, J.; Liu, J. Study on the TD-GC-MS to Detect HD in Exhaust Gas. In Proceedings from the ICERP 2016; De Gruyter: Warsaw, Poland, 2017; pp. 13–22. [Google Scholar] [CrossRef]
- Poojary, M.M.; Putnik, P.; Bursać Kovačević, D.; Barba, F.J.; Lorenzo, J.M.; Dias, D.A.; Shpigelman, A. Stability and Extraction of Bioactive Sulfur Compounds from Allium Genus Processed by Traditional and Innovative Technologies. J. Food Compos. Anal. 2017, 61, 28–39. [Google Scholar] [CrossRef]
- Choi, S.M.; Lee, D.J.; Kim, J.Y.; Lim, S.T. Volatile Composition and Sensory Characteristics of Onion Powders Prepared by Convective Drying. Food Chem. 2017, 231, 386–392. [Google Scholar] [CrossRef]
- Taglienti, A.; Araniti, F.; Piscopo, A.; Tiberini, A. Characterization of Volatile Organic Compounds in ‘Rossa Di Tropea’ Onion by Means of Headspace Solid-Phase Microextraction Gas Chromatography–Mass Spectrometry (Hs/Spme Gc–Ms) and Sensory Analysis. Agronomy 2021, 11, 874. [Google Scholar] [CrossRef]
- Boelends, H.; Brandsma, L. Formation of dialkylthiophenes by thermolysis of di(1-alkenyl) disulfides and alkyl 1-propenyl disulfides. Recueil des Travaux Chimiques des Pays-Bas 1972, 91, 141–145. [Google Scholar] [CrossRef]
- Woolfenden, E. Chapter 10—Thermal Desorption Gas Chromatography. In Handbooks in Separation Science; Poole, C.F., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 267–323. [Google Scholar] [CrossRef]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, E.; Barbero, G.F.; Palma, M.; Carrera, C. Optimization of a Microwave Assisted Extraction Method for Maximum Flavonols and Antioxidant Activity of Onion Extracts. Antioxidants 2022, 11, 2393. [Google Scholar] [CrossRef]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Carrera, C.; Espada-Bellido, E.; Ferreiro-González, E.; Barbero, G.F.; Palma, M. Development of a Rapid UHPLC-PDA Method for the Simultaneous Quantification of Flavonol Contents in Onions (Allium cepa L.). Pharmaceuticals 2021, 14, 310. [Google Scholar] [CrossRef] [PubMed]
- Machová, M.; Bajer, T.; Šilha, D.; Ventura, K.; Bajerová, P. Release of Volatile Compounds from Sliced Onion Analysed by Gas Chromatography Coupled to Mass Spectrometry and Its Antimicrobial Activity. J. Food Nutr. Res. 2019, 58, 393–400. [Google Scholar]
- Maran, J.; Manikandan, S.; Thirugnanasambandham, K.; Vigna Nivetha, C.; Dinesh, R. Box-Behnken Design Based Statistical Modeling for Ultrasound-Assisted Extraction of Corn Silk Polysaccharide. Carbohydr. Polym. 2013, 92, 604–611. [Google Scholar] [CrossRef]
- Eshel, D.; Teper-Bamnolker, P.; Vinokur, Y.; Saad, I.; Zutahy, Y.; Rodov, V. Fast Curing: A Method to Improve Postharvest Quality of Onions in Hot Climate Harvest. Postharvest Biol. Technol. 2014, 88, 34–39. [Google Scholar] [CrossRef]
- Marinković, V. A Novel Desirability Function for Multi-Response Optimization and Its Application in Chemical Engineering. Chem. Ind. Chem. Eng. Q. 2020, 26, 309–319. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cooj, C.M. Response Surface Methodology: Process and Products Optimization Using Designed Experiments; Wiley: Hoboken, NJ, USA, 2015; pp. 1–821. [Google Scholar]


| Name | Formula | N° CAS | Similarity (%) |
|---|---|---|---|
| Carboxylic acid | |||
| Acetic acid | C2H4O2 | 64-19-7 | 97 |
| Formic acid | CH2O2 | 64-18-6 | 98 |
| Propanoic acid | C3H6O2 | 79-9-4 | 83 |
| Ester | |||
| Oxiranylmethyl ester 2-propenoic acid | C6H8O3 | 106-90-1 | 90 |
| Methyl ester hexadecanoic acid | C17H34O2 | 112-39-0 | 90 |
| Ethyl ester 2-methyl-3-oxo-butanoic acid | C7H12O3 | 609-14-3 | 83 |
| 2-Hydroxy-gamma-butyrolactone | C4H6O3 | 19444-84-9 | 87 |
| 3,5-Dihydroxy-2-methyl-4H-pyran-4-one | C6H6O4 | 1073-96-7 | 92 |
| Alkane | |||
| 1-(1H-pyrrol-2-yl)-ethanone | C6H7NO | 1072-83-9 | 94 |
| Alcohol | |||
| Ethanol | C2H6O | 64-17-5 | 98 |
| 2,3-Butanediol | C4H10O2 | 513-85-9 | 92 |
| 3-Furanmethanol | C5H6O2 | 4412-91-3 | 94 |
| 2-Furanmethanol | C5H6O | 98-0-0 | 90 |
| 5-Methyl-2-furan methanol | C6H8O2 | 3857-25-8 | 95 |
| 3-Butene-1,2-diol | C4H8O2 | 497-6-3 | 88 |
| Aldehydes | |||
| Acetaldehyde | C2H4O | 75-7-0 | 98 |
| Propanal | C3H6O | 123-38-6 | 96 |
| 2-Methyl-propanal | C4H8O | 78-84-2 | 98 |
| 2-Methyl-butanal | C5H10O | 96-17-3 | 96 |
| 2-Methyl-2-butenal | C5H10O | 590-86-3 | 93 |
| 2-Methyl-2-pentenal | C6H10O | 14250-96-5 | 96 |
| Furfural | C5H4O2 | 98-1-1 | 98 |
| 2,2-Diethylbutyraldehyde | C8H16O | 26254-89-7 | 85 |
| Ketones | |||
| 2-Butanone | C4H8O | 78-93-3 | 86 |
| 2,3-Butanedione | C4H6O2 | 431-03-8 | 96 |
| Acetoin | C4H8O2 | 513-86-0 | 90 |
| 1-Hydroxy-2-propanone | C3H6O | 116-9-6 | 98 |
| 4,5-Dimethyl-1,3-dioxol-2-one | C5H6O3 | 37830-90-3 | 86 |
| Furans | |||
| 3-Methyl-furan | C5H6O | 930-27-8 | 95 |
| 2,4-Dimethylfuran | C6H8O | 3710-43-8 | 95 |
| Other compounds without S | |||
| Carbon dioxide | CO2 | 124-38-9 | 98 |
| Organosulfur compounds | |||
| Thiols | |||
| Methanethiol | CH4S | 74-93-1 | 98 |
| 1-Propanethiol | C3H8S | 107-3-9 | 95 |
| Allyl mercaptan | C3H6S | 870-23-5 | 94 |
| Monosulfide | |||
| Dimethyl sulfide | C2H6S | 75-18-3 | 98 |
| 1,1’-Thiobis-1-propene | C6H10S | 33922-80-4 | 89 |
| (Z)-Allyl(prop-1-en-1-yl)sulfane * | C6H10S | 104324-69-8 | 87 |
| (E)-Allyl(prop-1-en-1-yl)sulfane * | C6H10S | 104324-36-9 | 85 |
| Disulfides | |||
| Dimethyl disulfide | C2H6S2 | 624-92-0 | 95 |
| Methyl propyl disulfide | C4H10S2 | 2179-60-4 | 93 |
| (Z)-1-Methyl-2-(prop-1-en-1-yl)disulfane * | C4H8S2 | 23838-19-9 | 96 |
| (E)-1-Methyl-2-(prop-1-en-1-yl)disulfane * | C4H8S2 | 23838-18-8 | 97 |
| Trisulfide | |||
| Dimethyl trisulfide | C2H6S3 | 3658-80-8 | 97 |
| Episulfide | |||
| Methyl-thiirane | C3H6S | 1072-43-1 | 94 |
| Thiophene | |||
| 2-Methyl-thiophene | C5H6S | 554-14-3 | 93 |
| 3-Methyl-thiophene | C5H6S | 616-44-4 | 96 |
| 2,5-Dimethyl-thiophene | C6H8S | 638-02-8 | 91 |
| 3,4-Dimethyl-thiophene | C6H8S | 638-0-6 | 94 |
| 2,4-Dimethyl-thiophene | C6H8S | 638-00-6 | 95 |
| Sulfine | |||
| Propanethial S-oxide | C3H6OS | 32157-29-2 | 96 |
| Other S-Compounds | |||
| Sulfur dioxide | SO2 | 7446-09-5 | 96 |
| Source | Source Code | Coefficients | Sum of Squares | DF | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|---|
| A: Onion sample | X1 | −5.97 × 108 | 5.70 × 1018 | 1 | 5.70 × 1018 | 91.32 | <0.001 |
| B: Tube desorption temperature | X2 | 9.83 × 108 | 1.55 × 1019 | 1 | 1.55 × 1019 | 247.71 | <0.001 |
| C: Tube desorption time | X3 | 7.29 × 107 | 8.50 × 1016 | 1 | 8.50 × 1016 | 1.36 | 0.296 |
| D: Trap heat temperature | X4 | 9.92 × 107 | 1.57 × 1017 | 1 | 1.57 × 1017 | 2.52 | 0.173 |
| E: Trap heat time | X5 | −4.91 × 106 | 3.86 × 1014 | 1 | 3.86 × 1014 | 0.01 | 0.940 |
| AA | X12 | −6.81 × 108 | 4.05 × 1018 | 1 | 4.05 × 1018 | 64.89 | <0.001 |
| AB | X1X2 | −1.30 × 108 | 6.77 × 1016 | 1 | 6.77 × 1016 | 1.08 | 0.345 |
| AC | X1X3 | 1.88 × 108 | 1.42 × 1017 | 1 | 1.42 × 1017 | 2.27 | 0.192 |
| AD | X1 X4 | −1.37 × 107 | 7.52 × 1014 | 1 | 7.52 × 1014 | 0.01 | 0.917 |
| AE | X1X5 | −3.59 × 107 | 5.15 × 1015 | 1 | 5.15 × 1015 | 0.08 | 0.786 |
| BB | X22 | −4.13 × 108 | 1.49 × 1018 | 1 | 1.49 × 1018 | 23.8 | 0.00460 |
| BC | X2X3 | 5.12 × 108 | 1.05 × 1018 | 1 | 1.05 × 1018 | 16.78 | 0.00940 |
| BD | X2X4 | −7.35 × 107 | 2.16 × 1016 | 1 | 2.16 × 1016 | 0.35 | 0.582 |
| BE | X2X5 | −1.65 × 108 | 1.08 × 1017 | 1 | 1.08 × 1017 | 1.74 | 0.245 |
| CC | X32 | −6.09 × 108 | 3.24 × 1018 | 1 | 3.24 × 1018 | 51.89 | <0.001 |
| CD | X3X4 | −1.12 × 108 | 4.98 × 1016 | 1 | 4.98 × 1016 | 0.8 | 0.413 |
| CE | X3X5 | 3.62 × 108 | 5.25 × 1017 | 1 | 5.25 × 1017 | 8.41 | 0.0338 |
| DD | X42 | −5.44 × 108 | 2.58 × 1018 | 1 | 2.58 × 1018 | 41.4 | 0.00130 |
| DE | X4X5 | 2.90 × 108 | 3.37 × 1017 | 1 | 3.37 × 1017 | 5.4 | 0.0678 |
| EE | X52 | −6.77 × 108 | 4.00 × 1018 | 1 | 4.00 × 1018 | 64.12 | <0.001 |
| Lack-of-fit | 5.31 × 1018 | 20 | 2.65 × 1017 | 4.25 | 0.0577 | ||
| Residual | 5.62 × 1018 | 25 | 2.25 × 1017 | ||||
| Pure Error | 3.12 × 1017 | 5 | 6.24 × 1016 | ||||
| Cor Total | 3.71 × 1019 | 45 | |||||
| Model | 7.36 × 1009 |
| Source | Source Code | Coefficients | Sum of Squares | DF | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|---|
| A: Onion sample | X1 | −1.16 × 108 | 2.15 × 1017 | 1 | 2.15 × 1017 | 68.8 | 0.0004 |
| B: Tube desorption temperature | X2 | −4.60 × 107 | 3.39 × 1016 | 1 | 3.39 × 1016 | 10.83 | 0.0217 |
| C: Tube desorption time | X3 | 4.71 × 107 | 3.55 × 1016 | 1 | 3.55 × 1016 | 11.33 | 0.0200 |
| D: Trap heat temperature | X4 | 1.74 × 107 | 4.86 × 1015 | 1 | 4.86 × 1015 | 1.55 | 0.268 |
| E: Trap heat time | X5 | −3.29 × 107 | 1.73 × 1016 | 1 | 1.73 × 1016 | 5.53 | 0.0655 |
| AA | X12 | −1.27 × 108 | 1.40 × 1017 | 1 | 1.40 × 1017 | 44.6 | 0.00110 |
| AB | X1X2 | 1.22 × 108 | 5.92 × 1016 | 1 | 5.92 × 1016 | 18.89 | 0.00740 |
| AC | X1X3 | 3.05 × 107 | 3.72 × 1015 | 1 | 3.72 × 1015 | 1.19 | 0.325 |
| AD | X1X4 | −2.01 × 107 | 1.61 × 1015 | 1 | 1.61 × 1015 | 0.52 | 0.505 |
| AE | X1X5 | −4.36 × 107 | 7.62 × 1015 | 1 | 7.62 × 1015 | 2.43 | 0.180 |
| BB | X22 | −1.78 × 108 | 2.77 × 1017 | 1 | 2.77 × 1017 | 88.41 | <0.001 |
| BC | X2X3 | 4.26 × 107 | 7.27 × 1015 | 1 | 7.27 × 1015 | 2.32 | 0.188 |
| BD | X2X4 | 1.38 × 108 | 7.61 × 1016 | 1 | 7.61 × 1016 | 24.28 | 0.00440 |
| BE | X2X5 | −5.43 × 107 | 1.18 × 1016 | 1 | 1.18 × 1016 | 3.76 | 0.110 |
| CC | X32 | −8.15 × 107 | 5.80 × 1016 | 1 | 5.80 × 1016 | 18.53 | 0.00770 |
| CD | X3X4 | −7.83 × 107 | 2.45 × 1016 | 1 | 2.45 × 1016 | 7.83 | 0.0381 |
| CE | X3X5 | 9.73 × 106 | 3.79 × 1014 | 1 | 3.79 × 1014 | 0.12 | 0.742 |
| DD | X42 | −1.38 × 108 | 1.66 × 1017 | 1 | 1.66 × 1017 | 52.95 | <0.001 |
| DE | X4X5 | 1.06 × 107 | 4.47 × 1014 | 1 | 4.47 × 1014 | 0.14 | 0.721 |
| EE | X52 | −3.83 × 107 | 1.28 × 1016 | 1 | 1.28 × 1016 | 4.09 | 0.0992 |
| Lack-of-fit | 2.64 × 1017 | 20 | 1.32 × 1016 | 4.21 | 0.0586 | ||
| Residual | 2.80 × 1017 | 25 | 1.12 × 1016 | ||||
| Pure Error | 1.57 × 1016 | 5 | 3.13 × 1015 | ||||
| Cor Total | 1.18 × 1018 | 45 | |||||
| Model | 5.26 × 108 |
| Factors | Individual Optimization | MRO | |
|---|---|---|---|
| Total Area | Propanethial S-Oxide Area | ||
| X1: Onion sample (mg) | 46 | 47 | 46 |
| X2: Tube desorption temperature (°C) | 211 | 189 | 205 |
| X3: Tube desorption time (s) | 16 | 9 | 16 |
| X4: Trap heat temperature (°C) | 265 | 252 | 267 |
| X5: Trap heat time (s) | 3 | 2 | 3 |
| Code | Compound | Individual Relative Area (g−1) | Percentage Composition (%) |
|---|---|---|---|
| 1 | Methanethiol | 79,946,258 ± 5,037,254 | 0.8 ± 0.1 |
| 2 | Dimethyl sulfide | 368,819,241 ± 26,379,719 | 3.9 ± 0.3 |
| 3 | 1-Propanethiol | 520,814,238 ± 25,833,947 | 5.9 ± 1.2 |
| 4 | Sulfur dioxide | 741,589,894 ± 68,150,056 | 8.1 ± 0.3 |
| 5 | Methyl-thiirane | 356,083,962 ± 6,570,787 | 3.8 ± 0.2 |
| 6 | Allyl mercaptan | 925,374,280 ± 53,675,232 | 10.7 ± 0.6 |
| 7 | Dimethyl disulfide | 734,818,197 ± 68,447,885 | 7.0 ± 0.4 |
| 8 | 2-Methyl-thiophene | 79,399,050 ± 7,255,625 | 0.7 ± 0.04 |
| 9 | 3-Methyl-thiophene | 161,784,317 ± 15,999,654 | 1.6 ± 0.1 |
| 10 | 2,5-Dimethyl-thiophene | 46,771,965 ± 4,109,263 | 0.4 ± 0.03 |
| 11 | 3,4-Dimethyl-thiophene | 266,951,634 ± 26,402,475 | 3.2 ± 0.3 |
| 12 | 1,1’-Thiobis-1-propene | 12,137,629 ± 982,646 | 0.1 ± 0.01 |
| 13 | (Z)-Allyl(prop-1-en-1-yl)sulfane | 47,080,601 ± 3,942,928 | 0.5 ± 0.03 |
| 14 | (E)-Allyl(prop-1-en-1-yl)sulfane | 34,336,629 ± 1,522,835 | 0.4 ± 0.03 |
| 15 | Methyl propyl disulfide | 127,532,708 ± 1,832,161 | 1.4 ± 0.1 |
| 16 | Propanethial S-oxide | 385,303,062 ± 14,922,610 | 4.5 ± 0.3 |
| 17 | 2,4-Dimethyl-thiophene | 1,728,974,335 ± 94,208,493 | 19.4 ± 0.1 |
| 18 | (Z)-1-Methyl-2-(prop-1-en-1-yl)disulfane | 457,771,521 ± 11,134,133 | 5.2 ± 0.5 |
| 19 | (E)-1-Methyl-2-(prop-1-en-1-yl)disulfane | 983,648,059 ± 82,014,566 | 10.3 ± 0.4 |
| 20 | Dimethyl trisulfide | 839,379,417 ± 73,906,935 | 9.6 ± 1.0 |
| Total | 9,389,028,511 ± 908,282,182 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
V. González-de-Peredo, A.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; F. Barbero, G. Application of Direct Thermal Desorption–Gas Chromatography–Mass Spectrometry for Determination of Volatile and Semi-Volatile Organosulfur Compounds in Onions: A Novel Analytical Approach. Pharmaceuticals 2023, 16, 715. https://doi.org/10.3390/ph16050715
V. González-de-Peredo A, Vázquez-Espinosa M, Espada-Bellido E, Ferreiro-González M, Carrera C, Palma M, F. Barbero G. Application of Direct Thermal Desorption–Gas Chromatography–Mass Spectrometry for Determination of Volatile and Semi-Volatile Organosulfur Compounds in Onions: A Novel Analytical Approach. Pharmaceuticals. 2023; 16(5):715. https://doi.org/10.3390/ph16050715
Chicago/Turabian StyleV. González-de-Peredo, Ana, Mercedes Vázquez-Espinosa, Estrella Espada-Bellido, Marta Ferreiro-González, Ceferino Carrera, Miguel Palma, and Gerardo F. Barbero. 2023. "Application of Direct Thermal Desorption–Gas Chromatography–Mass Spectrometry for Determination of Volatile and Semi-Volatile Organosulfur Compounds in Onions: A Novel Analytical Approach" Pharmaceuticals 16, no. 5: 715. https://doi.org/10.3390/ph16050715
APA StyleV. González-de-Peredo, A., Vázquez-Espinosa, M., Espada-Bellido, E., Ferreiro-González, M., Carrera, C., Palma, M., & F. Barbero, G. (2023). Application of Direct Thermal Desorption–Gas Chromatography–Mass Spectrometry for Determination of Volatile and Semi-Volatile Organosulfur Compounds in Onions: A Novel Analytical Approach. Pharmaceuticals, 16(5), 715. https://doi.org/10.3390/ph16050715