Effective Generation of Functional Pancreatic β Cells from Human-Derived Dental Stem Cells of Apical Papilla and Bone-Marrow-Derived Stem Cells: A Comparative Study
Abstract
1. Introduction
2. Results
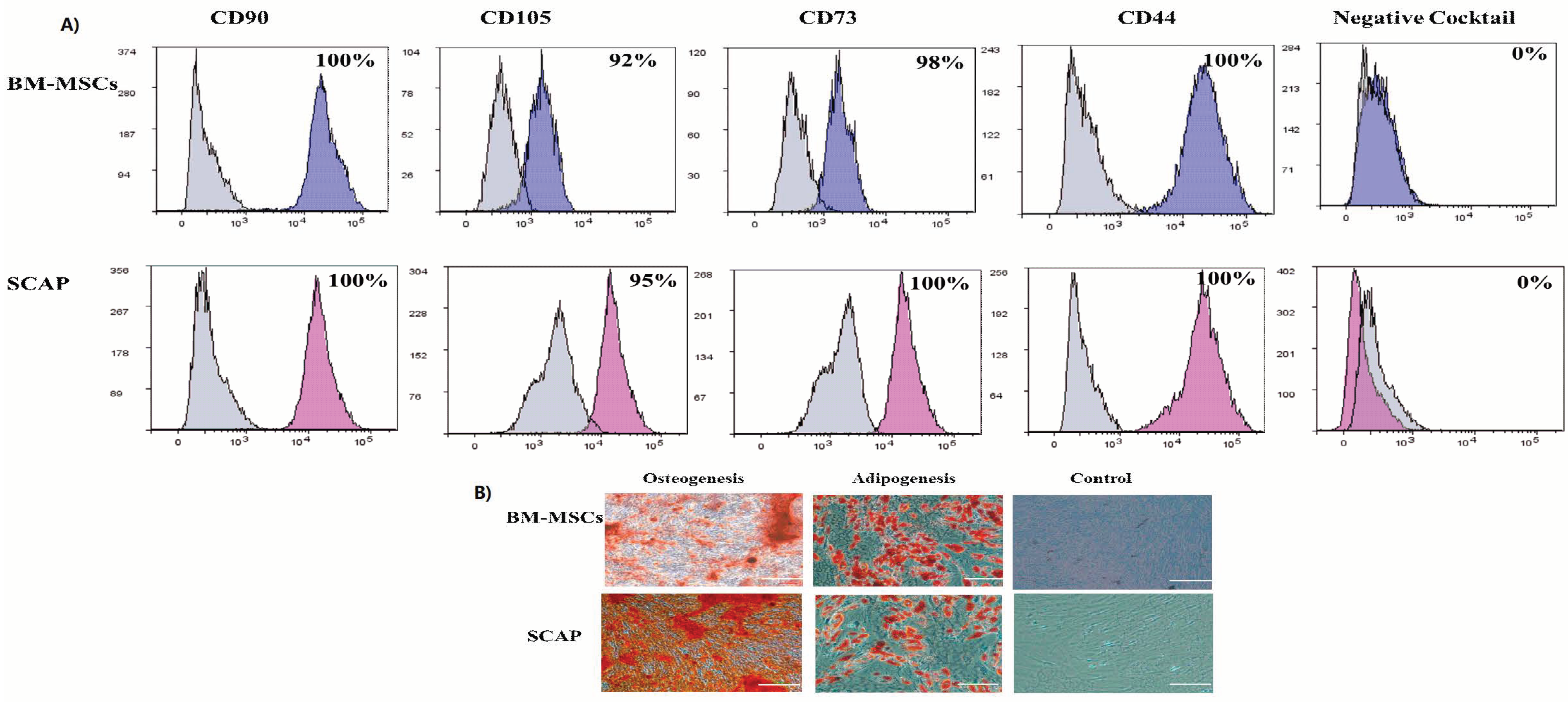
2.1. Characterization of Human MSCs
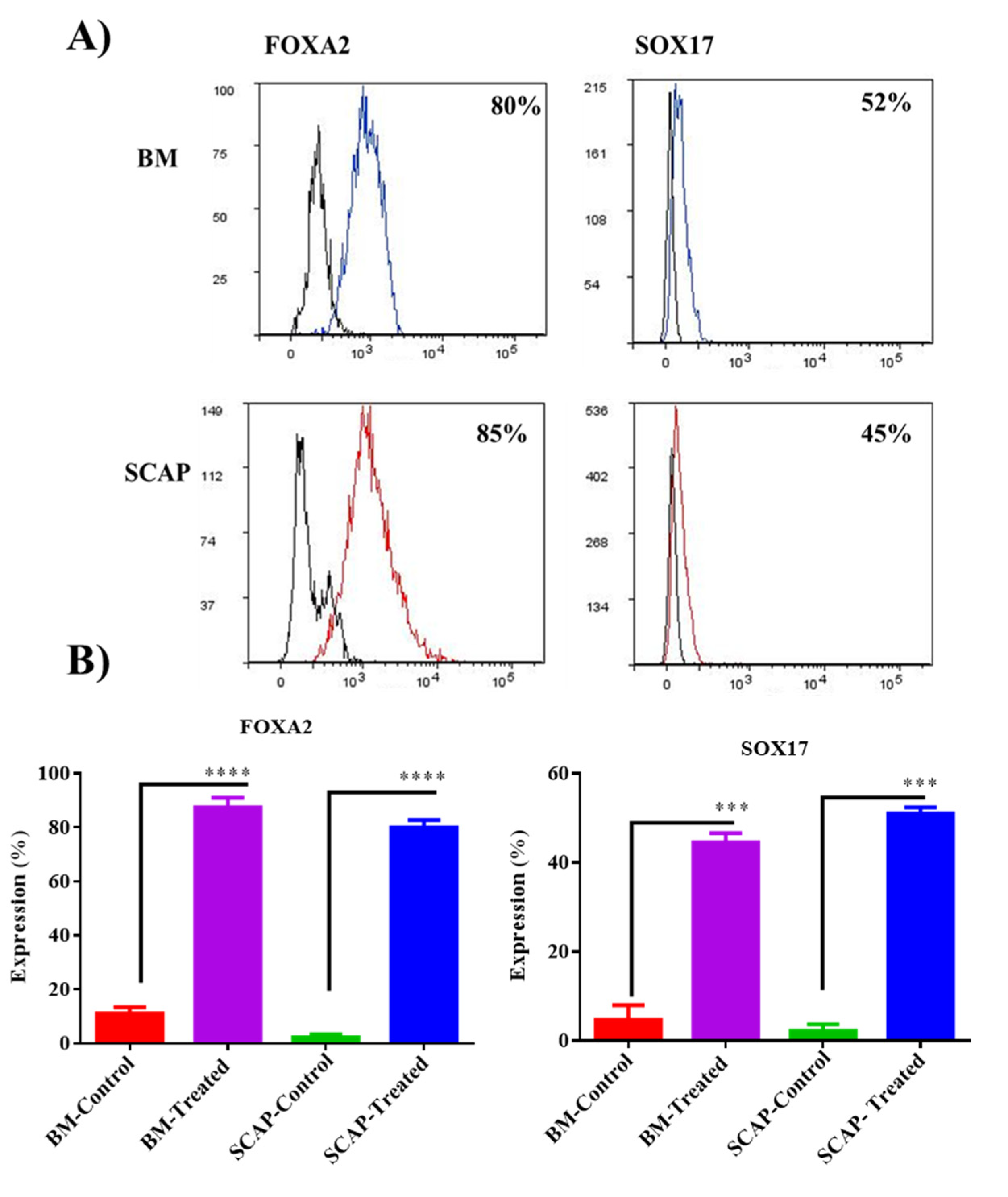
2.2. Validation of Definitive Endoderm Induction by Flow Cytometry
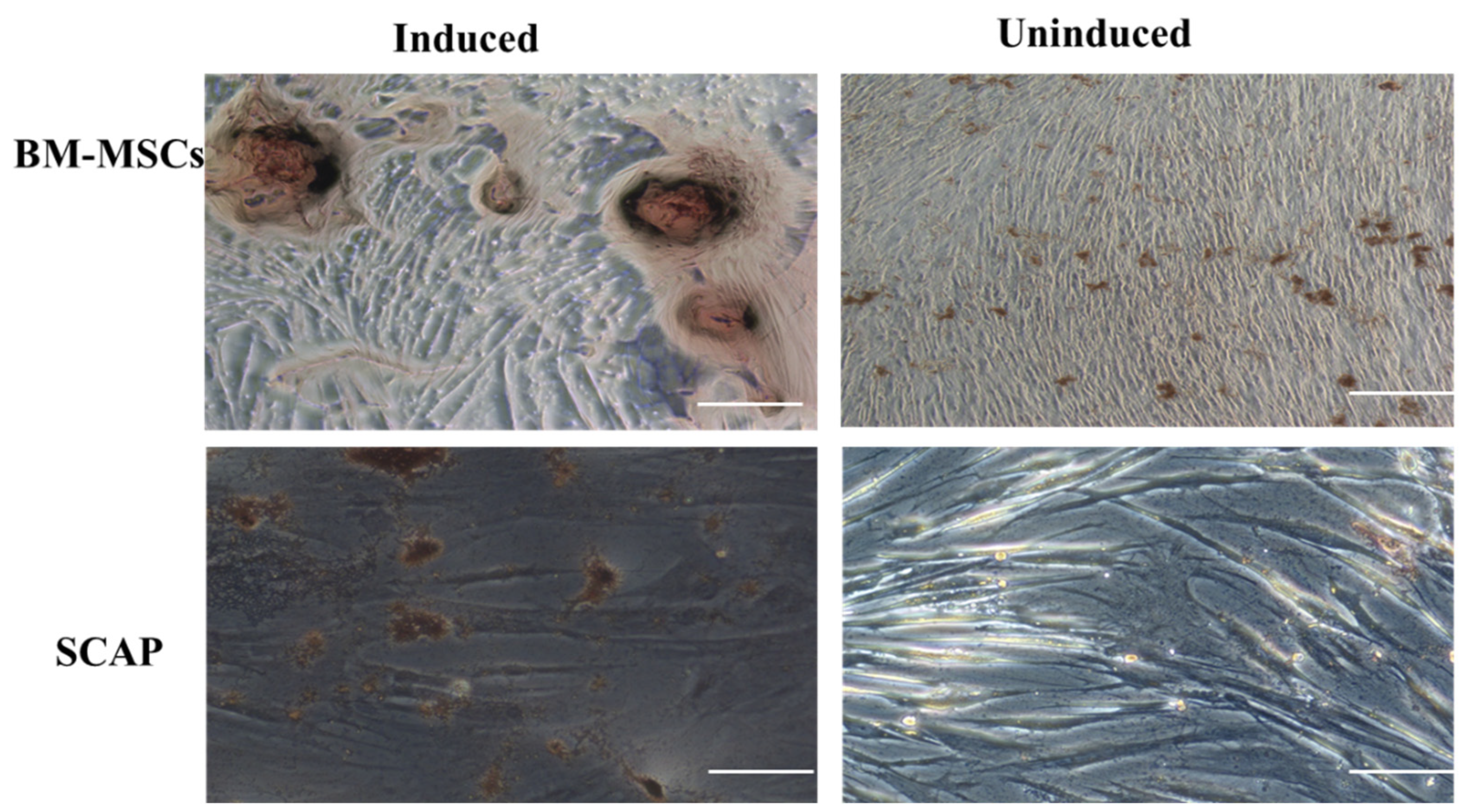
2.3. Diphenylthiocarbazone Staining (DTZ)
2.4. Secretion of Insulin and C-Peptide by Differentiated Cells by ELISA
2.5. Validation of Islet-like Clusters Maturation Markers; Insulin, C-peptide, Glucagon and PDX 1 by ICC
3. Discussion
4. Materials and Methods
4.1. Isolation of Mesenchymal Stem Cells (MSCs)
Subjects and Samples
4.2. Isolation and Expansion of Dental Stem Cells
4.3. Isolation and Expansion of Bone Marrow Stem Cells
4.4. Characterization of MSCs by Flow Cytometry
4.5. Multilineage Differentiation of MSCs
4.6. Assessment of Differentiation
4.6.1. Osteogenic Differentiation-Alizarin Red Stain
4.6.2. Adipogenic Differentiation and Oil Red O Stain
4.6.3. Endoderm Differentiation (β Cells)
4.7. Validation of Endodermal Differentiation
Definitive Endoderm Markers: FoxA2 and SOX17
4.8. Validation of the Formation and Maturation of Islet-like Clusters
Diphenylthiocarbazone Staining (DTZ)
4.9. Immunofluorescence Staining
4.9.1. Detection of Maturation Markers: Insulin, C-Peptide, Glucagon and PDX-1
4.9.2. Detection of Insulin and C-Peptide by ELISA
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARS | Alizarin Red Stain |
| BM-MSCs | Bone-marrow-derived stem cells |
| BSA | Bovine serum albumin |
| DE | Definitive Endoderm |
| DM | Diabetes Mellitus |
| DTZ | Diphenythiocarbazone. |
| FBS | Fetal Bovine Serum |
| GLP-1 | glucagon-like peptide-1 |
| ICA | Islet-like cell aggregates (ICAs) |
| IPC | insulin-producing beta cells |
| IRB | Institutional review board |
| ISCT | International Society for Cellular Therapy |
| ITS | Insulin Transferrin selenium |
| MNC | Mononuclear cells |
| MSC | Mesenchymal stem cells |
| PBS | Phosphate buffered saline |
| SCAP | Stem cells of apical papilla |
| T1DM | Type 1 Diabetes Mellitus |
References
- Ackermann, A.M.; Gannon, M. Molecular regulation of pancreatic β-cell mass development, maintenance, and expansion. J. Mol. Endocrinol. 2007, 38, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cohrs, C.M.; Stertmann, J.; Bozsak, R.; Speier, S. Human beta cell mass and function in diabetes: Recent advances in knowledge and technologies to understand disease pathogenesis. Mol. Metab. 2017, 6, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33 (Suppl. 1), S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.J.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Hering, B.J.; Clarke, W.R.; Bridges, N.D.; Eggerman, T.L.; Alejandro, R.; Bellin, M.D.; Chaloner, K.; Czarniecki, C.W.; Goldstein, J.S.; Hunsicker, L.G. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 2016, 39, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Hao, H.; Liu, J.; Li, Y.; Han, W.; Mu, Y. Mesenchymal stem cell therapy in type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2017, 9, 36. [Google Scholar] [CrossRef]
- Wan, X.-X.; Zhang, D.-Y.; Khan, M.A.; Zheng, S.-Y.; Hu, X.-M.; Zhang, Q.; Yang, R.-H.; Xiong, K. Stem Cell Transplantation in the Treatment of Type 1 Diabetes Mellitus: From Insulin Replacement to Beta-Cell Replacement. Front. Endocrinol. 2022, 13, 859638. [Google Scholar] [CrossRef]
- Yang, L.; Li, S.; Hatch, H.; Ahrens, K.; Cornelius, J.G.; Petersen, B.E.; Peck, A.B. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc. Natl. Acad. Sci. USA 2002, 99, 8078–8083. [Google Scholar] [CrossRef]
- Koblas, T.; Zacharovová, K.; Berková, Z.; Leontovic, I.; Dovolilová, E.; Zámecník, L.; Saudek, F. In vivo differentiation of human umbilical cord blood-derived cells into insulin-producing beta cells. Folia Biol. 2009, 55, 224–232. [Google Scholar]
- Chen, L.-B.; Jiang, X.-B.; Yang, L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J. Gastroenterol. WJG 2004, 10, 3016. [Google Scholar] [CrossRef]
- Li, J.; Zhu, L.; Qu, X.; Li, J.; Lin, R.; Liao, L.; Wang, J.; Wang, S.; Xu, Q.; Zhao, R.C. Stepwise differentiation of human adipose-derived mesenchymal stem cells toward definitive endoderm and pancreatic progenitor cells by mimicking pancreatic development in vivo. Stem Cells Dev. 2013, 22, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Fan, D.; Zhao, Y.; Li, J.; Wang, Z.; Wang, J.; Wang, X.; Guan, Z.; Niu, B. Three-dimensional culture promotes the differentiation of human dental pulp mesenchymal stem cells into insulin-producing cells for improving the diabetes therapy. Front. Pharmacol. 2020, 10, 1576. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J., Jr.; Lopes, H. Mechanisms of antimicrobial activity of calcium hydroxide: A critical review. Int. Endod. J. 1999, 32, 361–369. [Google Scholar] [CrossRef]
- Sun, Z.; Gou, W.; Kim, D.-S.; Dong, X.; Strange, C.; Tan, Y.; Adams, D.B.; Wang, H. Adipose stem cell therapy mitigates chronic pancreatitis via differentiation into acinar-like cells in mice. Mol. Ther. 2017, 25, 2490–2501. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, K.; Ohnishi, S.; Kuwatani, M.; Sakamoto, N. Mesenchymal stem cell therapy for acute and chronic pancreatitis. J. Gastroenterol. 2018, 53, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pavathuparambil Abdul Manaph, N.; Sivanathan, K.N.; Nitschke, J.; Zhou, X.-F.; Coates, P.T.; Drogemuller, C.J. An overview on small molecule-induced differentiation of mesenchymal stem cells into beta cells for diabetic therapy. Stem Cell Res. Ther. 2019, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Sağraç, D.; Şahin, F. Differentiation Potential of Mesenchymal Stem Cells into Pancreatic β-Cells. Cell Biol. Transl. Med. 2019, 1247, 135–156. [Google Scholar]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells-current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- Elahi, K.C.; Klein, G.; Avci-Adali, M.; Sievert, K.D.; MacNeil, S.; Aicher, W.K. Human mesenchymal stromal cells from different sources diverge in their expression of cell surface proteins and display distinct differentiation patterns. Stem Cells Int. 2016, 2016, 5646384. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Gabr, M.M.; Zakaria, M.M.; Refaie, A.F.; Abdel-Rahman, E.A.; Reda, A.M.; Ali, S.S.; Khater, S.M.; Ashamallah, S.A.; Ismail, A.M.; Ismail, H.E.-D.A. From human mesenchymal stem cells to insulin-producing cells: Comparison between bone marrow-and adipose tissue-derived cells. BioMed Res. Int. 2017, 2017, 3854232. [Google Scholar] [CrossRef]
- Murry, C.E.; Keller, G. Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell 2008, 132, 661–680. [Google Scholar] [CrossRef] [PubMed]
- Halpern, K.B.; Vana, T.; Walker, M.D. Paradoxical role of DNA methylation in activation of FoxA2 gene expression during endoderm development. J. Biol. Chem. 2014, 289, 23882–23892. [Google Scholar] [CrossRef]
- Shimoda, M.; Kanai-Azuma, M.; Hara, K.; Miyazaki, S.; Kanai, Y.; Monden, M.; Miyazaki, J.-I. Sox17 plays a substantial role in late-stage differentiation of the extraembryonic endoderm in vitro. J. Cell Sci. 2007, 120, 3859–3869. [Google Scholar] [CrossRef]
- Shiroi, A.; Yoshikawa, M.; Yokota, H.; Fukui, H.; Ishizaka, S.; Tatsumi, K.; Takahashi, Y. Identification of insulin-producing cells derived from embryonic stem cells by zinc-chelating dithizone. Stem Cells 2002, 20, 284–292. [Google Scholar] [CrossRef]
- Holland, A.M.; Hale, M.A.; Kagami, H.; Hammer, R.E.; MacDonald, R.J. Experimental control of pancreatic development and maintenance. Proc. Natl. Acad. Sci. USA 2002, 99, 12236–12241. [Google Scholar] [CrossRef]
- Johnson, J.D.; Ahmed, N.T.; Luciani, D.S.; Han, Z.; Tran, H.; Fujita, J.; Misler, S.; Edlund, H.; Polonsky, K.S. Increased islet apoptosis in Pdx1+/–mice. J. Clin. Investig. 2003, 111, 1147–1160. [Google Scholar] [CrossRef]
- Alpert, S.; Hanahan, D.; Teitelman, G. Hybrid insulin genes reveal a developmental lineage for pancreatic endocrine cells and imply a relationship with neurons. Cell 1988, 53, 295–308. [Google Scholar] [CrossRef]
- De Krijger, R.; Aanstoot, H.; Kranenburg, G.; Reinhard, M.; Visser, W.; Bruining, G. The midgestational human fetal pancreas contains cells coexpressing islet hormones. Dev. Biol. 1992, 153, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Herrera, P.-L.; Huarte, J.; Zufferey, R.; Nichols, A.; Mermillod, B.; Philippe, J.; Muniesa, P.; Sanvito, F.; Orci, L.; Vassalli, J.-D. Ablation of islet endocrine cells by targeted expression of hormone-promoter-driven toxigenes. Proc. Natl. Acad. Sci. USA 1994, 91, 12999–13003. [Google Scholar] [CrossRef]
- D’Amour, K.A.; Bang, A.G.; Eliazer, S.; Kelly, O.G.; Agulnick, A.D.; Smart, N.G.; Moorman, M.A.; Kroon, E.; Carpenter, M.K.; Baetge, E.E. Production of pancreatic hormone–expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 2006, 24, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Au, M.; Lu, K.; Eshpeter, A.; Korbutt, G.; Fisk, G.; Majumdar, A.S. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells 2007, 25, 1940–1953. [Google Scholar] [CrossRef] [PubMed]
- Rezania, A.; Riedel, M.J.; Wideman, R.D.; Karanu, F.; Ao, Z.; Warnock, G.L.; Kieffer, T.J. Production of functional glucagon-secreting α-cells from human embryonic stem cells. Diabetes 2011, 60, 239–247. [Google Scholar] [CrossRef]
- Riedel, M.; Asadi, A.; Wang, R.; Ao, Z.; Warnock, G.; Kieffer, T. Immunohistochemical characterisation of cells co-producing insulin and glucagon in the developing human pancreas. Diabetologia 2012, 55, 372–381. [Google Scholar] [CrossRef]
- Gabr, M.M.; Zakaria, M.M.; Refaie, A.F.; Ismail, A.M.; Abou-El-Mahasen, M.A.; Ashamallah, S.A.; Khater, S.M.; El-Halawani, S.M.; Ibrahim, R.Y.; Uin, G.S. Insulin-producing cells from adult human bone marrow mesenchymal stem cells control streptozotocin-induced diabetes in nude mice. Cell Transplant. 2013, 22, 133–145. [Google Scholar] [CrossRef]
- Abuarqoub, D.; Awidi, A.; Abuharfeil, N. Comparison of osteo/odontogenic differentiation of human adult dental pulp stem cells and stem cells from apical papilla in the presence of platelet lysate. Arch. Oral Biol. 2015, 60, 1545–1553. [Google Scholar] [CrossRef]
- Gnecchi, M.; Melo, L.G. Bone marrow-derived mesenchymal stem cells: Isolation, expansion, characterization, viral transduction, and production of conditioned medium. In Stem Cells in Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2009; pp. 281–294. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuarqoub, D.; Adwan, S.; Zaza, R.; Wehaibi, S.; Aslam, N.; Jafar, H.; Qinnah, N.; Awidi, A. Effective Generation of Functional Pancreatic β Cells from Human-Derived Dental Stem Cells of Apical Papilla and Bone-Marrow-Derived Stem Cells: A Comparative Study. Pharmaceuticals 2023, 16, 649. https://doi.org/10.3390/ph16050649
Abuarqoub D, Adwan S, Zaza R, Wehaibi S, Aslam N, Jafar H, Qinnah N, Awidi A. Effective Generation of Functional Pancreatic β Cells from Human-Derived Dental Stem Cells of Apical Papilla and Bone-Marrow-Derived Stem Cells: A Comparative Study. Pharmaceuticals. 2023; 16(5):649. https://doi.org/10.3390/ph16050649
Chicago/Turabian StyleAbuarqoub, Duaa, Sofia Adwan, Rand Zaza, Suha Wehaibi, Nazneen Aslam, Hanan Jafar, Nidal Qinnah, and Abdalla Awidi. 2023. "Effective Generation of Functional Pancreatic β Cells from Human-Derived Dental Stem Cells of Apical Papilla and Bone-Marrow-Derived Stem Cells: A Comparative Study" Pharmaceuticals 16, no. 5: 649. https://doi.org/10.3390/ph16050649
APA StyleAbuarqoub, D., Adwan, S., Zaza, R., Wehaibi, S., Aslam, N., Jafar, H., Qinnah, N., & Awidi, A. (2023). Effective Generation of Functional Pancreatic β Cells from Human-Derived Dental Stem Cells of Apical Papilla and Bone-Marrow-Derived Stem Cells: A Comparative Study. Pharmaceuticals, 16(5), 649. https://doi.org/10.3390/ph16050649





