Photomodulation Approaches to Overcome Antimicrobial Resistance
Abstract
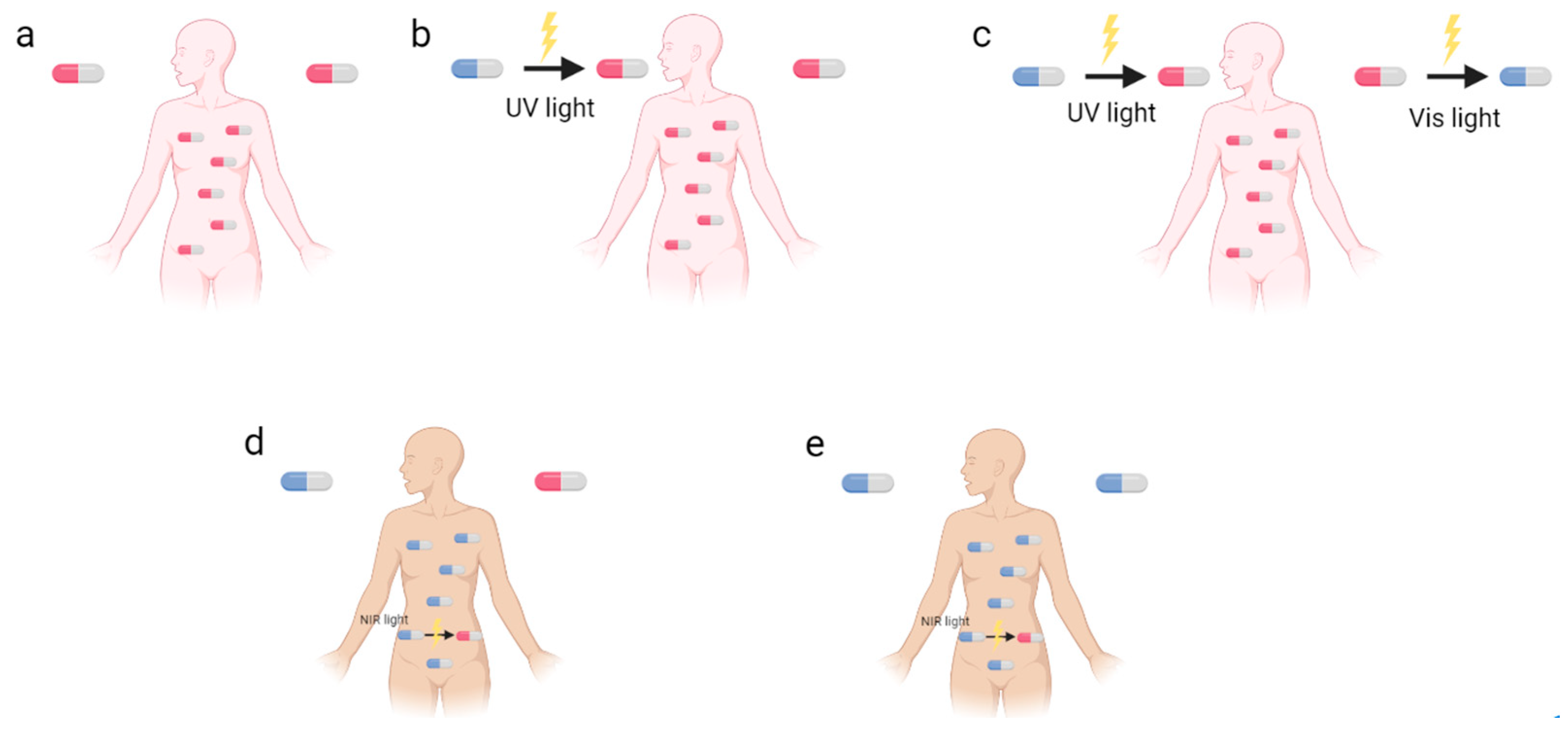
1. Introduction
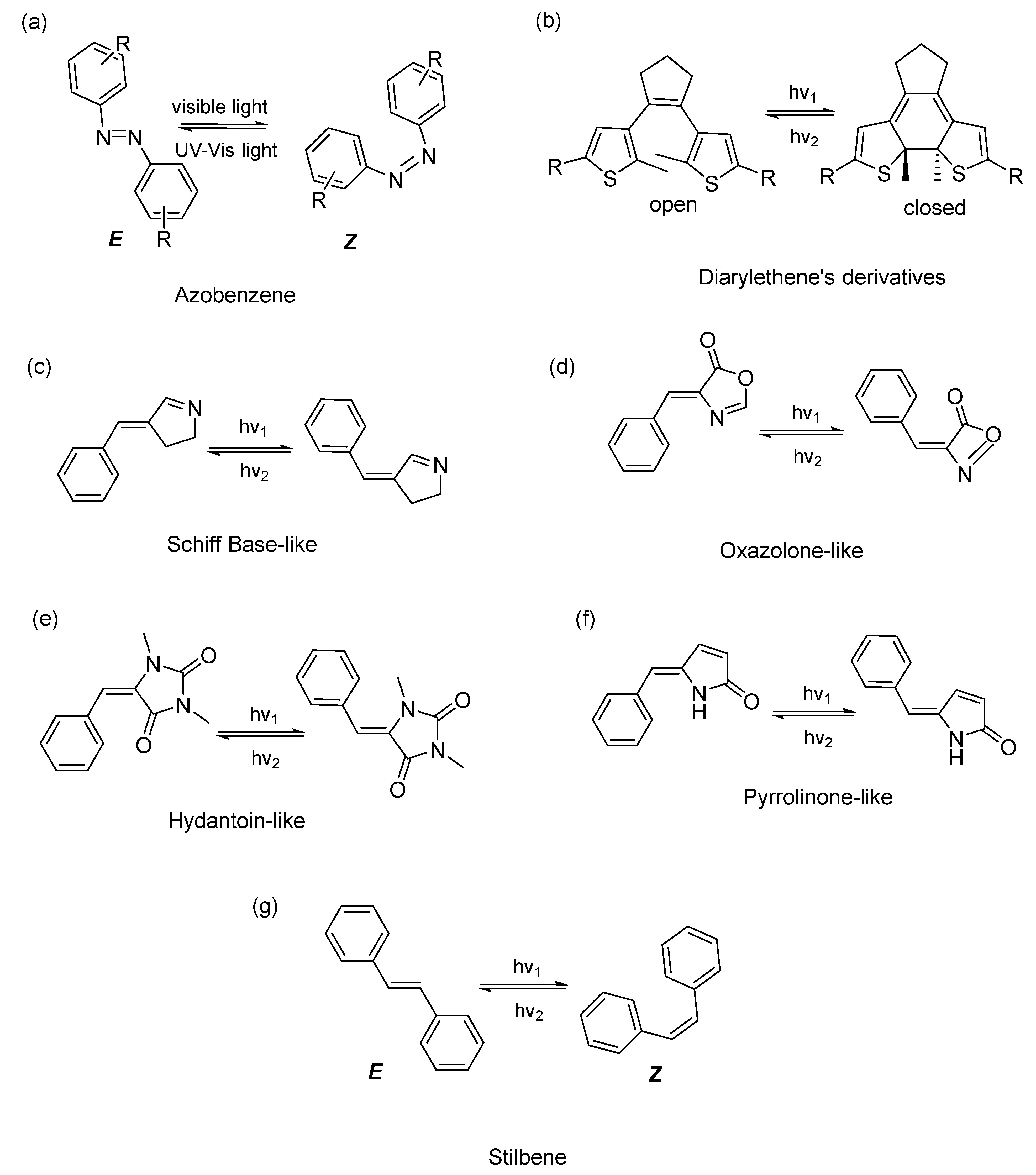
2. Different Classes of Photoswitches and Some Examples in Antibacterial Applications
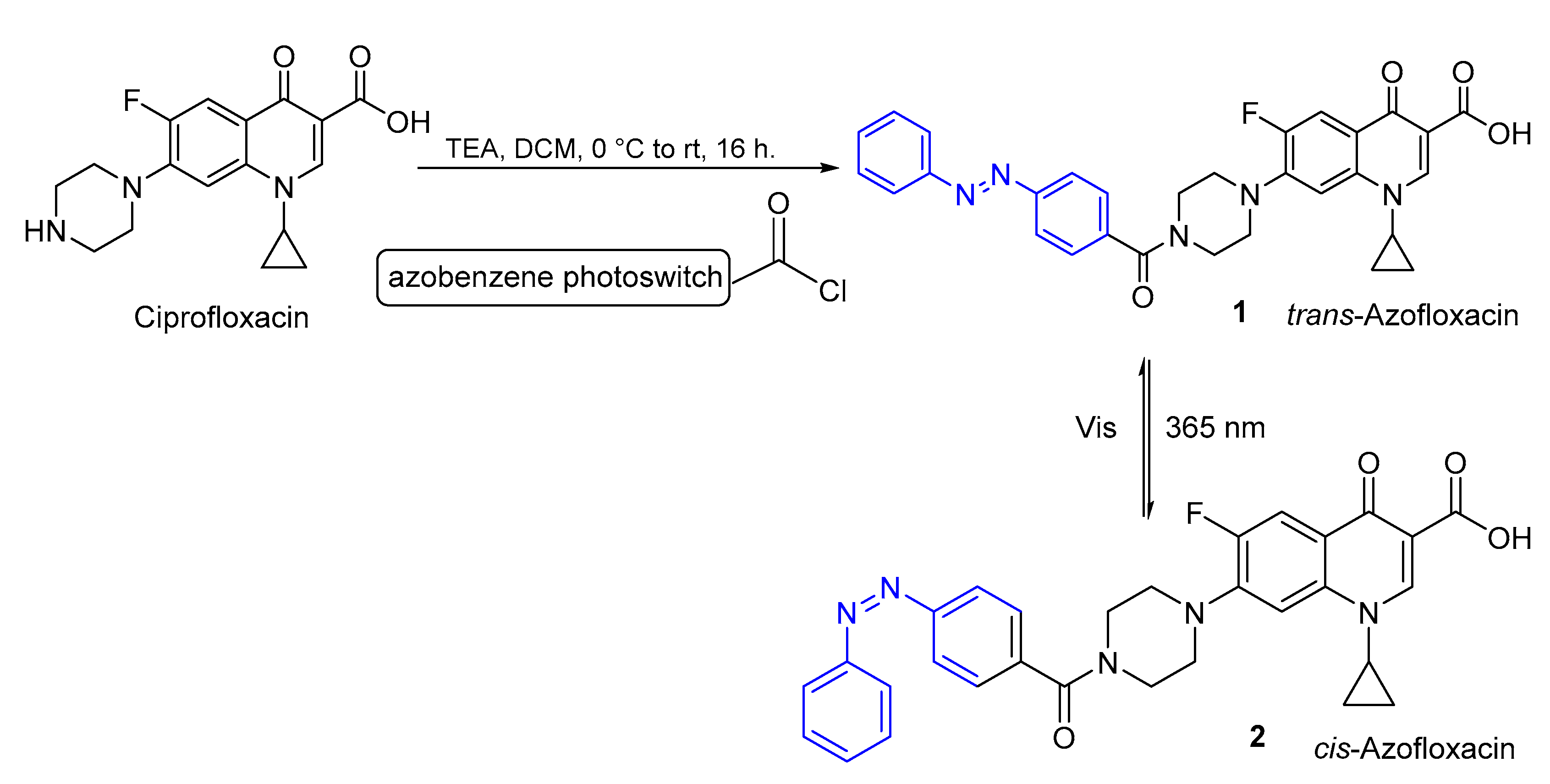
2.1. Azobenzenes
2.2. Diarylethenes
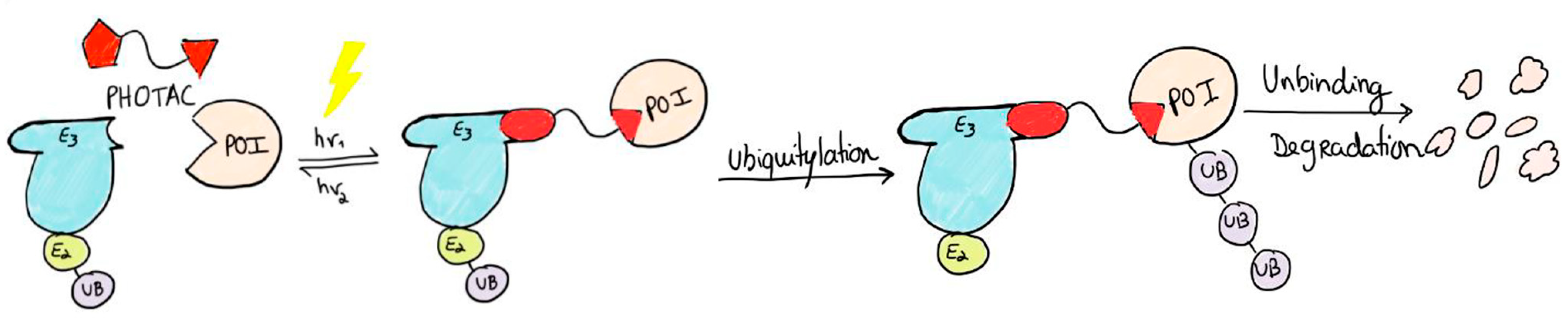
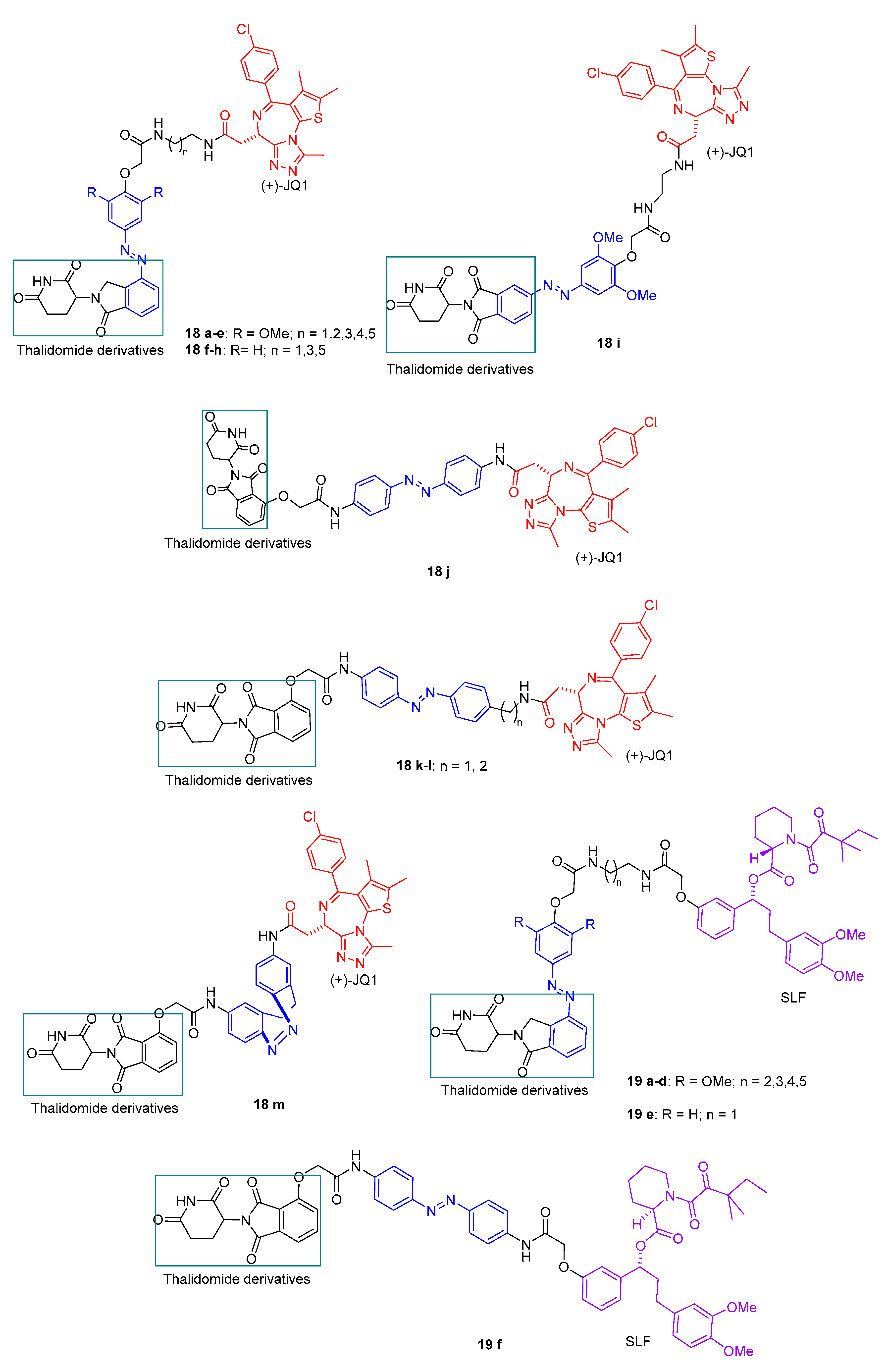
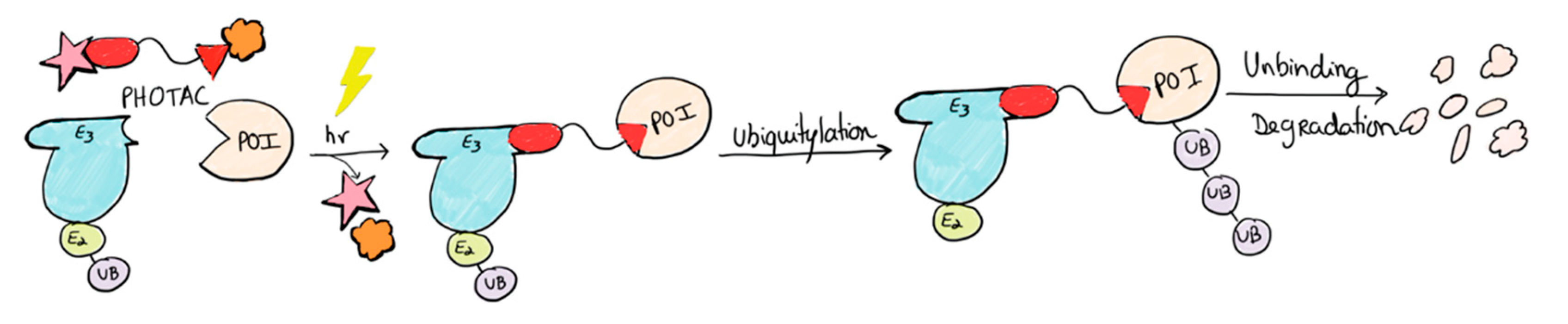
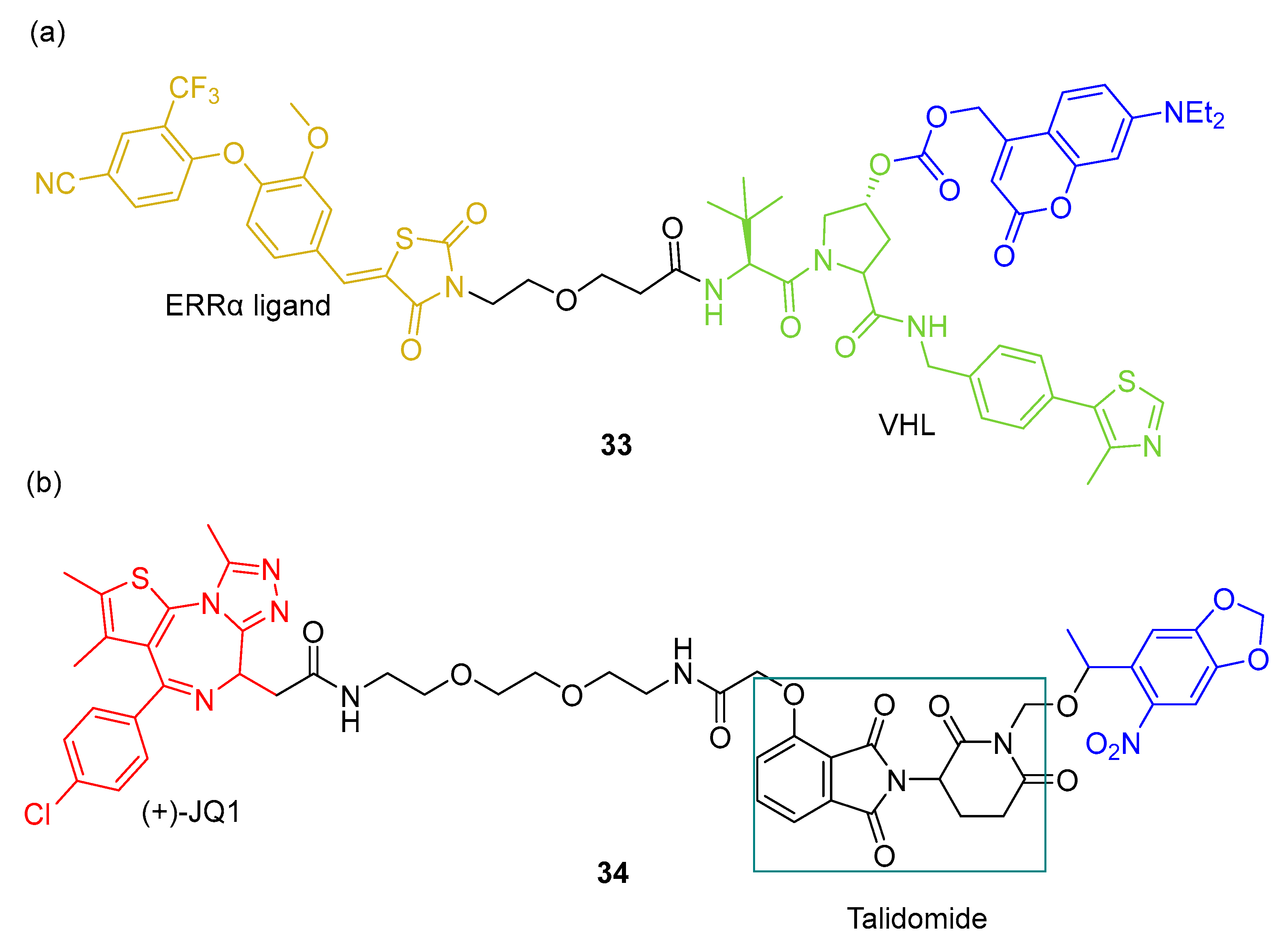
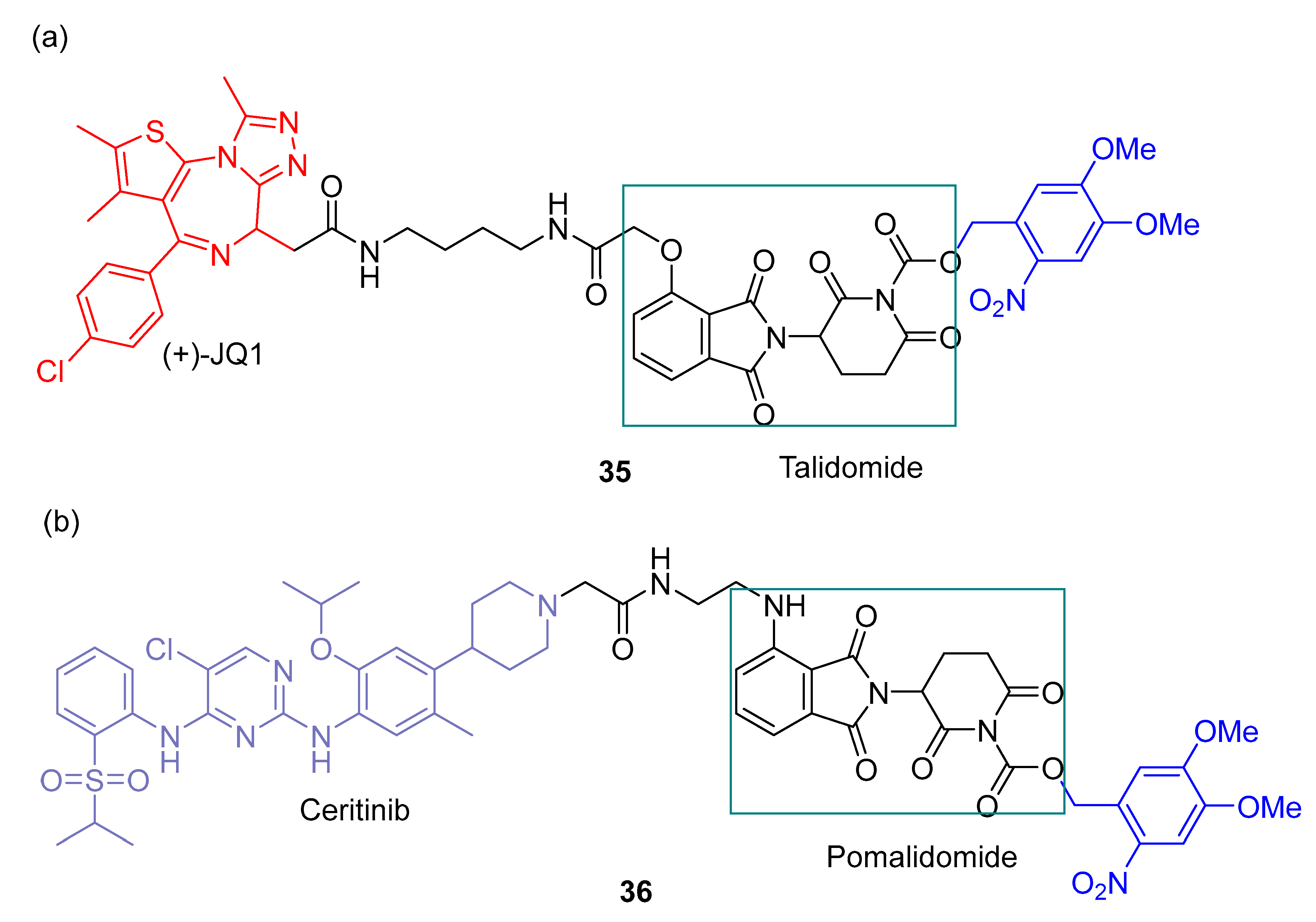
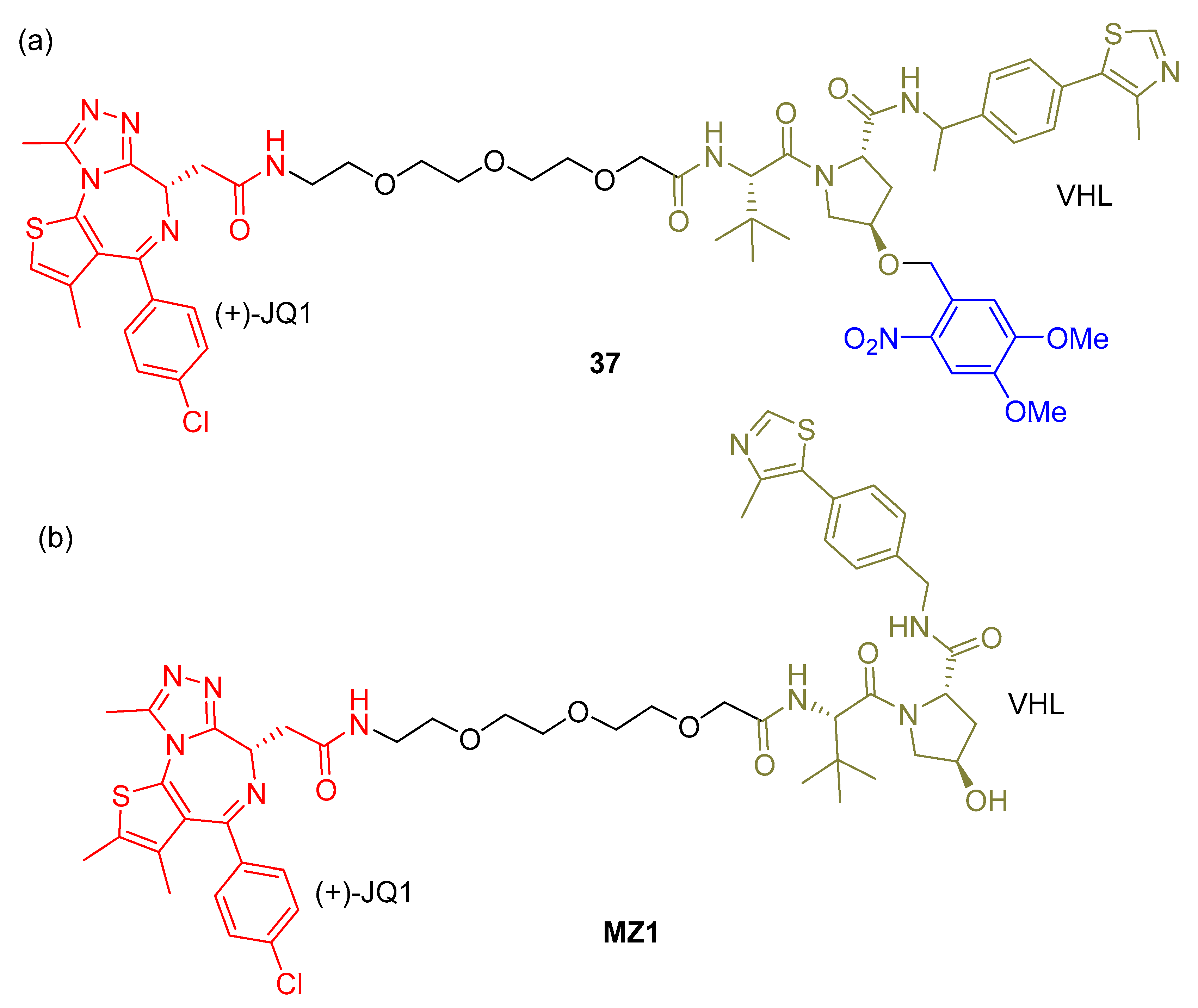
2.3. PHOTACs
3. Different Classes of Photocleavage and Some Examples in Antibacterial Applications
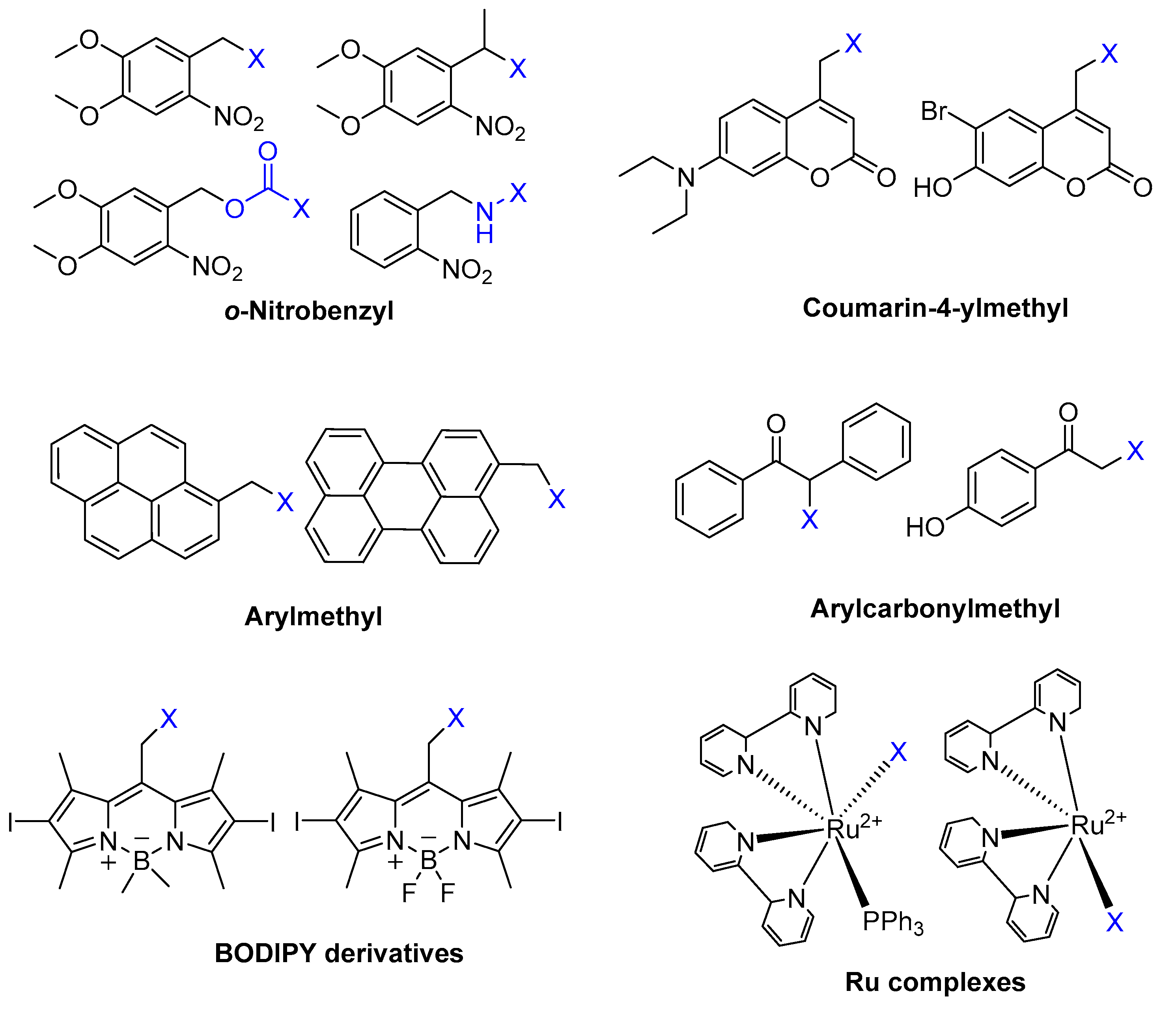
3.1. BODIPY
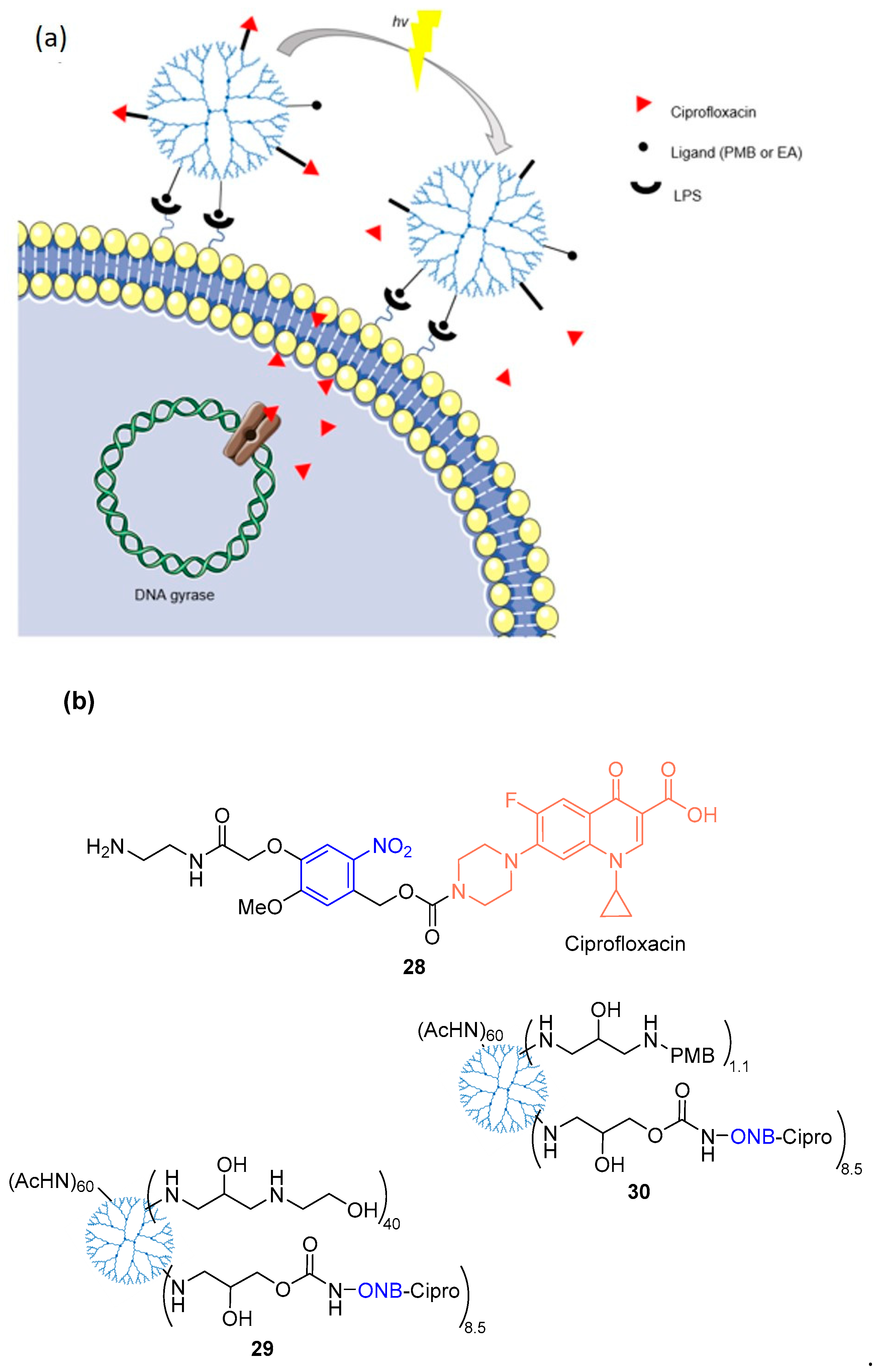
3.2. o-Nitrobenzyl Derivatives
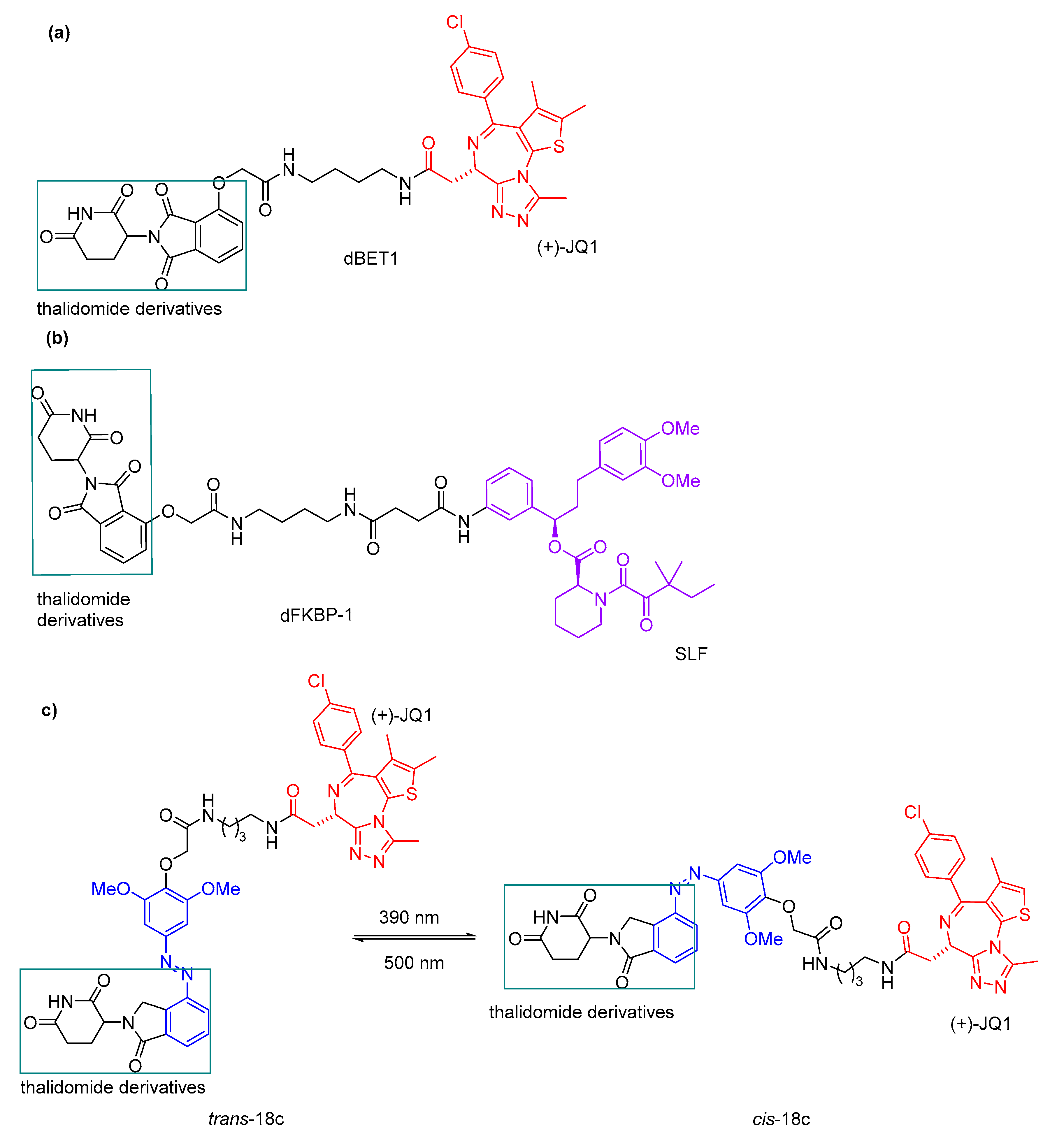
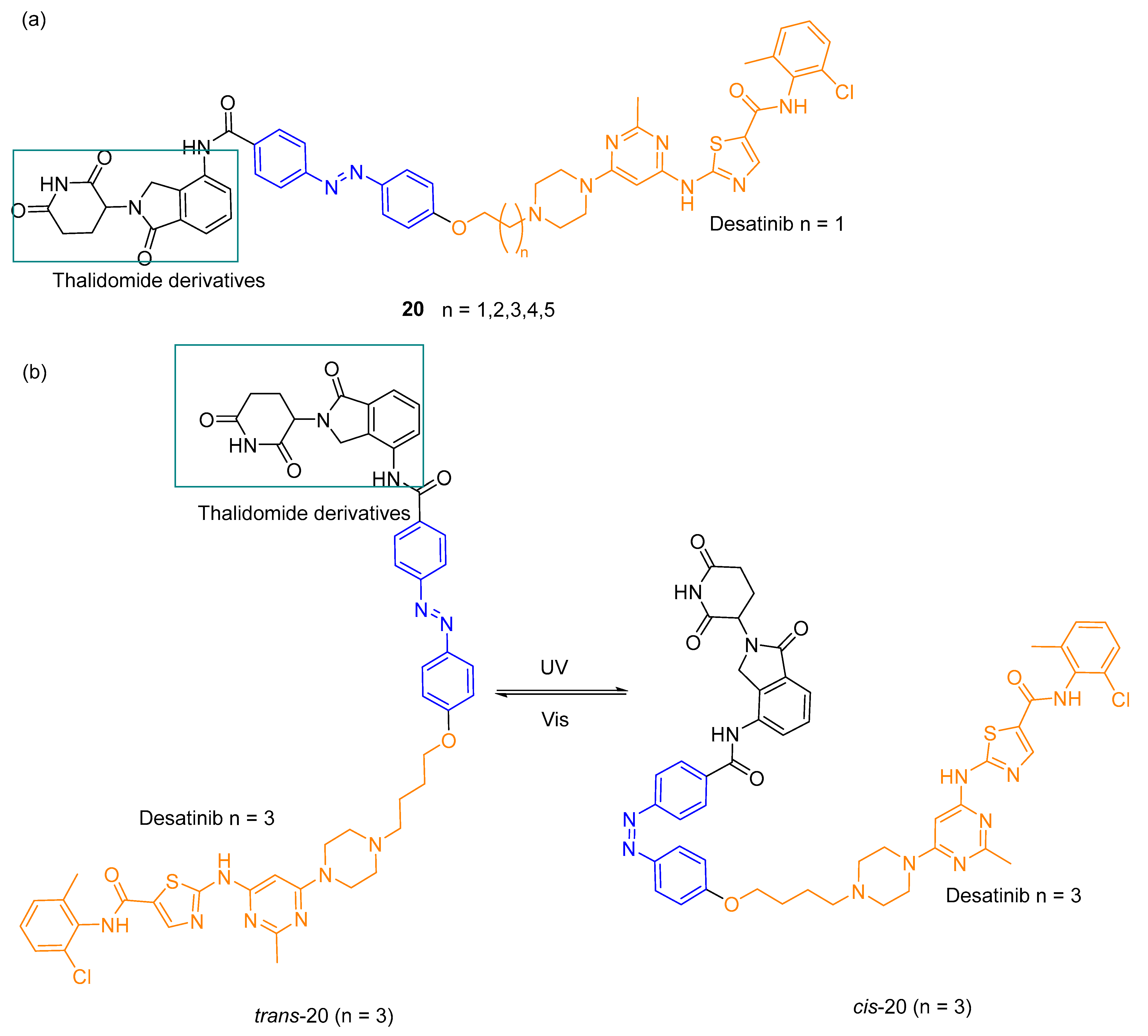
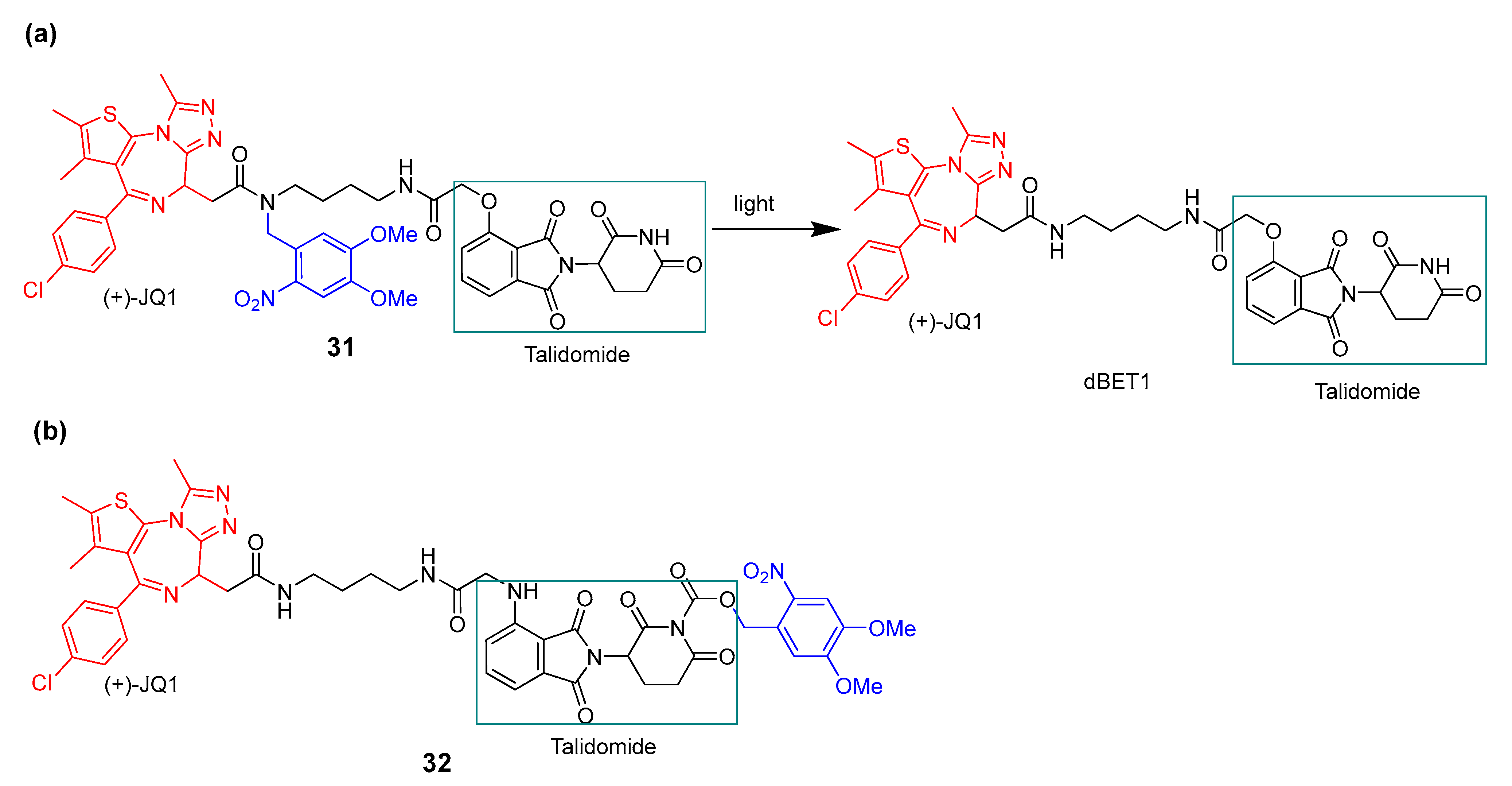
3.3. Photocaged PROTACs
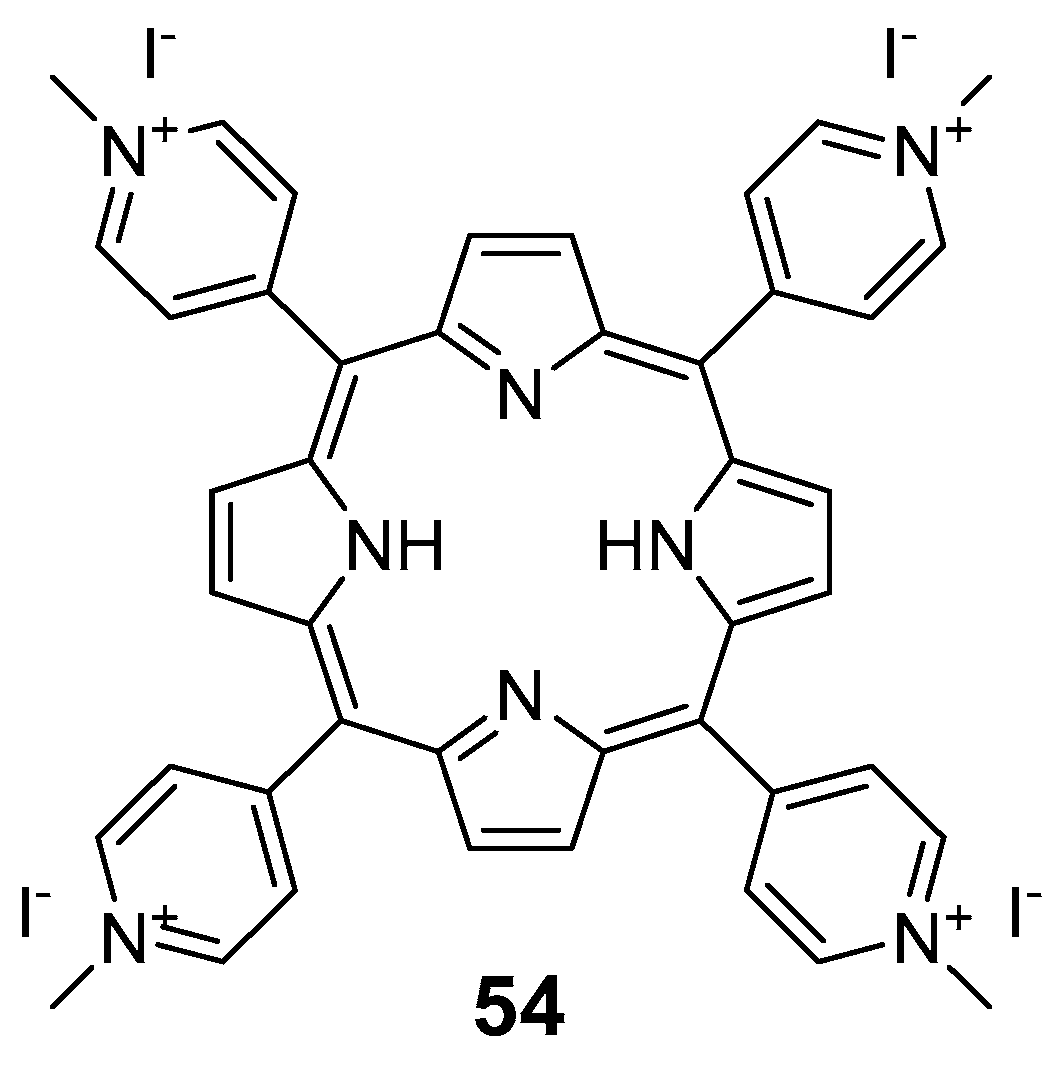
4. Porphyrins Combining Photoswitch Systems and Some Application Examples
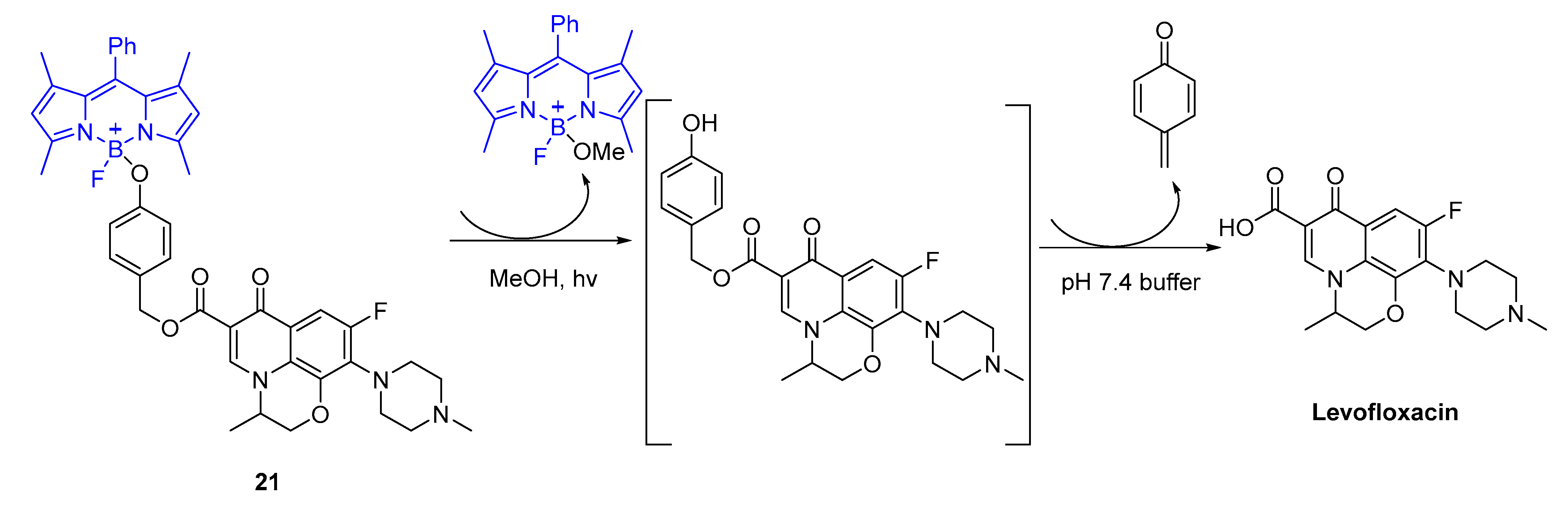
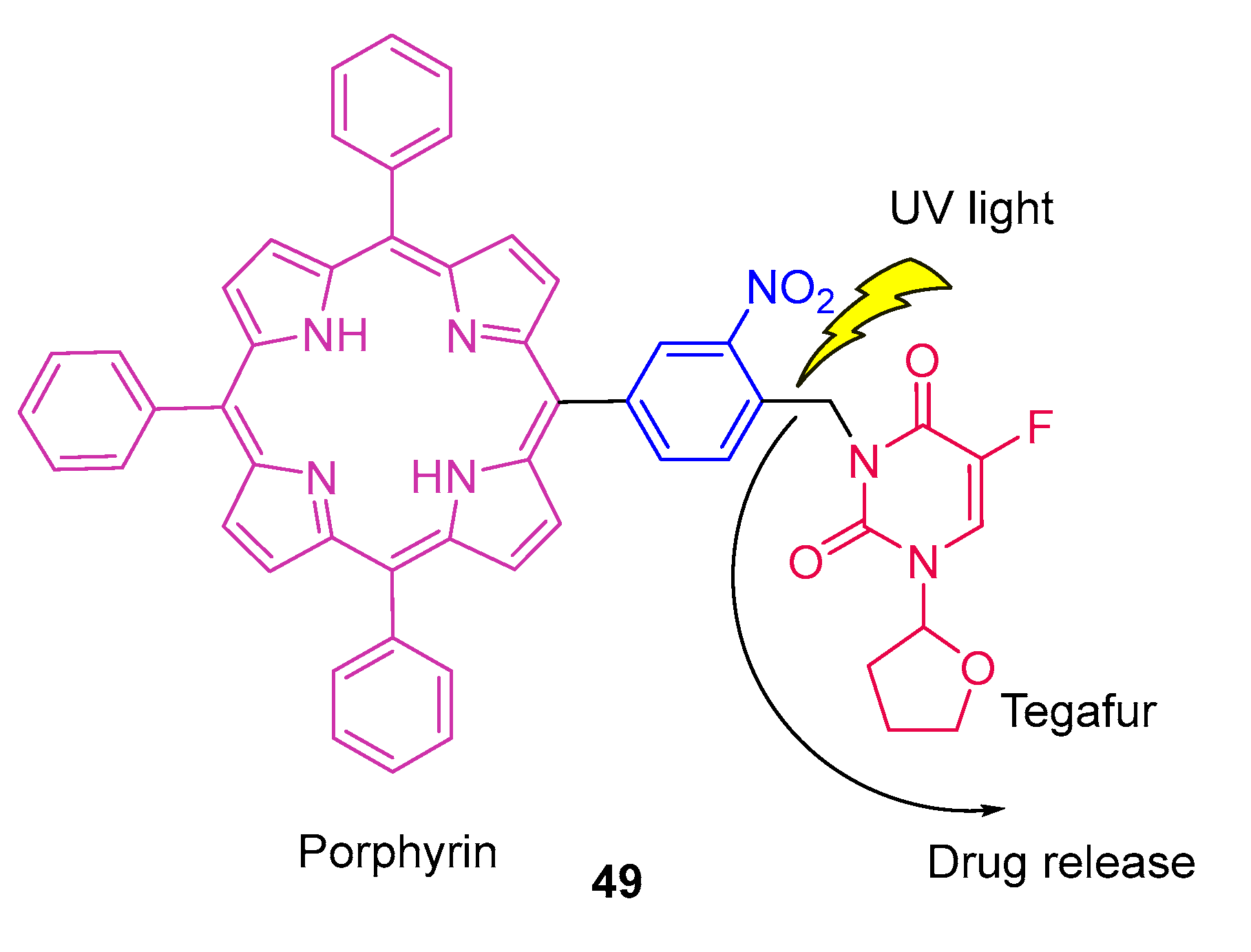
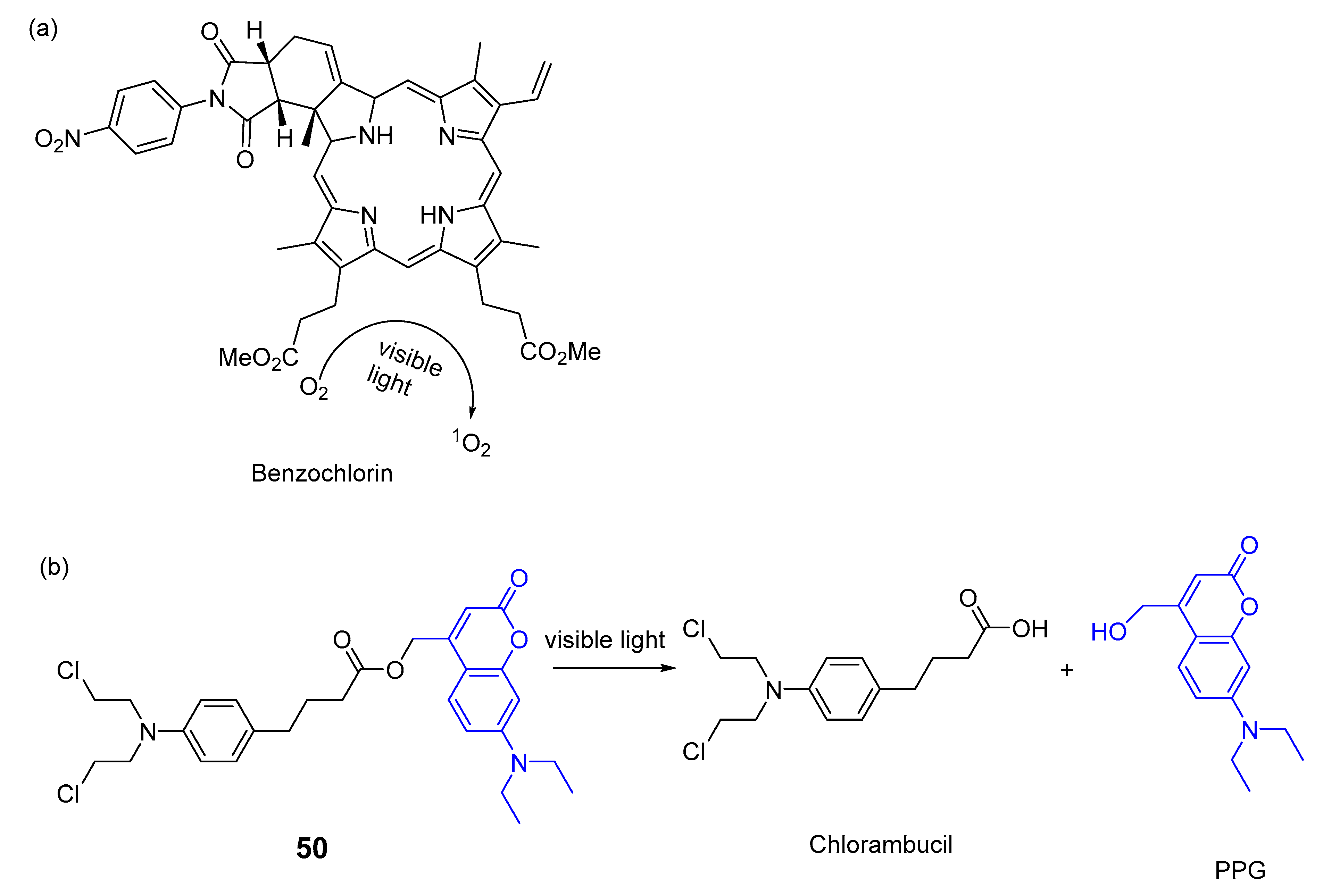
5. Porphyrins Combining Photocleavage Systems and Some Application Examples
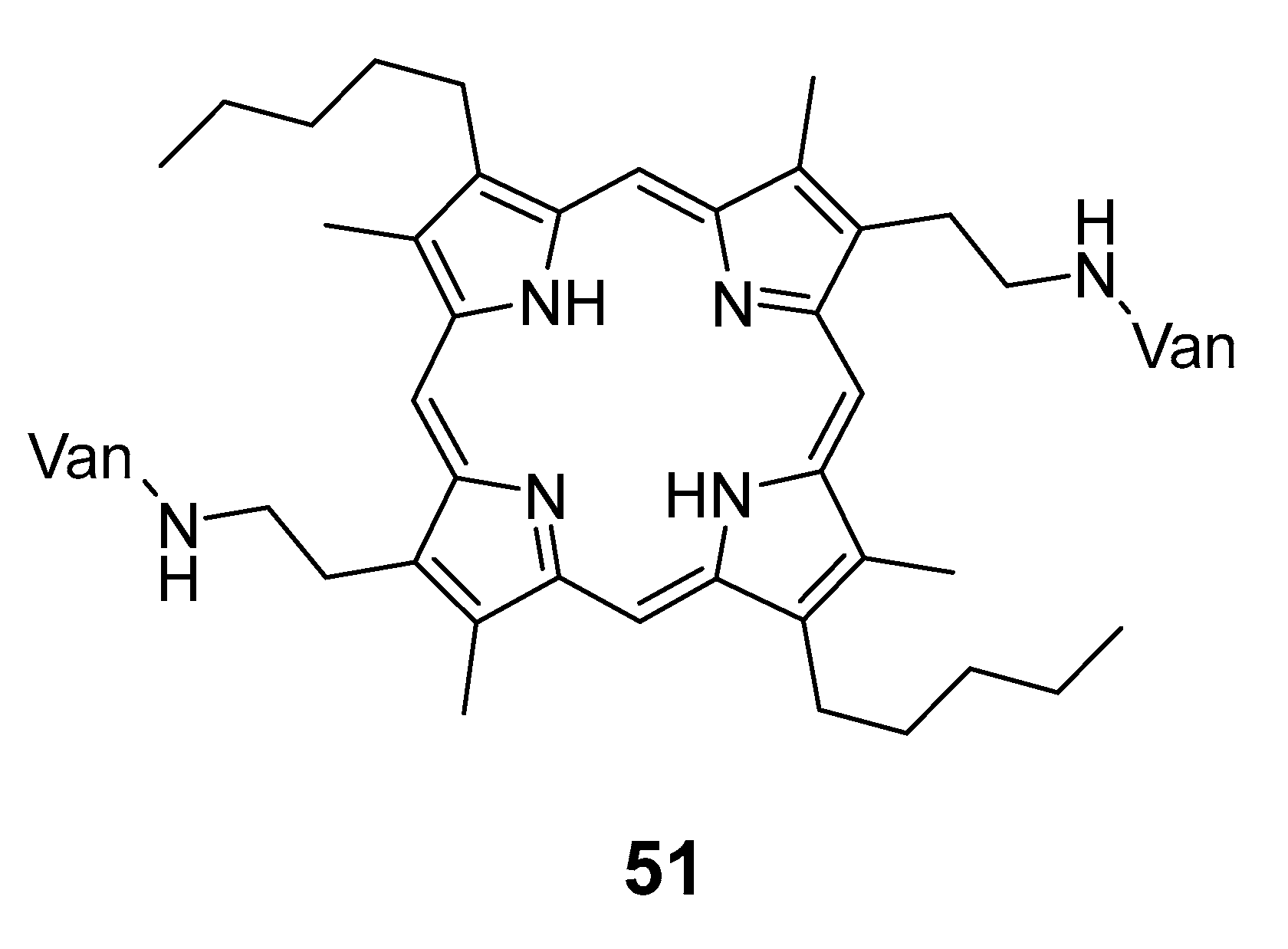
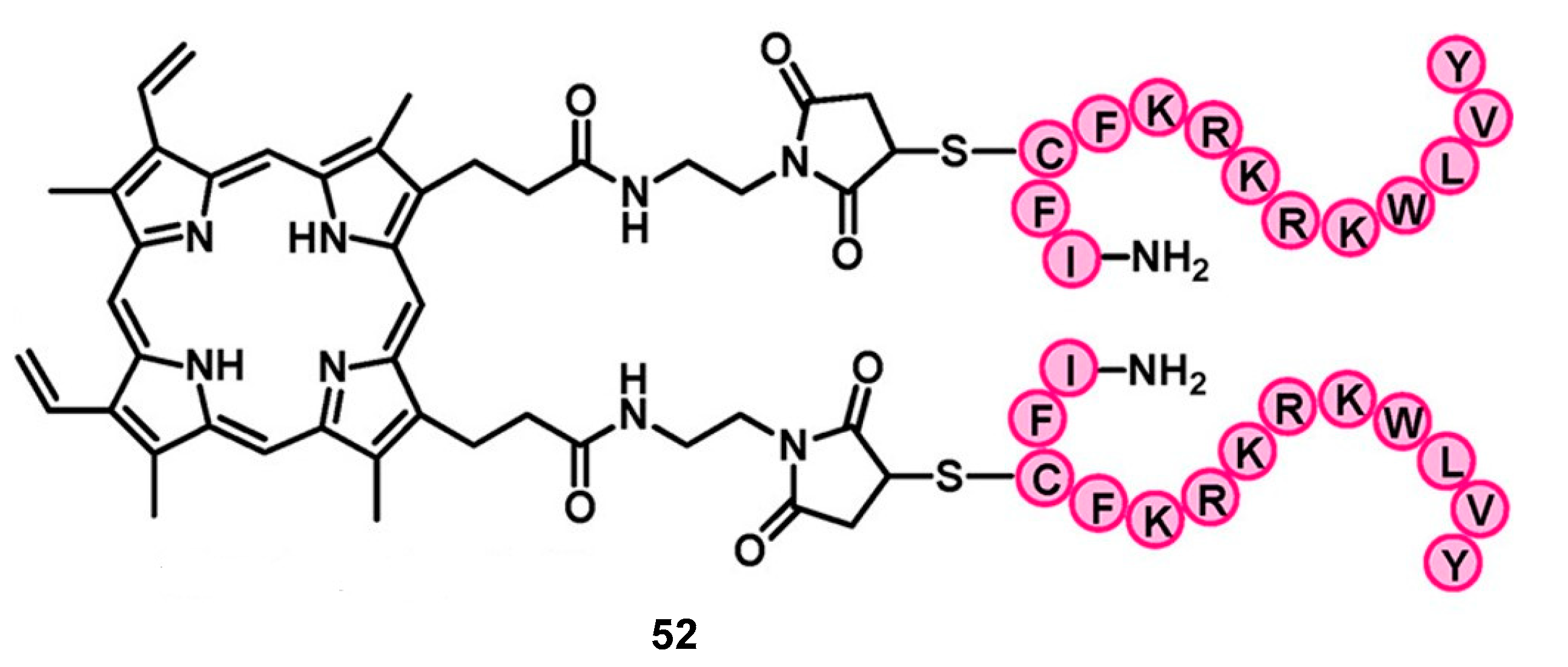
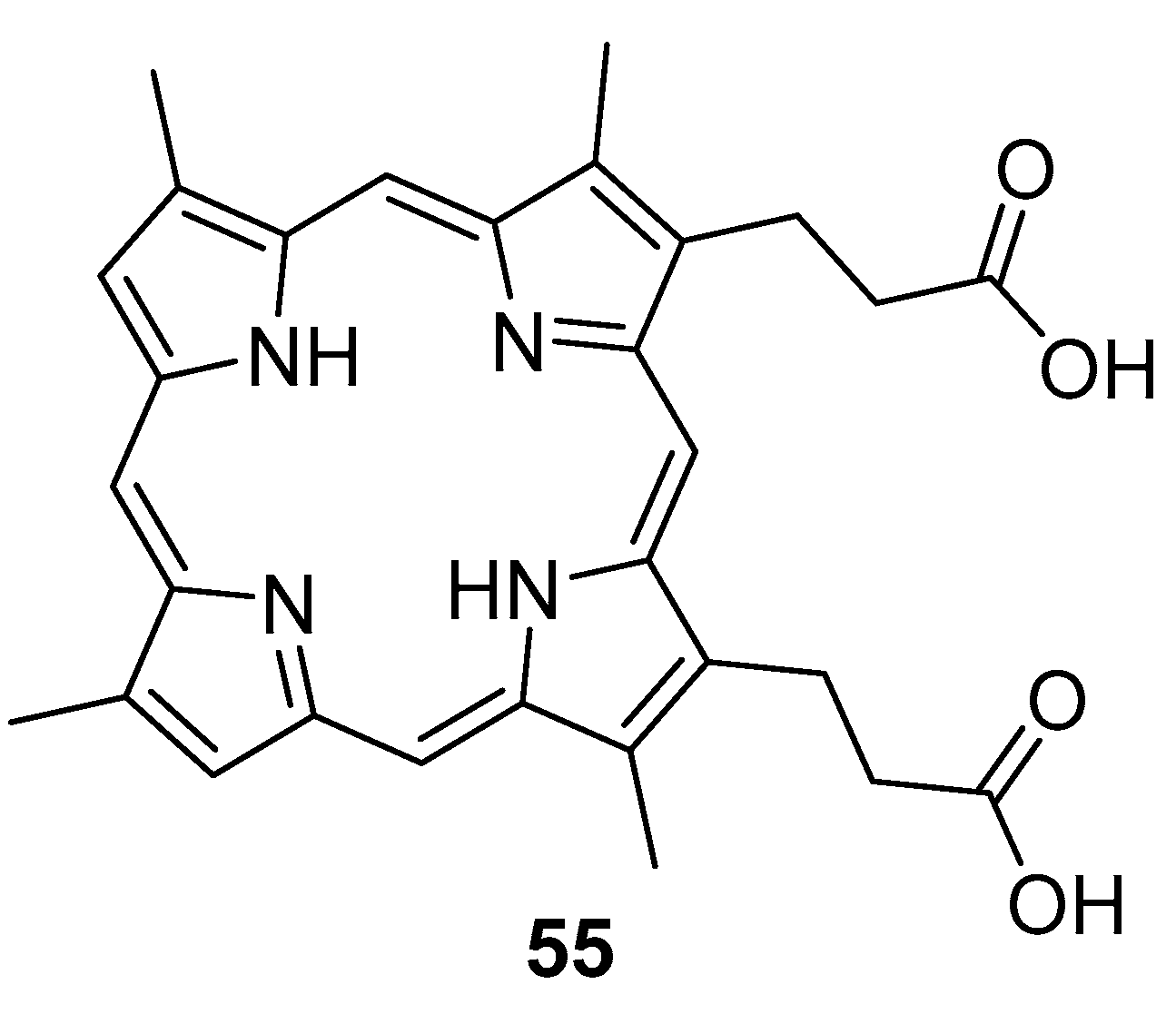
6. Porphyrins with Antibacterial Activity
7. Concluding Remarks and Future Challenges
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reessing, F.; Szymanski, W. Beyond Photodynamic Therapy: Light-Activated Cancer Chemotherapy. Curr. Med. Chem. 2017, 24, 4905–4950. [Google Scholar] [CrossRef]
- Jia, S.; Sletten, E.M. Spatiotemporal Control of Biology: Synthetic Photochemistry Toolbox with Far-Red and Near-Infrared Light. ACS Chem. Biol. 2022, 17, 3255–3269. [Google Scholar] [CrossRef] [PubMed]
- Broichhagen, J.; Frank, J.A.; Trauner, D. A Roadmap to Success in Photopharmacology. Acc. Chem. Res. 2015, 48, 1947–1960. [Google Scholar] [CrossRef] [PubMed]
- Velema, W.A.; Szymanski, W.; Feringa, B.L. Photopharmacology: Beyond Proof of Principle. J. Am. Chem. Soc. 2014, 136, 2178–2191. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization: Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 21 March 2023).
- Weinstain, R.; Slanina, T.; Kand, D.; Klan, P. Visible-to-NIR-Light Activated Release: From Small Molecules to Nanomaterials. Chem. Rev. 2020, 120, 13135–13272. [Google Scholar] [CrossRef]
- Liu, J.Z.; Kang, W.R.; Wang, W.P. Photocleavage-based Photoresponsive Drug Deliver. Photochem. Photobiol. 2022, 98, 288–302. [Google Scholar] [CrossRef]
- Hull, K.; Morstein, J.; Trauner, D. In Vivo Photopharmacology. Chem. Rev. 2018, 118, 10710–10747. [Google Scholar] [CrossRef]
- Velema, W.A.; Hansen, M.J.; Lerch, M.M.; Driessen, A.J.M.; Szymanski, W.; Feringa, B.L. Ciprofloxacin-Photoswitch Conjugates: A Facile Strategy for Photopharmacology. Bioconjugate Chem. 2015, 26, 2592–2597. [Google Scholar] [CrossRef]
- Mosinger, J.; Lang, K.; Kubát, P. Photoactivatable Nanostructured Surfaces for Biomedical Applications. In Light-Responsive Nanostructured Systems for Applications in Nanomedicine; Sortino, S., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 135–168. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Dias, C.J.; Neves, M.G.P.M.S.; Almeida, A.; Faustino, M.A.F. The Role of Photoactive Materials Based on Tetrapyrrolic Macrocycles in Antimicrobial Photodynamic Therapy. In Handbook of Porphyrin Science; World Scientific: Singapore, 2022; Volume 46, pp. 201–277. [Google Scholar]
- Q Mesquita, M.; J Dias, C.; P M S Neves, M.G.; Almeida, A.; F Faustino, M.A. Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy. Molecules 2018, 23, 2424. [Google Scholar] [CrossRef]
- Henderson, B.W. Photodynamic Therapy: Basic Principles and Clinical Applications; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- De Silva, P.; Saad, M.A.; Thomsen, H.C.; Bano, S.; Ashraf, S.; Hasan, T. Photodynamic therapy, priming and optical imaging: Potential co-conspirators in treatment design and optimization—A Thomas Dougherty Award for Excellence in PDT paper. J. Porphyr. Phthalocyanines 2020, 24, 1320–1360. [Google Scholar] [CrossRef]
- Santos, L.L.; Oliveira, J.; Monteiro, E.; Santos, J.; Sarmento, C. Treatment of Head and Neck Cancer with Photodynamic Therapy with Redaporfin: A Clinical Case Report. Case Rep. Oncol. 2018, 11, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and photochemistry of photodynamic therapy: Fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef]
- Rkein, A.M.; Ozog, D.M. Photodynamic Therapy. Dermatol. Clin. 2014, 32, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jung, H.Y.; Park, H.J. Topical PDT in the Treatment of Benign Skin Diseases: Principles and New Applications. Int. J. Mol. Sci. 2015, 16, 23259–23278. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Almeida, A.; Faustino, M.A.F.; Neves, M.G.P.M.S. Antimicrobial Photodynamic Therapy in the Control of COVID-19. Antibiotics 2020, 9, 320. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Nadais, H.; Almeida, A. Potential applications of porphyrins in photodynamic inactivation beyond the medical scope. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 34–57. [Google Scholar] [CrossRef]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef]
- Pucelik, B.; Dąbrowski, J.M. Chapter Three—Photodynamic inactivation (PDI) as a promising alternative to current pharmaceuticals for the treatment of resistant microorganisms. In Advances in Inorganic Chemistry; van Eldik, R., Hubbard, C.D., Eds.; Academic Press: Cambridge, MA, USA, 2022; Volume 79, pp. 65–108. [Google Scholar]
- Varzandeh, M.; Mohammadinejad, R.; Esmaeilzadeh-Salestani, K.; Dehshahri, A.; Zarrabi, A.; Aghaei-Afshar, A. Photodynamic therapy for leishmaniasis: Recent advances and future trends. Photodiagn. Photodyn. Ther. 2021, 36, 102609. [Google Scholar] [CrossRef] [PubMed]
- Sadraeian, M.; da Cruz, E.F.; Boyle, R.W.; Bahou, C.; Chudasama, V.; Janini, L.M.R.; Diaz, R.S.; Guimarães, F.E.G. Photoinduced Photosensitizer–Antibody Conjugates Kill HIV Env-Expressing Cells, Also Inactivating HIV. ACS Omega 2021, 6, 16524–16534. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hu, Y. Photodynamic Therapy for the Treatment of Fungal Infections. Infect. Drug Resist. 2022, 15, 3251–3266. [Google Scholar] [CrossRef]
- Barbosa, A.F.S.; Santos, I.P.; Santos, G.M.P.; Bastos, T.M.; Rocha, V.P.C.; Meira, C.S.; Soares, M.B.P.; Pitta, I.R.; Pinheiro, A.L.B. Anti–Trypanosoma cruzi effect of the photodynamic antiparasitic chemotherapy using phenothiazine derivatives as photosensitizers. Lasers Med. Sci. 2020, 35, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.W.; Oh, G.; Ahn, J.C.; Chung, E. Non-Oncologic Applications of Nanomedicine-Based Phototherapy. Biomedicines 2021, 9, 113. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Grumezescu, A.M. Photodynamic Therapy—An Up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Oyama, J.; Fernandes Herculano Ramos-Milaré, Á.C.; Lopes Lera-Nonose, D.S.S.; Nesi-Reis, V.; Galhardo Demarchi, I.; Alessi Aristides, S.M.; Juarez Vieira Teixeira, J.; Gomes Verzignassi Silveira, T.; Campana Lonardoni, M.V. Photodynamic therapy in wound healing in vivo: A systematic review. Photodiagn. Photodyn. Ther. 2020, 30, 101682. [Google Scholar] [CrossRef]
- Diogo, P.; F. Faustino, M.A.; PMS Neves, M.G.; Palma, P.J.; P. Baptista, I.; Gonçalves, T.; Santos, J.M. An Insight into Advanced Approaches for Photosensitizer Optimization in Endodontics—A Critical Review. J. Funct. Biomater. 2019, 10, 44. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; Zhang, P.; Wang, Y. Novel Approaches to Combat Medical Device-Associated BioFilms. Coatings 2021, 11, 294. [Google Scholar] [CrossRef]
- Almeida, A. Photodynamic Therapy in the Inactivation of Microorganisms. Antibiotics 2020, 9, 138. [Google Scholar] [CrossRef]
- Bartolomeu, M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Wastewater chemical contaminants: Remediation by advanced oxidation processes. Photochem. Photobiol. Sci. 2018, 17, 1573–1598. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeu, M.; Oliveira, C.; Pereira, C.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Antimicrobial Photodynamic Approach in the Inactivation of Viruses in Wastewater: Influence of Alternative Adjuvants. Antibiotics 2021, 10, 767. [Google Scholar] [CrossRef] [PubMed]
- Toro, P.M.; Jara, D.H.; Klahn, A.H.; Villaman, D.; Fuentealba, M.; Vega, A.; Pizarro, N. Spectroscopic Study of theE/ZPhotoisomerization of a New Cyrhetrenyl Acylhydrazone: A Potential Photoswitch and Photosensitizer(dagger). Photochem. Photobiol. 2021, 97, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.X.; Babalhavaeji, A.; Samanta, S.; Beharry, A.A.; Woolley, G.A. Red-Shifting Azobenzene Photoswitches for in Vivo Use. Acc. Chem. Res. 2015, 48, 2662–2670. [Google Scholar] [CrossRef] [PubMed]
- Ciminelli, C.; Granucci, G.; Persico, M. The photoisomerization mechanism of azobenzene: A semiclassical simulation of nonadiabatic dynamics. Chem. A Eur. J. 2004, 10, 2327–2341. [Google Scholar] [CrossRef] [PubMed]
- Gutzeit, V.A.; Acosta-Ruiz, A.; Munguba, H.; Hafner, S.; Landra-Willm, A.; Mathes, B.; Mony, J.; Yarotski, D.; Borjesson, K.; Liston, C.; et al. A fine-tuned azobenzene for enhanced photopharmacology in vivo. Cell Chem. Biol. 2021, 28, 1648–1663.e16. [Google Scholar] [CrossRef]
- Mukadum, F.; Nguyen, Q.; Adrion, D.M.; Appleby, G.; Chen, R.; Dang, H.; Chang, R.; Garnett, R.; Lopez, S.A. Efficient Discovery of Visible Light-Activated Azoarene Photoswitches with Long Half-Lives Using Active Search. J. Chem. Inf. Model. 2021, 61, 5524–5534. [Google Scholar] [CrossRef]
- Blanco-Lomas, M.; Martinez-Lopez, D.; Campos, P.J.; Sampedro, D. Tuning of the properties of rhodopsin-based molecular switches. Tetrahedron Lett. 2014, 55, 3361–3364. [Google Scholar] [CrossRef]
- Blanco-Lomas, M.; Funes-Ardoiz, I.; Campos, P.J.; Sampedro, D. Oxazolone-Based Photoswitches: Synthesis and Properties. Eur. J. Org. Chem. 2013, 2013, 6611–6618. [Google Scholar] [CrossRef]
- Martinez-Lopez, D.; Yu, M.L.; Garcia-Iriepa, C.; Campos, P.J.; Frutos, L.M.; Golen, J.A.; Rasapalli, S.; Sampedro, D. Hydantoin-Based Molecular Photoswitches. J. Org. Chem. 2015, 80, 3929–3939. [Google Scholar] [CrossRef]
- Carcia-Iriepa, C.; Ernst, H.A.; Liang, Y.; Unterreiner, A.N.; Frutos, L.M.; Sampedro, D. Study of Model Systems for Bilirubin and Bilin Chromophores: Determination and Modification of Thermal and Photochemical Properties. J. Org. Chem. 2016, 81, 6292–6302. [Google Scholar] [CrossRef]
- Waldeck, D.H. Photoisomerization dynamics of stilbenes. Chem. Rev. 1991, 91, 415–436. [Google Scholar] [CrossRef]
- Di Martino, M.; Sessa, L.; Di Matteo, M.; Panunzi, B.; Piotto, S.; Concilio, S. Azobenzene as Antimicrobial Molecules. Molecules 2022, 27, 5643. [Google Scholar] [CrossRef] [PubMed]
- Jerca, F.A.; Jerca, V.V.; Hoogenboom, R. Advances and opportunities in the exciting world of azobenzenes. Nat. Rev. Chem. 2022, 6, 51–69. [Google Scholar] [CrossRef]
- Wegener, M.; Hansen, M.J.; Driessen, A.J.M.; Szymanski, W.; Feringa, B. Photocontrol of Antibacterial Activity: Shifting from UV to Red Light Activation. J. Am. Chem. Soc. 2017, 139, 17979–17986. [Google Scholar] [CrossRef]
- Burchall, J.J. Mechanism of Action of Trimethoprim-Sulfamethoxazole—II. J. Infect. Dis. 1973, 128, S437–S441. [Google Scholar] [CrossRef]
- Hitchings, G.H. Mechanism of Action of Trimethoprim-Sulfamethoxazole—I. J. Infect. Dis. 1973, 128, S433–S436. [Google Scholar] [CrossRef] [PubMed]
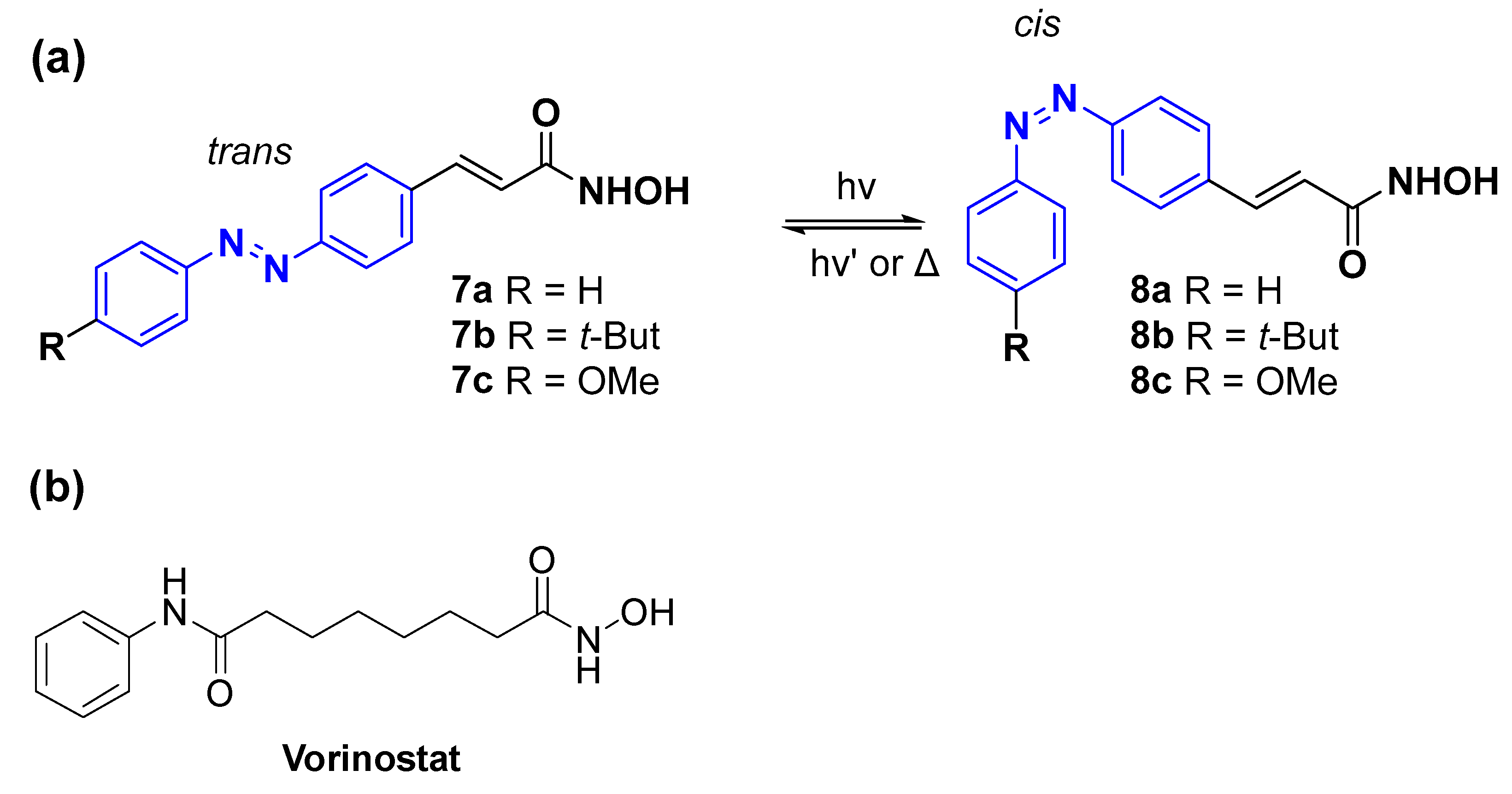
- Weston, C.E.; Kramer, A.; Colin, F.; Yildiz, O.; Baud, M.G.J.; Meyer-Almes, F.J.; Fuchter, M.J. Toward Photopharmacological Antimicrobial Chemotherapy Using Photoswitchable Amidohydrolase Inhibitors. ACS Infect. Dis. 2017, 3, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Hoffmann, K.; Brosch, G.; Loidl, P. Analogues of trichostatin A and trapoxin B as histone deacetylase inhibitors. Bioorg. Med. Chem. Lett. 1997, 7, 1655–1658. [Google Scholar] [CrossRef]
- Miller, T.A.; Witter, D.J.; Belvedere, S. Histone deacetylase inhibitors. J. Med. Chem. 2003, 46, 5097–5116. [Google Scholar] [CrossRef]
- Mai, A.; Massa, S.; Rotili, D.; Cerbara, I.; Valente, S.; Pezzi, R.; Simeoni, S.; Ragno, R. Histone deacetylation in epigenetics: An attractive target for anticancer therapy. Med. Res. Rev. 2005, 25, 261–309. [Google Scholar] [CrossRef] [PubMed]
- Vannini, A.; Volpari, C.; Filocamo, G.; Casavola, E.C.; Brunetti, M.; Renzoni, D.; Chakravarty, P.; Paolini, C.; De Francesco, R.; Gallinari, P.; et al. Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proc. Natl. Acad. Sci. USA 2004, 101, 15064–15069. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Herzer, J.; Overhage, J.; Meyer-Almes, F.J. Substrate specificity and function of acetylpolyamine amidohydrolases from Pseudomonas aeruginosa. BMC Biochem. 2016, 17, 4. [Google Scholar] [CrossRef]
- Hildmann, C.; Ninkovic, M.; Dietrich, R.; Wegener, D.; Riester, D.; Zimmermann, T.; Birch, O.M.; Bernegger, C.; Loidl, P.; Schwienhorst, A. A new amidohydrolase from Bordetella or Alcaligenes strain FB188 with similarities to histone deacetylases. J. Bacteriol. 2004, 186, 2328–2339. [Google Scholar] [CrossRef]
- Yao, H.; Wynendaele, E.; Xu, X.L.; Kosgei, A.; De Spiegeleer, B. Circular dichroism in functional quality evaluation of medicines. J. Pharm. Biomed. Anal. 2018, 147, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, Y.Q.; Yu, J.X.; Polyak, S.W.; Horsley, J.R.; Abell, A.D. Photopharmacological Control of Cyclic Antimicrobial Peptides. Chembiochem 2018, 19, 2591–2597. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.; Fulcaminato, T.; Matsuda, K.; Kobatake, S. Photochromism of Diarylethene Molecules and Crystals: Memories, Switches, and Actuators. Chem. Rev. 2014, 114, 12174–12277. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Irie, M. Diarylethene as a photoswitching unit. J. Photochem. Photobiol. C Photochem. Rev. 2004, 5, 169–182. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Wintner, A.; Seed, P.C.; Brauns, T.; Gelfand, J.A.; Hamblin, M.R. Antimicrobial photodynamic therapy mediated by methylene blue and potassium iodide to treat urinary tract infection in a female rat model. Sci. Rep. 2018, 8, 7257. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, J.; Zhong, K.H.; Tong, A.P.; Jia, D. Targeted protein degradation: Mechanisms, strategies and application. Signal Transduct. Target. Ther. 2022, 7, 113. [Google Scholar] [CrossRef]
- Wang, Z.W.; Liu, Y.; Zhu, X.Q. PhotoPROTACs: A Novel Biotechnology for Cancer Treatment. Trends Cell Biol. 2020, 30, 749–751. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, H.; Ma, L.N.; He, Z.X.; Wang, D.; Liu, Y.; Lin, Q.; Zhang, T.H.; Gray, N.; Kaniskan, H.U.; et al. Light-induced control of protein destruction by opto-PROTAC. Sci. Adv. 2020, 6, eaay5154. [Google Scholar] [CrossRef]
- Reynders, M.; Matsuura, B.S.; Berouti, M.; Simoneschi, D.; Marzio, A.; Pagano, M.; Trauner, D. PHOTACs enable optical control of protein degradation. Sci. Adv. 2020, 6, eaay5064. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Lu, M.C.; Wang, Y.; Shan, W.X.; Wang, X.Y.; You, Q.D.; Jiang, Z.Y. Azo-PROTAC: Novel Light-Controlled Small-Molecule Tool for Protein Knockdown. J. Med. Chem. 2020, 63, 4644–4654. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, P.; Samarasinghe, K.T.G.; Crews, C.; Carreira, E. Reversible Spatiotemporal Control of Induced Protein Degradation by Bistable PhotoPROTACs. ACS Cent. Sci. 2019, 5, 1682–1690. [Google Scholar] [CrossRef]
- Winter, G.E.; Buckley, D.L.; Paulk, J.; Roberts, J.M.; Souza, A.; Dhe-Paganon, S.; Bradner, J.E. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science 2015, 348, 1376–1381. [Google Scholar] [CrossRef]
- Sharma, M.; Friedman, S.H. The Issue of Tissue: Approaches and Challenges to the Light Control of Drug Activity. ChemPhotoChem 2021, 5, 611–618. [Google Scholar] [CrossRef]
- Yang, Y.M.; Mu, J.; Xing, B.G. Photoactivated drug delivery and bioimaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1408. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.A.; Wijesooriya, C.; Gehrmann, E.J.; Mahoney, K.M.; Goswami, P.P.; Albright, T.R.; Syed, A.; Dutton, A.S.; Smith, E.A.; Winter, A.H. Family of BODIPY Photocages Cleaved by Single Photons of Visible/Near-Infrared Light. J. Am. Chem. Soc. 2018, 140, 7343–7346. [Google Scholar] [CrossRef]
- Liu, M.; Meng, J.Q.; Bao, W.E.; Liu, S.Y.; Wei, W.; Ma, G.H.; Tian, Z.Y. Single-Chromophore-Based Therapeutic Agent Enables Green-Light-Triggered Chemotherapy and Simultaneous Photodynamic Therapy to Cancer Cells. ACS Appl. Bio Mater. 2019, 2, 3068–3076. [Google Scholar] [CrossRef]
- Silva, J.M.; Silva, E.; Reis, R.L. Light-triggered release of photocaged therapeutics—Where are we now? J. Control. Release 2019, 298, 154–176. [Google Scholar] [CrossRef]
- Kumari, P.; Kulkarni, A.; Sharma, A.K.; Chakrapani, H. Visible-Light Controlled Release of a Fluoroquinolone Antibiotic for Antimicrobial Photopharmacology. ACS Omega 2018, 3, 2155–2160. [Google Scholar] [CrossRef]
- Shchelik, I.S.; Tomio, A.; Gademann, K. Design, Synthesis, and Biological Evaluation of Light-Activated Antibiotics. ACS Infect. Dis. 2021, 7, 681–692. [Google Scholar] [CrossRef]
- Wong, P.T.; Tang, S.Z.; Mukherjee, J.; Tang, K.; Gam, K.; Isham, D.; Murat, C.; Sun, R.; Baker, J.R.; Choi, S.K. Light-controlled active release of photocaged ciprofloxacin for lipopolysaccharide-targeted drug delivery using dendrimer conjugates. Chem. Commun. 2016, 52, 10357–10360. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Manna, D. Controlling PROTACs with Light. ChemMedChem 2020, 15, 1258–1261. [Google Scholar] [CrossRef]
- Xue, G.; Wang, K.; Zhou, D.L.; Zhong, H.B.; Pan, Z.Y. Light-Induced Protein Degradation with Photocaged PROTACs. J. Am. Chem. Soc. 2019, 141, 18370–18374. [Google Scholar] [CrossRef]
- Naro, Y.; Darrah, K.; Deiters, A. Optical Control of Small Molecule-Induced Protein Degradation. J. Am. Chem. Soc. 2020, 142, 2193–2197. [Google Scholar] [CrossRef]
- Kounde, C.S.; Shchepinova, M.M.; Saunders, C.N.; Muelbaier, M.; Rackham, M.D.; Harling, J.D.; Tate, E.W. A caged E3 ligase ligand for PROTAC-mediated protein degradation with light. Chem. Commun. 2020, 56, 5532–5535. [Google Scholar] [CrossRef]
- Zengerle, M.; Chan, K.H.; Ciulli, A. Selective Small Molecule Induced Degradation of the BET Bromodomain Protein BRD4. ACS Chem. Biol. 2015, 10, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Dommaschk, M.; Grobner, J.; Wellm, V.; Hovener, J.B.; Riedel, C.; Herges, R. Dendronised Ni(II) porphyrins as photoswitchable contrast agents for MRI. Phys. Chem. Chem. Phys. 2019, 21, 24296–24299. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Busscher, H.J.; Liu, Y.; Zhang, Z.K.; van Kooten, T.G.; Su, L.Z.; Zhang, Y.M.; Liu, J.J.; Liu, J.F.; An, Y.L.; et al. Photoswitchable Micelles for the Control of Singlet-Oxygen Generation in Photodynamic Therapies. Biomacromolecules 2018, 19, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Peng, D.W.; Wang, B.; Long, L.; Guo, C.; Yuan, J. A model for light-triggered porphyrin anticancer prodrugs based on an o-nitrobenzyl photolabile group. Eur. J. Org. Chem. 2008, 2008, 793–796. [Google Scholar] [CrossRef]
- Tessaro, A.L.; Fraix, A.; Failla, M.; Cardile, V.; Graziano, A.C.E.; Estevao, B.M.; Rescifina, A.; Sortino, S. Light-Controlled Simultaneous “On Demand” Release of Cytotoxic Combinations for Bimodal Killing of Cancer Cells. Chem. A Eur. J. 2018, 24, 7664–7670. [Google Scholar] [CrossRef]
- Feng, Y.F.; Tonon, C.C.; Ashraf, S.; Hasan, T. Photodynamic and antibiotic therapy in combination against bacterial infections: Efficacy, determinants, mechanisms, and future perspectives. Adv. Drug Deliv. Rev. 2021, 177, 113941. [Google Scholar] [CrossRef]
- Branco, T.M.; Valerio, N.C.; Jesus, V.I.R.; Dias, C.J.; Neves, M.; Faustino, M.A.F.; Almeida, A. Single and combined effects of photodynamic therapy and antibiotics to inactivate Staphylococcus aureus on skin. Photodiagn. Photodyn. Ther. 2018, 21, 285–293. [Google Scholar] [CrossRef]
- Xing, B.G.; Jiang, T.T.; Bi, W.G.; Yang, Y.M.; Li, L.H.; Ma, M.L.; Chang, C.K.; Xu, B.; Yeow, E.K.L. Multifunctional divalent vancomycin: The fluorescent imaging and photodynamic antimicrobial properties for drug resistant bacteria. Chem. Commun. 2011, 47, 1601–1603. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ni, A.S.Y.; Lim, Y.; Mohanram, H.; Bhattacharjya, S.; Xing, B.G. Lipopolysaccharide Neutralizing Peptide-Porphyrin Conjugates for Effective Photoinactivation and Intracellular Imaging of Gram-Negative Bacteria Strains. Bioconjugate Chem. 2012, 23, 1639–1647. [Google Scholar] [CrossRef]
- Dastgheyb, S.S.; Eckmann, D.M.; Composto, R.J.; Hickok, N.J. Photo-activated porphyrin in combination with antibiotics: Therapies against Staphylococci. J. Photochem. Photobiol. B Biol. 2013, 129, 27–35. [Google Scholar] [CrossRef]
- Almeida, J.; Tome, J.P.C.; Neves, M.; Tome, A.C.; Cavaleiro, J.A.S.; Cunha, A.; Costa, L.; Faustino, M.A.F.; Almeida, A. Photodynamic inactivation of multidrug-resistant bacteria in hospital wastewaters: Influence of residual antibiotics. Photochem. Photobiol. Sci. 2014, 13, 626–633. [Google Scholar] [CrossRef]
- Iluz, N.; Maor, Y.; Keller, N.; Malik, Z. The synergistic effect of PDT and oxacillin on clinical isolates of Staphylococcus aureus. Lasers Surg. Med. 2018, 50, 535–551. [Google Scholar] [CrossRef]
- Thangudu, S.; Kaur, N.; Korupalli, C.; Sharma, V.; Kalluru, P.; Vankayala, R. Recent advances in near infrared light responsive multi-functional nanostructures for phototheranostic applications. Biomater. Sci. 2021, 9, 5432–5443. [Google Scholar] [CrossRef]
- Wang, X.; Xuan, Z.; Zhu, X.; Sun, H.; Li, J.; Xie, Z. Near-infrared photoresponsive drug delivery nanosystems for cancer photo-chemotherapy. J. Nanobiotechnol. 2020, 18, 108. [Google Scholar] [CrossRef]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Di, L. In vitro and in vivo methods to assess pharmacokinetic drug– drug interactions in drug discovery and development. Biopharm. Drug Dispos. 2020, 41, 3–31. [Google Scholar] [CrossRef] [PubMed]
- Said, S.S.; Campbell, S.; Hoare, T. Externally Addressable Smart Drug Delivery Vehicles: Current Technologies and Future Directions. Chem. Mater. 2019, 31, 4971–4989. [Google Scholar] [CrossRef]

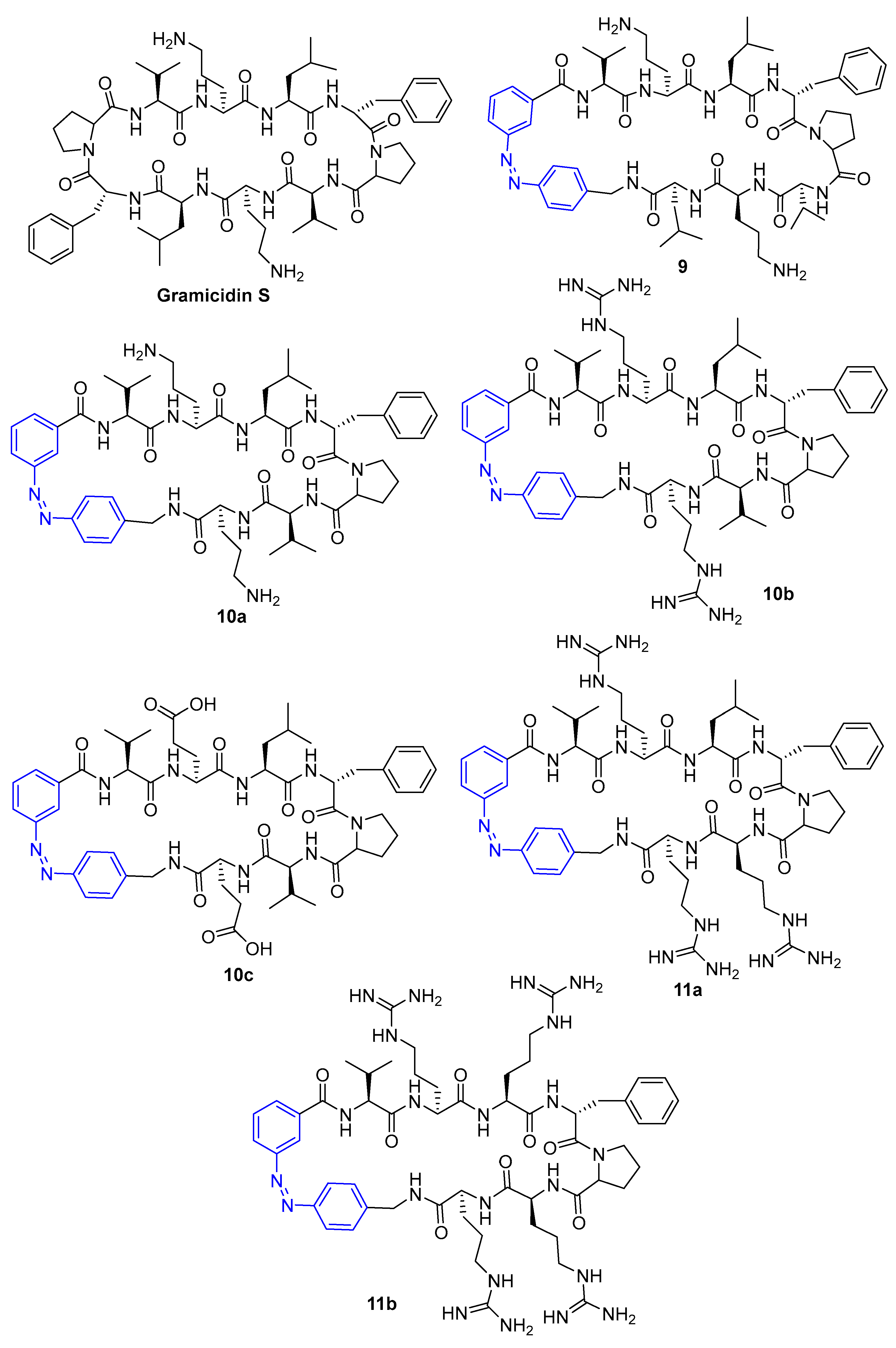
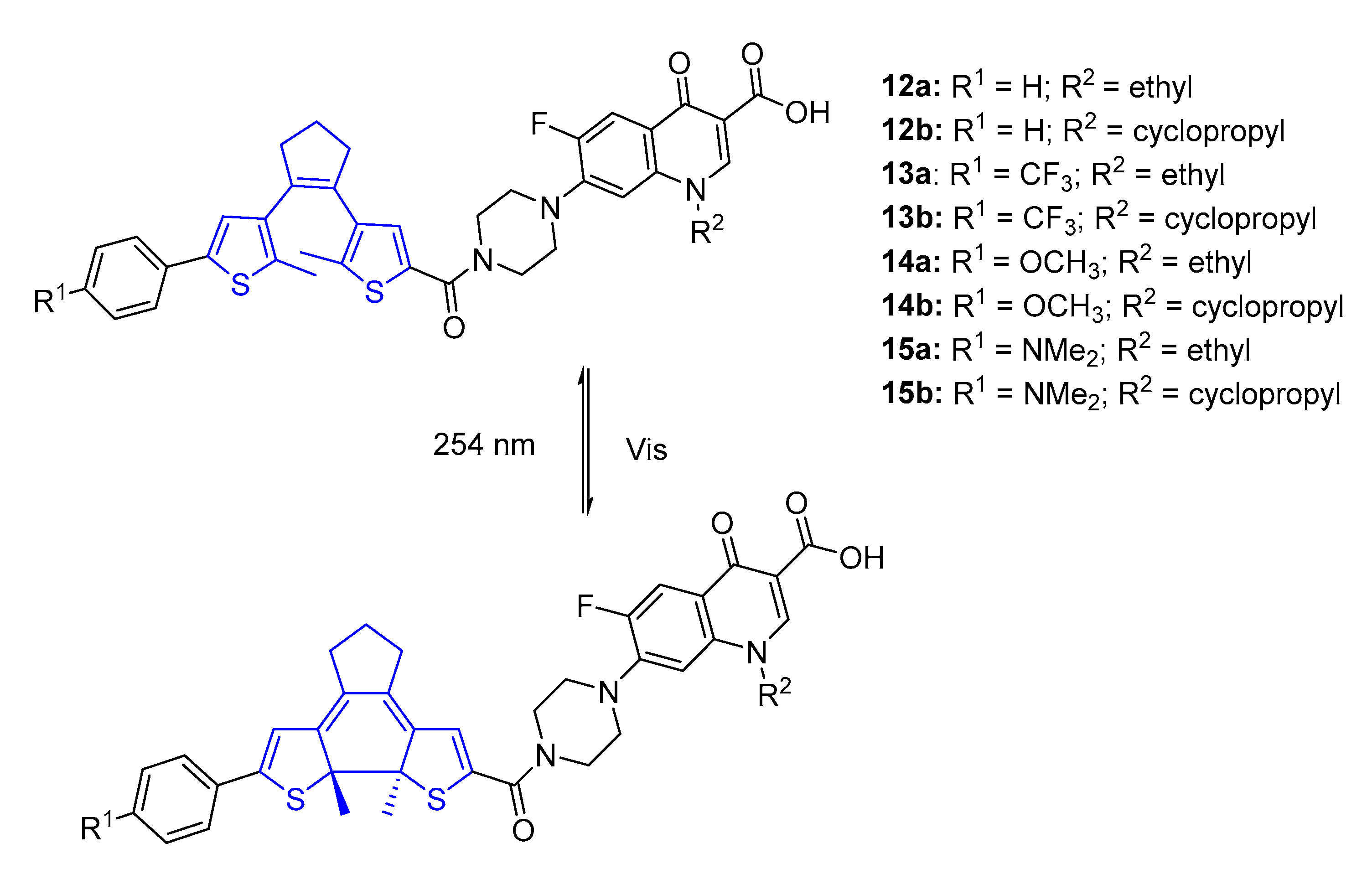

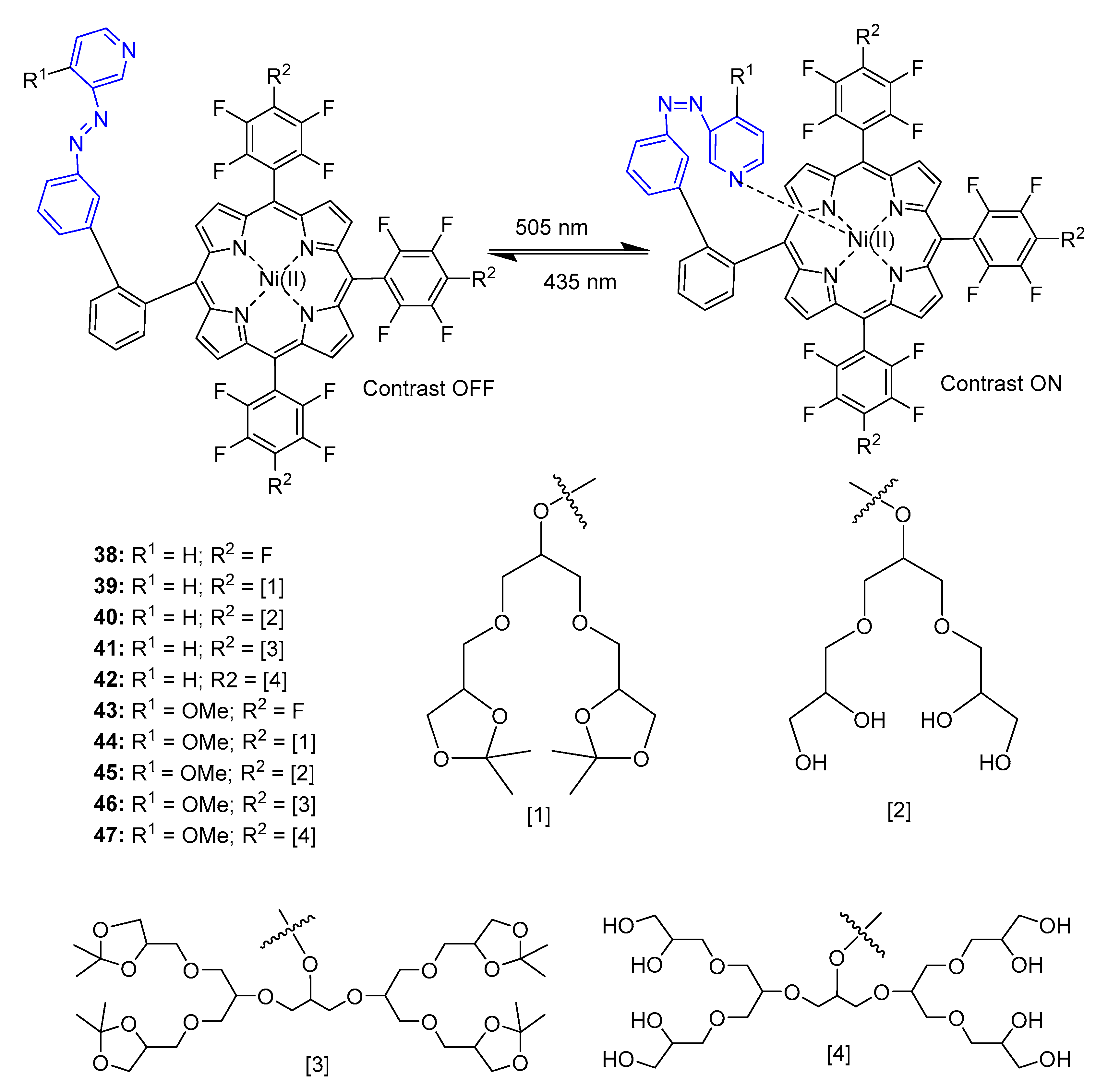
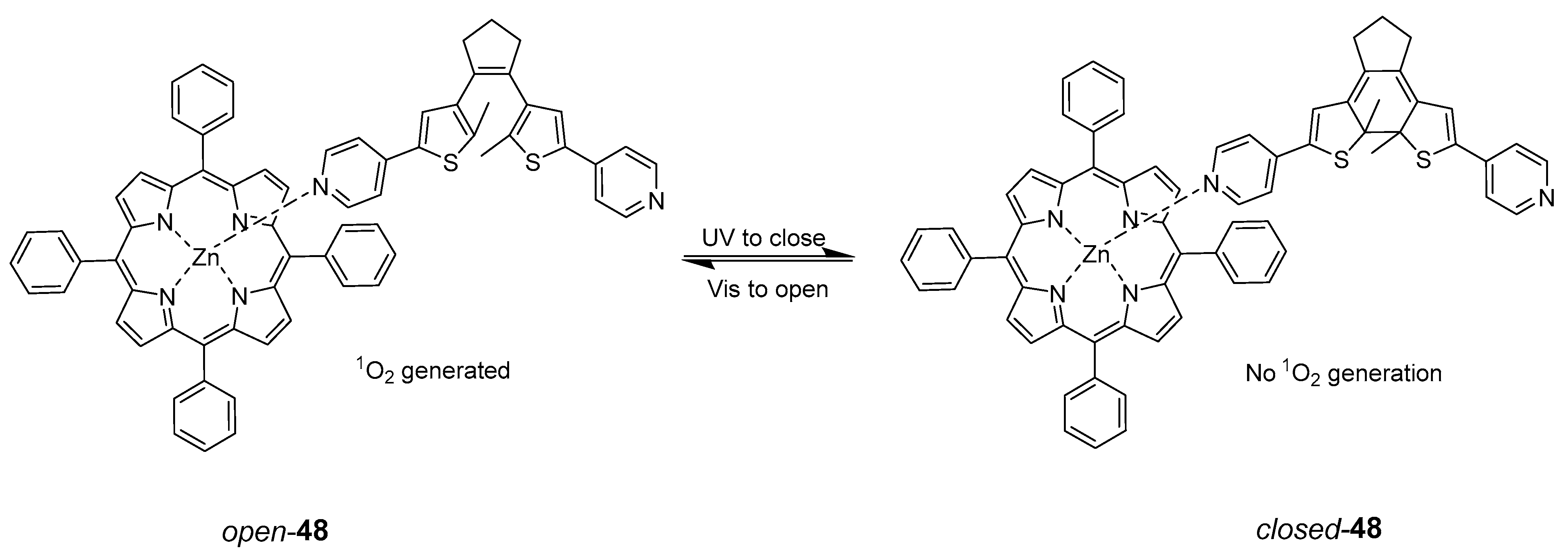

| Compound | MIC (μM) | |||
|---|---|---|---|---|
| Before Irradiation Compound 1 | After Irradiation Compound 2 | |||
| E. coli | M. luteus | E. coli | M. luteus | |
| Azofloxacin | 0.50 | 0.250 | 0.50 | 0.50 |
| Ciprofloxacin | 0.0125 | 12.0 | 0.0125 | 12.0 |
| IC50 (μM) | |||||
|---|---|---|---|---|---|
| Compound | R | B/A-HDAH | PA-HDAH | APAH PA0321 | APAH PA1409 |
| 7a (trans) | H | 0.042 | 0.0056 | 0.79 | 2.0 |
| 8a (cis) | H | 0.062 | 0.0066 | 0.40 | 0.21 |
| 7b (trans) | t-But | 0.37 | 0.072 | 2.2 | >25 |
| 8b (cis) | t-But | 0.38 | 0.094 | 1.8 | 3.5 |
| 7c (trans) | OMe | 0.059 | 0.051 | 2.3 | 5.1 |
| 8c (cis) | OMe | 0.075 | 0.090 | 1.4 | 0.65 |
| Vorinostat | 0.095 | 0.037 | 0.50 | 0.30 | |
| MIC (µg mL−1) | ||
|---|---|---|
| Compound | cis | trans |
| 9 | 64 | 64 |
| 10a | 64 | 256 |
| 10b | 32 | 128 |
| 10c | >256 | >256 |
| 11a | 256 | >256 |
| 11b | >256 | >256 |
| Gramicidin S | 2 | |
| Compound | MIC (μg mL−1) | |||
|---|---|---|---|---|
| Open-Isomer (before) | Closed-Isomer (after) | |||
| E. coli | S. aureus | E. coli | S. aureus | |
| 12a | 32 | 8 | 16 | 8 |
| 12b | 32 | 16 | 2 | 16 |
| 13a | 16 | 32 | 16 | 32 |
| 13b | 16 | 32 | 16 | 32 |
| 14a | 32 | 16 | 8 | 16 |
| 14b | 32 | 16 | 2 | 16 |
| 15a | 32 | 16 | 16 | 16 |
| 15b | 16 | 16 | 4 | 16 |
| Norfloxacin | 0.125 | 0.125 | 0.125 | 0.125 |
| Ciprofloxacin | 0.125 | 0.125 | 0.125 | 0.125 |
| Vancomycin Derivatives | Cephalosporin Derivatives | |
|---|---|---|
| Target Compounds | 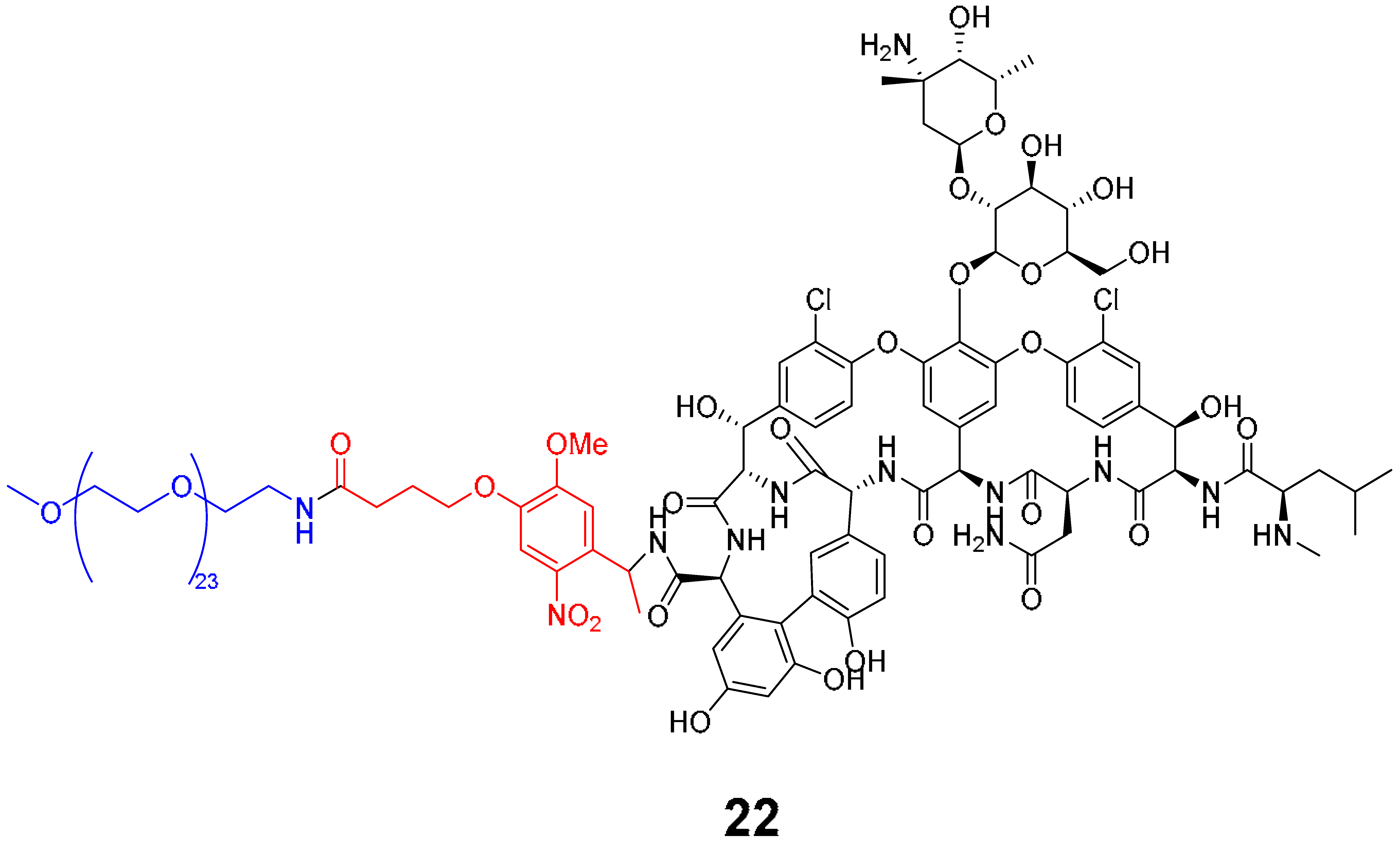 |  |
| Release Compounds | 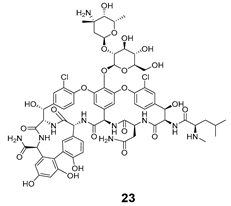 | 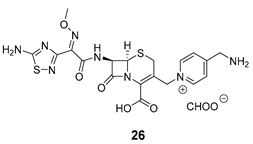 |
| Control Compounds | 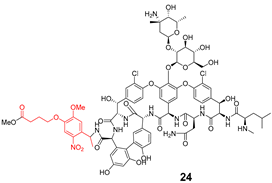 |  |
| Strains | MIC (µg mL−1) | |||||
|---|---|---|---|---|---|---|
| Vancomycin Series | Cephalosporin Series | |||||
| 22 | 23 | 24 | 25 | 26 | 27 | |
| E. coli ATCC 25922 | - | - | - | 8 | 1–2 | 1 |
| P. aeruginosa ATCC 27853 | - | - | - | 64 | 2–4 | 32 |
| B. subtilis ATCC 6633 | 32 | 0.06–0.125 | 0.125 | 8 | 2–4 | 1 |
| S. aureus ATCC 6633 | >64 | 0.5–1 | 0.5 | 32 | 8 | 4 |
| S. aureus ATCC 43300 | >64 | 1–2 | 1 | 64 | 32 | 16 |
| MIC Values (μM) | ||||
|---|---|---|---|---|
| E. coli DH5a | S. enterica | E. coli BL21 | K. pneumoniae | |
| PpIX−Peptide Conjugate | 2.0 | 4.0 | 4.0 | 8.0 |
| YI13WF | 8 | ~24 | 32 | 32 |
| PpIX | >64 | >64 | >64 | >64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarabando, S.N.; Palmeira, A.; Sousa, M.E.; Faustino, M.A.F.; Monteiro, C.J.P. Photomodulation Approaches to Overcome Antimicrobial Resistance. Pharmaceuticals 2023, 16, 682. https://doi.org/10.3390/ph16050682
Sarabando SN, Palmeira A, Sousa ME, Faustino MAF, Monteiro CJP. Photomodulation Approaches to Overcome Antimicrobial Resistance. Pharmaceuticals. 2023; 16(5):682. https://doi.org/10.3390/ph16050682
Chicago/Turabian StyleSarabando, Sofia N., Andreia Palmeira, Maria Emília Sousa, Maria Amparo F. Faustino, and Carlos J. P. Monteiro. 2023. "Photomodulation Approaches to Overcome Antimicrobial Resistance" Pharmaceuticals 16, no. 5: 682. https://doi.org/10.3390/ph16050682
APA StyleSarabando, S. N., Palmeira, A., Sousa, M. E., Faustino, M. A. F., & Monteiro, C. J. P. (2023). Photomodulation Approaches to Overcome Antimicrobial Resistance. Pharmaceuticals, 16(5), 682. https://doi.org/10.3390/ph16050682










