How to Promote Skin Repair? In-Depth Look at Pharmaceutical and Cosmetic Strategies
Abstract
1. Introduction
2. Results
2.1. Overview of the Use of Wound Repair Ingredients in Cosmetic Products, Medicines, and Medical Devices
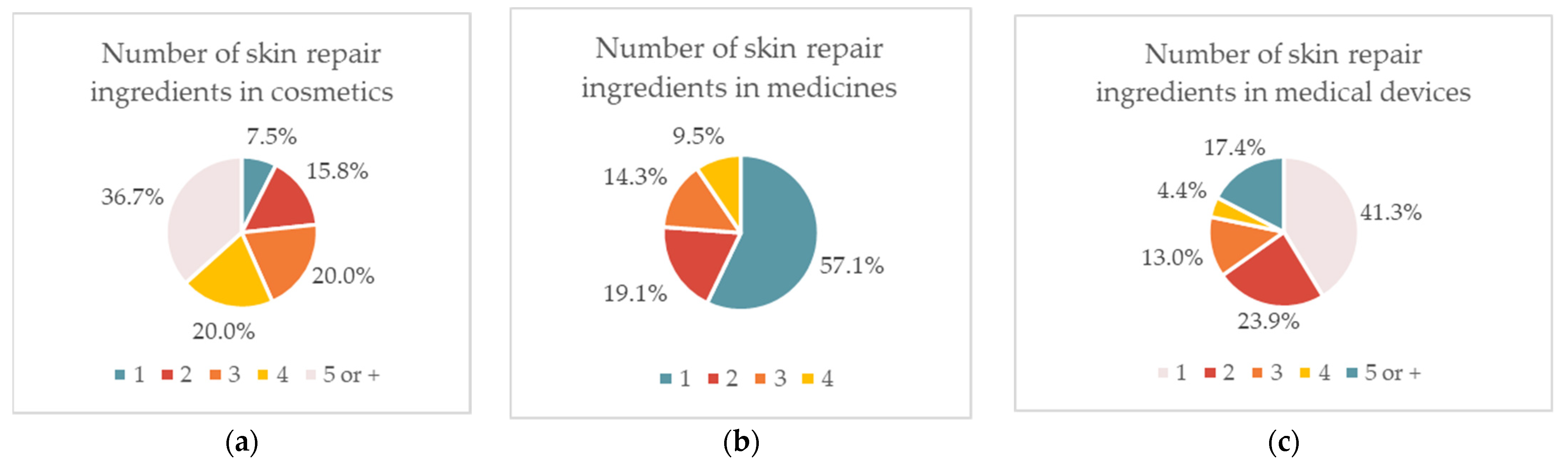
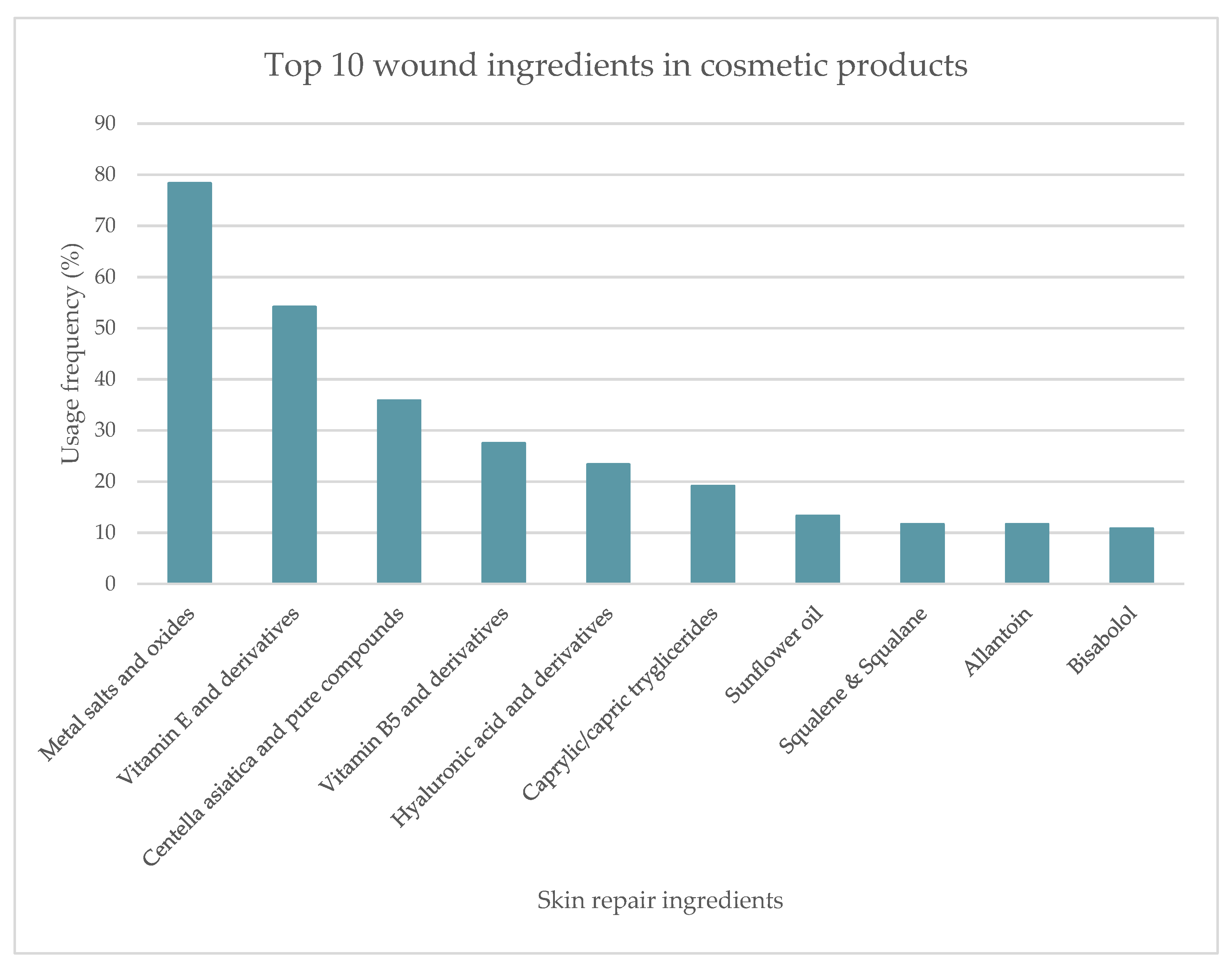
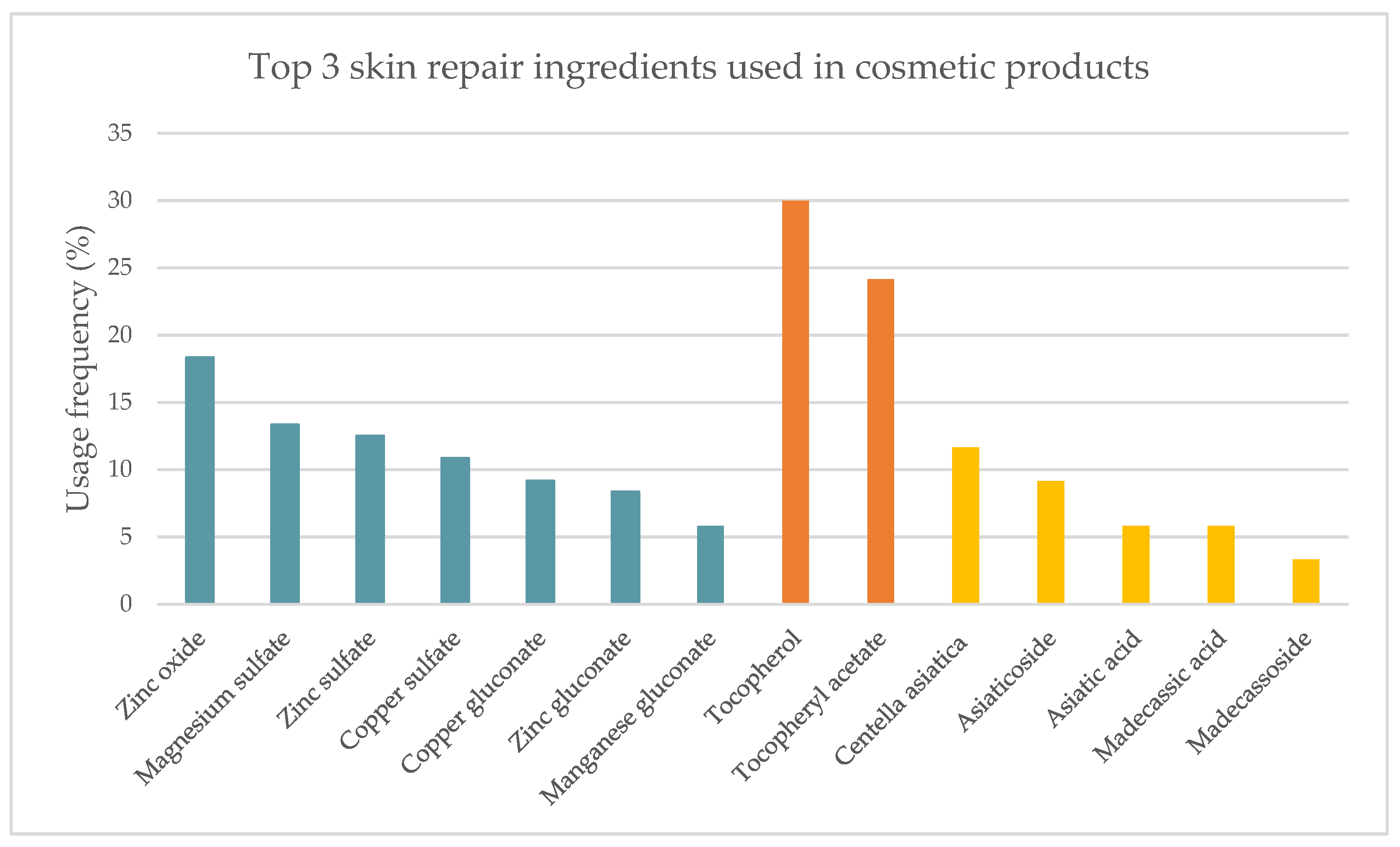
2.1.1. Cosmetic Products
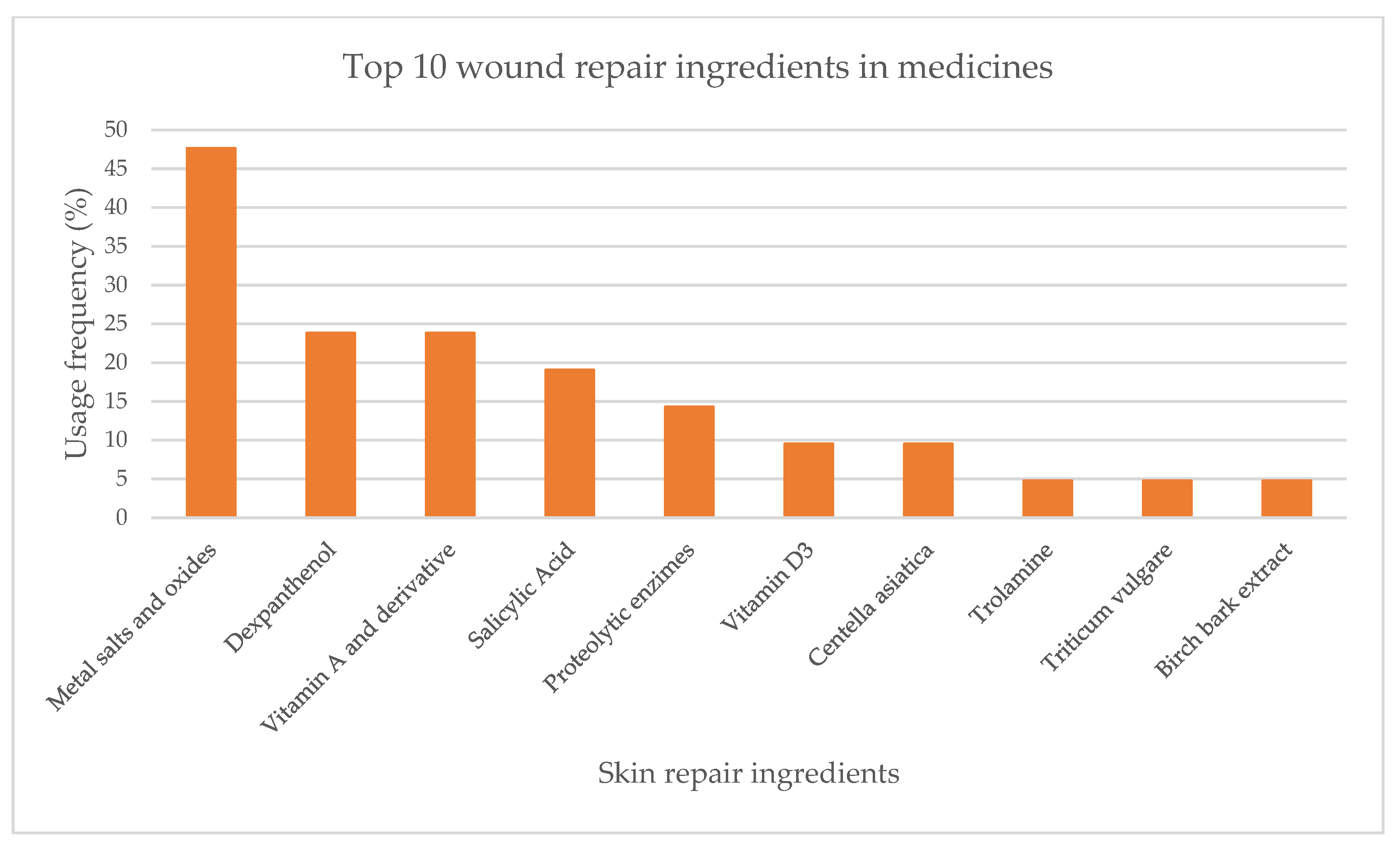
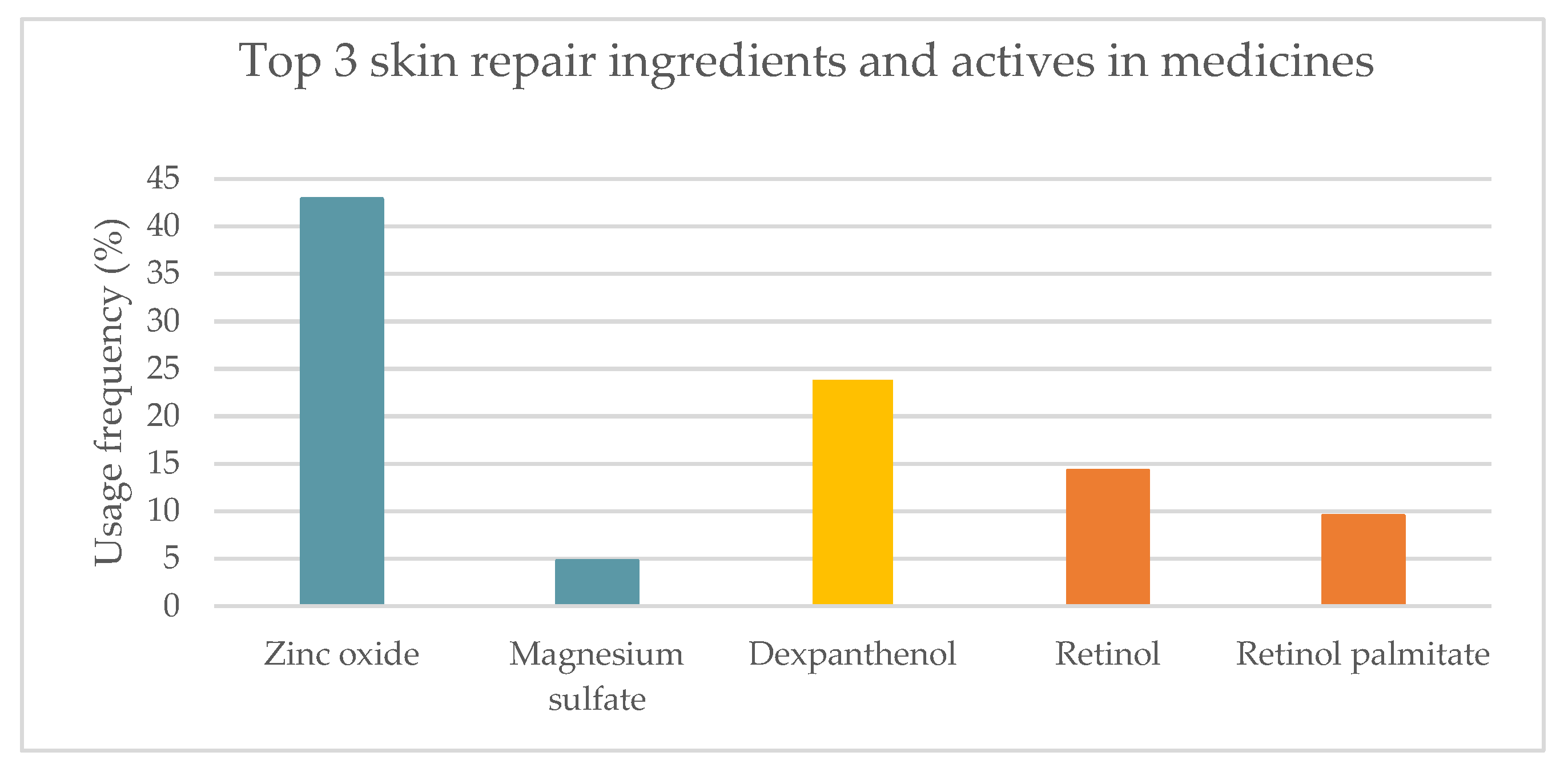
2.1.2. Topical Medicines
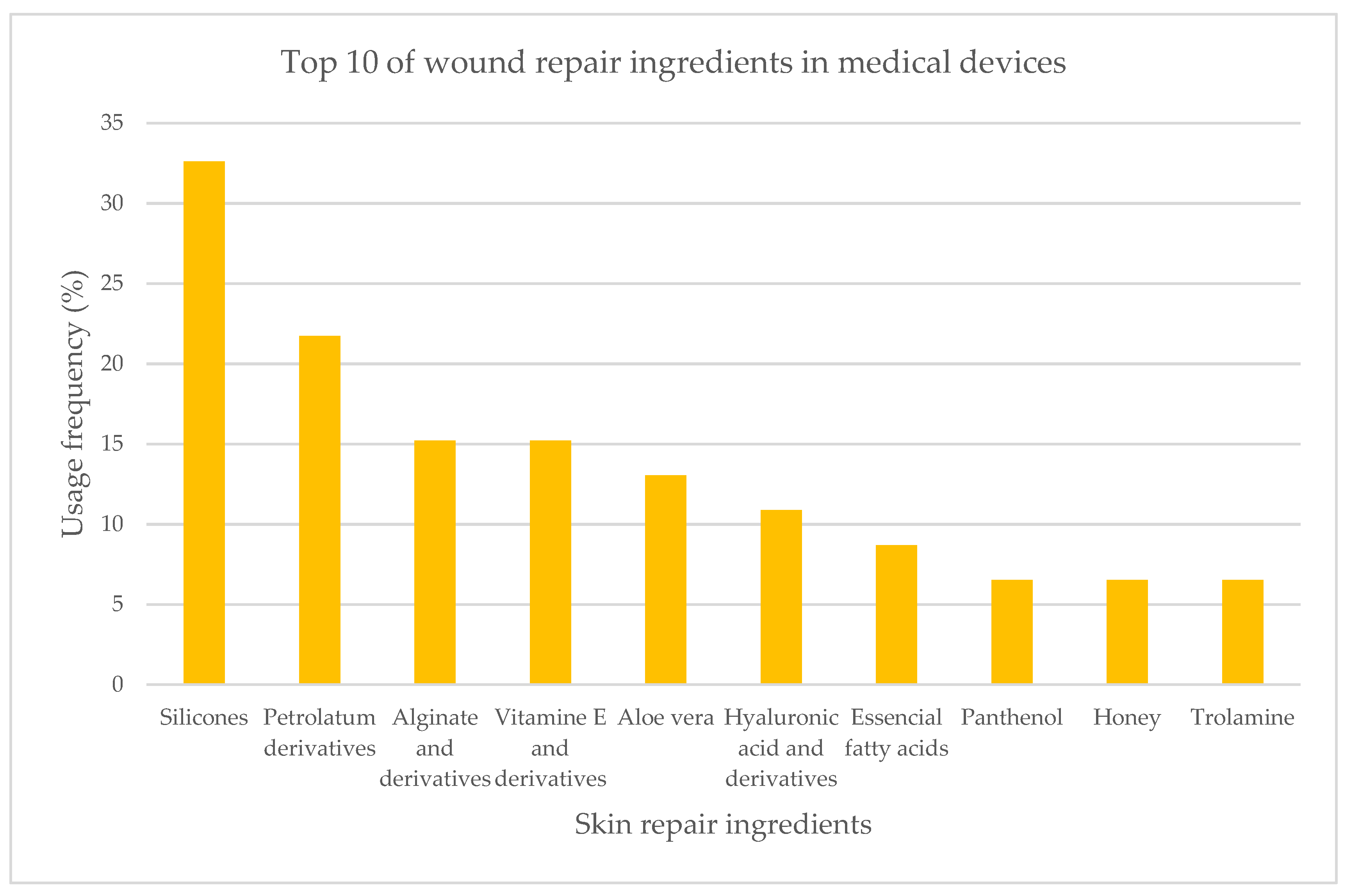
2.1.3. Medical Devices
2.2. Summary of Results
2.3. Scientific Evidence Supporting the Effectiveness of the Top Three Most Used Skin Repair Ingredients Used in Cosmetics, Medicines, and Medical Devices
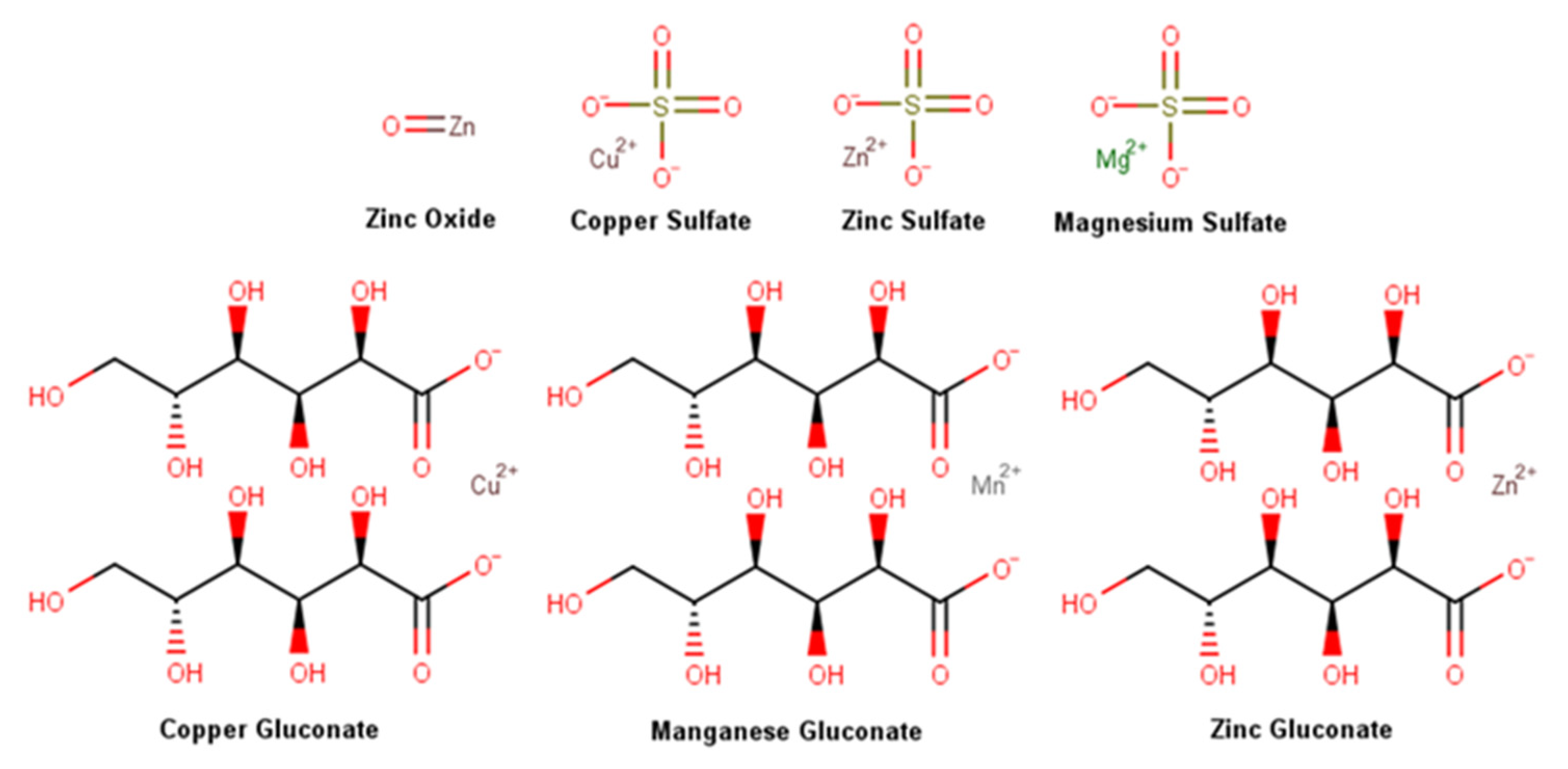
- Inorganic compounds
2.3.1. Metal Salts and Oxides
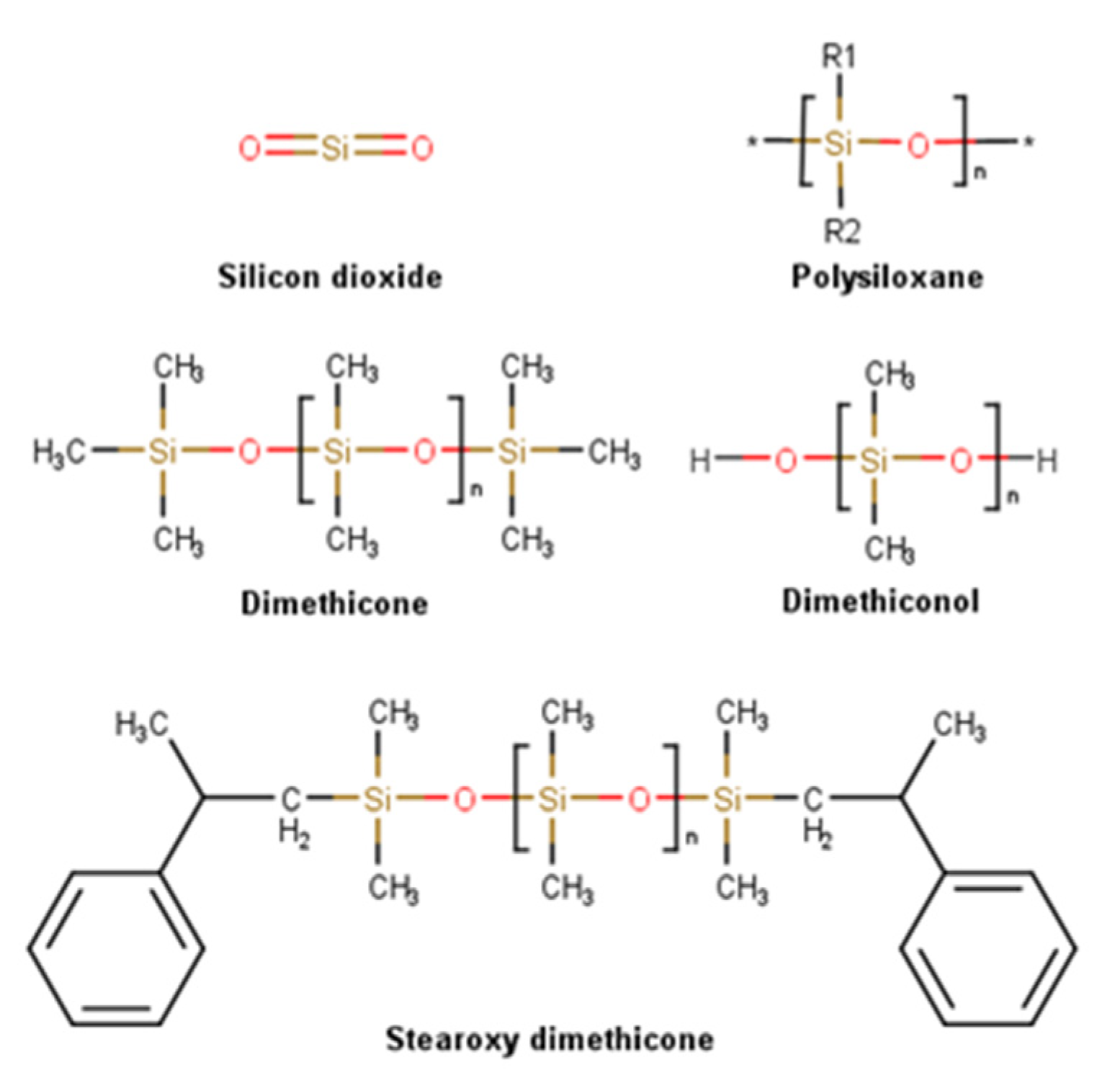
2.3.2. Silicones
- Hydrocarbon compounds
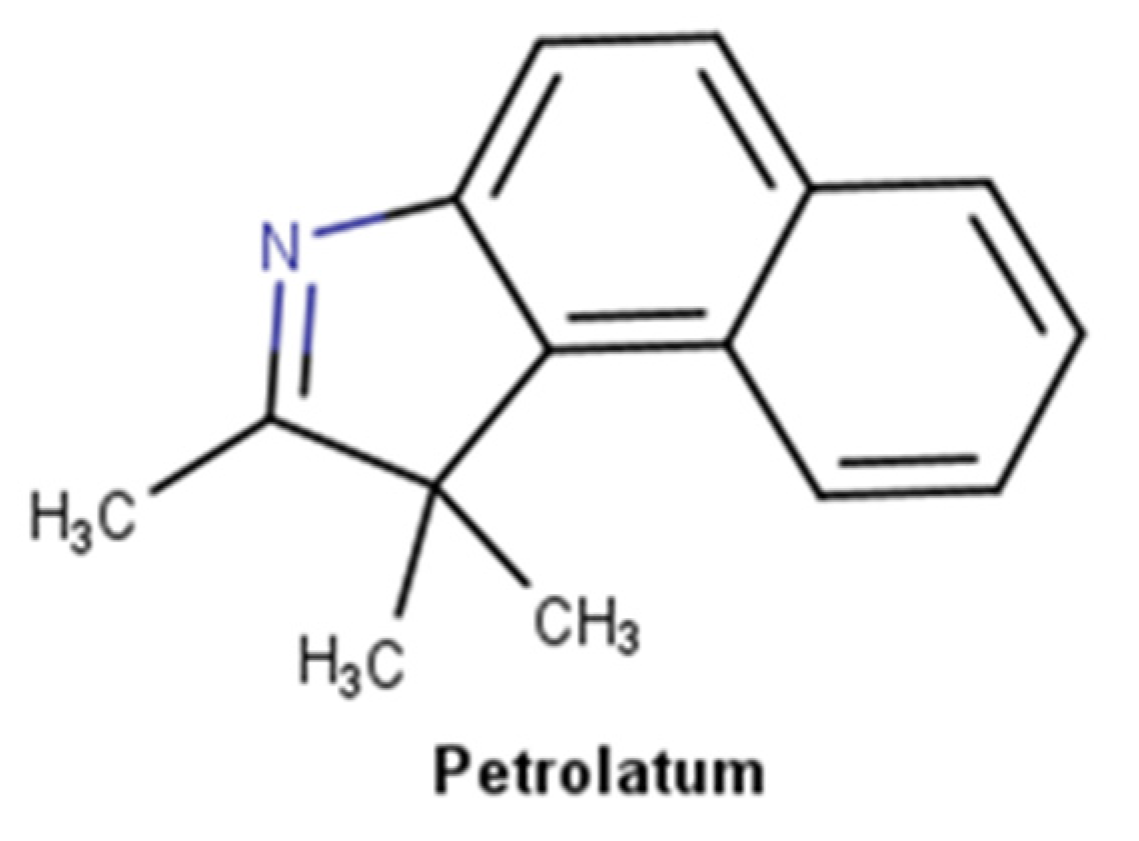
2.3.3. Petrolatum Derivatives
- Vitamins
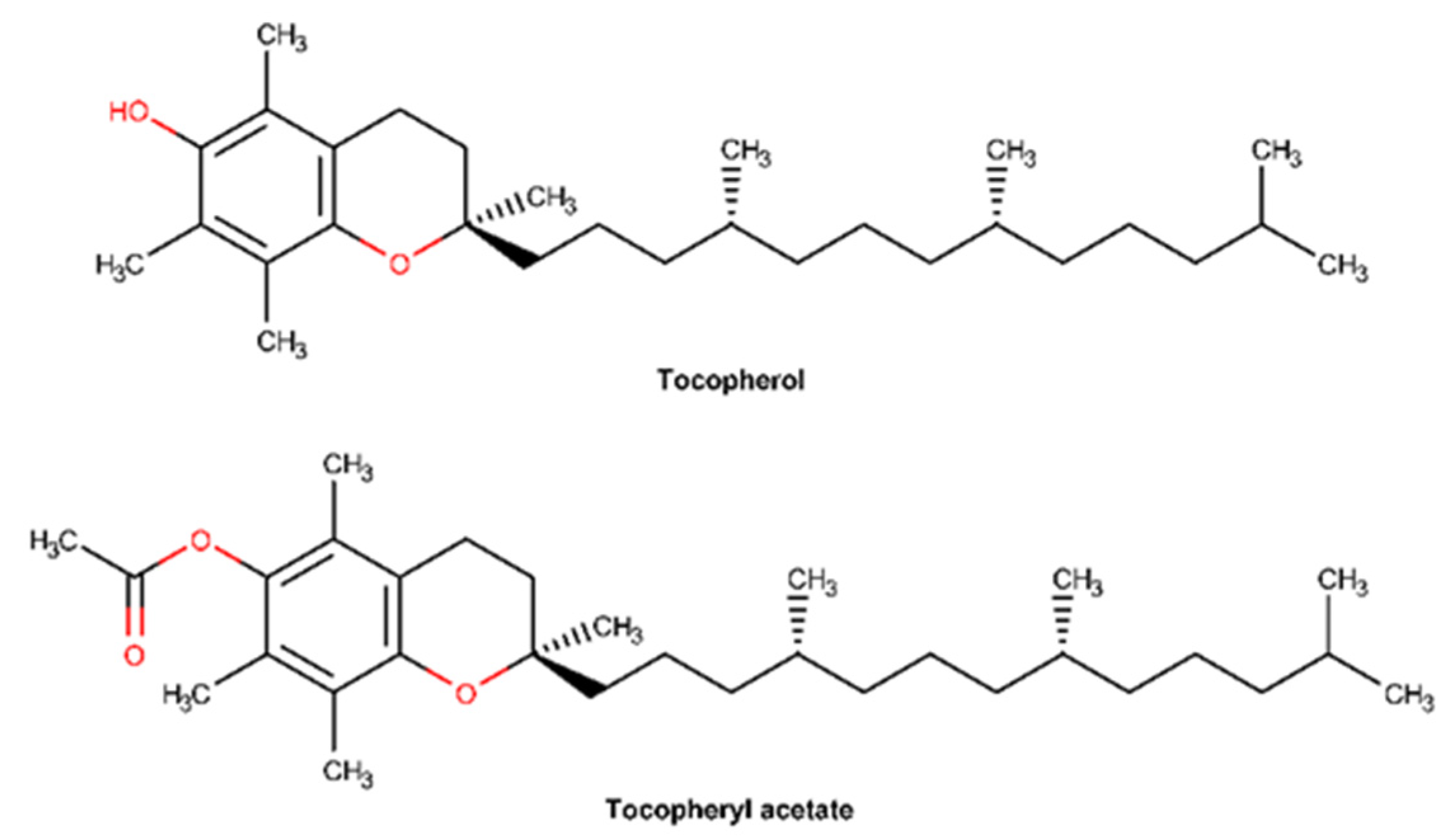
2.3.4. Vitamin E and Derivatives
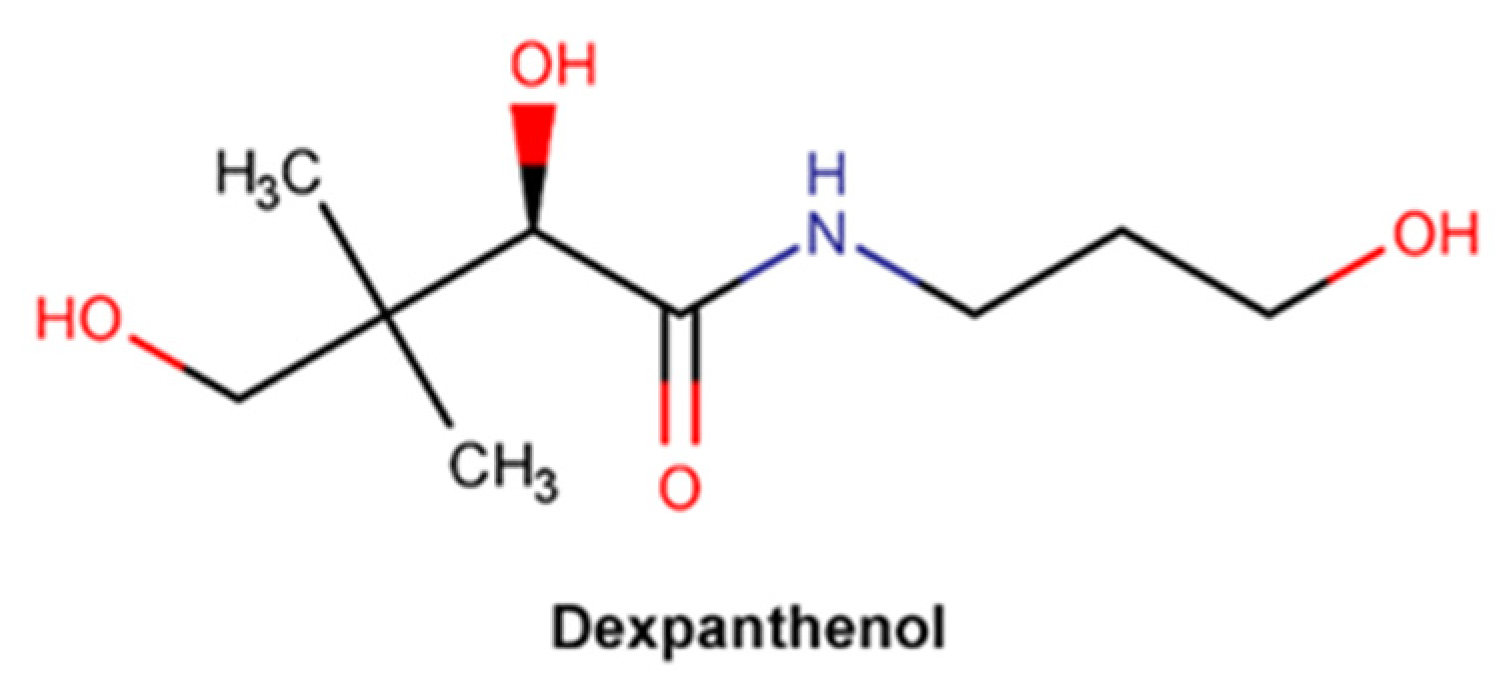
2.3.5. Dexpanthenol
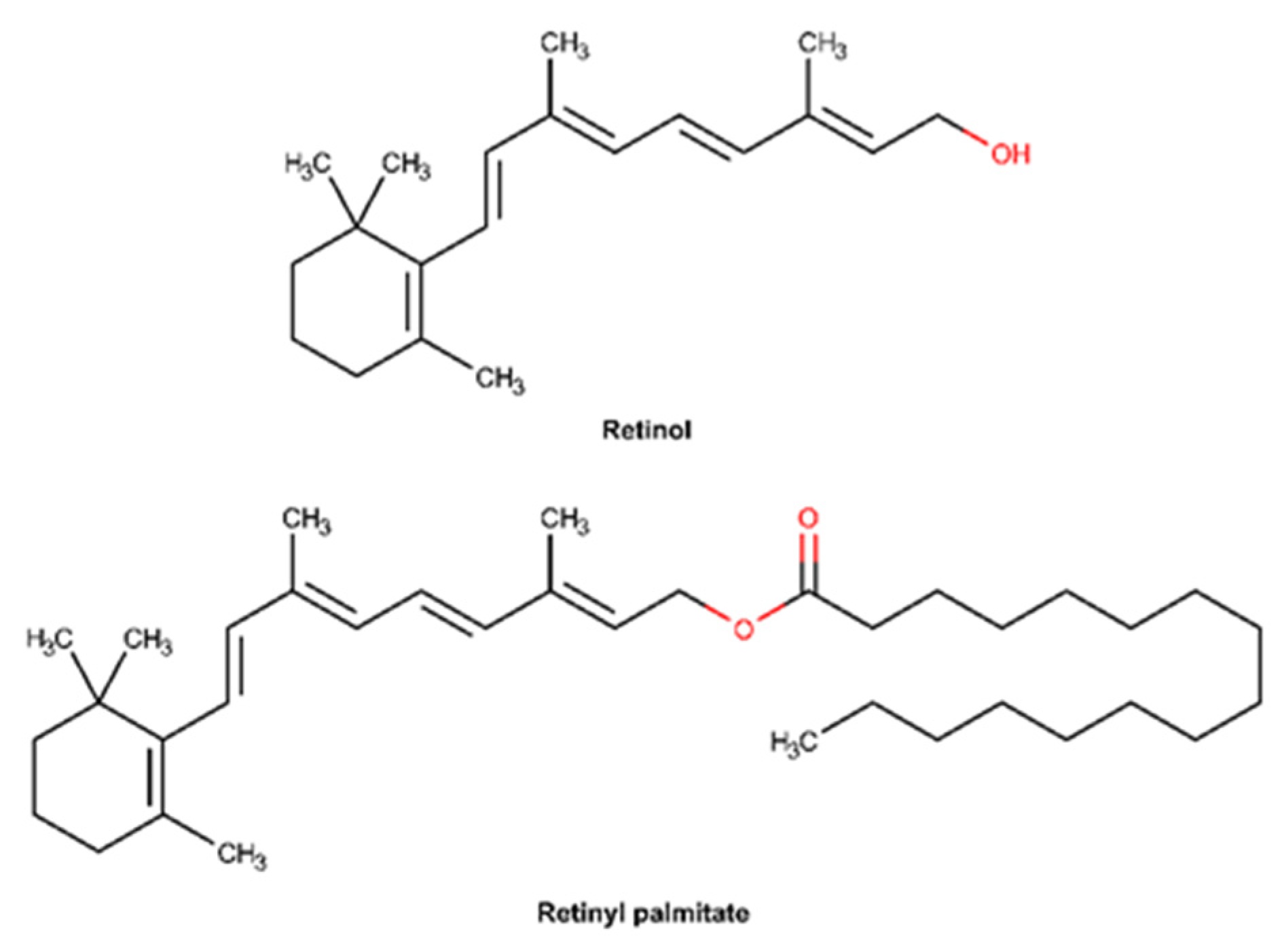
2.3.6. Vitamin A and Derivatives
- Botanic extracts
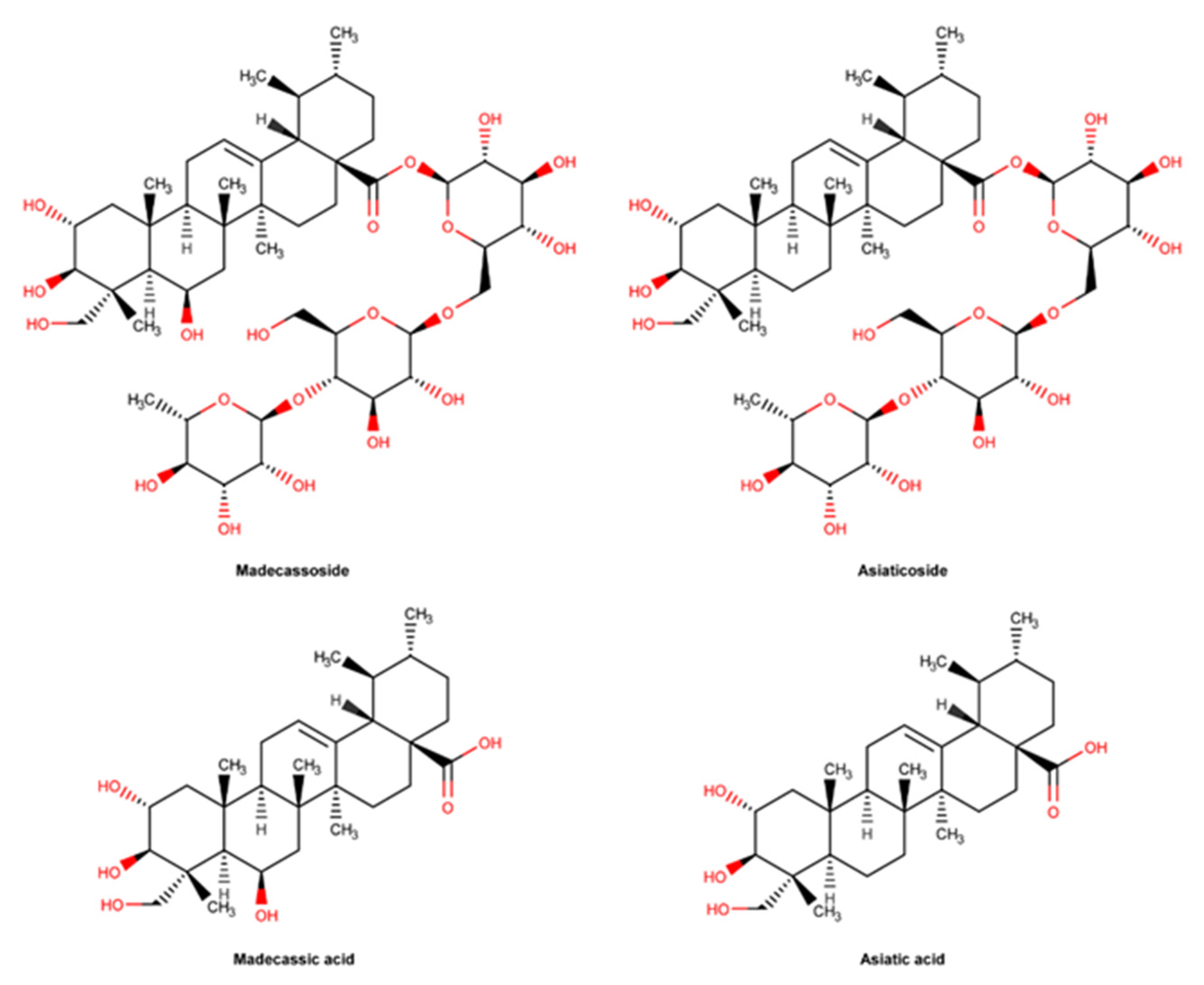
2.3.7. C. asiatica and Pure Compounds
- Marine ingredients
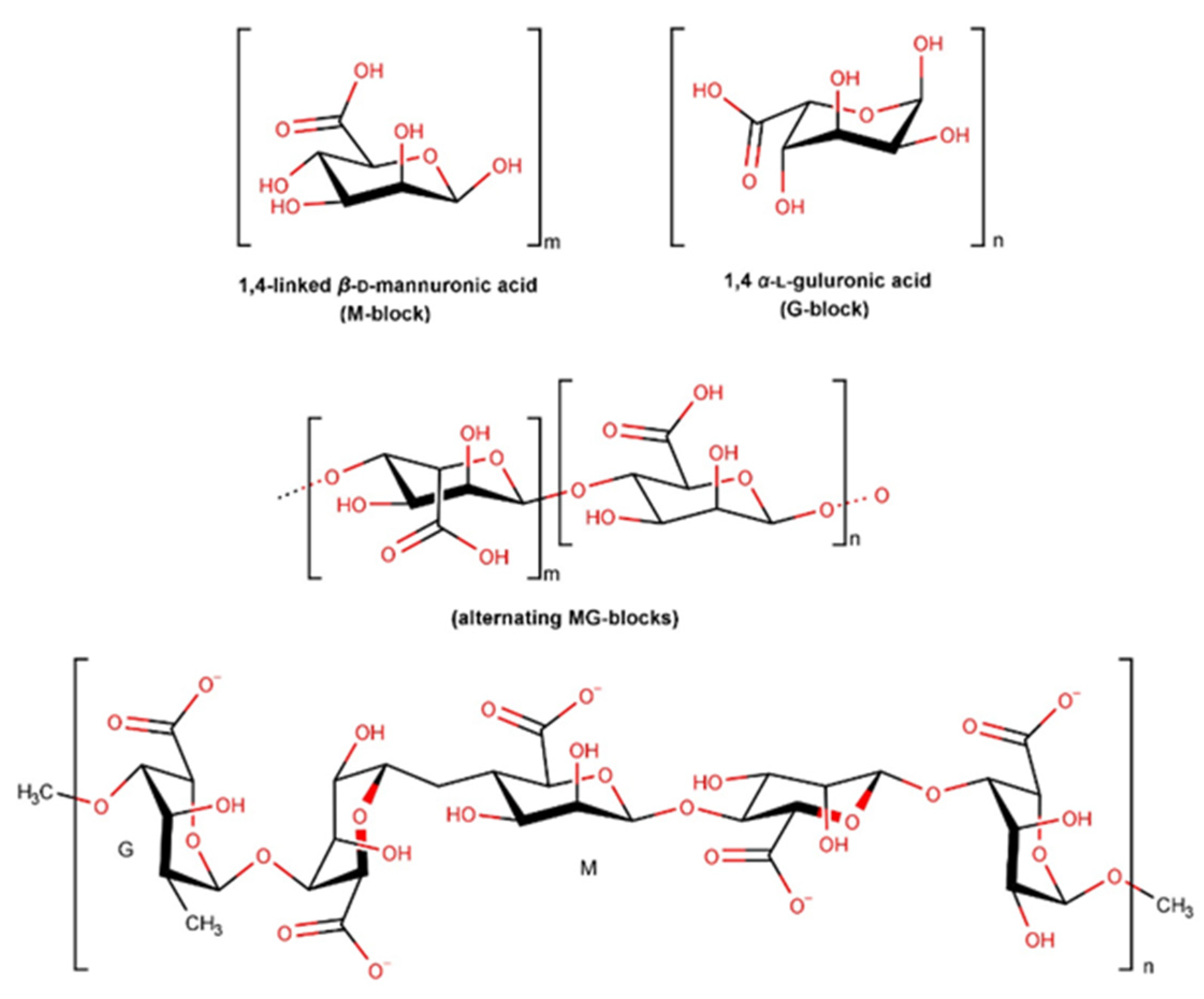
2.3.8. Alginate and Derivatives
3. Materials and Methods
3.1. Data Collection
3.2. Data Analysis
3.2.1. Skin Repair Ingredients or Active Substances Prevalence
3.2.2. Top of Most Used Skin Repair Ingredients or Active Substances
3.2.3. Scientific Evidence for Skin Repair Ingredients or Active Substances
4. Summary of the Mechanisms of Action
5. Conclusions
6. Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Čuříková-Kindlová, B.A.; Vovesná, A.; Nováčková, A.; Zbytovská, J. In Vitro Modeling of Skin Barrier Disruption and its Recovery by Ceramide-Based Formulations. AAPS PharmSciTech. 2021, 23, 21. [Google Scholar] [CrossRef]
- Maeno, K. Direct Quantification of Natural Moisturizing Factors in Stratum Corneum using Direct Analysis in Real Time Mass Spectrometry with Inkjet-Printing Technique. Sci. Rep. 2019, 9, 17789. [Google Scholar] [CrossRef] [PubMed]
- Rosso, J.D.; Zeichner, J.; Alexis, A.; Cohen, D.; Berson, D. Understanding the Epidermal Barrier in Healthy and Compromised Skin: Clinically Relevant Information for the Dermatology Practitioner: Proceedings of an Expert Panel Roundtable Meeting. J Clin Aesthet Dermatol. 2016, 9 (Suppl. S1), S2–S8. [Google Scholar] [PubMed]
- Lee, A.Y. Molecular Mechanism of Epidermal Barrier Dysfunction as Primary Abnormalities. Int. J. Mol. Sci. 2020, 21, 1194. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Gushiken, L.F.S.; Beserra, F.P.; Bastos, J.K.; Jackson, C.J.; Pellizzon, C.H. Cutaneous Wound Healing: An Update from Physiopathology to Current Therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef]
- Wilhelm, K.P.; Wilhelm, D.; Bielfeldt, S. Models of wound healing: An emphasis on clinical studies. Ski. Res. Technol. 2017, 23, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Zhang, C.; Wang, B.; Yin, J.; Yan, S. Progress in Hydrogels for Skin Wound Repair. Macromol. Biosci. 2022, 22, e2100475. [Google Scholar] [CrossRef]
- Baron, J.M.; Glatz, M.; Proksch, E. Optimal Support of Wound Healing: New Insights. Dermatology 2020, 236, 593–600. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid Based Complement Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef] [PubMed]
- Official Journal of the European Union. Regulation (EC) N. º 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products; Official Journal of the European Union: Brussels, Belgium, 2009. [Google Scholar]
- Official Journal of the European Union. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community Code Relating to Medicinal Products for Human Use; Official Journal of the European Union: Brussels, Belgium, 2001. [Google Scholar]
- Ministry of Health. Decree-Law no. 176/2006, of 30 August. Medicinal Products Statute. Diário da República. 2006. [Google Scholar]
- Official Journal of the European Union. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices; Official Journal of the European Union: Brussels, Belgium, 2017. [Google Scholar]
- Ruggeri, M.; Bianchi, E.; Rossi, S.; Vigani, B.; Bonferoni, M.C.; Caramella, C.; Sandri, G.; Ferrari, F. Nanotechnology-Based Medical Devices for the Treatment of Chronic Skin Lesions: From Research to the Clinic. Pharmaceutics 2020, 12, 815. [Google Scholar] [CrossRef]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front Bioeng Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Li, Q.; Wu, J. Application of metal-based biomaterials in wound repair. Eng. Regen. 2021, 2, 137–153. [Google Scholar] [CrossRef]
- Makpol, S.; Jam, F.A.; Khor, S.C.; Ismail, Z.; Mohd Yusof, Y.A.; Ngah, W.Z. Comparative effects of biodynes, tocotrienol-rich fraction, and tocopherol in enhancing collagen synthesis and inhibiting collagen degradation in stress-induced premature senescence model of human diploid fibroblasts. Oxid. Med. Cell Longev. 2013, 2013, 298574. [Google Scholar] [CrossRef] [PubMed]
- Duffy, E.; Guzman, K.D.; Wallace, R.; Murphy, R.; Morrin, A. Non-Invasive Assessment of Skin Barrier Properties: Investigating Emerging Tools for In Vitro and In Vivo Applications. Cosmetics 2017, 4, 44. [Google Scholar] [CrossRef]
- Liu, J.-K. Natural products in cosmetics. Nat. Prod. Bioprospecting 2022, 12, 40. [Google Scholar] [CrossRef]
- Elias, P.M. Optimizing emollient therapy for skin barrier repair in atopic dermatitis. Ann Allergy Asthma Immunol. 2022, 128, 505–511. [Google Scholar] [CrossRef]
- Lee, J.-O.; Hwang, S.-H.; Shen, T.; Kim, J.H.; You, L.; Hu, W.; Cho, J.Y. Enhancement of skin barrier and hydration-related molecules by protopanaxatriol in human keratinocytes. J. Ginseng. Res. 2021, 45, 354–360. [Google Scholar] [CrossRef]
- Deng, X.; Gould, M.; Ali, M.A. A review of current advancements for wound healing: Biomaterial applications and medical devices. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2542–2573. [Google Scholar] [CrossRef]
- Berg, J.M.; Shi, Y. The Galvanization of Biology: A Growing Appreciation for the Roles of Zinc. Science 1996, 271, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Festa, R.A.; Thiele, D.J. Copper: An essential metal in biology. Curr. Biol. 2011, 21, R877–R883. [Google Scholar] [CrossRef] [PubMed]
- Lippard, S.J. The inorganic side of chemical biology. Nat. Chem. Biol. 2006, 2, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.J. Metal-based antimicrobial strategies. Microb. Biotechnol. 2017, 10, 1062–1065. [Google Scholar] [CrossRef]
- Kogan, S.; Sood, A.; Garnick, M.S. Zinc and Wound Healing: A Review of Zinc Physiology and Clinical Applications. Wounds 2017, 29, 102–106. [Google Scholar]
- Lansdown, A.B.G.; Mirastschijski, U.; Stubbs, N.; Scanlon, E.; Agren, M.S. Zinc in wound healing: Theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007, 15, 2–16. [Google Scholar] [CrossRef]
- Agren, M.S. Zinc oxide increases degradation of collagen in necrotic wound tissue. Br. J. Dermatol. 1993, 129, 221. [Google Scholar] [CrossRef]
- Lansdown, A.B.; Sampson, B.; Rowe, A. Sequential changes in trace metal, metallothionein and calmodulin concentrations in healing skin wounds. J. Anat. 1999, 195 Pt 3, 375–386. [Google Scholar] [CrossRef]
- 34. Morellini, N.M.; Giles, N.L.; Rea, S.; Adcroft, K.F.; Falder, S.; King, C.E.; Fear, M.W. Exogenous metallothionein-IIA promotes accelerated healing after a burn wound. Wound Repair Regen. 2008, 16, 682–690. [Google Scholar] [CrossRef]
- Rembe, J.D.; Boehm, J.K.; Fromm-Dornieden, C.; Hauer, N.; Stuermer, E.K. Comprehensive analysis of zinc derivatives pro-proliferative, anti-apoptotic and antimicrobial effect on human fibroblasts and keratinocytes in a simulated, nutrient-deficient environment in vitro. Int. J. Mol. Cell Med. 2020, 9, 165–179. [Google Scholar]
- Lansdown, A.B.G. Influence of zinc oxide in the closure of open skin wounds. Int. J. Cosmet. Sci. 1993, 15, 83–85. [Google Scholar] [CrossRef]
- Ågren, M.S.; Chafranska, L.; Eriksen, J.O.; Forman, J.L.; Bjerrum, M.J.; Schjerling, P.; Larsen, H.F.; Cottarelli, E.; Jorgensen, L.N.; Gjerdrum, L.M. Spatial expression of metallothionein, matrix metalloproteinase-1 and Ki-67 in human epidermal wounds treated with zinc and determined by quantitative immunohistochemistry: A randomised double-blind trial. Eur. J. Cell Biol. 2021, 100, 151147. [Google Scholar] [CrossRef] [PubMed]
- Watson, B.R.; White, N.A.; Taylor, K.A.; Howes, J.-M.; Malcor, J.-D.M.; Bihan, D.; Sage, S.O.; Farndale, R.W.; Pugh, N. Zinc is a transmembrane agonist that induces platelet activation in a tyrosine phosphorylation-dependent manner. Metallomics. 2016, 8, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Sodhar, J.M.; Memon, Z.H.; Siddiqui, S.S.; Soomro, U.A.; Mawani, H.; Abbasi, A. Anti-Inflammatory role of low level laser therapy and zinc oxide in wound repair: A comparative study in rats. Med. Forum. Mon. 2021, 32, 11–14. [Google Scholar]
- Daghdari, S.G.; Ahmadi, M.; Saei, H.D.; Tehrani, A.A. The effect of ZnO nanoparticles on bacterial load of experimental infectious wounds contaminated with Staphylococcus aureus in mice. Nanomed. J. 2017, 4, 232–236. [Google Scholar]
- Gao, Y.; Han, Y.; Cui, M.; Tey, H.L.; Wang, L.; Xu, C. ZnO nanoparticles as an antimicrobial tissue adhesive for skin wound closure. J. Mater. Chem. B 2017, 5, 4535–4541. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, B.J.; Atasoy, N.; Omer, A.K. Evaluate the effects of platelet rich plasma (PRP) and zinc oxide ointment on skin wound healing. Ann Med Surg. 2019, 37, 30–37. [Google Scholar] [CrossRef]
- Arslan, K.; Karahan, O.; Okuş, A.; Unlu, Y.; Eryilmaz, M.A.; Ay, S.; Sevinc, B. Comparison of topical zinc oxide and silver sulfadiazine in burn wounds: An experimental study. Ulus. Travma. Acil. Cerrahi. Derg. 2012, 18, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, K.L.; Mann, F.A.; Kim, D.Y.; Lee, S.; Yoon, H.Y. Evaluation of the Effects of Topical Zinc Gluconate in Wound Healing. Vet. Surg. 2014, 43, 972–982. [Google Scholar] [CrossRef]
- Larsen, H.F.; Ahlström, M.G.; Gjerdrum, L.M.; Mogensen, M.; Ghathian, K.; Calum, H.; Sørensen, A.L.; Forman, J.L.; Vandeven, M.; Holerca, M.N.; et al. Noninvasive measurement of reepithelialization and microvascularity of suction-blister wounds with benchmarking to histology. Wound Repair Regen. 2017, 25, 984–993. [Google Scholar] [CrossRef]
- Ågren, M.S.; Ostenfeld, U.; Kallehave, F.; Gong, Y.; Raffn, K.; Crawford, M.E.; Kiss, K.; Friis-Møller, A.; Gluud, C.; Jorgensen, L.N. A randomized, double-blind, placebo-controlled multicenter trial evaluating topical zinc oxide for acute open wounds following pilonidal disease excision. Wound Repair Regen. 2006, 14, 526–535. [Google Scholar] [CrossRef]
- Bhat, R.M.; Chavda, R.; Ribet, V. To Evaluate the Efficacy and Safety of “RV2427B” cream in Irritant dermatitis care. Indian Derm. Online J. 2013, 4, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Dalisson, B.; Barralet, J. Bioinorganics and Wound Healing. Adv. Healthc. Mater. 2019, 8, 1900764. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Magnesium Sulfate as Used in Cosmetics. Int. J. Toxicol. 2018, 37, 47s–54s. [Google Scholar] [CrossRef]
- Topical Agents Containing Magnesium Sulfate & Wound Healing in the Rat Model. Available online: https://clinicaltrials.gov/ct2/show/NCT04886882 (accessed on 14 January 2023).
- Proksch, E.; Nissen, H.P.; Bremgartner, M.; Urquhart, C. Bathing in a magnesium-rich Dead Sea salt solution improves skin barrier function, enhances skin hydration, and reduces inflammation in atopic dry skin. Int. J. Dermatol. 2005, 44, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Morison, A.E. The treatment of infected war wounds by magnesium sulphate. Br. Med. J. 1918, 1, 342–344. [Google Scholar] [CrossRef]
- Andreasen, A.T. Magnesium Sulphate Powder in the Treatment of Wounds and Ulcers. Ind. Med. Gaz. 1942, 77, 129–131. [Google Scholar]
- Linder, M.C.; Wooten, L.; Cerveza, P.; Cotton, S.; Shulze, R.; Lomeli, N. Copper transport. Am. J. Clin. Nutr. 1998, 67, 71S–965S. [Google Scholar] [CrossRef]
- Ellingsen, D.G.; Møller, L.B.; Aaseth, J. Chapter 35—Copper. In Handbook on the Toxicology of Metal, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 765–786. [Google Scholar]
- Kornblatt, A.P.; Nicoletti, V.G.; Travaglia, A. The neglected role of copper ions in wound healing. J. Inorg. Biochem. 2016, 161, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ozumi, K.; Sudhahar, V.; Kim, H.W.; Chen, G.F.; Kohno, T.; Finney, L.; Vogt, S.; McKinney, R.D.; Ushio-Fukai, M.; Fukai, T. Role of copper transport protein antioxidant 1 in angiotensin II-induced hypertension: A key regulator of extracellular superoxide dismutase. Hypertension 2012, 60, 476–486. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Finley, J.C.; Ali, S.S.; Patel, H.H.; Howell, S.B. Copper influx transporter 1 is required for FGF, PDGF and EGF-induced MAPK signaling. Biochem. Pharmacol. 2012, 84, 1007–1013. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Venojarvi, M.; Trikha, P.; Ellison, E.C.; Hunt, T.K.; Roy, S. Copper-induced vascular endothelial growth factor expression and wound healing. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1821–H1827. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Samuel, P.; Parakandi, H.; Gopal, S.; Siomyk, H.; Ministro, A.; Thompson, T.; Borkow, G. Beneficial regulation of fibrillar collagens, heat shock protein-47, elastin fiber components, transforming growth factor-β1, vascular endothelial growth factor and oxidative stress effects by copper in dermal fibroblasts. Connect Tissue Res. 2012, 53, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Bordeleau, L.J.; Barralet, J.; Doillon, C.J. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials 2010, 31, 824–831. [Google Scholar] [CrossRef]
- Ogen-Shtern, N.; Chumin, K.; Cohen, G.; Borkow, G. Increased pro-collagen 1, elastin, and TGF-β1 expression by copper ions in an ex-vivo human skin model. J. Cosmet. Dermatol. 2020, 19, 1522–1527. [Google Scholar] [CrossRef]
- Kothapalli, C.R.; Ramamurthi, A. Copper nanoparticle cues for biomimetic cellular assembly of crosslinked elastin fibers. Acta Biomater. 2009, 5, 541–553. [Google Scholar] [CrossRef]
- Tenaud, I.; Sainte-Marie, I.; Jumbou, O.; Litoux, P.; Dréno, B. In vitro modulation of keratinocyte wound healing integrins by zinc, copper and manganese. Br. J. Dermatol. 1999, 140, 26–34. [Google Scholar] [CrossRef]
- Philips, N.; Hwang, H.; Chauhan, S.; Leonardi, D.; Gonzalez, S. Stimulation of Cell Proliferation and Expression of Matrixmetalloproteinase-1 and Interluekin-8 Genes in Dermal Fibroblasts by Copper. Connect. Tissue Res. 2010, 51, 224–229. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Copper as a biocidal tool. Curr. Med. Chem. 2005, 12, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Barnabas, J.; Miraftab, M.; Qinand, Y.; Changjun, Z. Evaluating the antibacterial properties of chitosan fibres embedded with copper ions for wound dressing applications. J. Ind. Text. 2014, 44, 232–244. [Google Scholar] [CrossRef]
- Tenaud, I.; Leroy, S.; Chebassier, N.; Dreno, B. Zinc, copper and manganese enhanced keratinocyte migration through a functional modulation of keratinocyte integrins. Exp. Dermatol. 2000, 9, 407–416. [Google Scholar] [CrossRef]
- Borkow, G. Using Copper to Improve the Well-Being of the Skin. Curr. Chem. Biol. 2014, 8, 89–102. [Google Scholar] [CrossRef]
- Mesa, M.; Becerra, N.Y. Silica/Protein and Silica/Polysaccharide Interactions and Their Contributions to the Functional Properties of Derived Hybrid Wound Dressing Hydrogels. Int. J. Biomater. 2021, 2021, 6857204. [Google Scholar] [CrossRef] [PubMed]
- Hacker, M.C.; Mikos, A.G. Chapter 33—Synthetic Polymers. In Principles of Regenerative Medicine, 2nd ed.; Atala, A., Lanza, R., Thomson, J.A., Nerem, R., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 587–622. [Google Scholar]
- Marchioretto, S., Plotzke, K., Eds.; The Power of Silicones in Cosmetic Applications: The Science behind the Performance. SOFW Journal 12/21 2021, 147, 1–7. [Google Scholar]
- Goussard, V.; Aubry, J.-M.; Nardello-Rataj, V. Bio-based alternatives to volatile silicones: Relationships between chemical structure, physicochemical properties and functional performances. Adv. Colloid Interface Sci. 2022, 304, 102679. [Google Scholar] [CrossRef]
- Mojsiewicz-Pieńkowska, K.; Jamrógiewicz, M.; Szymkowska, K.; Krenczkowska, D. Direct Human Contact with Siloxanes (Silicones)—Safety or Risk Part 1. Characteristics of Siloxanes (Silicones). Front. Pharmacol. 2016, 7, 132. [Google Scholar] [CrossRef]
- Draelos, Z.D. New treatments for restoring impaired epidermal barrier permeability: Skin barrier repair creams. Clin. Dermatology. 2012, 30, 345–348. [Google Scholar] [CrossRef]
- Patil, G.; Torris, A.; Suresha, P.R.; Jadhav, S.; Badiger, M.V.; Ghormade, V. Design and synthesis of a new topical agent for halting blood loss rapidly: A multimodal chitosan-gelatin xerogel composite loaded with silica nanoparticles and calcium. Colloids Surf. B Biointerfaces 2021, 198, 111454. [Google Scholar] [CrossRef]
- Sun, X.; Fang, Y.; Tang, Z.; Wang, Z.; Liu, X.; Liu, H. Mesoporous silica nanoparticles carried on chitosan microspheres for traumatic bleeding control. Int. J. Biol. Macromol. 2019, 127, 311–319. [Google Scholar] [CrossRef]
- Zirak Hassan Kiadeh, S.; Ghaee, A.; Mashak, A.; Mohammadnejad, J. Preparation of chitosan–silica/PCL composite membrane as wound dressing with enhanced cell attachment. Polym. Adv. Technol. 2017, 28, 1396–1408. [Google Scholar] [CrossRef]
- Oh, J.-S.; Lee, E.-J. Engineered dressing of hybrid chitosan-silica for effective delivery of keratin growth factor and acceleration of wound healing. Mater. Sci. Eng. C 2019, 103, 109815. [Google Scholar] [CrossRef]
- Chan, K.Y.; Lau, C.L.; Adeeb, S.M.; Somasundaram, S.; Nasir-Zahari, M. A randomized, placebo-controlled, double-blind, prospective clinical trial of silicone gel in prevention of hypertrophic scar development in median sternotomy wound. Plast Reconstr Surg. 2005, 116, 1013–1020, discussion 21–22. [Google Scholar] [CrossRef]
- Atiyeh, B.S. Nonsurgical management of hypertrophic scars: Evidence-based therapies, standard practices, and emerging methods. Aesthetic Plast Surg. 2007, 31, 468–492, discussion 93–94. [Google Scholar] [CrossRef]
- Gold, M.H. A controlled clinical trial of topical silicone gel sheeting in the treatment of hypertrophic scars and keloids. J. Am. Acad. Dermatology. 1994, 30, 506–507. [Google Scholar] [CrossRef]
- Musgrave, M.A.; Umraw, N.; Fish, J.S.; Gomez, M.; Cartotto, R.C. The effect of silicone gel sheets on perfusion of hypertrophic burn scars. J. Burn. Care Rehabil. 2002, 23, 208–214. [Google Scholar] [CrossRef]
- Carney, S.; Cason, C.; Gowar, J.; Stevenson, J.; McNee, J.; Groves, A.; Thomas, S.; Hart, N.; Auclair, P. Cica-Care gel sheeting in the management of hypertrophic scarring. Burns 1994, 20, 163–167. [Google Scholar] [CrossRef]
- Khamthara, J.; Kumtornrut, C.; Pongpairoj, K.; Asawanonda, P. Silicone gel enhances the efficacy of Er:YAG laser treatment for atrophic acne scars: A randomized, split-face, evaluator-blinded, placebo-controlled, comparative trial. J. Cosmet. Laser Therapy. 2018, 20, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Yeh, L.C.; Gonzalez, N.; Goldberg, D.J. Comparison of a novel wound dressing vs current clinical practice after laser resurfacing. J Cosmet Dermatol. 2019, 18, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, V.; Sestini, S.; Mannone, F.; Papi, F.; Alfaioli, B.; Gori, A.; Lotti, T. The use of silicone gel in the treatment of fresh surgical scars: A randomized study. Clin. Exp. Dermatol. 2009, 34, 688–693. [Google Scholar] [CrossRef]
- Quinn, K.J.; Evans, J.H.; Courtney, J.M.; Gaylor, J.D.S.; Reid, W.H. Non-pressure treatment of hypertrophic scars. Burns 1985, 12, 102–108. [Google Scholar] [CrossRef]
- Chang, C.-C.; Kuo, Y.-F.; Chiu, H.-C.; Lee, J.-L.; Wong, T.-W.; Jee, S.-H. Hydration, Not Silicone, Modulates the Effects of Keratinocytes on Fibroblasts. J. Surg. Res. 1995, 59, 705–711. [Google Scholar] [CrossRef]
- Kuhn, M.A.; Moffit, M.R.; Smith, P.D.; Lyle, W.G.; Ko, F.; Meltzer, D.D.; Robson, M.C. Silicone sheeting decreases fibroblast activity and downregulates TGFbeta2 in hypertrophic scar model. Int. J. Surg. Investig. 2001, 2, 467–474. [Google Scholar]
- Bikash, C.; Sarkar, R. Topical management of acne scars: The uncharted terrain. J. Cosmet. Dermatol. 2023, 22, 1196–1911. [Google Scholar] [CrossRef]
- Hanasono, M.M.; Lum, J.; Carroll, L.A.; Mikulec, A.A.; Koch, R.J. The effect of silicone gel on basic fibroblast growth factor levels in fibroblast cell culture. Arch. Facial Plast. Surg. 2004, 6, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, C.H.; Martin, L.; Faria, D.T.; Saed, G.M.; Fivenson, D.P. Cytokine mRNA changes during the treatment of hypertrophic scars with silicone and nonsilicone gel dressings. Derm. Surg. 1996, 22, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Jakasa, I.; Kezic, S.; Boogaard, P.J. Dermal uptake of petroleum substances. Toxicol. Lett. 2015, 235, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Kaur, T.; Malhotra, S.K.; Gambhir, M.L. Moisturizers: The Slippery Road. Indian J. Dermatol. 2016, 61, 279–287. [Google Scholar] [CrossRef]
- Raymond; Rowe, C.; Sheskey, P.J.; Weller, P.J. (Eds.) Handbook of Pharmaceutical Excipients, 4th ed.; American Pharmaceutical Association: Washington, DC, USA, 2003. [Google Scholar]
- Lodén, M. The increase in skin hydration after application of emollients with different amounts of lipids. Acta Derm. Venereol. 1992, 72, 327–330. [Google Scholar]
- Lodén, M.; Bárány, E. Skin-identical lipids versus petrolatum in the treatment of tape-stripped and detergent-perturbed human skin. Acta Derm Venereol. 2000, 80, 412–415. [Google Scholar]
- Ghadially, R.; Halkier-Sorensen, L.; Elias, P.M. Effects of petrolatum on stratum corneum structure and function. J. Am. Acad. Dermatol. 1992, 26, 387–396. [Google Scholar] [CrossRef]
- Draelos, Z.D. The science behind skin care: Moisturizers. J. Cosmet. Dermatol. 2018, 17, 138–144. [Google Scholar] [CrossRef]
- Food and Drug Administration. Skin protectant drug products for over-the-counter human use; Final Monograph. Fed. Regist. 2003, 68, 33362–33381. [Google Scholar]
- Campbell, R.M.; Perlis, C.S.; Fisher, E.; Gloster, H.M. Gentamicin ointment versus petrolatum for management of auricular wounds. Dermatol. Surg. 2005, 31, 664–669. [Google Scholar] [CrossRef]
- Tóth, T.; Broström, H.; Båverud, V.; Emanuelson, U.; Bagge, E.; Karlsson, T.; Bergvall, K. Evaluation of LHP® (1% hydrogen peroxide) cream versus petrolatum and untreated controls in open wounds in healthy horses: A randomized, blinded control study. Acta Vet Scand. 2011, 53, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sarnoff, D.S. A comparison of wound healing between a skin protectant ointment and a medical device topical emulsion after laser resurfacing of the perioral area. J. Am. Acad. Dermatol. 2011, 64 (Suppl. S1), S36–S43. [Google Scholar] [CrossRef]
- Alonso, C.; Larburu, I.; Bon, E.; González, M.M.; Iglesias, M.T.; Urreta, I.; Emparanza, J.I. Efficacy of petrolatum jelly for the prevention of diaper rash: A randomized clinical trial. J. Spec. Pediatr. Nurs. 2013, 18, 123–132. [Google Scholar] [CrossRef]
- Smack, D.P.; Harrington, A.C.; Dunn, C.; Howard, R.S.; Szkutnik, A.J.; Krivda, S.J.; Caldwell, J.B.; James, W.D. Infection and Allergy Incidence in Ambulatory Surgery Patients Using White Petrolatum vs Bacitracin Ointment: A Randomized Controlled Trial. JAMA 1996, 276, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D.; Rizer, R.L.; Trookman, N.S. A comparison of postprocedural wound care treatments: Do antibiotic-based ointments improve outcomes? J. Am. Acad. Dermatol. 2011, 64, S23–S29. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, D.Y.; Yoon, S.Y.; Park, H.S.; Yoon, H.S.; Cho, S. Retrospective Clinical Trial of Fusidic Acid versus Petrolatum in the Postprocedure Care of Clean Dermatologic Procedures. Ann. Dermatol. 2015, 27, 15–20. [Google Scholar] [CrossRef]
- Trookman, N.S.; Rizer, R.L.; Weber, T. Treatment of minor wounds from dermatologic procedures: A comparison of three topical wound care ointments using a laser wound model. J. Am. Acad. Dermatol. 2011, 64 (Suppl. S1), S8–S15. [Google Scholar] [CrossRef]
- Saco, M.; Howe, N.; Nathoo, R.; Cherpelis, B. Topical antibiotic prophylaxis for prevention of surgical wound infections from dermatologic procedures: A systematic review and meta-analysis. J. Dermatol. Treat. 2015, 26, 151–158. [Google Scholar] [CrossRef]
- Taylor, S.C.; Averyhart, A.N.; Heath, C.R. Postprocedural wound-healing efficacy following removal of dermatosis papulosa nigra lesions in an African American population: A comparison of a skin protectant ointment and a topical antibiotic. J. Am. Acad. Dermatol. 2011, 64 (Suppl. S1), S30–S35. [Google Scholar] [CrossRef] [PubMed]
- Bolognia, J.L.; Schaffer, J.V.; Cerroni, L. Dermatology, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2018; 2880p. [Google Scholar]
- Malave, G.S.; James, W.D. Petrolatum Is Effective as a Moisturizer, But There Are More Uses for It. Cutis 2022, 110, 175–176. [Google Scholar] [CrossRef]
- Czarnowicki, T.; Malajian, D.; Khattri, S.; da Rosa, J.C.; Dutt, R.; Finney, R.; Guttman-Yassky, E. Petrolatum: Barrier repair and antimicrobial responses underlying this “inert”moisturizer. J. Allergy Clin. Immunol. 2016, 137, 1091–1102.e7. [Google Scholar] [CrossRef]
- Thiele, J.J.; Hsieh, S.N.; Ekanayake-Mudiyanselage, S. Vitamin E: Critical review of its current use in cosmetic and clinical dermatology. Dermatol. Surg. 2005, 31 Pt 2, 805–813, Discussion 13. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.; Mota, S.; Torres, A.; Cruz, M.T.; Sousa, E.; Almeida, I.F.; Cidade, H. Antioxidants in Sunscreens: Which and What For? Antioxidants 2023, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Podda, M.; Rallis, M.; Thiele, J.J.; Traber, M.G.; Packer, L. Efficacy of topically applied tocopherols and tocotrienols in protection of murine skin from oxidative damage induced by UV-irradiation. Free Radic. Biol. Med. 1997, 22, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.S.; Spencer, J. The effects of topical vitamin E on the cosmetic appearance of scars. Derm. Surg. 1999, 25, 311–315. [Google Scholar] [CrossRef]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Ghazali, N.I.; Mohd Rais, R.Z.; Makpol, S.; Chin, K.Y.; Yap, W.N.; Goon, J.A. Effects of tocotrienol on aging skin: A systematic review. Front. Pharmacol. 2022, 13, 1006198. [Google Scholar] [CrossRef] [PubMed]
- Nachbar, F.; Korting, H.C. The role of vitamin E in normal and damaged skin. J. Mol. Med. 1995, 73, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Tanaydin, V.; Conings, J.; Malyar, M.; van der Hulst, R.; van der Lei, B. The Role of Topical Vitamin E in Scar Management: A Systematic Review. Aesthet. Surg. J. 2016, 36, 959–965. [Google Scholar] [CrossRef]
- Pinto, C.; Martins, T.; Martinez, R.; Freire, T.; Velasco, M.; Baby, A. Vitamin E in Human Skin: Functionality and Topical Products; IntechOpen: London, UK, 2021. [Google Scholar]
- Zampieri, N.; Zuin, V.; Burro, R.; Ottolenghi, A.; Camoglio, F.S. A prospective study in children: Pre- and post-surgery use of vitamin E in surgical incisions. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Cuba, L.; Braga Filho, A.; Cherubini, K.; Salum, F.G.; Figueiredo, M.A. Topical application of Aloe vera and vitamin E on induced ulcers on the tongue of rats subjected to radiation: Clinical and histological evaluation. Support Care Cancer 2016, 24, 2557–2564. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, E.; Watanabe, T.; Takata, J.; Yamazaki, A.; Karube, Y.; Kobayashi, S. Topical Application of a Novel, Hydrophilic γ-Tocopherol Derivative Reduces Photo-Inflammation in Mice Skin. J. Investig. Dermatol. 2006, 126, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Stanizzi, A.; Bottoni, M.; Torresetti, M.; Campanati, A.; Di Benedetto, G. Topical use of α-tocopherol acetate in delayed wound healing. Int. Wound J. 2015, 12, 746–747. [Google Scholar] [CrossRef] [PubMed]
- Testa, D.; Marcuccio, G.; Panin, G.; Bianco, A.; Tafuri, D.; Thyrion, F.Z.; Motta, G. Nasal mucosa healing after endoscopic sinus surgery in chronic rhinosinusitis of elderly patients: Role of topic alpha-tocopherol acetate. Aging Clin. Exp. Res. 2017, 29 Suppl. S1, 191–195. [Google Scholar] [CrossRef]
- Gehring, W.; Fluhr, J.; Gloor, M. Influence of vitamin E acetate on stratum corneum hydration. Arzneimittelforschung 1998, 48, 772–775. [Google Scholar]
- Hobson, R. Vitamin E and wound healing: An evidence-based review. Int. Wound J. 2016, 13, 331–335. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Maroni, P.; Ozer, N.; Zingg, J.M.; Azzi, A. Age-dependent increase of collagenase expression can be reduced by alpha-tocopherol via protein kinase C inhibition. Free Radic Biol. Med. 1999, 27, 729–737. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 131204, Dexpanthenol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Dexpanthenol (accessed on 5 February 2023).
- Cho, Y.S.; Kim, H.O.; Woo, S.M.; Lee, D.H. Use of Dexpanthenol for Atopic Dermatitis-Benefits and Recommendations Based on Current Evidence. J Clin Med. 2022, 11, 3943. [Google Scholar] [CrossRef]
- Proksch, E.; de Bony, R.; Trapp, S.; Boudon, S. Topical use of dexpanthenol: A 70th anniversary article. J. Dermatol. Treat. 2017, 28, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Gehring, W.; Gloor, M. Effect of topically applied dexpanthenol on epidermal barrier function and stratum corneum hydration. Results A Hum. Vivo Study. Arzneim. 2000, 50, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.; Jackowski, S. Biosynthesis of Pantothenic Acid and Coenzyme A. EcoSal Plus 2007. [Google Scholar] [CrossRef]
- Stettler, H.; de Salvo, R.; Olsavszky, R.; Nanu, E.A.; Dumitru, V.; Trapp, S. Performance and Tolerability of a New Topical Dexpanthenol-Containing Emollient Line in Subjects with Dry Skin: Results from Three Randomized Studies. Cosmetics 2021, 8, 18. [Google Scholar] [CrossRef]
- Schmid, D.A.; Domingues, M.P.; Nanu, A.; Kluger, N.; de Salvo, R.; Trapp, S. Exploratory evaluation of tolerability, performance, and cosmetic acceptance of dexpanthenol-containing dermo-cosmetic wash and sun-care products for tattoo aftercare. Health Sci. Rep. 2022, 5, e635. [Google Scholar] [CrossRef] [PubMed]
- Addor, F.A.S.A.; de Souza, M.C.; Trapp, S.; Peltier, E.; Canosa, J.M. Efficacy and Safety of Topical Dexpanthenol-Containing Spray and Cream in the Recovery of the Skin Integrity Compared with Petroleum Jelly after Dermatologic Aesthetic Procedures. Cosmetics 2021, 8, 87. [Google Scholar] [CrossRef]
- Stettler, H.; Kurka, P.; Wagner, C.; Sznurkowska, K.; Czernicka, O.; Böhling, A.; Bielfeldt, S.; Wilhelm, K.-P.; Lenz, H. A new topical panthenol-containing emollient: Skin-moisturizing effect following single and prolonged usage in healthy adults, and tolerability in healthy infants. J. Dermatol. Treat. 2017, 28, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Stettler, H.; Kurka, P.; Lunau, N.; Manger, C.; Böhling, A.; Bielfeldt, S.; Wilhelm, K.-P.; Dähnhardt-Pfeiffer, S.; Dähnhardt, D.; Brill, F.H.H.; et al. A new topical panthenol-containing emollient: Results from two randomized controlled studies assessing its skin moisturization and barrier restoration potential, and the effect on skin microflora. J. Dermatol. Treat. 2017, 28, 173–180. [Google Scholar] [CrossRef]
- Björklund, S.; Pham, Q.D.; Jensen, L.B.; Knudsen, N.Ø.; Nielsen, L.D.; Ekelund, K.; Ruzgas, T.; Engblom, J.; Sparr, E. The effects of polar excipients transcutol and dexpanthenol on molecular mobility, permeability, and electrical impedance of the skin barrier. J. Colloid. Interface Sci. 2016, 479, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Nissen, H.P. Dexpanthenol enhances skin barrier repair and reduces inflammation after sodium lauryl sulphate-induced irritation. J Dermatol. Treat. 2002, 13, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Biro, K.; Thaçi, D.; Ochsendorf, F.R.; Kaufmann, R.; Boehncke, W.H. Efficacy of dexpanthenol in skin protection against irritation: A double-blind, placebo-controlled study. Contact Dermat. 2003, 49, 80–84. [Google Scholar] [CrossRef]
- Heise, R.; Skazik, C.; Marquardt, Y.; Czaja, K.; Sebastian, K.; Kurschat, P.; Gan, L.; Denecke, B.; Ekanayake-Bohlig, S.; Wilhelm, K.-P.; et al. Dexpanthenol modulates gene expression in skin wound healing in vivo. Ski. Pharm. Physiol. 2012, 25, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Bjerke, D.L.; Li, R.; Price, J.M.; Dobson, R.L.M.; Rodrigues, M.; Tey, C.; Vires, L.; Adams, R.L.; Sherrill, J.D.; Styczynski, P.B.; et al. The vitamin A ester retinyl propionate has a unique metabolic profile and higher retinoid-related bioactivity over retinol and retinyl palmitate in human skin models. Exp. Dermatol. 2021, 30, 226–236. [Google Scholar] [CrossRef]
- Khalil, S.; Bardawil, T.; Stephan, C.; Darwiche, N.; Abbas, O.; Kibbi, A.G.; Nemer, G.; Kurban, M. Retinoids: A journey from the molecular structures and mechanisms of action to clinical uses in dermatology and adverse effects. J. Dermatol. Treat. 2017, 28, 684–696. [Google Scholar] [CrossRef]
- Zasada, M.; Budzisz, E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postep. Derm. Alergol. 2019, 36, 392–397. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Prado, A.H.; Bernegossi, J.; Sato, C.S.; Lourenço Brunetti, I.; Scarpa, M.V.; Leonardi, G.R.; Friberg, S.E.; Chorilli, M. Topical application of retinyl palmitate-loaded nanotechnology-based drug delivery systems for the treatment of skin aging. Biomed. Res. Int. 2014, 2014, 632570. [Google Scholar] [CrossRef]
- Counts, D.; Skreko, F.; McBee, J.; Wich, A.G. The effect of retinyl palmitate on skin composition and morphometry. J. Soc. Cosmet. Chem. 1988, 39, 235–240. [Google Scholar]
- Polcz, M.E.; Barbul, A. The Role of Vitamin A in Wound Healing. Nutr. Clin. Pract. 2019, 34, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Cui, Y.; Fisher, G.J.; Wang, X.; Chen, Y.; Schneider, L.M.; Majmudar, G. A comparative study of the effects of retinol and retinoic acid on histological, molecular, and clinical properties of human skin. J. Cosmet. Dermatol. 2016, 15, 49–57. [Google Scholar] [CrossRef]
- Kafi, R.; Kwak, H.S.R.; Schumacher, W.E.; Cho, S.; Hanft, V.N.; Hamilton, T.A.; King, A.L.; Neal, J.D.; Varani, J.; Fisher, G.J.; et al. Improvement of Naturally Aged Skin with Vitamin A (Retinol). Arch. Dermatol. 2007, 143, 606–612. [Google Scholar] [CrossRef]
- Piérard-Franchimont, C.; Castelli, D.; Cromphaut, I.V.; Bertin, C.; Ries, G.; Cauwenbergh, G.; Piérard, G.E. Tensile properties and contours of aging facial skin. A controlled double-blind comparative study of the effects of retinol, melibiose-lactose and their association. Ski. Res Technol. 1998, 4, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Warner, R.L.; Gharaee-Kermani, M.; Phan, S.H.; Kang, S.; Chung, J.; Wang, Z.; Datta, S.C.; Fisher, G.J.; Voorhees, J.J. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J. Invest Dermatol. 2000, 114, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Duell, E.A.; Derguini, F.; Kang, S.; Elder, J.T.; Voorhees, J.J. Extraction of human epidermis treated with retinol yields retro-retinoids in addition to free retinol and retinyl esters. J. Invest Dermatol. 1996, 107, 178–182. [Google Scholar] [CrossRef]
- Kang, S.; Duell, E.A.; Fisher, G.J.; Datta, S.C.; Wang, Z.Q.; Reddy, A.P.; Tavakkol, A.; Jong, Y.Y.; Griffiths, C.E.; Elder, J.T.; et al. Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid binding proteins characteristic of retinoic acid but without measurable retinoic acid levels or irritation. J. Invest. Dermatol. 1995, 105, 549–556. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Vienne, M.P.; Lauze, C.; Dupuy, P.; Gehring, W.; Gloor, M. Tolerance profile of retinol, retinaldehyde and retinoic acid under maximized and long-term clinical conditions. Dermatology 1999, 199 (Suppl. S1), 57–60. [Google Scholar] [CrossRef]
- Neghab, H.K.; Soheilifar, M.H.; Djavid, G.E. An in vitro model for investigation of vitamin A effects on wound healing. Int. J. Vitam. Nutr. Res. 2021, 91, 385–390. [Google Scholar] [CrossRef]
- Demetriou, A.A.; Levenson, S.M.; Rettura, G.; Seifter, E. Vitamin A and retinoic acid: Induced fibroblast differentiation in vitro. Surgery 1985, 98, 931–934. [Google Scholar]
- Elson, M.L. The role of retinoids in wound healing. J. Am. Acad. Dermatol. 1998, 39, S79–S81. [Google Scholar] [CrossRef]
- Roche, F.C.; Harris-Tryon, T.A. Illuminating the Role of Vitamin A in Skin Innate Immunity and the Skin Microbiome: A Narrative Review. Nutrients 2021, 13, 302. [Google Scholar] [CrossRef] [PubMed]
- Bylka, W.; Znajdek-Awiżeń, P.; Studzińska-Sroka, E.; Brzezińska, M. Centella asiatica in cosmetology. Postepy Dermatol Alergol. 2013, 30, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Luthra, R.; Satija, G.; Roy, A. Centellosides: Pharmaceutical applications and production enhancement strategies. Plant Cell Tissue Organ Cult. (PCTOC) 2022, 149, 25–39. [Google Scholar] [CrossRef]
- Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. A Systematic Review of the Effect of Centella asiatica on Wound Healing. Int. J. Environ. Res. Public Health 2022, 19, 3266. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 119034, Asiatic Acid” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Asiatic-acid (accessed on 13 February 2023).
- Xia, B.; Bai, L.; Li, X.; Xiong, J.; Xu, P.; Xue, M. Structural Analysis of Metabolites of Asiatic Acid and Its Analogue Madecassic Acid in Zebrafish Using LC/IT-MSn. Molecules 2015, 20, 3001–3019. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Goyal, S.N.; Suchal, K.; Sharma, C.; Patil, C.R.; Ojha, S.K. Pharmacological Properties, Molecular Mechanisms, and Pharmaceutical Development of Asiatic Acid: A Pentacyclic Triterpenoid of Therapeutic Promise. Front. Pharmacol. 2018, 9, 892. [Google Scholar] [CrossRef]
- Jenwitheesuk, K.; Rojsanga, P.; Chowchuen, B.; Surakunprapha, P. A Prospective Randomized, Controlled, Double-Blind Trial of the Efficacy Using Centella Cream for Scar Improvement. Evid Based Complement Altern. Med. 2018, 2018, 9525624. [Google Scholar] [CrossRef]
- Somboonwong, J.; Kankaisre, M.; Tantisira, B.; Tantisira, M.H. Wound healing activities of different extracts of Centella asiatica in incision and burn wound models: An experimental animal study. BMC Complement Altern Med. 2012, 12, 103. [Google Scholar] [CrossRef]
- Damkerngsuntorn, W.; Rerknimitr, P.; Panchaprateep, R.; Tangkijngamvong, N.; Kumtornrut, C.; Kerr, S.J.; Asawanonda, P.; Tantisira, M.H.; Khemawoot, P. The Effects of a Standardized Extract of Centella asiatica on Postlaser Resurfacing Wound Healing on the Face: A Split-Face, Double-Blind, Randomized, Placebo-Controlled Trial. J. Altern. Complement Med. 2020, 26, 529–536. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, X.; Chen, L. Asiaticoside delays senescence and attenuate generation of ROS in UV-exposure cells through regulates TGF-β1/Smad pathway. Exp. Med. 2022, 24, 667. [Google Scholar] [CrossRef]
- Shukla, A.; Rasik, A.M.; Dhawan, B.N. Asiaticoside-induced elevation of antioxidant levels in healing wounds. Phytother Res. 1999, 13, 50–54. [Google Scholar] [CrossRef]
- Tang, B.; Zhu, B.; Liang, Y.; Bi, L.; Hu, Z.; Chen, B.; Zhang, K.; Zhu, J. Asiaticoside suppresses collagen expression and TGF-β/Smad signaling through inducing Smad7 and inhibiting TGF-βRI and TGF-βRII in keloid fibroblasts. Arch. Dermatol. Res. 2011, 303, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Ying, K.; Wei, S.; Liu, Y.; Lin, H.; Mao, Y. Dermal fibroblast-associated gene induction by asiaticoside shown in vitro by DNA microarray analysis. Br. J. Dermatol. 2004, 151, 571–578. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, H.-L.; Lee, M.H.; You, K.E.; Kwon, B.-J.; Seo, H.J.; Park, J.-C. Asiaticoside enhances normal human skin cell migration, attachment and growth in vitro wound healing model. Phytomedicine 2012, 19, 1223–1227. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Samukawa K-I Satake, N.; Sakanaka, M. Facilitating action of asiaticoside at low doses on burn wound repair and its mechanism. Eur. J. Pharmacol. 2008, 584, 415–423. [Google Scholar] [CrossRef]
- Liu, M.; Dai, Y.; Li, Y.; Luo, Y.; Huang, F.; Gong, Z.; Meng, Q. Madecassoside isolated from Centella asiatica herbs facilitates burn wound healing in mice. Planta Med. 2008, 74, 809–815. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 9035. [Google Scholar] [CrossRef] [PubMed]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Barbu, A.; Neamtu, B.; Zăhan, M.; Iancu, G.M.; Bacila, C.; Mireșan, V. Current Trends in Advanced Alginate-Based Wound Dressings for Chronic Wounds. J. Pers. Med. 2021, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, P.; He, F.; Zhang, C. Application of Alginate-Based Hydrogels in Hemostasis. Gels 2022, 8, 109. [Google Scholar] [CrossRef]
- Lee, W.R.; Park, J.H.; Kim, K.H.; Kim, S.J.; Park, D.H.; Chae, M.H.; Suh, S.H.; Jeong, S.W.; Park, K.K. The biological effects of topical alginate treatment in an animal model of skin wound healing. Wound Repair Regen. 2009, 17, 505–510. [Google Scholar] [CrossRef]
- Segal, H.C.; Hunt, B.J.; Gilding, K. The Effects of Alginate and Non-Alginate Wound Dressings on Blood Coagulation and Platelet Activation. J. Biomater. Appl. 1998, 12, 249–257. [Google Scholar] [CrossRef]

| Ingredient | Cosmetics | Medicines | Medical Devices | Concentration |
|---|---|---|---|---|
| Allantoin | x | N/A | ||
| Alginate and derivatives | x | N/A | ||
| Aloe vera | x | N/A | ||
| Birch bark extract | x | 100 mg/g | ||
| Bisabolol | x | N/A | ||
| Caprylic/Capric triglycerides | x | N/A | ||
| C. asiatica and pure compounds | x | x | N/A | |
| Essential fatty acids | x | N/A | ||
| Honey | x | N/A | ||
| Metal salts and oxides | x | x | Zinc oxide: 150–500 mg/g 1 | |
| Proteolytic enzymes | x | Proteolytic enzymes of Ananas comosus (L.) Merr.: 2–5 g/22 g 1 Collagenase: 0,6 U/g 1 | ||
| Petrolatum derivatives | x | N/A | ||
| Salicylic acid | x | 5 mg/g 1 | ||
| Silicones | x | N/A | ||
| Squalene or squalane | x | N/A | ||
| Sunflower oil | x | N/A | ||
| T. vulgare | x | 150 mg/g 1 | ||
| Trolamine | x | x | 6.7 mg/g 1 | |
| Vitamin A and derivative | x | 212.5 U.I./g 1 | ||
| Vitamin B5 and/or derivatives | x | x | x | Dexpanthenol: 50 mg/g 1 |
| Vitamin D3 | x | 21.25 U.I./g 1 | ||
| Vitamin E and derivative | x | x | N/A |
| Ingredients | Wound Healing Phase Targeted | Skin Repair Mechanism | References |
|---|---|---|---|
| Metal salts and oxides | Hemostasis phase Inflammatory phase Proliferation phase Remodeling phase | Antioxidant effect
Restoring dermal matrix
Antinociceptive effect
| [31,32,35,36,37,39,40,41,46,47,49,51,52,58,59,60,61,62,63,64,65,66,67,68,69,70] |
| Silicones | Hemostasis phase Proliferation phase Remodeling phase | Hemostatic effect:
| [81,84,86,87,88,89,90,91,92,93,94] |
| Petrolatum derivatives | Inflammatory phase Proliferation phase Remodeling phase | Occlusive effect
| [96,98,99,100,101,103,104,105,106,107,108,109,110,111,112,113,114,115] |
| Vitamin E and derivatives | Inflammatory phase Remodeling phase | Antioxidant effect
↓PGE2 levels; inhibit iNOS mRNA expression, NO production and COX-2 activity; restoring dermal matrix:
| [20,117,124,125,126,127,128,129,130,131,132] |
| Dexpanthenol | Proliferation phase | Moisturizing effect:
| [135,136,138,139,140,141,142,143,144,145,146] |
| Vitamin A and derivatives | Inflammatory phase Proliferation phase Remodeling phase | Anti-inflammatory effect:
| [153,163] |
| Alginate and derivatives | Hemostasis phase Inflammatory phase | Antioxidant effect Anti-inflammatory effect Antimicrobial effect Moisturizing effect Exudate absorption | [181,184,185] |
| C. asiatica and pure compounds | Inflammatory phase Proliferation phase Remodeling phase | Antioxidant effect: Anti-inflammatory effect:
| [166,170,171,172,173,174,175,176,177,178,179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, A.; Rego, L.; Martins, M.S.; Ferreira, M.S.; Cruz, M.T.; Sousa, E.; Almeida, I.F. How to Promote Skin Repair? In-Depth Look at Pharmaceutical and Cosmetic Strategies. Pharmaceuticals 2023, 16, 573. https://doi.org/10.3390/ph16040573
Torres A, Rego L, Martins MS, Ferreira MS, Cruz MT, Sousa E, Almeida IF. How to Promote Skin Repair? In-Depth Look at Pharmaceutical and Cosmetic Strategies. Pharmaceuticals. 2023; 16(4):573. https://doi.org/10.3390/ph16040573
Chicago/Turabian StyleTorres, Ana, Liliana Rego, Márcia S. Martins, Marta S. Ferreira, Maria T. Cruz, Emília Sousa, and Isabel F. Almeida. 2023. "How to Promote Skin Repair? In-Depth Look at Pharmaceutical and Cosmetic Strategies" Pharmaceuticals 16, no. 4: 573. https://doi.org/10.3390/ph16040573
APA StyleTorres, A., Rego, L., Martins, M. S., Ferreira, M. S., Cruz, M. T., Sousa, E., & Almeida, I. F. (2023). How to Promote Skin Repair? In-Depth Look at Pharmaceutical and Cosmetic Strategies. Pharmaceuticals, 16(4), 573. https://doi.org/10.3390/ph16040573










