Fabrication and Characterization of Oxygen-Generating Polylactic Acid/Calcium Peroxide Composite Filaments for Bone Scaffolds
Abstract
1. Introduction
2. Results and Discussion
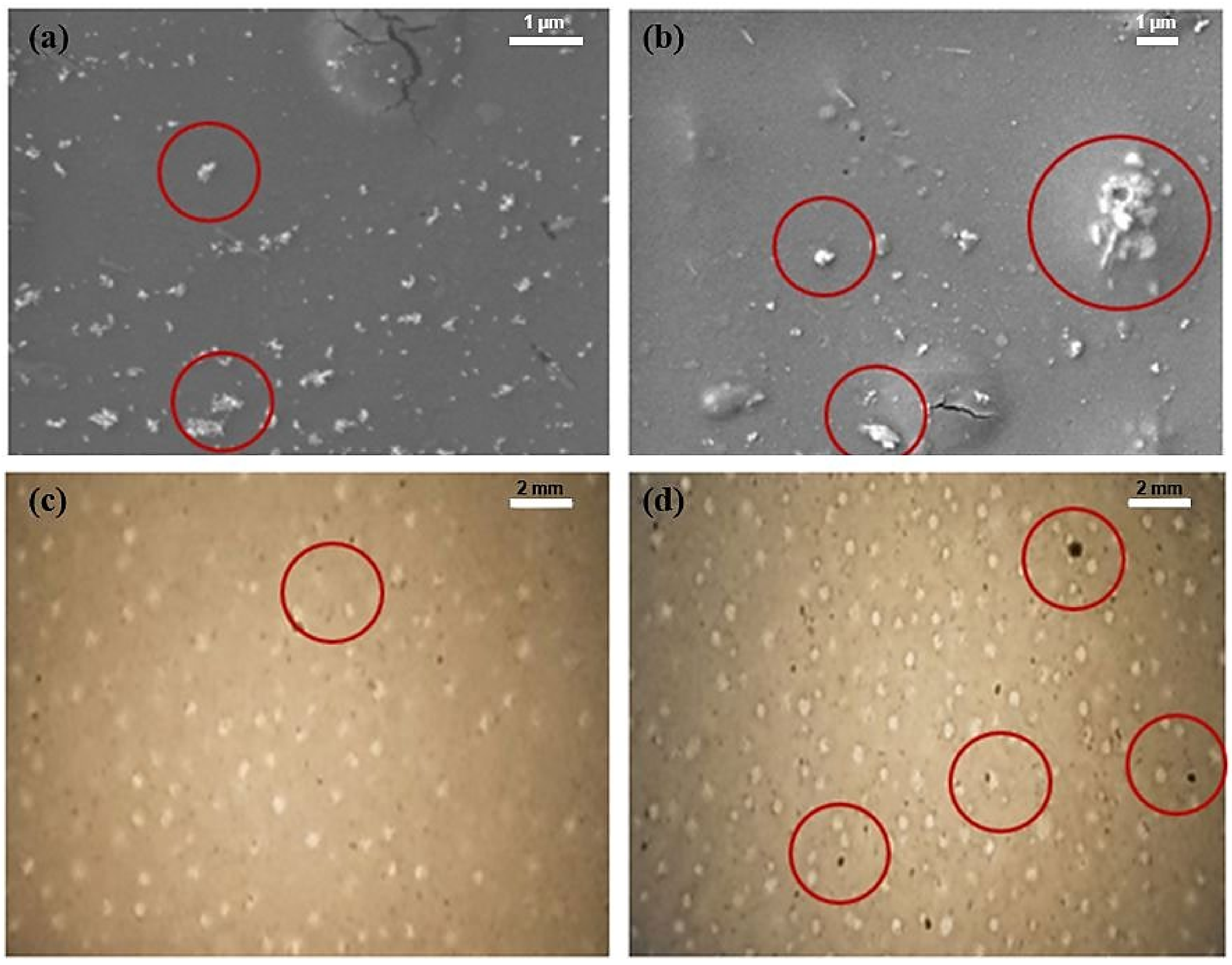
2.1. Presence of CPO and Printability of Filament
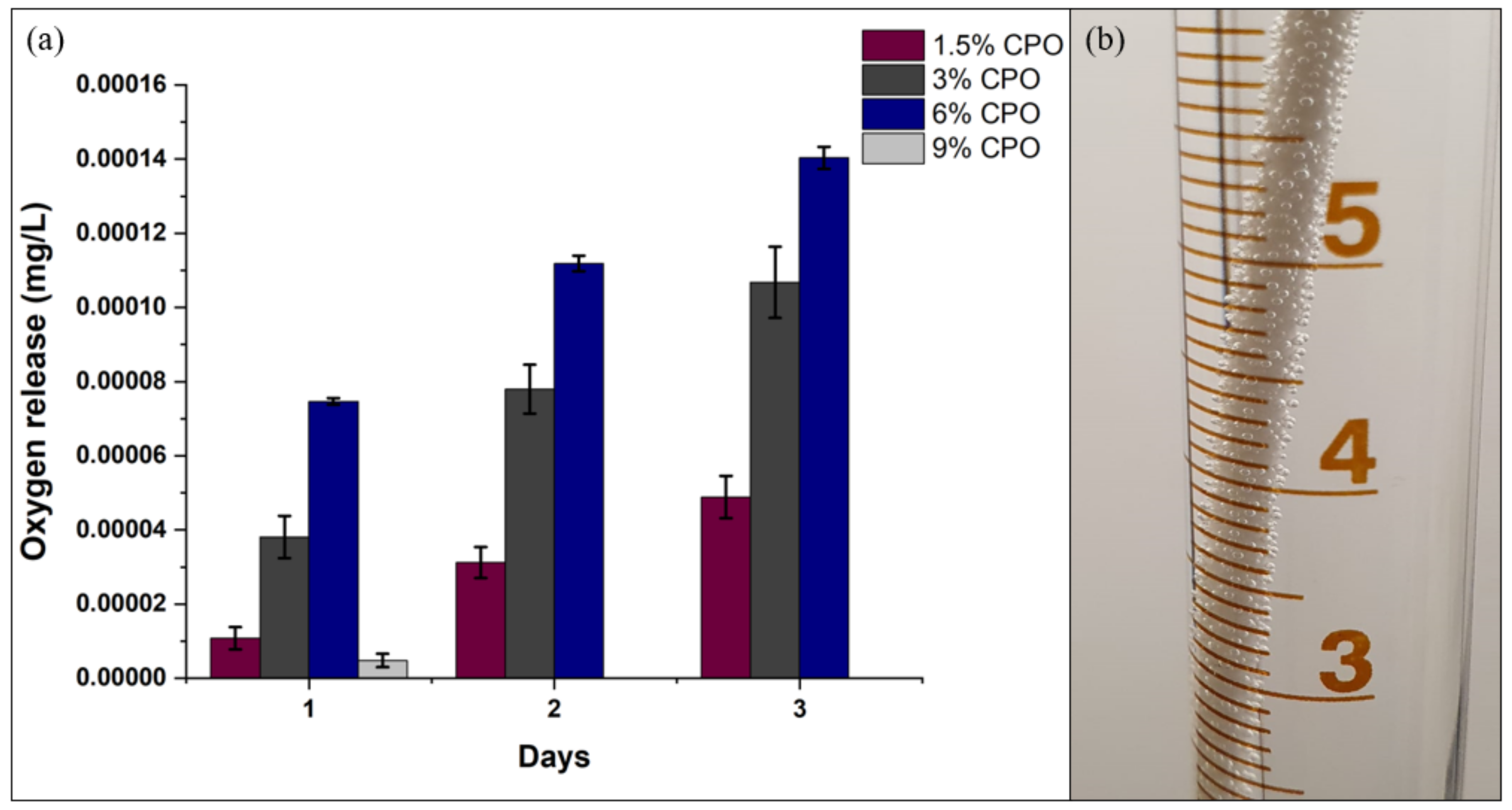
2.2. Filament Degradation, Oxygen and Calcium Ion Release
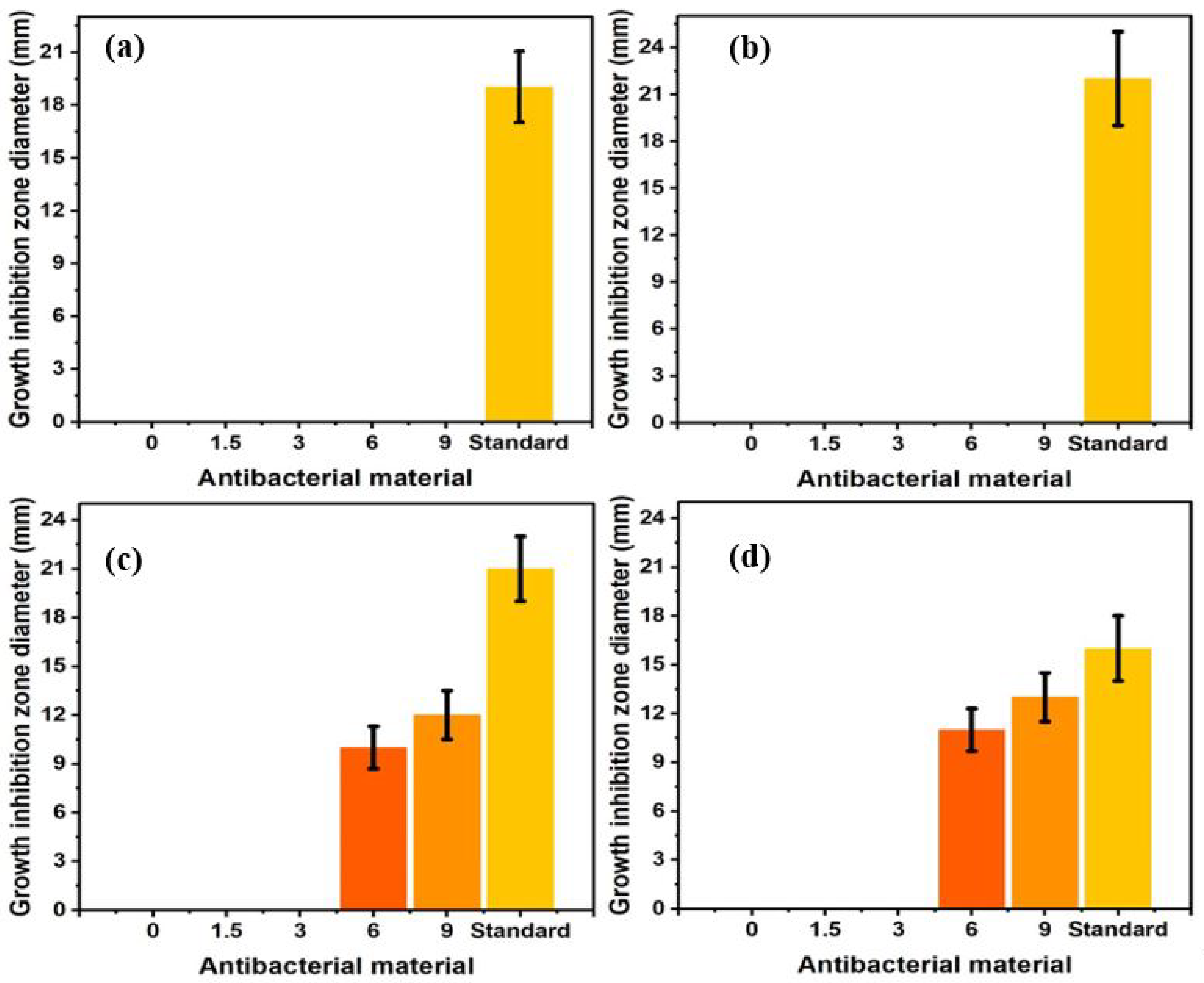
2.3. Antibacterial Activities
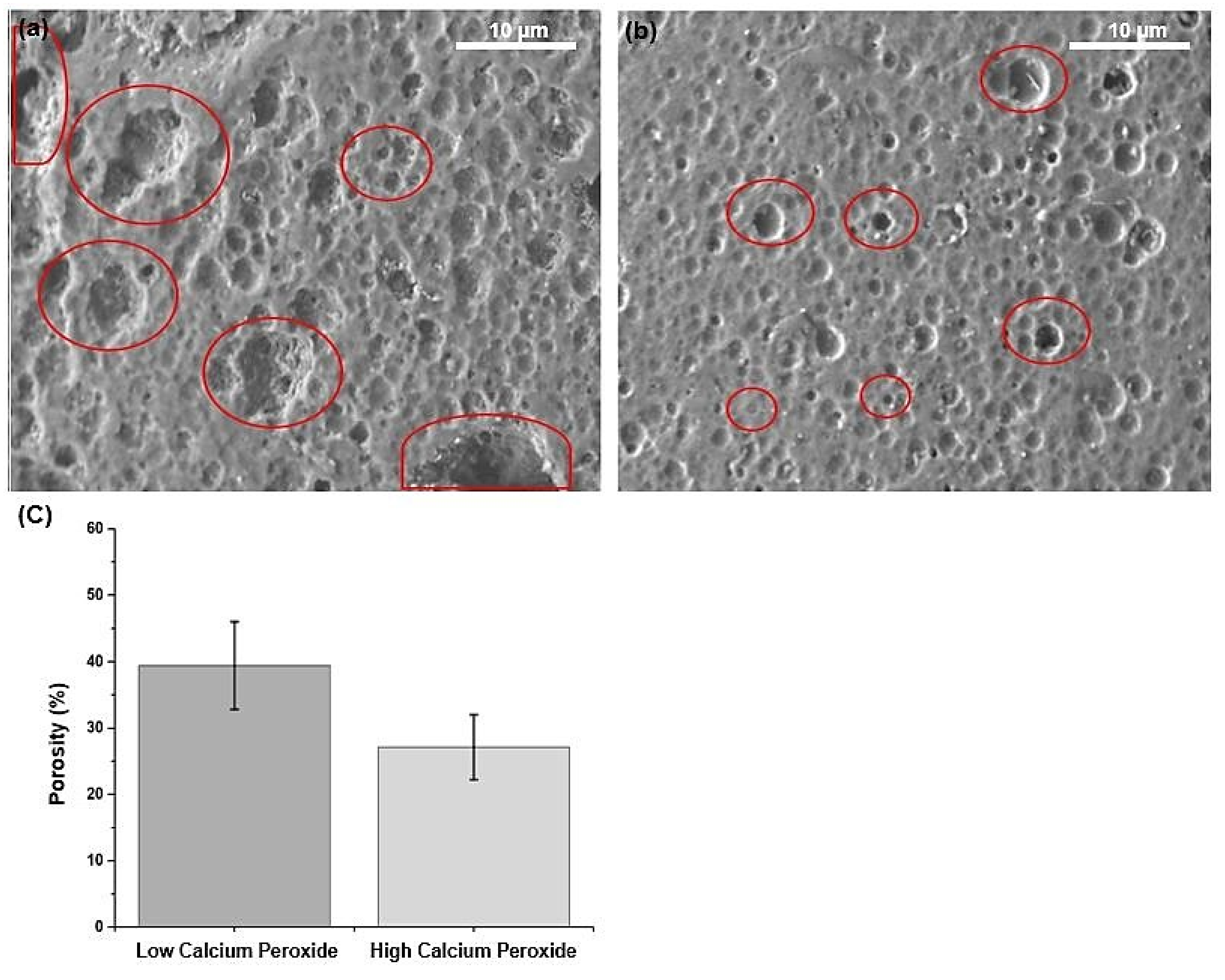
2.4. Porosity
3. Materials and Methods
3.1. Materials
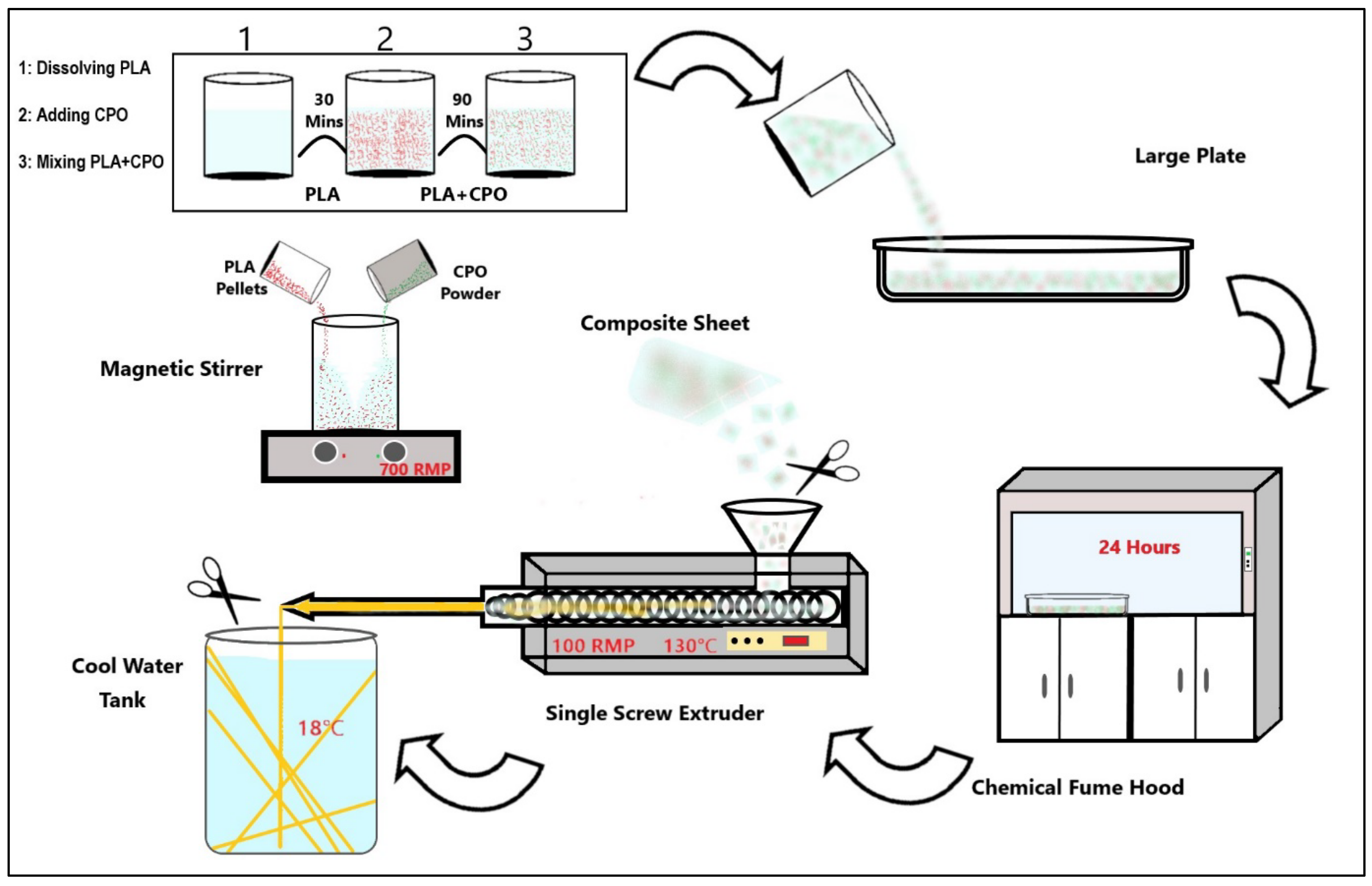
3.2. Preparation of PLA/CPO Filaments
3.3. 3D Printing of Bone Scaffolds
3.4. Characterisations
3.5. Degradation and Oxygen Release
3.6. Porosity Measurements
3.7. Antibacterial Activity
3.8. Statistics Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United States Department of Health and Human Services. Organ Donation Statistics. Available online: https://www.organdonor.gov/learn/organ-donation-statistics (accessed on 15 January 2023).
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/transplantsafety/overview/key-facts.html#:~:text=In%20the%20U.S%2C%20the%20most,1%20million%20grafts%20are%20transplanted (accessed on 27 December 2022).
- Sharifi, M.; Kheradmandi, R.; Salehi, M.; Alizadeh, M.; Ten Hagen, T.L.; Falahati, M. Criteria, Challenges, and Opportunities for Acellularized Allogeneic/Xenogeneic Bone Grafts in Bone Repairing. ACS Biomater. Sci. Eng. 2022, 8, 3199–3219. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, P.; Przekora, A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Barua, S.; Chattopadhyay, P.; Aidew, L.; Buragohain, A.K.; Karak, N. Infection-resistant hyperbranched epoxy nanocomposite as a scaffold for skin tissue regeneration. Polym. Int. 2014, 64, 303–311. [Google Scholar] [CrossRef]
- Suvarnapathaki, S.; Wu, X.; Lantigua, D.; Nguyen, M.; Camci-Unal, G. Breathing life into engineered tissues using oxygen-releasing biomaterials. NPG Asia Mater. 2019, 11, 65. [Google Scholar] [CrossRef]
- Xiao, Y.; Ahadian, S.; Radisic, M. Biochemical and Biophysical Cues in Matrix Design for Chronic and Diabetic Wound Treatment. Tissue Eng. Part B Rev. 2017, 23, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Ke, D.; Sahasrabudhe, H.; Bandyopadhyay, A. Additive manufacturing of biomaterials. Prog. Mater. Sci. 2018, 93, 45–111. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Baumann, M.; Coenen, C.; Frank, D.; Hennen, L.; Moniz, A.; Torgersen, H.; Torgersen, J.; van Bodegom, L.; van Duijne, F.; et al. Additive Bio-Manufacturing: 3D Printing for Medical Recovery and Human Enhancement; European Parliament: Strasburg, France, 2018. [Google Scholar]
- Felfel, R.M.; Poocza, L.; Gimeno-Fabra, M.; Milde, T.; Hildebrand, G.; Ahmed, I.; Scotchford, C.; Sottile, V.; Grant, D.M.; Liefeith, K. In vitro degradation and mechanical properties of PLA-PCL copolymer unit cell scaffolds generated by two-photon polymerization. Biomed. Mater. 2016, 11, 015011. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Raeisdasteh Hokmabad, V.; Davaran, S.; Ramazani, A.; Salehi, R. Design and fabrication of porous biodegradable scaffolds: A strategy for tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 1797–1825. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly (lactic acid)/poly (lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, Y.; Zhang, X. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater. Des. 2021, 201, 109490. [Google Scholar] [CrossRef]
- Radwan-Pragłowska, J.; Janus, Ł.; Piątkowski, M.; Bogdał, D.; Matysek, D. 3D hierarchical, nanostructured chi-tosan/PLA/HA scaffolds doped with TiO2/Au/Pt NPs with tunable properties for guided bone tissue engineering. Polymers 2020, 12, 792. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Zhao, S.; Harrison, B.S.; Mozafari, M.; Seifalian, A.M. Oxygen-Generating Biomaterials: A New, Viable Paradigm for Tissue Engineering? Trends Biotechnol. 2016, 34, 1010–1021. [Google Scholar] [CrossRef]
- Augustine, R.; Gezek, M.; Bostanci, N.S.; Nguyen, A.; Camci-Unal, G. Oxygen-generating scaffolds: One step closer to the clinical translation of tissue engineered products. Chem. Eng. J. 2023, 455, 140783. [Google Scholar] [CrossRef]
- Javid, N.; Honarmandrad, Z.; Malakootian, M. Ciprofloxacin removal from aqueous solutions by ozonation with calcium peroxide. Desalination Water Treat. 2020, 174, 178–185. [Google Scholar] [CrossRef]
- Mallepally, R.R.; Parrish, C.C.; Mc Hugh, M.A.; Ward, K.R. Hydrogen peroxide filled poly(methyl methacrylate) microcapsules: Potential oxygen delivery materials. Int. J. Pharm. 2014, 475, 130–137. [Google Scholar] [CrossRef]
- Abdullah, T.; Gauthaman, K.; Hammad, A.H.; Navare, K.J.; Alshahrie, A.A.; Bencherif, S.A.; Tamayol, A.; Memic, A. Oxygen-Releasing Antibacterial Nanofibrous Scaffolds for Tissue Engineering Applications. Polymers 2020, 12, 1233. [Google Scholar] [CrossRef]
- Steg, H.; Buizer, A.T.; Woudstra, W.; Veldhuizen, A.G.; Bulstra, S.K.; Grijpma, D.W.; Kuijer, R. Control of oxygen release from peroxides using polymers. J. Mater. Sci. Mater. Med. 2015, 26, 207. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, N.; Leijten, J.; Camci-Unal, G.; Hjortnaes, J.; Ribas, J.; Paul, A.; Mostafalu, P.; Gaharwar, A.K.; Qiu, Y.; Sonkusale, S.; et al. Oxygen-Generating Photo-Cross-Linkable Hydrogels Support Cardiac Progenitor Cell Survival by Reducing Hypoxia-Induced Necrosis. ACS Biomater. Sci. Eng. 2016, 3, 1964–1971. [Google Scholar] [CrossRef]
- Feng, P.; Jia, J.; Liu, M.; Peng, S.; Zhao, Z.; Shuai, C. Degradation mechanisms and acceleration strategies of poly (lactic acid) scaffold for bone regeneration. Mater. Des. 2021, 210, 110066. [Google Scholar] [CrossRef]
- Donate, R.; Monzón, M.; Alemán-Domínguez, M.E.; Ortega, Z. Enzymatic degradation study of PLA-based composite scaffolds. Rev. Adv. Mater. Sci. 2020, 59, 170–175. [Google Scholar] [CrossRef]
- Touri, M.; Moztarzadeh, F.; Abu Osman, N.A.; Dehghan, M.M.; Mozafari, M. 3D–printed biphasic calcium phosphate scaffolds coated with an oxygen generating system for enhancing engineered tissue survival. Mater. Sci. Eng. C 2018, 84, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Kiratiwongwan, T.; Shen, W. Oxygen-releasing polycaprolactone/calcium peroxide composite microspheres. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 108, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Seekell, R.P.; Lock, A.T.; Peng, Y.; Cole, A.R.; Perry, D.A.; Kheir, J.N.; Polizzotti, B.D. Oxygen delivery using engineered microparticles. Proc. Natl. Acad. Sci. USA 2016, 113, 12380–12385. [Google Scholar] [CrossRef]
- Ward, C.L.; Corona, B.T.; Yoo, J.J.; Harrison, B.S.; Christ, G.J. Oxygen Generating Biomaterials Preserve Skeletal Muscle Homeostasis under Hypoxic and Ischemic Conditions. PLoS ONE 2013, 8, e72485. [Google Scholar] [CrossRef]
- Cook, C.A.; Hahn, K.C.; Morrissette-McAlmon, J.B.; Grayson, W.L. Oxygen delivery from hyperbarically loaded microtanks extends cell viability in anoxic environments. Biomaterials 2015, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, L.; Hojjati-Emami, S.; Bonakdar, S.; Tahamtani, Y.; Hajizadeh-Saffar, E.; Noori-Keshtkar, M.; Najar-Asl, M.; Ashtiani, M.K.; Baharvand, H. Improvement of islet engrafts by enhanced angiogenesis and microparticle-mediated oxygenation. Biomaterials 2016, 89, 157–165. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Kim, H.-W.; Lee, J.H.; Oh, S.H. Controlling oxygen release from hollow microparticles for prolonged cell survival under hypoxic environment. Biomaterials 2015, 53, 583–591. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Med. Chem. 2014, 6, S14459. [Google Scholar] [CrossRef] [PubMed]
- Sladdin, M.; Lynch, J.M. Antimicrobial Properties of Calcium Peroxide in Relation to Its Potential Use as a Seed Dressing. Microbiology 1983, 129, 2307–2314. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Lopes, H.P. Mechanisms of antimicrobial activity of calcium hydroxide: A critical review. Int. Endod. J. 1999, 32, 361–369. [Google Scholar] [CrossRef] [PubMed]
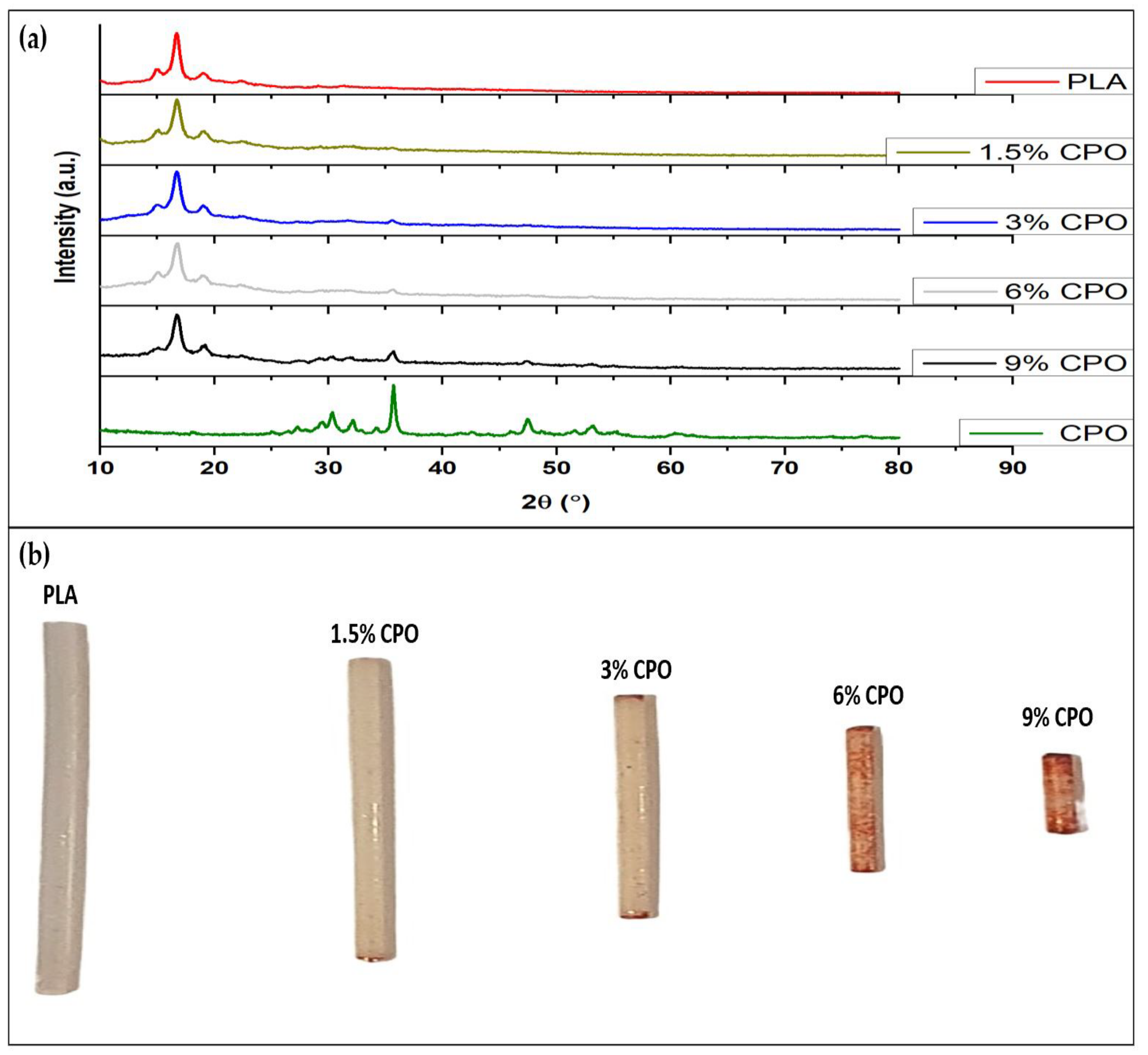
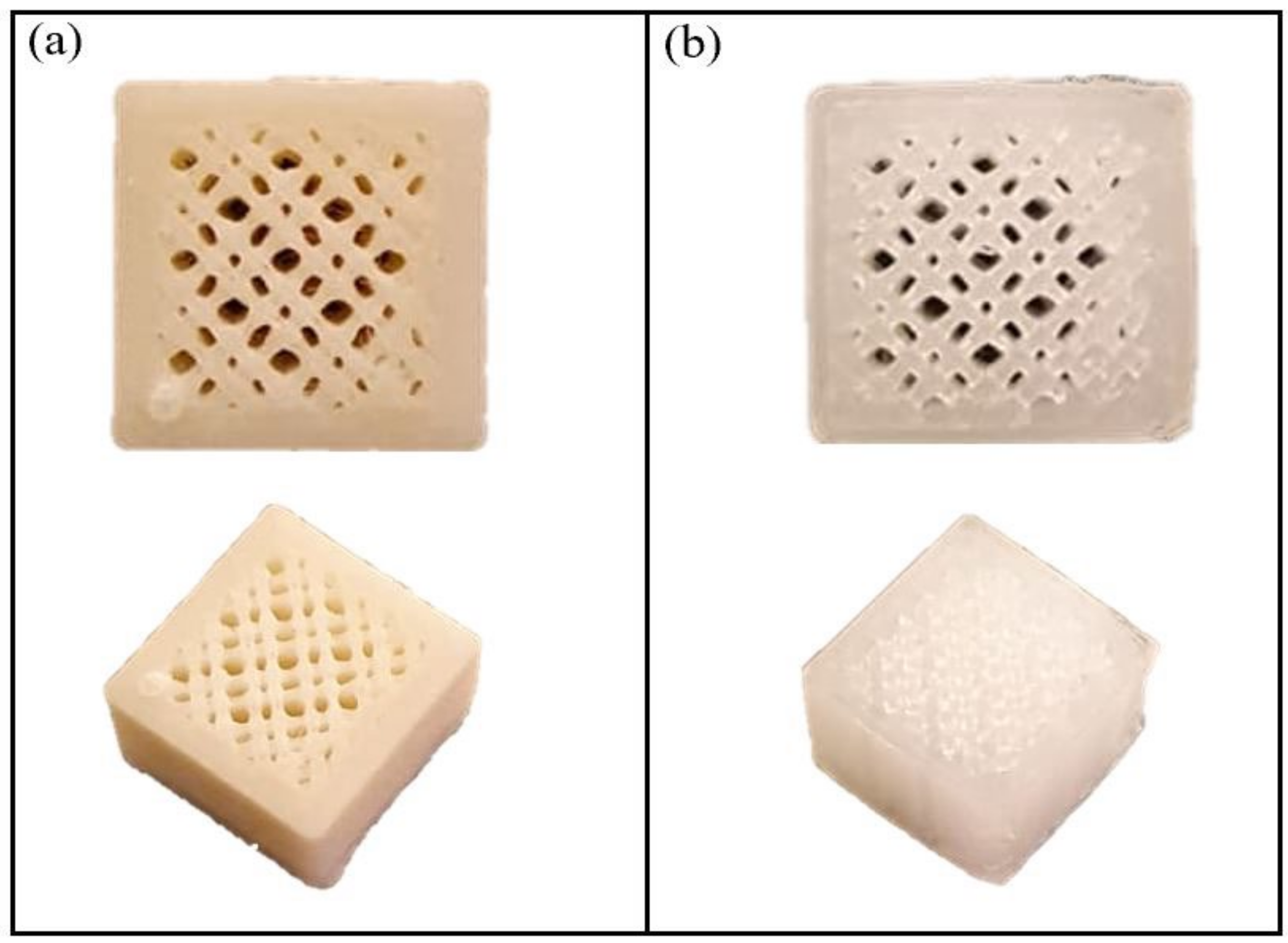
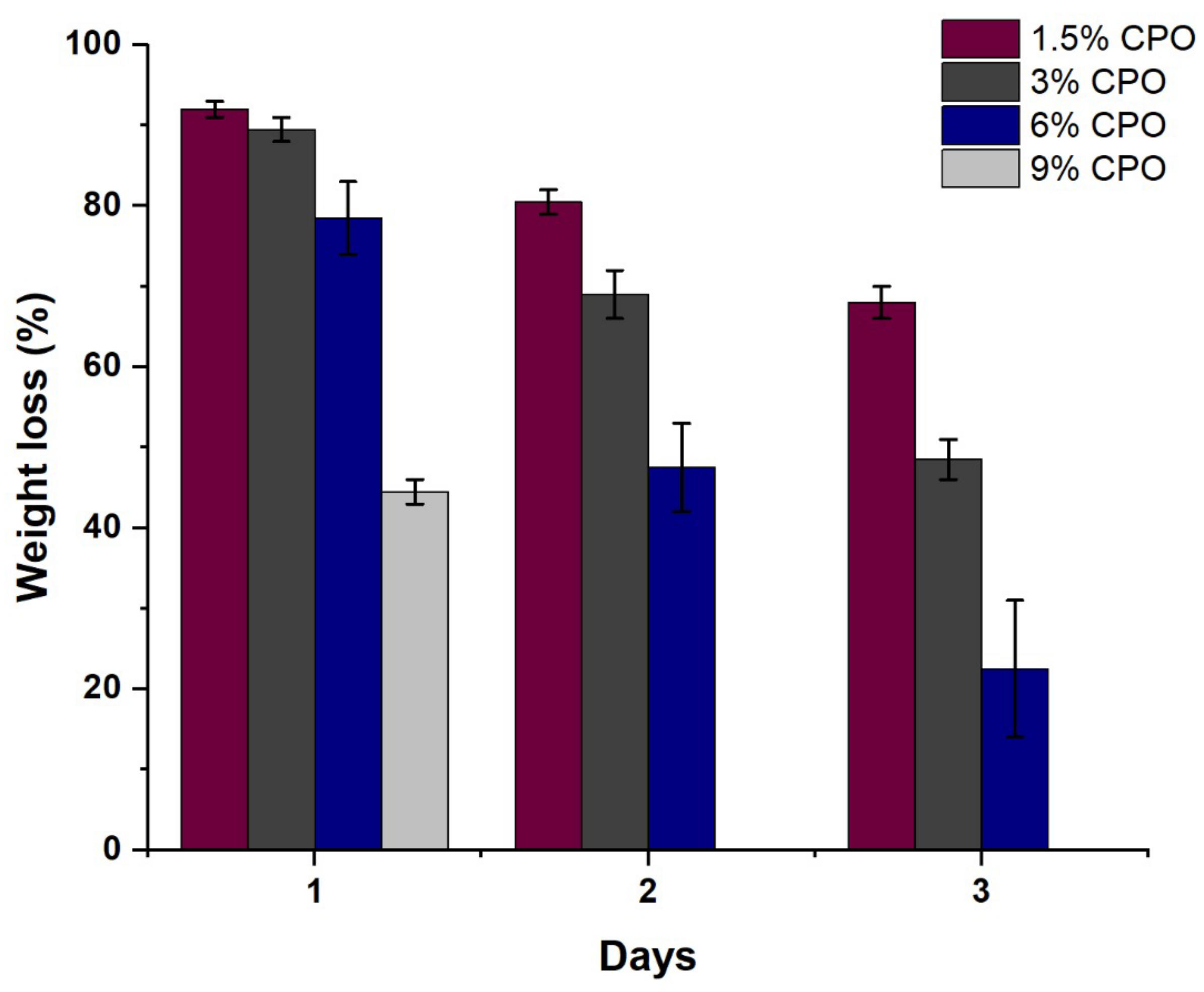
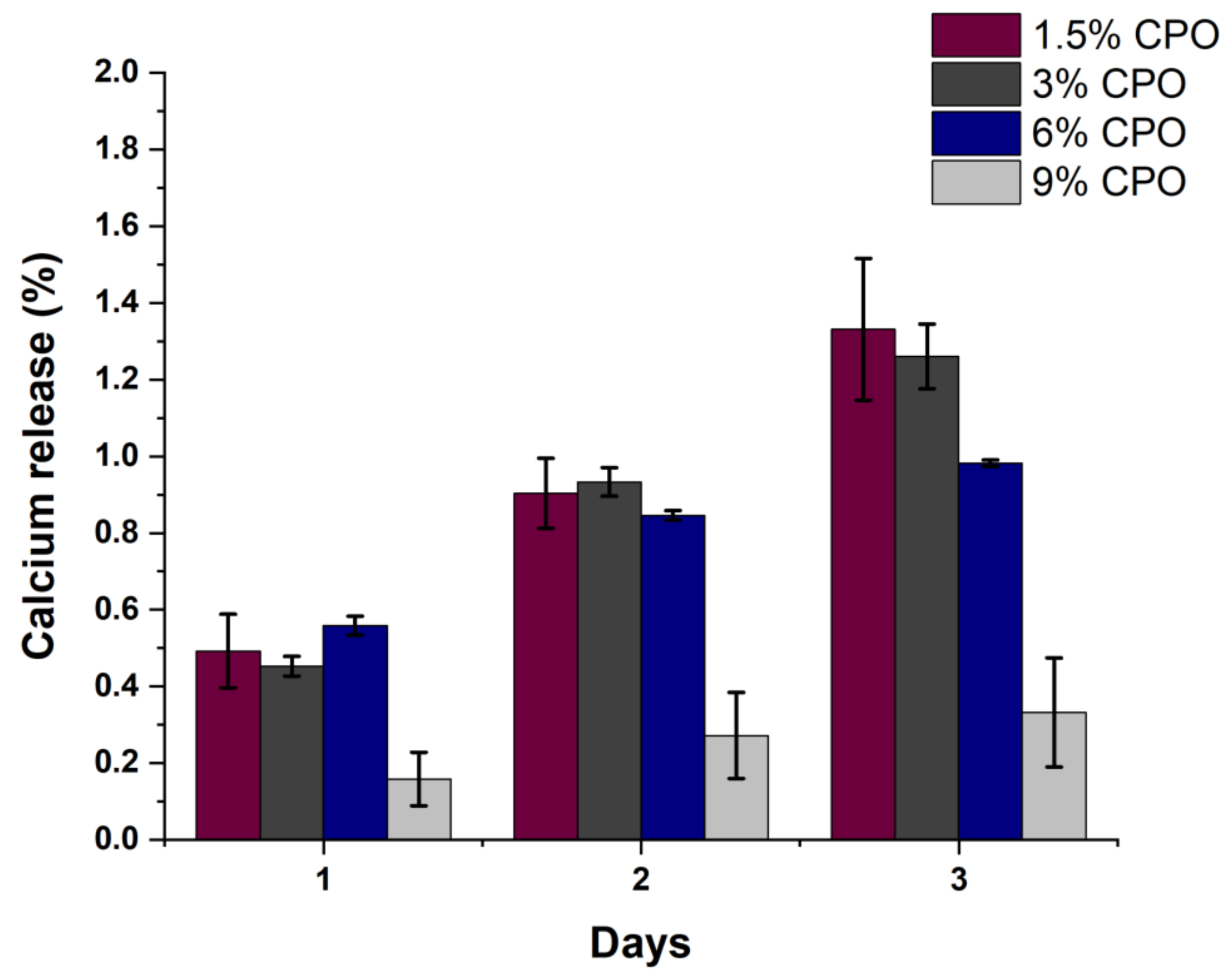
| Sample No. | Samples Name | PLA (%wt./v) | CPO (%wt./v) |
|---|---|---|---|
| 1 | 0% CPO | 100 | 0 |
| 2 | 1.5% CPO | 98.5 | 1.5 |
| 3 | 3% CPO | 97 | 3 |
| 4 | 6% CPO | 94 | 6 |
| 5 | 9% CPO | 91 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, A.; Saeed, A.; Elshaer, A.; Melaibari, A.A.; Memić, A.; Hassanin, H.; Essa, K. Fabrication and Characterization of Oxygen-Generating Polylactic Acid/Calcium Peroxide Composite Filaments for Bone Scaffolds. Pharmaceuticals 2023, 16, 627. https://doi.org/10.3390/ph16040627
Mohammed A, Saeed A, Elshaer A, Melaibari AA, Memić A, Hassanin H, Essa K. Fabrication and Characterization of Oxygen-Generating Polylactic Acid/Calcium Peroxide Composite Filaments for Bone Scaffolds. Pharmaceuticals. 2023; 16(4):627. https://doi.org/10.3390/ph16040627
Chicago/Turabian StyleMohammed, Abdullah, Abdu Saeed, Amr Elshaer, Ammar A. Melaibari, Adnan Memić, Hany Hassanin, and Khamis Essa. 2023. "Fabrication and Characterization of Oxygen-Generating Polylactic Acid/Calcium Peroxide Composite Filaments for Bone Scaffolds" Pharmaceuticals 16, no. 4: 627. https://doi.org/10.3390/ph16040627
APA StyleMohammed, A., Saeed, A., Elshaer, A., Melaibari, A. A., Memić, A., Hassanin, H., & Essa, K. (2023). Fabrication and Characterization of Oxygen-Generating Polylactic Acid/Calcium Peroxide Composite Filaments for Bone Scaffolds. Pharmaceuticals, 16(4), 627. https://doi.org/10.3390/ph16040627










