Preparation and Bioevaluation of a Novel 99mTc-Labeled Glucose Derivative Containing Cyclohexane as a Promising Tumor Imaging Agent
Abstract
1. Introduction
2. Results
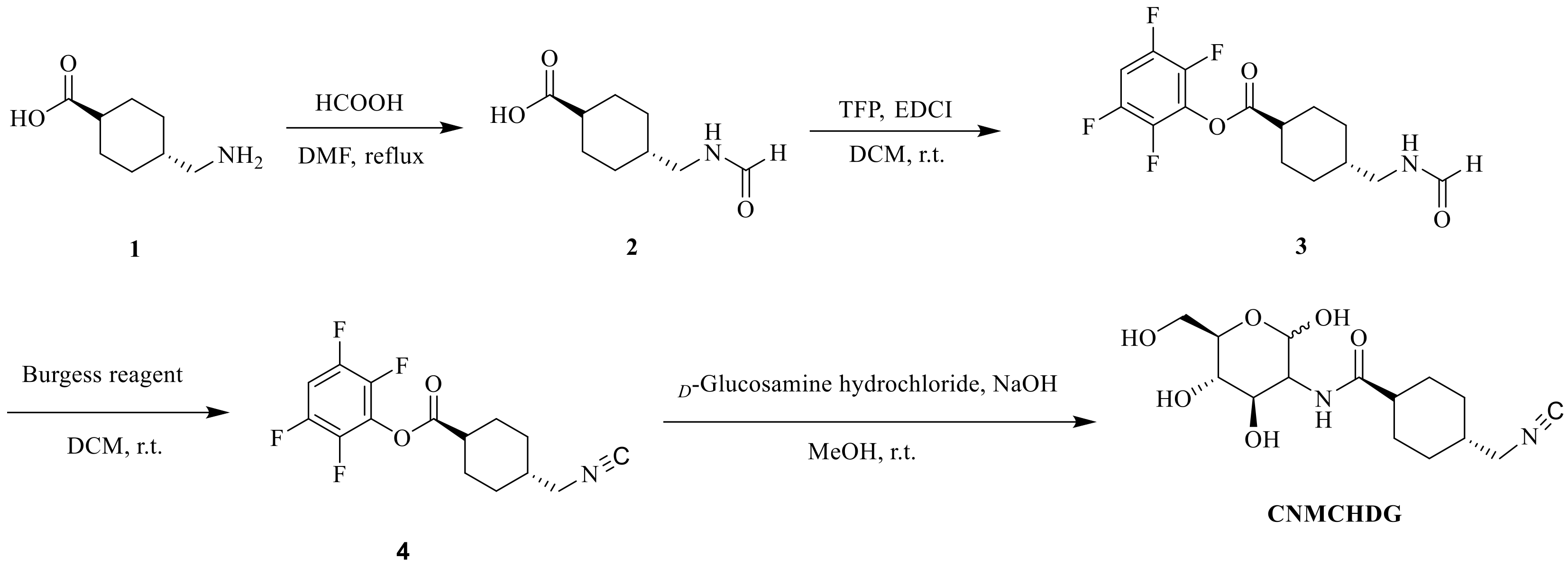
2.1. Synthesis of CNMCHDG
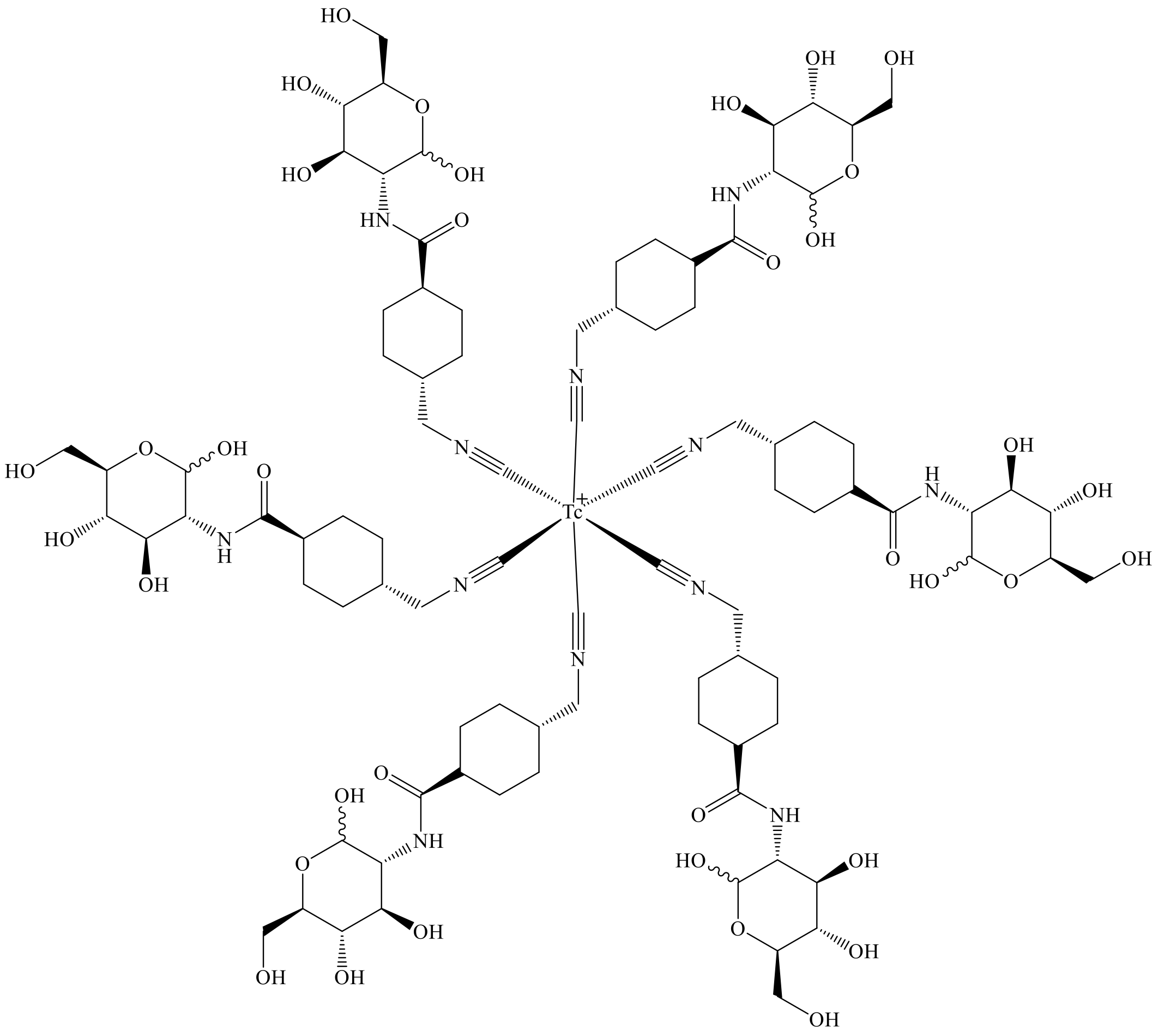
2.2. Radiolabeling and Quality Control
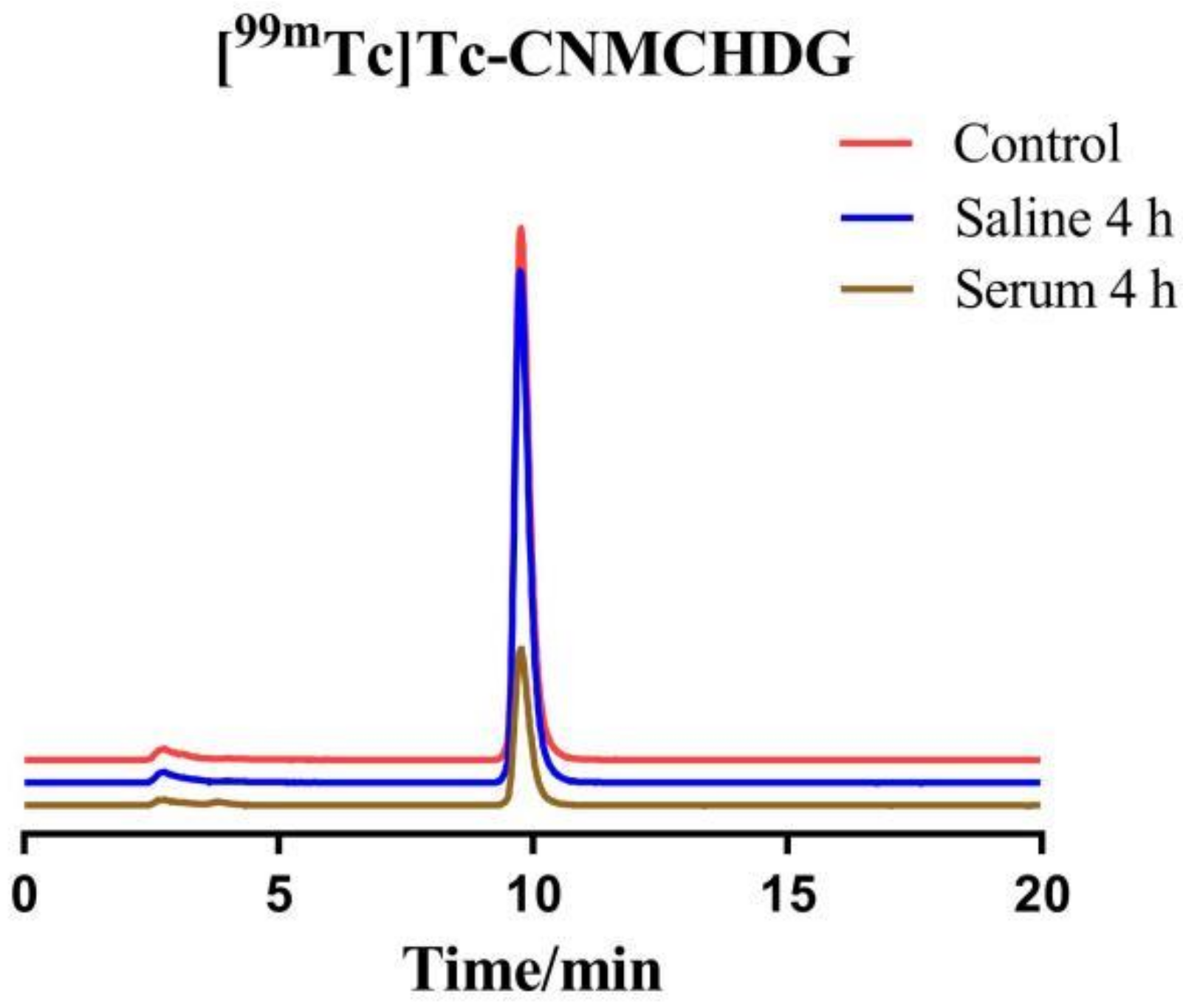
2.3. In Vitro Stability Studies and Partition Coefficient
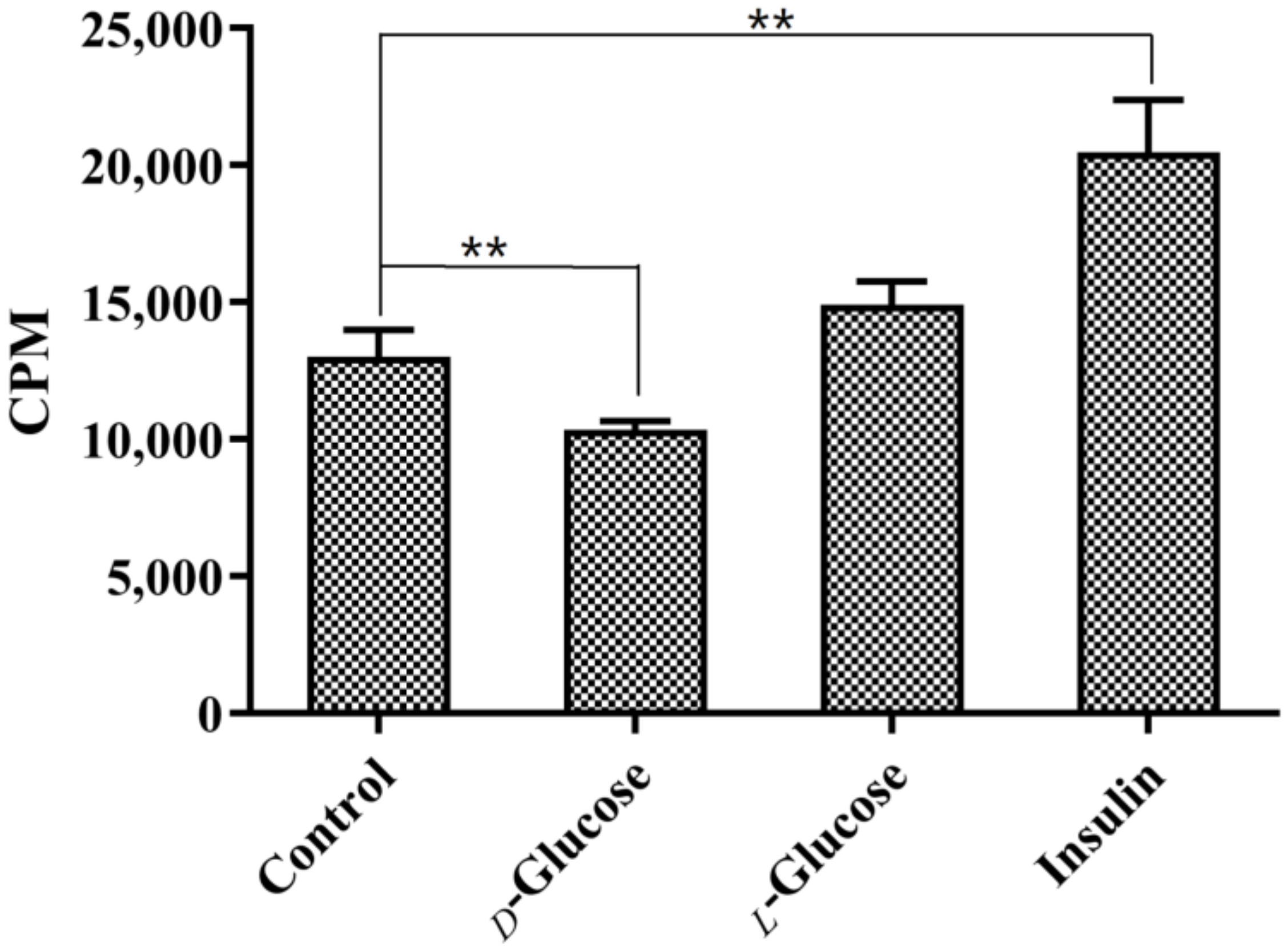
2.4. In Vitro Cellular Uptake Studies
2.5. Biodistribution Studies
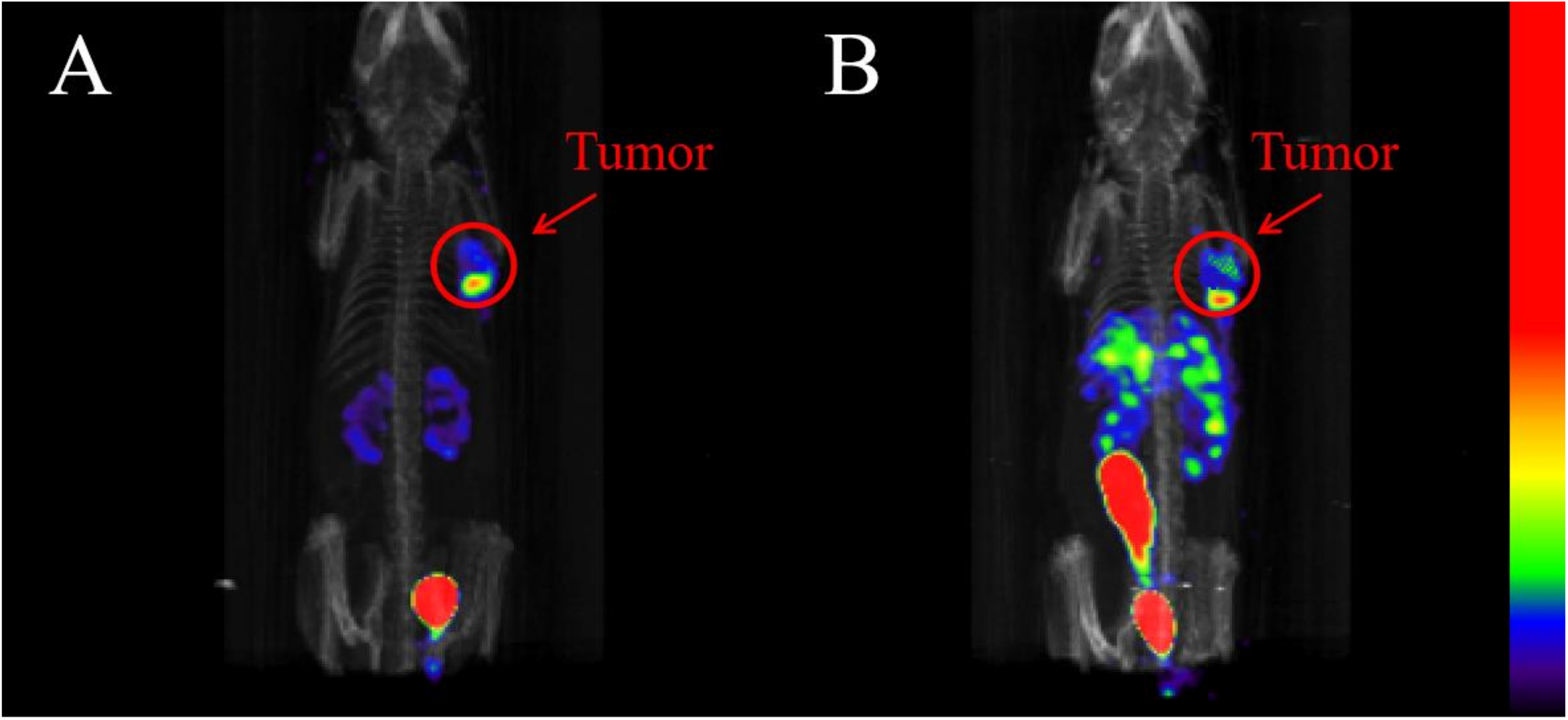
2.6. SPECT/CT Imaging Studies
2.7. Pharmacokinetic Characteristics
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of CNMCHDG
4.3. Radiolabeling and Quality Control
4.4. In Vitro Stability Studies
4.5. Determination of the Partition Coefficient (log P)
4.6. In Vitro Cellular Uptake Studies
4.7. Tumor Models and Biodistribution Studies
4.8. SPECT/CT Imaging Studies
4.9. Pharmacokinetic Characteristics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, B.; Lazarus, N.R. An in vitro system for studying insulin release: Effects of glucose and glucose-6-phosphate. J. Physiol. 1977, 271, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D.; Morin, A.M. Brain uptake of glucose in diabetes mellitus: The role of glucose transporters. Am. J. Med. Sci. 1991, 301, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Riby, L.M.; van Eekelen, J.A.M.; Foster, J.K. Glucose enhancement of human memory: A comprehensive research review of the glucose memory facilitation effect. Neurosci. Biobehav. Rev. 2011, 35, 770–783. [Google Scholar] [CrossRef]
- Sędzikowska, A.; Szablewski, L. Human glucose transporters in renal glucose homeostasis. Int. J. Mol. Sci. 2021, 22, 13522–13538. [Google Scholar] [CrossRef] [PubMed]
- Kalhan, S.C.; Oliven, A.; King, K.C.; Lucero, C. Role of glucose in the regulation of endogenous glucose production in the human newborn. Pediatr. Res. 1986, 20, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Dart, A. Cancer goes tick tock. Nat. Rev. Cancer 2016, 16, 409. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, A.; Pawar, S.; Govender, P.; Berman, J.; Ruberg, F.L.; Miller, E.J. The response of FDG uptake to immunosuppressive treatment on FDG PET/CT imaging for cardiac sarcoidosis. J. Nucl. Cardiol. 2017, 24, 413–424. [Google Scholar] [CrossRef]
- Zhai, G.; Zhang, M.; Xu, H.; Zhu, C.; Li, B. The role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography whole body imaging in the evaluation of focal thyroid incidentaloma. J. Endocrinol. Investig. 2010, 33, 151–155. [Google Scholar] [CrossRef]
- Allegra, E.; Cristofaro, M.G.; Cascini, L.G.; Lombardo, N.; Tamburrini, O.; Garozzo, A.; April, M.M. 18FDG uptake in sinonasal inverted papilloma detected by positron emission tomography/computed tomography. Sci. World J. 2012, 2012, 943412–943415. [Google Scholar] [CrossRef]
- Shaw, T.B.; Jeffree, R.L.; Thomas, P.; Goodman, S.; Debowski, M.; Lwin, Z.; Chua, B. Diagnostic performance of 18F-fluorodeoxyglucose positron emission tomography in the evaluation of glioma. J. Med. Imaging Radiat. Oncol. 2019, 63, 650–656. [Google Scholar] [CrossRef]
- Park, J.H.; Pahk, K.; Kim, S.; Lim, S.M.; Cheon, G.J.; Park, Y.H.; Lee, S.S.; Choe, J.G. Fluorine-18 fluorodeoxyglucose positron emission tomography imaging of T-lymphoblastic lymphoma patients. Oncol. Lett. 2016, 12, 1620–1622. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.W.; So, Y.; Kang, S.Y.; So, M.K.; Kim, H.; Chung, H.W.; Kim, W.S.; Kim, S.E. F-18 fluorodeoxyglucose positron emission tomography for differential diagnosis and prognosis prediction of vascular tumors. Oncol. Lett. 2017, 14, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, B.M.; Fowler, J.S.; Gutterson, N.I.; MacGregor, R.R.; Wan, C.N.; Wolf, A.P. Metabolic trapping as a principle of oradiopharmaceutical design: Some factors resposible for the biodistribution of [18F] 2-deoxy-2-fluoro-D-glucose. J. Nucl. Med. 1978, 19, 1154–1161. [Google Scholar] [PubMed]
- Páez, D.; Orellana, P.; Gutiérrez, C.; Ramirez, R.; Mut, F.; Torres, L. Current status of nuclear medicine practice in latin america and the caribbean. J. Nucl. Med. 2015, 56, 1629–1634. [Google Scholar] [CrossRef]
- Van den Wyngaert, T.; Elvas, F.; de Schepper, S.; Kennedy, J.A.; Israel, O. SPECT/CT: Standing on the shoulders of giants, it is time to reach for the sky! J. Nucl. Med. 2020, 61, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Kim, C.; Schechter, N.R.; Azhdarinia, A.; Yu, D.; Oh, C.; Bryant, J.L.; Won, J.; Kim, E.E.; Podoloff, D.A. Imaging with 99mTc ECDG targeted at the multifunctional glucose transport system: Feasibility study with rodents. Radiology 2003, 226, 465–473. [Google Scholar] [CrossRef]
- De Barros, A.L.B.; Cardoso, V.N.; Mota, L.D.G.; Leite, E.A.; Oliveira, M.C.D.; Alves, R.J. Synthesis and biological evaluation of technetium-labeled D-glucose-MAG3 derivative as agent for tumor diagnosis. Bioorg. Med. Chem. Lett. 2009, 19, 2497–2499. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.W.; He, L.; Zheng, S.L.; Li, J.L.; Qin, D.L. Synthesis and evaluation of a technetium-99m-labeled diethylenetriaminepentaacetate-deoxyglucose complex ([99mTc]-DTPA-DG) as a potential imaging modality for tumors. Appl. Radiat. Isot. 2006, 64, 342–347. [Google Scholar] [CrossRef]
- Zhang, X.; Ruan, Q.; Duan, X.; Gan, Q.; Song, X.; Fang, S.; Lin, X.; Du, J.; Zhang, J. Novel 99mTc-labeled glucose derivative for single photon emission computed tomography: A promising tumor imaging agent. Mol. Pharm. 2018, 15, 3417–3424. [Google Scholar] [CrossRef]
- Gan, Q.; Zhang, X.; Ruan, Q.; Fang, S.A.; Zhang, J. 99mTc-CN7DG: A highly expected spect imaging agent of cancer with satisfactory tumor uptake and tumor-to-nontarget ratios. Mol. Pharm. 2021, 18, 1356–1363. [Google Scholar] [CrossRef]
- Fernández, S.; Crócamo, N.; Incerti, M.; Giglio, J.; Scarone, L.; Rey, A. Preparation and preliminary bioevaluation of a 99mTc(CO)3-glucose derivative prepared by a click chemistry route. J. Labelled Compd. Radiopharm. 2012, 55, 274–280. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, J.; Lin, X.; Wang, X. Synthesis and biological evaluation of a novel 99mTc nitrido radiopharmaceutical with deoxyglucose dithiocarbamate, showing tumor uptake. Bioorg. Med. Chem. Lett. 2009, 19, 2752–2754. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gan, Q.; Zhang, J. Macrocyclic triamine derived glucose analogues for 99mTc(CO)3 labeling: Synthesis and biological evaluation as potential tumor-imaging agents. Chem. Biol. Drug Des. 2017, 89, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gan, Q.; Zhang, J.; Jin, Z.; Zhang, W.; Zhang, Y. Synthesis and biodistribution of novel 99mTcN complexes of glucose dithiocarbamate as potential probes for tumor imaging. Med. Chem. Comm. 2016, 7, 1381–1386. [Google Scholar] [CrossRef]
- Lin, X.; Jin, Z.; Ren, J.; Pang, Y.; Zhang, W.; Huo, J.; Wang, X.; Zhang, J.; Zhang, Y. Synthesis and biodistribution of a new 99mTc-oxo complex with deoxyglucose dithiocarbamate for tumor imaging. Chem. Biol. Drug Des. 2012, 79, 239–245. [Google Scholar] [CrossRef]
- Oh, S.J.; Ryu, J.S.; Yoon, E.J.; Bae, M.S.; Choi, S.J.; Park, K.B.; Moon, D.H. 99mTc-labeled 1-thio-β-D-glucose as a new tumor-seeking agent: Synthesis and tumor cell uptake assay. Appl. Radiat. Isot. 2006, 64, 207–215. [Google Scholar]
- Yang, D.; Yukihiro, M.; Yu, D.; Ito, M.; Oh, C.; Kohanim, S.; Azhdarinia, A.; Kim, C.; Bryant, J.; Kim, E.E.; et al. Assessment of therapeutic tumor response using 99mTc-ethylenedicysteine-glucosamine. Cancer Biother. Radiopharm. 2004, 19, 443–456. [Google Scholar] [CrossRef]
- Schechter, N.R.; Erwin, W.D.; Yang, D.J.; Kim, E.E.; Munden, R.F.; Forster, K.; Taing, L.C.; Cox, J.D.; Macapinlac, H.A.; Podoloff, D.A. Radiation dosimetry and biodistribution of 99mTc-ethylene dicysteine-deoxyglucose in patients with non-small-cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1583–1591. [Google Scholar] [CrossRef]
- Dai, D.; Rollo, F.D.; Bryant, J.; Kim, E.E.; Schirrmacher, R. Noninferiority of 99mtc-ethylenedicysteine-glucosamine as an alternative analogue to 18F-fluorodeoxyglucose in the detection and staging of non-small cell lung cancer. Contrast Media Mol. Imaging 2018, 2018, 8969714–8969723. [Google Scholar] [CrossRef]
- Omae, I. Applications of six-membered ring products from cyclometalation reactions. J. Organomet. Chem. 2017, 848, 184–195. [Google Scholar] [CrossRef]
- Testa, B.; Kier, L.B. The concept of molecular structure in structure-activity relationship studies and drug design. Med. Res. Rev. 1991, 11, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.F.; Erlandsson, K.; Thielemans, K. Advances in clinical molecular imaging instrumentation. Clin. Transl. Imaging 2018, 6, 31–45. [Google Scholar] [CrossRef]
- Lalumera, E.; Fanti, S.; Boniolo, G. Reliability of molecular imaging diagnostics. Synthese 2021, 198, 5701–5717. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, H.; Lin, X. Synthesis and evaluation of 111In-labeled D-glucose as a potential spect imaging agent. J. Radioanal. Nucl. Chem. 2013, 295, 1371–1375. [Google Scholar] [CrossRef]
- Neal, T.R.; Schumann, W.C.; Berridge, M.S.; Landau, B.R. Synthesis of [18F]-6-deoxy-6-fluoro-d-glucose ([18F]6FDG), a potential tracer of glucose transport. J. Labelled Compd. Radiopharm. 2005, 48, 845–854. [Google Scholar] [CrossRef]
- Qi, C.; He, Y.; Wang, X.; Feng, M.; Xu, J.; Ding, R.; Liu, H.; Chen, Y.; Li, F.; Zhu, Z.; et al. Synthesis and evaluation of n-(2-[18F]fluoro-4-nitrobenzoyl)glucosamine: A preliminary report. J. Radioanal. Nucl. Chem. 2011, 287, 913–920. [Google Scholar] [CrossRef]
- Tishchenko, V.K.; Petriev, V.M.; Kuzenkova, K.A.; Zavestovskaya, I.N.; Shegai, P.V.; Ivanov, S.A.; Kaprin, A.D. Biological behavior of a new 68Ga-labelled glucose derivative as a potential agent for tumor imaging. J. Phys. 2021, 2058, 012037–012041. [Google Scholar] [CrossRef]
- Bayly, S.R.; King, R.C.; Honess, D.J.; Barnard, P.J.; Betts, H.M.; Holland, J.P.; Hueting, R.; Bonnitcha, P.D.; Dilworth, J.R.; Aigbirhio, F.I.; et al. In vitro and in vivo evaluations of a hydrophilic 64Cu-bis(thiosemicarbazonato)-glucose conjugate for hypoxia imaging. J. Nucl. Med. 2008, 49, 1862–1868. [Google Scholar] [CrossRef]
- Taillefer, R.; Primeau, M.; Costi, P.; Lambert, R.; Léveillé, J.; Latour, Y. Technetium-99m-sestamibi myocardial perfusion imaging in detection of coronary artery disease: Comparison between initial (1 h) and delayed(3 h) post-exercise images. J. Nucl. Med. 1991, 32, 1961–1965. [Google Scholar]
- Tzonevska, A.; Sergieva, S.; Pipercova, E.; Timcheva, K.; Trifonova, I. 99mTc-MIBI myocardial perfusion scintigraphy in the assessment of early cardiac effects of anthracycline cancer therapy. Eur. J. Cancer 2001, 37, s359. [Google Scholar] [CrossRef]
- Cwikla, J.B.; Kolasinska, A.D.; Buscombe, J.R.; Hilson, A.J.W. Tc-99m MIBI in suspected recurrent breast cancer. Cancer Biother. Radiopharm. 2000, 15, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Asiri, J.A.; Kulaybi, S.A.; Daghas, F.A. Incidental pituitary adenoma on MIBI parathyroid imaging. Clin. Nucl. Med. 2022, 47, e63–e65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ruan, Q.; Jiang, Y.; Gan, Q.; Zhang, J. Evaluation of 99mTc-CN5DG as a broad-spectrum spect probe for tumor imaging. Transl. Oncol. 2021, 14, 100966–100971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gan, Q.; Ruan, Q.; Xiao, D.; Zhang, J. Evaluation and comparison of 99mTc-labeled d-glucosamine derivatives with different 99mTc cores as potential tumor imaging agents. Appl. Organomet. Chem. 2020, 34, 6008–6015. [Google Scholar] [CrossRef]
- Choi, W.H.; Yoo, I.R.; O, J.H.; Kim, T.J.; Lee, K.Y.; Kim, Y.K. Is the glut expression related to FDG uptake in PRT/CT of non-small cell lung cancer patients? Technol. Health Care 2015, 23, S311–S318. [Google Scholar] [CrossRef] [PubMed]
- Suzawa, N.; Ito, M.; Qiao, S.; Uchida, K.; Takao, M.; Yamada, T.; Takeda, K.; Murashima, S. Assessment of factors influencing FDG uptake in non-small cell lung cancer on PET/CT by investigating histological differences in expression of glucose transporters 1 and 3 and tumour size. Lung Cancer 2011, 72, 191–198. [Google Scholar] [CrossRef]
- Han, X.; Ren, H.; Nandi, A.; Fan, X.; Koehler, R.C. Analysis of glucose metabolism by 18F-FDG-PET imaging and glucose transporter expression in a mouse model of intracerebral hemorrhage. Sci. Rep. 2021, 11, 10885–10897. [Google Scholar] [CrossRef]

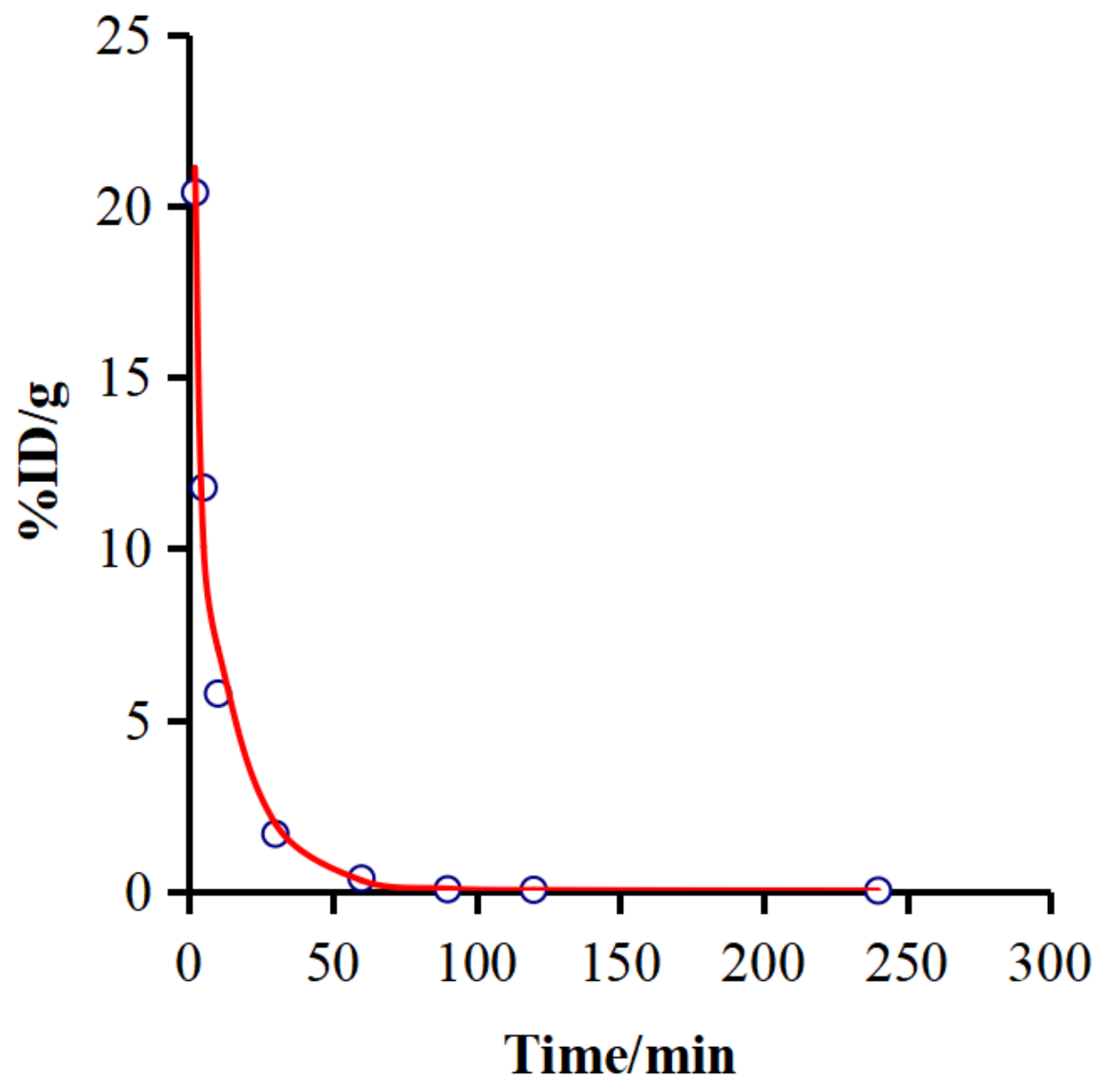
| Parameter | Unit | Value |
|---|---|---|
| AUC(0-t) | %ID/g × min | 298.528 |
| AUC(0-∞) | %ID/g × min | 353.484 |
| t1/2α | min | 0.598 |
| t1/2β | min | 10.706 |
| t1/2γ | min | 218.932 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Zhang, X.; Jiang, Y.; Ruan, Q.; Wang, Q.; Zhang, J. Preparation and Bioevaluation of a Novel 99mTc-Labeled Glucose Derivative Containing Cyclohexane as a Promising Tumor Imaging Agent. Pharmaceuticals 2023, 16, 612. https://doi.org/10.3390/ph16040612
Feng J, Zhang X, Jiang Y, Ruan Q, Wang Q, Zhang J. Preparation and Bioevaluation of a Novel 99mTc-Labeled Glucose Derivative Containing Cyclohexane as a Promising Tumor Imaging Agent. Pharmaceuticals. 2023; 16(4):612. https://doi.org/10.3390/ph16040612
Chicago/Turabian StyleFeng, Junhong, Xuran Zhang, Yuhao Jiang, Qing Ruan, Qianna Wang, and Junbo Zhang. 2023. "Preparation and Bioevaluation of a Novel 99mTc-Labeled Glucose Derivative Containing Cyclohexane as a Promising Tumor Imaging Agent" Pharmaceuticals 16, no. 4: 612. https://doi.org/10.3390/ph16040612
APA StyleFeng, J., Zhang, X., Jiang, Y., Ruan, Q., Wang, Q., & Zhang, J. (2023). Preparation and Bioevaluation of a Novel 99mTc-Labeled Glucose Derivative Containing Cyclohexane as a Promising Tumor Imaging Agent. Pharmaceuticals, 16(4), 612. https://doi.org/10.3390/ph16040612







