Abstract
A small library of 6-O-sucrose monoester surfactants has been synthesized and tested against various microorganisms. The synthetic procedure involved a modified Mitsunobu reaction, which showed improved results compared to those present in the literature (higher yields and larger scope). The antifungal activities of most of these glycolipids were satisfactory. In particular, sucrose palmitoleate (URB1537) showed good activity against Candida albicans ATCC 10231, Fusarium spp., and Aspergillus fumigatus IDRAH01 (MIC value: 16, 32, 64 µg/mL, respectively), and was further characterized through radical scavenging, anti-inflammatory, and biocompatibility tests. URB1537 has been shown to control the inflammatory response and to have a safe profile.
1. Introduction
Fungal invasive infections represent a serious problem in human health and are associated with at least 1.5–2 million deaths worldwide [1,2,3,4]. In this scenario, opportunistic mycotic pathogens such as Candida spp., A. fumigatus and C. neoformans are the most important causes of invasive fungal infections, accounting for more than 90% of fungal deaths [4]. These infections are mainly common in immunocompromised patients subjected to anticancer therapy, long-term corticosteroid treatments or organ transplant as well as immunosuppressive infections. Moreover, fungi are responsible for infections on skin and mucosal surfaces with a greater incidence compared to invasive fungal ones. In addition, the development of antifungal drugs faces off with the fact that fungi are eukaryotes as their hosts and thus the potential targets may also be found in human cells with a substantial toxicity risk. Nowadays, the antifungals on the market are represented by various classes of drugs called polyenes (i.e., amphotericin B), azoles (i.e., fluconazole), allyl-/benzylamines (i.e., terbinafine), benzofurancyclohexenes (i.e., griseofulvin), echinocandins (i.e., caspofungin), antimetabolites (flucytosine), nikkomycines (nikkomycine Z), thiocarbamates (tolnaftate), and morpholines (amorolfine) [5,6]. Unfortunately, most of the antifungals and antibiotics actually commercially available, in particular those systemically and per os administered, show some limits, such as toxicity, the spectrum of activity, safety, pharmacokinetic properties, and drug-resistant strains [7]. Consequently, there is an urgent need to develop new antifungals as well as new antibiotics to fill this therapeutic gap.
Sucrose fatty acid esters (SFAE) are the most common glycolipids derived from polyalcohol sucrose (polar head) and naturally occurring fatty acid (hydrophobic tail). They are non-ionic surfactants and considered “green” because they are non-toxic and biodegradable. Moreover, they are non-irritant, odorless, and possess a safer biocompatibility profile in comparison with other amphiphiles such as polysorbates and sulfates [8]. Most of the time, SFAEs are synthesized from inexpensive and renewable agricultural products [8,9], and some of them are commercially available [8]. Moreover, they possess excellent emulsifying characteristics [10,11,12], antibacterial [8,10,13], antifungal [8] and insecticidal [8,14] activities, and permeability-enhancing properties [10]. For all these reasons, sucrose esters are very important commodity compounds in the agriculture, food, nutraceutical, cosmetic, dental, and pharmaceutical industries. In the 2010s, the world production of SFAE was above 6000 tons per year [8] and their market is projected to grow from USD 76 million in 2019 to USD 106 million by 2025 [15].
As part of our ongoing investigations on the synthesis, biological activities, and applications of sugar derivatives [16,17,18,19,20,21,22,23,24], a series of 6-O-sucrose monoesters were designed and synthesized by using an opportunely modified Mitsunobu-type methodology [25] starting from free sucrose and lipophilic acids. This coupling, compared to the others reported in the literature [8,10], such as esterification (acyl chlorides, anhydrides) and transesterification with activated esters (vinyl or enol esters) [26,27,28,29], and/or enzymatic preparation methods [30,31,32], showed higher conversions/yields, high regioselectivity and larger scope including saturated, mono- and polyunsaturated fatty acids or aromatic and alkyl aromatic acids.
The small library of sucrose esters was explored for antibacterial and antifungal activities and the profile of the most interesting glycolipid (URB1537) was deepened through the study of its antioxidant, anti-inflammatory, and biocompatibility properties.
2. Results and Discussion
2.1. Chemistry
A series of glycolipids characterized by the 6-O-sucrose hydrophilic head and a lipophilic tail of various sizes and nature has been designed and synthesized with the aim of exploring the influence of the hydrophobic portion on their biological properties. For this purpose, both linear saturated (caprylic (C8)), monounsaturated (undecylenic (C11:1), undecylinic (C11:1), palmitoleic (C16:1), oleic (C18:1), and nervonic (C24:1)) or polyunsaturated (linoleic (C18:2) and linolenic (C18:3)), and aromatic (benzoic and p-phenylbenzoic) or alkyl aromatic (phenyl-, p-biphenyl- and p-triphenylacetic) fatty acid derivatives substituents have been used. The most important physicochemical constants in the landscape of surfactants are hydrophilic–lipophilic balance (HLB), octanol–water portion coefficient (logP) and the polar surface area (TPSA). However, TPSA could not constitute a discriminant indicator when the polar head is maintained constant in all the designed surfactants, as in our case (calculated TPSA = 196 Å2 for sucrose esters). The glycolipids were indeed designed with the aim of keeping the HLB and logP values in a non-strict range to try to understand if a relationship between the biological activity and physicochemical properties is present.
With the only exception of sucrose nervonate (HLB = 8.6, Table 1, Entry 8), all the derivatives have HLB values greater than 9.5 (9.7–13.4) classifying the molecules as hydrophilic surfactants and are consequently able to act as oil-in-water emulsifiers. On the other hand, the designed sucrose-based glycolipids are molecules showing a widespread calculated logP (clogP) ranging from a negative value −2.7 (sucrose benzoate, Entry 9) to a positive 2.9 (sucrose oleate, Entry 5). As above, the only exception of the series is the sucrose nervonate that showed a clogP out of the Lipinski’s rules of five (5.9, Entry 8) due to its very lipophilic long tail.

Table 1.
Calculated physicochemical properties of the sucrose-based ester surfactants.
The designed 6-O-sucrose-based monoesters could be synthesized by several reported procedures starting from unprotected sucrose [8,10]. We tried several of them by using classical chemical esterification and transesterification procedures and also using appropriate enzymatic methodologies but without satisfactory results in terms of regioselectivity and conversion/yield (data not shown). On the contrary, the Mitsunobu methodology reported by Molinier et al. [25] immediately proved to be suitable for our purposes. Indeed, not protected sucrose (1) and undecylenic acid (C11:1) (2b) under the reported Mitsunobu conditions gave the corresponding 6-O-sucrose ester 3b in 34% yield (Table 1, Scheme 1 and Entry 1) and other known side products including the diester 4 [25]. With the aim of ameliorating the yield of 3b, the reaction reported in Scheme 1 was studied as a model reaction. All the conditions were investigated in detail and the results are reported in Table 2.
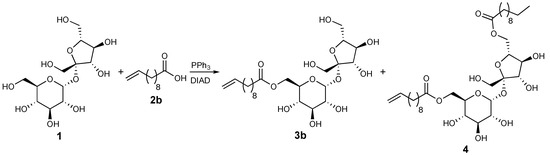
Scheme 1.
Model reaction for methodology studies.

Table 2.
Investigation of the conditions for Mitsunobu-type reaction between sucrose and undecylenic acid.
As reported in Table 2 (Entry 1), the diester 4 represents the main product of the first attempt (38% yield vs. 34% of the desired product). A reduction of the acid could be decisive, with 1 equivalent of 2b, almost no diester was obtained; however, the yield of 6-O-sucrose monoester was reduced to 19% (Entry 2). A 1.5 equivalent of the model acid gave the best result in term of yield of the desired product 3b (39%, Entry 3) and lower production of 4 (15%). With the aim to reduce the 6-O-undecylenyl-3′,6′-anhydrosucrose side product [25], the equivalents of the Mitsunobu reagents DIAD and PPh3 were also decreased (Entries 4 and 5). However, no good results were obtained. The yield of 3b did not also increase with a higher amount of DIAD and PPh3 (Entry 6). Increasing the temperature (Entry 7) or the reaction time to 48 h (data not shown) did not ameliorate the production of 3b but enhanced the formation of the diester 4. Moreover, the reduction of the reaction time worsened the yield of the desired monoester (Entries 8 and 9). Finally, other polar aprotic solvents, such as THF and dioxane, did not solubilize well sucrose and the reaction gave only a trace amount of the product (Entries 10 and 11). Other azodicarboxylates were also used under optimal reaction conditions as comparison with DIAD. By the use of DEAD, the reaction gave the desired product 3b with comparable yield, albeit slightly lower, but with a higher production of diester 4 (Entry 12). Instead, the use of DCAD led to a clear lowering of the yield of 6-O-sucrose undecylenate (Entry 13).
The improved Mitsunobu-type procedure (Entry 3) was then used to synthesize all the designed 6-O-sucrose monoester surfactants starting from not protected sucrose and the corresponding commercially available acids (Scheme 2).
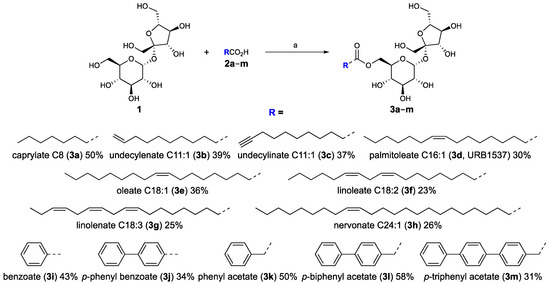
Scheme 2.
Sucrose ester synthesis. Substrate scopes, reagents, conditions, and yields: (a) PPh3, DIAD, dry DMF, rt, 24–30 h.
The ameliorated methodology worked well for all the acid substrates giving the corresponding products in low to moderate yields (Scheme 2). Although the yields for some substrates are not exciting, it should be noted that the procedure uses commercially available, inactivated, or unprotected reagents, avoiding the activation or protection and deprotection steps. Moreover, the reaction is regioselective for the six-position of sucrose and, conveniently, the desired products are easily purified from the side ones by a short flash column chromatography. Therefore, this improved Mitsunobu-type esterification has shown to be versatile and has widespread substrate scope, and could be used for the production of any kind of 6-O-sucrose monoesters in low to medium scale.
2.2. Antibacterial Activity
The antimicrobial activity of the different sucrose esters is summarized in Table 3. As shown, the activity of the tested compounds was prevalently observed against Gram-positive bacteria (Enterococcus faecalis ATCC 29212, Staphylococcus aureus ATCC 43387, S. aureus ATCC 43300, Listeria monocytogenes ATCC 7644) but not against Gram-negative ones (E. coli O157:H7 ATCC 35150, Klebsiella pneumoniae ATCC 13883, Pseudomonas aeruginosa ATCC 9027, Salmonella enteritidis ATCC 13076). In detail, sucrose palmitoleate (3d) and sucrose oleate (3e) showed minimum inhibitory concentration (MIC) values of 1024 µg/mL against E. faecalis ATCC 29212, S. aureus ATCC 43387, S. aureus ATCC 43300 (MRSA) and L. monocytogenes ATCC 7644, while sucrose linoleate (3f) and sucrose linolenate (3g) reached MIC of 256 µg/mL and 512 µg/mL against E. faecalis ATCC 29212 and 1024 µg/mL against the other Gram-positive microorganisms. Sucrose nervonate (3h), sucrose benzoate (3i), and sucrose phenyl acetate (3k) were active only against E. faecalis ATCC 29212 (MIC 1024 µg/mL), while sucrose p-phenyl benzoate (3j) and sucrose undecylenate (3b) were active also against L. monocytogenes ATCC 7644 (in both cases MIC 1024 µg/mL). Sucrose caprylate (3a), sucrose undecylenate (3c), p-biphenyl acetate (3l), and p-triphenyl acetate (3m) were always inactive.

Table 3.
Antibacterial activity of sucrose-based compounds (MIC values are reported in µg/mL).
The mechanism of antibacterial action of sugar fatty acid esters is mainly based on the increase in permeability of the cell membrane and the consequent leakage of some cellular constituents, in particular proteins and sugars [35]. Polar compounds interact better with the bacterial cell wall, especially in Gram-positive bacteria, causing their damage. Effectively, our data showed that Gram-negative bacteria were more resistant to the examined sucrose esters compared to Gram-positive bacteria, probably due to their outer membrane, which limits the diffusion of sucrose fatty acid esters through the lipopolysaccharide covering. On the other hand, the literature data reports that the antibacterial activity of sugar fatty acid esters decreases rapidly as the chain length of fatty acid increases [36]. However, such a situation is not appreciable in this study because of the low activities of our tested compounds. Moreover, for the same reason, it is not possible to rationalize a correlation between the activity of the compounds and their physicochemical characteristics and lipophilic tails.
2.3. Antifungal Activity
The antifungal activity of the different sucrose esters is illustrated in Table 4. As a general trend, the tested compounds showed good antifungal activity and, interestingly, some of them (3b, 3d–f) resulted active against all the selected fungi with MIC ranging from 16 to 1024 µg/mL. Among the molecules analyzed, the lowest MIC value of 16 µg/mL was observed for sucrose palmitoleate (C16:1) (3d), 3e against Candida albicans ATCC 10231 and Aspergillus fumigatus IDRAH01, respectively, followed by 3f (MIC 32 µg/mL against A. fumigatus IDRAH01). Further, 3b resulted active against Aspergillus niger ATCC 9642 and Fusarium spp. (MIC 512 µg/mL) and 3g showed MIC values of 128 µg/mL and 512 µg/mL for Fusarium spp. and C. albicans ATCC 10231, respectively. Similarly, 3i and 3k were active against Fusarium spp. and C. albicans ATCC 10231 with a MIC ranging from 256 to 1024 µg/mL. These two microorganisms showed to be sensitive also to the remaining sucrose esters, specifically 3b, 3j, 3l (MIC 1024 µg/mL), confirming 3b antimicrobial activity against C. albicans, reported in some previous research [37] As observed for bacteria, 3m was inactive against all the examined fungi.

Table 4.
Antifungal activity of sucrose-based compounds (MIC values are reported in µg/mL).
The best series of these sucrose esters against the tested fungi were the mono and polyunsaturated fatty acid-containing tail compounds. In detail, 3d showed a relevant activity against all four fungi strains (MIC from 16 to 512 µg/mL). By decreasing the chain length, 3b is less active showing higher MIC in the range of 512–1024 µg/mL. The molecule obtained by substitution of the terminal double bond with a triple bond, that is 3c, was detrimental to the activity (MIC 1024 or >1024 µg/mL). From a general point of view, an increase in chain length seems to produce sucrose ester surfactants less effective against the tested fungi strains. Indeed, 3e–h showed higher MIC values than 3d with only one exception. Indeed, 3e and 3f seem selective and very active against A. fumigatus (MIC 16 and 32 µg/mL, respectively). The only saturated fatty acid containing tail compound, that is 3a is less effective than 3d (MIC 1024 µg/mL or greater). Alkyl aromatic and aromatic sucrose esters (3i–m) are also less effective against the tested fungi compared to 3d. They showed MIC of 1024 or >1024 µg/mL with the only exception of 3i and 3k which showed interesting activities against Fusarium spp. (MIC 512 and 256 µg/mL, respectively), and C. albicans (MIC 256 and 1024 µg/mL, respectively), strains.
2.4. Radical Scavenging Activity
The antioxidant activity of sucrose palmitoleate (3d, URB1537) was investigated by the 2,2-diphenyl-1-picrylhydrazyl hydrate (DPPH) radical scavenging assay. As indicated in Figure 1, URB1537 did not show scavenger effects against DPPH radicals within the range of concentrations tested (EC50 >> 512 µg/mL). This evidence agrees with previous findings showing that sugar esters possess lower radical scavenging properties than the reference antioxidant molecules [24,38,39], such as quercetin (calculated EC50 equal to 1.74 µg/mL). In particular, sugar esters show scavenger abilities at concentrations ranging from 1 to 10 mg/mL, higher than those herein tested for URB1537 (up to 512 µg/mL).
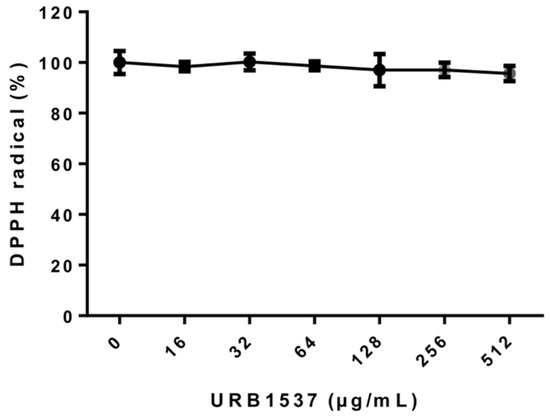
Figure 1.
Evaluation of URB1537 scavenger activity against DPPH radicals. Data are expressed as the mean ± SD (n = 3).
2.5. Biocompatibility Assay
The evaluation of URB1537 cytotoxicity by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay revealed a significant reduction of cultured human keratinocyte (HaCaT) cell viability with 256 and 512 µg/mL concentrations (Figure 2). The calculated IC50 value was equal to 230 µg/mL. Based on cytotoxicity criteria, URB1537 could be classified as weakly cytotoxic [40]. This finding confirms that both carbon chain length and sucrose ester concentration may influence cell viability, as previously evidenced for lactose-based fatty acid monoesters [19]. However, the cytotoxicity of URB1537 in HaCaT is still higher than the MIC showed against most of the selected fungi strains and the compound is indeed safe at least up to 128 µg/mL.
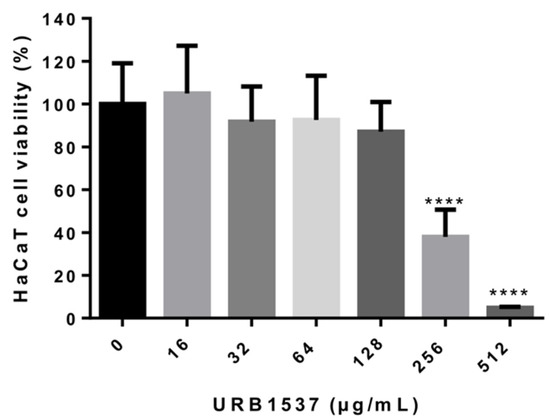
Figure 2.
Cell viability evaluation after URB1537 administration to HaCaT cells for 24 h. Data are expressed as the mean ± SD (n = 3). **** p < 0.0001 vs. untreated control cells (Tukey’s post hoc test).
2.6. Anti-Inflammatory Activity
As reported in Figure 3, the stimulation of RAW 264.7 cells (murine macrophages) by lipopolysaccharide (LPS) (CTR+) led to a strong extracellular release of the inflammatory response mediator nitric oxide (NO) as compared to untreated control cells (CTR-). When LPS-exposed cells were co-incubated with URB1537, a dose-dependent decrement of NO production was observed. The anti-inflammatory activity of URB1537 128 µg/mL was comparable to that of the reference drug dexamethasone 2 µg/mL, suggesting that this sugar ester might help to control the inflammatory response. NO release was not increased in non-stimulated RAW 264.7 cells after URB1537 administration, indicating that the compound did not lead to immune system activation (data not shown).
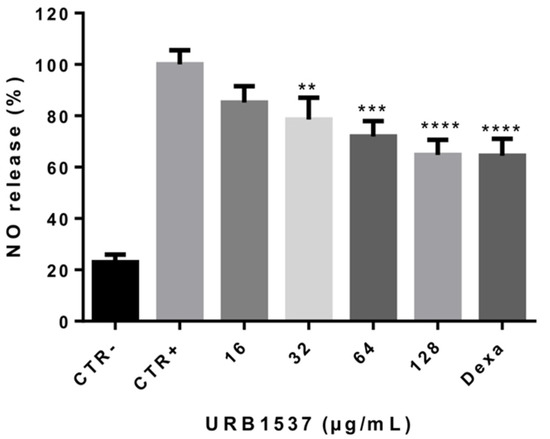
Figure 3.
Extracellular NO release after RAW 264.7 stimulation by LPS for 24 h in the presence of URB1537 (16–128 µg/mL). CTR-: negative control (untreated cells); CTR+: positive control (LPS); Dexa: dexamethasone 2 µg/mL. Data are expressed as the mean ± SD (n = 3). ** p < 0.01, *** p < 0.001, **** p < 0.0001 vs. CTR+ (Tukey’s post hoc test).
Overall, these data agree with previous observations demonstrating that sucrose fatty acid esters exhibit anti-inflammatory effects through different mechanisms, such as the inhibition of NF-κB activation involved in immunity and inflammation [41]. Similarly, we have recently observed that some lactose-based fatty acid esters may counteract NO production in LPS-stimulated macrophages [24].
The anti-inflammatory properties of URB1537 were also compared to those of its single constituents, i.e., sucrose (S) and palmitoleic acid (C16). As indicated in Figure 4, URB1537 and C16 presented a similar anti-inflammatory activity (p = ns URB1537 vs. C16), significantly reducing LPS-induced NO production. Sucrose alone did not show anti-inflammatory properties. This finding suggests that the anti-inflammatory capacity of URB1537 could be linked to the presence of the palmitoleate portion. Accordingly, it has been previously demonstrated that palmitoleic acid promotes anti-inflammatory effects in macrophages exposed to LPS by inhibiting the inflammasome pathway [42]. However, its real application could be limited by its oily nature, low solubility, and tendency to irritate (Safety Data Sheet). Indeed, URB1537 could be considered a safe potential prodrug of palmitoleic acid with better physicochemical and pharmacokinetic properties.
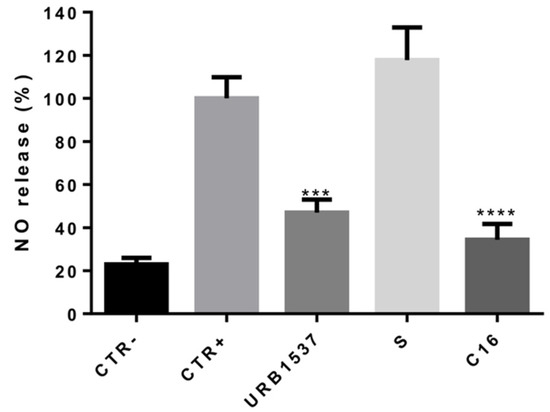
Figure 4.
Extracellular NO release after RAW 264.7 stimulation by LPS for 24 h in the presence of URB1537, S, and C16 (128 µg/mL). CTR-: negative control (untreated cells); CTR+: positive control (LPS). Data are expressed as the mean ± SD (n = 3). *** p < 0.001, **** p < 0.0001 vs. CTR+ (Tukey’s post hoc test).
Noteworthily, no cytotoxic effects were observed after LPS, URB1537, and dexamethasone administration to RAW 264.7 cells compared to untreated control cells (Figure 5), confirming that URB1537 was safe at concentrations up to 128 µg/mL, as also evidenced on HaCaT cells.
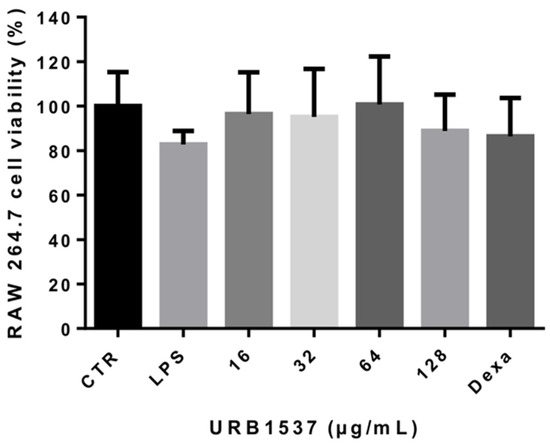
Figure 5.
Cell viability evaluation after LPS (1 µg/mL), URB1537 (16–128 µg/mL), and dexamethasone (2 µg/mL) administration to RAW 264.7 cells for 24 h. Data are expressed as the mean ± SD (n = 3).
3. Materials and Methods
3.1. Chemicals
Caprylic and undecynoic acids were purchased from TCI (Zwijndrecht, Belgium). Undecylenic, palmitoleic, oleic, linoleic, linolenic, and nervonic acids, and sucrose were purchased from Fluorochem (Hadfield, UK). Diisopropyl azodicarboxylate (DIAD) and triphenylphosphine (PPh3) were purchased from Alpha Aesar (Ward Hill, MA, USA). Benzoic, phenylacetic, phenylbenzoic, and biphenylacetic acids, dichloromethane (CH2Cl2), acetone [CH3C(O)CH3], methanol (CH3OH), dimethyl sulfoxide (DMSO and DMSO-d6) were purchased from Sigma-Aldrich (Milan, Italy). The structures of compounds were unambiguously assessed by MS, 1H NMR, 13C NMR, and IR. ESI-MS spectra were recorded with a Waters Micromass ZQ spectrometer in a negative or positive mode using nebulizing nitrogen gas at 400 L/min and a temperature of 250 °C, cone flow 40 mL/min, capillary 3.5 kV and cone voltage 60 V; only molecular ions [M-H]− or [M + NH4]+ or [M + Na]+ are given. HRMS spectra were performed by slow direct infusion (5 μL/min) of ≈ 0.1 μg/mL solution (acetonitrile/0.1% aqueous formic acid 1:1) of new compounds, using Orbitrap Exploris 240 mass spectrometer (Thermo Scientific, Waltham, MA, USA); only molecular ions [M + Na]+ are given. 1H NMR and 13C NMR spectra were recorded on a Bruker AC 400 or 101, respectively, spectrometer and analyzed using the TopSpin 1.3 software package. Chemical shifts were measured by using the central peak of the solvent. Column chromatography purifications were performed under “flash” conditions using Merck 230–400 mesh silica gel. TLC was carried out on Merck silica gel 60 F254 plates, which were visualized by exposure to ultraviolet light and by exposure to an aqueous solution of ceric ammonium molybdate.
3.2. General Procedure for the Synthesis of Sucrose Ester Surfactants (3a–m, URB1480-1482, URB1534-1543)
Sucrose (1) (0.342 g, 1 mmol) was dissolved in dry DMF (7.9 mL) at 70 °C and stirred under N2 atmosphere. The mixture was cooled to room temperature and then PPh3 (0.656 g, 2.5 mmol), the appropriate carboxylic acid (2a–m) (1.5 mmol), and DMF (2.1 mL) were added. After complete dissolution, the mixture was cooled to 0 °C and DIAD (0.508 g, 0.493 mL, 2.5 mmol) was introduced. The mixture was stirred at room temperature until the total consumption of sucrose (24–30 h) and concentrated. Purification of the residue by column chromatography (CH2Cl2/CH3C(O)CH3/MeOH/H2O 78:10:10:1.5) gave 3a–m as solids.
β-d-Fructofuranosyl 6-O-octanoyl-α-d-glucopyranoside, sucrose caprylate (3a, URB1534) [43].
White solid. Yield: 50%. MS (ESI): 467 [M–H]−, 486 [M + NH4]+, 491 [M + Na]+. 1H NMR (DMSO-d6): δ = 0.86 (t, 3H, J = 7.0 Hz, CH3) 1.22–1.30 [m, 8H, (CH2)4], 1.48–1.55 (m, 2H, CH2CH2COOR), 2.29–2.33 (m, 2H, CH2COOR), 3.06 (ddd, 1H, JH4-OH4 = 6.0 Hz, JH4-H5 = 9.0 Hz, JH4-H3 = 9.5 Hz, H4), 3.21 (ddd, 1H, JH2-H1 = 3.5 Hz, JH2-OH2 = 6.0 Hz, JH2-H3 = 9.5 Hz, H2), 3.38–3.41 (m, 2H, H1′a, H1′b), 3.49 (ddd, 1H, JH3-OH3 = 5.0 Hz, JH3-H2 ≈ JH3-H4 = 9.5 Hz, H3), 3.53–3.60 (m, 3H, H5′, H6′a, H6′b), 3.70–3.76 (m, 1H, H4′), 3.88 (dd, 1H, JH3′-OH3′ ≈ JH3′-H4′= 8.0 Hz, H3′), 3.90 (ddd, 1H, JH5-H6b = 1.5 Hz, JH5-H6a = 6.0 Hz, JH5-H4 = 9.0 Hz, H5), 4.02 (dd, 1H, JH6a-H5 = 6.0 Hz, JH6a-H6b = 11.5 Hz, H6a), 4.23 (dd, 1H, JH6b-H5 = 1.5 Hz, JH6b-H6a = 11.5 Hz, H6b), 4.38 (dd, 1H, JOH6′-H6′a ≈ JOH6′-H6′b = 6.0 Hz, OH6′), 4.55 (d, 1H, JOH3′-H3′ = 8.0 Hz, OH3′), 4.80 (dd, 1H, JOH1′-H1′a ≈ JOH1′-H1′b = 6.5 Hz, OH1′), 4.88 (d, 1H, JOH3-H3 = 5.0 Hz, OH3), 5.00 (d, 1H, JOH4-H4 = 6.0 Hz, OH4), 5.12 (d, 1H, JOH2-H2 = 6.0 Hz, OH2), 5.15 (d, 1H, JOH4′-H4′ = 6.0 Hz, OH4′), 5.18 (d, 1H, JH1-H2 = 3.5 Hz, H1) ppm. 13C NMR (DMSO-d6): δ = 14.4, 22.5, 24.9, 28.8, 28.9, 31.6, 33.8, 62.6, 63.1, 64.0, 70.4, 70.6, 72.0, 73.1, 75.0, 77.3, 83.2, 91.9, 104.3, 173.5 ppm.
β-d-Fructofuranosyl 6-O-undec-10-enoyl-α-d-glucopyranoside, sucrose undec-10-enoate (3b, URB1535).
White solid. Yield: 39%. MS (ESI): 507 [M–H]−, 526 [M + NH4]+, 531 [M + Na]+. 1H NMR (DMSO-d6): δ = 1.24–1.27 [m, 8H, (CH2)4], 1.33–1.35 (m, 2H, CH2CH2CH=CH2), 1.49–1.53 (m, 2H, CH2CH2COOR), 1.98–2.04 (m, 2H, CH2CH=CH2), 2.28–2.32 (m, 2H, CH2COOR), 3.06 (ddd, 1H, JH4-OH4 = 5.0 Hz, JH4-H3 ≈ JH4-H5 = 9.5 Hz, H4), 3.20 (ddd, 1H, JH2-H1 = 3.5 Hz, JH2-OH2 = 6.0 Hz, JH2-H3 = 9.5 Hz, H2), 3.37–3.40 (m, 2H, H1′a, H1′b), 3.48 (m, 1H, H3), 3.53–3.62 (m, 3H, H5′, H6′a, H6′b), 3.70–3.76 (m, 1H, H4′), 3.88 (dd, 1H, JH3′-OH3′ ≈ JH3′-H4′= 8.0 Hz, H3′), 3.91 (ddd, 1H, JH5-H6b = 1.5 Hz, JH5-H6a = 6.0 Hz, JH5-H4 = 9.5 Hz, H5), 4.01 (dd, 1H, JH6a-H5 = 6.0 Hz, JH6a-H6b = 11.5 Hz, H6a), 4.23 (dd, 1H, JH6b-H5 = 1.5 Hz, JH6b-H6a = 11.5 Hz, H6b), 4.39 (dd, 1H, JOH6′-H6′a ≈ JOH6′-H6′b = 5.0 Hz, OH6′), 4.56 (d, 1H, JOH3′-H3′ = 8.0 Hz, OH3′), 4.81 (dd, 1H, JOH1′-H1′a ≈ JOH1′-H1′b = 6.0 Hz, OH1′), 4.89 (brs, 1H, OH3), 4.94 (dddd, 1H, Jgem ≈ J1 = 1.5 Hz, J2 = 4.0 Hz, Jcis = 10.0 Hz, HCH=CHCH2), 5.00 (dddd, 1H, Jgem ≈ J1 ≈ J2 = 1.5 Hz, Jtrans = 17.0 Hz, HCH=CHCH2), 5.01 (d, 1H, JOH4-H4 = 5.0 Hz, OH4), 5.13 (d,1H, JOH2-H2 = 6.0 Hz, OH2), 5.16 (d, 1H, JOH4′-H4′ = 6.0 Hz, OH4′), 5.18 (d, 1H, JH1-H2 = 3.5 Hz, H1), 5.80 (dddd, 1H, J1 ≈ J2 = 7.0 Hz, Jcis = 10.0 Hz, Jtrans = 17.0 Hz, CH2=CHCH2) ppm. 13C NMR (DMSO-d6): δ = 24.9, 28.7, 28.9, 29.1, 29.1, 29.2, 33.6, 33.8, 62.6, 63.1, 64.0, 70.5, 70.6, 72.0, 73.1, 75.0, 77.4, 83.2, 91.9, 104.4, 115.1, 139.3, 173.5 ppm. HRMS (ESI) m/z for C23H40NaO12 [M + Na]+: calcd 531.2412; found 531.2411.
β-d-Fructofuranosyl 6-O-undec-10-inoyl-α-d-glucopyranoside, sucrose undec-10-inoate (3c, URB1536).
White solid. Yield: 37%. MS (ESI): 505 [M–H]−, 524 [M + NH4]+, 529 [M + Na]+. 1H NMR (DMSO-d6): δ = 1.23–1.27 [m, 6H, (CH2)3], 1.30–1.37 (m, 2H, CH2CH2CH2CCH), 1.40–1.47 (m, 2H, CH2CH2CCH), 1.49–1.55 (m, 2H, CH2CH2COOR), 2.14 (td, 2H, J1 = 2.5 Hz, J2 = 7.0 Hz, CH2CCH), 2.28–2.33 (m, 2H, CH2COOR), 2.72 (t, 1H, J = 2.5 Hz, CCH), 3.06 (ddd, 1H, JH4-OH4 = 5.0 Hz, JH4-H3 ≈ JH4-H5 = 9.5 Hz, H4), 3.21 (ddd, 1H, JH2-H1 = 3.5 Hz, JH2-OH2 = 6.0 Hz, JH2-H3 = 9.5 Hz, H2), 3.36–3.41 (m, 2H, H1′a, H1′b), 3.49 (ddd, 1H, JH3-OH3 = 5.0 Hz, JH3-H2 ≈ JH3-H4 = 9.5 Hz, H3), 3.53–3.62 (m, 3H, H5′, H6′a, H6′b), 3.70–3.76 (m, 1H, H4′), 3.88 (dd, 1H, JH3′-OH3′ ≈ JH3′-H4′ = 8.0 Hz, H3′), 3.90 (ddd, 1H, JH5-H6b = 1.5 Hz, JH5-H6a = 6.0 Hz, JH5-H4 = 9.5 Hz, H5), 4.02 (dd, 1H, JH6a-H5 = 6.0 Hz, JH6a-H6b = 11.5 Hz, H6a), 4.23 (dd, 1H, JH6b-H5 = 1.5 Hz, JH6b-H6a = 11.5 Hz, H6b), 4.38 (dd, 1H, JOH6′-H6′a ≈ JOH6′-H6′b = 5.0 Hz, OH6′), 4.56 (d, 1H, JOH3′-H3′ = 8.0 Hz, OH3′), 4.80 (dd, 1H, JOH1′-H1′a ≈ JOH1′-H1′b = 6.0 Hz, OH1′), 4.88 (d, 1H, JOH3-H3 = 5.0 Hz, OH3), 5.00 (d, 1H, JOH4-H4 = 5.0 Hz, OH4), 5.13 (d, 1H, JOH2-H2 = 6.0 Hz, OH2), 5.16 (d, 1H, JOH4′-H4′ = 6.0 Hz, OH4′), 5.18 (d, 1H, JH1-H2 = 3.5 Hz, H1) ppm. 13C NMR (DMSO-d6) δ 18.1, 24.9, 28.4, 28.5, 28.8, 28.9, 29.1, 33.8, 62.7, 63.0, 64.0, 70.5, 70.6, 71.5, 72.0, 73.1, 75.0, 77.4, 83.2, 85.0, 91.9, 104.4, 173.5 ppm. HRMS (ESI) m/z for C23H38NaO12 [M + Na]+: calcd 529.2256; found 529.2253.
β-d-Fructofuranosyl 6-O-hexadec-9-enoyl-α-d-glucopyranoside, sucrose palmitoleate (3d, URB1537) [25].
White solid. Yield: 30%. MS (ESI): 577 [M–H]−, 596 [M + NH4]+, 601 [M + Na]+. 1H NMR (DMSO-d6): δ = 0.86 (t, 3H, J = 7.0 Hz, CH3), 1.26–1.30 [m, 16H, (CH2)8], 1.50–1.53 (m 2H, CH2CH2COOR), 1.97–2.00 (m, 4H, CH2CH=CHCH2), 2.28–2.32 (m, 2H, CH2COOR), 3.06 (ddd, 1H, JH4-OH4 = 5.0 Hz, JH4-H3 ≈ JH4-H5 = 9.5 Hz, H4), 3.19 (ddd, 1H, JH2-H1 = 3.5 Hz, JH2-OH2 = 4.0 Hz, JH2-H3 = 9.5 Hz, H2), 3.38–3.39 (m, 2H, H1′a, H1′b), 3.49 (m, 1H, H3), 3.54–3.62 (m, 3H, H5′, H6′a, H6′b), 3.71–3.76 (m, 1H, H4′), 3.88 (dd, 1H, JH3′-OH3′ ≈ JH3′-H4′ = 8.0 Hz, H3′), 3.91 (ddd, 1H, JH5-H6b = 1.5 Hz, JH5-H6a = 6.0 Hz, JH5-H4 = 9.5 Hz, H5), 4.03 (dd, 1H, JH6a-H5 = 6.0 Hz, JH6a-H6b = 11.5 Hz, H6a), 4.23 (dd, 1H, JH6b-H5 = 1.5 Hz, JH6b-H6a = 11.5 Hz, H6b), 4.38 (dd, 1H, JOH6′-H6′a ≈ JOH6′-H6′b = 5.0 Hz, OH6′), 4.55 (d, 1H, JOH3′-H3′ = 8.0 Hz, OH3′), 4.80 (dd, 1H, JOH1′-H1′a ≈ JOH1′-H1′b = 6.0 Hz, OH1′), 4.88 (brs, 1H, OH3), 5.00 (d, 1H, JOH4-H4 = 5.0 Hz, OH4), 5.12 (d, 1H, JOH2-H2 = 4.0 Hz, OH2), 5.14 (d, 1H, JOH4′-H4′ = 6.0 Hz, OH4′), 5.18 (d, 1H, JH1-H2 = 3.5 Hz, H1), 5.31 (ddd, 1H, J1 ≈ J2 = 6.0 Hz, J3 = 11.0 Hz, CH=CH), 5.34 (ddd, 1H, J1 ≈ J2 = 6.0 Hz, J3 = 11.0 Hz, CH=CH) ppm. 13C NMR (DMSO-d6): δ = 14.4, 22.5, 24.8, 27.1 (2C), 28.7, 29.0, 29.1, 29.6, 31.6, 33.8, 62.6, 63.1, 64.0, 70.4, 70.6, 72.0, 73.1, 75.0, 77.4, 83.2, 91.9, 104.4, 130.1 (2C), 173.5 ppm.
β-d-Fructofuranosyl 6-O-octadec-9-enoyl-α-d-glucopyranoside, sucrose oleate (3e, URB1538) [44].
White solid. Yield: 36%. MS (ESI): 605 [M–H]−, 624 [M + NH4]+, 629 [M + Na]+. 1H NMR (DMSO-d6): δ = 0.86 (t, 3H, J = 6.5 Hz, CH3) 1.22–1.33 [m, 20H, (CH2)10], 1.47–1.55 (m, 2H, CH2CH2COOR), 1.95–2.01 (m, 4H, CH2CH=CHCH2), 2.28–2.32 (m, 2H, CH2COOR), 3.06 (ddd, 1H, JH4-OH4 = 6.0 Hz, JH4-H3 ≈ JH4-H5 = 9.5 Hz, H4), 3.20 (ddd, 1H, JH2-H1 = 3.5 Hz, JH2-OH2 = 6.0 Hz, JH2-H3 = 9.5 Hz, H2), 3.39–3.40 (m, 2H, H1′a, H1′b), 3.48 (ddd, 1H, JH3-OH3 = 5.0 Hz, JH3-H2 ≈ JH3-H4 = 9.5 Hz, H3), 3.52–3.62 (m, 3H, H5′, H6′a, H6′b), 3.70–3.76 (m, 1H, H4′), 3.87 (dd, 1H, JH3′-OH3′ ≈ JH3′-H4′= 8.0 Hz, H3′), 3.91 (ddd, 1H, JH5-H6b = 1.5 Hz, JH5-H6a = 6.0 Hz, JH5-H4 = 9.5 Hz, H5), 4.02 (dd, 1H, JH6a-H5 = 6.0 Hz, JH6a-H6b = 11.5 Hz, H6a), 4.23 (dd, 1H, JH6b-H5 = 1.5 Hz, JH6b-H6a = 11.5 Hz, H6b), 4.39 (dd, 1H, JOH6′-H6′a ≈ JOH6′-H6′b = 5.0 Hz, OH6′), 4.56 (d, 1H, JOH3′-H3′ = 8.0 Hz, OH3′), 4.81 (dd, 1H, JOH1′-H1′a ≈ JOH1′-H1′b = 6.0 Hz, OH1′), 4.88 (d, 1H, JOH3-H3 = 5.0 Hz, OH3), 5.00 (d, 1H, JOH4-H4 = 6.0 Hz, OH4), 5.13 (d, 1H, JOH2-H2 = 6.0 Hz, OH2), 5.16 (d, 1H, JOH4′-H4′ = 6.0 Hz, OH4′), 5.18 (d, 1H, JH1-H2 = 3.5 Hz, H1), 5.31 (ddd, 1H, J1 ≈ J2 = 6.0 Hz, J3 = 11.0 Hz, CH=CH), 5.35 (ddd, 1H, J1 ≈ J2 = 6.0 Hz, J3 = 11.0 Hz, CH=CH) ppm. 13C NMR (DMSO-d6): δ = 14.4, 22.5, 24.9, 27.0, 27.1, 28.95, 28.97, 29.03, 29.06, 29.12, 29.3, 29.6, 31.7, 33.7, 62.6, 63.0, 64.0, 70.4, 70.6, 72.0, 73.1, 75.0, 77.4, 83.2, 92.0, 104.4, 130.1(2C), 173.5 ppm.
β-d-Fructofuranosyl 6-O-octadec-9,12-enoyl-α-d-glucopyranoside, sucrose linoleate (3f, URB1539) [41].
White solid. Yield: 23%. MS (ESI): 603 [M–H]−, 622 [M + NH4]+, 627 [M + Na]+. 1H NMR (DMSO-d6): δ = 0.86 (t, 3H, J = 6.5 Hz, CH3), 1.22–1.34 [m, 14H, (CH2)7], 1.49–1.54 (m 2H, CH2CH2COOR), 2.00–2.05 (m, 4H, CH2CH=CHCH2CH=CHCH2), 2.28–2.32 (m, 2H, CH2COOR), 2.74 (m, 2H, CH=CHCH2CH=CH), 3.06 (ddd, 1H, JH4-OH4 = 5.5 Hz, JH4-H3≈JH4-H5 = 9.0 Hz, H4), 3.20 (ddd, 1H, JH2-H1 = 3.5 Hz, JH2-OH2 = 6.0 Hz, JH2-H3 = 9.0 Hz, H2), 3.39–3.40 (m, 2H, H1′a, H1′b), 3.48 (m, 1H, H3), 3.53–3.61 (m, 3H, H5′, H6′a, H6′b), 3.71–3.77 (m, 1H, H4′), 3.87 (dd, 1H, JH3′-OH3′ ≈ JH3′-H4′ = 8.0 Hz, H3′), 3.90–3.93 (m, 1H, H5), 4.02 (dd, 1H, JH6a-H5 = 6.0 Hz, JH6a-H6b = 11.5 Hz, H6a), 4.22 (dd, 1H, JH6b-H6a = 11.5 Hz, H6b), 4.38 (dd, 1H, JOH6′-H6′a ≈ JOH6′-H6′b = 5.0 Hz, OH6′), 4.56 (d, 1H, JOH3′-H3′ = 8.0 Hz, OH3′), 4.81 (dd, 1H, JOH1′-H1′a ≈ JOH1′-H1′b = 6.0 Hz, OH1′), 4.89 (d, 1H, JOH3-H3 = 4.5 Hz, OH3), 5.00 (d, 1H, JOH4-H4 = 5.5 Hz, OH4), 5.12 (d,1H, JOH2-H2 = 6.0 Hz, OH2), 5.14 (d, 1H, JOH4′-H4′ = 6.5 Hz, OH4′), 5.18 (d, 1H, JH1-H2 = 3.5 Hz, H1), 5.27–5.38 (m, 4H, CH=CHCH2CH=CH) ppm. 13C NMR (DMSO-d6): δ = 14.4, 22.4, 24.9, 25.7, 27.0, 27.1, 28.95, 29.0, 29.1, 29.2, 29.5, 31.3, 33.8, 62.6, 63.0, 64.0, 70.4, 70.6, 72.0, 73.0, 75.0, 77.4, 83.2, 91.9, 104.4, 128.2(2C), 130.2(2C), 173.5 ppm.
β-d-Fructofuranosyl 6-O-octadec-9,12,15-enoyl-α-d-glucopyranoside, sucrose linolenate (3g, URB1540).
White solid. Yield: 25%. MS (ESI): 601 [M–H]–, 620 [M + NH4]+, 625 [M + Na]+. 1H NMR (DMSO-d6): δ = 0.93 (t, 3H, J = 7.5 Hz, CH3) 1.19–1.26 [m, 8H, (CH2)4], 1.49–1.54 (m 2H, CH2CH2COOR), 2.02–2.08 (m, 4H, CH2CH=CHCH2CH=CHCH2CH=CHCH2), 2.28–2.32 (m, 2H, CH2COOR), 2.73–2.79 (m, 4H, CH=CHCH2CH=CHCH2CH=CH), 3.06 (ddd, 1H, JH4-OH4 = 6.0 Hz, JH4-H3 ≈ JH4-H5 = 9.5 Hz, H4), 3.21 (ddd, 1H, JH2-H1 = 3.5 Hz, JH2-OH2 = 6.0 Hz, JH2-H3 = 9.5 Hz, H2), 3.38–3.39 (m, 2H, H1′a, H1′b), 3.49 (ddd, 1H, JH3-OH3 = 5.0 Hz, JH3-H2 ≈ JH3-H4 = 9.5 Hz, H3), 3.54–3.61 (m, 3H, H5′, H6′a, H6′b), 3.70–3.76 (m, 1H, H4′), 3.86 (dd, 1H, JH3′-OH3′ ≈ JH3′-H4′ = 8.0 Hz, H3′), 3.90–3.93 (m, 1H, H5), 4.02 (dd, 1H, JH6a-H5 = 6.0 Hz, JH6a-H6b = 11.5 Hz, H6a), 4.23 (dd, 1H, JH6b-H5 = 1.5 Hz, JH6b-H6a = 11.5 Hz, H6b), 4.38 (dd, 1H, JOH6′-H6′a ≈ JOH6′-H6′b = 5.0 Hz, OH6′), 4.56 (d, 1H, JOH3′-H3′ = 8.0 Hz, OH3′), 4.80 (dd, 1H, JOH1′-H1′a ≈ JOH1′-H1′b = 6.5 Hz, OH1′), 4.89 (d, 1H, JOH3-H3 = 5.0 Hz, OH3), 5.00 (d, 1H, JOH4-H4 = 6.00 Hz, OH4), 5.13 (d, 1H, JOH2-H2 = 6.0 Hz, OH2), 5.15 (d, 1H, JOH4′-H4′ = 6.0 Hz, OH4′), 5.18 (d, 1H, JH1-H2 = 3.5 Hz, H1), 5.25–5.40 (m, 6H, CH=CHCH2CH=CHCH2CH=CH) ppm. 13C NMR (DMSO-d6): δ = 14.6, 20.5, 24.8, 25.6, 25.7, 27.1, 28.9, 29.0, 29.1, 29.5, 33.8, 62.6, 63.0, 64.0, 70.4, 70.6, 72.0, 73.1, 75.0, 77.4, 83.2, 92.0, 104.4, 127.4, 128.0, 128.3, 128.4, 130.4, 132.0, 173.5 ppm. HRMS (ESI) m/z for C30H50NaO12 [M + Na]+: calcd 625.3195; found 625.3191.
β-d-Fructofuranosyl 6-O-tetracos-15-enoyl-α-d-glucopyranoside, sucrose nervonate (3h, URB1541).
White solid. Yield: 26%. MS (ESI): 689 [M–H]− 708 [M + NH4]+, 713 [M + Na]+. 1H NMR (DMSO-d6): δ = 0.86 (t, 3H, J = 7.0 Hz, CH3), 1.24–1.29 [m, 32H, (CH2)16], 1.49–1.53 (m 2H, CH2CH2COOR), 1.96–2.00 (m, 4H, CH2CH=CHCH2), 2.30 (t, 2H, J = 7.5 Hz, CH2COOR), 3.06 (ddd, 1H, JH4-OH4 = 5.5 Hz, JH4-H3 ≈ JH4-H5 = 9.5 Hz, H4), 3.21 (ddd, 1H, JH2-H1 = 3.5 Hz, JH2-OH2 = 6.0 Hz, JH2-H3 = 9.5 Hz, H2), 3.38–3.40 (m, 2H, H1′a, H1′b), 3.49 (dd, 1H, JH3-H2 ≈ JH3-H4 = 9.5 Hz, H3), 3.54–3.62 (m, 3H, H5′, H6′a, H6′b), 3.71–3.76 (m, 1H, H4′), 3.88 (dd, 1H, JH3′-OH3′ ≈ JH3′-H4′ = 8.0 Hz, H3′), 3.89–3.93 (m, 1H, H5), 4.02 (dd, 1H, JH6a-H5 = 6.0 Hz, JH6a-H6b = 11.5 Hz, H6a), 4.23 (dd, 1H, JH6b-H5 = 1.5 Hz, JH6b-H6a = 11.5 Hz, H6b), 4.37 (dd, 1H, JOH6′-H6′a ≈ JOH6′-H6′b = 5.5 Hz, OH6′), 4.56 (d, 1H, JOH3′-H3′ = 8.0 Hz, OH3′), 4.80 (dd, 1H, JOH1′-H1′a ≈ JOH1′-H1′b = 6.5 Hz, OH1′), 4.89 (d, 1H, JOH3-H3 = 3.5 Hz, OH3), 5.00 (d, 1H, JOH4-H4 = 5.5 Hz, OH4), 5.13 (d, 1H, JOH2-H2 = 6.0 Hz, OH2), 5.15 (d, 1H, JOH4′-H4′ = 6.0 Hz, OH4′), 5.18 (d, 1H, JH1-H2 = 3.5 Hz, H1), 5.31 (ddd, 1H, J1 ≈ J2 = 6.0 Hz, J3 = 11.0 Hz, CH=CH), 5.34 (ddd, 1H, J1 ≈ J2 = 6.0 Hz, J3 = 11.0 Hz CH=CH) ppm 13C NMR (DMSO-d6): δ = 14.3, 22.6, 24.9, 27.0, 29.0, 29.1, 29.29, 29.31, 29.4, 29.5, 31.75, 33.8, 62.7, 63.0, 64.0, 70.5, 70.6, 72.0, 73.1, 75.0, 77.4, 83.2, 92.0, 104.4, 130.1, 173.5 ppm. HRMS (ESI) m/z for C36H66NaO12 [M + Na]+: calcd 713.4447; found 713.4443.
β-d-Fructofuranosyl 6-O-benzoyl-α-d-glucopyranoside, sucrose benzoate (3i, URB1542) [45].
White solid. Yield: 43%. MS (ESI): 445 [M–H]−, 428 [M + NH4]+, 433 [M + Na]+. 1H NMR (DMSO-d6): δ = 3.26 (ddd, 1H, JH4-OH4 = 6.0 Hz, JH4-H3 ≈ JH4-H5 = 9.5 Hz, H4), 3.28 (ddd, 1H, JH2-H1 = 3.5 Hz, JH2-OH2 = 6.0 Hz, JH2-H3 = 9.5 Hz, H2), 3.41–3.43 (m, 2H, H1′a, H1′b), 3.50–3.61 (m, 4H, H3, H5′, H6′a, H6′b), 3.79 (ddd, 1H, JH4′-OH4 = 6.00 Hz, JH4′-H3′ ≈ JH4′-H5′ = 8.00 Hz, H4′), 3.91 (dd, 1H, JH3′-OH3′ ≈ JH3′-H4′ = 8.0 Hz, H3′), 4.04–4.11 (m, 1H, H5), 4.34 (dd, 1H, JH6a-H5 = 5.0 Hz, JH6a-H6b = 11.5 Hz, H6a), 4.37 (dd, 1H, JOH6′-H6′a ≈ JOH6′-H6′b = 5.5 Hz, OH6′), 4.45 (dd, 1H, JH6b-H5 = 1.5 Hz, JH6b-H6a = 11.5 Hz, H6b), 4.65 (d, 1H, JOH3′-H3′ = 8.0 Hz, OH3′), 4.83 (dd, 1H, JOH1′-H1′a ≈ JOH1′-H1′b = 6.0 Hz, OH1′), 4.92 (d, 1H, JOH3-H3 = 5.0 Hz, OH3), 5.14 (d, 1H, JOH4-H4 = 6.0 Hz, OH4), 5.15 (d, 1H, JOH2-H2 = 6.0 Hz, OH2), 5.19 (d, 1H, JOH4′-H4′ = 6.0 Hz, OH4′), 5.23 (d, 1H, JH1-H2 = 3.5 Hz, H1), 7.51–7.57 (m, 2H, ArH), 7.67 [dddd, 1H, J1 ≈ J2 = 1.0 Hz, J3 ≈ J4 = 8.5 Hz, ArH(p)], 7.97–8.01 (m, 2H, ArH) ppm. 13C NMR (DMSO-d6): δ = 62.6, 63.0, 64.8, 70.5, 72.0, 73.1, 75.0, 77.4, 83.1, 92.2, 104.5, 129.2, 129.7, 130.1, 133.7, 173.5 ppm.
β-d-Fructofuranosyl 6-O-[2-(4-phenyl)benzoyl]-α-d-glucopyranoside, sucrose p-phenyl benzoate (3j, URB1481).
Pale yellow solid. Yield: 34%. MS (ESI): 521 [M-H]−, 540 [M + NH4]+, 545 [M + Na]+. 1H NMR (DMSO-d6): δ = 3.25–3.32 (m, 2H, H4, H2), 3.40–3.44 (m, 2H, H1′a, H1′b), 3.48 (ddd, 1H, JH3-OH3 = 5.0 Hz, JH3-H2 ≈ JH3-H4 = 9.0 Hz, H3), 3.52–3.62 (m, 3H, H5′, H6′a, H6′b), 3.78–3.83 (m, 1H, H4′), 3.92 (dd, 1H, JH3′-OH3′ ≈ JH3′-H4′ = 8.0 Hz, H3′), 4.09 (ddd, 1H, JH5-H6b = 1.5 Hz, JH5-H6a = 5.0 Hz, JH5-H4 = 9.0 Hz, H5), 4.36 (dd, 1H, JH6a-H5 = 5.0 Hz, JH6a-H6b = 12.0 Hz, H6a), 4.41 (dd, 1H, JOH6′-H6′a ≈ JOH6′-H6′b = 6.0 Hz, OH6′), 4.47 (dd, 1H, JH6b-H5 = 1.5 Hz, JH6b-H6a = 12.0 Hz, H6b), 4.68 (d, 1H, JOH3′-H3′ = 8.0 Hz, OH3′), 4.85 (dd, 1H, JOH1′-H1′a ≈ JOH1′-H1′b = 6.5 Hz, OH1′), 4.96 (d, 1H, JOH3-H3 = 5.0 Hz, OH3), 5.17 (d, 1H, JOH4-H4 = 5.0 Hz, OH4), 5.18 (d,1H, JOH2-H2 = 6.0 Hz, OH2), 5.21 (d, 1H, JOH4′-H4′ = 5.5 Hz, OH4′), 5.24 (d, 1H, JH1-H2 = 3.5 Hz, H1), 7.42–7.46 (m, 1H, ArH), 7.50–7.54 (m, 2H, ArH), 7.74–7.76 (m, 2H, ArH), 7.82–7.85 (m, 2H, ArH), 8.05–8.08 (m, 2H, ArH) ppm. 13C NMR (DMSO-d6): δ = 62.5, 63.0, 64.8, 70.5, 70.6, 72.0, 73.1, 74.9, 77.3, 83.1, 92.2, 104.5, 127.4, 127.5, 128.9, 129.0, 129.6, 130.4, 139.4, 145.1, 166.1 ppm. HRMS (ESI) m/z for C25H30NaO12 [M + Na]+: calcd 545.1630; found 545.1636.
β-d-Fructofuranosyl 6-O-(2-phenylethanoyl)-α-d-glucopyranoside, sucrose phenyl acetate (3k, URB1480) [46].
White solid. Yield: 50%. MS (ESI): 459 [M–H]−, 478 [M + NH4]+, 483 [M + Na]+. 1H NMR (DMSO-d6): δ = 3.07 (ddd, 1H, JH4-OH4 = 6.0 Hz, JH4-H3 ≈ JH4-H5 = 9.5 Hz, H4), 3.21 (ddd, 1H, JH2-H1 = 3.5 Hz, JH2-OH2 = 6.0 Hz, JH2-H3 = 9.5 Hz, H2), 3.39–3.43 (m, 2H, H1′a, H1′b), 3.50 (ddd, 1H, JH3-OH3 = 5.0 Hz, JH3-H2 ≈ JH3-H4 = 9.5 Hz, H3), 3.58–3.64 (m, 3H, H5′, H6′a, H6′b), 3.67 (d, J = 16.0 Hz, 1H, HCHAr), 3.72 (d, J = 16.0 Hz, 1H, HCHAr), 3.76–3.82 (m, 1H, H4′), 3.90 (dd, 1H, JH3′-OH3′ ≈ JH3′-H4′ = 8.0 Hz, H3′), 3.95 (m, 1H, H5), 4.05 (dd, 1H, JH6a-H5 = 6.0 Hz, JH6a-H6b = 11.5 Hz, H6a), 4.28 (dd, 1H, JH6b-H5 = 1.5 Hz, JH6b-H6a = 11.5 Hz, H6b), 4.43 (dd, 1H, JOH6′-H6′a ≈ JOH6′-H6′b = 5.5 Hz, OH6′), 4.60 (d, 1H, JOH3′-H3′ = 8.0 Hz, OH3′), 4.83 (dd, 1H, JOH1′-H1′a ≈ JOH1′-H1′b = 6.5 Hz, OH1′), 4.90 (d, 1H, JOH3-H3 = 5.0 Hz, OH3), 5.03 (d, 1H, JOH4-H4 = 6.0 Hz, OH4), 5.13 (d, 1H, JOH2-H2 = 6.0 Hz, OH2), 5.19 (d, 1H, JH1-H2 = 3.5 Hz, H1), 5.20 (d, 1H, JOH4′-H4′ = 6.0 Hz, OH4′), 7.24–7.35 (m, 5H, ArH) ppm. 13C NMR (DMSO-d6): δ = 21.2, 62.7, 63.1, 64.6, 70.6, 72.0, 73.1, 75.0, 77.4, 83.2, 92.0, 104.4, 127.2, 128.7, 129.9, 134.8, 171.7 ppm.
β-d-Fructofuranosyl 6-O-[2-(4-phenyl)phenylethanoyl]-α-d-glucopyranoside, sucrose p-biphenyl acetate (3l, URB1482).
White solid. Yield: 58%. MS (ESI): 527 [M–H]–, 536 [M + NH4]+, 541 [M + Na]+. 1H NMR (DMSO-d6): δ = 3.03 (ddd, 1H, JH4-OH4 = 6.0 Hz, JH4-H3 ≈ JH4-H5 = 9.5 Hz, H4), 3.13 (ddd, 1H, JH2-H1 = 3.5 Hz, JH2-OH2 = 6.0 Hz, JH2-H3 = 9.5 Hz, H2), 3.37–3.41 (m, 2H, H1′a, H1′b), 3.48 (ddd, 1H, J H3-OH3 = 5.0 Hz, JH3-H2 ≈ JH3-H4 = 9.5 Hz, H3), 3.56–3.63 (m, 3H, H5′, H6′a, H6′b), 3.76–3.81 (m, 1H, H4′), 3.88–3.96 (m, 2H, H3′, H5), 4.14 (dd, 1H, JH6a-H5 = 5.0 Hz, JH6a-H6b = 11.5 Hz, H6a), 4.33 (dd, 1H, JH6b-H5 = 1.0 Hz, JH6b-H6a = 11.5 Hz, H6b), 4.42 (dd, 1H, JOH6′-H6′a ≈ JOH6′-H6′b = 5.5 Hz, OH6′), 4.62 (d, 1H, JOH3′-H3′ = 8.0 Hz, OH3′), 4.82 (dd, 1H, JOH1′-H1′a ≈ JOH1′-H1′b = 6.0 Hz, OH1′), 4.89 (d, 1H, JOH3-H3 = 5.0 Hz, OH3), 5.02 (d, 1H, JOH4-H4 = 6.0 Hz, OH4), 5.13 (d, 1H, JOH2-H2 = 6.0 Hz, OH2), 5.17 (d, 1H, JH1-H2 = 3.5 Hz, H1) 5.20 (d, 1H, JOH4′-H4′ = 5.5 Hz, OH4′), 7.24–7.28 (m, 2H, ArH), 7.29–7.37 (m, 7H, ArH) ppm. 13C NMR (DMSO-d6): δ = 56.1, 62.5, 63.0, 64.6, 70.3, 70.4, 71.9, 73.1, 75.0, 77.4, 83.2, 92.0, 104.5, 127.4, 127.5, 128.9, 130.0, 139.48, 139.52, 172.4 ppm. HRMS (ESI) m/z for C26H32NaO12 [M + Na]+: calcd 559.1786; found 559.1790.
β-d-Fructofuranosyl 6-O-[2-(4,4′-biphenyl)phenylethanoyl]-α-d-glucopyranoside, sucrose p-triphenylacetate (3m, URB1543).
White solid. Yield: 31%. MS (ESI): 611 [M–H]−, 630 [M + NH4]+, 635 [M + Na]+.1H NMR (DMSO-d6) δ: 3.08 (ddd, 1H, JH4-OH4 = 5.0 Hz, JH4-H3 ≈ JH4-H5 = 9.5 Hz, H4), 3.23 (ddd, 1H, JH2-H1 = 4.0 Hz, JH2-OH2 = 6.0 Hz, JH2-H3 = 9.5 Hz, H2), 3.38–3.43 (m, 2H, H1′a, H1′b), 3.46–3.54 (m, 1H, H3), 3.57–3.66 (m, 3H, H5′, H6′a, H6′b), 3.73 (d, J = 16.0 Hz, 1H, HCHAr), 3.77 (d, J = 16.0 Hz, 1H, HCHAr), 3.79–3.82 (m, 1H, H4′), 3.90 (dd, 1H, JH3′-OH3′ ≈ JH3′-H4′ = 8.0 Hz, H3′), 3.97 (m, 1H, H5), 4.08 (dd, 1H, JH6a-H5 = 6.0 Hz, JH6a-H6b = 11.5 Hz, H6a), 4.31 (dd, 1H, JH6b-H5 = 1.5 Hz, JH6b-H6a = 11.5 Hz, H6b), 4.46 (dd, 1H, JOH6′-H6′a ≈ JOH6′-H6′b = 5.5 Hz, OH6′), 4.62 (d, 1H, JOH3′-H3′ = 8.0 Hz, OH3′), 4.83 (dd, 1H, JOH1′-H1′a ≈ JOH1′-H1′b = 6.0 Hz, OH1′), 4.84 (brs, 1H, OH3), 5.06 (d, 1H, JOH4-H4 = 5.0 Hz, OH4), 5.15 (d, 1H, JOH2-H2 = 6.0 Hz, OH2), 5.21 (m, 2H, H1, OH4′), 7.35–7.42 (m, 3H, ArH), 7.45–7.51 (m, 2H, ArH), 7.66–7.78 (m, 8H, ArH) ppm. 13C NMR (DMSO-d6) δ: 62.7, 63.1, 64.7, 70.5, 70.6, 72.0, 73.1, 75.0, 77.3, 83.2, 91.9, 104.4, 126.9, 127.0, 127.5, 127.55, 127.65, 128.0, 129.4, 130.6, 134.2, 138.5, 139.3, 139.6, 140.1, 171.7 ppm. HRMS (ESI) m/z for C32H36NaO12 [M + Na]+: calcd 635.2099; found 635.2104.
3.3. Bacterial Strains and Culture Conditions
Eight reference human pathogens were used in this study: E. faecalis ATCC 29212, E. coli O157:H7 ATCC 35150, S. aureus ATCC 43387, S. aureus ATCC 43300 (MRSA), L. monocytogenes ATCC 7644, K. pneumoniae ATCC 13883, P. aeruginosa ATCC 9027, and S. enteritidis ATCC 13076. All the strains were maintained in TSA (tryptone soy agar) (VWR, Milan, Italy) at 37 °C, while the stock cultures were kept at −80 °C in nutrient broth with glycerol 15%.
3.4. Fungi and Culture Conditions
Three pathogenic filamentous fungi belonging to the strain collection of Pharmacology and Hygiene Section (Department of Biomolecular Sciences, University of Urbino Carlo Bo) A. niger ATCC 9642, A. fumigatus IDRAH01 and Fusarium spp., as well as the reference strain C. albicans ATCC 10231, were included. All the filamentous fungi were maintained on potato dextrose agar (PDA) (VWR) at 30 °C for 7 days, while C. albicans was grown at 37 °C for 24 h.
3.5. Minimum Inhibitory Concentration (MIC)
MIC of each compound was determined by standard micro-dilution method according to the National Committee for NCCLS (Clinical Laboratory Standards) document M100-S12 method (CLSI, 2016). First, stock solution of each compound (~12 mg) was prepared in 50:50 (v/v) of distilled sterile water and biological grade DMSO (final volume 1 mL). Several colonies of each bacterial strain were inoculated in 10 mL of sterile Mueller–Hinton broth II (MHB II) (VWR) and incubated at 37 °C for 24 h. At the end of the incubation period, each suspension was diluted in MHB II to obtain ca. 105 cfu/mL and 100 µL was added in wells of the 96-well plate together with the appropriate volumes of the test solutions (final concentration range: 4–1024 µg/mL). Two rows were used for positive (bacteria alone) and negative controls (MHB II alone), respectively. Preliminary assays with DMSO were carried out to exclude its possible bacteriostatic and/or bactericidal activity; in any case, the quantity of DMSO added in each well never exceeded 5% (v/v) of the final total volume. MIC was defined as the lowest concentration of compound able to inhibit bacterial growth after 24 h of incubation at 37 °C. All the experiments were performed in duplicate.
Regarding the filamentous fungi, spores were harvested from PDA plates by adding 2 mL of sterile 0.85% saline solution supplemented with 0.05% Tween 80; the surface was scraped with a sterile cotton swab and the suspension, transferred in a sterile tube, was left at room temperature for 5 min to allow the sedimentation of hyphal fragments. The upper homogeneous suspension was transferred into a new sterile tube, vortexed for 15 s and adjusted to an optical density (OD 530 nm) between 0.09 to 0.4 (about 106 spores/mL). For C. albicans the suspension was adjusted with the spectrophotometer to a turbidity of 0.12 (about 106 cfu/mL). Successively, 100 μL of each fungal suspension was diluted 1:50 in standard RPMI 1640 medium (Sigma-Aldrich, Milan, Italy) and inoculated into 96-well plates together with the appropriate volumes of each test solution as described above. Two rows were left for positive control growth and negative controls (medium only), respectively. Plates were incubated at 30 °C for 72 h; in the case of C. albicans plates were incubated at 37 °C for 24–48 h. MIC is defined as the lowest drug concentration that inhibits visible growth compared to the untreated control. In addition, the turbidity of the 96-wells plate was assessed by spectrophotometer (530 nm) (Multiskan EX, Thermo Scientific, Waltham, MA, USA).
3.6. DPPH Assays
The antioxidant capacity of URB1537 was evaluated in a cell-free system by the DPPH radical scavenging assay, as previously described (Verboni 2022). Concentrations up to 512 µg/mL were tested, and the EC50 value (i.e., the concentration necessary to achieve a 50% antioxidant effect) was calculated. Quercetin (up to 4.5 µg/mL) was used as a reference antioxidant molecule to check the procedure’s correctness.
3.7. Cytotoxicity Assay
The cytotoxicity of URB1537 was investigated on HaCaT cells (immortalized human keratinocytes) from CLS-Cell Lines Service GmbH (Eppelheim, Germany) in vitro. Cells (5 × 103/well) were seeded in 96-well plates and incubated for 24 h with S-C16 (16–512 µg/mL). After incubation, cell viability was analyzed by the MTT assay. Color development was monitored at 570 nm in a multi-well plate reader (Thermo Scientific, Waltham, MA, USA), and data were expressed as cell viability (%) vs. untreated control cells. The IC50 value (i.e., the concentration required to reduce cell viability by 50%) was then calculated.
3.8. Griess Assay
The anti-inflammatory properties of URB1537 were evaluated in RAW 264.7 cells (murine macrophages) stimulated by lipopolysaccharide (LPS) (Sigma-Aldrich, Milan, Italy). Cells (3 × 104/well) were seeded in 96-well plates and treated for 24 h with LPS (1 µg/mL) in the presence of URB1537 (16–128 µg/mL). After that, NO release was determined in the culture medium by the Griess reagent (Sigma-Aldrich, Milan, Italy), as recently reported (Verboni 2022). The anti-inflammatory effects of URB1537 (128 µg/mL) in comparison to sucrose and palmitoleic acid were also assessed. Dexamethasone (2 µg/mL) was used as a reference anti-inflammatory drug. RAW 264.7 cell viability after LPS, URB1537, and dexamethasone administration were assessed by the MTT assay as described above.
3.9. Statistical Analysis
Comparisons between multiple means were performed via ANOVA followed by Tukey’s post hoc test. Significance was set at p < 0.05. GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA, USA) was used for statistical analysis.
4. Conclusions
An effective variation of a Mitsunobu-type methodology reported in the literature has allowed obtaining a small library of 6-O-sucrose-based surfactants. The ameliorated procedure proved to be versatile and to have a large scope, starting from non-protected and commercially available substrates. The compounds were then screened for antimicrobic and antimycotic activities. Interestingly, some of the herein-reported sucrose monoesters showed interesting antifungal activities. In particular, sucrose palmitoleate (3d, URB1537) shows promising antifungal activity against C. albicans, Fusarium spp. and A. fumigatus with MIC values of 16, 32, and 64 μg/mL, respectively. For this reason, URB1537 was selected for further biological characterizations. The new surfactant is shown to be safe for human keratinocytes and murine macrophages up to 128 µg/mL. Although URB1537 did not show any antioxidant properties within the range of tested concentrations (up to 512 µg/mL), 6-O-sucrose palmitoleate presents an appreciable anti-inflammatory activity in LPS-activated macrophages, suggesting that the compound might help to control the inflammatory response. Thus, URB1537 could have therapeutic potential and could also be used in designing and developing innovative drugs such as those based on liposomes.
Author Contributions
Conceptualization, S.L. and A.D.; methodology, M.V., M.S., R.C., S.B., F.P. and L.P.; formal analysis, M.V.; investigation, M.V., M.S., R.C., S.B., F.P. and L.P.; resources, S.L. and A.D.; data curation, M.V., R.C., F.P. and L.P.; writing—original draft preparation, R.C., L.P., S.L. and A.D.; writing—review and editing, M.V., M.S., R.C., S.B., F.P., L.P., S.L. and A.D.; supervision, S.L. and A.D.; funding acquisition, S.L. and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Urbino Carlo Bo.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank Michele Menotta and Federica Biancucci for HRMS technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Almeida, F.; Rodrigues, M.L.; Coelho, C. The still underestimated problem of fungal diseases worldwide. Front. Microbiol. 2019, 10, 214. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, L.; Wang, S. Recent progress in the discovery of antifungal agents targeting the cell wall. J. Med. Chem. 2020, 63, 12429–12459. [Google Scholar] [CrossRef] [PubMed]
- Heard, S.C.; Wu, G.; Winter, J.M. Antifungal natural products. Curr. Opin. Biotechnol. 2021, 69, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-K.; Dan, W.-J.; Wan, J.-B. Natural and synthetic β-carboline as a privileged antifungal scaffolds. J. Med. Chem. 2022, 229, 114057. [Google Scholar] [CrossRef]
- Van Daele, R.; Spriet, I.; Wauters, J.; Maertens, J.; Mercier, T.; Van Hecke, S.; Brüggemann, R. Antifungal drugs: What brings the future? Med. Mycol. 2019, 57, S328–S343. [Google Scholar] [CrossRef]
- Bouz, G.; Doležal, M. Advances in antifungal drug development: An up-to-date mini review. Pharmaceuticals 2021, 14, 1312. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Teng, Y.; Stewart, S.G.; Hai, Y.W.; Li, X.; Banwell, M.G.; Lan, P. Sucrose fatty acid esters: Synthesis, emulsifying capacities, biological activities and structure-property profiles. Crit. Rev. Food Sci. Nutr. 2021, 61, 3297–3317. [Google Scholar] [CrossRef]
- Farran, A.; Cai, C.; Sandoval, M.; Xu, Y.; Liu, J.; Hernaiz, M.J.; Linhardt, R.J. Green solvents in carbohydrate chemistry: From raw materials to fine chemicals. Chem. Rev. 2015, 115, 6811–6853. [Google Scholar] [CrossRef]
- Polat, T.; Linhardt, R.J. Syntheses and applications of sucrose-based esters. J. Surf. Deterg. 2001, 4, 415–421. [Google Scholar] [CrossRef]
- Garti, N.; Clement, V.; Fanun, M.; Leser, M.E. Some characteristics of sugar ester nonionic microemulsions in view of possible food applications. J. Agric. Food Chem. 2000, 48, 3945–3956. [Google Scholar] [CrossRef] [PubMed]
- Megahed, M.G. Preparation of sucrose fatty acid esters as food emulsifiers and evaluation of their surface active and emulsification properties. Grasas Aceites 1999, 50, 280–282. [Google Scholar] [CrossRef]
- Devulapalle, K.S.; Gomez de Segura, A.; Ferrer, M.; Alcalde, M.; Mooser, G.; Plou, F. Effect of carbohydrate fatty acid esters on Streptococcus sobrinus and glucosyltransferase activity. Carbohydr. Res. 2004, 339, 1029–1034. [Google Scholar] [CrossRef]
- Chortyk, O.T.; Pomonis, J.G.; Johnson, A.W. Syntheses and characterizations of insecticidal sucrose esters. J. Agric. Food Chem. 1996, 44, 1551–1557. [Google Scholar] [CrossRef]
- Markets and Markets. Sucrose Esters Market Worth $106 Million by 2025. Available online: https://www.marketsandmarkets.com/PressReleases/sucrose-esters.asp (accessed on 23 November 2022).
- Verboni, M.; Lucarini, S.; Duranti, A. 6’-O-Lactose ester surfactants as an innovative opportunity in the pharmaceutical field: From synthetic methods to biological applications. Pharmaceuticals 2021, 14, 1306. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, S.; Fagioli, L.; Campana, R.; Cole, H.; Duranti, A.; Baffone, W.; Vllasaliu, D.; Casettari, L. Unsaturated fatty acids lactose esters: Cytotoxicity, permeability enhancement and antimicrobial activity. Eur. J. Pharm. Biopharm. 2016, 107, 88–96. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Lucarini, S.; Fagioli, L.; Campana, R.; Vllasaliu, D.; Duranti, A.; Casettari, L. Lactose oleate as new biocompatible surfactant for pharmaceutical applications. Eur. J. Pharm. Biopharm. 2018, 124, 55–62. [Google Scholar] [CrossRef]
- Lucarini, S.; Fagioli, L.; Cavanagh, R.; Liang, W.; Perinelli, D.; Campana, M.; Stolnik, S.; Lam, J.; Casettari, L.; Duranti, A. Synthesis, structure–activity relationships and in vitro toxicity profile of lactose-based fatty acid monoesters as possible drug permeability enhancers. Pharmaceutics 2018, 10, 81. [Google Scholar] [CrossRef]
- Campana, R.; Merli, A.; Verboni, M.; Biondo, F.; Favi, G.; Duranti, A.; Lucarini, S. Synthesis and evaluation of saccharide-based aliphatic and aromatic esters as antimicrobial and antibiofilm agents. Pharmaceuticals 2019, 12, 186. [Google Scholar] [CrossRef]
- Lucarini, S.; Ciulla, M.G.; Mestichelli, P.; Duranti, A. Total Synthesis of Natural Disaccharide Sambubiose. Pharmaceuticals 2020, 13, 198. [Google Scholar] [CrossRef]
- McCartney, F.; Perinelli, D.R.; Tiboni, M.; Cavanagh, R.; Lucarini, S.; Palmieri, G.P.; Casettari, L.; Brayden, D.J. Permeability-enhancing effects of three laurate-disaccharide monoesters across isolated rat intestinal mucosae. Int. J. Pharm. 2021, 601, 120593. [Google Scholar] [CrossRef]
- Tiboni, M.; Elmowafy, E.; El-Derany, M.O.; Benedetti, S.; Campana, R.; Verboni, M.; Potenza, L.; Palma, F.; Citterio, B.; Sisti, M.; et al. A combination of sugar esters and chitosan to promote in vivo wound care. Int. J. Pharm. 2022, 616, 121508. [Google Scholar] [CrossRef]
- Verboni, M.; Benedetti, S.; Campana, R.; Palma, F.; Potenza, L.; Sisti, M.; Duranti, A.; Lucarini, S. Synthesis and Biological Characterization of the New Glycolipid Lactose Undecylenate (URB1418). Pharmaceuticals 2022, 15, 456. [Google Scholar] [CrossRef] [PubMed]
- Molinier, V.; Fitremann, J.; Bouchu, A.; Queneau, Y. Sucrose esterification under Mitsunobu conditions: Evidence for the formation of 6-O-acyl-3’,6’-anhydrosucrose besides mono and diesters of fatty acids. Tetrahedron Asymmetry 2004, 15, 1753–1762. [Google Scholar] [CrossRef]
- Vlahov, I.R.; Vlahova, P.I.; Linhardt, R.J. Regioselective synthesis of sucrose monoesters as surfactants. J. Carb. Chem. 1997, 16, 1–10. [Google Scholar] [CrossRef]
- Thévenet, S.; Wernicke, A.; Belniak, S.; Descotes, G.; Bouchu, A.; Queneau, Y. Esterification of unprotected sucrose with acid chlorides in aqueous medium: Kinetic reactivity versus acyl- or alkyloxycarbonyl-group migrations. Carb. Res. 1999, 318, 52–66. [Google Scholar] [CrossRef]
- Cruces, M.A.; Plou, F.J.; Ferrer, M.; Bernabé, M.; Ballesteros, A. Improved synthesis of sucrose fatty acid monoesters. J. Am. Oil Chem. Soc. 2001, 78, 541–546. [Google Scholar] [CrossRef]
- Jarosz, S.; Mach, M. Regio- and stereoselective transformations of sucrose at the terminal positions. Eur. J. Org. Chem. 2002, 2002, 769–780. [Google Scholar] [CrossRef]
- Ferrer, M.; Cruces, M.A.; Bernabé, M.; Ballesteros, A.; Plou, F.J. Lipase-catalyzed regioselective acylation of sucrose in two solvent mixtures. Biotechnol. Bioeng. 1999, 65, 10–16. [Google Scholar] [CrossRef]
- Shi, Y.-G.; Li, J.R.; Chu, Y.-H. Enzyme-catalyzed regioselective synthesis of sucrose-based esters. J Chem. Tec. Biotechnol. 2011, 86, 1457–1468. [Google Scholar] [CrossRef]
- Possiel, C.; Bäuerle, A.; Seibel, J.J. A chemoenzymatic route to a class of sucrose esters. Eur. J. Org. Chem. 2017, 2017, 6335–6337. [Google Scholar] [CrossRef]
- Griffin, W.C. Calculation of HLB values of non-ionic surfactants. J. Soc. Cosmet. Chem. 1954, 5, 249–256. [Google Scholar]
- Organic Chemistry Portal. Available online: https://www.organic-chemistry.org/prog/ (accessed on 20 December 2022).
- Zhao, L.; Zhang, H.; Hao, T.; Li, S. In vitro antibacterial activities and mechanism of sugar fatty acid esters against five food-related bacteria. Food Chem. 2015, 187, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Karlová, T.; Poláková, L.; Šmidrkal, J.; Filip, V. Antimicrobial effects of fatty acid fructose esters. Czech J. Food Sci. 2010, 28, 146–149. [Google Scholar] [CrossRef]
- Petkova, N.; Vassilev, D.; Arabadzhieva, R.; Tumbarski, Y.; Vasileva, I.; Koleva, M.; Denev, P. “Green” synthesis of sucrose octaacetate and characterization of its physicochemical properties and antimicrobial activity. Chem. Biochem. Eng. Q. 2017, 31, 395–402. [Google Scholar] [CrossRef]
- Merghni, A.; Dallel, I.; Noumi, E.; Kadmi, Y.; Hentati, H.; Tobji, S.; Ben Amor, A.; Mastouri, M. Antioxidant and antiproliferative potential of biosurfactants isolated from Lactobacillus casei and their anti-biofilm effect in oral Staphylococcus aureus strains. Microb. Pathog. 2017, 104, 84–89. [Google Scholar] [CrossRef]
- Giri, S.S.; Ryu, E.C.; Sukumaran, V.; Park, S.C. Antioxidant, antibacterial, and anti-adhesive activities of biosurfactants isolated from Bacillus strains. Microb. Pathog. 2019, 132, 66–72. [Google Scholar] [CrossRef]
- Geran, R.I.; Greenberg, N.H.; Macdonald, M.M.; Schumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological systems (Third Edition). Cancer Chemother. Rep. 1972, 3, 59–61. [Google Scholar]
- Li, W.; Sun, Y.N.; Yan, X.T.; Yang, S.Y.; Song, S.B.; Lee, Y.M.; Kim, Y.H. NF-κB Inhibitory activity of sucrose fatty acid esters and related constituents from Astragalus membranaceus. J. Agric. Food Chem. 2013, 61, 7081–7088. [Google Scholar] [CrossRef]
- Souza, C.O.; Teixeira, A.A.S.; Biondo, L.A.; Silveira, L.S.; Calder, F.P.; Neto, J.C.R. Palmitoleic acid reduces the inflammation in LPS-stimulated macrophages by inhibition of NFκB, independently of PPARs. Clin. Exp. Pharmacol. Physiol. 2017, 44, 566–575. [Google Scholar] [CrossRef]
- Abran, D.; Boucher, F.; Hamanaka, T.; Hiraki, K.; Kito, Y.; Koyama, K.; Leblanc, R.M.; Machida, H.; Munger, G.; Seidou, M.; et al. On some physicochemical properties of sucrose esters and the stability they confer to membrane proteins. J. Colloid Interface Sci. 1989, 128, 230–236. [Google Scholar] [CrossRef]
- Youan, B.-B.C.; Hussain, A.; Nguyen, N.T. Evaluation of sucrose esters as alternative surfactants in microencapsulation of proteins by the solvent evaporation method. AAPS PharmSci. 2003, 5, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, Y.; Tu, P.-F. Tricornoses A−L, oligosaccharide multi-esters from the roots of Polygala tricornis. J. Nat. Prod. 2005, 68, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Furuya, T.; Ushiyama, M.; Asada, Y.; Yoshikawa, T.; Orihara, Y. Biotransformation of phenylacetic acid and 2-phenyl-propionic acid in suspension culture of offea arabica. Phytochemistry 1988, 27, 803–807. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).