Effects of Statin Dose, Class, and Use Intensity on All-Cause Mortality in Patients with Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Results
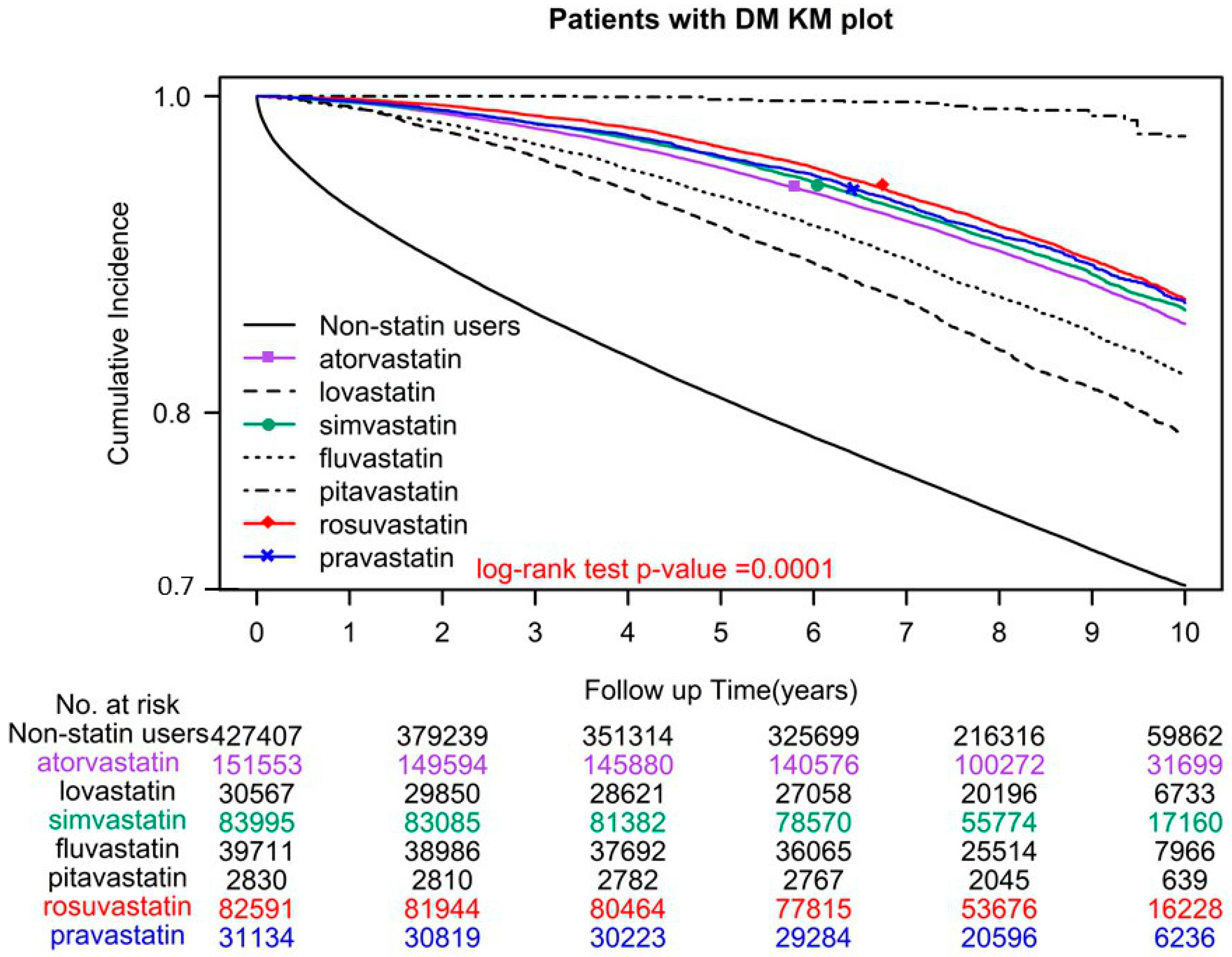
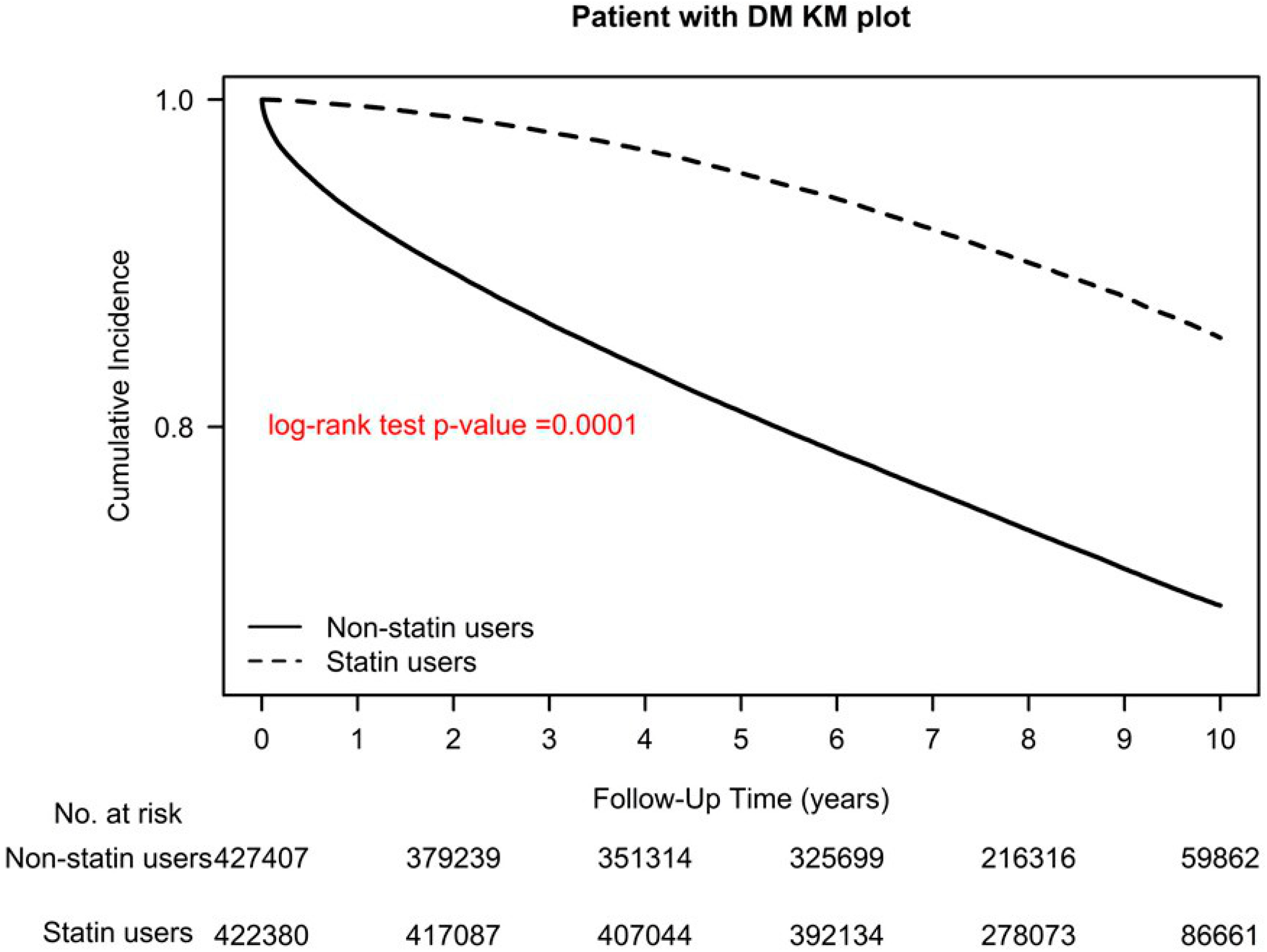
2.1. Association of All-Cause Mortality with Different Statin Dosages and Classes
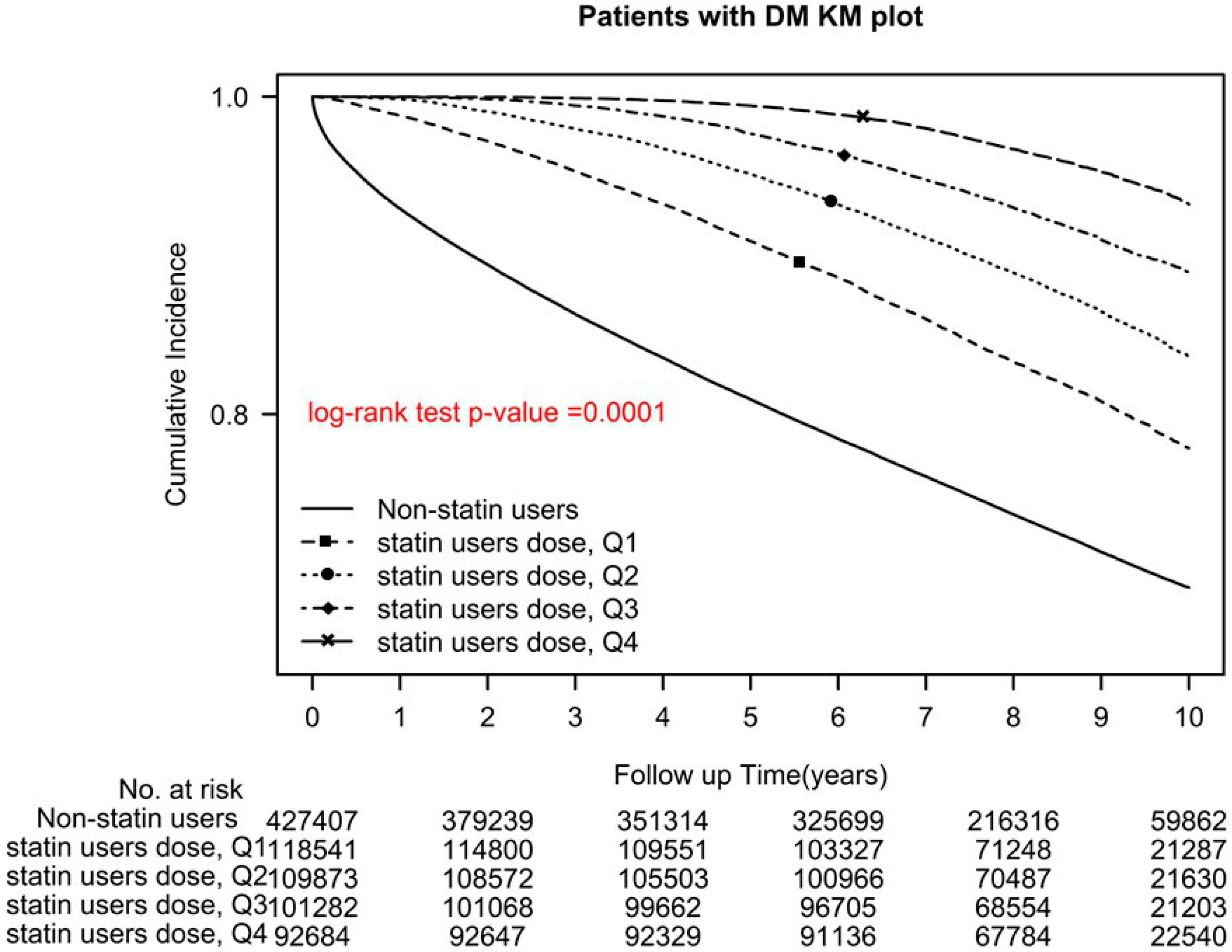
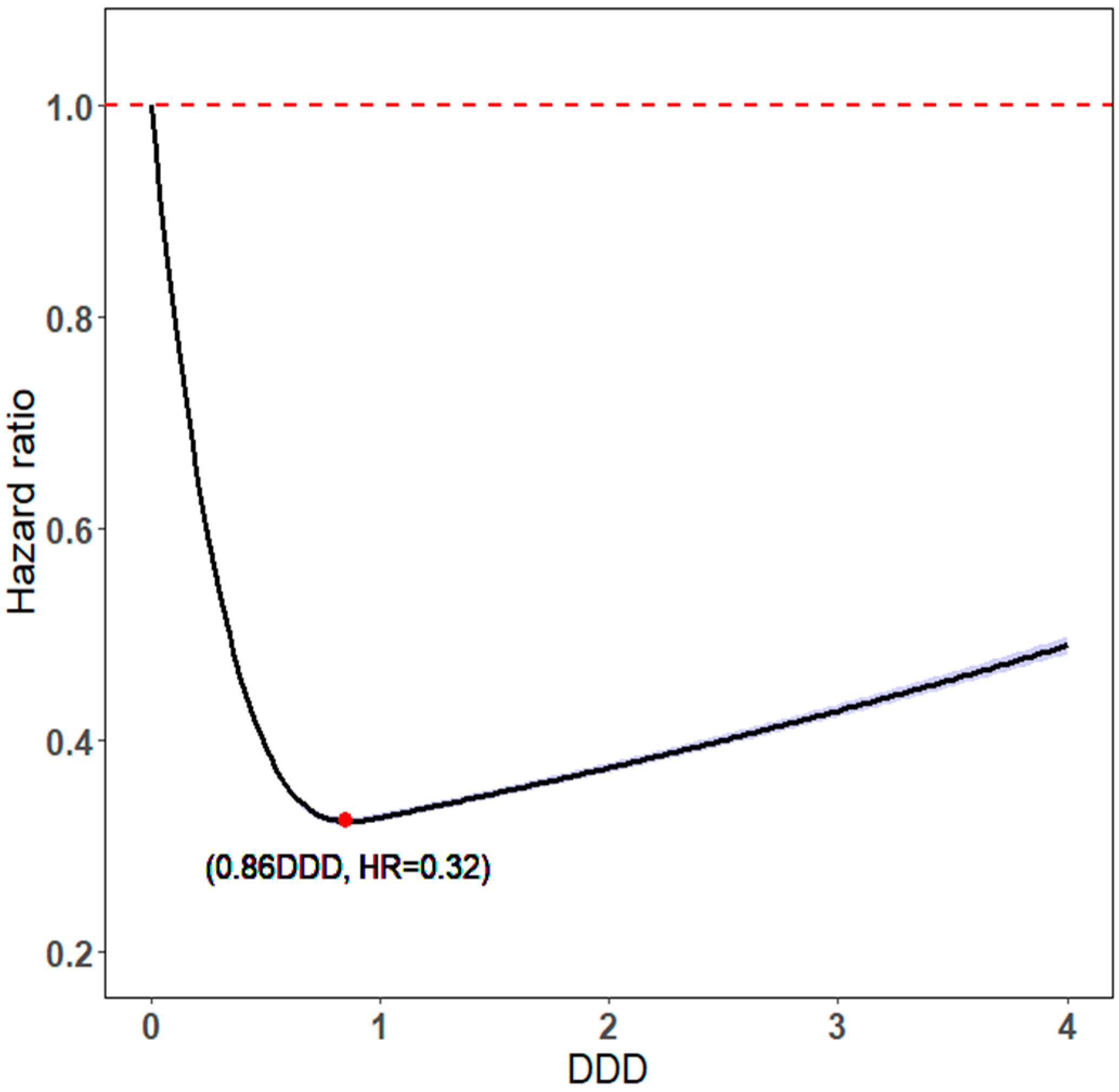
2.2. Statin Use Intensity
2.3. Sensitivity Analysis
3. Discussion
4. Methods
4.1. Study Population
4.2. Study Covariates
4.3. Outcome Variables
4.4. Statin Use
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991, 14, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.I.; Barker, D.J.; Hales, C.N.; Hirst, S.; Osmond, C. Thinness at birth and insulin resistance in adult life. Diabetologia 1994, 37, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Valdez, R.; Athens, M.A.; Thompson, G.H.; Bradshaw, B.S.; Stern, M.P. Birthweight and adult health outcomes in a biethnic population in the USA. Diabetologia 1994, 37, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Low Wang, C.C.; Hess, C.N.; Hiatt, W.R.; Goldfine, A.B. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus—Mechanisms, Management, and Clinical Considerations. Circulation 2016, 133, 2459–2502. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.J.; Deedwania, P.; Acharya, T.; Aguilar, D.; Bhatt, D.L.; Chyun, D.A.; Di Palo, K.E.; Golden, S.H.; Sperling, L.S.; American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health; et al. Comprehensive Management of Cardiovascular Risk Factors for Adults with Type 2 Diabetes: A Scientific Statement from the American Heart Association. Circulation 2022, 145, e722–e759. [Google Scholar] [CrossRef]
- Linkeviciute-Ulinskiene, D.; Kaceniene, A.; Dulskas, A.; Patasius, A.; Zabuliene, L.; Smailyte, G. Increased Mortality Risk in People with Type 2 Diabetes Mellitus in Lithuania. Int. J. Environ. Res. Public Health 2020, 17, 6870. [Google Scholar] [CrossRef]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef]
- Cavender, M.A.; Steg, P.G.; Smith, S.C., Jr.; Eagle, K.; Ohman, E.M.; Goto, S.; Kuder, J.; Im, K.; Wilson, P.W.; Bhatt, D.L.; et al. Impact of Diabetes Mellitus on Hospitalization for Heart Failure, Cardiovascular Events, and Death: Outcomes at 4 Years from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation 2015, 132, 923–931. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 15. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S173–S181. [Google Scholar] [CrossRef] [PubMed]
- Martin-Timon, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; Del Canizo-Gomez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes 2014, 5, 444–470. [Google Scholar] [CrossRef] [PubMed]
- Keech, A.; Colquhoun, D.; Best, J.; Kirby, A.; Simes, R.J.; Hunt, D.; Hague, W.; Beller, E.; Arulchelvam, M.; Baker, J.; et al. Secondary prevention of cardiovascular events with long-term pravastatin in patients with diabetes or impaired fasting glucose: Results from the LIPID trial. Diabetes Care 2003, 26, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Pyorala, K.; Pedersen, T.R.; Kjekshus, J.; Faergeman, O.; Olsson, A.G.; Thorgeirsson, G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care 1997, 20, 614–620. [Google Scholar] [CrossRef]
- Downs, J.R.; Clearfield, M.; Weis, S.; Whitney, E.; Shapiro, D.R.; Beere, P.A.; Langendorfer, A.; Stein, E.A.; Kruyer, W.; Gotto, A.M., Jr. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998, 279, 1615–1622. [Google Scholar] [CrossRef]
- Goldberg, R.B.; Mellies, M.J.; Sacks, F.M.; Moye, L.A.; Howard, B.V.; Howard, W.J.; Davis, B.R.; Cole, T.G.; Pfeffer, M.A.; Braunwald, E. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: Subgroup analyses in the cholesterol and recurrent events (CARE) trial. The Care Investigators. Circulation 1998, 98, 2513–2519. [Google Scholar] [CrossRef]
- Hoogwerf, B.J.; Waness, A.; Cressman, M.; Canner, J.; Campeau, L.; Domanski, M.; Geller, N.; Herd, A.; Hickey, A.; Hunninghake, D.B.; et al. Effects of aggressive cholesterol lowering and low-dose anticoagulation on clinical and angiographic outcomes in patients with diabetes: The Post Coronary Artery Bypass Graft Trial. Diabetes 1999, 48, 1289–1294. [Google Scholar] [CrossRef]
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group; The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major Outcomes in Moderately Hypercholesterolemic, Hypertensive Patients Randomized to Pravastatin vs. Usual Care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA 2002, 288, 2998–3007. [Google Scholar] [CrossRef]
- Serruys, P.W.; de Feyter, P.; Macaya, C.; Kokott, N.; Puel, J.; Vrolix, M.; Branzi, A.; Bertolami, M.C.; Jackson, G.; Strauss, B.; et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: A randomized controlled trial. JAMA 2002, 287, 3215–3222. [Google Scholar] [CrossRef]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Bollen, E.L.; Buckley, B.M.; Cobbe, S.M.; Ford, I.; Gaw, A.; Hyland, M.; Jukema, J.W.; et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomised controlled trial. Lancet 2002, 360, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.; Armitage, J.; Parish, S.; Sleigh, P.; Peto, R.; Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: A randomised placebo-controlled trial. Lancet 2003, 361, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Tognoni, G.; et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Callahan, A.; Amarenco, P.; Goldstein, L.B.; Sillesen, H.; Messig, M.; Samsa, G.P.; Altafullah, I.; Ledbetter, L.Y.; MacLeod, M.J.; Scott, R.; et al. Risk of stroke and cardiovascular events after ischemic stroke or transient ischemic attack in patients with type 2 diabetes or metabolic syndrome: Secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Arch. Neurol. 2011, 68, 1245–1251. [Google Scholar] [CrossRef]
- Tajima, N.; Kurata, H.; Nakaya, N.; Mizuno, K.; Ohashi, Y.; Kushiro, T.; Teramoto, T.; Uchiyama, S.; Nakamura, H.; Primary Prevention Group of Adult Japanese (MEGA) Study. Pravastatin reduces the risk for cardiovascular disease in Japanese hypercholesterolemic patients with impaired fasting glucose or diabetes: Diabetes subanalysis of the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA) Study. Atherosclerosis 2008, 199, 455–462. [Google Scholar] [CrossRef]
- Ramos, R.; Comas-Cufi, M.; Marti-Lluch, R.; Ballo, E.; Ponjoan, A.; Alves-Cabratosa, L.; Blanch, J.; Marrugat, J.; Elosua, R.; Grau, M.; et al. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: Retrospective cohort study. BMJ 2018, 362, k3359. [Google Scholar] [CrossRef]
- Saeed, O.; Castagna, F.; Agalliu, I.; Xue, X.; Patel, S.R.; Rochlani, Y.; Kataria, R.; Vukelic, S.; Sims, D.B.; Alvarez, C.; et al. Statin Use and In-Hospital Mortality in Patients With Diabetes Mellitus and COVID-19. J. Am. Heart Assoc. 2020, 9, e018475. [Google Scholar] [CrossRef]
- Olafsdottir, E.; Aspelund, T.; Sigurdsson, G.; Thorsson, B.; Eiriksdottir, G.; Harris, T.B.; Launer, L.J.; Benediktsson, R.; Gudnason, V. Effects of statin medication on mortality risk associated with type 2 diabetes in older persons: The population-based AGES-Reykjavik Study. BMJ Open 2011, 1, e000132. [Google Scholar] [CrossRef]
- Castro, M.R.; Simon, G.; Cha, S.S.; Yawn, B.P.; Melton, L.J., 3rd; Caraballo, P.J. Statin Use, Diabetes Incidence and Overall Mortality in Normoglycemic and Impaired Fasting Glucose Patients. J. Gen. Intern. Med. 2016, 31, 502–508. [Google Scholar] [CrossRef]
- Wen, C.P.; Tsai, S.P.; Chung, W.S. A 10-year experience with universal health insurance in Taiwan: Measuring changes in health and health disparity. Ann. Intern. Med. 2008, 148, 258–267. [Google Scholar] [CrossRef]
- Robins, J.M.; Hernan, M.A.; Brumback, B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000, 11, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Schachter, M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: An update. Fundam. Clin. Pharmacol. 2005, 19, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. U-shaped dose-responses in biology, toxicology, and public health. Annu. Rev. Public Health 2001, 22, 15–33. [Google Scholar] [CrossRef]
- Yang, X.H.; Zhang, B.L.; Cheng, Y.; Fu, S.K.; Jin, H.M. Statin use and the risk of CVD events, stroke, and all-cause mortality in patients with diabetes: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2470–2482. [Google Scholar] [CrossRef]
- Rosenson, R.S. Rosuvastatin: A new inhibitor of HMG-coA reductase for the treatment of dyslipidemia. Expert Rev. Cardiovasc. Ther. 2003, 1, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.H.; Davidson, M.H.; Stein, E.A.; Bays, H.E.; McKenney, J.M.; Miller, E.; Cain, V.A.; Blasetto, J.W.; Group, S.S. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR * Trial). Am. J. Cardiol. 2003, 92, 152–160. [Google Scholar] [CrossRef]
- Brown, A.S.; Bakker-Arkema, R.G.; Yellen, L.; Henley, R.W., Jr.; Guthrie, R.; Campbell, C.F.; Koren, M.; Woo, W.; McLain, R.; Black, D.M. Treating patients with documented atherosclerosis to National Cholesterol Education Program-recommended low-density-lipoprotein cholesterol goals with atorvastatin, fluvastatin, lovastatin and simvastatin. J. Am. Coll. Cardiol. 1998, 32, 665–672. [Google Scholar] [CrossRef]
- Barter, P.J.; Brandrup-Wognsen, G.; Palmer, M.K.; Nicholls, S.J. Effect of statins on HDL-C: A complex process unrelated to changes in LDL-C: Analysis of the VOYAGER Database. J. Lipid Res. 2010, 51, 1546–1553. [Google Scholar] [CrossRef]
- Shitara, Y.; Maeda, K.; Ikejiri, K.; Yoshida, K.; Horie, T.; Sugiyama, Y. Clinical significance of organic anion transporting polypeptides (OATPs) in drug disposition: Their roles in hepatic clearance and intestinal absorption. Biopharm. Drug Dispos. 2013, 34, 45–78. [Google Scholar] [CrossRef]
- Neuvonen, P.J. Drug interactions with HMG-CoA reductase inhibitors (statins): The importance of CYP enzymes, transporters and pharmacogenetics. J. Curr. Opin. Investig. Drugs 2010, 11, 323–332. [Google Scholar]
- Eidelman, R.S.; Lamas, G.A.; Hennekens, C.H. The new National Cholesterol Education Program guidelines: Clinical challenges for more widespread therapy of lipids to treat and prevent coronary heart disease. Arch. Intern. Med. 2002, 162, 2033–2036. [Google Scholar] [CrossRef] [PubMed]
- Hudzik, B.; Szkodzinski, J.; Polonski, L.J.C. Statins: The good, the bad and the ugly. CMAJ 2012, 184, 1175. [Google Scholar] [CrossRef] [PubMed]
- Mulder, A.B.; van Lijf, H.J.; Bon, M.A.; van den Bergh, F.A.; Touw, D.J.; Neef, C.; Vermes, I. Association of polymorphism in the cytochrome CYP2D6 and the efficacy and tolerability of simvastatin. Clin. Pharmacol. Ther. 2001, 70, 546–551. [Google Scholar] [CrossRef]
- Liao, J.K. Safety and efficacy of statins in Asians. Am. J. Cardiol. 2007, 99, 410–414. [Google Scholar] [CrossRef]
- Chasman, D.I.; Posada, D.; Subrahmanyan, L.; Cook, N.R.; Stanton, V.P., Jr.; Ridker, P.M. Pharmacogenetic study of statin therapy and cholesterol reduction. JAMA 2004, 291, 2821–2827. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.F.; Hristeva, N.; Chang, J.; Liang, X.; Li, R.; Frassetto, L.; Benet, L.Z. Rosuvastatin Pharmacokinetics in Asian and White Subjects Wild Type for Both OATP1B1 and BCRP Under Control and Inhibited Conditions. J. Pharm. Sci. 2017, 106, 2751–2757. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, P.; Cameli, M.; Maiello, M.; Modesti, P.A.; Muiesan, M.L.; Novo, S.; Palmiero, P.; Saba, P.S.; Pedrinelli, R.; Ciccone, M.M. Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J. Funct. Foods 2014, 6, 11–32. [Google Scholar] [CrossRef]
- The Republic of China Yearbook; Executive Yuan Press Office: Taipei, Taiwan, 2016; pp. 10–11.
- Bhatt, D.L.; Steg, P.G.; Ohman, E.M.; Hirsch, A.T.; Ikeda, Y.; Mas, J.L.; Goto, S.; Liau, C.S.; Richard, A.J.; Rother, J.; et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006, 295, 180–189. [Google Scholar] [CrossRef]
| Nonusers | Users | p | ASMD | |||
|---|---|---|---|---|---|---|
| N = 427,407 | N = 422,380 | |||||
| Characteristic | n | % | n | % | ||
| Age, mean ± SD, years | 56.85 ± 20.97 | 56.92 ± 19.24 | 0.8520 | |||
| Age, median (IQR), years | 56.00 (46.00, 68.00) | 56.00 (48.00, 68.00) | 0.9999 | |||
| Age group, years | 0.0844 | 0.0046 | ||||
| ≤50 | 143,911 | 33.67% | 141,194 | 33.43% | ||
| 51–60 | 112,251 | 26.26% | 111,046 | 26.29% | ||
| 61–70 | 86,430 | 20.22% | 86,057 | 20.37% | ||
| ≥71 | 84,815 | 19.84% | 84,083 | 19.91% | ||
| Sex | 0.6946 | 0.0004 | ||||
| Female | 202,041 | 47.27% | 199,485 | 47.23% | ||
| Male | 225,366 | 52.73% | 222,895 | 52.77% | ||
| Income levels (NTD) | 0.6213 | 0.0008 | ||||
| Low income | 6860 | 1.61% | 6702 | 1.59% | ||
| Financially dependent | 135,057 | 31.60% | 133,548 | 31.62% | ||
| ≤20,000 | 202,250 | 47.32% | 200,462 | 47.46% | ||
| 20,001–30,000 | 38,833 | 9.09% | 38,088 | 9.02% | ||
| 30,001–45,000 | 28,027 | 6.56% | 27,510 | 6.51% | ||
| >45,000 | 16,380 | 3.83% | 16,070 | 3.80% | ||
| Urbanization | 0.9444 | 0.0001 | ||||
| Rural | 121,995 | 28.54% | 120,589 | 28.55% | ||
| Urban | 305,412 | 71.46% | 301,791 | 71.45% | ||
| Number of antidiabetic drug types used | 0.0701 | 0.0009 | ||||
| 0 | 156,611 | 36.64% | 155,804 | 36.89% | ||
| 1 | 105,742 | 24.74% | 104,725 | 24.79% | ||
| 2 | 105,362 | 24.65% | 103,280 | 24.45% | ||
| 3 | 43,350 | 10.14% | 42,551 | 10.07% | ||
| ≥4 | 16,342 | 3.82% | 16,020 | 3.79% | ||
| Antidiabetic drugs used | ||||||
| Insulin | 45,219 | 10.58% | 44,743 | 10.59% | 0.9485 | 0.0002 |
| Metformin | 183,186 | 43.86% | 181,487 | 42.97% | 0.5920 | 0.0007 |
| SU | 206,950 | 48.42% | 204,777 | 48.48% | 0.3972 | 0.0006 |
| AGI | 3479 | 0.81% | 3473 | 0.82% | 0.4462 | 0.0001 |
| TZD | 27,054 | 6.33% | 26,950 | 6.38% | 0.6642 | 0.0002 |
| DPP4i | 21,071 | 4.93% | 20,903 | 4.95% | 0.7950 | 0.0002 |
| SGLT2i | 488 | 0.11% | 464 | 0.11% | 0.9429 | 0.0001 |
| Others | 24,661 | 5.78% | 24,412 | 5.78% | 0.9652 | 0.0001 |
| aDCSI score | ||||||
| Mean ± SD | 1.00 ± 1.89 | 1.03 ± 1.44 | 0.5461 | |||
| Median (IQR) | 0.00 (0.00, 2.00) | 0.00 (0.00, 2.00) | 0.5659 | |||
| aDCSI score | 0.7967 | 0.0059 | ||||
| 0 | 219,618 | 51.38% | 217,419 | 51.47% | ||
| 1 | 89,009 | 20.83% | 87,662 | 20.75% | ||
| 2 | 65,173 | 15.25% | 64,273 | 15.22% | ||
| ≥3 | 53,607 | 12.54% | 53,026 | 12.55% | ||
| Retinopathy | 24,661 | 5.77% | 24,395 | 5.78% | 0.8936 | 0.0004 |
| Nephropathy | 50,647 | 11.85% | 50,118 | 11.87% | 0.8851 | 0.0002 |
| Neuropathy | 44,450 | 10.40% | 44,130 | 10.45% | 0.3064 | 0.0008 |
| Cerebrovascular | 45,518 | 10.65% | 45,226 | 10.71% | 0.7408 | 0.0002 |
| Cardiovascular | 113,946 | 26.66% | 113,559 | 26.89% | 0.8863 | 0.0002 |
| Peripheral vascular disease | 16,113 | 3.77% | 15,914 | 3.77% | 0.9940 | 0.0001 |
| Metabolic disorder | 7738 | 1.81% | 7734 | 1.83% | 0.8046 | 0.0001 |
| Comorbidities | ||||||
| Hypertension | 219,833 | 51.43% | 217,360 | 51.46% | 0.8053 | 0.0003 |
| Coronary artery disease | 96,754 | 22.64% | 95,261 | 22.55% | 0.3541 | 0.0008 |
| Stroke | 62,388 | 14.60% | 61,602 | 14.58% | 0.8697 | 0.0001 |
| Depression | 28,112 | 6.58% | 28,035 | 6.64% | 0.2645 | 0.0006 |
| Anxiety | 59,006 | 13.81% | 58,624 | 13.88% | 0.3245 | 0.0007 |
| Heart failure | 28,686 | 6.71% | 28,508 | 6.75% | 0.4897 | 0.0004 |
| Peripheral vascular disease | 9221 | 2.16% | 9091 | 2.15% | 0.8691 | 0.0001 |
| COPD | 88,209 | 20.64% | 86,839 | 20.56% | 0.3698 | 0.0008 |
| Atrial fibrillation | 9495 | 2.22% | 9328 | 2.21% | 0.6841 | 0.0001 |
| Traumatic head injury | 26,003 | 6.08% | 25,696 | 6.08% | 0.9955 | 0.0000 |
| Hearing loss | 11,359 | 2.66% | 11,365 | 2.69% | 0.3464 | 0.0003 |
| Sleep apnea | 2423 | 0.57% | 2349 | 0.56% | 0.5036 | 0.0001 |
| Liver cirrhosis | 119,973 | 28.07% | 118,674 | 28.10% | 0.2204 | 0.0023 |
| SLE | 6592 | 1.54% | 6547 | 1.55% | 0.7749 | 0.0001 |
| CCI scores | ||||||
| Mean ± SD | 1.10 ± 2.10 | 1.20 ± 1.58 | 0.1397 | |||
| Median (Q1, Q3) | 0.00 (0.00, 2.00) | 1.00 (0.00, 2.00) | 0.9628 | |||
| CCI scores | 0.0785 | 0.0019 | ||||
| 0 | 229,905 | 53.79% | 226,397 | 53.60% | ||
| ≥1 | 197,503 | 46.21% | 195,983 | 46.40% | ||
| Different classes of statins | ||||||
| Lipophilic statins | ||||||
| Atorvastatin | 0 | 0.00% | 151,553 | 35.88% | ||
| Lovastatin | 0 | 0.00% | 30,567 | 7.24% | ||
| Simvastatin | 0 | 0.00% | 83,995 | 19.89% | ||
| Fluvastatin | 0 | 0.00% | 39,711 | 9.40% | ||
| Pitavastatin | 0 | 0.00% | 2830 | 0.67% | ||
| Hydrophilic statins | ||||||
| Rosuvastatin | 0 | 0.00% | 82,591 | 19.55% | ||
| Pravastatin | 0 | 0.00% | 31,134 | 7.37% | ||
| cDDD-year of statins | ||||||
| Q1 | 0 | 0.00% | 118,541 | 28.06% | ||
| Q2 | 0 | 0.00% | 109,873 | 26.01% | ||
| Q3 | 0 | 0.00% | 101,282 | 23.98% | ||
| Q4 | 0 | 0.00% | 98,684 | 21.94% | ||
| DDD | ||||||
| ≤1 | 0 | 0.00% | 143,141 | 33.89% | ||
| >1 | 0 | 0.00% | 279,239 | 66.11% | ||
| Stain use | ||||||
| New use (after type 2 diabetes diagnosis) | 0 | 0.00% | 384,108 | 90.94% | ||
| Prevalent use (before type 2 diabetes diagnosis) | 0 | 0.00% | 38,272 | 9.06% | ||
| Time from type 2 diabetes diagnosis to statins exposure | ||||||
| Mean ±SD follow-up | 2.42 ± 2.69 | |||||
| Median (IQR) follow-up | 1.33 (0.07, 4.19) | |||||
| Follow-up duration | ||||||
| Mean ± SD follow-up | 8.04 ± 3.12 | 9.48 ± 1.76 | <0.0001 | |||
| Median (IQR) follow-up | 8.97 (5.66, 9.33) | 9.65 (7.58, 9.76) | <0.0001 | |||
| All-cause mortality | <0.0001 | |||||
| No | 308,643 | 72.21% | 371,576 | 87.97% | ||
| Yes | 118,765 | 27.79% | 50,804 | 12.03% | ||
| Variables | Crude HR (95%CI) | p | aHR (95%CI) * | p | ||
|---|---|---|---|---|---|---|
| Stain user or nonusers | ||||||
| Nonusers | Reference | |||||
| Users | 0.37 | (0.36, 0.37) | <0.0001 | 0.32 | (0.31, 0.33) | <0.0001 |
| Different classes of statins | ||||||
| Nonusers | Reference | |||||
| Hydrophilic statins | ||||||
| Rosuvastatin | 0.32 | (0.31, 0.34) | <0.0001 | 0.29 | (0.28, 0.31) | <0.0001 |
| Pravastatin | 0.31 | (0.3, 0.32) | <0.0001 | 0.28 | (0.27, 0.29) | <0.0001 |
| Lipophilic statins | ||||||
| Atorvastatin | 0.05 | (0.03, 0.07) | <0.0001 | 0.06 | (0.04, 0.09) | <0.0001 |
| Lovastatin | 0.47 | (0.45, 0.48) | <0.0001 | 0.36 | (0.35, 0.38) | <0.0001 |
| Simvastatin | 0.34 | (0.33, 0.35) | <0.0001 | 0.31 | (0.30, 0.32) | <0.0001 |
| Fluvastatin | 0.58 | (0.56, 0.61) | <0.0001 | 0.48 | (0.47, 0.50) | <0.0001 |
| Pitavastatin | 0.36 | (0.36, 0.37) | <0.0001 | 0.31 | (0.31, 0.32) | <0.0001 |
| cDDD-year of statins | ||||||
| Nonusers | Reference | |||||
| Q1 | 0.61 | (0.6, 0.62) | <0.0001 | 0.51 | (0.5, 0.52) | <0.0001 |
| Q2 | 0.41 | (0.4, 0.42) | <0.0001 | 0.36 | (0.35, 0.37) | <0.0001 |
| Q3 | 0.27 | (0.26, 0.27) | <0.0001 | 0.24 | (0.23, 0.25) | <0.0001 |
| Q4 | 0.15 | (0.14, 0.15) | <0.0001 | 0.13 | (0.13, 0.14) | <0.0001 |
| p for trend | <0.0001 | <0.0001 | ||||
| Subpopulation or Exposure | No. of Patients | All-Cause Mortality | |||
|---|---|---|---|---|---|
| No. of Deaths | aHR * | 95% CI | p | ||
| Age group, years | |||||
| ≤50 | 285,105 | 23,316 | 0.29 | (0.28–0.30) | <0.0001 |
| 51–60 | 223,297 | 27,319 | 0.31 | (0.30–0.32) | <0.0001 |
| 61–70 | 172,487 | 37,672 | 0.33 | (0.32–0.34) | <0.0001 |
| ≥71 | 168,898 | 81,260 | 0.32 | (0.32–0.33) | <0.0001 |
| Sex | |||||
| Female | 401,526 | 68,131 | 0.3 | (0.30–0.31) | <0.0001 |
| Male | 448,261 | 101,438 | 0.33 | (0.33–0.34) | <0.0001 |
| Income levels (NTD) | |||||
| Low income | 13,562 | 4936 | 0.35 | (0.32–0.38) | <0.0001 |
| Financially dependent | 268,604 | 62,198 | 0.33 | (0.32–0.33) | <0.0001 |
| ≤20,000 | 402,713 | 90,946 | 0.31 | (0.31–0.32) | <0.0001 |
| 20,001–30,000 | 76,921 | 5847 | 0.34 | (0.31–0.36) | <0.0001 |
| 30,001–45,000 | 55,537 | 3713 | 0.32 | (0.29–0.35) | <0.0001 |
| >45,000 | 32,450 | 1928 | 0.41 | (0.36–0.47) | <0.0001 |
| Urbanization | |||||
| Rural | 242,584 | 58,568 | 0.31 | (0.30–0.32) | <0.0001 |
| Urban | 607,203 | 111,001 | 0.33 | (0.32–0.33) | <0.0001 |
| Number of antidiabetic drug types used | |||||
| 0 | 312,415 | 50,615 | 0.33 | (0.32–0.34) | <0.0001 |
| 1 | 210,467 | 43,730 | 0.30 | (0.29–0.31) | <0.0001 |
| 2 | 208,642 | 40,260 | 0.33 | (0.32–0.34) | <0.0001 |
| 3 | 85,901 | 24,499 | 0.31 | (0.30–0.32) | <0.0001 |
| ≥4 | 32,362 | 10,464 | 0.33 | (0.31–0.35) | <0.0001 |
| aDCSI score | |||||
| 0 | 437,037 | 53,522 | 0.31 | (0.31–0.32) | <0.0001 |
| 1 | 176,671 | 27,167 | 0.36 | (0.34–0.37) | <0.0001 |
| 2 | 129,446 | 39,528 | 0.29 | (0.28–0.30) | <0.0001 |
| ≥3 | 106,633 | 49,352 | 0.33 | (0.32–0.34) | <0.0001 |
| CCI scores | |||||
| 0 | 437,037 | 53,522 | 0.31 | (0.31–0.32) | <0.0001 |
| ≥1 | 393,486 | 105,134 | 0.30 | (0.29–0.30) | <0.0001 |
| Coexisting comorbidities | |||||
| Hypertension | 437,193 | 112,774 | 0.33 | (0.32–0.34) | <0.0001 |
| Coronary artery disease | 192,015 | 50,785 | 0.30 | (0.29–0.31) | <0.0001 |
| Stroke | 123,990 | 58,964 | 0.34 | (0.33–0.34) | <0.0001 |
| Depression | 56,147 | 13,352 | 0.32 | (0.30–0.34) | <0.0001 |
| Anxiety | 117,630 | 24,118 | 0.33 | (0.31–0.34) | <0.0001 |
| Heart failure | 57,194 | 27,547 | 0.32 | (0.31–0.34) | <0.0001 |
| Peripheral vascular disease | 18,312 | 6472 | 0.34 | (0.31–0.36) | <0.0001 |
| COPD | 175,048 | 56,398 | 0.31 | (0.30–0.32) | <0.0001 |
| Atrial fibrillation | 18,823 | 10,415 | 0.35 | (0.33–0.37) | <0.0001 |
| Traumatic head injury | 51,699 | 15,134 | 0.29 | (0.27–0.30) | <0.0001 |
| Hearing loss | 22,724 | 6519 | 0.32 | (0.30–0.35) | <0.0001 |
| Sleep apnea | 4772 | 840 | 0.33 | (0.27–0.42) | <0.0001 |
| Liver cirrhosis | 237,795 | 46,407 | 0.29 | (0.28–0.30) | <0.0001 |
| SLE | 13,139 | 2879 | 0.31 | (0.27–0.34) | <0.0001 |
| DDD | |||||
| ≤1 | 560,998 | 137,268 | 0.36 | (0.35–0.37) | <0.0001 |
| >1 | 288,789 | 32,300 | 0.50 | (0.46–0.53) | <0.0001 |
| Stain use | |||||
| New use (after type 2 diabetes diagnosis) | 803,889 | 159,321 | 0.31 | (0.31–0.32) | <0.0001 |
| Prevalent use (before type 2 diabetes diagnosis) | 45,898 | 10,247 | 0.28 | (0.26–0.29) | <0.0001 |
| Metformin use | 357,572 | 69,229 | 0.35 | (0.34–0.36) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.-M.; Chen, W.-M.; Chen, M.; Shia, B.-C.; Wu, S.-Y. Effects of Statin Dose, Class, and Use Intensity on All-Cause Mortality in Patients with Type 2 Diabetes Mellitus. Pharmaceuticals 2023, 16, 507. https://doi.org/10.3390/ph16040507
Yu J-M, Chen W-M, Chen M, Shia B-C, Wu S-Y. Effects of Statin Dose, Class, and Use Intensity on All-Cause Mortality in Patients with Type 2 Diabetes Mellitus. Pharmaceuticals. 2023; 16(4):507. https://doi.org/10.3390/ph16040507
Chicago/Turabian StyleYu, Jung-Min, Wan-Ming Chen, Mingchih Chen, Ben-Chang Shia, and Szu-Yuan Wu. 2023. "Effects of Statin Dose, Class, and Use Intensity on All-Cause Mortality in Patients with Type 2 Diabetes Mellitus" Pharmaceuticals 16, no. 4: 507. https://doi.org/10.3390/ph16040507
APA StyleYu, J.-M., Chen, W.-M., Chen, M., Shia, B.-C., & Wu, S.-Y. (2023). Effects of Statin Dose, Class, and Use Intensity on All-Cause Mortality in Patients with Type 2 Diabetes Mellitus. Pharmaceuticals, 16(4), 507. https://doi.org/10.3390/ph16040507







