Protective Effect of Hydrogen-Rich Saline on Spinal Cord Damage in Rats
Abstract
1. Introduction
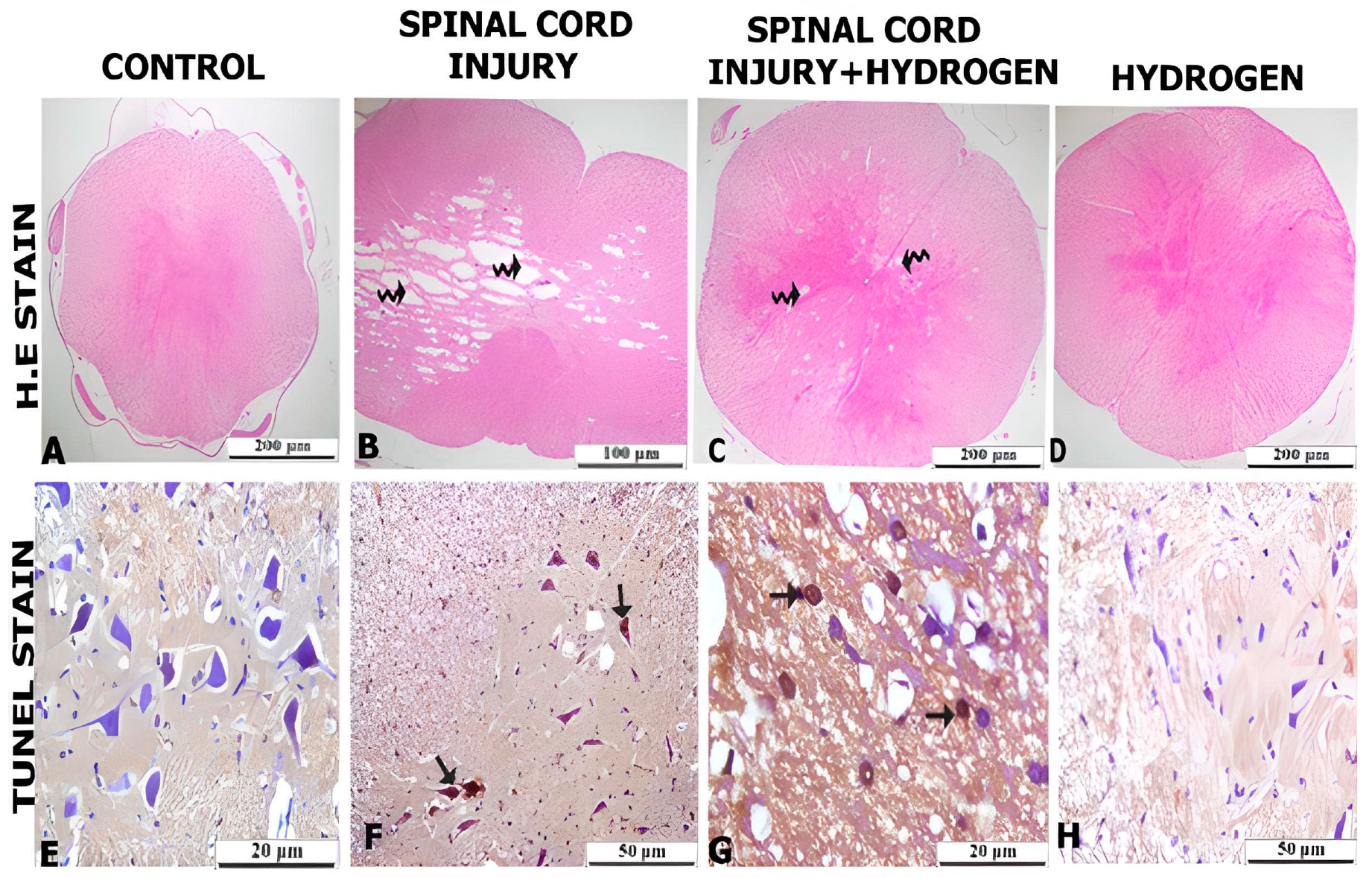
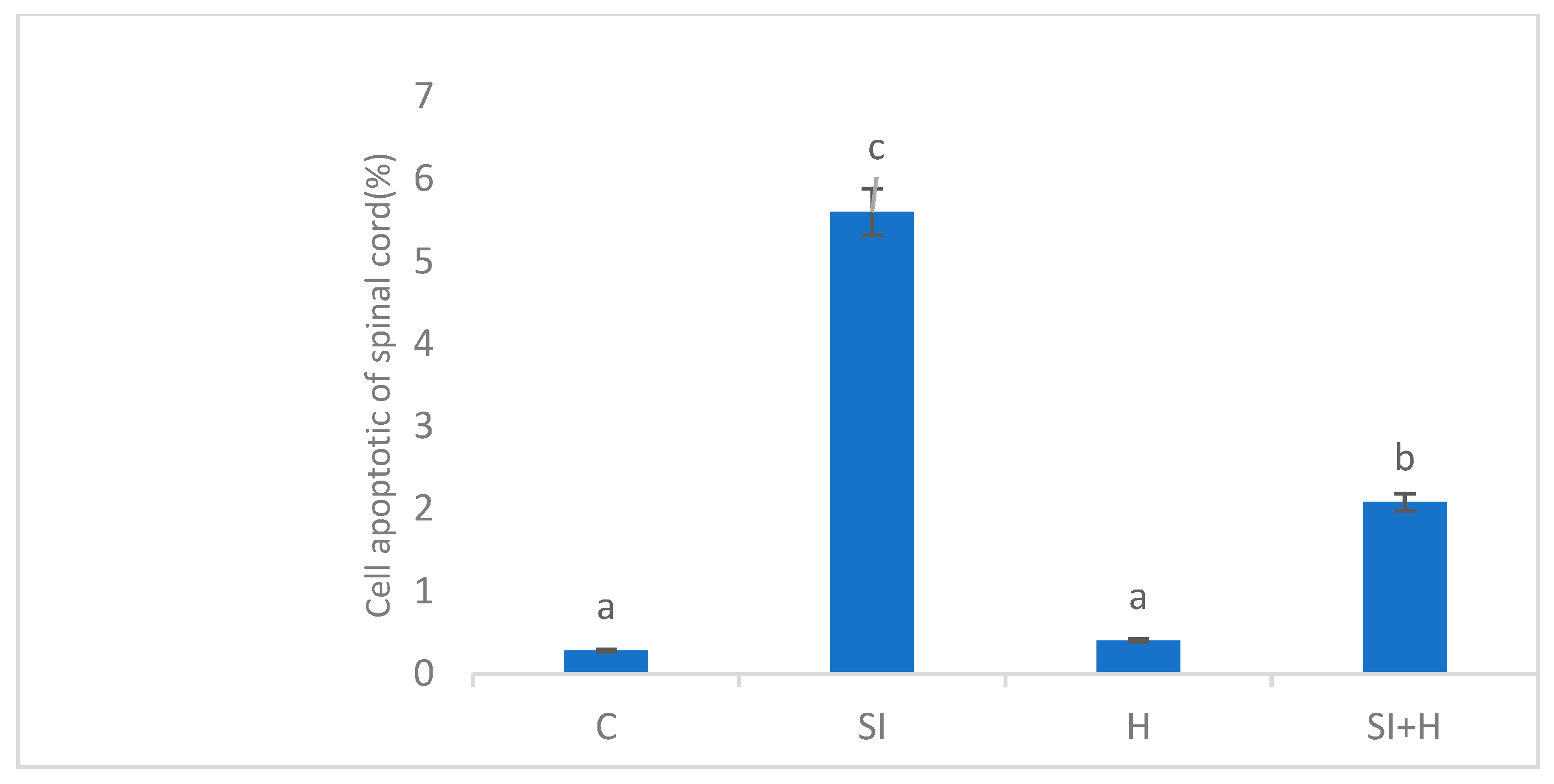
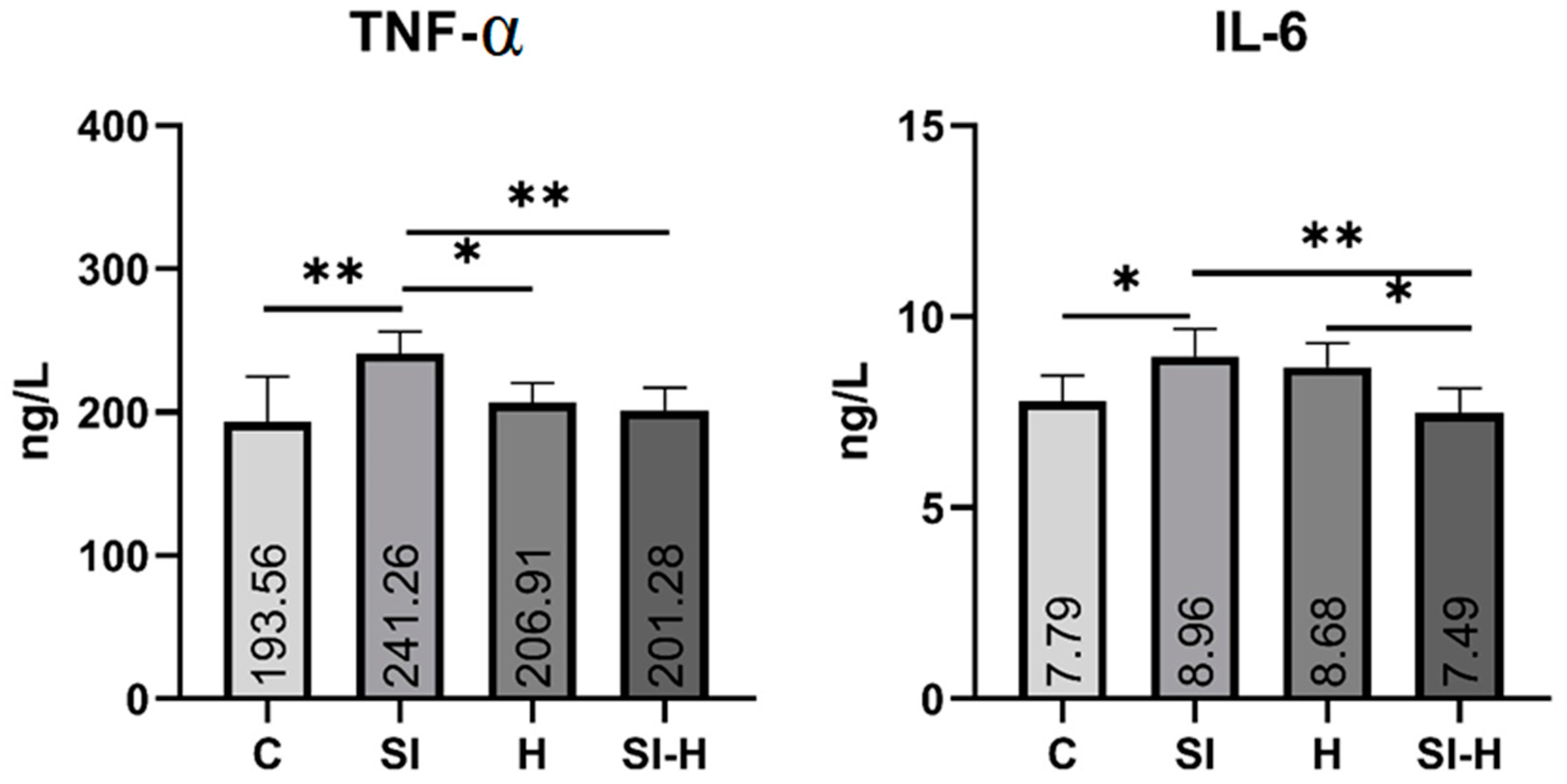
2. Results
3. Discussion
4. Materials and Methods
4.1. Ethics and Study Design
- Control (C): laminectomy only.
- Spinal cord injury (SI): after laminectomy at T7–T10, the dura was left intact and the Tator and Rivlin clip compression model was applied to the spinal cord for 1 min; no other treatment was applied.
- Hydrogen (H): laminectomy at the T7–T10 level followed by treatment with i.p. hydrogen-rich saline for 7 days.
- Spinal cord injury-hydrogen (SI-H): hydrogen-rich saline administered i.p. for 7 days after laminectomy at T7–T10, leaving the dura intact and applying the Tator and Rivlin clip compression model to the spinal cord for 1 minute.
4.2. Surgical Procedure
4.3. Hydrogen-Rich Saline Procedure
4.4. Treatment
4.5. Histological Analyses
4.6. Biochemical Measurements
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, B.B.; Cripps, R.A.; Fitzharris, M.; Wing, P.C. The global map for traumatic spinal cord injury epidemiology: Update 2011, global incidence rate. Spinal Cord 2014, 52, 110–116. [Google Scholar] [CrossRef]
- Li, Y.; Cao, T.; Ritzelet, R.M.; He, J.; Faden, A.I.; Wu, J. Dementia, depression, and associated brain inflammatory mechanisms after spinal cord injury. Cells 2020, 9, 1420. [Google Scholar] [CrossRef]
- Dumont, R.J.; Okonkwo, D.O.; Verma, S.; Hurlbert, R.J.; Boulos, P.T.; Ellegala, D.B.; Dumont, A.S. Acute spinal cord injury, part I: Pathophysiologic mechanisms. Clin. Neuropharmacol. 2001, 24, 254–264. [Google Scholar] [CrossRef]
- Coyoy-Salgado, A.; Segura-Uribe, J.J.; Guerra-Araiza, C.; Orozco-Suárez, S.; Salgado-Ceballos, H.; Feria-Romero, I.A.; Gallardo, J.M.; Orozco-Barrios, C.E. The importance of natural antioxidants in the treatment of spinal cord injury in animal models: An overview. Oxidative Med. Cell. Longev. 2019, 2019, 3642491. [Google Scholar] [CrossRef]
- Karsy, M.; Gregory, H. Modern medical management of spinal cord injury. Curr. Neurol. Neurosci. Rep. 2019, 19, 65. [Google Scholar] [CrossRef]
- Alizadeh, A.; Scott, M.D.; Soheila, K.A. Traumatic spinal cord injury: An overview of pathophysiology, models and acute injury mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Pei, J.; Fan, L.; Nan, K.; Li, J.; Dang, X.Q.; Wang, K.Z. HSYA alleviates secondary neuronal death through attenuating oxidative stress, inflammatory response, and neural apoptosis in SD rat spinal cord compression injury. J. Neuroinflamm. 2017, 14, 97. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Genovese, T. Role of free radicals and poly (ADP-ribose) polymerase-1 in the development of spinal cord injury: New potential therapeutic targets. Curr. Med. Chem. 2008, 15, 477–487. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Q.; Mao, Y.; Xu, S.; Xia, C.; Shi, X.; Zhang, J.H.; Yuan, H.; Sun, X. Hydrogen-rich saline protects against spinal cord injury in rats. Neurochem. Res. 2010, 35, 1111–1118. [Google Scholar] [CrossRef]
- Li, Q.S.; Jia, Y.J. Ferroptosis: A critical player and potential therapeutic target in traumatic brain injury and spinal cord injury. Neural Regen. Res. 2023, 18, 506–512. [Google Scholar]
- Genovese, T.; Mazzon, E.; Menegazzi, M.; Di Paola, R.; Muià, C.; Crisafulli, C.; Bramanti, P.; Suzuki, H.; Cuzzocrea, S. Neuroprotection and enhanced recovery with hypericum perforatum extract after experimental spinal cord injury in mice. Shock 2006, 25, 608–617. [Google Scholar] [CrossRef]
- Xiong, Y.; Hall, E.D. Pharmacological evidence for a role of peroxynitrite in the pathophysiology of spinal cord injury. Exp. Neurol. 2009, 216, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Chen, S.; Zhang, J.M. Hydrogen as a selective antioxidant: A review of clinical and experimental studies. J. Int. Med. Res. 2010, 38, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.; Nenov, A.; Kisher, H.; Hancock, J.T. Molecular hydrogen as medicine: An assessment of administration methods. Hydrogen 2021, 2, 444–460. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Liu, W.; Xie, K.; Liu, W.; Qu, Y.; Chao, X.; Chen, T.; Zhou, J.; Fei, Z. Beneficial effects of hydrogen gas in a rat model of traumatic brain injury via reducing oxidative stress. Brain Res. 2010, 1354, 196–205. [Google Scholar] [CrossRef]
- Slezak, J.; Kura, B.; LeBaron, T.W.; Singal, P.K.; Buday, J.; Barancik, M. Oxidative stress and pathways of molecular hydrogen effects in medicine. Curr. Pharm. Des. 2021, 27, 610–625. [Google Scholar] [CrossRef]
- Ono, H.; Nishijima, Y.; Adachi, N.; Tachibana, S.; Chitoku, S.; Mukaihara, S.; Nawashiro, H. Improved brain MRI indices in the acute brain stem infarct sites treated with hydroxyl radical scavengers, Edaravone and hydrogen, as compared to Edaravone alone. A non-controlled study. Med. Gas Res. 2011, 1, 12. [Google Scholar] [CrossRef]
- Cai, J.; Kang, Z.; Liu, W.W.; Luo, X.; Qiang, S.; Zhang, J.H.; Li, R. Hydrogen therapy reduces apoptosis in neonatal hypoxia–ischemia rat model. Neurosci. Lett. 2008, 441, 167–172. [Google Scholar] [CrossRef]
- Shen, L.; Wang, J.; Liu, K.; Wang, C.; Wang, C.; Wu, H.; Jing, H. Hydrogen-rich saline is cerebroprotective in a rat model of deep hypothermic circulatory arrest. Neurochem. Res. 2011, 36, 1501–1511. [Google Scholar] [CrossRef]
- Tan, X.; Shen, F.; Dong, W.L.; Yang, Y.; Chen, G. The role of hydrogen in Alzheimer’s disease. Med. Gas Res. 2018, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, C.; Zhang, J.H.; Cai, J.M.; Cao, Y.P.; Sun, X.J. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced Alzheimer′s disease by reduction of oxidative stress. Brain Res. 2010, 1328, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhou, B.; Li, W.; Sun, X.; Luo, D. Hydrogen–rich saline protects against ultraviolet B radiation injury in rats. J. Biomed. Res. 2012, 26, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Sano, M.; Ohsawa, I.; Shinmura, K.; Tamaki, K.; Kimura, K.; Endo, J.; Katayama, T.; Kawamura, A.; Kohsaka, S.; et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia–reperfusion injury. Biochem. Biophys. Res. Commun. 2008, 373, 30–35. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017, 3, 17018. [Google Scholar] [CrossRef]
- Sng, K.S.; Li, G.; Zhou, L.Y.; Song, Y.J.; Chen, X.Q.; Wang, Y.J.; Yao, M.; Cui, X.J. Ginseng extract and ginsenosides improve neurological function and promote antioxidant effects in rats with spinal cord injury: A meta-analysis and systematic review. J. Ginseng Res. 2021, 46, 11–22. [Google Scholar] [CrossRef]
- Ren, H.; Chen, X.; Tian, M.; Zhou, J.; Ouyang, H.; Zhang, Z. Regulation of inflammatory cytokines for spinal cord injury repair through local delivery of therapeutic agents. Adv. Sci. 2018, 5, 1800529. [Google Scholar] [CrossRef]
- Gao, J.; Khang, M.; Liao, Z.; Detloff, M.; Lee, J.S. Therapeutic targets and nanomaterial-based therapies for mitigation of secondary injury after spinal cord injury. Nanomedicine 2021, 16, 2013–2028. [Google Scholar] [CrossRef]
- Tohda, C.; Kuboyama, T. Current and future therapeutic strategies for functional repair of spinal cord injury. Pharmacol. Ther. 2011, 132, 57–71. [Google Scholar] [CrossRef]
- Bi, Y.; Duan, W.; Chen, J.; You, T.; Li, S.; Jiang, W.; Li, M.; Wang, G.; Pan, X.; Wu, J.; et al. Neutrophil decoys with anti-inflammatory and anti-oxidative properties reduce secondary spinal cord injury and improve neurological functional recovery. Adv. Funct. Mater. 2021, 31, 2102912. [Google Scholar] [CrossRef]
- Tyler, J.Y.; Xu, X.M.; Cheng, J.X. Nanomedicine for treating spinal cord injury. Nanoscale 2013, 5, 8821–8836. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhu, H.; Li, J.; Wang, X.; Misra, H.; Li, Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord 2012, 50, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Gao, J. Macrophage polarization: A key event in the secondary phase of acute spinal cord injury. J. Cell. Mol. Med. 2017, 21, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jones, N.R.; Blumbergs, P.C.; Van Den Heuvel, C.; Moore, E.J.; Manavis, J.; Sarvestani, G.T.; Ghabriel, M.N. Severity-dependent expression of pro-inflammatory cytokines in traumatic spinal cord injury in the rat. J. Clin. Neurosci. 2005, 12, 276–284. [Google Scholar] [CrossRef]
- Davies, A.L.; Hayes, K.C.; Dekaban, G.A. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch. Phys. Med. Rehabil. 2007, 88, 1384–1393. [Google Scholar] [CrossRef]
- Dyck, S.; Kataria, H.; Alizadeh, A.; Santhosh, K.T.; Lang, B.; Silver, J.; Karimi-Abdolrezaee, S. Perturbing chondroitin sulfate proteoglycan signaling through LAR and PTPσ receptors promotes a beneficial inflammatory response following spinal cord injury. J. Neuroinflamm. 2018, 15, 90. [Google Scholar] [CrossRef]
- Reier, P.J. Gliosis following CNS injury: The anatomy of astrocytic scars and their influences on axonal elongation. Astrocytes 2012, 3, 263–324. [Google Scholar]
- Gao, Z.; Zhu, Q.; Zhang, Y.; Zhao, Y.; Cai, L.; Shields, C.B.; Cai, J. Reciprocal modulation between microglia and astrocyte in reactive gliosis following the CNS injury. Mol. Neurobiol. 2013, 48, 690–701. [Google Scholar] [CrossRef]
- Duncan, G.J.; Manesh, S.B.; Hilton, B.J.; Assinck, P.; Plemel, J.R.; Tetzlaff, W. The fate and function of oligodendrocyte progenitor cells after traumatic spinal cord injury. GLIA 2020, 68, 227–245. [Google Scholar] [CrossRef]
- Pineau, I.; Lacroix, S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J. Compd. Neurol. 2007, 500, 267–285. [Google Scholar] [CrossRef]
- Seekamp, A.; Warren, J.S.; Remick, D.G.; Till, G.O.; Ward, P.A. Requirements for tumor necrosis factor-alpha and interleukin-1 in limb ischemia/reperfusion injury and associated lung injury. Am. J. Pathol. 1993, 143, 453–463. [Google Scholar] [PubMed]
- Korkmaz, K.; Gedik, H.S.; Budak, A.B.; Erdem, H.; Lafci, G.; Karakilic, E.; Nacar, O.A.; Yildirim, L.; Ankarali, H. Effect of heparin on neuroprotection against spinal cord ischemia and reperfusion in rats. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 522–530. [Google Scholar] [PubMed]
- Wang, T.; He, C. TNF-α and IL-6: The link between immune and bone system. Current Drug Targets 2020, 21, 213–227. [Google Scholar] [CrossRef]
- Mudter, J.; Neurath, M.F. Il-6 signaling in inflammatory bowel disease: Pathophysiological role and clinical relevance. Inflamm. Bowel Dis. 2007, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Blumbergs, P.C.; Jones, N.R.; Manavis, J.; Sarvestani, G.T.; Ghabriel, M.N. Early expression and cellular localization of proinflammatory cytokines interleukin-1β, interleukin-6, and tumor necrosis factor-α in human traumatic spinal cord injury. Spine 2004, 29, 966–971. [Google Scholar] [CrossRef]
- Okada, S.; Nakamura, M.; Mikami, Y.; Shimazaki, T.; Mihara, M.; Ohsugi, Y.; Iwamoto, Y.; Yoshizaki, K.; Kishimoto, T.; Toyama, Y.; et al. Blockade of interleukin-6 receptor suppresses reactive astrogliosis and ameliorates functional recovery in experimental spinal cord injury. J. Neurosci. Res. 2004, 76, 265–276. [Google Scholar] [CrossRef]
- Frankola, K.A.; Greig, N.H.; Luo, W.; Tweedie, D. Targeting TNF-alpha to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol. Disord.-Drug Targets Former. Curr. Drug Targets-CNS Neurol. Disord. 2011, 10, 391–403. [Google Scholar] [CrossRef]
- Akçapınar, R.; Garipcan, B.; Goodarzi, V.; Uzun, L. Designing of various biosensor devices for determination of apoptosis: A comprehensive review. Biochem. Biophys. Res. Commun. 2021, 578, 42–62. [Google Scholar] [CrossRef]
- Ren, X.; Wan, C.; Niu, Y. Overexpression of lnc RNA TCTN 2 protects neurons from apoptosis by enhancing cell autophagy in spinal cord injury. FEBS Open Bio 2019, 9, 1223–1231. [Google Scholar] [CrossRef]
- Solaroglu, I.; Kaptanoglu, E.; Okutan, O.; Beskonakli, E.; Attar, A.; Kilinc, K. Magnesium sulfate treatment decreases caspase-3 activity after experimental spinal cord injury in rats. Surg. Neurol. 2005, 64, S17–S21. [Google Scholar] [CrossRef]
- Chen, M.; Gao, Y.; Li, W.; Wang, J.C.; He, Y.P.; Li, Z.W.; Gan, G.S.; Yuan, B. FBW7 protects against spinal cord injury by mitigating inflammation-associated neuronal apoptosis in mice. Biochem. Biophys. Res. Commun. 2020, 532, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, E.E.; Scott, A.L. Purinergic signaling systems across comparative models of spinal cord injury. Neural Regen. Res. 2022, 17, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Inukai, T.; Uchida, K.; Nakajima, H.; Yayama, T.; Kobayashi, S.; Mwaka, E.S.; Guerrero, A.R.; Baba, H. Tumor necrosis factor-alpha and its receptors contribute to apoptosis of oligodendrocytes in the spinal cord of spinal hyperostotic mouse (twy/twy) sustaining chronic mechanical compression. Spine 2009, 34, 2848–2857. [Google Scholar] [CrossRef]
- Wang, J.; Ren, C.; Feng, J.; Ou, C.; Liu, L. Oleanolic acid inhibits mouse spinal cord injury through suppressing inflammation and apoptosis via the blockage of p38 and JNK MAPKs. Biomed. Pharmacother. 2020, 123, 109752. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xie, K.; Li, J.; Xu, N.; Gong, G.; Wang, G.; Yu, Y.; Dong, H.; Xiong, L. Beneficial effects of hydrogen gas against spinal cord ischemia–reperfusion injury in rabbits. Brain Res. 2011, 1378, 125–136. [Google Scholar] [CrossRef]
- Leveque, C.; Mrakic-Sposta, S.; Lafère, P.; Vezzoli, A.; Germonpré, P.; Beer, A.; Mievis, S.; Virgili, F.; Lambrechts, K.; Theunissen, S.; et al. Oxidative Stress Response’s Kinetics after 60 Minutes at Different (30% or 100%) Normobaric Hyperoxia Exposures. Int. J. Mol. Sci. 2023, 24, 664. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cui, J.; Zhai, X.; Zhang, J.; Gu, Z.; Zhi, X.; Weng, W.; Pan, P.; Cao, L.; Ji, F.; et al. Inhalation of hydrogen of different concentrations ameliorates spinal cord injury in mice by protecting spinal cord neurons from apoptosis, oxidative injury and mitochondrial structure damages. Cell. Physiol. Biochem. 2018, 47, 176–190. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhu, K.; Sun, J.F.; Zhang, Z.P.; Sun, J.W.; Zhang, K.X. Hydrogen-rich saline injection into the subarachnoid cavity within 2 weeks promotes recovery after acute spinal cord injury. Neural Regen. Res. 2015, 10, 958. [Google Scholar] [CrossRef]
- Stoica, S.I.; Onose, G.; Hoteteu, M.; Munteanu, C. Effects of ethanol and deferoxamine on rat primary glial cell cultures, in regard with ischemia induced by traumatic spinal cord injury. Balneo PRM Res. J. 2022, 13, 502. [Google Scholar] [CrossRef]
- Mukaino, M.; Nakamura, M.; Yamada, O.; Okada, S.; Morikawa, S.; Renault-Mihara, F.; Iwanami, A.; Ikegami, T.; Ohsugi, Y.; Tsuji, O.; et al. Anti-IL-6-receptor antibody promotes repair of spinal cord injury by inducing microglia-dominant inflammation. Exp. Neurol. 2010, 224, 403–414. [Google Scholar] [CrossRef]
- Rivlin, A.S.; Tator, C.H. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg. Neurol. 1978, 10, 38–43. [Google Scholar] [PubMed]
- Bulut, M.; Sezer, Y.Ç.; Ceylan, M.M.; Alwazeer, D.; Koyuncu, M. Hydrogen-rich water can reduce the formation of biogenic amines in butter. Food Chem. 2022, 384, 132613. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Tian, Y.; Xie, K.; Liu, W.; Qu, Y.; Fei, Z. Protective effects of hydrogen-rich saline in a rat model of traumatic brain injury via reducing oxidative stress. J. Surg. Res. 2012, 178, e9–e16. [Google Scholar] [CrossRef]
- Le Baron, T.W.; Sharpe, R. ORP should not be used to estimate or compare concentrations of aqueous H2: An in-silico analysis and narrative synopsis. Front. Food Sci. Technol. 2022, 2, 1007001. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayabaş, M.; Şahin, L.; Makav, M.; Alwazeer, D.; Aras, L.; Yiğit, S.; LeBaron, T.W. Protective Effect of Hydrogen-Rich Saline on Spinal Cord Damage in Rats. Pharmaceuticals 2023, 16, 527. https://doi.org/10.3390/ph16040527
Kayabaş M, Şahin L, Makav M, Alwazeer D, Aras L, Yiğit S, LeBaron TW. Protective Effect of Hydrogen-Rich Saline on Spinal Cord Damage in Rats. Pharmaceuticals. 2023; 16(4):527. https://doi.org/10.3390/ph16040527
Chicago/Turabian StyleKayabaş, Murat, Levent Şahin, Mustafa Makav, Duried Alwazeer, Levent Aras, Serdar Yiğit, and Tyler W. LeBaron. 2023. "Protective Effect of Hydrogen-Rich Saline on Spinal Cord Damage in Rats" Pharmaceuticals 16, no. 4: 527. https://doi.org/10.3390/ph16040527
APA StyleKayabaş, M., Şahin, L., Makav, M., Alwazeer, D., Aras, L., Yiğit, S., & LeBaron, T. W. (2023). Protective Effect of Hydrogen-Rich Saline on Spinal Cord Damage in Rats. Pharmaceuticals, 16(4), 527. https://doi.org/10.3390/ph16040527








