SPECT Imaging with Tc-99m-Labeled HYNIC-FAPI-04 to Extend the Differential Time Window in Evaluating Tumor Fibrosis
Abstract
1. Introduction
2. Results
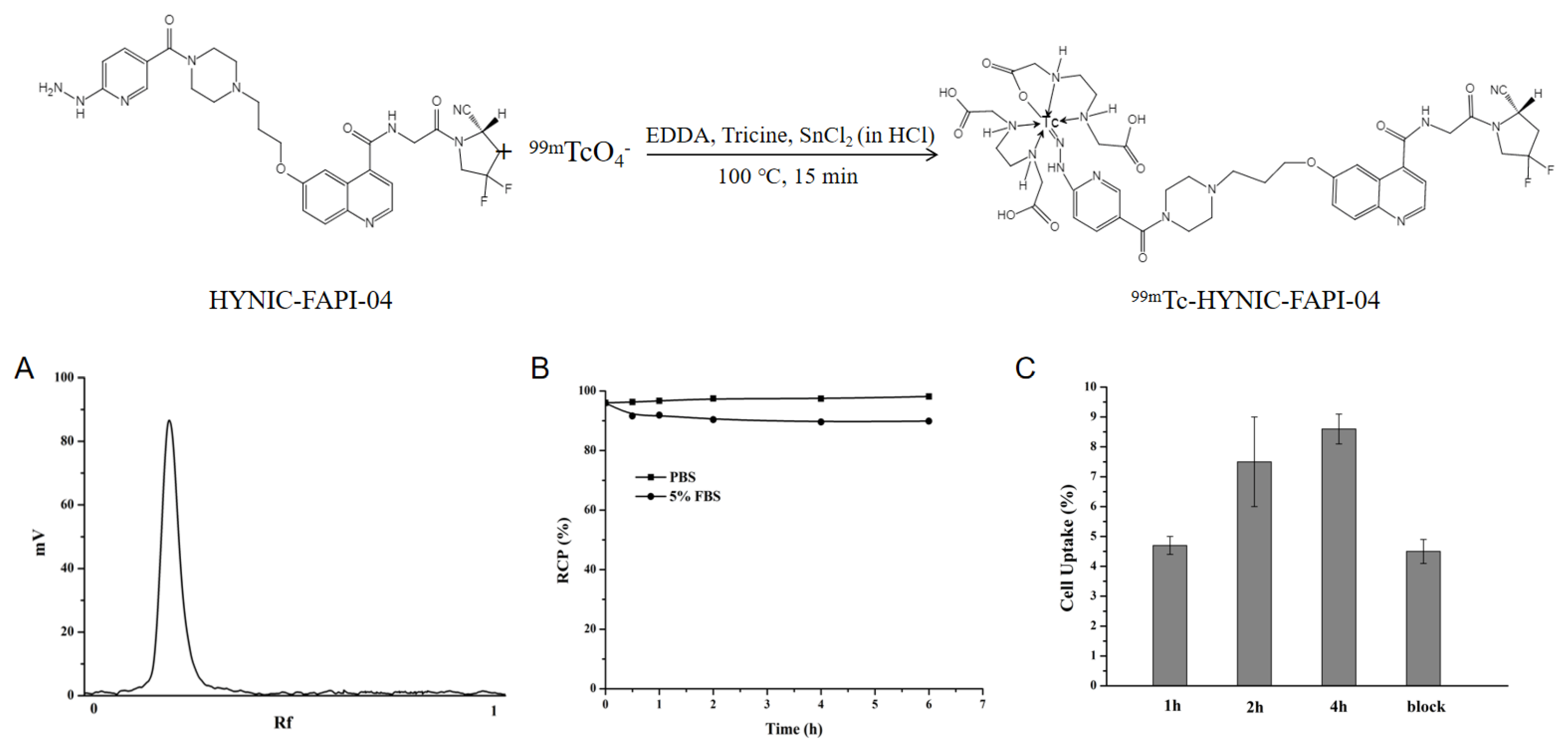
2.1. Radiolabeling and Stability
2.2. In Vitro Binding Ability
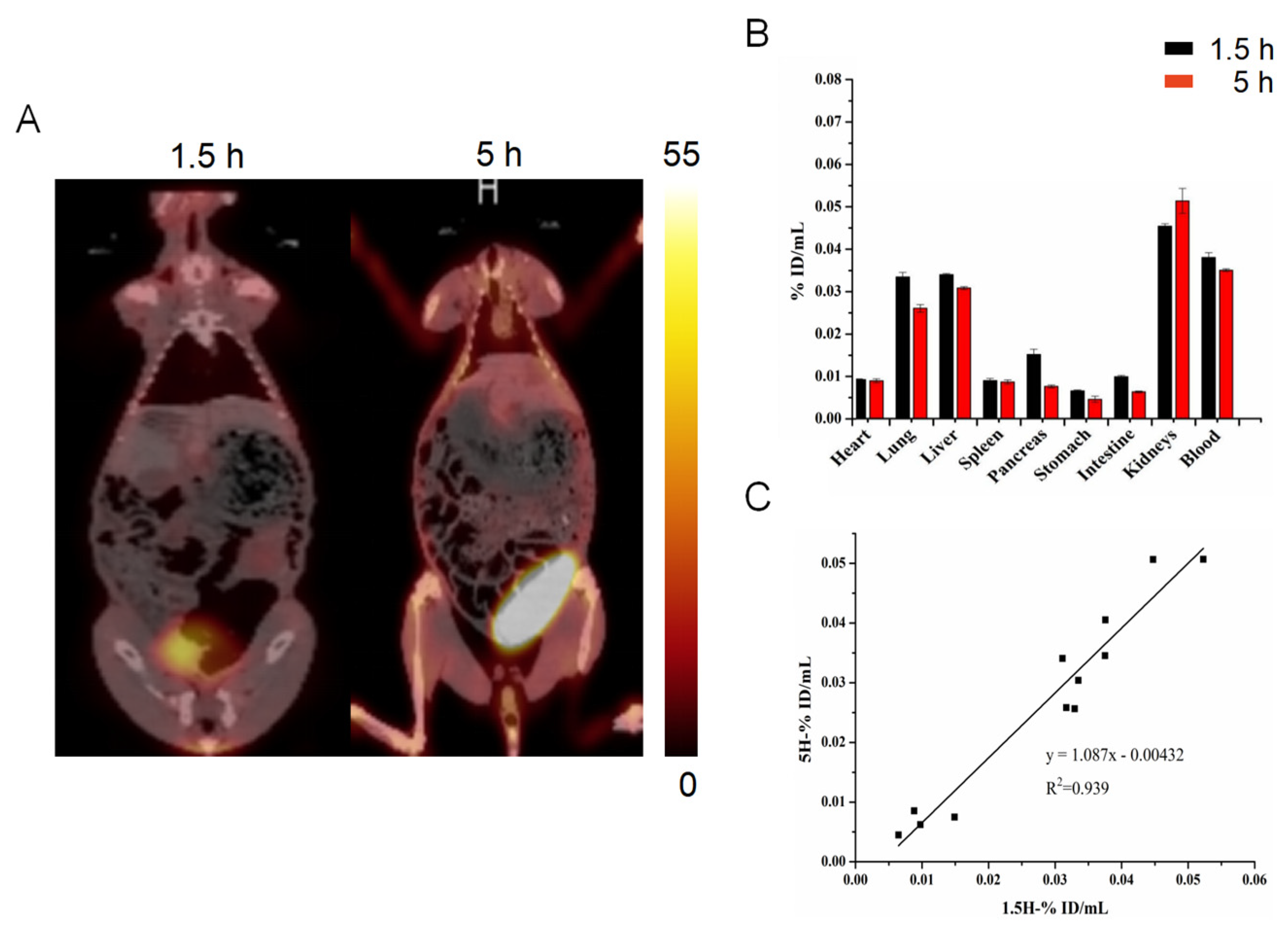
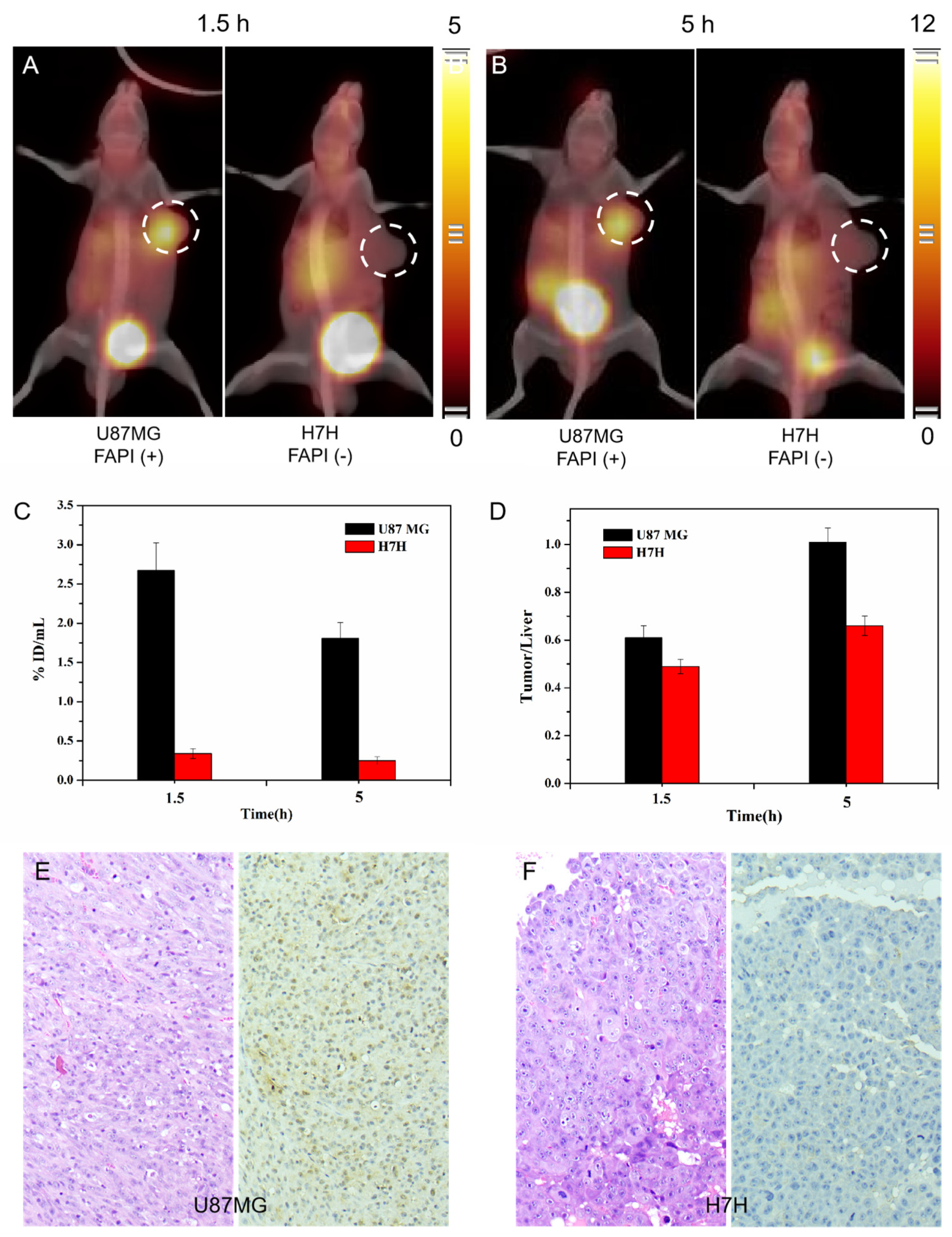
2.3. SPECT/CT Imaging with 99mTc-HYNIC-FAPI-04
2.4. Comparison with Other Imaging Modalities
2.4.1. Comparison with 18F-FDG PET
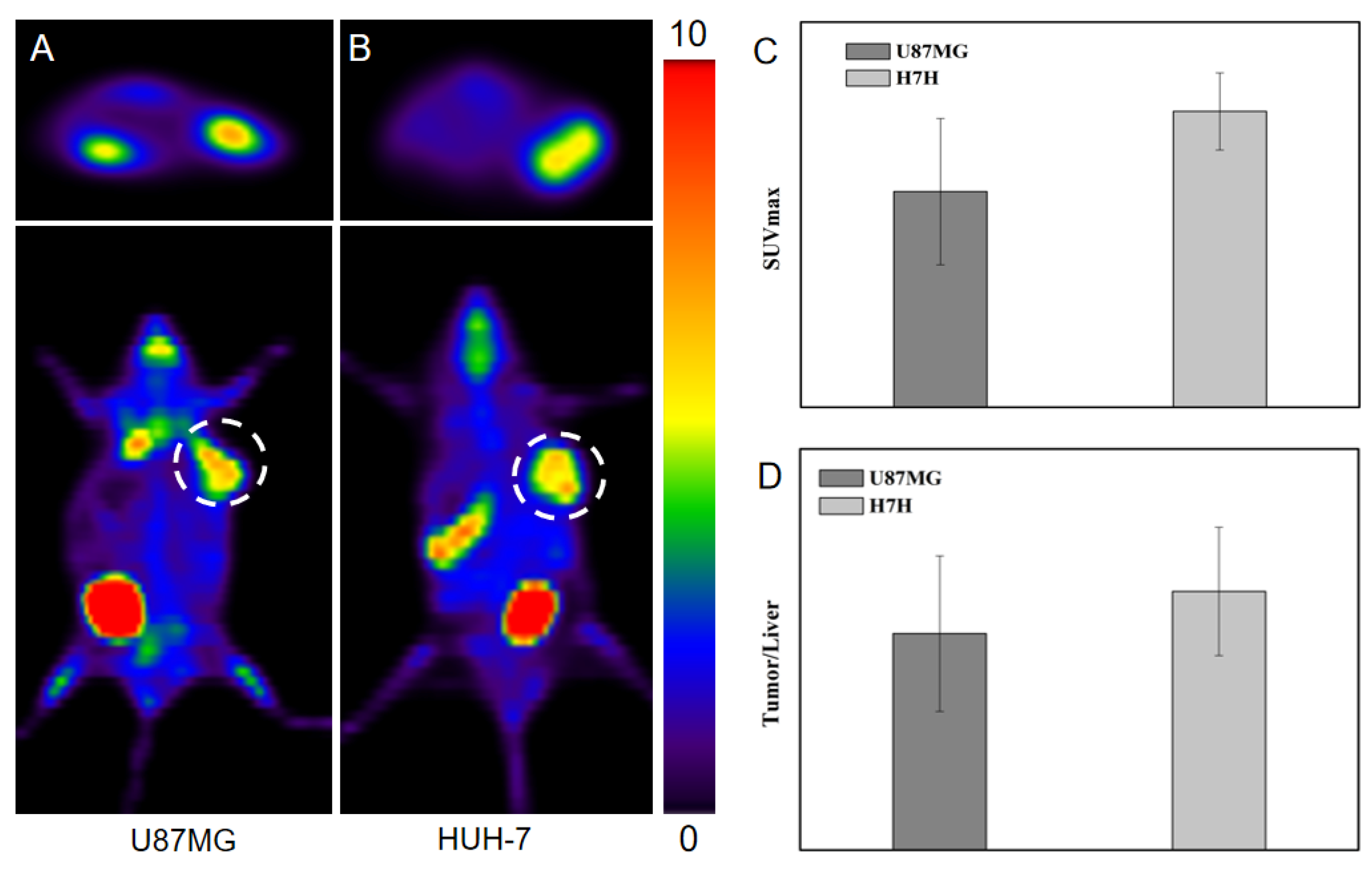
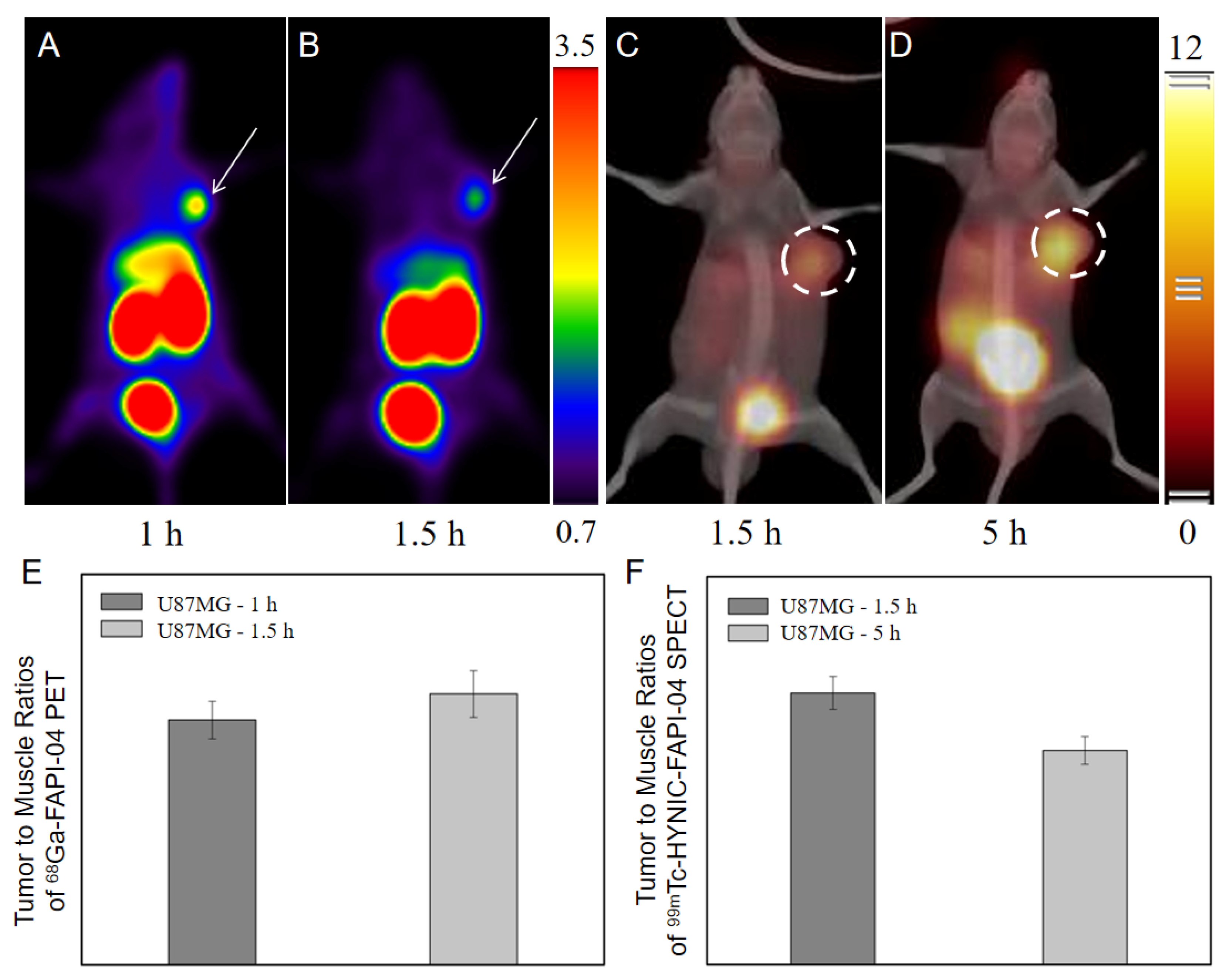
2.4.2. Comparison with 68Ga-FAPI-04 PET
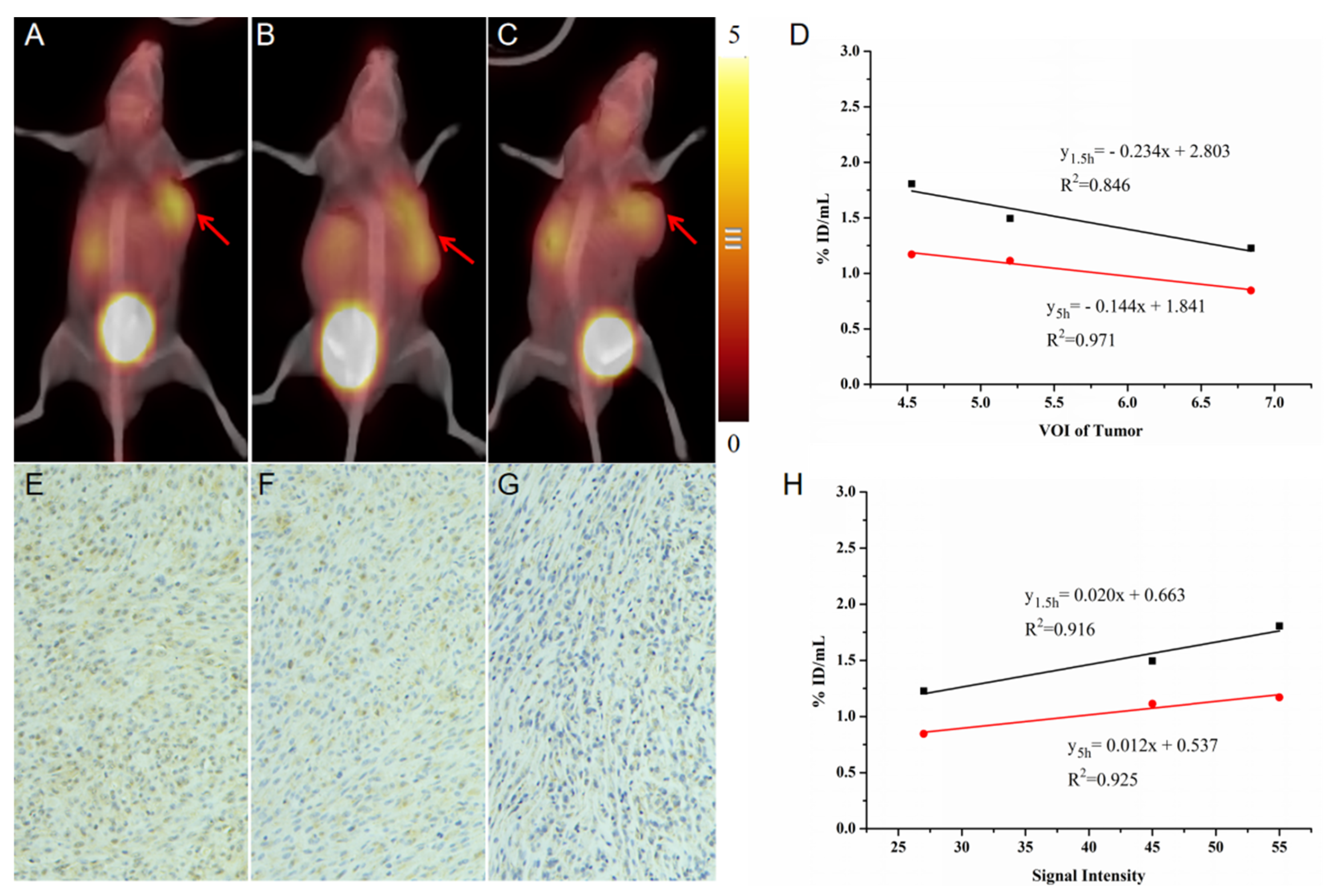
2.5. Validation of Fibrosis with a Longer Differential Time Window
3. Discussion
4. Materials and Methods
4.1. Reagents and Equipment
4.2. Radiopharmaceuticals and Quality Control
4.3. Cell Culture and In Vitro Binding Efficiency
4.4. Animal Models
4.5. SPECT/CT Imaging
4.6. PET/CT Imaging
4.7. Definition and Quantification of ROIs
4.8. Immunohistochemistry and Quantification
4.9. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scanlan, M.J.; Mohan, B.K.; Calvo, B.; Garin-Chesa, P.; Sanz-Moncasi, M.P.; Healey, J.H.; Old, L.J.; Rettig, W.J. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc. Natl. Acad. Sci. USA 1994, 91, 5657–5661. [Google Scholar] [CrossRef] [PubMed]
- Hamson, E.J.; Keane, F.M.; Tholen, S.; Schilling, O.; Gorrell, M.D. Understanding fibroblast activation protein (FAP): Substrates, activities, expression and targeting for cancer therapy. Proteom. Clin. Appl. 2014, 8, 454–463. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer uptake in 28 different kinds of cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Coto-Llerena, M.; Ercan, C.; Kancherla, V.; Taha-Mehlitz, S.; Eppenberger-Castori, S.; Soysal, S.D.; Charlotte, K.Y.; Bolli, M.; Flüe, M.V.; Nicolas, G.P.; et al. High expression of FAP in colorectal cancer is associated with angiogenesis and immunoregulation processes. Front. Oncol. 2020, 10, 979–991. [Google Scholar] [CrossRef]
- Wikberg, M.L.; Edin, S.; Lundberg, I.V.; Guelpen, B.V.; Dahlin, A.M.; Rutegard, J.; Stenling, R.; Öberg, A.; Palmqvist, R. High intratumoral expression of fibroblast activation protein (FAP) in colon cancer is associated with poorer patient prognosis. Tumor Biol. 2013, 34, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Simkova, A.; Busek, P.; Sedo, A.; Konvalinka, J. Molecular recognition of fibroblast activation protein for diagnostic and therapeutic applications. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140409. [Google Scholar] [CrossRef]
- Boonkawin, N.; Chotipanich, C. The first radiolabeled 68Ga-FAPI-46 for clinical PET applications using a fully automated iQS-TS synthesis system in Thailand. J. Chulabhorn R. Acad. 2021, 3, 180–188. Available online: https://he02.tci-thaijo.org/index.php/jcra/article/view/249531 (accessed on 22 July 2021).
- Loktev, A.; Lindner, T.; Mier, W.; Debus, J.; Altmann, A.; Jäger, D.; Giesel, F.; Kratochwil, C.; Barthe, P.; Roumestand, C.; et al. A tumor-imaging method targeting cancer-associated fibroblasts. J. Nucl. Med. 2018, 59, 1423–1429. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Burger, E.M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marmé, F.; Jäger, D.; Mier, W.; et al. Development of fibroblast activation protein-targeted radiotracers with improved tumor retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. 68Ga-FAPI PET/CT: Biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J. Nucl. Med. 2019, 60, 386–392. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Zhao, L.; Ruan, D.; Pang, Y.Z.; Hao, B.; Dai, Y.Q.; Wu, X.R.; Guo, W.; Fan, C.L.; Wu, J.X.; et al. Usefulness of [68Ga]Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive [18F]FDG PET/CT findings. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.M.; Xing, H.Q.; Yang, X.B.; Li, F.; Yao, S.B.; Jia, C.W.; Zhao, H.T.; Hacker, M.; Huo, L.; Li, X. Comparison of PET imaging of activated fibroblasts and 18F-FDG for diagnosis of primary hepatic tumours: A prospective pilot study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Pang, Y.Z.; Wu, J.X.; Zhao, L.; Hao, B.; Wu, J.; Wei, J.H.; Wu, S.M.; Zhao, L.; Luo, Z.M.; et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1820–1832. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Zhou, X.; Xu, X.X.; Ding, J.; Liu, S.; Hou, X.G.; Li, N.; Zhu, H.; Yang, Z. Clinical translational evaluation of Al18F-NOTA-FAPI for fibroblast activation protein-targeted tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4259–4271. [Google Scholar] [CrossRef]
- Hicks, R.J.; Roselt, P.J.; Kallur, K.G.; Tothill, R.W.; Mileshkin, L. FAPI PET/CT: Will It End the Hegemony of 18F-FDG in Oncology? J. Nucl. Med. 2021, 62, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Eryilmaz, K.; Kilbas, B. Fully-automated synthesis of 177Lu labelled FAPI derivatives on the module modular lab-Eazy. EJNMMI Radiopharm. Chem. 2021, 6, 16–25. [Google Scholar] [CrossRef]
- Watabe, T.; Liu, Y.; Kaneda-Nakashima, K.; Shirakami, Y.; Lindner, T.; Ooe, K.; Toyoshima, A.; Nagata, K.; Shimosegawa, E.; Haberkorn, U.; et al. Theranostics targeting fibroblast activation protein in the tumor stroma: 64Cu- and 225Ac-labeled FAPI-04 in pancreatic cancer xenograft mouse models. J. Nucl. Med. 2020, 61, 563–569. [Google Scholar] [CrossRef]
- Giglio, J.; Rey, A. 99mTc labelling strategies for the development of potential nitroimidazolic hypoxia imaging agents. Inorganics 2019, 7, 128. [Google Scholar] [CrossRef]
- Ahmadpour, S.; Noaparast, Z.; Abedi, S.M.; Hosseinimehr, S.J. 99mTc-HYNIC-(tricine/EDDA)-FROP peptide for MCF-7 breast tumor targeting and imaging. J. Biomed. Sci. 2018, 25, 17–28. [Google Scholar] [CrossRef]
- Guggenberg, E.V.; Behe, M.; Behr, T.M.; Saurer, M.; Seppi, T.; Decristoforo, C. 99mTc-labeling and in vitro and in vivo evaluation of HYNIC- and (Nα-His)acetic acid-modified [D-Glu1]-minigastrin. Bioconjugate Chem. 2004, 15, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Gandomkar, M.; Najafi, R.; Shafiei, M.; Mazidi, M.; Goudarzi, M.; Mirfallah, S.H.; Ebrahimi, F.; Heydarpor, H.R.; Abdie, N. Clinical evaluation of antimicrobial peptide [99mTc/Tricine/HYNIC0] ubiquicidin 29-41 as a human-specific infection imaging agent. Nucl. Med. Biol. 2009, 36, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Zhang, J.G.; Xu, X.P.; Lu, L.J.; Hu, S.L.; Liu, C.; Cheng, J.Y.; Song, S.L.; Zhang, Y.J.; Shi, L.Q. Evaluation of Radiation dosimetry of 99mTc-HYNIC-PSMA and imaging in prostate cancer. Sci. Rep. 2020, 10, 4179–4188. [Google Scholar] [CrossRef] [PubMed]
- Franzese, E.; Falco, S.D.; Laterza, M.M.; Montella, L.; Facchini, S.; Liguori, C.; Coppola, P.; Diessa, Y.; Berretta, M.; Pisconti, S.; et al. The use of 68Ga prostate-specific membrane antigen PET-CT in prostate cancer: Diagnostic challenges and therapeutic opportunities. Future Sci. OA 2021, 7, FSO705. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Feng, J.H.; Jiang, Y.H.; Zhang, X.; Duan, X.J.; Wang, Q.N.; Yin, G.X.; Xiao, D.; Zhang, J.B. Preparation and bioevaluation of Tc-99m-labeled FAP inhibitors as tumor radiotracers to target the fibroblast activation protein. Mol. Pharm. 2022, 19, 160–171. [Google Scholar] [CrossRef]
- Bernardes, E.; Caravan, P.; Dam, R.M.; Deuther-Conrad, W.; Ellis, B.; Furumoto, S.; Guillet, B.; Huang, Y.; Jia, H.; Laverman, P.; et al. Highlight selection of radiochemistry and radiopharmacy developments by editorial board. EJNMMI Radiopharm. Chem. 2022, 7, 9–26. [Google Scholar] [CrossRef]
- Eary, J.F. Nuclear medicine in cancer diagnosis. Lancet 1999, 354, 853–857. [Google Scholar] [CrossRef]
- Kramer-Marek, G.; Capala, J. The role of nuclear medicine in modern therapy of cancer. Tumor Biol. 2012, 33, 629–640. [Google Scholar] [CrossRef]
- Kang, P.M.; Seo, W.I.; Lee, S.S.; Bae, S.K.; Kwak, H.S.; Min, K.; Kim, W.; Kang, D.I. Incidental abnormal FDG uptake in the prostate on 18-fluoro2-deoxyglucose positron emission tomography-computed tomography scans. Asian Pac. J. Cancer Prev. 2014, 15, 8699–8703. [Google Scholar] [CrossRef]
- Dolan, R.D.; McLees, N.G.; Irfan, A.; McSorley, S.T.; Horgan, P.G.; Colville, D.; McMillan, D.C. The relationship between tumor glucose metabolism and host systemic inflammatory responses in patients with cancer: A systematic review. J. Nucl. Med. 2019, 60, 467–471. [Google Scholar] [CrossRef]
- Valkenburg, K.C.; de Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Lavely, W.C.; Goetze, S.; Friedman, K.P.; Leal, J.P.; Zhang, Z.; Garret-Mayer, E.; Dackiw, A.P.; Tufano, R.P.; Zeiger, M.A.; Ziessman, H.A. Comparison of SPECT/CT, SPECT, and planar imaging with single- and dual-phase 99mTc-sestamibi parathyroid scintigraphy. J. Nucl. Med. 2007, 48, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lu, D.Y.; Xia, L.; Cheng, X.J. Diagnosis performance of 99mTc-MIBI and multimodality imaging for hyperparathyroidism. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 582–586. [Google Scholar] [CrossRef]
- Castano, A.; DeLuca, A.; Weinberg, R.; Pozniakof, T.; Blaner, W.S.; Pirmohamed, A.; Bettencourt, B.; Gollob, J.; Karsten, V.; Vest, J.A.; et al. Serial scanning with technetium pyrophosphate (99mTc-PYP) in advanced ATTR cardiac amyloidosis. J. Nucl. Cardiol. Off. Publ. Am. Soc. Nucl. Cardiol. 2016, 23, 1355–1363. [Google Scholar] [CrossRef]
- Albalooshi, B.; Sharhan, M.A.; Bagheri, F.; Miyanath, S.; Ray, B.; Muhasin, M.; Zakavi, S.R. Direct comparison of 99mTc-PSMA SPECT/CT and 68Ga-PSMA PET/CT in patients with prostate cancer. Asia Ocean. J. Nucl. Med. Biol. 2020, 8, 1–7. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jia, G.R.; Pan, G.X.; Cao, K.; Yang, Q.Q.; Meng, H.Y.; Yang, J.; Zhang, L.; Wang, T.; Cheng, C.; et al. Comparison of the diagnostic efficacy of 68Ga-FAPI-04 PET/MR and 18F-FDG PET/CT in patients with pancreatic cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2877–2888. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Zhang, Z.; Cheng, C.; Wang, T.; Fang, D.; Zuo, C.; Yuan, G.; Li, R.; Li, X. SPECT Imaging with Tc-99m-Labeled HYNIC-FAPI-04 to Extend the Differential Time Window in Evaluating Tumor Fibrosis. Pharmaceuticals 2023, 16, 423. https://doi.org/10.3390/ph16030423
Luo X, Zhang Z, Cheng C, Wang T, Fang D, Zuo C, Yuan G, Li R, Li X. SPECT Imaging with Tc-99m-Labeled HYNIC-FAPI-04 to Extend the Differential Time Window in Evaluating Tumor Fibrosis. Pharmaceuticals. 2023; 16(3):423. https://doi.org/10.3390/ph16030423
Chicago/Turabian StyleLuo, Xiu, Zhe Zhang, Chao Cheng, Tao Wang, Danzhou Fang, Changjing Zuo, Gengbiao Yuan, Rou Li, and Xiao Li. 2023. "SPECT Imaging with Tc-99m-Labeled HYNIC-FAPI-04 to Extend the Differential Time Window in Evaluating Tumor Fibrosis" Pharmaceuticals 16, no. 3: 423. https://doi.org/10.3390/ph16030423
APA StyleLuo, X., Zhang, Z., Cheng, C., Wang, T., Fang, D., Zuo, C., Yuan, G., Li, R., & Li, X. (2023). SPECT Imaging with Tc-99m-Labeled HYNIC-FAPI-04 to Extend the Differential Time Window in Evaluating Tumor Fibrosis. Pharmaceuticals, 16(3), 423. https://doi.org/10.3390/ph16030423





