Abstract
In the context of a continuously increasing global cancer risk, the search for new effective and affordable anticancer drugs remains a constant demand. This study describes chemical experimental drugs able to destroy cancer cells by arresting their growth. New hydrazones with quinoline, pyridine, benzothiazole and imidazole moieties have been synthesized and evaluated for their cytotoxic potential against 60 cancer cell lines. 7-Chloroquinolinehydrazones were the most active in the current study and exhibited good cytotoxic activity with submicromolar GI50 values on a large panel of cell lines from nine tumor types (leukemia, non-small cell lung cancer, colon cancer, CNS cancer, melanoma, ovarian cancer, renal cancer, prostate cancer and breast cancer). This study provided consistent structure-activity relationships in this series of experimental antitumor compounds.
1. Introduction
The burden of cancer continues to grow globally. In addition to quality and timely diagnosis, access to adapted treatments remains an important priority in order to increase the survival rate of many types of cancer and defeat cancer [1]. The discovery of new anticancer experimental drugs remains a major issue in medical chemistry.
7-Chloroquinoline hydrazones have been previously developed and successfully tested in vitro for many biological activities [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. The major part of the studies has been carried out to identify their antimalarial properties [2,3,4,5,6,7,8,9,10]. In addition, these hydrazones were explored for their anti-leishmanial [10,11,12,13], anti-tubercular [3,14,15,16,17,18], anti-bacterial [19,20], anti-oxidant [21,22], anti-fungal [23,24], anti-viral [25], anti-inflammatory [26], anti-prion disease [27], anti-convulsant [28], antinociceptive [29], but also anticancer activities [30,31,32,33]. Many chemical modulations have been realized on 7-chloroquinoline hydrazones. Most of the reported derivatives contain a differently substituted aryl unit, and fewer derivatives have another heterocycle in their structure. In search of new anticancer agents, in this study, we were interested in obtaining 7-chloroquinoline hydrazones with anti-proliferative activity against several cancer cell lines and establish a structure–activity relationship (SAR) in these series (Figure 1). The most promising 7-chloroquinoline hydrazones as anticancer agents reported so far are presented in Figure 1. Hydrazone I inhibited the growth of SF-295 CNS cancer cells with an IC50 value of 0.688 µg/cm−3 (Figure 1) [30]. Only one report described molecules containing a heterocyclic unit in addition to the 7-chloroquinoline ring as promising anti-proliferative agents, with notable cell growth inhibition of melanoma MDA-MB-435 cells [33]. The best antitumor activity was obtained using a pyrrole unit (compound II, Figure 1). The latter compound was four times more active than reference Doxorubicin [33]. This work aims to explore additional heterocyclic units for the improvement of the antitumoral activity of final compounds on the NCI-60 cancer cell line panel. Three types of chemical modulations have been envisaged on the target compounds 1–27, first on the 7-chloroquinoline unit (A-ring): (1) the importance of the position of the chloro substituent and (2) the replacement of the chloroquinoline unit by another heterocycle (pyridine, benzothiazole and dihydroimidazole) or by a substituted phenyle (Figure 1); and finally, (3) aromatic and heteroaromatic (phenothiazine, indole and thiophene) units have been designed for the B-ring of target molecules 1–27 (Figure 1 and Scheme 1).
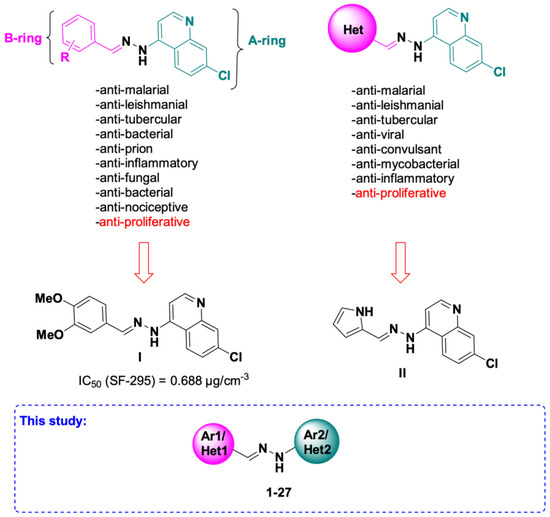
Figure 1.
General structure of previously reported 7-chloroquinoline hydrazones and target compounds 1–27 for antitumor evaluation on NCI-60 cancer cell line panel.
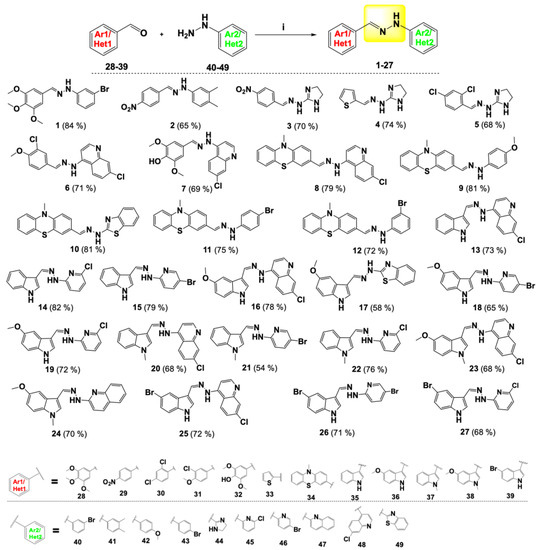
Scheme 1.
Reagents and conditions: (i) EtOH, reflux, 4–8 h.
2. Results and Discussion
2.1. Synthetic Strategy
A series of aldehydes 28–39 was reacted with substituted (hetero)arylhydrazines 40–49 in ethanol at reflux. All starting aldehydes were commercially available except aldehyde 34, which was obtained in 70% yield by formylation of N-methylphenothiazine 50 with Vilsmeier reagent in dichloroethane [34,35]. Final condensation products were easily obtained in good yields (54–84%) and confirmed as hydrazone derivatives 1–27 as E-isomers (Scheme 1).
2.2. Biological Evaluation
All synthesized compounds 1–27 have been submitted to the National Cancer Institute (NCI) (Germantown, MD, USA) and have been selected for evaluation in the NCI-60 cell-line screen. Compounds are generally selected for screening based on their ability to add diversity to the NCI small molecule compound collection [36]. The NCI-60 panel includes cell lines from nine tumor types (leukemia, non-small cell lung cancer, colon cancer, CNS cancer, melanoma, ovarian cancer, renal cancer, prostate cancer and breast cancer) and is extremely well characterized at the molecular level with both in-house and crowd-sourced characterization, including exome sequence for mutations, SNPs, DNA methylation, metabolome, mRNA, microRNA, and protein expression. This molecular characterization dataset enables interrogation of patterns of growth inhibition by the investigational drug set looking for characteristics of the cell lines that determine sensitivity.
Selected compounds 1–27 have been tested initially at a single high dose (10 µM) in the full NCI 60-cell panel. The one-dose data is reported as an average of the percent growth of treated cells. The number reported for the single dose (10 µM) test is the cell growth relative to the no-drug control and relative to the cell count at time zero. This allows the detection of both cell growth inhibition (values between 0 and 100, cytostatic effect) and cell lethality (values less than 0, cytotoxic effect). The same screening methodology is applied for the 5-dose test, also realized by NCI on the most promising compounds identified in the one-dose test. To the best of our knowledge, this is the first study of 7-chloroquinolinehydrazones on such a large number of cancer cell lines. Among tested compounds 1–27 in the one-dose screen, seventeen hydrazones 1, 2, 6, 7, 9, 12–20, 22, 23 and 25 were the most active, while the others did not have any impact on cancer cell growth. The cell growth inhibition values induced by these compounds is presented in Table 1. Interestingly, six compounds, 6, 13, 16, 20, 23 and 25, displayed significant cytotoxic effects in the one-dose screen on almost all tested cell lines (Figure 2). However, not only these molecules have progressed to the full 5-dose assay. A total of ten compounds, 6, 7, 13–16, 18, 20, 23 and 25, with both promising cytostatic and cytotoxic effects, have been further selected by the NCI for 5-dose in vitro human cancer cell growth inhibition in order to obtain their GI50 values (Table 2). Compounds 6, 7, 14, 15 and 18 displayed GI50 values in the micromolar range, while compounds 13, 16, 20, 23 and 25 were more active with GI50 values in the submicromolar range up to 120 nM (e.g., hydrazone 16 effect on SR leukemic cells, Table 2). The most effective cancer cell growth inhibition has been achieved with hydrazone 23 (Table 2). Therefore, several structure-activity relationships could be determined.

Table 1.
Results of the in vitro human cancer cell growth inhibition a for compounds 1, 2, 6, 7, 9, 12–20, 22, 23 and 25.
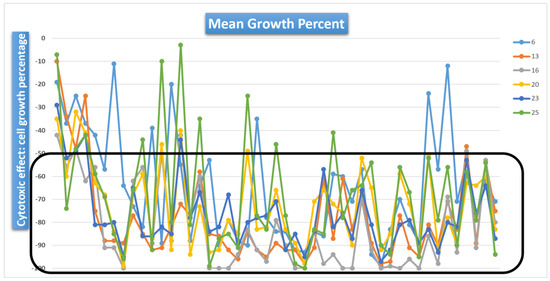
Figure 2.
Cytotoxic activity of compounds 6, 13, 16, 20, 23 and 25 on NCI-60 cancer cell lines at 10 µM concentration.

Table 2.
5-Dose in vitro human cancer cell growth inhibition for compounds 6, 7, 13–16, 18, 20, 23 and 25.
2.3. Structure-Activity Relationships
Dihydroimidazole derivatives 3–5 were completely ineffective and did not deserve future development as anticancer agents. In the same way, hydrazones bearing two differently substituted aryl units 1 and 2 failed to achieve effective cancer cell growth inhibition (Table 1). The combination of two heterocyclic units was highly more tolerated (e.g., 7-chloroquinoline and 1-methyl-5-methoxyindole in the structure of hydrazone 23, Scheme 1 and Table 2) and proved to be the best pharmacomodulation in the current study. Additional chemical modulations were performed on these specific heterocyclic units.
Concerning the 1-methyl-5-methoxyindole unit (B-ring of target compounds): (1) the replacement of the indole unit with an aromatic unit (3-chloro-4-methoxyphenyl in hydrazone 6 and 4-hydroxy-3,5-dimethoxyphenyl in hydrazone 7) conserved antitumor efficiency (GI50 values in the micromolar range) but resulted in diminished antitumor effect compared to indole-hydrazones 16 and 23 (Table 2). Hydrazones 6 and 7 displayed similar overall efficiency, and only two differences have been observed on SR (leukemia) and SK-MEL-2 (melanoma) cell lines, hydrazone 6 being slightly more active (6: GI50 (SR) = 0.7 µM vs. 7: GI50 (SR) = 1.9 µM and 6: GI50 (SK-MEL-2) = 1.79 µM vs. 7: GI50 (SK-MEL-2) = 16.3 µM, Table 2); (2) the replacement of the indole unit by a 10-methyl-phenothiazine group in hydrazone 8 was unfavorable to the biological activity, and no inhibition effect was obtained with the latter compound. In order to rule out the potential influence of the overall volume of molecule 8 on the lack of biological effect, smaller-size phenothiazine hydrazones have been synthesized (hydrazones 9–12, Scheme 1). None of these hydrazones were active on the NCI-60 screen. Some modest cytostatic effects were however registered in the one-dose screen with hydrazone 9, decorated with a 4-methoxyphenyl unit, and hydrazone 12, decorated with a 3-bromophenyl unit (Table 1); (3) the suppression of the methyl substituent on the indole nitrogen was tolerated but resulted in slightly reduced overall cancer cell growth inhibition (compare GI50 values of methylated-compound 23 vs. non-methylated analog 16, Table 2); finally, (4) the importance of the 5-methoxy substituent on the indole ring was also explored. The absence of the 5-methoxy substituent in hydrazone 13 conserved the antitumor efficiency on a large number of cell lines but displayed slightly reduced efficiency compared with 5-methoxy-analogue hydrazone 16, especially on ovarian, renal, prostate and breast cancer cell lines (Table 2). A similar activity profile has been observed with hydrazone 20 compared to 5-methoxy analog hydrazone 23, underlining the added value of the 5-methoxy group (Table 2). Finally, the replacement of the electro-donating M+ methoxy group (hydrazone 16) by an electro-withdrawing -I bromo substituent in hydrazone 25 conserved the antitumor effect on a large number of cancer cell lines and showed some decreased effect on ovarian, renal, prostate and breast cancer cell lines (compare hydrazones 16 and 25, Table 2).
The chemical modulations performed also concerned the 7-chloroquinoline unit (A-ring of target compounds): the replacement of the 7-chloroquinoline unit in hydrazones 13, 16, 20 and 25 by a chloro- or bromopyridine moiety in hydrazones 14, 15, 18, 19, 21, 22, 26 and 27 resulted in global diminished antitumor activity but displayed some interesting effects on CNS (14: GI50 (SNB-75) = 0.14 µM; 15: GI50 (SNB-75) = 0.38 µM) and breast (14: GI50 (BT-549) = 0.24 µM; 15: GI50 (BT-549) = 0.17 µM) cancer cells and were slightly more active than 7-chloroquinoline congener 13 (GI50 (SNB-75) = 1.38 µM; GI50 (BT-549) = 1.55 µM) (Table 2). The same particularity was observed between hydrazones 16 (with a 7-chloroquinoline unit) and 18 (with a 5-bromopyridin-2-yl group). Despite slightly diminished cell growth inhibition potential on the major part of tested cell lines, hydrazone 18 inhibited more effectively the growth of SNB-75 and BT-549 cells compared to hydrazone 16 (16: GI50 (SNB-75) = 1.58 µM; 18: GI50 (SNB-75) = 0.17 µM); 16: GI50 (BT-549) = 1.76 µM; 18: GI50 (BT-549) = 0.17 µM) (Table 2). Compound 19 did not enter 5-dose testing and was not able to be compared with related analogs 16 and 18. Based solely on the data from the one-dose screen (Table 1), compound 19 with a 6-chloropyridin-2-yl group seemed slightly less active than compound 18 with a 5-bromopyridin-2-yl group. Going further with the comparison of 7-chloroquinoline hydrazone 20, bromopyridine hydrazone 21 and chloropyridine hydrazone 22, in this case, the activity of the first compound 20 was largely superior to that of analogs 21 and 22, underlining the importance of the 7-chloroquinoline unit when the other part of the molecule was an N-methylindole. Compounds 21 and 22 did not satisfy pre-determined threshold inhibition criteria in a minimum number of cell lines and consequently did not progress to the full 5-dose assay. This was also supported by comparing the activity of hydrazone 25 with that of hydrazones 26 and 27. While the 7-chloroquinoline hydrazone 25 showed one of the best cancer cell growth inhibitions in the current study, bromopyridine hydrazone 26 and chloropyridine 27 were completely inactive. Two additional heterocyclic units have been used to substitute the 7-chloroquinoline unit. First, the replacement by a benzothiazole ring in hydrazone 17 was not tolerated and abolished the antitumor effect compared to 7-chloroquinoline hydrazone 16 (Table 1 and Table 2). Finally, the importance of the 7-chloroquinoline moiety was once again demonstrated by using a non-chlorinated quinoline in hydrazone 24 (Scheme 1).
The absence of the chlorine atom concomitant with the change of the substitution position on the quinolinic ring (from 4 in hydrazone 23 to 2 in hydrazone 24) was detrimental to the antitumor activity; this compound being stopped in the one-dose screen for lack of activity.
A global visualization of the structure-activity relationships for hydrazones obtained in this study has been proposed in Figure 3.
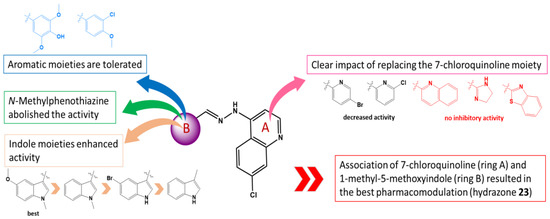
Figure 3.
Summary of structure-activity relationships observed in the current study.
2.4. Conclusions
To conclude, twenty-seven hydrazones 1–27 were synthesized in this work and screened for their potential to inhibit the NCI-60 cancer cell panel. As oncology treatment moves toward personalized, targeted therapeutic agents, the NCI-60 human tumor cell line panel remains an ideal community-wide tool to further understand the disease targets of new agents. Two main points of chemical modulation of the model molecules I and II (Figure 1) have been studied and were realized on their A and B rings. This allowed to establish several structure-activity relationships in this series. The best pharmacomodulation was obtained by associating two heterocyclic units on the hydrazone bridge: 7-chloroquinoline as the A-ring and 1-methyl-5-methoxyindole as the B-ring. The resulting hydrazone 23 showed very promising antitumor activity on the NCI-60 cancer cell lines (GI50 values in the submicromolar range). While the 1-methyl-5-methoxyindole tolerated different substitutions (suppression of the methyl, substitution or suppression of the 5-methoxy group), the 7-chloroquinoline ring was found to be essential for antitumor activity. Interestingly, promising cell growth inhibition was detected for chloropyridine and bromopyridine hydrazones 14, 15 and 18 on SNB-75 (CNS cancer) and BT-549 (breast cancer) cell lines. These compounds outperformed the most active hydrazone 23 in this study on these particular cancer cell lines and deserved future biological investigation.
The study resulted in a new collection of 7-chloroquinoline hydrazones with promising potential for further development of compounds for oncology. Their mechanism of action is currently under study and will be reported in due course.
3. Materials and Methods for Synthesis and Characterizations
Starting materials are commercially available and were used without further purification (suppliers: Carlo Erba Reagents S.A.S., Val de Reuil, France, Thermo Fisher Scientific Inc., Illkirch-Graffenstaden, France, and Sigma-Aldrich Co., Saint-Quentin-Fallavier, France). Nuclear magnetic resonance (NMR) spectra were acquired at 500 MHz for 1H-NMR and at 125 MHz for 13C-NMR on a Bruker Avance III 500 MHz spectrometer (Bruker, Mannheim, Germany) with tetramethylsilane (TMS) as internal standard, at room temperature (RT). All spectra have been realized using deuterated solvents (CDCl3 99.8%D + 0.03% TMS v/v or DMSO-d6 99.8%D + 0.03% TMS v/v), purchased from Eurisotop, Saint-Aubin, France. The calibration has been realized using TMS pic as the 0.00 ppm value in the registered spectra. Chemical shifts (δ) are expressed in ppm relative to TMS. Splitting patterns are designed: s, singlet; d, doublet; dd, doublet of doublets; t, triplet; q, quadruplet; quint, quintuplet; m, multiplet; sym m, symmetric multiplet; br s, broaden singlet; br t, broaden triplet. Coupling constants (J) are reported in Hertz (Hz). 1H and 13C NMR spectra for all synthesized hydrazones 1–27 are provided in the Supplementary Materials section. Melting points were measured on an MPA 100 OptiMelt® apparatus (Stanford Research Systems, Sunnyvale, CA, USA) and are uncorrected. Thin layer chromatography (TLC) was realized on Macherey Nagel silica gel plates (Macherey Nagel, Hœrdt, France) with fluorescent indicator and were visualized under a UV lamp at 254 nm and 365 nm. IR spectra were recorded on an FTIR Bruker Tensor 27 Spectrometer. Elemental analyses (C, H, N) of new compounds were determined on a Thermo Electron apparatus (Thermo Fisher Scientific Inc., Illkirch, France) by “Pôle Chimie Moléculaire-Welience”, Faculté des Sciences Mirande, Dijon, France.
3.1. Synthesis of 10-Methyl-10H-phenothiazine-3-carbaldehyde (34)
The Vilsmeier reagent was generated by stirring a mixture of DMF (11.64 mL, 8 equiv.) and POCl3 (5.8 mL, 4 equiv.) at 0 °C for 30 min. Then, a solution of 10-methyl-10H-phenothiazine (4 g, 1 equiv.) solubilized in 10 mL dichloroethane was added, and the mixture was stirred at 80 °C for 46 h. After cooling to room temperature, the reaction mixture was poured into an ice–water mixture and treated with aqueous NaOH (32%) until pH = 6 and then extracted with EtOAc. The organic phase was dried with Na2SO4 and concentrated in vacuo to obtain a crude mixture that was purified by column chromatography (EtOAc:cyclohexane 1:5 as eluent) to afford the pure product 34 (3.16 g, 70% yield).
10-methyl-10H-phenothiazine-3-carbaldehyde (34) has the same physico-chemical properties as described previously [37].
3.2. General Procedure for the Synthesis of Hydrazone Derivatives (1–27)
A solution of aldehyde 28–39 (1 equiv.) and hydrazine derivative 40–49 (1 equiv.) in ethanol (5–10 mL) was stirred at reflux for 4–8 h. When the reaction was completed (TLC monitoring), the reaction mixture was cooled to room temperature, the product precipitated and was collected by filtration, washed with ethanol and purified by recrystallization from ethanol to obtain pure target hydrazone derivative as E-isomers (1–27).
3.2.1. 1-(3-Bromophenyl)-2-(3,4,5-trimethoxybenzylidene)hydrazine (1)
The general procedure was used with 3,4,5-trimethoxybenzaldehyde (0.26 g, 1.3 mmol) and 3-bromophenylhydrazine (0.28 g, 1.3 mmol) to obtain pure compound 1 as a white solid (0.40 g, 1.0 mmol, 84% yield); mp (EtOH) 146–148 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.63. 1H NMR (DMSO-d6, 500 MHz) δ ppm 3.88 (s, 3H, OCH3), 3.92 (s, 6H, 2OCH3), 6.97 (s, 2H, 2ArH), 6.93–7.01 (m, 2H, 2ArH), 7.11 (t, J = 8.0 Hz, 1H, ArH), 7.29 (t, J = 2.0 Hz, 1H, ArH), 7.58 (s, 1H, =CH), 7.67 (br s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 56.3 (2OCH3), 61.1 (OCH3), 103.5 (2CH), 111.5 (CH), 115.7 (CH), 122.9 (CH), 123.4 (C), 130.6 (C), 130.7 (CH), 138.5 (=CH), 138.9 (C), 145.9 (C), 153.6 (2C). IR ν (cm−1): 3245, 1618, 1625, 1585, 1455, 1379, 1357, 1278, 1098, 1072, 1043, 972, 878, 856, 827, 794, 733, 660. Elemental analysis calcd (%) for C16H17BrN2O3: C, 52.62; H, 4.69; N, 7.67; found: C, 52.89; H, 4.92; N, 7.88.
3.2.2. 1-(3,4-Dimethylphenyl)-2-(4-nitrobenzylidene)hydrazine (2)
The general procedure was used with 4-nitrobenzaldehyde (0.20 g, 1.3 mmol) and 3,4-dimethylphenylhydrazine (0.22 g, 1.3 mmol) to obtain pure compound 2 as a dark red solid (0.23 g, 0.8 mmol, 65% yield); mp (EtOH) 118–120 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.36. 1H NMR (CDCl3, 500 MHz) δ ppm 2.22 (s, 3H, CH3), 2.28 (s, 3H, CH3), 6.88 (d, J = 8.0 Hz, 1H, ArH), 6.89 (s, 1H, ArH), 7.06 (d, J = 8.0 Hz, 1H, ArH), 7.64 (s, 1H, =CH), 7.75 (d, J = 8.5 Hz, 2H, 2ArH), 7.91 (br s, 1H, NH), 8.21 (d, J = 8.5 Hz, 2H, 2ArH). 13C NMR (CDCl3, 125 MHz) δ ppm 19.1 (CH3), 20.2 (CH3), 110.7 (CH), 114.6 (CH), 124.2 (2CH), 126.2 (2CH), 129.6 (C), 130.6 (CH), 133.2 (=CH), 137.9 (C), 141.7 (C), 142.1 (C), 146.9 (C). IR ν (cm−1): 3292, 1616, 1597, 1555, 1531, 1497, 1406, 1323, 1265, 1169, 1106, 912, 842, 812, 746, 690. Elemental analysis calcd (%) for C15H15N3O2: C, 66.90; H, 5.61; N, 15.60; found: C, 67.22; H, 5.90; N, 15.76.
3.2.3. 2-(2-(4-Nitrobenzylidene)hydrazinyl)-4,5-dihydro-1H-imidazole (3)
The general procedure was used with 4-nitrobenzaldehyde (0.20 g, 1.3 mmol) and 2-hydrazinyl-4,5-dihydro-1H-imidazole (0.24 g, 1.3 mmol) to obtain pure compound 3 as a dark red solid (0.22 g, 0.9 mmol, 70% yield) with the same physico-chemical properties as described previously [38]; mp (EtOH) >250 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.13. 1H NMR (DMSO-d6, 500 MHz) δ ppm 3.45 (br s, 4H, 2CH2), 6.87 (s, 1H, NH), 7.20 (s, 1H, NH), 7.91 (d, J = 8.5 Hz, 2H, 2ArH), 8.05 (s, 1H, =CH), 8.17 (d, J = 8.5 Hz, 2H, 2ArH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 41.8 (CH2), 42.3 (CH2), 123.7 (2CH), 126.6 (2CH), 141.6 (=CH), 143.9 (C), 146.0 (C), 166.5 (C). IR ν (cm−1): 3109, 1622, 1590, 1539, 1495, 1481, 1398, 1375, 1320. 1288, 1167, 1103, 1055, 987, 923, 844, 777, 748, 690, 621. Elemental analysis calcd (%) for C10H11N5O2: C, 51.50; H, 4.75; N, 30.03; found: C, 51.74; H, 4.98; N, 30.29.
3.2.4. 2-(2-(Thiophen-2-ylmethylene)hydrazinyl)-4,5-dihydro-1H-imidazole (4)
The general procedure was used with 2-thiophenecarboxaldehyde (0.20 g, 1.7 mmol) and 2-hydrazinyl-4,5-dihydro-1H-imidazole (0.32 g, 1.7 mmol) to obtain pure compound 4 as a white solid (0.26 g, 1.3 mmol, 74% yield) with the same physico-chemical properties as described previously [39]; mp (EtOH) >250 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.1. 1H NMR (DMSO-d6, 500 MHz) δ ppm 3.70 (br s, 4H, 2CH2), 7.16 (t, J = 4.0 Hz, 1H, ArH), 7.54 (d, J = 4.0 Hz, 1H, ArH), 7.74 (d, J = 4.0 Hz, 1H, ArH), 8.41 (s, 1H, =CH), 8.52 (br s, 1H, NH), 12.26 (br s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 42.8 (2CH2), 128.0 (CH), 130.1 (CH), 132.1 (CH), 137.5 (C), 143.5 (=CH), 157.5 (C). IR ν (cm−1): 3112, 1616, 1585, 1525, 1493, 1479, 1395, 1370, 1325, 1103, 1055, 985, 844, 748, 690, 621. Elemental analysis calcd (%) for C8H10N4S: C, 49.46; H, 5.19; N, 28.84; found: C, 49.76; H, 5.39; N, 29.01.
3.2.5. 2-(2-(2,4-Dichlorobenzylidene)hydrazinyl)-4,5-dihydro-1H-imidazole (5)
The general procedure was used with 2,4-dichlorobenzaldehyde (0.30 g, 1.7 mmol) and 2-hydrazinyl-4,5-dihydro-1H-imidazole (0.31 g, 1.7 mmol) to obtain pure compound 5 as a white solid (0.30 g, 1.1 mmol, 68% yield); mp (EtOH) >250 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.09. 1H NMR (DMSO-d6, 500 MHz) δ ppm 3.75 (br s, 4H, 2CH2), 7.56 (dd, J = 8.5, 2.0 Hz, 1H, ArH), 7.74 (d, J = 2.0 Hz, 1H, ArH), 7.24 (d, J = 8.5 Hz, 1H, ArH), 8.54 (s, 1H, =CH), 8.91 (br s, 1H, NH), 12.61 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 42.8 (2CH2), 127.9 (CH), 128.6 (CH), 129.4 (CH), 129.7 (C), 134.1 (C), 135.8 (C), 142.9 (=CH), 157.6 (C). IR ν (cm−1): 3130, 1641, 1603, 1584, 1456, 1379, 1357, 1278, 1205, 1097, 1070, 1046, 997, 929, 877, 856, 827, 794, 736, 661. Elemental analysis calcd (%) for C10H10Cl2N4: C, 46.71; H, 3.92; N, 21.79; found: C, 47.02; H, 4.16; N, 22.04.
3.2.6. 7-Chloro-4-(2-(3-chloro-4-methoxybenzylidene)hydrazinyl)quinoline (6)
The general procedure was used with 4-chloro-3-methoxybenzaldehyde (0.20 g, 1.1 mmol) and 7-chloro-4-hydrazinoquinoline (0.23 g, 1.1 mmol) to obtain pure compound 6 as a yellow solid (0.28 g, 0.8 mmol, 71% yield); mp (EtOH) 147–148 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.31. 1H NMR (DMSO-d6, 500 MHz) δ ppm 3.92 (s, 3H, OCH3), 7.23 (d, J = 8.0 Hz, 1H, ArH), 7.36–7.45 (m, 1H, ArH), 7.54–7.63 (m, 1H, ArH), 7.71 (dd, J = 8.0, 2.0 Hz, 1H, ArH), 7.89 (d, J = 2.0 Hz, 2H, 2ArH), 8.25–8.44 (m, 2H, 2ArH), 8.58 (s, 1H, =CH), 11.24 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 56.3 (OCH3), 101.3 (CH), 113.0 (CH), 115.5 (C), 121.8 (C), 124.0 (CH), 124.9 (CH), 127.2 (CH), 127.4 (CH), 127.7 (CH), 128.4 (C), 133.8 (C), 141.8 (=CH), 147.0 (C), 149.2 (C), 152.1 (CH), 155.4 (C). IR ν (cm−1): 3232, 1616, 1572, 1534, 1440, 1420, 1366, 1192, 1025, 912, 850, 820, 752. Elemental analysis calcd (%) for C17H13Cl2N3O: C, 58.98; H, 3.78; N, 12.14; found: C, 59.25; H, 3.95; N, 12.37.
3.2.7. 4-((2-(7-Chloroquinolin-4-yl)hydrazono)methyl)-2,6-dimethoxyphenol (7)
The general procedure was used with 3,5-dimethoxy-4-hydroxybenzaldehyde (0.30 g, 1.6 mmol) and 7-chloro-4-hydrazinoquinoline (0.31 g, 1.6 mmol) to obtain pure compound 7 as a yellow solid (0.59 g, 1.6 mmol, 69% yield); mp (EtOH) 220–222 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.32. 1H NMR (DMSO-d6, 500 MHz) δ ppm 3.85 (s, 6H, 2OCH3), 7.06 (s, 2H, 2ArH), 7.21–7.30 (m, 1H, ArH), 7.31–7.60 (m, 1H, ArH), 7.71–8.01 (m, 1H, ArH), 8.28 (s, 1H, ArH), 8.36 (d, J = 9.0 Hz, 1H, ArH), 8.53 (br s, 1H, OH), 8.85 (s, 1H, =CH), 11.11 (br s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 56.1 (2OCH3), 101.0 (CH), 104.3 (2CH), 115.6 (C), 124.2 (CH), 124.6 (CH), 125.1 (2C), 127.6 (CH), 133.8 (C), 137.5 (C), 144.1 (=CH), 147.1 (C), 148.2 (2C), 152.0 (CH). IR ν (cm−1): 3292, 3213, 1616, 1574, 1502, 1447, 1416, 1366, 1302, 1194, 1094, 1026, 910, 858, 806, 756. Elemental analysis calcd (%) for C18H16ClN3O3: C, 60.42; H, 4.51; N, 11.74; found: C, 60.78; H, 4.69; N, 12.01.
3.2.8. 3-((2-(7-Chloroquinolin-4-yl)hydrazono)methyl)-10-methyl-10H-phenothiazine (8)
The general procedure was used with 3-formyl-10-methylphenothiazine (0.25 g, 1 mmol) and 7-chloro-4-hydrazinoquinoline (0.20 g, 1 mmol) to obtain pure compound 8 as a yellow solid (0.34 g, 0.8 mmol, 79% yield); mp (EtOH) 239–241 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.23. 1H NMR (DMSO-d6, 500 MHz) δ ppm 3.35 (s, 3H, NCH3), 6.96–7.20 (m, 3H, 3ArH), 7.18 (d, J = 7.0 Hz, 1H, ArH), 7.23 (t, J = 7.0 Hz, 1H, ArH), 7.35–7.42 (m, 1H, ArH), 7.53–7.61 (m, 3H, 3ArH), 7.89 (s, 1H, ArH), 8.27 (s, 1H, =CH), 8.35 (d, J = 9.0 Hz, 1H, ArH), 8.53–8.61 (m, 1H, ArH), 11.16 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 35.4 (NCH3), 101.3 (CH), 114.7 (CH), 114. 9 (CH), 115.6 (C), 121.6 (C), 122.7 (C), 122.9 (CH), 123.9 (CH), 124.3 (CH), 124.8 (CH), 126.8 (CH), 126.9 (CH), 127.7 (CH), 127.9 (CH), 129.2 (C), 133.8 (C), 142.4 (=CH), 144.7 (C), 146.2 (C), 147.0 (C), 149.2 (C), 152.0 (CH). IR ν (cm−1): 3404, 1618, 1570, 1460, 1427, 1354, 1331, 1248, 1209, 1127, 1089, 1049, 881, 812, 748, 638, 606. Elemental analysis calcd (%) for C23H17ClN4S: C, 66.26; H, 4.11; N, 13.44; found: C, 66.51; H, 4.28 N, 13.69.
3.2.9. 3-((2-(4-Methoxyphenyl)hydrazono)methyl)-10-methyl-10H-phenothiazine (9)
The general procedure was used with 3-formyl-10-methylphenothiazine (0.25 g, 1 mmol) and 4-methoxyphenylhydrazine (0.18 g, 1 mmol) to obtain pure compound 9 as a yellow solid (0.30 g, 0.8 mmol, 81% yield); mp (EtOH) 188–190 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.3. 1H NMR (DMSO-d6, 500 MHz) δ ppm 3.34 (s, 3H, NCH3), 3.69 (s, 3H, OCH3), 6.84 (d, J = 8 Hz, 2H, 2ArH), 6.80–7.03 (m, 5H, 5ArH), 7.18 (d, J = 7.5 Hz, 1H, ArH), 7.24 (d, J = 7.5 Hz, 1H, ArH), 7.38–7.47 (m, 2H, 2ArH), 7.71 (s, 1H, =CH), 10.02 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 35.3 (NCH3), 55.3 (OCH3), 112.9 (2CH), 114.6 (2CH), 114.7 (CH), 116.8 (CH), 121.7 (C), 122.5 (C), 122.6 (CH), 123.1 (CH), 125.2 (CH), 126.8 (CH), 127.8 (CH), 130.7 (C), 134.4 (=CH), 139.5 (C), 144.6 (C), 145.0 (C), 152.5 (C). IR ν (cm−1): 3281, 1683, 1601, 1584, 1522, 1501, 1466, 1448, 1352, 1331, 1259, 1243, 1142, 1034, 911, 827, 765, 609. Elemental analysis calcd (%) for C21H19N3OS: C, 66.26; H, 4.11; N, 13.44; found: C, 66.50; H, 4.33; N, 13.69.
3.2.10. 3-((2-(Benzo[d]thiazol-2-yl)hydrazono)methyl)-10-methyl-10H-phenothiazine (10)
The general procedure was used with 3-formyl-10-methylphenothiazine (0.30 g, 1.3 mmol) and 2-hydrazinobenzothiazole (0.20 g, 1.3 mmol) to obtain pure compound 10 as a yellow solid (0.38 g, 0.9 mmol, 81% yield); mp (EtOH) >250 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.36. 1H NMR (DMSO-d6, 500 MHz) δ ppm 3.51 (s, 3H, NCH3), 6.96–7.05 (m, 3H, 3ArH), 7.10 (t, J = 7.5 Hz, 1H, ArH), 7.19 (d, J = 7.5 Hz, 1H, ArH), 7.24 (t, J = 7.0 Hz, 1H, ArH), 7.29 (t, J = 7.0 Hz, 1H, ArH), 7.35–7.54 (m, 3H, ArH), 7.70–7.82 (m, 1H, ArH), 8.03 (s, 1H, =CH), 12.23 (br s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 35.6 (NCH3), 114.8 (CH), 114.9 (2CH), 121.5 (CH), 121.6 (C), 122.6 (C), 122.9 (2CH), 124.1 (CH), 126.0 (CH), 126.7 (CH), 126.9 (2CH), 128.0 (=CH), 128.9 (C), 144.6 (2C), 146.3 (2C), 167.0 (C). IR ν (cm−1): 3090, 1624, 1578, 1464, 1439, 1335, 1254, 1219, 1113, 923, 883, 800, 736, 715. Elemental analysis calcd (%) for C21H16N4S2: C, 64.92; H, 4.15; N, 14.42; found: C, 64.78; H, 4.03; N, 14.23.
3.2.11. 3-((2-(4-Bromophenyl)hydrazono)methyl)-10-methyl-10H-phenothiazine (11)
The general procedure was used with 3-formyl-10-methylphenothiazine (0.30 g, 1.3 mmol) and 4-bromophenylhydrazine (0.28 g, 1.3 mmol) to obtain pure compound 11 as a yellow solid (0.38 g, 0.9 mmol, 75% yield); mp (EtOH) 203–204 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.32. 1H NMR (DMSO-d6, 500 MHz) δ ppm 3.34 (s, 3H, NCH3), 6.93–7.02 (m, 5H, 5ArH), 7.18 (d, J = 8.0 Hz, 1H, ArH), 7.23 (td, J = 8.0, 2.0 Hz, 1H, ArH), 7.34 (d, J = 8.0 Hz, 2H, 2ArH), 7.42–7.48 (m, 2H, 2ArH), 7.77 (s, 1H, =CH), 10.39 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 35.3 (NCH3), 109.3 (C), 113.9 (2CH), 114.7 (CH), 114.8 (CH), 121.6 (C), 122.6 (C), 122.7 (CH), 123.5 (CH), 125.7 (CH), 126.9 (CH), 127.9 (CH), 130.2 (C), 131.7 (2CH), 136.5 (=CH), 144.7 (C), 144.9 (C), 145.2 (C). IR ν (cm−1): 3273, 1683, 1548, 1453, 1331, 1257, 1331, 1257, 1139, 1068, 912, 831, 810, 756, 601. Elemental analysis calcd (%) for C20H16BrN3S: C, 58.54; H, 3.93; N, 10.24; found: C, 58.72; H, 4.16; N, 10.44.
3.2.12. 3-((2-(3-Bromophenyl)hydrazono)methyl)-10-methyl-10H-phenothiazine (12)
The general procedure was used with 3-formyl-10-methylphenothiazine (0.30 g, 1.3 mmol) and 3-bromophenylhydrazine (0.28 g, 1.3 mmol) to obtain pure compound 12 as a yellow solid (0.36 g, 0.8 mmol, 72% yield); mp (EtOH) 128–130 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.3. 1H NMR (CDCl3, 500 MHz) δ ppm 3.37 (s, 3H, NCH3), 6.76 (d, J = 8.0 Hz, 1H, ArH), 6.82 (d, J = 8.0 Hz, 1H, ArH), 6.90- 6.98 (m, 3H, 3ArH), 7.10 (t, J = 8.0 Hz, 1H, ArH), 7.14- 7.21 (m, 2H, 2ArH), 7.29 (s, 1H, ArH), 7.38 (dd, J = 8.2 Hz, 1H, ArH), 7.45 (d, J = 2.0 Hz, 1H, ArH), 7.47–7.55 (m, 2H, =CH+ NH). 13C NMR (CDCl3, 125 MHz) δ ppm 35.6 (NCH3), 111.4 (CH), 114.1 (CH), 114.4 (CH), 115.6 (CH), 122.8 (CH), 122.9 (CH), 123.0 (C), 123.4 (C), 123.9 (C), 124.7 (CH), 126.1 (CH), 127.4 (CH), 127.7 (CH), 129.6 (C), 130.7 (CH), 137.7 (=CH), 145.4 (C), 146.1 (C), 146.3 (C). IR ν (cm−1): 3297, 1686, 1586, 1464, 1336, 1257, 1236, 1257, 1236, 1123, 1082, 1063, 987, 916, 847, 810, 746, 680. Elemental analysis calcd (%) for C20H16BrN3S: C, 58.54; H, 3.93; N, 10.24; found: C, 58.36; H, 3.87; N, 10.15.
3.2.13. 4-(2-((1H-Indol-3-yl)methylene)hydrazinyl)-7-chloroquinoline (13)
The general procedure was used with indole-3-carboxaldehyde (0.20 g, 1.3 mmol) and 7-chloro-4-hydrazinoquinoline (0.26 g, 1.3 mmol) to obtain pure compound 13 as a yellow solid (0.32 g, 0.9 mmol, 73% yield) with the same physico-chemical properties as described previously [28]; mp (EtOH) >250 °C; Rf (EtOAc:Cyclohexane 1:1) = 0. 1H NMR (DMSO-d6, 500 MHz) δ ppm 7.21–7.28 (m, 2H, 2ArH), 7.35 (d, J = 4.5 Hz, 1H, ArH), 7.43–7.51 (m, 1H, ArH), 7.55 (d, J = 8.5 Hz, 1H, ArH), 7.83–7.91 (m, 2H, 2ArH), 8.30–8.35 (m, 1H, ArH), 8.40 (d, J = 8.5 Hz, 1H, ArH), 8.60 (d, J = 4.5 Hz, 1H, ArH), 8.63 (s, 1H, =CH), 10.94 (s, 1H, NH), 11.61 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 100.3 (CH), 112.0 (CH), 115.6 (C), 120.6 (CH), 121.7 (CH), 122.6 (CH), 124.1 (CH), 124.2 (C), 124.4 (CH), 127.6 (CH), 130.0 (CH), 133.6 (C), 137.2 (2C), 141.5 (=CH), 147.2 (C), 149.3 (C), 152.1 (CH). IR ν (cm−1): 3332, 1614, 1572, 1483, 1447, 1416, 1356, 1317, 1277, 1244, 1198, 1123, 1080, 1022, 860, 810, 747. Elemental analysis calcd (%) for C18H13ClN4: C, 67.40; H, 4.08; N, 17.47; found: C, 67.72; H, 4.35; N, 17.39.
3.2.14. 3-((2-(6-Chloropyridin-2-yl)hydrazono)methyl)-1H-indole (14)
The general procedure was used with indole-3-carboxaldehyde (0.25 g, 1.7 mmol) and 2-chloro-6-hydrazinopyridine (0.25 g, 1.7 mmol) to obtain pure compound 14 as a beige solid (0.38 g, 1.4 mmol, 82% yield); mp (EtOH) 206–208 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.32. 1H NMR (DMSO-d6, 500 MHz) δ ppm 6.71 (d, J = 7.5 Hz, 1H, ArH), 7.12–7.23 (m, 3H, 3ArH), 7.43 (d, J = 7.5 Hz, 1H, ArH), 7.68 (t, J = 7.5 Hz, 1H, ArH), 7.72 (d, J = 2.0 Hz, 1H, ArH), 8.22 (d, J = 7.5 Hz, 1H, ArH), 8.25 (s, 1H, =CH), 10.89 (s, 1H, NH), 11.46 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 104.1 (CH), 111.8 (CH), 112.1 (C), 112.3 (CH), 120.3 (CH), 121.6 (CH), 122.5 (CH), 124.1 (C), 129.0 (CH), 137.1 (C), 138.6 (=CH), 141.0 (CH), 148.2 (C), 157.5 (C). IR ν (cm−1): 3375, 3273, 1612, 1587, 1639, 1508, 1454, 1418, 1311, 1242, 1089, 1072, 975, 929, 771, 739, 704, 630. Elemental analysis calcd (%) for C14H11ClN4: C, 62.11; H, 4.10; N, 20.70; found: C, 62.37; H, 4.32; N, 20.96.
3.2.15. 3-((2-(5-Bromopyridin-2-yl)hydrazono)methyl)-1H-indole (15)
The general procedure was used with indole-3-carboxaldehyde (0.25 g, 1.7 mmol) and 5-bromo-2-hydrazinopyridine (0.33 g, 1.7 mmol) to obtain pure compound 15 as a beige solid (0.54 g, 1.7 mmol, 79% yield); mp (EtOH) 244–246 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.56. 1H NMR (DMSO-d6, 500 MHz) δ ppm 7.12–7.23 (m, 3H, 3ArH), 7.43 (d, J = 7.5 Hz, 1H, ArH), 7.69 (d, J = 2.5 Hz, 1H, ArH), 7.82 (dd, J = 7.5, 2.5 Hz, 1H, ArH), 8.15 (d, J = 2.5 Hz, 1H, ArH), 8.20 (d, J = 7.5 Hz, 1H, ArH), 8.26 (s, 1H, =CH), 10.69 (s, 1H, NH), 11.44 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 106.8 (C), 107.5 (CH), 111.8 (CH), 112.2 (C), 120.3 (CH), 121.6 (CH), 122.4 (CH), 124.1 (C), 128.7 (CH), 137.1 (C), 138.1 (=CH), 140.2 (CH), 148.0 (CH), 156.3 (C). IR ν (cm−1): 3378, 3180, 1616, 1585, 1537, 1445, 1386, 1362, 1304, 1243, 1130, 1083, 999, 808, 785, 743, 642, 619. Elemental analysis calcd (%) for C14H11BrN4: C, 53.35; H, 3.52; N, 17.78; found: C, 53.50; H, 3.77; N, 17.95.
3.2.16. 7-Chloro-4-(2-((5-methoxy-1H-indol-3-yl)methylene)hydrazinyl)quinoline (16)
The general procedure was used with 5-methoxyindole-3-carboxaldehyde (0.30 g, 1.7 mmol) and 7-chloro-4-hydrazinoquinoline (0.33 g, 1.7 mmol) to obtain pure compound 16 as a yellow solid (0.46 g, 1.3 mmol, 78% yield) with the same physico-chemical properties as described previously [40]; mp (EtOH) > 255 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.32. 1H NMR (DMSO-d6, 500 MHz) δ ppm 3.88 (s, 3H, OCH3), 6.89 (dd, J = 8.5, 2.0 Hz, 1H, ArH), 7.29 (d, J = 5.0 Hz, 1H, ArH), 7.37 (d, J = 8.5 Hz, 1H, ArH), 7.55 (d, J = 2.0 Hz, 1H, ArH), 7.72–7.95 (m, 3H, 3ArH), 8.39 (d, J = 5.0 Hz, 1H, ArH), 8.58 (d, J = 5.0 Hz, 1H, ArH), 8.61 (s, 1H, =CH), 10.92 (s, 1H, NH), 11.47 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 55.2 (OCH3), 100.0 (CH), 103.6 (CH), 111.8 (C), 112.2 (CH), 112.6 (CH), 115.6 (C), 124.2 (CH), 124.8 (CH), 127.5 (CH), 130.3 (CH), 132.1 (2C), 133.7 (C), 141.6 (=CH), 147.2 (C), 149.3 (C), 152.0 (CH), 154.5 (C). IR ν (cm−1): 3355, 3235, 1616, 1581, 1429, 1423, 1369, 1292, 1209, 1123, 1076, 1028, 921, 873, 839, 794, 761, 721, 621. Elemental analysis calcd (%) for C19H15ClN4O: C, 65.05; H, 4.31; N, 15.97; found: C, 65.31; H, 4.59; N, 14.98.
3.2.17. 2-(2-((5-Methoxy-1H-indol-3-yl)methylene)hydrazinyl)benzo[d]thiazole (17)
The general procedure was used with 5-methoxyindole-3-carboxaldehyde (0.30 g, 1.7 mmol) and 2-hydrazinobenzothiazole (0.28 g, 1.7 mmol) to obtain pure compound 17 as a yellow solid (0.32 g, 0.9 mmol, 58% yield); mp (EtOH) >250 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.04. 1H NMR (DMSO-d6, 500 MHz) δ ppm 3.91 (s, 3H, OCH3), 6.88 (dd, J = 8.5, 2.0 Hz, 1H, ArH), 7.06 (t, J = 7.5 Hz, 1H, ArH), 7.27 (t, J = 7.5 Hz, 1H, ArH), 7.35 (d, J = 8.5 Hz, 1H, ArH), 7.39 (d, J = 2.0 Hz, 1H, ArH), 7.75 (d, J = 2.0 Hz, 1H, ArH), 7.77 (d, J = 7.5 Hz, 1H, ArH), 7.83 (d, J = 2.0 Hz, 1H, ArH), 8.33 (s, 1H, =CH), 11.43 (s, 1H, NH), 11.93 (br s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 55.4 (OCH3), 103.7 (CH), 111.4 (C), 112.4 (2CH), 112.6 (CH), 117, 4 (C), 121.0 (CH), 121.5 (CH), 124.7 (C), 125.9 (CH), 129.1 (C), 130.3 (CH), 132.0 (C), 141.8 (=CH), 154.5 (C), 166.5 (C). IR ν (cm−1): 3427, 1614, 1576, 1485, 1440, 1418, 1261, 1207, 1121, 1096, 1022, 921, 889, 858, 810, 796, 738, 715. Elemental analysis calcd (%) for C17H14N4OS: C, 63.33; H, 4.38; N, 17.38; found: C, 63.38; H, 4.26; N, 17.17.
3.2.18. 3-((2-(5-Bromopyridin-2-yl)hydrazono)methyl)-5-methoxy-1H-indole (18)
The general procedure was used with 5-methoxyindole-3-carboxaldehyde (0.25 g, 1.4 mmol) and 5-bromo-2-hydrazinopyridine (0.27 g, 1.4 mmol) to obtain pure compound 18 as a white solid (0.32 g, 0.9 mmol, 65% yield); mp (EtOH) 212–214 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.36. 1H NMR (DMSO-d6, 500 MHz) δ ppm 3.83 (s, 3H, OCH3), 6.85 (dd, J = 9.0, 2.5 Hz, 1H, ArH), 7.13 (d, J = 9.0 Hz, 1H, ArH), 7.33 (d, J = 9.0 Hz, 1H, ArH), 7.65 (d, J = 2.5 Hz, 1H, ArH), 7.71 (d, J = 2.5 Hz, 1H, ArH), 7.84 (dd, J = 9.0, 2.5 Hz, 1H, ArH), 8.15 (d, J = 2.5 Hz, 1H, ArH), 8.25 (s, 1H, =CH), 10.68 (s, 1H, NH), 11.31 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 55.5 (OCH3), 103.5 (CH), 106.7 (C), 107.3 (CH), 111.9 (C), 112.0 (CH), 112.4 (CH), 124.6 (C), 129.0 (CH), 132.0 (C), 138.2 (=CH), 140.2 (CH), 148.1 (CH), 154.2 (C), 156.3 (C). IR ν (cm−1): 3321, 3217, 1618, 1588, 1537, 1489, 1436, 1381, 1300, 1258, 1211, 1107, 1076, 1026, 993, 921, 846, 776, 729, 611. Elemental analysis calcd (%) for C15H13BrN4O: C, 52.19; H, 3.80; N, 16.23; found: C, 52.50; H, 4.06; N, 16.42.
3.2.19. 3-((2-(6-Chloropyridin-2-yl)hydrazono)methyl)-5-methoxy-1H-indole (19)
The general procedure was used with 5-methoxyindole-3-carboxaldehyde (0.25 g, 1.4 mmol) and 2-chloro-6-hydrazinopyridine (0.21 g, 1.4 mmol) to obtain pure compound 19 as a white solid (0.31 g, 1.0 mmol, 72% yield); mp (EtOH) 200–202 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.32. 1H NMR (DMSO-d6, 500 MHz) δ ppm 3.84 (s, 3H, OCH3), 6.71 (d, J = 8.5 Hz, 1H, ArH), 6.85 (dd, J = 8.5, 2.0 Hz, 1H, ArH), 7.09 (d, J = 8.5 Hz, 1H, ArH), 7.33 (d, J = 8.5 Hz, 1H, ArH), 7.64–7.71 (m, 2H, 2ArH), 7.73 (d, J = 2.0 Hz, 1H, ArH), 8.23 (s, 1H, =CH), 10.87 (s, 1H, NH), 11.33 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 55.2 (OCH3), 103.5 (CH), 103.8 (CH), 111.8 (C), 112.1 (CH), 112.2 (CH), 112.5 (CH), 124.6 (C), 129.3 (CH), 132.0 (C), 138.8 (=CH), 141.0 (CH), 148.3 (C), 154.3 (C), 157.5 (C). IR ν (cm−1): 3420, 3222, 1620, 1593, 1556, 1483, 1428, 1306, 1256, 12071093, 1067, 1022, 979, 912, 844, 802, 763, 698, 616. Elemental analysis calcd (%) for C15H13ClN4O: C, 59.91; H, 4.36; N, 18.63; found: C, 60.18; H, 4.44; N, 18.79.
3.2.20. 7-Chloro-4-(2-((1-methyl-1H-indol-3-yl)methylene)hydrazinyl)quinoline (20)
The general procedure was used with 1-methyl-1H-indole-3-carbaldehyde (0.25 g, 1.6 mmol) and 7-chloro-4-hydrazinoquinoline (0.30 g, 1.6 mmol) to obtain pure compound 20 as a yellow solid (0.36 g, 1.0 mmol, 68% yield); mp (EtOH) >250 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.32. 1H NMR (DMSO-d6, 500 MHz) δ ppm 3.84 (s, 3H, NCH3), 7.22–7.37 (m, 3H, 3ArH), 7.46–7.61 (m, 2H, 2ArH), 7.81–7.91 (m, 2H, 2ArH), 8.32 (d, J = 8.0 Hz, 1H, ArH), 8.38 (d, J = 8 Hz, 1H, ArH), 8.59 (s, 2H, =CH + ArH), 10.92 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 32.8 (NCH3),100.3 (CH), 110.3 (CH), 111.0 (C), 115.5 (C), 120.8 (CH), 121.9 (CH), 122.7 (CH), 124.0 (CH), 124.4 (CH), 124.5 (C), 127.6 (CH), 133.6 (CH), 138.7 (2C), 140.9 (=CH), 147.1 (C), 149.3 (C), 152.1 (CH). IR ν (cm−1): 3216, 1618, 1570, 1493, 1421, 1371, 1248, 1200, 1124, 1078, 862, 814, 739, 617. Elemental analysis calcd (%) for C19H15ClN4: C, 68.16; H, 4.52; N, 16.73; found: C, 68.34; H, 4.60; N, 16.91.
3.2.21. 3-((2-(5-Bromopyridin-2-yl)hydrazono)methyl)-1-methyl-1H-indole (21)
The general procedure was used with 1-methyl-1H-indole-3-carbaldehyde (0.25 g, 1.6 mmol) and 5-bromo-2-hydrazinopyridine (0.29 g, 1.6 mmol) to obtain pure compound 21 as a beige solid (0.28 g, 0.8 mmol, 54% yield); mp (EtOH) 204–206 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.3. 1H NMR (CDCl3, 500 MHz) δ ppm 3.81 (s, 3H, CH3), 7.24 (s, 1H, ArH), 7.27–7.36 (m, 4H, 4ArH), 7.70 (dd, J = 8.0, 2.0 Hz, 1H, ArH), 7.97 (s, 1H, =CH), 8.16 (d, J = 2.0 Hz, 1H, ArH), 8.24 (br s, 1H, NH), 8.32 (d, J = 8.0 Hz, 1H, ArH). 13C NMR (CDCl3, 125 MHz) δ ppm 33.3 (CH3), 108.8 (CH), 108.9 (C), 109.6 (CH), 111.9 (C), 121.1 (CH), 122.4 (CH), 123.3 (CH), 125.2 (C), 131.0 (CH), 136.7 (CH), 137.9 (C), 140.5 (=CH), 148.3 (CH), 155.9 (C). IR ν (cm−1): 3190, 1622, 1586, 1537, 1444, 1375, 1302, 1124, 1074, 996, 919, 815, 740, 686, 634. Elemental analysis calcd (%) for C15H13BrN4: C, 54.73; H, 3.98; N, 17.02; found: C, 54.59; H, 3.86; N, 16.94.
3.2.22. 3-((2-(6-Chloropyridin-2-yl)hydrazono)methyl)-1-methyl-1H-indole (22)
The general procedure was used with 1-methyl-1H-indole-3-carbaldehyde (0.25 g, 1.6 mmol) and 2-chloro-6-hydrazinopyridine (0.23 g, 1.6 mmol) to obtain pure compound 22 as a beige solid (0.34 g, 1.1 mmol, 76% yield); mp (EtOH) 205–207 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.3. 1H NMR (CDCl3, 500 MHz) δ ppm 3.80 (s, 3H, NCH3), 6.73 (d, J = 7.0 Hz, 1H, ArH), 7.23 (s, 1H, ArH), 7.25–7.37 (m, 4H, 4ArH), 7.56 (t, J = 8.0 Hz, 1H, ArH), 7.94 (s, 1H, =CH), 8.25 (s, 1H, NH), 8.33 (d, J = 8.0 Hz, 1H, ArH). 13C NMR (CDCl3, 125 MHz) δ ppm 33.2 (NCH3), 105.2 (CH), 109.6 (CH), 111.8 (C), 114.0 (CH), 121.1 (CH), 122.4 (CH), 123.2 (CH), 125.2 (C), 131.2 (CH), 137.2 (=CH), 137.9 (C), 140.5 (CH), 149.0 (C), 157.1 (C). IR ν (cm−1): 3307, 1618, 1586, 1553, 1472, 1425, 1312, 1192, 1091, 977, 933, 773, 749, 698, 619. Elemental analysis calcd (%) for C15H13ClN4: C, 63.27; H, 4.60; N, 19.68; found: C, 63.47; H, 4.91; N, 19.95.
3.2.23. 7-Chloro-4-(2-((5-methoxy-1-methyl-1H-indol-3-yl)methylene)hydrazinyl)quinoline (23)
The general procedure was used with 5-bromo-1-methyl-1H-indole-3-carbaldehyde (0.40 g, 2.1 mmol) and 7-chloro-4-hydrazinoquinoline (0.40 g, 2.1 mmol) to obtain pure compound 23 as a yellow solid (0.52 g, 1.4 mmol, 68% yield); mp (EtOH) 221–223 °C; Rf (EtOAc:Cyclohexane 1:1) = 0. 1H NMR (DMSO-d6, 500 MHz) δ ppm 3.81 (s, 3H, NCH3), 3.89 (s, 3H, OCH3), 6.94 (d, J = 8.0 Hz, 1H, ArH), 7.28 (d, J = 5.0 Hz, 1H, ArH), 7.44 (d, J = 8.0 Hz, 1H, ArH), 7.55 (d, J = 8.5 Hz, 1H, ArH), 7.78- 7.85 (m, 2H, 2ArH), 7.86 (s, 1H, ArH), 8.38 (d, J = 8.5 Hz, 1H, ArH), 8.53–8.64 (m, 2H, ArH + =CH), 10.92 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 33.0 (NCH3), 55.3 (OCH3), 100.1 (CH), 103.7 (CH), 110.5 (C), 111.2 (CH), 112.2 (CH), 115.6 (C), 124.0 (CH), 124.5 (CH), 125.1 (C), 127.6 (CH), 132.8 (CH), 133.6 (C), 133.8 (C), 141.1 (=CH), 147.1 (C), 149.3 (C), 152.1 (CH), 154.7 (C). IR ν (cm−1): 3168, 1612, 1565, 1527, 1425, 1372, 1360, 1305, 1251, 1115, 854, 784, 637, 604. Elemental analysis calcd (%) for C20H17ClN4O: C, 65.84; H, 4.70; N, 15.36; found: C, 65.67; H, 4.51; N, 15.22.
3.2.24. 2-(2-((5-Methoxy-1-methyl-1H-indol-3-yl)methylene)hydrazinyl)quinoline (24)
The general procedure was used with 5-bromo-1-methyl-1H-indole-3-carbaldehyde (0.30 g, 1.6 mmol) and 2-hydrazinoquinoline (0.25 g, 1.6 mmol) to obtain pure compound 24 as a white solid (0.37 g, 1.1 mmol, 70% yield); mp (EtOH) 171–173 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.23. 1H NMR (DMSO-d6, 500 MHz) δ ppm 3.78 (s, 3H, CH3), 3.89 (s, 3H, OCH3), 6.92 (d, J = 8.0 Hz, 1H, ArH), 7.17–7.31 (m, 1H, ArH), 7.40 (d, J = 8.0 Hz, 1H, ArH), 7.47–7.62 (m, 3H, 3ArH), 7.66–7.70 (m, 1H, ArH), 7.73 (d, J = 8.0 Hz, 1H, ArH), 7.82 (s, 1H, ArH), 8.20 (d, J = 8.0 Hz, 1H, ArH), 8.26 (s, 1H, =CH), 10.99 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 32.9 (CH3), 55.2 (OCH3), 103.6 (CH), 109.0 (CH), 111.0 (CH), 112.1 (CH), 121.9 (CH), 123.8 (C), 125.0 (CH), 125.5 (C), 127.8 (CH), 129.6 (CH), 132.6 (CH), 132.7 (2C), 137.3 (CH), 137.9 (CH), 147.5 (C), 154.5 (C), 156.0 (C). IR ν (cm−1): 3309, 1655, 1604, 1568, 1541, 1504, 1430, 1345, 1256, 1114, 1040, 931, 862, 815, 788, 763, 678, 643. Elemental analysis calcd (%) for C20H18N4O: C, 72.71; H, 5.49; N, 16.96; found: C, 73.04; H, 5.71; N, 17.13.
3.2.25. 4-(2-((5-Bromo-1H-indol-3-yl)methylene)hydrazinyl)-7-chloroquinoline (25)
The general procedure was used with 5-bromoindole-3-carboxaldehyde (0.25 g, 1.1 mmol) and 7-chloro-4-hydrazinoquinoline (0.22 g, 1.1 mmol) to obtain pure compound 25 as a yellow solid (0.32 g, 0.8 mmol, 72% yield); mp (EtOH) 247–249 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.3. 1H NMR (DMSO-d6, 500 MHz) δ ppm 7.20–7.81 (m, 4H, 4ArH), 7.85–8.20 (m, 2H, 2ArH), 8.21–8.50 (m, 2H, 2ArH), 8.60 (s, 2H, =CH + ArH), 10.97 (s, 1H, NH), 11.80 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 100.1 (CH), 111.5 (C), 113.1 (C), 114.0 (CH), 115.5 (C), 123.7 (CH), 124.0 (CH), 124.5 (CH), 125.1 (CH), 125.8 (C), 127.6 (CH), 131.2 (CH), 133.7 (C), 135.9 (C), 141.0 (=CH), 147.1 (C), 149.3 (C), 152.0 (CH). IR ν (cm−1): 3348, 3033, 1614, 1580, 1483, 1447, 1420, 1368, 1291, 1236, 1200, 1135, 1078, 1022, 929, 854, 798, 669, 622. Elemental analysis calcd (%) for C18H12BrClN4: C, 54.09; H, 3.03; N, 14.02; found: C, 54.34; H, 3.23; N, 14.19.
3.2.26. 5-Bromo-3-((2-(5-bromopyridin-2-yl)hydrazono)methyl)-1H-indole (26)
The general procedure was used with 5-bromoindole-3-carboxaldehyde (0.25 g, 1.1 mmol) and 5-bromo-2-hydrazinopyridine (0.20 g, 1.1 mmol) to obtain pure compound 26 as a beige solid (0.31 g, 0.7 mmol, 71% yield); mp (EtOH) 209–211 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.23. 1H NMR (DMSO-d6, 500 MHz) δ ppm 7.06 (d, J = 9.0 Hz, 1H, ArH), 7.32 (dd, J = 8.5, 2.5 Hz, 1H, ArH), 7.41 (d, J = 8.5 Hz, 1H, ArH), 7.78 (d, J = 3.0 Hz, 1H, ArH), 7.87 (dd, J = 9.0, 2.5 Hz, 1H, ArH), 8.16 (d, J = 2.5 Hz, 1H, ArH), 8.24 (s, 1H, =CH), 8.30 (d, J = 3.0 Hz, 1H, ArH) 10.75 (s, 1H, NH), 11.65 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 107.1 (CH), 107.2 (C), 111.8 (C), 112.8 (C), 113.9 (CH), 123.6 (CH), 124.9 (CH), 125.8 (C), 130.0 (CH), 135.7 (C), 137.7 (=CH), 140.3 (CH), 148.2 (CH), 156.1 (C). IR ν (cm−1): 3406, 3192, 1620, 1590, 1543, 1514, 1444, 1425,1383, 1290, 1134, 1086, 997, 873, 819, 786, 664. Elemental analysis calcd (%) for C14H10Br2N4: C, 42.67; H, 2.56; N, 14.22; found: C, 42.91; H, 2.72; N, 14.36.
3.2.27. 5-Bromo-3-((2-(6-chloropyridin-2-yl)hydrazono)methyl)-1H-indole (27)
The general procedure was used with 5-bromoindole-3-carboxaldehyde (0.25 g, 1.1 mmol) and 2-chloro-6-hydrazinopyridine (0.16 g, 1.1 mmol) to obtain pure compound 27 as a beige solid (0.26 g, 0.7 mmol, 68% yield); mp (EtOH) 214–216 °C; Rf (EtOAc:Cyclohexane 1:1) = 0.3. 1H NMR (DMSO-d6, 500 MHz) δ ppm 6.73 (d, J = 7.5 Hz, 1H, ArH), 7.12 (d, J = 7.5 Hz, 1H, ArH), 7.33 (dd, J = 8.5, 2.0 Hz, 1H, ArH), 7.41 (d, J = 8.5 Hz, 1H, ArH), 7.72 (d, J = 7.5 Hz, 1H, ArH), 7.79 (d, J = 2.0 Hz, 1H, ArH), 8.22 (s, 1H, =CH), 8.32 (d, J = 2.0 Hz, 1H, ArH), 10.95 (s, 1H, NH), 11.67 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) δ ppm 103.7 (CH), 111.6 (C), 112.5 (CH), 112.8 (C), 113.9 (CH), 123.6 (CH), 125.0 (CH), 125.8 (C), 130.3 (CH), 135.8 (C), 138.2 (=CH), 141.1 (CH), 148.3 (C), 157.4 (C). IR ν (cm−1): 3342, 3160, 1622, 1593, 1556, 1531, 1474, 1425, 1317, 1283, 1234, 1097, 983, 920, 876, 773, 612. Elemental analysis calcd (%) for C14H10BrClN4: C, 48.10; H, 2.88; N, 16.03; found: C, 48.42; H, 2.96; N, 16.27.
3.3. Cell Proliferation Assay
Compounds 1–27 were tested on a panel of 60 human cancer cell lines at the National Cancer Institute, Germantown, MD [41]. The cytotoxicity studies were conducted using a 48h exposure protocol using the sulforhodamine B assay [42,43].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph16050691/s1, Copies of 1H and 13C NMR spectra for all synthesized hydrazones 1–27 are provided in this section.
Author Contributions
A.G. and E.B.: Conceptualization, resources, supervision, data curation, project administration, validation. A.G.: writing—original draft, writing—review and editing. G.N.: data curation, formal analysis, investigation, methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Executive Unit for Financing Higher Education, Research, Development and Innovation (UEFISCDI), Bucharest, Romania, grant number PN-III-P4-ID-PCE-2020-0818, acronym: REPAIR. The APC was also funded by UEFISCDI.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors. Supplementary Materials include copies of NMR spectra of all synthesized compounds.
Acknowledgments
The authors acknowledge the Executive Unit for Financing Higher Education, Research, Development and Innovation (UEFISCDI), Romania, for financial support of this work which is part of the project PN-III-P4-ID-PCE-2020-0818 (REPAIR). The authors gratefully acknowledge the National Cancer Institute (NCI) for the biological evaluation of compounds on their 60-cell panel. The testing was performed by the Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis (the URL to the Program’s website: http://dtp.cancer.gov, accessed 12 December 2022). The authors also thank the CERNESIM Center within the Interdisciplinary Research Institute at “Alexandru Ioan Cuza” University of Iasi, Romania for the infrastructure used in recording NMR experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 10 April 2023).
- Fattorusso, C.; Campiani, G.; Kukreja, G.; Persico, M.; Butini, S.; Romano, M.P.; Altarelli, M.; Ros, S.; Brindisi, M.; Savini, L.; et al. Design, synthesis, and structure-activity relationship studies of 4-quinolinyl- and 9-acrydinylhydrazones as potent antimalarial agents. J. Med. Chem. 2008, 51, 1333–1343. [Google Scholar] [CrossRef]
- Patel, A.J.; Patel, M.P.; Dholakia, A.B.; Patel, V.C.; Patel, D.S. Antitubercular, Antimalarial Activity and Molecular Docking Study of New Synthesized 7-Chloroquinoline Derivatives. Polycyl. Aromat. Compd. 2022, 42, 4717–4725. [Google Scholar] [CrossRef]
- Kalita, J.; Chetia, D.; Rudrapal, M. Design, Synthesis, Antimalarial Activity and Docking Study of 7-Chloro-4-(2-(substituted benzylidene)hydrazineyl)quinolines. Med. Chem. 2020, 16, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Gut, J.; Rosenthal, P.J.; Kumar, V. 1H-1,2,3-Triazole-tethered isatin-7-chloroquinoline and 3-hydroxy-indole-7-chloroquinoline conjugates: Synthesis and antimalarial evaluation. Bioorg. Med. Chem. Lett. 2014, 24, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Avula, S.R.; Palnati, G.R.; Singh, S.V.; Srivastava, K.; Puri, S.K.; Saxena, J.K. Synthesis and in vitro evaluation of new chloroquine-chalcone hybrids against chloroquine-resistant strain of Plasmodium falciparum. Bioorg. Med. Chem. Lett. 2012, 22, 5455–5459. [Google Scholar] [CrossRef] [PubMed]
- Thuy, L.T.; Tien, H.X.; Hoang, V.D.; Vu, T.K. Design, Synthesis and In Vitro Antimalarial Evaluation of New Quinolinylhydrazone Derivatives. Lett. Drug Des. Discov. 2012, 9, 163–168. [Google Scholar] [CrossRef]
- Luo, W.; Lu, W.-Q.; Cui, K.-Q.; Liu, Y.; Wang, J.; Guo, C. N1-{4-[(10S)-Dihydroartemisinin-10-oxyl]}phenylmethylene-N2-(2-methylquinoline-4-yl)hydrazine derivatives as antiplasmodial falcipain-2 inhibitors. Med. Chem. Res. 2012, 21, 3073–3079. [Google Scholar] [CrossRef]
- Singh, T.; Stein, R.G.; Biel, J.H. Antimalarials. 4-Proximal hydrazine derivatives of 7-chloroquinoline. J. Med. Chem. 1969, 12, 801–803. [Google Scholar] [CrossRef]
- Soares, R.R.; Antinarelli, L.M.R.; de O Souza, I.; Lopes, F.V.; Scopel, K.K.G.; Coimbra, E.S.; da Silva, A.D.; Abramo, C. In Vivo Antimalarial and In Vitro Antileishmanial Activity of 4-Aminoquinoline Derivatives Hybridized to Isoniazid or Sulfa or Hydrazine Groups. Lett. Drug Des. Discov. 2017, 14, 597–604. [Google Scholar] [CrossRef]
- Coimbra, E.S.; Antinarelli, L.M.R.; da Silva, A.D.; Bispo, M.L.F.; Kaiser, C.R.; de Souza, M.V.N. 7-Chloro-4-quinolinyl Hydrazones: A Promising and Potent Class of Antileishmanial Compounds. Chem. Biol. Drug Des. 2013, 81, 658–665. [Google Scholar] [CrossRef]
- Antinarelli, L.M.R.; Dias, R.M.P.; Souza, I.O.; Lima, W.P.; Gameiro, J.; da Silva, A.D.; Coimbra, E.S. 4-Aminoquinoline Derivatives as Potential Antileishmanial Agents. Chem. Biol. Drug Des. 2015, 86, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Antinarelli, L.M.R.; de Oliveira Souza, I.; Glanzmann, N.; das Chagas Almeida, A.; Porcino, G.N.; Vasconcelos, E.G.; da Silva, A.D. Aminoquinoline compounds: Effect of 7-chloro-4-quinolinylhydrazone derivatives against Leishmania amazonensis. Exp. Parasitol. 2016, 171, 10–16. [Google Scholar] [CrossRef]
- de L Ferreira, M.; Gonçalves, R.S.B.; de F Cardoso, L.N.; Kaiser, C.R.; Candéa, A.L.P.; das Graças M de O Henriques, M.; Lourenço, M.C.S.; Bezerra, F.A.F.M.; de Souza, M.V.N. Synthesis and Antitubercular Activity of Heteroaromatic Isonicotinoyl and 7-Chloro-4-Quinolinyl Hydrazone Derivatives. Sci. World J. 2010, 10, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Kremer, L.; Louw, S.; Guéradel, Y.; Chibale, K.; Biot, C. Synthesis and in vitro antitubercular activity of ferrocene-based hydrazones. Bioorg. Med. Chem. Lett. 2011, 21, 2866–2868. [Google Scholar] [CrossRef] [PubMed]
- Maguene, G.M.; Jakhlal, J.; Ladyman, M.; Vallin, A.; Ralambomanana, D.A.; Bousquet, T.; Maugein, J.; Lebibi, J.; Pelinski, L. Synthesis and antimycobacterial activity of a series of ferrocenyl derivatives. Eur. J. Med. Chem. 2010, 46, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Candea, A.L.P.; de L Ferreira, M.; Pais, K.C.; de F Cardoso, L.N.; Kaiser, C.R.; das Graças M de O Henriques, M.; Lourenço, M.C.S.; Bezerra, F.A.F.M.; de Souza, M.V.N. Synthesis and antitubercular activity of 7-chloro-4-quinolinylhydrazones derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 6272–6274. [Google Scholar] [CrossRef] [PubMed]
- Salve, P.S.; Alegaon, S.G.; Sriram, D. Three-component, one-pot synthesis of anthranilamide Schiff bases bearing 4-aminoquinoline moiety as Mycobacterium tuberculosis gyrase inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 1859–1866. [Google Scholar] [CrossRef]
- Pyta, K.; Janas, A.; Szukowska, M.; Pecyna, P.; Jaworska, M.; Gajecka, M.; Bartl, F.; Przybylski, P. Synthesis, docking and antibacterial studies of more potent amine and hydrazone rifamycin congeners than rifampicin. Eur. J. Med. Chem. 2019, 167, 96–104. [Google Scholar] [CrossRef]
- Al-Shaalan, N.H. Synthesis, characterization and biological activities of Cu(II), Co(II), Mn(II), Fe(II), and UO2(VI) complexes with a new Schiff base hydrazone: o-hydroxyacetophenone-7-chloro-4-quinoline hydrazone. Molecules 2011, 16, 8629–8645. [Google Scholar] [CrossRef] [PubMed]
- Khizhan, E.I.; Khizhan, A.I.; Tikhonova, G.A.; Maslova, V.Y. Antioxidant Activity of Arylhydrazones in Sunflower Oil Oxidation. Russ. J. Appl. Chem. 2012, 85, 460–464. [Google Scholar] [CrossRef]
- Khizhan, E.I.; Vinogradov, V.V.; Morenko, V.V.; Nikolaevskii, A.N.; Khizhan, A.I.; Zarechnaya, O.M.; Dmitruk, A.F. Antiradical Activity of Aryl- and Hetarylhydrazones in Ethylbenzene Oxidation. Russ. J. Gen. Chem. 2013, 83, 1529–1536. [Google Scholar] [CrossRef]
- Duval, A.R.; Carvalho, P.H.; Soares, M.C.; Gouvea, D.P.; Siqueira, G.M.; Lund, R.G.; Cunico, W. 7-Chloroquinolin-4-yl arylhydrazone derivatives: Synthesis and antifungal activity. Sci. World J. 2011, 11, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.H.D.A.; Duval, A.R.; Leite, F.R.M.; Nedel, F.; Cunico, W.; Lund, R.G. (7-Chloroquinolin-4-yl)arylhydrazones: Candida albicans enzymatic repression and cytotoxicity evaluation, Part 2. J. Enz. Inhib. Med. Chem. 2016, 31, 126–131. [Google Scholar] [CrossRef]
- Thomas, J.; Berkoff, C.E.; Flagg, W.B.; Gallo, J.J.; Haff, R.F.; Pinto, C.A.; Pellerano, C.; Savini, L. Antiviral quinolinehydrazones. Modified Free-Wilson analysis. J. Med. Chem. 1975, 18, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Debnath, U.; Mukherjee, S.; Joardar, N.; Sinha Babu, S.P.; Jana, K.; Misra, A.K. Aryl quinolinyl hydrazone derivatives as anti-inflammatory agents that inhibit TLR4 activation in the macrophages. Eur. J. Pharm. Sci. 2019, 134, 102–115. [Google Scholar] [CrossRef]
- Zaccagnini, L.; Rossetti, G.; Tran, T.H.; Salzano, G.; Gandini, A.; Colini Baldeschi, A.; Bolognesi, M.L.; Carloni, P.; Legname, G. In silico/in vitro screening and hit evaluation identified new phenothiazine anti-prion derivatives. Eur. J. Med. Chem. 2020, 196, 112295. [Google Scholar] [CrossRef]
- Popp, F.D. Potential anticonvulsants. VIII. Some hydrazones of indole-3-carboxaldehyde. J. Heterocycl. Chem. 1984, 21, 617–619. [Google Scholar] [CrossRef]
- Gomes, I.; Fujita, W.; Gupta, A.; Saldanha, S.A.; Negri, A.; Pinello, C.E.; Eberhart, C.; Roberts, E.; Filizola, M.; Hodder, P.; et al. Identification of a μ-δ opioid receptor heteromer-biased agonist with antinociceptive activity. Proc. Natl. Acad. Sci. USA 2013, 110, 12072–12077. [Google Scholar] [CrossRef]
- de L. F. Bispo, M.; de Alcantara, C.C.; de Moraes, M.O.; do O Pessoa, C.; Rodrigues, F.A.R.; Kaiser, C.R.; Wardell, S.M.S.V.; Wardell, J.L.; de Souza, M.V.N. A new and potent class of quinoline derivatives against cancer. Monatsh. Chem. 2015, 146, 2041–2052. [Google Scholar] [CrossRef]
- Montenegro, R.C.; Lotufo, L.V.; Odorico de Moraes, M.; do O Pessoa, C.; Rodrigues, F.A.R.; de Lima Ferreira Bispo, M.; Freire, B.A.; Kaiser, C.R.; de Souza, M.V.N. Cytotoxic Activity of Polysubstituted 7-chloro-4-quinolinylhydrazone Derivatives. Lett. Drug Des. Discov. 2012, 9, 251–256. [Google Scholar] [CrossRef]
- Montenegro, R.C.; Lotufo, L.V.; Odorico de Moraes, M.; do O Pessoa, C.; Rodrigues, F.A.R.; de Lima Ferreira Bispo, M.; de Faria Cardoso, L.N.; Kaiser, C.R.; de Souza, M.V.N. Synthesis and antitumoral evaluation of 7-chloro-4-quinolinylhydrazones derivatives. Med. Chem. 2011, 7, 599–604. [Google Scholar] [CrossRef]
- Montenegro, R.C.; Lotufo, L.V.; Odorico de Moraes, M.; do O Pessoa, C.; Rodrigues, F.A.R.; de Lima Ferreira Bispo, M.; de Alcantara, C.C.; Kaiser, C.R.; de Souza, M.V.N. 1-(7-Chloroquinolin-4-yl)-2-[(1H-pyrrol-2-yl)methylene]hydrazine. A potent compound against cancer. Med. Chem. Res. 2012, 21, 3615–3619. [Google Scholar] [CrossRef]
- Moise, I.-M.; Ghinet, A.; Belei, D.; Dubois, J.; Farce, A.; Bîcu, E. New indolizine-chalcones as potent inhibitors of human farnesyltransferase: Design, synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2016, 26, 3730–3734. [Google Scholar] [CrossRef] [PubMed]
- Bâcu, E.; Petrovanu, M.; Grandclaudon, P.; Couture, A. Diamides et dipeptides lies au cycle phenothiazinique. Rev. Roum. Chim. 1999, 44, 699–703. [Google Scholar]
- Available online: https://dtp.cancer.gov/organization/dscb/compoundSubmission/structureSelection.htm (accessed on 21 March 2023).
- Spreitzer, H.; Scholz, M.; Gescheidt, G.; Daub, J. Electron-Transfer Chemistry and Redox-Switching of Stilbene-Like Heteroaromatic Compounds—Syntheses, Optoelectrochemical and ESR/ENDOR Studies. Liebigs Ann. 1996, 2069–2077. [Google Scholar] [CrossRef]
- Scott, F.L.; O’Halloran, J.K.; O’Driscoll, J.; Hegarty, A.F. Synthesis and solvolysis of a new group of reactive halides, the imidazolin-2-ylidenehydrazonyl chlorides; a route to 6,7-dihydro-3-aryl-5H-imidazolo[2,1-c]-s-triazoles. J. Chem. Soc. Perkin Trans. 1972, 1, 2224–2231. [Google Scholar] [CrossRef]
- Sandoz-Wander Inc. Heterocyclic Substituted Midazoline Hydrazones. Patent US3528968 A, 15 September 1970. [Google Scholar]
- Purgatorio, R.; Gambacorta, N.; Catto, M.; de Candia, M.; Pisani, L.; Espargaró, A.; Sabaté, R.; Cellamare, S.; Nicolotti, O.; Altomare, C.D. Pharmacophore Modeling and 3D-QSAR Study of Indole and Isatin Derivatives as Antiamyloidogenic Agents Targeting Alzheimer’s Disease. Molecules 2020, 25, 5773. [Google Scholar] [CrossRef]
- Boyd, R.B. The NCI in vitro Anticancer Drug Discovery Screen. In Anticancer Drug Development Guide; Preclinical Screening, Clinical Trials, and Approval; Teicher, B., Ed.; Humana Press Inc.: Totowa, NJ, USA, 1997; pp. 23–42. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesh, H.; Kennedy, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Available online: https://dtp.cancer.gov/discovery_development/nci-60/methodology.htm (accessed on 3 April 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).