Characterization of Non-Specific Uptake and Retention Mechanisms of [177Lu]Lu-PSMA-617 in the Salivary Glands
Abstract
1. Introduction
2. Results
2.1. Radiolabeling of PSMA-617 with Lutetium-177
2.2. Characterization of Pig Salivary Gland Sections by Immunohistochemistry
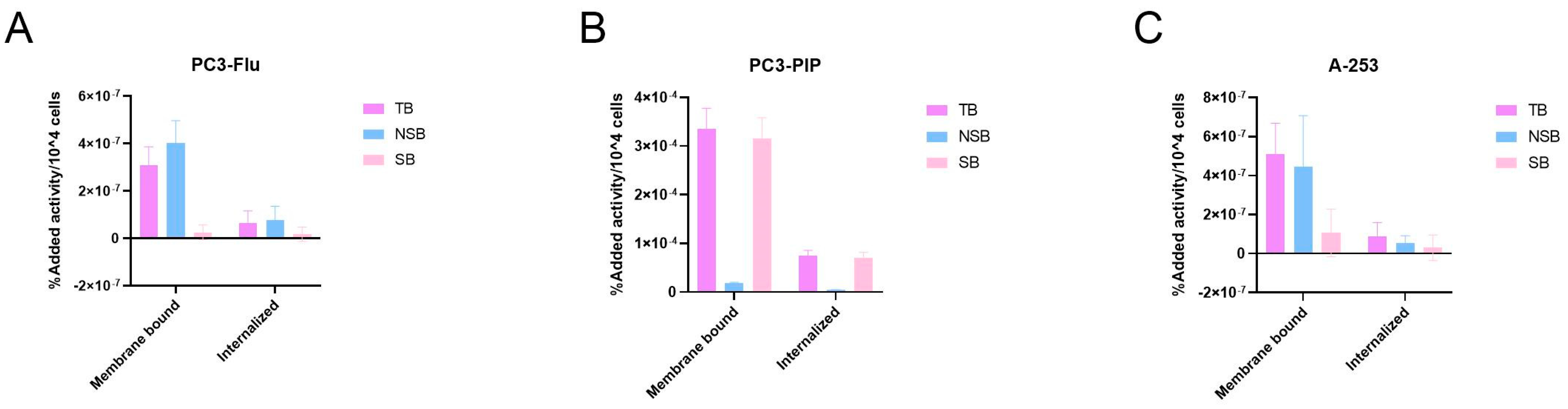
2.3. Characterization of [177Lu]Lu-PSMA-617 Binding and Internalization in Salivary Gland Cells
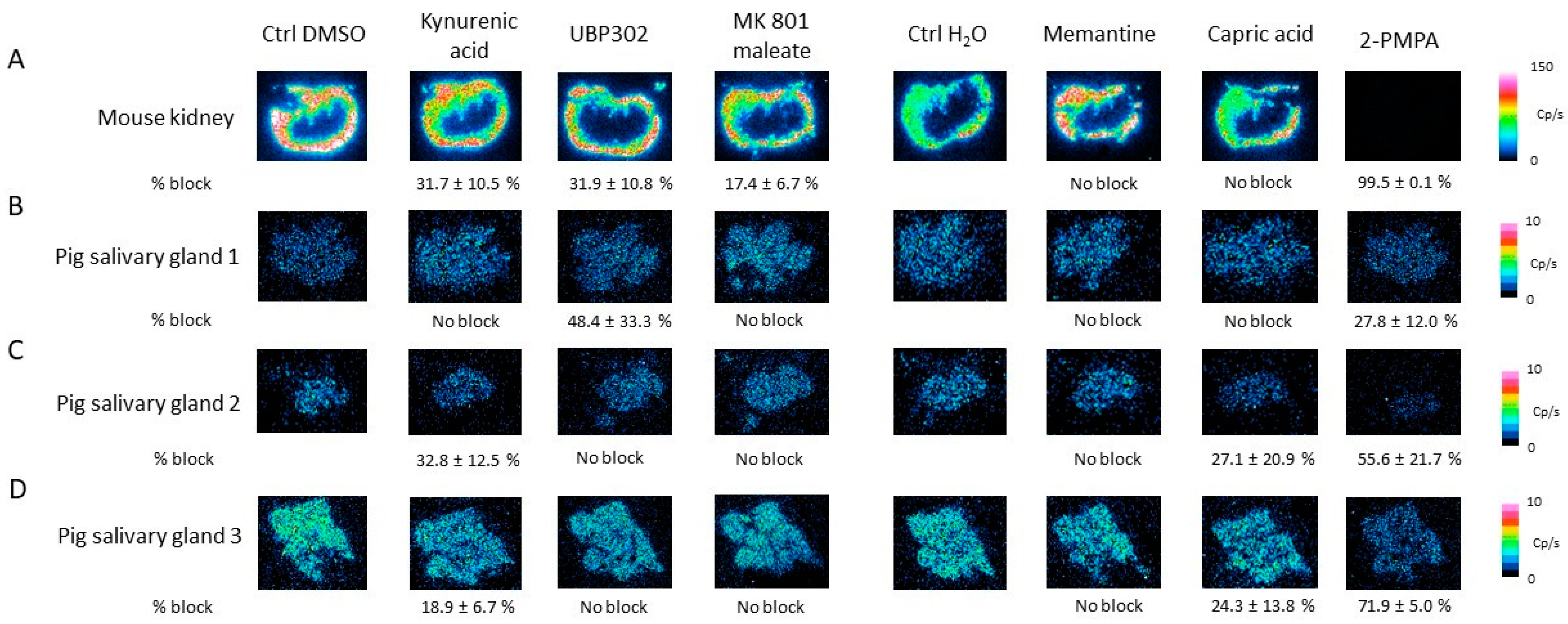
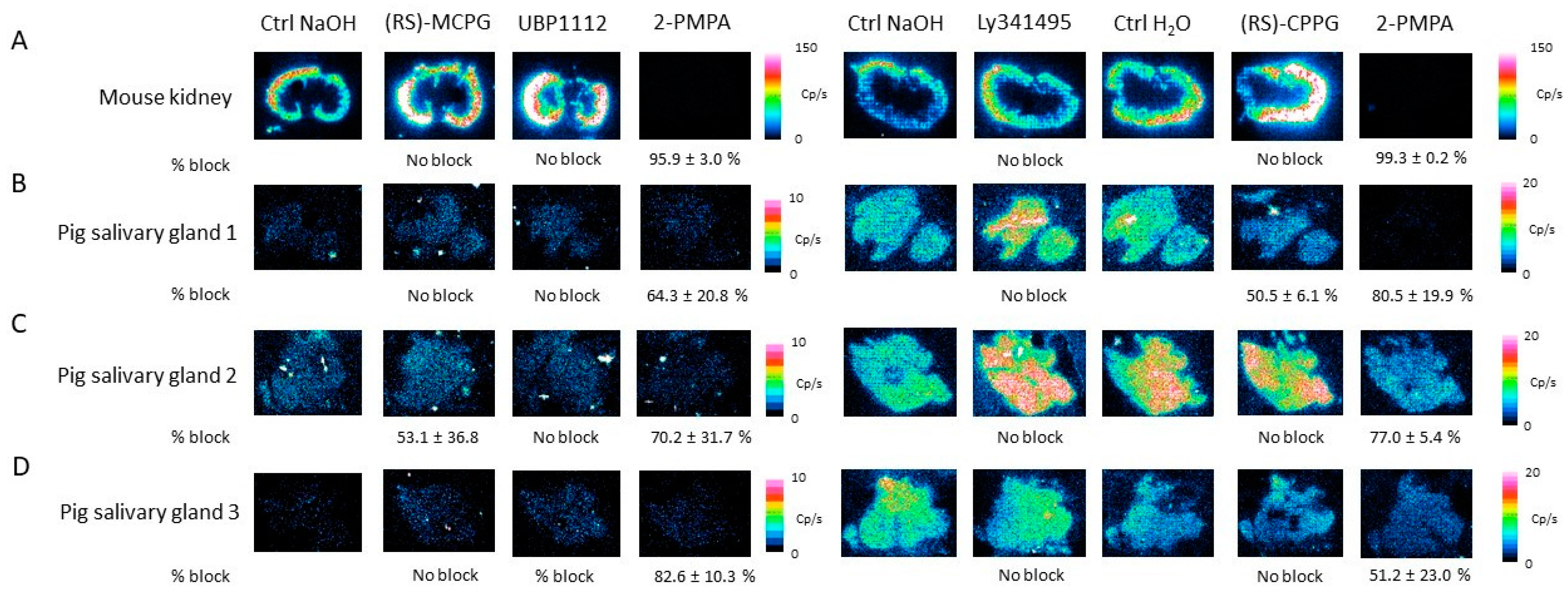
2.4. Characterization of [177Lu]Lu-PSMA-617 Binding on Salivary Gland Tissue
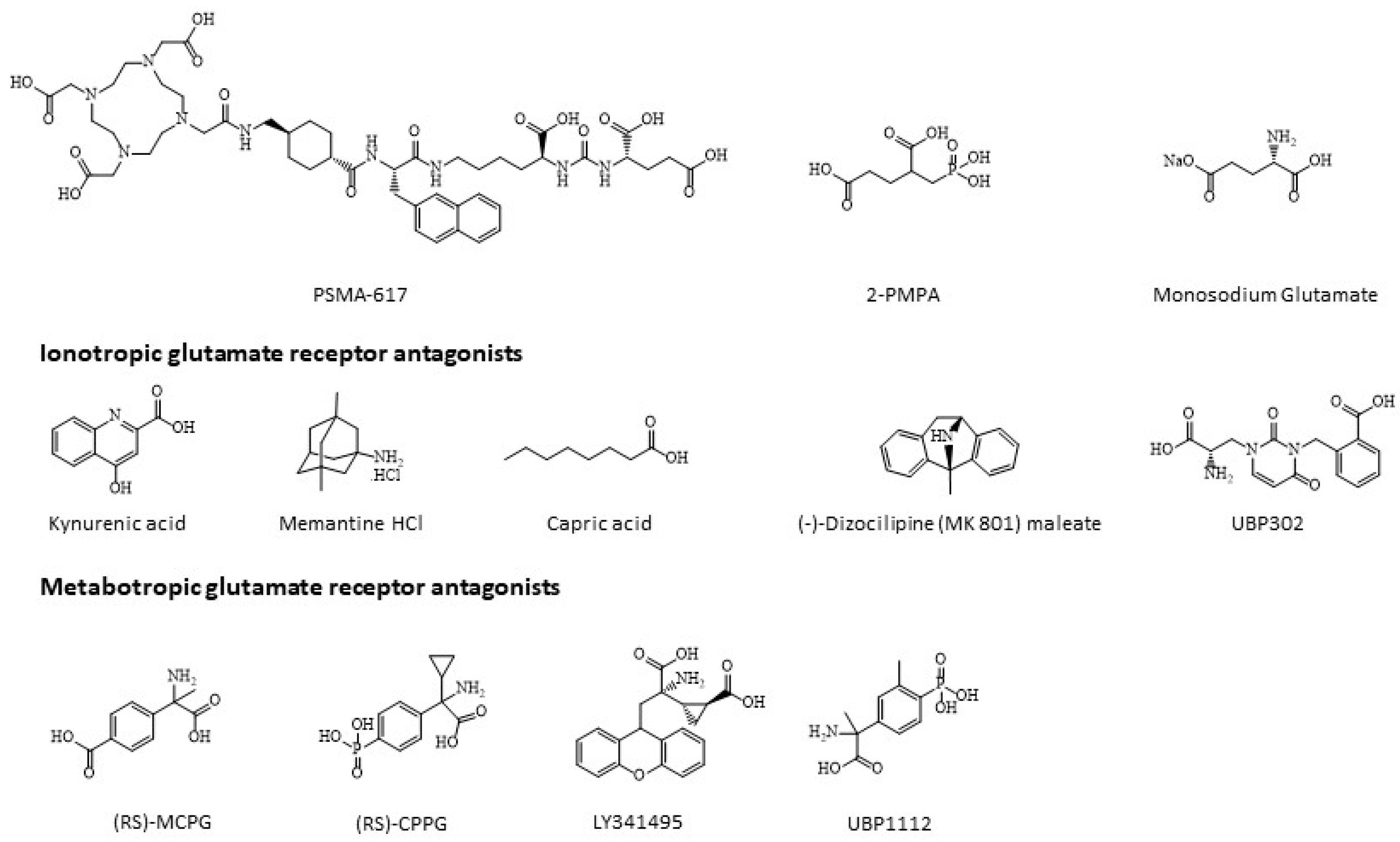
2.5. Cytotoxicity of Monosodium Glutamate, Ionotropic and Metabotropic Glutamate Receptor Antagonists
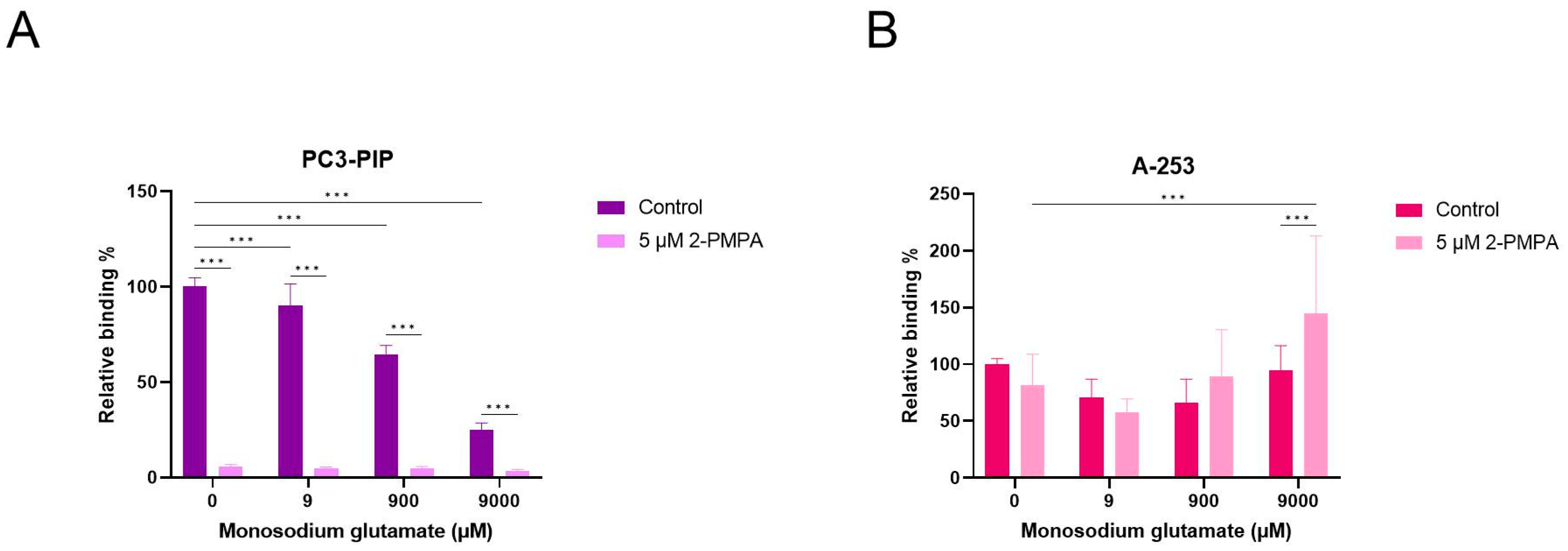
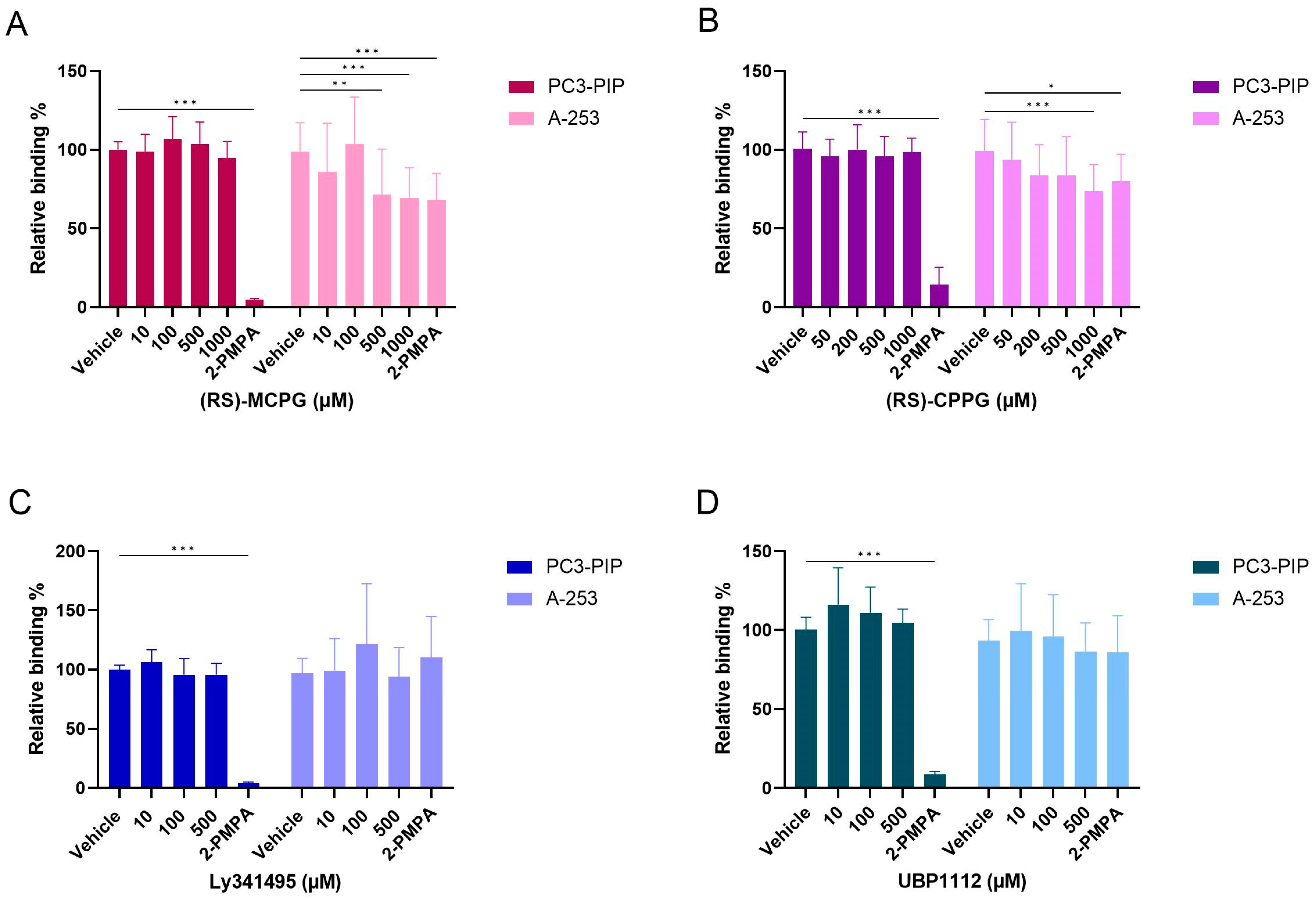
2.6. Competition Study with Monosodium Glutamate
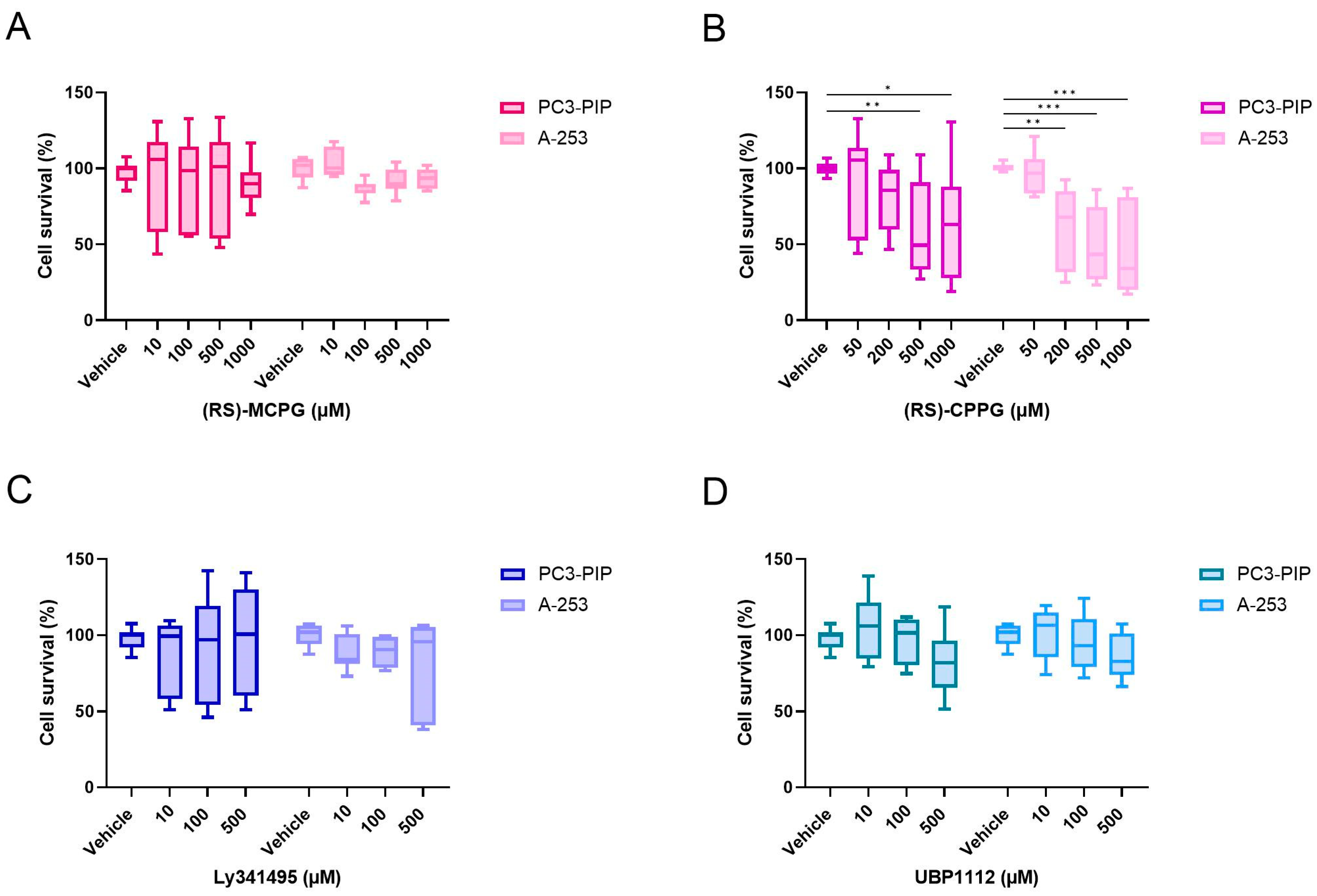
2.7. Competition Study with Ionotropic Glutamate Receptor Antagonists
2.8. Competition Study with Metabotropic Glutamate Receptor Antagonists
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. In Vitro Tissue Models
4.4. Radiolabeling of PSMA-617 with Lutetium-177
4.5. Cell Binding and Internalization Assays
4.6. Tissue Saturation Binding Assay
4.7. Cytotoxicity Assay of Monosodium Glutamate, Ionotropic and Metabotropic Glutamate Receptor Antagonists
4.8. Blocking Studies Using Monosodium Glutamate, Ionotropic and Metabotropic Glutamate Receptor Antagonists on Cells
4.9. Blocking Studies Using Monosodium Glutamate, Ionotropic and Metabotropic Glutamate Receptor Antagonists on Tissue
4.10. Immunohistochemistry
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries from 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef]
- Czerwińska, M.; Bilewicz, A.; Kruszewski, M.; Wegierek-Ciuk, A.; Lankoff, A. Targeted Radionuclide Therapy of Prostate Cancer—From Basic Research to Clinical Perspectives. Molecules 2020, 25, 1743. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Minner, S.; Wittmer, C.; Graefen, M.; Salomon, G.; Steuber, T.; Haese, A.; Huland, H.; Bokemeyer, C.; Yekebas, E.; Dierlamm, J.; et al. High level PSMA expression is associated with early psa recurrence in surgically treated prostate cancer. Prostate 2011, 71, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Kuratsukuri, K.; Landas, S.; Imaida, K.; Rovito, P.M., Jr.; Wang, C.Y.; Haas, G.P. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J. Surg. 2006, 30, 628–636. [Google Scholar] [CrossRef]
- Heynickx, N.; Herrmann, K.; Vermeulen, K.; Baatout, S.; Aerts, A. The salivary glands as a dose limiting organ of PSMA- targeted radionuclide therapy: A review of the lessons learnt so far. Nucl. Med. Biol. 2021, 98–99, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Rupp, N.J.; Umbricht, C.A.; Pizzuto, D.A.; Lenggenhager, D.; Töpfer, A.; Müller, J.; Muehlematter, U.J.; Ferraro, D.A.; Messerli, M.; Morand, G.B.; et al. First Clinicopathologic Evidence of a Non–PSMA-Related Uptake Mechanism for 68Ga-PSMA-11 in Salivary Glands. J. Nucl. Med. 2019, 60, 1270. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941. [Google Scholar] [CrossRef]
- Navrátil, M.; Ptáček, J.; Šácha, P.; Starková, J.; Lubkowski, J.; Bařinka, C.; Konvalinka, J. Structural and biochemical characterization of the folyl-poly-γ-l-glutamate hydrolyzing activity of human glutamate carboxypeptidase II. FEBS J. 2014, 281, 3228–3242. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Byun, Y.; Barinka, C.; Pullambhatla, M.; Bhang, H.E.; Fox, J.J.; Lubkowski, J.; Mease, R.C.; Pomper, M.G. Bioisosterism of urea-based GCPII inhibitors: Synthesis and structure-activity relationship studies. Bioorg. Med. Chem. Lett. 2010, 20, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, E.; Lau, J.; Kuo, H.T.; Zhang, Z.; Merkens, H.; Hundal-Jabal, N.; Colpo, N.; Lin, K.S.; Bénard, F. Monosodium Glutamate Reduces (68)Ga-PSMA-11 Uptake in Salivary Glands and Kidneys in a Preclinical Prostate Cancer Model. J. Nucl. Med. 2018, 59, 1865–1868. [Google Scholar] [CrossRef] [PubMed]
- Harsini, S.; Saprunoff, H.; Alden, T.; Mohammadi, B.; Wilson, D.; Bénard, F. The Effects of Monosodium Glutamate on PSMA Radiotracer Uptake in Men with Recurrent Prostate Cancer: A Prospective, Randomized, Double-Blind, Placebo-Controlled Intraindividual Imaging Study. J. Nucl. Med. 2021, 62, 81. [Google Scholar] [CrossRef] [PubMed]
- Tönnesmann, R.; Meyer, P.T.; Eder, M.; Baranski, A.C. [(177)Lu]Lu-PSMA-617 Salivary Gland Uptake Characterized by Quantitative In vitro Autoradiography. Pharmaceuticals 2019, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Hetzheim, H.; Kratochwil, C.; Benesova, M.; Eder, M.; Neels, O.C.; Eisenhut, M.; Kübler, W.; Holland-Letz, T.; Giesel, F.L.; et al. The Theranostic PSMA Ligand PSMA-617 in the Diagnosis of Prostate Cancer by PET/CT: Biodistribution in Humans, Radiation Dosimetry, and First Evaluation of Tumor Lesions. J. Nucl. Med. 2015, 56, 1697. [Google Scholar] [CrossRef] [PubMed]
- Klein Nulent, T.J.W.; Valstar, M.H.; de Keizer, B.; Willems, S.M.; Smit, L.A.; Al-Mamgani, A.; Smeele, L.E.; van Es, R.J.J.; de Bree, R.; Vogel, W.V. Physiologic distribution of PSMA-ligand in salivary glands and seromucous glands of the head and neck on PET/CT. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, H.; Yang, G.; Wang, X.; Sun, Y.; Song, T.; Zhang, C.; Wang, S. Histological and Ultrastructural Characterization of Developing Miniature Pig Salivary Glands. Anat. Rec. 2010, 293, 1227–1239. [Google Scholar] [CrossRef]
- Ghannam, M.G.; Singh, P. Anatomy, Head and Neck, Salivary Glands; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Felber, V.B.; Valentin, M.A.; Wester, H.-J. Design of PSMA ligands with modifications at the inhibitor part: An approach to reduce the salivary gland uptake of radiolabeled PSMA inhibitors? EJNMMI Radiopharm. Chem. 2021, 6, 10. [Google Scholar] [CrossRef]
- Jeitner, T.M.; Babich, J.W.; Kelly, J.M. Advances in PSMA theranostics. Transl. Oncol. 2022, 22, 101450. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.A.; Coleman, R.E.; Goldsmith, S.J.; Vallabhajosula, S.; Petry, N.A.; Cho, S.; Armor, T.; Stubbs, J.B.; Maresca, K.P.; Stabin, M.G.; et al. First-in-Man Evaluation of 2 High-Affinity PSMA-Avid Small Molecules for Imaging Prostate Cancer. J. Nucl. Med. 2013, 54, 380. [Google Scholar] [CrossRef]
- Armstrong, W.R.; Gafita, A.; Zhu, S.; Thin, P.; Nguyen, K.; Alano, R.; Lira, S.; Booker, K.; Gardner, L.; Grogan, T.; et al. The Impact of Monosodium Glutamate on (68)Ga-PSMA-11 Biodistribution in Men with Prostate Cancer: A Prospective Randomized, Controlled Imaging Study. J. Nucl. Med. 2021, 62, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liu, X.; Sun, Q.; Fan, Z.; Xia, D.; Ding, G.; Ong, H.L.; Adams, D.; Gahl, W.A.; Zheng, C.; et al. Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 13434–13439. [Google Scholar] [CrossRef]
- Holland, J.P.; Divilov, V.; Bander, N.H.; Smith-Jones, P.M.; Larson, S.M.; Lewis, J.S. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J. Nucl. Med. 2010, 51, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Bakht, M.K.; Hayward, J.J.; Shahbazi-Raz, F.; Skubal, M.; Tamura, R.; Stringer, K.F.; Meister, D.; Venkadakrishnan, V.B.; Xue, H.; Pillon, A.; et al. Identification of alternative protein targets of glutamate-ureido-lysine associated with PSMA tracer uptake in prostate cancer cells. Proc. Natl. Acad. Sci. USA 2022, 119, e2025710119. [Google Scholar] [CrossRef] [PubMed]
- Lucaroni, L.; Georgiev, T.; Prodi, E.; Puglioli, S.; Pellegrino, C.; Favalli, N.; Prati, L.; Manz, M.G.; Cazzamalli, S.; Neri, D.; et al. Cross-reactivity to glutamate carboxypeptidase III causes undesired salivary gland and kidney uptake of PSMA-targeted small-molecule radionuclide therapeutics. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 957–961. [Google Scholar] [CrossRef]
- Marmary, Y.; He, X.J.; Hand, A.R.; Ship, J.A.; Wellner, R.B. Beta-adrenergic responsiveness in a human submandibular tumor cell line (A253). Vitr. Cell. Dev. Biol. 1989, 25, 951–958. [Google Scholar] [CrossRef]
- Knedlík, T.; Vorlová, B.; Navrátil, V.; Tykvart, J.; Sedlák, F.; Vaculín, Š.; Franěk, M.; Šácha, P.; Konvalinka, J. Mouse glutamate carboxypeptidase II (GCPII) has a similar enzyme activity and inhibition profile but a different tissue distribution to human GCPII. FEBS Open Bio 2017, 7, 1362–1378. [Google Scholar] [CrossRef]
- Halsted, C.H.; Ling, E.H.; Luthi-Carter, R.; Villanueva, J.A.; Gardner, J.M.; Coyle, J.T. Folylpoly-gamma-glutamate carboxypeptidase from pig jejunum. Molecular characterization and relation to glutamate carboxypeptidase II. J. Biol. Chem. 1998, 273, 20417–20424. [Google Scholar] [CrossRef]
- Štembírek, J.; Kyllar, M.; Putnová, I.; Stehlík, L.; Buchtová, M. The pig as an experimental model for clinical craniofacial research. Lab. Anim. 2012, 46, 269–279. [Google Scholar] [CrossRef]
- Roy, J.; Warner, B.M.; Basuli, F.; Zhang, X.; Wong, K.; Pranzatelli, T.; Ton, A.T.; Chiorini, J.A.; Choyke, P.L.; Lin, F.I.; et al. Comparison of Prostate-Specific Membrane Antigen Expression Levels in Human Salivary Glands to Non-Human Primates and Rodents. Cancer Biother. Radiopharm. 2020, 35, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Benešová, M.; Umbricht, C.A.; Schibli, R.; Müller, C. Albumin-Binding PSMA Ligands: Optimization of the Tissue Distribution Profile. Mol. Pharm. 2018, 15, 934–946. [Google Scholar] [CrossRef]






| 0 h | 24 h | 48 h | 72 h | |
|---|---|---|---|---|
| Radiolabeling buffer | 98.7% | 92.1% | 85.0% | 86.9% |
| Phosphate-buffered saline | 94.3% | 92.7% | 92.7% | |
| Fetal bovine serum | 97.0% | 96.0% | 97.7% | |
| Complete growth media | 98.8% | 97.9% | 98.1% |
| Kd (nM) | Bmax (fmol/mm2) | BP | |
|---|---|---|---|
| Mouse kidney | 2.8686 | 0.2356 | 4.1065 |
| Pig salivary gland 1 | 4.6148 | 0.0029 | 0.0317 |
| Pig salivary gland 2 | 146.6207 | 0.0178 | 0.0061 |
| Pig salivary gland 3 | 8.6022 | 0.0059 | 0.0341 |
| Compound | Receptor Class Affinity | Investigated Treatment Concentrations (µM) |
|---|---|---|
| Ionotropic Glutamate Receptor Antagonists | ||
| Kynurenic acid | NMDA, AMPA and kainate | 0, 50, 500 and 1000 |
| Memantine | NMDA | 0, 50, 150 and 300 |
| Capric acid | AMPA | 0, 100, 500 and 1000 |
| UBP 302 | Kainate | 0, 25, 100 and 250 |
| MK 801 maleate | NMDA | 0, 50, 200 and 500 |
| Metabotropic glutamate receptor antagonists | ||
| (RS)-MCPG | Group I + Group II | 0, 10, 100, 500 and 1000 |
| (RS)-CPPG | Group II + Group III | 0, 50, 200, 500 and 1000 |
| Ly341495 | Group II | 0, 10, 100 and 500 |
| UBP1112 | Group III | 0, 10, 100 and 500 |
| Compound | Investigated Treatment Concentrations (µM) |
|---|---|
| 2-PMPA | 5 |
| Monosodium glutamate | 0, 9, 900 and 9000 |
| Kynurenic acid | 0, 50, 500 and 1000 |
| Memantine | 0, 50, 150 and 300 |
| Capric acid | 0, 100, 500 and 1000 |
| UBP 302 | 0, 25, 100 and 250 |
| MK 801 maleate | 0, 50, 200 and 500 |
| (RS)-MCPG | 0, 10, 100, 500 and 1000 |
| (RS)-CPPG | 0, 50, 200, 500 and 1000 |
| Ly341495 | 0, 10, 100 and 500 |
| UBP1112 | 0, 10, 100 and 500 |
| Compound | Investigated Treatment Concentrations (µM) |
|---|---|
| 2-PMPA | 100 |
| Monosodium glutamate | 0, 9, 900 and 9000 |
| Kynurenic acid | 0 and 1000 |
| Memantine | 0 and 300 |
| Capric acid | 0 and 1000 |
| UBP 302 | 0 and 250 |
| MK 801 maleate | 0 and 500 |
| (RS)-MCPG | 0 and 1000 |
| (RS)-CPPG | 0 and 1000 |
| L341495 | 0 and 500 |
| UBP1112 | 0 and 500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heynickx, N.; Segers, C.; Coolkens, A.; Baatout, S.; Vermeulen, K. Characterization of Non-Specific Uptake and Retention Mechanisms of [177Lu]Lu-PSMA-617 in the Salivary Glands. Pharmaceuticals 2023, 16, 692. https://doi.org/10.3390/ph16050692
Heynickx N, Segers C, Coolkens A, Baatout S, Vermeulen K. Characterization of Non-Specific Uptake and Retention Mechanisms of [177Lu]Lu-PSMA-617 in the Salivary Glands. Pharmaceuticals. 2023; 16(5):692. https://doi.org/10.3390/ph16050692
Chicago/Turabian StyleHeynickx, Nathalie, Charlotte Segers, Amelie Coolkens, Sarah Baatout, and Koen Vermeulen. 2023. "Characterization of Non-Specific Uptake and Retention Mechanisms of [177Lu]Lu-PSMA-617 in the Salivary Glands" Pharmaceuticals 16, no. 5: 692. https://doi.org/10.3390/ph16050692
APA StyleHeynickx, N., Segers, C., Coolkens, A., Baatout, S., & Vermeulen, K. (2023). Characterization of Non-Specific Uptake and Retention Mechanisms of [177Lu]Lu-PSMA-617 in the Salivary Glands. Pharmaceuticals, 16(5), 692. https://doi.org/10.3390/ph16050692






