Natural Coumarin Derivatives Activating Nrf2 Signaling Pathway as Lead Compounds for the Design and Synthesis of Intestinal Anti-Inflammatory Drugs
Abstract
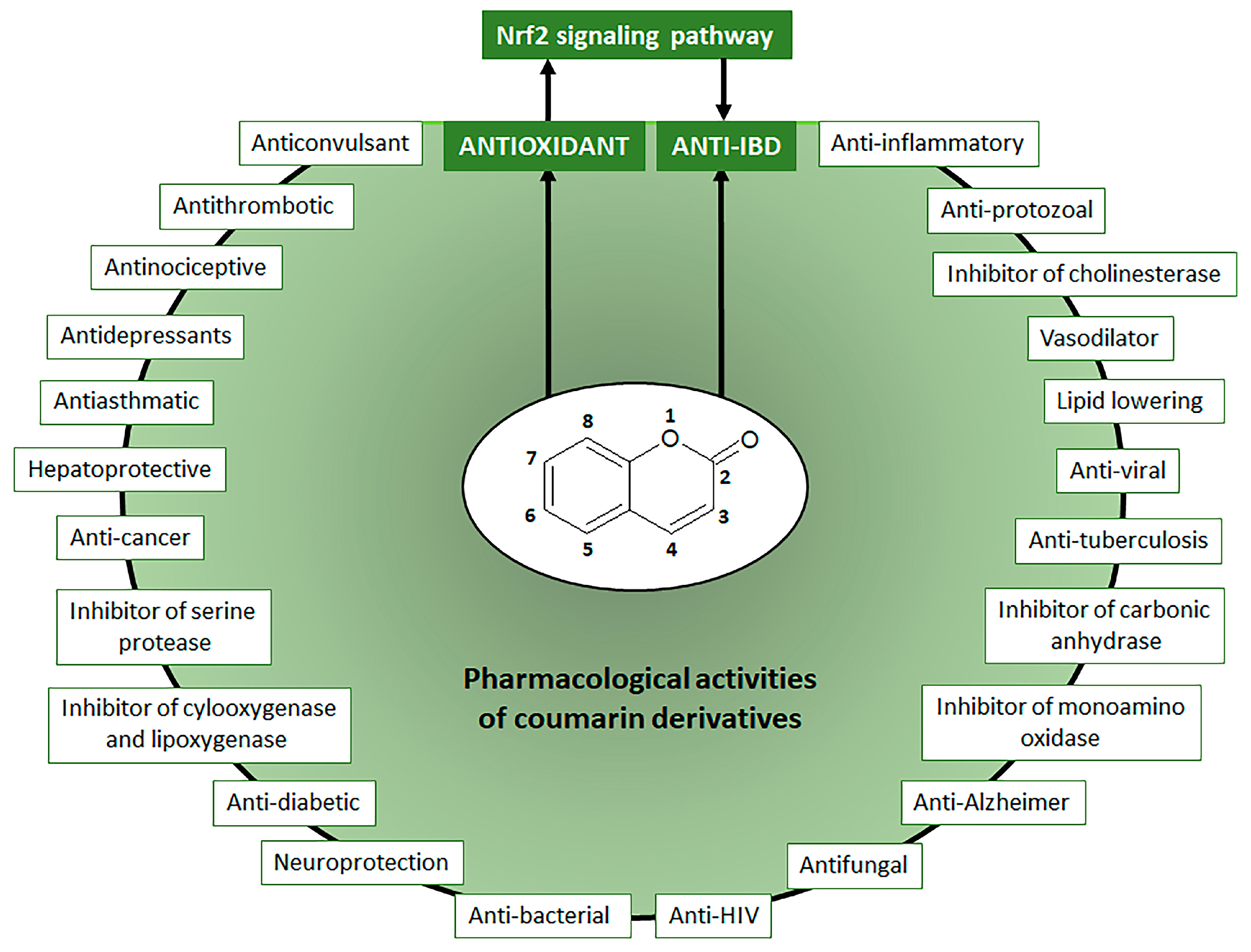
1. Introduction
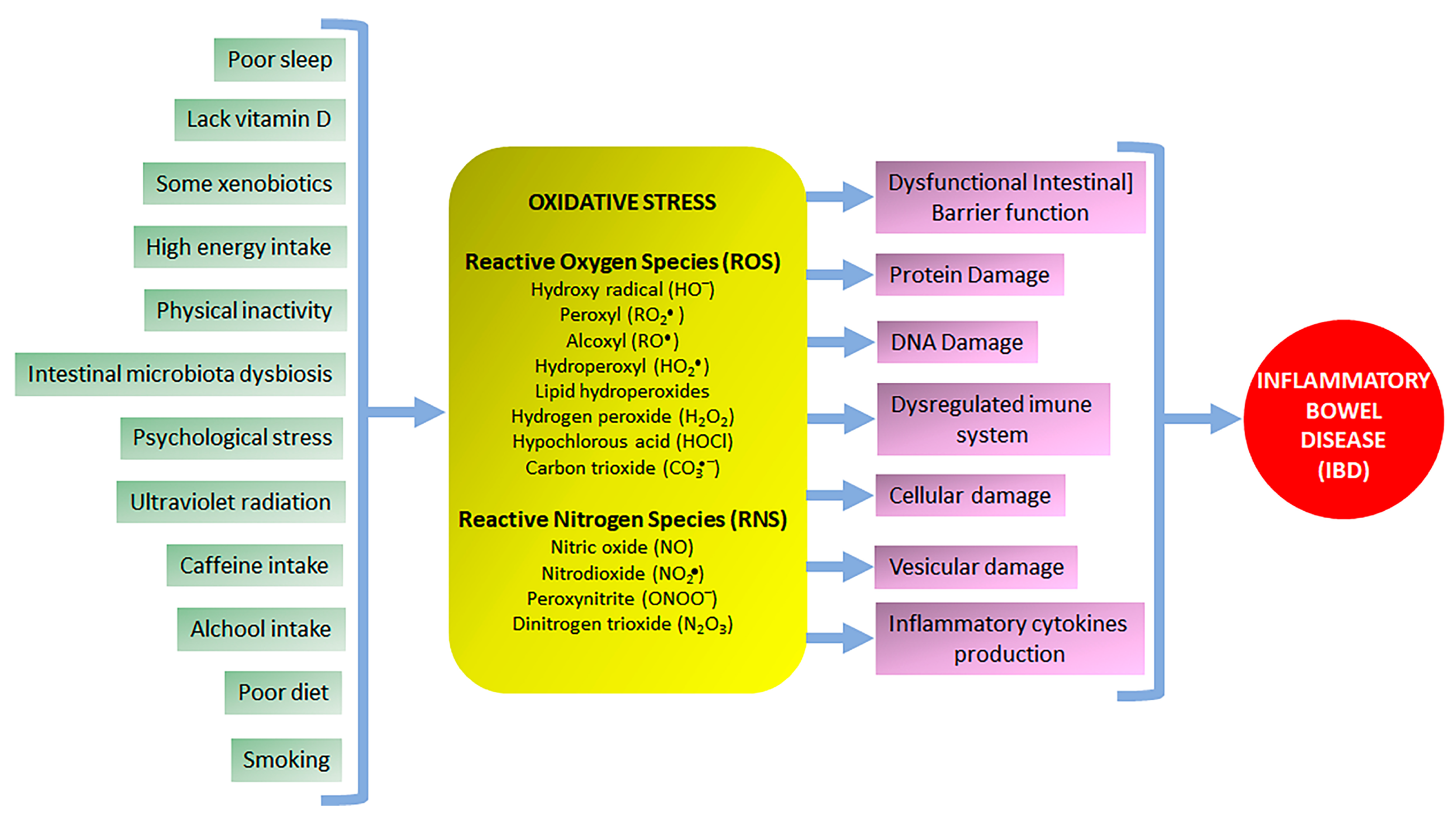
2. The Role of Oxidative Stress in NCDs Focusing on IBD
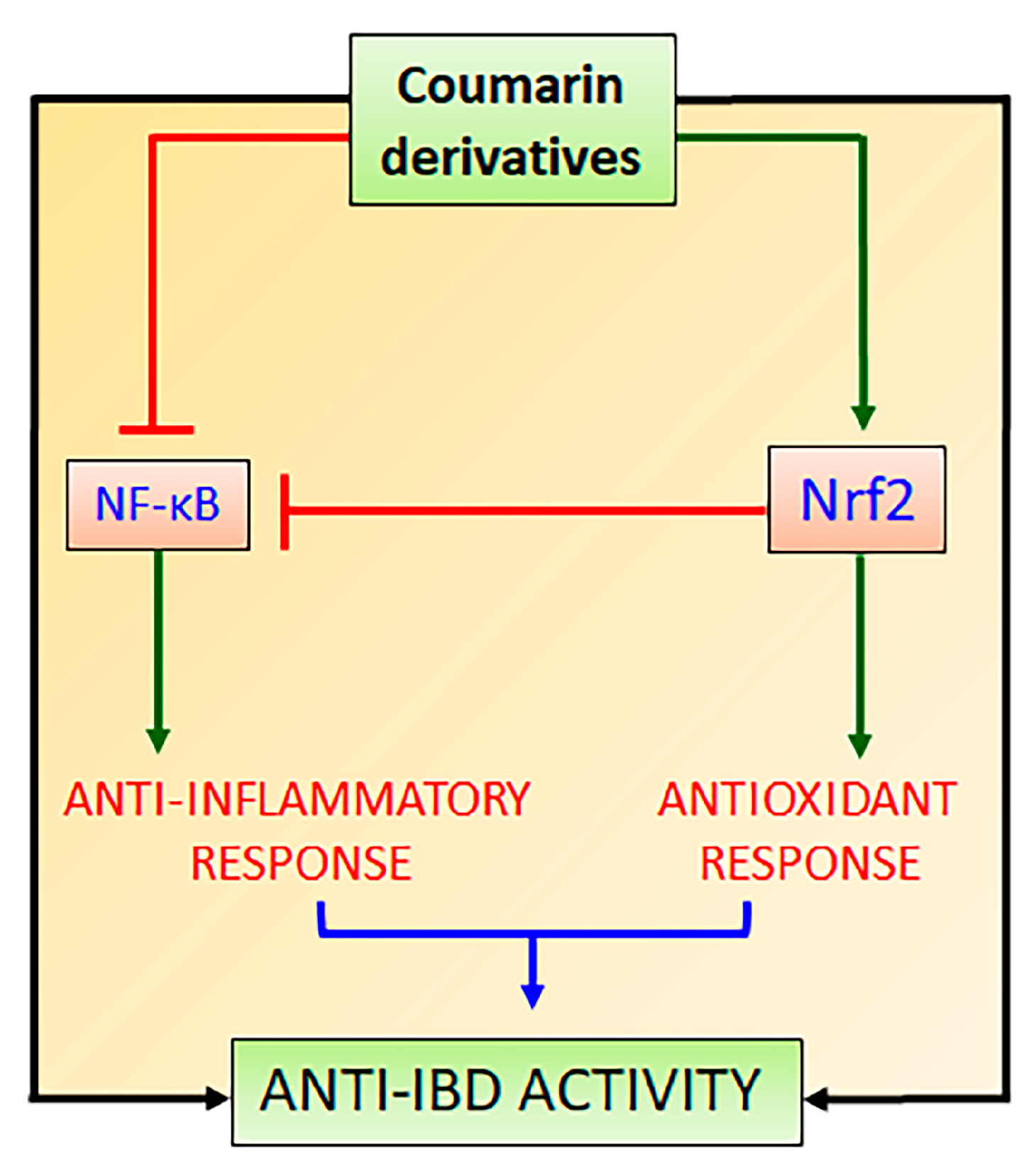
3. Nrf2 and Its Interaction with NF-κB Signaling Pathways to Control Oxidative Stress and Promote Intestinal Anti-Inflammatory Activity
4. Intestinal Anti-Inflammatory Coumarin Derivatives Targeting Nrf2-Keap1 Signaling Pathway
4.1. Simple Intestinal Anti-Inflammatory Coumarin Derivatives Targeting Nrf2 Signaling
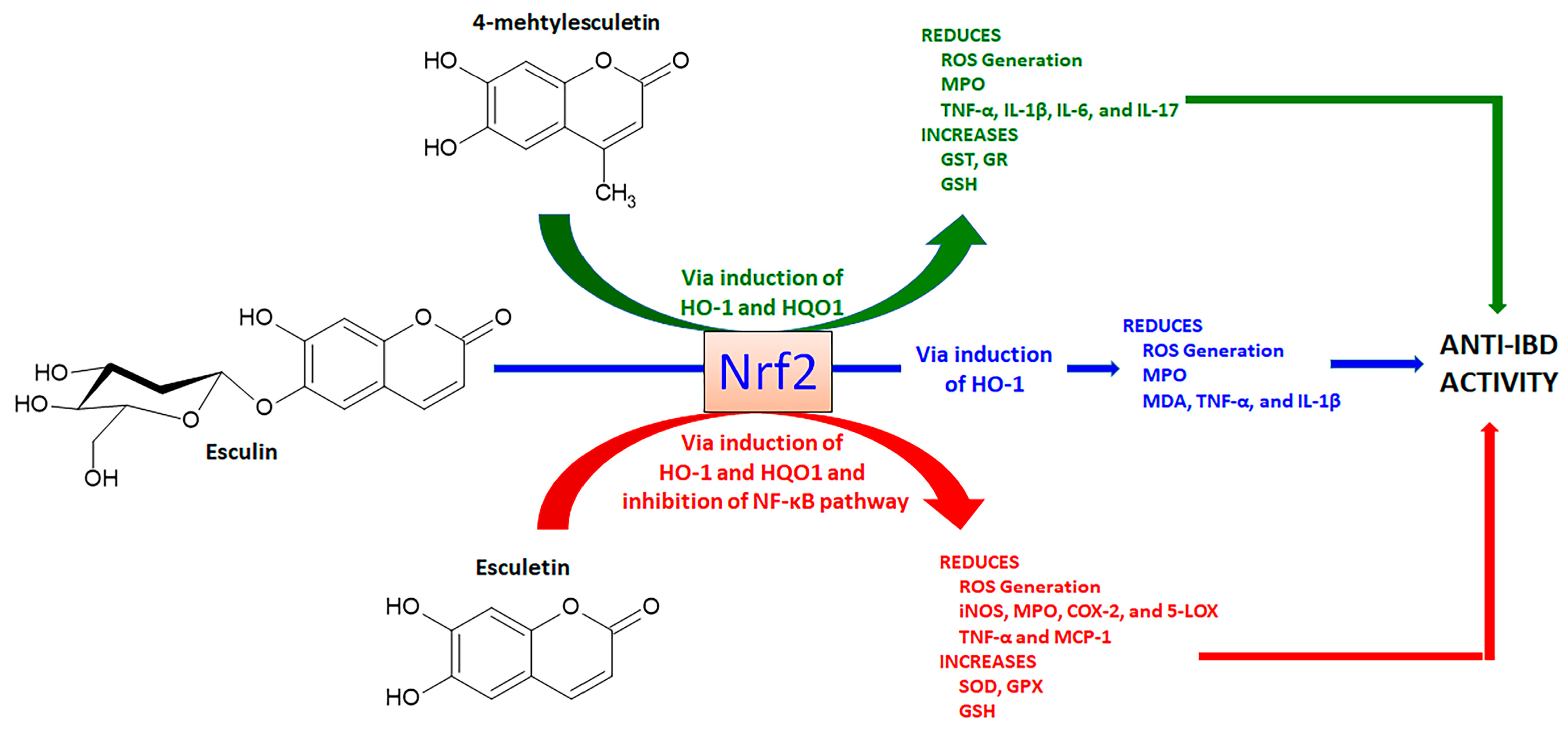
4.1.1. Esculetin, 4-Methylesculetin, and Esculin
4.1.2. Daphnetin
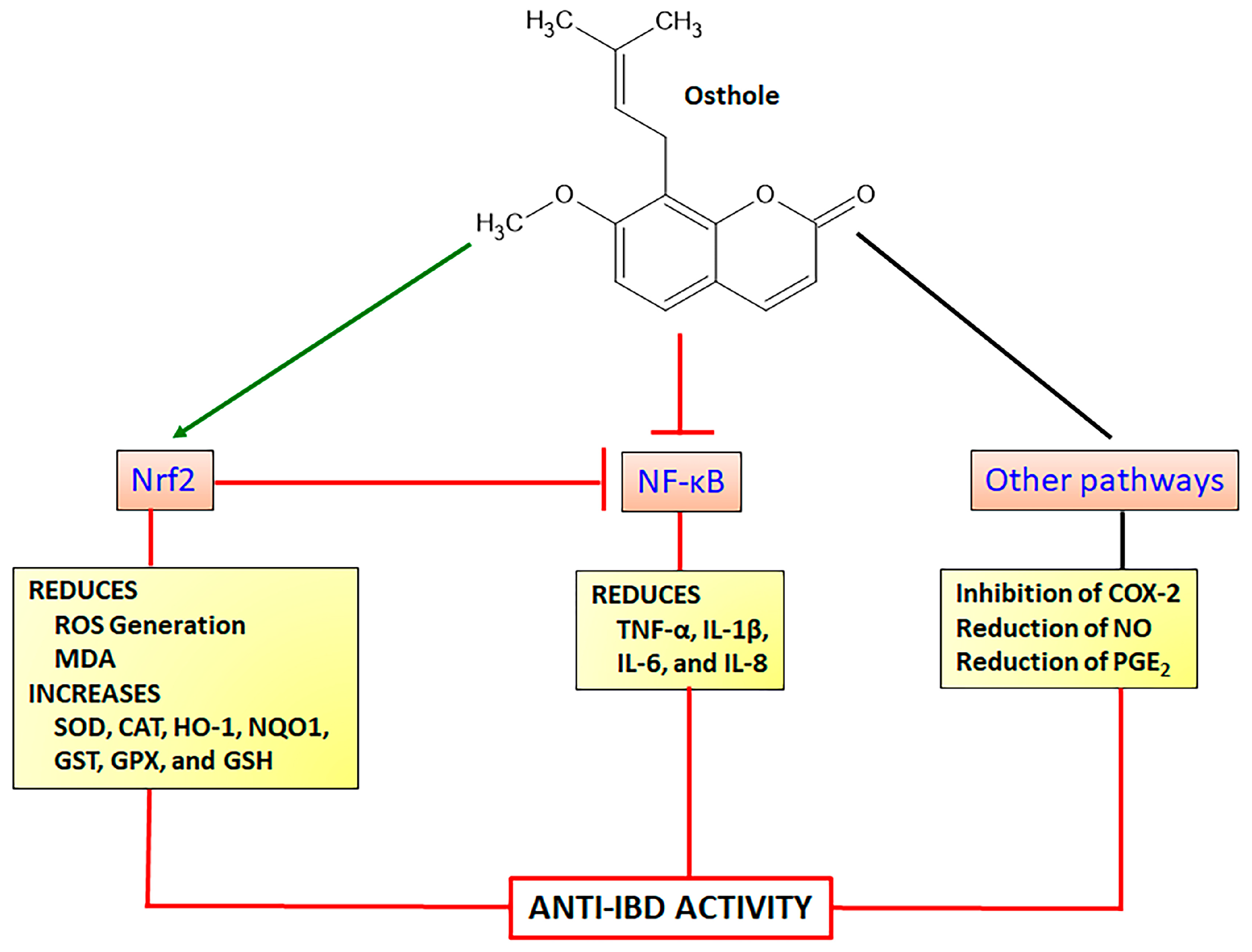
4.1.3. Osthole
4.1.4. Umbelliferone
4.1.5. Fraxetin
4.1.6. Scopoletin and Scoparone
4.2. Intestinal Anti-Inflammatory Furanocoumarin Derivatives Targeting Nrf2 Signaling
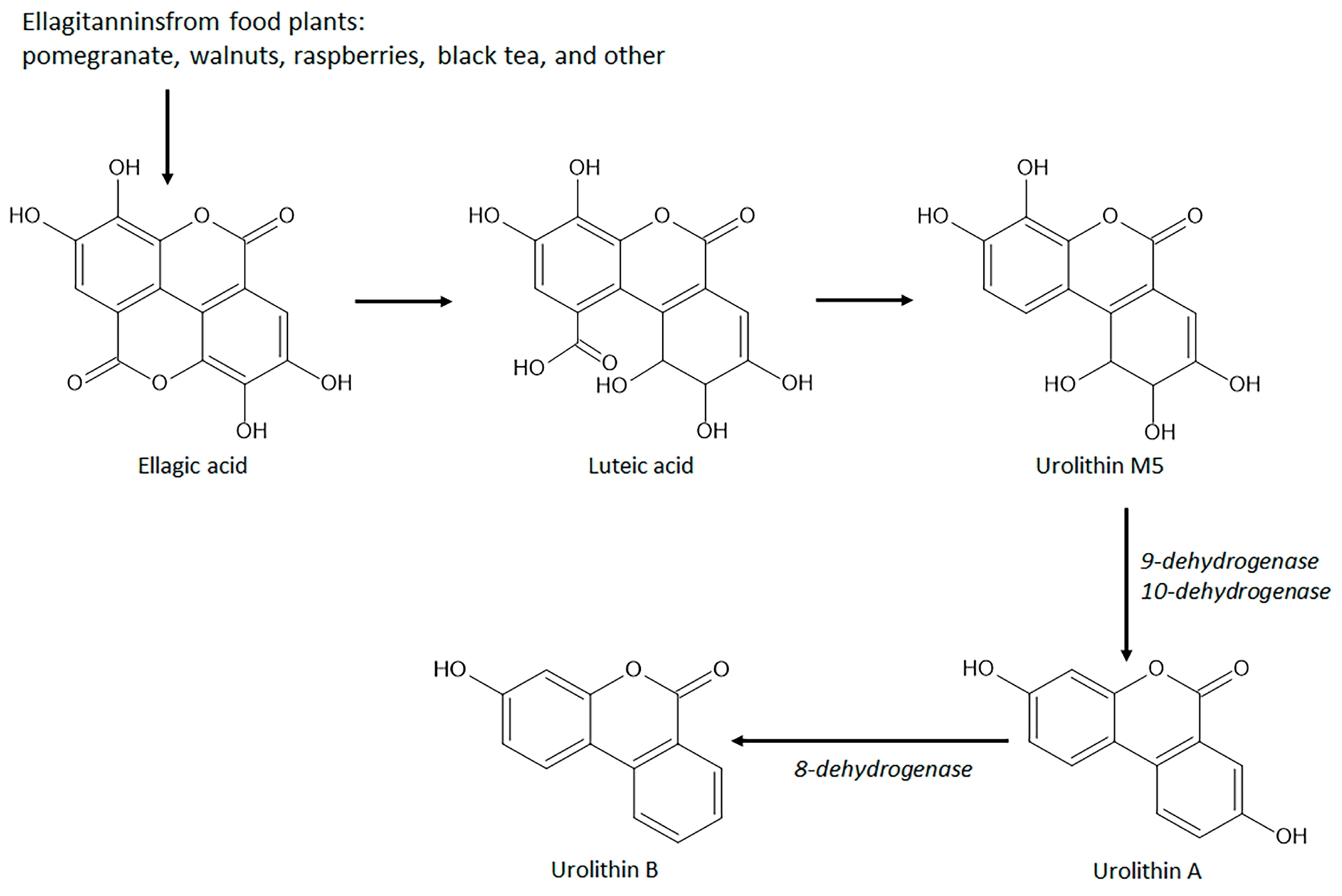
4.3. Intestinal Anti-Inflammatory Gut Microbial Coumarins Targeting Nrf2 Signaling
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jain, P.K.; Joshi, H. Coumarin: Chemical and pharmacological profile. J. Appl. Pharm. Sci. 2012, 2, 236–240. [Google Scholar]
- Lake, B.G. Coumarin metabolism, toxicity, and carcinogenicity: Relevance for human risk assessment. Food Chem. Toxicol. 1999, 37, 423–453. [Google Scholar] [CrossRef]
- Costa, T.M.; Tavares, L.B.B.; Oliveira, D. Fungi as a source of natural coumarins production. Appl. Microbiol. Biotechnol. 2016, 100, 6571–6584. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.B. Warfarin: From rat poison to clinical use. Nat. Rev. Cardiol. 2017. [Google Scholar] [CrossRef]
- Bush, E.; Trager, W.F. High-yield synthesis of warfarin and its phenolic metabolites: New compounds. J. Pharm. Sci. 1983, 72, 830–831. [Google Scholar] [CrossRef]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef] [PubMed]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Song, X.F.; Fan, J.; Liou, L.; Liu, X.F.; Gao, F. Coumarin derivatives with anticancer activities: An update. Arch. Pharm. 2020, 353, e22000025. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, A.; Yang, Y.; Yang, P. Coumarin-containing hybrids and their antibacterial activities. Arch. Pharm. 2020, 353, e1900380. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Q.; Zhang, Y.; Liang, C. Coumarin-based derivatives with potential anti-HIV activity. Fitoterapia 2021, 150, 104863. [Google Scholar] [CrossRef]
- Reddy, D.S.; Kongot, M.; Kumar, A. Coumarin hybrid derivatives as promising leads to treat tuberculosis: Recent developments and critical aspects of structural design to exhibit anti-tubercular activity. Tuberculosis 2021, 127, 102050. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. Coumarin-containing hybrids and their anticancer activities. Eur. J. Med. Chem. 2019, 181, 111587. [Google Scholar] [CrossRef]
- Carneiro, A.; Matos, M.J.; Uriarte, E.; Santana, L. Trending topics on coumarin and its derivatives in 2020. Molecules 2021, 26, 501. [Google Scholar] [CrossRef]
- Supuran, C.T. Coumarin carbonic anhydrase inhibitors from natural sources. J. Enz. Inhib. Med. Chem. 2020, 35, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Di Stasi, L.C. Coumarin derivatives in inflammatory bowel disease. Molecules 2021, 26, 422. [Google Scholar] [CrossRef]
- Jameel, E.; Ulmar, T.; Kumar, J.; Hoda, N. Coumarin: A privileged scaffold for the design and development of antineurodegenerative agents. Chem. Biol. Drug Des. 2016, 87, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.S.; Gupta, J.; Sharma, S.; Sahu, D. An insight into the therapeutic applications of coumarin compounds and their mechanisms of action. Eur. J. Pharm. Sci. 2020, 152, 105424. [Google Scholar] [CrossRef] [PubMed]
- Al-Warhi, T.; Sabt, A.; Elkaeed, E.B.; Eldehna, W.M. Recent advancements of coumarin-based anticancer agents: An up-to-date review. Bioog. Chem. 2020, 103, 104163. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Sayed, A.M.; Hussein, O.E.; Mahmoud, A.M. Coumarins as modulators of the keap1/Nrf2/ARE signaling pathway. Oxidat. Med. Cell. Long. 2020, 2020, 167957. [Google Scholar] [CrossRef]
- Bubols, G.B.; Vianna, D.R.; Medina-Remón, A.; von Poser, G.; Lamuela-Raventos, R.M.; Eifler-Lima, V.L.; Garcia, S.C. The antioxidant activity of coumarins and flavonoids. Minirev. Med. Chem. 2013, 13, 318–334. [Google Scholar]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E. Study of antioxidant properties of agents from the perspective of their action mechanisms. Molecules 2020, 25, 4251. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, W.; Su, Z.; Kong, A.T. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsfoled, A.M.; Sanderson, C.M. Dissecting molecular crosstalk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Opara, E.C. Oxidative stress. Dis. Mon. 2006, 52, 183–198. [Google Scholar] [CrossRef]
- GDB 2017. Burden Disease Collaborators. Global, regional, and national age-sex specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Bloom, D.E.; Cafiero, E.T.; Jané-Llopis, E.; Abrahams-Gessel, S.; Bloom, L.R.; Fathima, S.; Feigl, A.B.; Gaziano, T.; Mowafi, M.; Pandya, A.; et al. The Global Economic Burden of Non-Communicable Diseases; World Economic Forum: Geneva, Switzerland, 2011. [Google Scholar]
- WHO. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Beard, J.R.; Officer, A.; De Carvalho, I.A.; Sadana, R.; Pot, A.M.; Michel, J.P.; Lloyd-Sherlock, P.; Epping-Jordan, J.E.; Peeters, G.M.E.E.; Mahanani, W.R.; et al. The World report on ageing and health: A policy framework for healthy ageing. Lancet 2016, 387, 2145–2154. [Google Scholar] [CrossRef]
- Seyedsadjadi, N.; Grant, R. The potential benefit of monitoring oxidative stress and inflammation in the prevention of non-communicable diseases (NCDs). Antioxidants 2021, 10, 15. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P.; Rahman, H.S. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2015, 9, 1162. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Gastroenterol. Hepatol. 2015, 12, 205–2017. [Google Scholar] [CrossRef]
- Ghouri, Y.A.; Tahan, V.; Shen, B. Secondary causes of inflammatory bowel diseases. World J. Gastroenterol. 2020, 26, 3998–4017. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhanf, J. Pathomechanisms of oxidative stress in Inflammatory Bowel Disease and potential antioxidant therapies. Oxidat. Med. Cell. Long. 2017, 2017, 4535194. [Google Scholar] [CrossRef] [PubMed]
- Piechota-Polanczyk, A.; Fichna, J. Review article: The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn-Schimiedeberg’s Arch. Pharmacolol. 2014, 387, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Krzystek-Korpacka, M.; Kempinski, R.; Bromke, M.A.; Meubauer, K. Oxidative stress markers in Inflammatory Bowel Diseases: Systematic review. Diagnostics 2020, 10, 601. [Google Scholar] [CrossRef] [PubMed]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Diseases: Clinical aspects and animal models. Saudi J. Gastroenterol. 2016, 22, 3–17. [Google Scholar]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative stress and redox-modulating therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- Dziabowska-Grabias, K.; Sztanke, M.; Zajac, P.; Celejawski, M.; Kurek, K.; Szkutnicki, S.; Korga, P.; Bulikowski, W.; Sztanke, K. Antioxidant therapy in inflammatory bowel diseases. Antioxidants 2021, 10, 412. [Google Scholar] [CrossRef]
- Quaglio, A.E.V.; Castilho, A.C.S.; Di Stasi, L.C. Experimental evidence of heparanase, Hsp70 and NF-κB gene expression on the response of anti-inflammatory drugs in TNBS-induced colonic inflammation. Life Sci. 2015, 141, 179–187. [Google Scholar] [CrossRef]
- Quaglio, A.E.V.; Castilho, A.C.S.; Di Stasi, L.C. Experimental evidence of MAP kinase gene expression on the response of anti-inflammatory drugs. Life Sci. 2015, 136, 60–66. [Google Scholar] [CrossRef]
- Beijani, F.; Evanno, E.; Zibara, K.; Piechaczyk, M. The AP-1 transcriptional complex: Local switch or remote command? BBA Rev. Cancer 2019, 1872, 11–23. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Sign. Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Reddy, R.C.; Standiford, T.J. Nrf2 and PPARγ: PPARtnering against oxidant-induced lung injury. Am. J. Resp. Crit. Care Med. 2010, 182, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Collaborative power of Nrf2 and PPARγ activators against metabolic and drug-induced oxidative injury. Oxidat. Med. Cell. Long. 2017, 2017, 1378175. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Lu, M.C.; Ji, J.A.; Jiang, Z.Y.; You, Q.D. The keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: Un update. Med. Res. Rev. 2016, 5, 924–963. [Google Scholar] [CrossRef] [PubMed]
- Dovinova, I.; Kvandova, M.; Balis, P.; Gresova, L.; Majzunova, M.; Horakova, L.; Chan, J.Y.H.; Barancik, M. The role of Nrf2 and PPAR-γ in the improvement of oxidative stress in hypertension and cardiovascular diseases. Physiol. Res. 2020, 69, S541–S553. [Google Scholar] [CrossRef]
- Taguchi, K.; Motohaschi, H.; Yamamoto, M. Molecular mechanism of the keap-1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011, 16, 123–140. [Google Scholar] [CrossRef]
- Uruno, A.; Yagishita, Y.; Yamamoto, M. The Keap-1-Nrf2 system and diabetes mellitus. Arch. Biochem. Biophys. 2015, 566, 76–85. [Google Scholar] [CrossRef]
- Vasileva, L.V.; Savova, M.S.; Amirova, K.M.; Dinkova-Kostova, A.T.; Georgiev, M.L. Obesity and Nrf2-mediated cytoprotection: Where is the missing link? Pharmacol. Res. 2020, 156, 104760. [Google Scholar] [CrossRef]
- Peña-Oyarzun, D.; Bravo-Sagua, R.; Diaz, A.; Aleman, L.; Chiong, M.; Gracia, L.; Bambs, C.; Troncoso, R.; Cifuentes, M.; Morselli, E.; et al. Autophagy and oxidative stress in non-communicable diseases: A matter of the inflammatory state? Free Rad. Biol. Med. 2018, 124, 61–78. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative diseases. Free Rad. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef]
- Piotrowska, M.; Swierczynski, M.; Fichna, J.; Piechota-Polanczyk, A. The Nrf2 in the pathophysiology of the intestine: Molecular mechanisms and therapeutic implications for inflammatory bowel diseases. Pharmacol. Res. 2021, 163, 105243. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, D.K.; Eden, K.; Ringel, V.M.; Allen, I.C. Emerging roles for noncanonical NF-κB signaling in the modulation of inflammatory bowel disease pathobiology. Inflamm. Bowel Dis. 2016, 22, 2265–2279. [Google Scholar] [CrossRef] [PubMed]
- Spehlman, M.E.; Eckmann, L. Nuclear factor-kappa B in intestinal protection and destruction. Curr. Opin. Gastroenterol. 2009, 25, 92–99. [Google Scholar] [CrossRef]
- Karrash, T.; Jobin, C. NF-_B and the intestine: Friend or foe? Inflamm. Bowel Dis. 2008, 14, 114–124. [Google Scholar] [CrossRef]
- Rogler, G.; Brand, K.; Vogl, D.; Page, S.; Hofmeister, R.; Andus, T.; Knuechel, R.; Baeuerle, P.A.; Scholmerich, J.; Gross, V. Nuclear factor κB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterol. 1998, 115, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Yerra, V.G.; Negi, G.; Sharma, S.S.; Kumar, A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef]
- Kim, J.E.; You, D.J.; Lee, C.; Ahn, C.; Seong, J.Y.; Hwang, J.I. Suppression of NF-κB signaling by Keap1 regulation of IKKβ activity through autophagic degradation and inhibition of phosphorylation. Cell. Sign. 2010, 22, 1645–1654. [Google Scholar] [CrossRef]
- Soares, M.P.; Seldon, M.P.; Gregoire, I.P.; Vassilevskaia, T.; Berberat, P.O.; Yu, J.; Tsui, T.Y.; Bach, F.H. Heme-oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J. Immunol. 2004, 172, 3553–3563. [Google Scholar] [CrossRef]
- Sun, Z.; Chin, E.; Zhang, D.D. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during antioxidant response. Mol. Cell. Biol. 2009, 29, 2658–2672. [Google Scholar] [CrossRef]
- Liu, G.H.; Qu, J.; Shen, X. NF-κB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta 2008, 1783, 713–727. [Google Scholar] [CrossRef]
- Yu, M.; Li, H.; Liu, Q.; Liu, F.; Tang, L.; Li, C.; Yuan, Y.; Zhan, Y.; Xu, W.; Li, W.; et al. Nuclear factor p65 interacts with keap1 to repress the Nrf2-ARE pathway. Cell. Sign. 2011, 23, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the Keap1-Nrf2 pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Sawney, S.; Saini, V.; Steffi, C.; Tiwari, M.; Saluja, D. Esculetn induces antiproliferative and apoptotic response in pancreatic cancer cells by directly binding to Keap1. Mol. Cancer 2016, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, Y.; Namkoong, S.; Lee, J. Esculetin inhibits the inflammatory response by inducing heme-oxygenase-1 in co-cultured macrophages and adipocytes. Food Funct. 2014, 5, 2371. [Google Scholar] [CrossRef] [PubMed]
- Rubio, V.; García-Pérez, A.I.; Herráez, A.; Diez, J.C. Different roles of Nrf2 and NFκB in the antioxidant imbalance produced by esculetin or quercetin on NB4 Leukemia cells. Chemico-Biol. Interac. 2018, 294, 158–166. [Google Scholar] [CrossRef]
- Zhang, Y.; An, Y.; He, X.; Zhang, D.; He, W. Esculetin protects human corneal epithelial cells from oxidative stress through Nrf2 signaling pathway. Exp. Eye Res. 2021, 202, 108360. [Google Scholar] [CrossRef]
- Han, M.H.; Park, C.; Lee, D.S.; Hong, S.H.; Choi, I.W.; Kim, G.Y.; Choi, S.H.; Shim, J.H.; Chae, J.I.; Yoo, Y.H.; et al. Cutoprotective effects of esculetin against oxidative stress are associated with the upregulation of Nrf2-mediated NQO1 expression via the activation of the ERK pathway. Int. J. Mol. Sci. 2017, 39, 380–386. [Google Scholar]
- Pruccoli, L.; Morroni, F.; Sita, G.; Hrelia, P.; Tarozzi, A. Esculetin as a bifunctional antioxidant prevents and counteracts the oxidative stress and neuronal death induced by amyloid protein in SH-SY5Y cells. Antioxidants 2020, 9, 551. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Wu, H.; Wang, J.; Zhang, S. Esculetin alleviates murine lupus nephritis by inhibiting complement activation and enhancing Nrf2 signaling pathway. J. Ethnopharmacol. 2022, 288, 115004. [Google Scholar] [CrossRef]
- Türk, E.; Tekeli, I.O.; Özkan, H.; Uyar, A.; Cellat, M.; Kuzu, M.; Yava, I.; Yegani, A.A.; Yaman, T.; Güvenç, M. The protective effect of esculetin against aluminium chloride-induced reproductive toxicity in rats. Andrologia 2021, 53, e13930. [Google Scholar] [CrossRef]
- Bingru, X.; Liyang, Z.; Jin, C.; Zhanqiang, M.; Qiang, F.; Wei, W.; Xueyang, D.; Shiping, M. Esculetin improves cognitive impairments induced by transient cerebral ischaemia and reperfusion in mice via regulation of mitochondrial fragmentation and mitophagy. Behav. Brain Res. 2019, 372, 112007. [Google Scholar]
- Li, L.; Zhu, G.; Fu, G.; Zha, W.; Li, H. Metabolic syndrome ameliorated by 4-methylesculetin by reducing hepatic lipid accumulation. Int. J. Mol. Sci. 2022, 23, 10465. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Park, H.; Park, H.J.; Choi, K.H.; Sadikot, R.T.; Cha, J.; Joo, M. Glycosylation enables aesculin to activate Nrf2. Sci. Rep. 2016, 6, 29956. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, A.; Nagarajan, r.; Sundarraj, K.; Palanisamy, K.; Perumal, E. Identification of compounds that inhibit the binding of Keap1a/Keap1b Kelch DGR domain with Nrf2 ETGE/DLG motifsin zebrafish. Basic Clin. Pharmacol. Toxicol. 2019, 125, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Shen, Y.; Du, Y.; Chen, J.; Pei, F.; Fu, W.; Qiao, J. Esculin prevents lipopolysaccharide/D-galactosamine-induced acute liver injury in mice. Microb. Pathog. 2018, 125, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Witaicenis, A.; Seito, L.N.; Di Stasi, L.C. Intestinal anti-inflammatory activity of esculetin and 4-methylesculetin in the trinitrobenzenesulphonic acid model of rat colitis. Chemico-Biol. Interact. 2010, 186, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Witaicenis, A.; Luchini, A.C.; Hiruma-Lima, C.A.; Felisbino, S.L.; Garrido-Mesa, N.; Utrilla, P.; Gálvez, J.; Di Stasi, L.C. Suppression of TNBS-induced colitis in rats by 4-methylesculetin, a natural coumarin? Comparison with prednisolone and sulphasalazine. Chemico-Biol. Interact. 2012, 195, 76–85. [Google Scholar] [CrossRef]
- Witaicenis, A.; Oliveira, E.C.S.; Tanimoto, A.; Zorzella-Pezavento, S.F.G.; Oliveira, S.L.; Sartori, A.; Di Stasi, L.C. 4-methylesculetin, a coumarin derivative, ameliorates dextran sulfate sodium-induced intestinal inflammation. Chemico-Biol. Interact. 2018, 280, 59–63. [Google Scholar] [CrossRef]
- Yum, S.; Jeong, S.; Lee, S.; Kim, W.; Nam, J.; Jung, Y. HIF-prolyl hydroxylase is a potential molecular target for esculetin-mediated anti-colitic effects. Fitoterapia 2015, 103, 55–62. [Google Scholar] [CrossRef]
- Wang, S.K.; Chen, T.X.; Wang, W.; Xu, L.L.; Zhang, Y.Q.; Jin, Z.; Liu, Y.B.; Tang, Y.Z. Aesculetin exhibited anti-inflammatory activities through inhibiting NF-κB and MAPKs pathway in vitro and in vivo. J. Ethnopharmacol. 2022, 296, 115489. [Google Scholar] [CrossRef]
- Tanimoto, A.; Witaicenis, A.; Caruso, I.P.; Piva, H.M.R.; Araujo, G.C.; Moraes, F.R.; Fossey, M.C.; Cornélio, M.L.; Souza, F.P.; Di Stasi, L.C. 4-methylesculetin, a natural coumarin with intestinal anti-inflammatory activity, elicits a glutathione antioxidant response by different mechanisms. Chemico-Biol. Interact. 2020, 315, 108876. [Google Scholar] [CrossRef] [PubMed]
- Witaicenis, A.; Seito, L.N.; Chagas, A.S.; Almeida-Junior, L.D.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Di Stasi, L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine 2014, 21, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Peng, Z.; Luo, S.; Zhang, S.; Li, B.; Zhou, C.; Fan, H. Aesculin protects against DSS-induced colitis through activating PPAR-γ and inhibiting NF-κB pathway. Eur. J. Pharmacol. 2019, 857, 172453. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jha, S.; Pattanayak, S.P. Daphnetin ameliorates 7,12-dimethylbenz[a]anthracene-induced mammary carcinogenesis through Nrf2-Keap1 and NF-κB pathways. Biomed. Pharmacother. 2016, 82, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, Y.; Li, H.; Cao, H.; Liu, B.; Zhang, H.; Shao, F. Daphnetin protects against cisplatin-induced nephrotoxicity by inhibiting inflammatory and oxidative response. Int. Immunopharmacol. 2018, 65, 402–407. [Google Scholar] [CrossRef]
- Yang, Y.; Sheng, Q.; Nie, Z.; Liu, L.; Zhang, W.; Chen, G.; Ye, F.; Shi, L.; Lv, Z.; Wang, D. Daphnetin inhibits spinal glial activation via Nrf2/HO-1/NF-κB signaling pathway and attenuates CFA-induced inflammatory pain. Int. Immunopharmacol. 2021, 98, 107882. [Google Scholar] [CrossRef]
- Tian, B.; Ma, X.; Jiang, R. Daphnetin mitigates ovalbumin-induced allergic rhinitis in mice by regulating Nrf2/HO-1 and TLR4/NF-κB signaling. Am. J. Rhinol. Allergy 2023, 37, 19–25. [Google Scholar] [CrossRef]
- Syed, A.M.; Kundu, S.; Ran, C.; Kulhari, U.; Kumar, A.; Mugale, M.N.; Mohapatra, P.; Murty, U.S.; Sahu, B.D. Up-regulation of Nrf2/HO-1 and inhibition of TGF-β1/Smad2/3 signaling axis by daphnetin alleviates transverse aortic constriction-induced cardiac remodeling in mice. Free Rad. Biol. Med. 2022, 186, 17–30. [Google Scholar] [CrossRef]
- Xu, K.; Guo, L.; Bu, H.; Wang, H. Daphnetin inhibits high glucose-induced extracellular matrix accumulation, oxidative stress and inflammation in human glomerular mesangial cells. J. Pharm. Sci. 2019, 139, 91–97. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, L.; Chen, Y.; Han, F. Effects of daphnetin on lipid metabolism, insulin resistance and oxidative stress in OA-treated HepG2 cells. Mol. Med. Rep. 2019, 19, 4673–4684. [Google Scholar] [CrossRef]
- Lv, H.; Zhu, C.; Wei, W.; Lv, X.; Yu, Q.; Deng, X.; Ci, X. Enhanced keap1-Nrf2/Trx-1 axis by daphnetin protects against oxidative stress-driven hepatotoxicity via inhibiting ASK1/JNK and Txnip/NLRP3 inflammasome activation. Phytomedicine 2020, 71, 153241. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Li, Y.; Xiao, Q.; Li, D. Daphnetin activates the Nrf2-dependent antioxidant response to prevent arsenic-induced oxidative insult in human lung epithelial cells. Chemico-Biol. Interac. 2019, 302, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Zhi, J.; Duan, B.; Peo, J.; Wu, S.; Wei, J. Daphnetin protects hippocampal neurons from oxygen-glucose deprivation-induced injury. J. Cell. Biochem. 2019, 120, 4132–4139. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Gu, W.; Gao, Y.; Ma, N.; Fan, C.; Ci, X. Daphnetin ameliorated GM-induced renal injury through the suppression of oxidative stress and apoptosis in mice. Int. Immunopharmacol. 2021, 96, 107601. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, B.; Liu, X.; Han, Q.; Ge, W.; Zhanf, W.; Lu, Y.; Wu, Q.; Shi, L. Daphnetin ameliorates experimental autoimmune encephalomyelitis through regulating heme-oxygenase-1. Neurochem. Res. 2020, 45, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Liu, Q.; Zhou, J.; Tan, G.; Deng, X.; Ci, X. Daphentin-mediated Nrf2 antioxidant signaling pathways ameliorate tert-butyl hydroperoxide (t-BHP)-induced mitochondrial dysfunction and cell death. Free Rad. Biol. Med. 2017, 106, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Singh, A.P.; Bhatti, R. Mechanistic interplay of various mediators involved in mediating the neuroprotective effect of daphnetin. Pharmacol. Rep. 2021, 53, 1220–1229. [Google Scholar] [CrossRef]
- Ji, J.; Ge, X.; Chen, Y.; Zhu, B.; Wu, Q.; Zhang, J.; Shan, J.; Cheng, H.; Shi, L. Daphnetin ameliorates experimental colitis by modulating microbiota composition and Treg/Th17 balance. FASEB J. 2019, 3, 9308–9322. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Leung, W.N.; Cheung, H.Y.; Chan, C.W. Osthole: A review on its bioactivities, pharmacological properties, and potential as alternative medicine. Evid.-Based Complem. Alt. Med. 2015, 2015, 919616. [Google Scholar] [CrossRef]
- Yang, S.; Dai, W.; Wang, J.; Zhang, X.; Zheng, Y.; Bi, S.; Pang, L.; Ren, T.; Yang, Y.; Sun, Y.; et al. Osthole: An up-to-date review of its anticancer potential and mechanism of action. Front. Pharmacol. 2022, 13, 945627. [Google Scholar] [CrossRef]
- Su, J.; Zhang, F.; Li, X.; Liu, Z. Osthole promotes the suppressive effects of cisplatin on Nrf2 expression to prevent drug-resistant cervical cancer progression. Biochem. Biophys. Res. Commun. 2019, 514, 510–517. [Google Scholar] [CrossRef]
- Yang, S.M.; Cham, Y.L.; Hua, H.F.; Chang, J.M.; Chen, H.L.; Tsai, Y.J.; Hsu, Y.J.; Chao, L.K.; Feng-Ling, Y.; Tsai, U.L.; et al. Osthole improves an accelerated focal segmental glomerulosclerosis model in the early stage by activating the Nrf2 antioxidant pathway and subsequently inhibiting NF-κB-mediated COX-2 expression and apoptosis. Free Rad. Biol. Med. 2014, 73, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W.; Mao, X.X.; Lin, A.M.; Gao, X.Y.; Chen, X.H.; Ye, M.Z.; Ye, J.T.; Liu, P.Q.; Xu, S.W.; Liu, J.X.; et al. Osthole, a natural coumarin improves cognitive impairments and BBB dysfunction after transient global brain ischemia in C57BL/6J mice: Involvement of Nrf2 pathway. Neurochem. Res. 2015, 40, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Zhu, Y.; Cao, T.; Zhang, Y.; Chang, Z.; Liu, Y.; Lu, J.; Zhang, Y. Studies on the neuroprotection of osthole on glutamate-induced apoptotic cells and an Alzheimer’s disease mouse model via modulation oxidative stress. Appl. Biochem. Biotechnol. 2020, 190, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Li, K.; Dou, X.; Zhao, Y.; Huang, C.; Shu, F. The neuroprotective effect of osthole against chronic sleep deprivation (CSD)-induced memory impairment in rats. Life Sci. 2020, 263, 118524. [Google Scholar] [CrossRef]
- García-Arroyo, F.E.; Gonzaga-Sánchez, G.; Tapia, E.; Muñoz-Jiménez, I.; Manterola-Romero, L.; Osorio-Alonso, H.; Arellano-Buendía, A.S.; Pedraza-Chaverri, J.; Roncal-Jiménez, C.A.; Lanaspa, M.A.; et al. Osthol ameliorates kidney damage and metabolic syndrome induced by a high-fat/high-sugar diet. Int. J. Mol. Sci. 2021, 22, 2431. [Google Scholar] [CrossRef]
- Hua, K.F.; Yang, S.M.; Kao, T.Y.; Chang, J.M.; Chen, H.L.; Tsai, Y.J.; Chen, A.; Yang, S.S.; Chao, L.K.; Ka, S.M. Osthole mitigates progressive IgA nephropathy by inhibiting reactive oxygen species generation and NF-κB/NLRP3 pathway. PLoS ONE 2013, 8, 77794. [Google Scholar] [CrossRef]
- Bao, Y.; Meng, X.; Liu, F.; Wang, F.; Yang, J.; Wang, H.; Xie, G. Protective effects of osthole against inflammation induced by lipopolysaccharide in BV2 cells. Mol. Med. Rep. 2018, 17, 4561–4566. [Google Scholar] [CrossRef]
- Wu, S.J. Osthole attenuates inflammatory responses and regulates the expression of inflammatory mediators in Hep22 celss grown in differentiated medium from 3T3-L1 preadipocytes. J. Med. Food 2015, 18, 972–979. [Google Scholar] [CrossRef]
- Kordulewska, N.K.; Topa, J.; Tanska, M.; Cieslinska, A.; Fiedorowicz, E.; Savelkoul, H.E.J.; Jarmolowska, B. Modulatory effect of osthole on lipopolysaccharides-induced inflammation in Caco-2 cell monolayer and co-cultures with THP-1 and THP-1-derived macrophages. Nutrients 2021, 13, 123. [Google Scholar] [CrossRef]
- Sun, W.; Cai, Y.; Zhang, X.; Chen, H.; Lin, Y.; Li, H. Osthole pretreatment alleviates TNBS-induced colitis in mice via both cAMP/PKA-dependent and independent pathways. Acta Pharmacol. Sin. 2017, 38, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Khairy, H.; Saleh, H.; Badr, A.M.; Marie, M.S. Therapeutic efficacy of osthole against dinitrobenzene sulphonic acid induced-clitis in rats. Biomed. Pharmacother. 2018, 100, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Gao, Z.; Ji, K.; Li, X.; Wu, J.; Liu, Y.; Wang, X.; Liang, H.; Liu, Y.; Li8, X.; et al. The in vitro and in vivo anti-inflammatory effect of osthole, the major natural coumarin from Cnidium monnieri (L.) Cuss, via the blocking of the activation of the NF-κB and MAPK/p38 pathways. Phytomedicine 2019, 58, 152864. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.R.; Emam, M.A.; Hassan, N.S.; Mogaden, A.I. Umbelliferone and daphnetin ameliorate carbon tetrachloride-induced hepatotoxicity in rats via nuclear factor erythroid 2-related factor 2-mediated heme-oxygenase-1 expression. Environ. Toxicol. Pharmacol. 2014, 38, 531–541. [Google Scholar] [CrossRef]
- Yin, J.; Wang, H.; Lu, G. Umbelliferone alleviates hepatic injury in diabetic db/db mice by inhibiting inflammatory response and activating Nrf2-mediated antioxidant. Biosci. Rep. 2018, 38, BSR20180444. [Google Scholar] [CrossRef]
- Khan, A.; Shehzad, O.; Seo, E.K.; Onder, A.; Khan, S. Anti-allergic activities of umbelliferone against histamine- and picryl chloride-induced ear edema by targeting Nrf2/iNOS signaling in mice. BMC Complem. Med. Ther. 2021, 21, 215. [Google Scholar]
- Althunibat, O.Y.; Abduh, M.S.; Abukhalil, M.H.; Aladaileh, S.H.; Hanieh, H.; Mahmoud, A.M. Umbelliferone prevents isoproterenol-induced myocardial injury by upregulating Nrf2/HO-1 signaling, and attenuating oxidative stress, inflammation, and cell death in rats. Biomed. Pharmacother. 2022, 149, 112900. [Google Scholar] [CrossRef]
- Shalkami, A.G.S.; Hassanein, E.H.M.; Sayed, A.M.; Moohamed, W.R.; Khalaf, M.M.; Hmeida, R.A.M. Hepatoprotective effects of phytochemicals berberine and umbelliferone against methotrexate-induced hepatic intoxication: Experimental studies and in silico evidence. Environ. Sci. Pollut. Res. 2021, 28, 67593–67607. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Khader, H.R.; Elmansy, R.A.; Seleem, H.S.; Elfiky, M.; Mohammedsaleh, Z.M.; Ali, F.E.M.; Abd-Elhamid, T.H. Umbelliferone alleviates hepatic ischemia/reperfusion-induced oxidative stress injury via keap-1/Nrf2/ARE and TLR4/NF-κB-p65 signaling pathway. Environ. Sci. Pollut. Res. 2021, 28, 67863–67879. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Germoush, M.O.; Alotaibi, M.F.; Hussein, O.E. Possible involvement of Nrf2 and PPAR-γ up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed. Pharmacother. 2017, 86, 297–306. [Google Scholar] [CrossRef]
- Subramaniam, S.R.; Ellis, E.M. Umbelliferone and esculetin protect agains N-nitrosodiethylamine-induced hepatoxicity in rats. Cell Biol. Int. 2016, 40, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.E.M.; Hassanein, E.H.M.; El-Bahrawy, A.H.; Omar, Z.M.M.; Rashwan, E.K.; Abdel-Wahab, B.A.; Abd-Elhamid, T.H. Neprhoprotective effect of umbelliferone against cisplatin-induced kidney damage is mediated by regulation of Nrf2, cytoglobin, SIRT1/FOXO-3, and NF-κB-p65 signaling pathways. Biochem. Mol. Toxicol. 2021, 35, e22738. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E.H.M.; Mohamed, W.R.; Shalkami, A.G.S.; Khalaf, M.M.; Hemeida, R.A.M. Renoprotective effects of umbelliferone on methotrexate-induced renal injury through regulation of Nrf2/Keap-1, p38MAPK/NF-κB, and apoptosis signaling pathways. Food Chem. Toxicol. 2018, 116, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Chen, C. Umbelliferone delays the progression of diabetic nephropathy by inhibiting ferroptosis through activation of the Nrf2/HO-1 pathway. Food Chem. Toxicol. 2022, 163, 112892. [Google Scholar] [CrossRef] [PubMed]
- Hindam, M.O.; Sayed, R.H.; Skalicka-Wozniak, K.; Budzynska, B.; El Sayed, N.S. Xanthotoxin and umbelliferone attenuate cognitive dysfunction in a streptozotocin-induced rat model of sporadic Alzheimer’s disease: The role of JAK2/STAT3 and Nrf2/HO-1 signalling pathway modulation. Phytother. Res. 2020, 34, 2351–2365. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, B.A.; Alkahtani, S.A.; Alqahtani, A.A.; Hassanein, E.H.M. Umbelliferone ameliorates ulcerative colitis induced by acetic acid via modulation of TLR4/NF-κB-p65/iNOS and SIRT1/PPARγ signaling pathways in rats. Environ. Sci. Pollut. Res. Int. 2022, 29, 37644–37659. [Google Scholar] [CrossRef]
- Kundu, J.; Chae, I.G.; Chun, K.S. Fraxetin induces heme oxygenase-1 expression by activation of Akt/Nrf2 or AMP-activated protein kinase α/Nrf2 pathway in HaCaT cells. J. Canc. Prev. 2016, 21, 135–143. [Google Scholar] [CrossRef]
- Singh, D.K.; Cheema, H.S.; Saxena, A.; Jyotshana; Singh, S.; Darokar, M.P.; Bawankule, D.U.; Shanker, K.; Luqman, S. Fraxetin and ethyl acetate extract from Lawsonia inermis L. ameliorate oxidative stress in P. berguei infected mice by augmenting antioxidant defence system. Phytomedicine 2017, 36, 262–272. [Google Scholar] [CrossRef]
- Chen, X.; Ying, X.; Sun, W.; Zhu, H.; Jiang, X.; Chen, B. The therapeutic effect of fraxetin on ethanol-induced hepatic fibrosis by enhancing ethanol metabolism, inhibiting oxidative stress and modulating inflammatory mediators in rats. Int. Immunopharmacol. 2018, 56, 98–104. [Google Scholar] [CrossRef]
- Chang, W.C.; Wu, S.C.; Xu, K.D.; Liao, B.C.; Wu, J.F.; Cheng, A.S. Scopoletin protects against methylglyoxal-induced hyperglycemia and insulin resistance mediated by suppression of advanced glycation end products (AGEs) generation and anti-glycation. Molecules 2015, 20, 2786–2801. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Hassan, S.M.; Sayed, A.M.; Abo-Youssef, A.M. Ameliorate impacts of scopoletin against vancomycin-induced intoxication in rat model through modulation of keap1-Nrf2/HO-1 and IκBα-p65 NF-κB/p38 MAPK signaling pathways: Molecular study, molecular docking evidence and network pharmacology analysis. Int. Immunopharmacol. 2022, 102, 108382. [Google Scholar]
- Liu, B.; Deng, X.; Jiang, Q.; Li, G.; Zhang, J.; Zhang, N.; Xin, S. Scoparone improves hepatic inflammation and autophagy in mice with nonalcoholic steatohepatitis by regulating the ROS/p38/Nrf2 axis and P13K/Akt/mTOR pathway in macrophages. Biomed. Pharmacother. 2020, 125, 109895. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.F.; Li, Z.L.; Yan, Z.Q.; Gui, Z.; Liang, J.W.; Wang, Q.; Zhao, Z.D.; Li, P.L.; Hao, R.C.; Han, M.Y.; et al. Psoralen alleviates radiation-induced bone injury by rescuing skeletal stem cell stemness through AKT-mediated upregulation of GSK-3β and Nrf2. Stem Cell Res. Ther. 2022, 13, 241. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Luo, Y. Imperatorin relieved ulcerative colitis by regulating the Nrf2/ARE/HO-1 pathway in rats. Inflammation 2021, 44, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Fang, C.; Ding, S.; Liu, Z.; Hu, J.; Xu, J.; Mei, Q. PAP-1 ameliorates DSS-induced colitis with involvement of NLRP3 inflammasome pathway. Int. Immunopharmacol. 2019, 75, 105776. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, X.; Zhao, G.; Xu, D.; Jiang, Z.; Zhang, L.; Wang, T. Psoralen induced liver injury by attenuating liver regenerative capability. Front. Pharmacol. 2018, 9, 1179. [Google Scholar] [CrossRef]
- Yu, R.; Yu, Y.; Su, S.; Zhao, L.; Wang, Q.; Zhang, Y.; Song, L.; Zhu, K. Psoralen induces liver injuries through endoplasmatic reticulum stress signaling in female mice. Drug Chem. Toxicol. 2022, 45, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, X.; Lv, B.; Lu, Y.; Ma, X.; Liu, W.; Bai, G.; Gao, X. Psoralen induces hepatotoxicity by covalently binding to glutathione-S-transferases and the hepatic cytochrome P450. Phytomedicine 2022, 104, 154165. [Google Scholar] [CrossRef]
- Hu, L.; Sun, J.; Li, H.; Wang, L.; Wei, Y.; Wang, Y.; Zhu, Y.; Huo, H.; Tan, Y. Differential mechanistic investigation of protective effects from imperatorin and sec-O-glucosylhamaudol against arsenic trioxide-induced cytotoxicity in vitro. Toxicol. Vitr. 2016, 37, 97–105. [Google Scholar] [CrossRef]
- Xian, Z.; Jin, G.; Li, H.; Jiang, J.; Wang, C.; Zhu, L.; Jin, Z.; Li, L.; Piao, H.; Zheng, M.; et al. Imperatorin suppresses anaphylactic reaction and IgE-mediated allergic responses by inhibiting multiple steps of FceRI signaling in mast cells: IMP alleviates allergic responses in PCA. BioMed. Res. Int. 2019, 2019, 7823761. [Google Scholar] [CrossRef]
- Prasartthong, P.; Pakdeechote, P.; Maneesai, P.; Meephat, S.; Rattanakanokchai, S.; Wunpathe, C.; Apaijit, K.; Bunbupha, S. Imperatorin attenuates cardiac remodelling and dysfunction in high-fat/high-fructose diet-fed rats by modulating oxidative stress, inflammation and Nrf2 expression. Tissue Cell 2022, 75, 101728. [Google Scholar] [CrossRef] [PubMed]
- Xian, Z.; Choi, Y.H.; Zheng, M.; Jiang, J.; Zhao, Y.; Wang, C.; Li, J.; Li, Y.; Li, L.; Piao, H.; et al. imperatorin alleviates ROS-mediated airway remodeling by targeting the Nrf2/HO-1 signaling pathway. Biosci. Biotechnol. Biochem. 2020, 84, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, G.; Zheng, C.; Song, M.; Liu, F.; Huang, X.; Bai, S.; Huang, X.; Lin, C.; Zhu, C.; et al. Activating the pregnane X receptor by imperatorin attenuates dextran sulphate sodium-induced colitis in mice. Brit. J. Pharmacol. 2018, 175, 3563–3580. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Yang, H.J.; Ma, J.Y. Simultaneous determination of three furanocoumarins by UPLC/MS/MS: Application to pharmacokinetic study of Angelica dahurica radix after oral administration to normal and experimental colitis-induced rats. Molecules 2017, 22, 416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, L.; Pan, Y. detection of the content of two coumarins, IM and ISOIM, and their mechanism of action on colitis rats in Angelica albicans. Comp. Math. Meth Med. 2022, 2022, 5475559. [Google Scholar] [CrossRef] [PubMed]
- Djedjibegovic, J.; Marjanovic, A.; Panieri, E.; Saso, L. Ellagic acid-derived urolithins as modulators of oxidative stress. Oxidat. Med. Cell. Long. 2020, 2020, 5194508. [Google Scholar] [CrossRef]
- Lu, C.; Li, Z.; Gao, Z.; Song, Y.; Shen, Y. Urolithins and intestinal health. Drug. Disc. Ther. 2022, 16, 105–111. [Google Scholar] [CrossRef]
- Tow, W.K.; Chee, P.Y.; Sundralingam, U.; Palanisamy, U.D. The therapeutic relevance of urolithins, intestinal metabolites of ellagitannin-rich food: A systematic review of in vivo studies. Nutrients 2022, 14, 3494. [Google Scholar] [CrossRef]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortéz-Martín, A.; Ávila-Gálvez, M.A.; Tomás-Barberan, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Utolithins: A comprehensive update on their metabolism, bioactivity, and associated gut microbiota. Mol. Nutr. Food Res. 2022, 696, 2101019. [Google Scholar] [CrossRef]
- Xu, Z.; Li, S.; Li, K.; Wang, X.; Li, X.; An, M.; Yu, X.; Long, X.; Zhong, R.; Liu, Q.; et al. Urolithin A ameliorates diabetic retinopathy via activation of the Nrf2/HO-1 pathway. Endocrine J. 2022, 69, 971–982. [Google Scholar] [CrossRef]
- Liu, W.; Yang, F.; Xu, Z.; Chen, Q.; Ren, J.; Wang, Q.; Chen, L.; Ying, J.; Liu, Z.; Zhao, J.; et al. Urolithin A protects human dermal fibroblasts from UVA-induced photoaging through Nrf2 activation and mitophagy. J. Photochem. Photobiol. Biol. 2022, 232, 112462. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yi, W.; Tang, J.; Sun, Y.; Huang, J.; Lan, T.; Dai, X.; Xu, S.; Jin, Z.G.; Wu, X. Urolithin A protects against acetaminophen-induced liver injury in mice via sustained activation of Nrf2. Int. J. Bio. Sci. 2022, 18, 2146–2162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, M.; Zhang, Y.; Tian, M.; Chen, P.; Lan, Y.; Zhou, B. Urolithin A alleviates acute kidney injury induced by renal ischemia reperfusion through the p62-keap1-Nrf2 signaling pathway. Phytother. Res. 2022, 36, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.H.; Chen, W.Q.; Shen, Z.Y. Urolithin A shows anti-atherosclerotic activity via activation of class B scavenger receptor and activation of Nrf2 signaling pathway. Pharmacol. Rep. 2018, 70, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Peng, C.; Gu, R.; Yan, X.; Ye, J.; Zu, Z.; Sheng, X.; Huang, G.; Guo, Y. Urolithin A attenuates RANKL-induced osteoclastogenesis by co-regulating the p38 MAPK and Nrf2 signaling pathway. Eur. J. Pharmacol. 2022, 921, 174865. [Google Scholar] [CrossRef]
- Lee, G.; Park, J.S.; Lee, E.J.; Ahn, S.H.; Kim, H.S. Anti-inflammatory and antioxidant mechanisms of urolithin B in activated microglia. Phytomedicine 2019, 55, 50–57. [Google Scholar] [CrossRef]
- Qu, Z.; An, H.; Feng, M.; Huang, W.; Wang, D.; Zhang, Z.; Yan, L. Urolithin B suppresses osteoclastogenesis via inhibiting RANKL-induced signaling pathways and attenuating ROS activities. J. Cell. Mol. Med. 2022, 26, 4428–4439. [Google Scholar] [CrossRef]
- Liu, C.F.; Li, X.L.; Zhang, Z.L.; Qiu, L.; Ding, S.X.; Xue, J.X.; Zhao, G.P.; Li, J. Anti-aging effects of urolithin A on replicative senescence human skin fibroblasts. Rejuven. Res. 2019, 22, 191–200. [Google Scholar] [CrossRef]
- Zheng, D.; Liu, Z.; Zhou, Y.; Hou, N.; Yan, W.; Qin, Y.; Ye, Q.; Chen, X.Y.; Xiao, Q.; Bao, Y.; et al. Urolithin B, a gut microbiota metabolite, protects against myocardial ischemia/reperfusion injury via p62/keap1/Nrf2 signaling pathway. Pharmacol. Res. 2020, 153, 104655. [Google Scholar] [CrossRef]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Bárberan, F.; Dolara, P.; Espín, J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef]
- Pernonian, L.; Duarte-Silva, M.; Cardoso, C.R.B. The aryl hydrocarbon receptor (AHR) as a potential target for the control of intestinal inflammation: Insights from an immune and bacteria sensor receptor. Clin. Rev. Allergy Immunol. 2020, 59, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bastida, J.A.; Larrosa, M.; González-Sarrías, A.; Tomás-Barberan, F.; Espín, J.C.; García-Conesa, M.T. Intestinal ellagitannin metabolites ameliorate cytokine-induced inflammation and associated molecular markers in human colon fibroblasts. J. Agric. Food Chem. 2012, 60, 8866–8876. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.M.; Chen, H.H.; Wang, T.C.; Chen, I.L.; Chen, Y.T.; Yang, S.C.; Chen, Y.L.; Chang, H.H.; Huang, C.H.; Chang, J.Y.; et al. Novel oxime-bearing coumarin derivatives act as potent Nrf2/ARE activators in vitro and in mouse model. Eur. J. Med. Chem. 2015, 106, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Chiu, Y.J.; Yang, S.M.; Chen, C.M.; Huang, C.C.; Lee-Chen, G.J.; Lin, W.; Chang, K.H. Novel synthetic chalcone-coumarin hydrib for Aβ aggregation reduction, antioxidation, and neuroprotection. CNS Neurosci. Ther. 2018, 24, 1286–1298. [Google Scholar] [CrossRef]
- Wei, B.; Zhou, J.; Xu, J.J.; Cui, J.; Ping, F.F.; Ling, J.J.; Chen, Y.J. Discovery of coumarin-derived imino sulfonates as a novel class of potential cardioprotective agents. Eur. J. Med. Chem. 2019, 184, 111779. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.; Cui, F.; Liao, Y.; Wang, X. Synthesis, docking studies, biological activity of carbon monoxide release molecules based on coumarin derivatives. Front. Chem. 2022, 10, 996079. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Huant, Y.; Cui, J.; Wu, Y.; Zhu, F.; Huang, J.; Ma, L. Design and synthesis of osthole-based compounds as potential Nrf2 agonists. Bioorg. Med. Chem. Lett. 2022, 61, 128547. [Google Scholar] [CrossRef]
- Sun, M.; Sun, M.; Zhang, J. Osthole: An overview of its sources, biological activities, and modification development. Med. Chem. Res. 2021, 30, 1767–1794. [Google Scholar] [CrossRef]
- Bansal, Y.; Sethi, P.; Bansal, G. Coumarin: A potential nucleus for anti-inflammatory molecules. Med. Chem. Res. 2013, 22, 3049–3060. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stasi, L.C. Natural Coumarin Derivatives Activating Nrf2 Signaling Pathway as Lead Compounds for the Design and Synthesis of Intestinal Anti-Inflammatory Drugs. Pharmaceuticals 2023, 16, 511. https://doi.org/10.3390/ph16040511
Di Stasi LC. Natural Coumarin Derivatives Activating Nrf2 Signaling Pathway as Lead Compounds for the Design and Synthesis of Intestinal Anti-Inflammatory Drugs. Pharmaceuticals. 2023; 16(4):511. https://doi.org/10.3390/ph16040511
Chicago/Turabian StyleDi Stasi, Luiz C. 2023. "Natural Coumarin Derivatives Activating Nrf2 Signaling Pathway as Lead Compounds for the Design and Synthesis of Intestinal Anti-Inflammatory Drugs" Pharmaceuticals 16, no. 4: 511. https://doi.org/10.3390/ph16040511
APA StyleDi Stasi, L. C. (2023). Natural Coumarin Derivatives Activating Nrf2 Signaling Pathway as Lead Compounds for the Design and Synthesis of Intestinal Anti-Inflammatory Drugs. Pharmaceuticals, 16(4), 511. https://doi.org/10.3390/ph16040511




