A Real-Life Study on the Use of Tildrakizumab in Psoriatic Patients
Abstract
1. Introduction
2. Results
2.1. Demographic Characteristics of Enrolled Patients
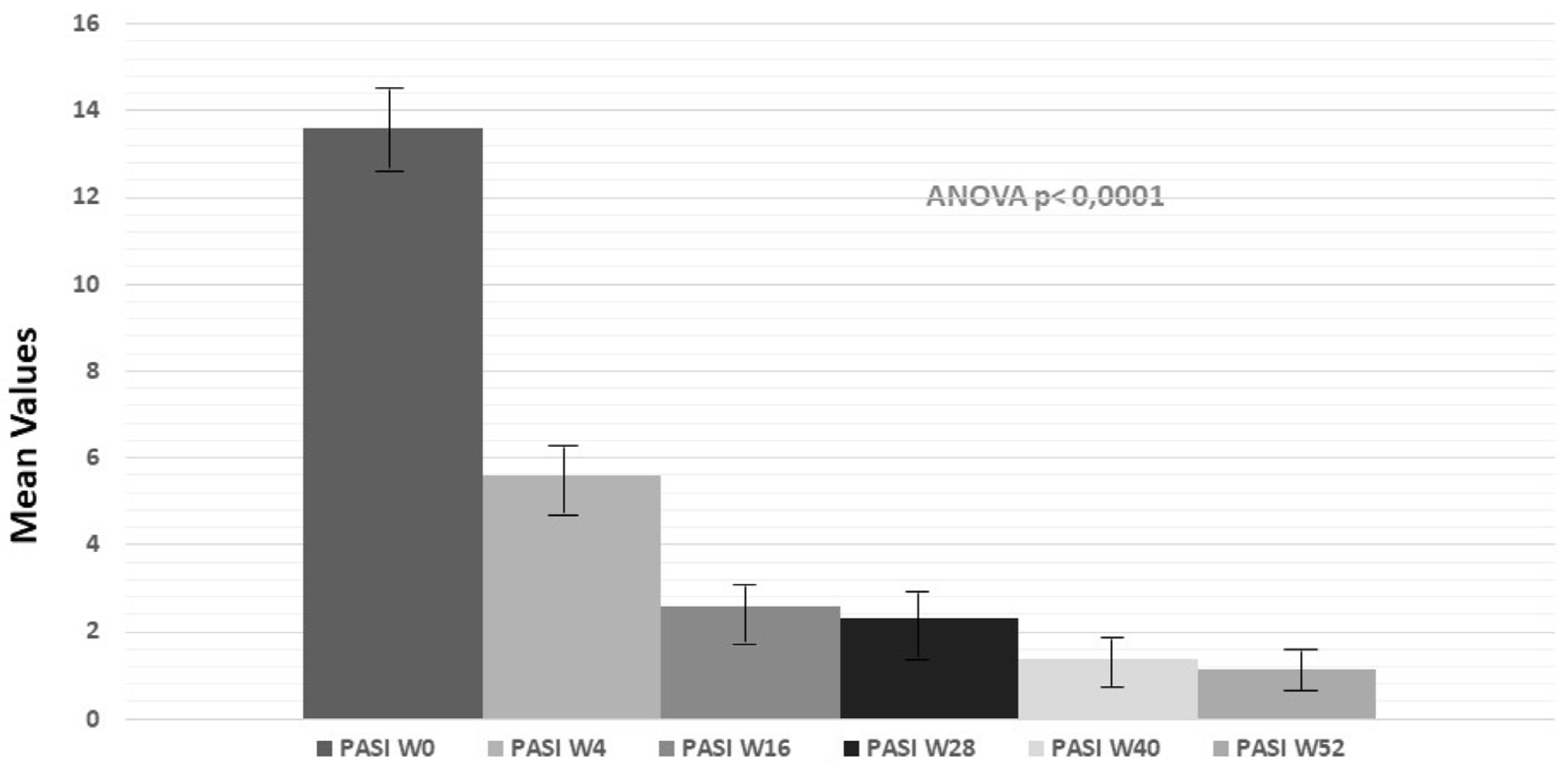
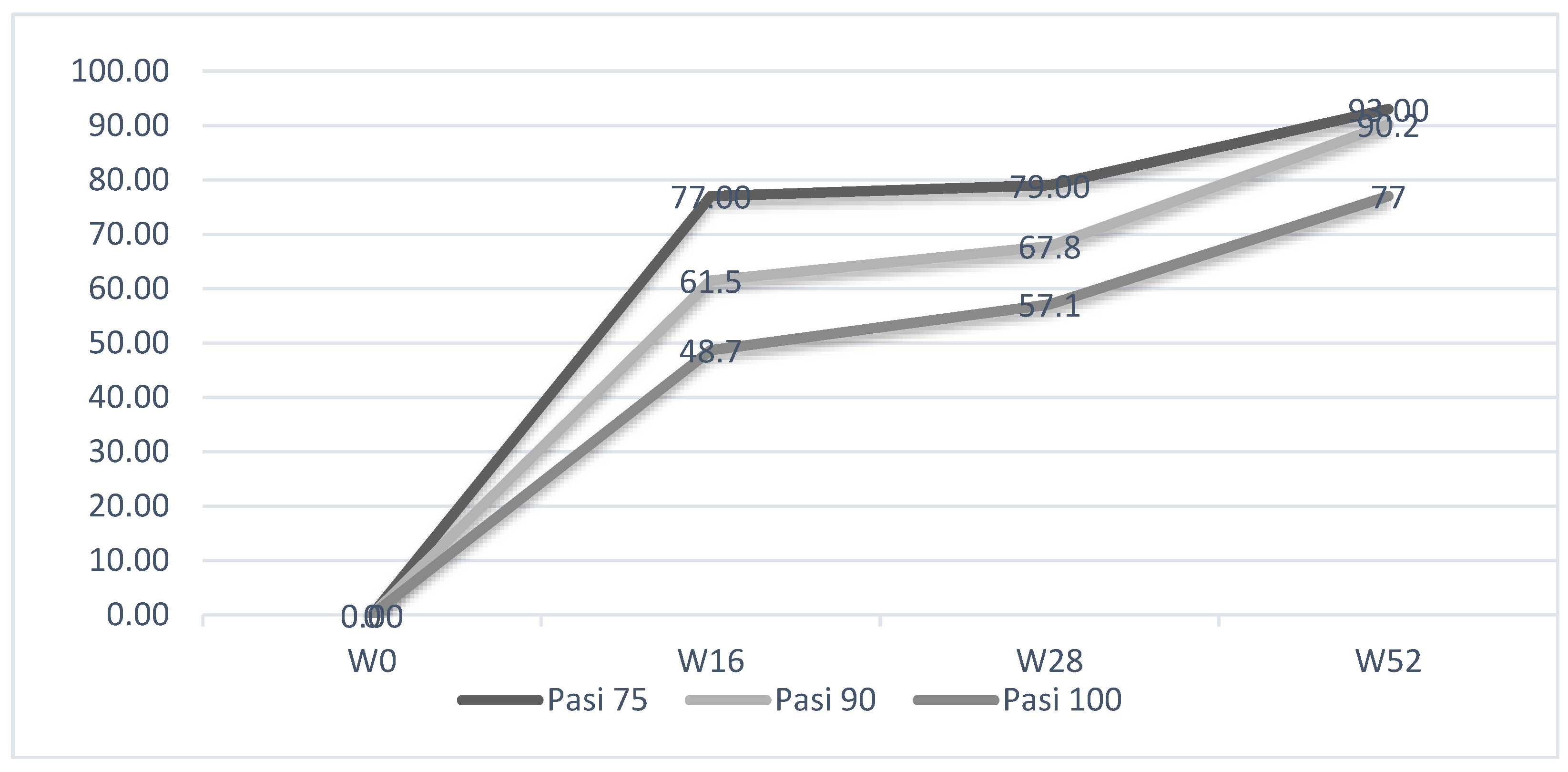
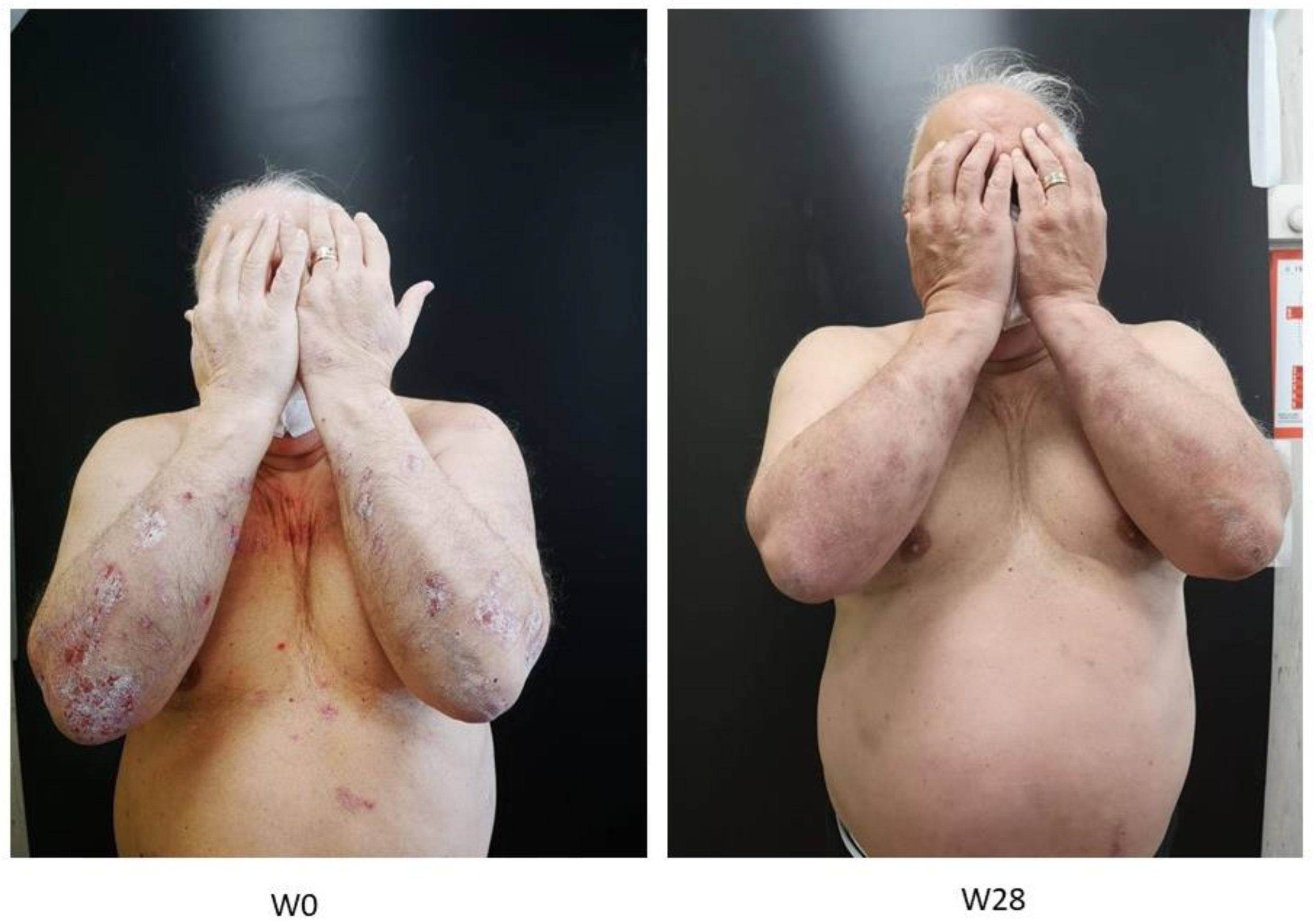
2.2. Evaluation of PASI, PPPGA and DLQL Indexes
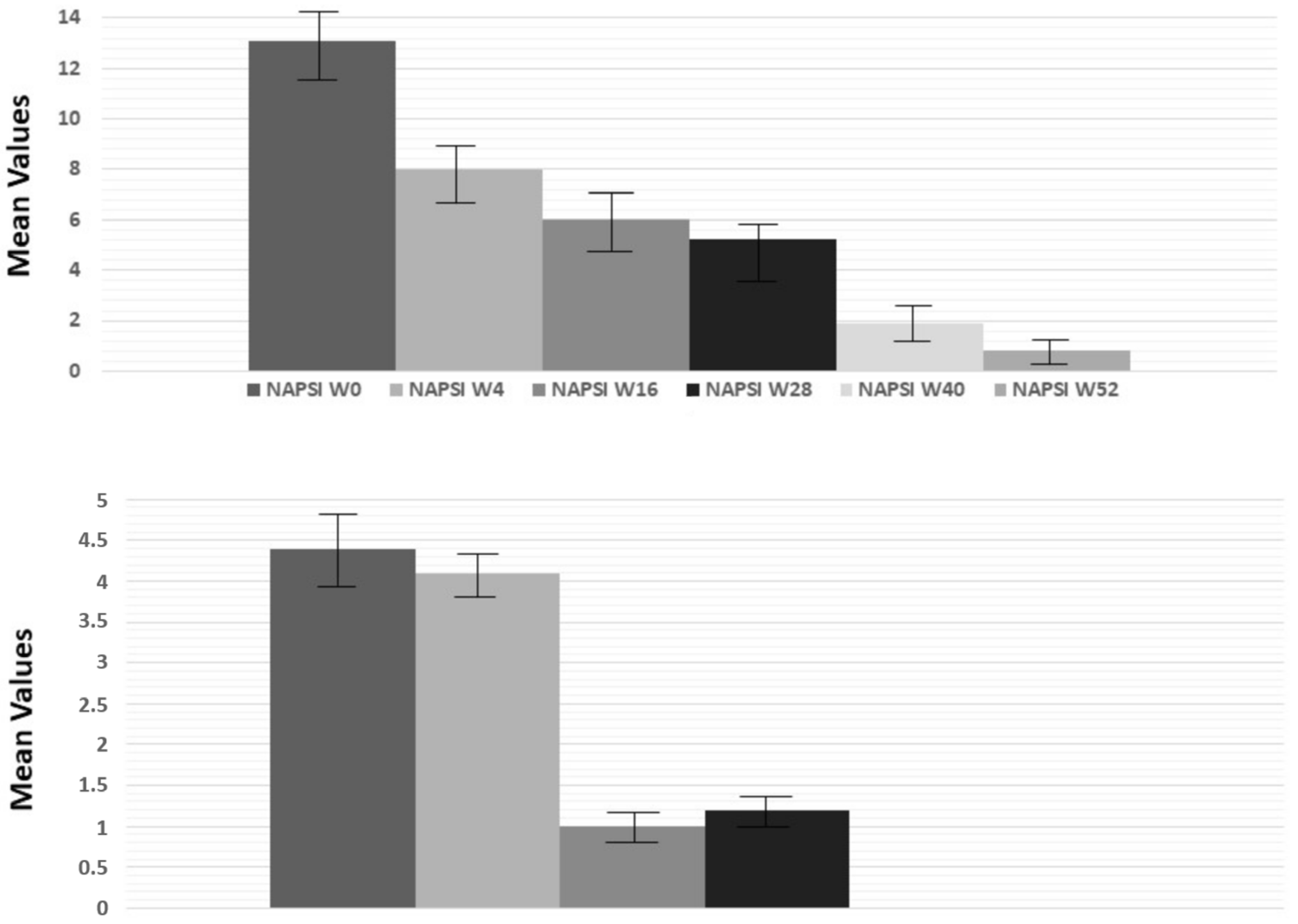
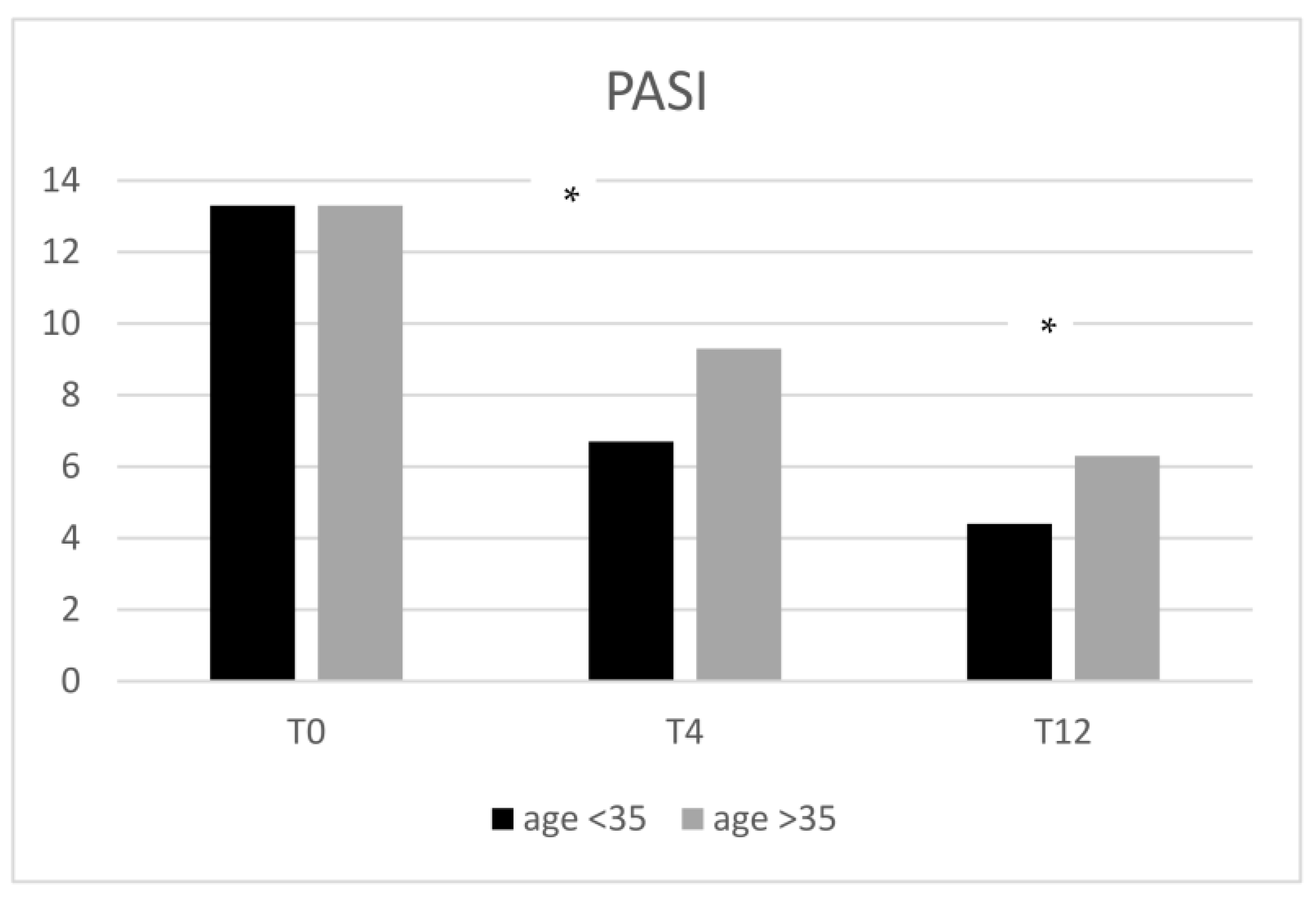
2.3. Stratification of Patients Considering the Age of Onset of Psoriasis, PASI, and the Involvement of Difficult-to-Treat Sites
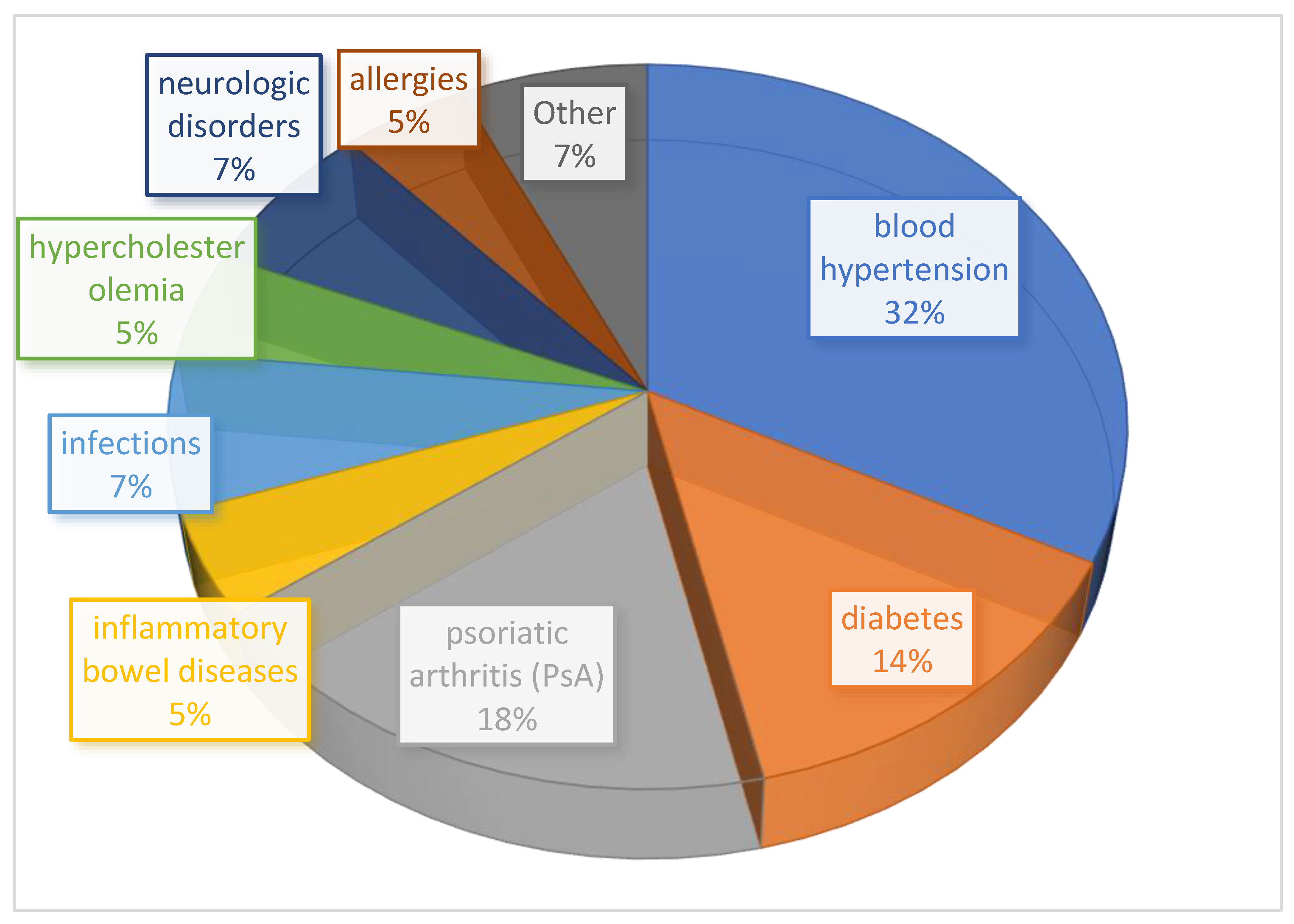
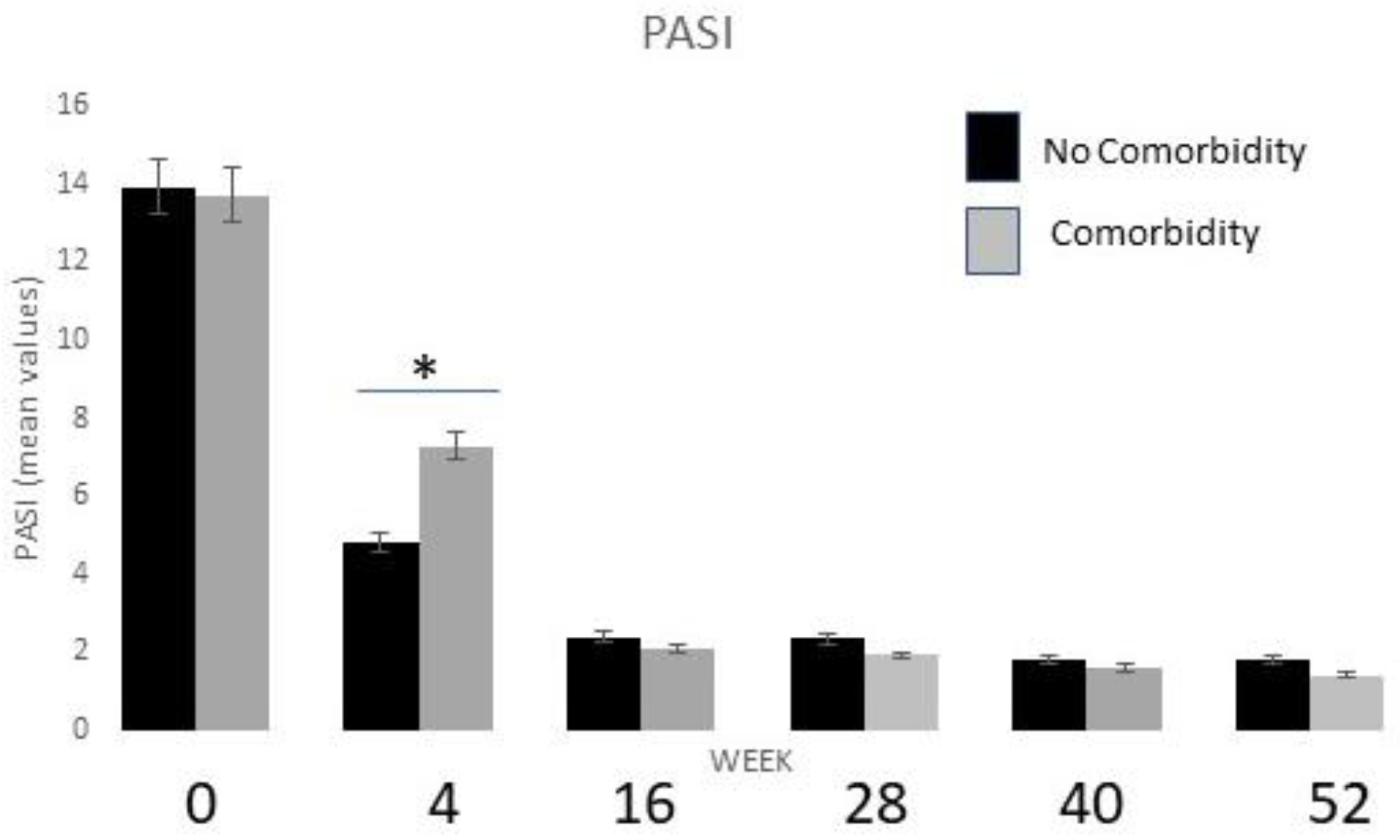
2.4. Stratification by Comorbidities and Drug Safety Profile
3. Discussion
4. Materials and Methods
4.1. Enrolled Patients and Study Design
4.2. Score and Laboratory Analyses Evaluation
4.3. Safety
4.4. Statistical Analysis
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caputo, V.; Strafella, C.; Termine, A.; Dattola, A.; Mazzilli, S.; Lanna, C.; Cosio, T.; Campione, E.; Novelli, G.; Giardina, E.; et al. Overview of the molecular determinants contributing to the expression of Psoriasis and Psoriatic Arthritis phenotypes. J. Cell Mol. Med. 2020, 24, 13554–13563. [Google Scholar] [CrossRef] [PubMed]
- Mastorino, L.; Cariti, C.; Susca, S.; Sciamarrelli, N.; Borriello, S.; Ortoncelli, M.; Stroppiana, E.; Verrone, A.; Dapavo, P.; Quaglino, P.; et al. Tildrakizumab in real-life shows good efficacy in moderate-to-severe psoriasis regardless of previous use of biologic drugs and joint involvement. Dermatol. Ther. 2022, 35, e15818. [Google Scholar] [CrossRef] [PubMed]
- Lanna, C.; Mancini, M.; Gaziano, R.; Cannizzaro, M.V.; Galluzzo, M.; Talamonti, M.; Rovella, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Wang, Y.; et al. Skin immunity and its dysregulation in psoriasis. Cell Cycle 2019, 18, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Ghamrawi, R.I.; Ghiam, N.; Wu, J.J. Comparison of psoriasis guidelines for use of IL-23 inhibitors in the United States and United Kingdom: A critical appraisal and comprehensive review. J. Dermatol. Treat. 2020, 33, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Leonardi, C.L.; Davis, D.M.; Gelfand, J.M.; Lichten, J.; Mehta, N.N.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Elewski, B.E.; et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J. Am. Acad. Dermatol. 2019, 80, 1073–1113. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Kumar, B.; Saraswat, A.; Kaur, I. Palmoplantar lesions in psoriasis: A study of 3065 patients. Acta Derm. Venereol. 2002, 82, 192–195. [Google Scholar] [CrossRef]
- Lillis, J.V.; Guo, C.-S.; Lee, J.J.; Blauvelt, A. Increased IL-23 expression in palmoplantar psoriasis and hyperkeratotic hand dermatitis. Arch. Dermatol. 2010, 146, 918–919. [Google Scholar] [CrossRef]
- Wiedemann, J.; Billi, A.C.; Bocci, F.; Kashgari, G.; Xing, E.; Tsoi, L.C.; Meller, L.; Swindell, W.R.; Wasikowski, R.; Xing, X.; et al. Differential cell composition and split epidermal differentiation in human palm, sole, and hip skin. Cell Rep. 2023, 42, 111994. [Google Scholar] [CrossRef]
- Lebwohl, M.G.; Leonardi, C.L.; Mehta, N.N.; Gottlieb, A.B.; Mendelsohn, A.M.; Parno, J.; Rozzo, S.J.; Menter, M.A. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: Long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J. Am. Acad. Dermatol. 2021, 84, 398–407. [Google Scholar] [CrossRef]
- Megna, M.; Potestio, L.; Fabbrocini, G.; Camela, E. Treating psoriasis in the elderly: Biologics and small molecules. Expert Opin. Biol. Ther. 2022, 22, 1503–1520. [Google Scholar] [CrossRef]
- Burlando, M.; Castelli, R.; Cozzani, E.; Parodi, A. Treatment of moderate-to-severe plaque psoriasis with tildrakizumab in the real-life setting. Drugs Context 2021, 10, 2021-2-6. [Google Scholar] [CrossRef]
- Reich, K.; Warren, R.; Iversen, L.; Puig, L.; Pau-Charles, I.; Igarashi, A.; Ohtsuki, M.; Falqués, M.; Harmut, M.; Rozzo, S.; et al. Long-term efficacy and safety of tildrakizumab for moderate-to-severe psoriasis: Pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2) through 148 weeks. Br. J. Dermatol. 2020, 182, 605–617. [Google Scholar] [CrossRef]
- Thaci, D.; Piaserico, S.; Warren, R.; Gupta, A.; Cantrell, W.; Draelos, Z.; Foley, P.; Igarashi, A.; Langley, R.; Asahina, A.; et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: Pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2). Br. J. Dermatol. 2021, 185, 323–334. [Google Scholar] [CrossRef]
- Galluzzo, M.; Chiricozzi, A.; Cinotti, E.; Brunasso, G.; Congedo, M.; Esposito, M.; Franchi, C.; Malara, G.; Narcisi, A.; Piaserico, S.; et al. Tildrakizumab for treatment of moderate to severe psoriasis: An expert opinion of efficacy, safety, and use in special populations. Expert Opin. Biol. Ther. 2022, 22, 367–376. [Google Scholar] [CrossRef]
- Nast, A.; Smith, C.; Spuls, P.; Valle, G.A.; Bata-Csörgö, Z.; Boonen, H.; De Jong, E.; Garcia-Doval, I.; Gisondi, P.; Kaur-Knudsen, D.; et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris-Part 1: Treatment and monitoring recommendations. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2461–2498. [Google Scholar] [CrossRef]
- Ricceri, F.; Chiricozzi, A.; Peris, K.; Prignano, F. Successful use of anti-IL-23 molecules in overweight-to-obese psoriatic patients: A multicentric retrospective study. Dermatol. Ther. 2022, 35, e15793. [Google Scholar] [CrossRef]
- Yan, J.; Smyth, M.J.; Teng, M.W.L. Interleukin (IL)-12 and IL-23 and Their Conflicting Roles in Cancer. Review. Cold Spring Harb. Perspect. Biol. 2018, 10, a028530. [Google Scholar] [CrossRef]
- Prignano, F.; Brunasso, A.M.G.; Fabbrocini, G.; Argenziano, G.; Bardazzi, F.; Borroni, R.G.; Burlando, M.; Cagni, A.E.; Campione, E.; Cinotti, E.; et al. Sharing Patient and Clinician Experiences of Moderate-to-Severe Psoriasis: A Nationwide Italian Survey and Expert Opinion to Explore Barriers Impacting upon Patient Wellbeing. J. Clin. Med. 2022, 11, 2801. [Google Scholar] [CrossRef]
- Bonifati, C.; Morrone, A.; Cristaudo, A.; Graceffa, D. Effectiveness of anti-interleukin 23 biologic drugs in psoriasis patients who failed anti-interleukin 17 regimens. A real-life experience. Dermatol. Ther. 2021, 34, e14584. [Google Scholar] [CrossRef]
- Drerup, K.A.; Seemann, C.; Gerdes, S.; Mrowietz, U. Effective and Safe Treatment of Psoriatic Disease with the Anti-IL-23p19 Biologic Tildrakizumab: Results of a Real-World Prospective Cohort Study in Nonselected Patients. Dermatology 2022, 238, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Galluzzo, M.; Talamonti, M.; Cioni, A.; Maffei, V.; Shumak, R.G.; Tofani, L.; Bianchi, L.; Campione, E. Efficacy of Tildrakizumab for the treatment of Difficult-to-Treat areas: Scalp, Nail, Palmoplantar and Genital Psoriasis. J. Clin. Med. 2022, 11, 2631. [Google Scholar] [CrossRef] [PubMed]
- Megna, M.; Tommasino, N.; Potestio, L.; Battista, T.; Ruggiero, A.; Noto, M.; Fabbrocini, G.; Genco, L. Real-world practice indirect comparison between guselkumab, risankizumab and tildrakizumab: Results from an Italian 28-week retrospective study. J. Dermatol. Treat. 2022, 33, 2813–2820. [Google Scholar] [CrossRef] [PubMed]
- Megna, M.; Potestio, L.; Fabbrocini, G.; Cinelli, E. Tildrakizumab: A new therapeutic option for erythrodermic psoriasis? Dermatol. Ther. 2021, 34, e15030. [Google Scholar] [CrossRef]
- Narcisi, A.; Valenti, M.; Gargiulo, L.; Ibba, L.; Amoruso, F.; Argenziano, G.; Bardazzi, F.; Burlando, M.; Carrera, C.G.; Damiani, G.; et al. Real-life effectiveness of tildrakizumab in chronic plaque psoriasis: A 52-week multicentre retrospective study-IL PSO (Italian landscape psoriasis). J. Eur. Acad. Dermatol. Venereol. 2023, 37, 93–103. [Google Scholar] [CrossRef]
- Bernardini, N.; Skroza, N.; Rossi, G.; Mambrin, A.; Tolino, E.; Marraffa, F.; Caviglia, M.; Guardo, A.D.; Volpe, S.; Proietti, I.; et al. Long term efficacy, safety, and tolerability of tildrakizumab in epileptic patient with psoriasis and eczema. Dermatol. Rep. 2022, 14, 9447. [Google Scholar] [CrossRef]
- Tsianakas, A.; Schwichtenberg, U.; Pierchalla, P.; Hinz, T.; Diemert, S.; Korge, B. Real-world effectiveness and safety of tildrakizumab in long-term treatment of plaque psoriasis: Results from the non-interventional, prospective, multicentre study TILOT. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 85–92. [Google Scholar] [CrossRef]
- Whitley, S.K.; Li, M.; Kashem, S.W.; Hirai, T.; Igyártó, B.Z.; Knizner, K.; Ho, J.; Ferris, L.K.; Weaver, C.T.; Cua, D.J.; et al. Local IL-23 is required for proliferation and retention of skin-resident memory TH17 cells. Sci. Immunol. 2022, 7, eabq3254. [Google Scholar] [CrossRef]
- Menter, M.A.; Mehta, N.N.; Lebwohl, M.G.; Gottlieb, A.B.; Mendelsohn, A.M.; Rozzo, S.J.; Leonardi, C. The Effect of Tildrakizumab on Cardiometabolic Risk Factors in Psoriasis by Metabolic Syndrome Status: Post Hoc Analysis of Two Phase 3 Trials (ReSURFACE 1 and ReSURFACE 2). J. Drugs Dermatol. 2020, 19, 703–708. [Google Scholar] [CrossRef]
- Augustin, M.; Sommer, R.; Daudén, E.; Laws, P.; de Jong, E.; Fabbrocini, G.; Naldi, L.; Navarini, A.; Lambert, J.; Reguiai, Z.; et al. Patient-reported well-being in value-based care using tildrakizumab in a real-world setting: Protocol of a multinational, phase IV, 1-cohort prospective observational study (the POSITIVE study). BMJ Open 2023, 13, e060536. [Google Scholar] [CrossRef]
- Gottlieb, A.B.; Wu, J.J.; Griffiths, C.E.M.; Marfo, K.; Muscianisi, E.; Meng, X.; Frueh, J.; Lebwohl, M. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: Pooled analysis of 4 phase 3 clinical trials. J. Dermatol. Treat. 2022, 33, 1482–1490. [Google Scholar] [CrossRef]
- de Carvalho, A.V.E.; Alegre, B.S.C.D.M.D.P.; Romiti, R.; Souza, C.D.S.; Paschoal, R.S.; Milman, L.D.M.; Meneghello, L.P. Psoriasis comorbidities: Complications and benefits of immunobiological treatment. An. Bras. Dermatol. 2016, 91, 781–789. [Google Scholar] [CrossRef]
- Viganò, M.; Degasperi, E.; Aghemo, A.; Lampertico, P.; Colombo, M. Anti-TNF drugs in patients with hepatitis B or C virus infection: Safety and clinical management. Expert Opin. Biol. Ther. 2012, 12, 193–207. [Google Scholar] [CrossRef]
- Minozzi, S.; Bonovas, S.; Lytras, T.; Pecoraro, V.; González-Lorenzo, M.; Bastiampillai, A.J.; Gabrielli, E.M.; Lonati, A.C.; Moja, L.; Cinquini, M.; et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: A systematic review and meta-analysis. Expert Opin. Drug Saf. 2016, 15 (Suppl. S1), 11–34. [Google Scholar] [CrossRef]
- Campione, E.; Cosio, T.; Lanna, C.; Mazzilli, S.; Ventura, A.; Dika, E.; Gaziano, R.; Dattola, A.; Candi, E.; Bianchi, L. Predictive role of vitamin A serum concentration in psoriatic patients treated with IL-17 inhibitors to prevent skin and systemic fungal infections. J. Pharmacol. Sci. 2020, 144, 52–56. [Google Scholar] [CrossRef]
- Bianchi, L.; Costanza, G.; Campione, E.; Ruzzetti, M.; Di Stefani, A.; Diluvio, L.; Giardina, E.; Cascella, R.; Cordiali-Fei, P.; Bonifati, C.; et al. Biomolecular index of therapeutic efficacy in psoriasis treated with anti-TNF-α agents. G. Ital. Dermatol. Venereol. 2018, 153, 316–325. [Google Scholar] [CrossRef]
- Ventura, A.; Mazzeo, M.; Gaziano, R.; Galluzzo, M.; Bianchi, L.; Campione, E. New insight into the pathogenesis of nail psoriasis and overview of treatment strategies. Drug Des. Dev. Ther. 2017, 11, 2527–2535. [Google Scholar] [CrossRef]
- Frampton, J.E. Tildrakizumab: A Review in Moderate-to-Severe Plaque Psoriasis. Am. J. Clin. Dermatol. 2019, 20, 295–306. [Google Scholar] [CrossRef]
- Bangert, C.; Kopp, T. Tildrakizumab for the treatment of psoriasis. Immunotherapy 2018, 10, 1105–1122. [Google Scholar] [CrossRef]
- Ruggiero, A.; Fabbrocini, G.; Cinelli, E.; Ocampo Garza, S.S.; Camela, E.; Megna, M. Anti-interleukin-23 for psoriasis in elderly patients: Guselkumab, risankizumab and tildrakizumab in real-world practice. Clin. Exp. Dermatol. 2022, 47, 561–567. [Google Scholar] [CrossRef]
- Crowley, J.J.; Warren, R.B.; Cather, J.C. Safety of selective IL-23p19 inhibitors for the treatment of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Costanza, G.; Doldo, E.; Ferlosio, A.; Tarquini, C.; Passeri, D.; Cascella, R.; Bavetta, M.; Di Stefani, A.; Bonifati, C.; Agostinelli, S.; et al. Expression and potential role of cellular retinol binding protein I in psoriasis. Oncotarget 2018, 9, 36736–36749. [Google Scholar] [CrossRef] [PubMed]

| n (%) 53 (100%) | |
|---|---|
| Mean age, yrs: 51.2 (range 22–79) | |
| Male | 34 (64.1%) |
| Female | 19 (35.9%) |
| Smokers | 25 (47.1%) |
| Family history | 24 (45.2%) |
| Disease duration | 16 yrs (range 1–46) |
| Mean age of onset, yrs: 34.6 (range 8–73) |
| Previous Treatments | n (%) 77 (100) |
|---|---|
| Topical treatments | 10 (12.9) |
| Methotrexate | 14 (18.2) |
| Cyclosporine | 21 (27.3) |
| Acitretin | 3 (3.9) |
| NB-UVB | 4 (5.2) |
| Dimethyl fumarate | 3 (3.9) |
| Apremilast | 8 (10.4) |
| Adalimumab | 2 (2.6) |
| Ustekinumab | 2 (2.6) |
| Secukinumab | 7 (9.1) |
| Others (etanercept, brodalumab) | 3 (3.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campione, E.; Lambiase, S.; Gaeta Shumak, R.; Galluzzo, M.; Lanna, C.; Costanza, G.; Borselli, C.; Artosi, F.; Cosio, T.; Tofani, L.; et al. A Real-Life Study on the Use of Tildrakizumab in Psoriatic Patients. Pharmaceuticals 2023, 16, 526. https://doi.org/10.3390/ph16040526
Campione E, Lambiase S, Gaeta Shumak R, Galluzzo M, Lanna C, Costanza G, Borselli C, Artosi F, Cosio T, Tofani L, et al. A Real-Life Study on the Use of Tildrakizumab in Psoriatic Patients. Pharmaceuticals. 2023; 16(4):526. https://doi.org/10.3390/ph16040526
Chicago/Turabian StyleCampione, Elena, Sara Lambiase, Ruslana Gaeta Shumak, Marco Galluzzo, Caterina Lanna, Gaetana Costanza, Cristiana Borselli, Fabio Artosi, Terenzio Cosio, Lorenzo Tofani, and et al. 2023. "A Real-Life Study on the Use of Tildrakizumab in Psoriatic Patients" Pharmaceuticals 16, no. 4: 526. https://doi.org/10.3390/ph16040526
APA StyleCampione, E., Lambiase, S., Gaeta Shumak, R., Galluzzo, M., Lanna, C., Costanza, G., Borselli, C., Artosi, F., Cosio, T., Tofani, L., Dattola, A., Di Daniele, F., & Bianchi, L. (2023). A Real-Life Study on the Use of Tildrakizumab in Psoriatic Patients. Pharmaceuticals, 16(4), 526. https://doi.org/10.3390/ph16040526









