Azithromycin and Sildenafil May Have Protective Effects on Retinal Ganglion Cells via Different Pathways: Study in a Rodent Microbead Model
Abstract
1. Introduction
2. Results
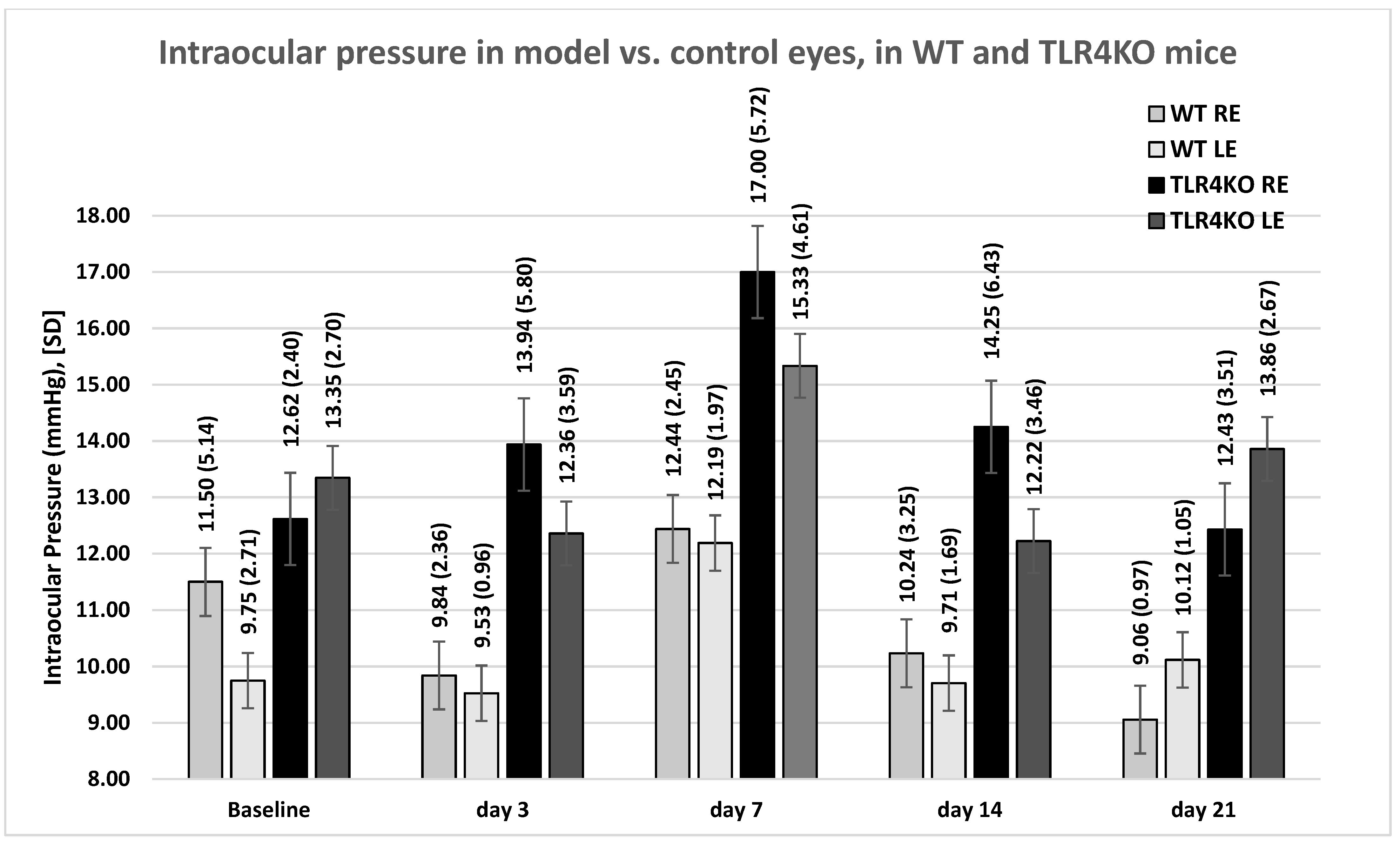
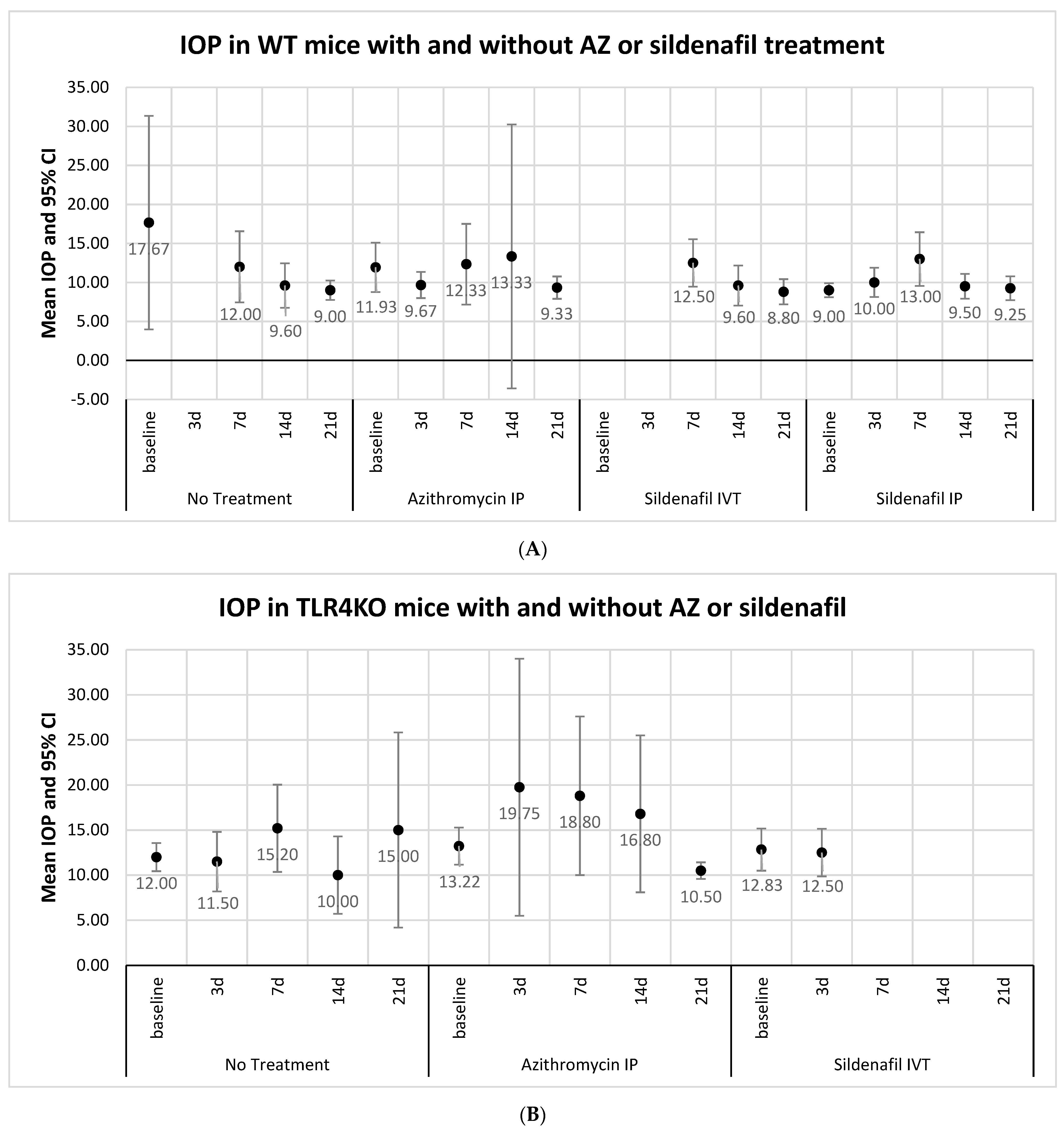
2.1. IOP Measurements
2.2. Histology
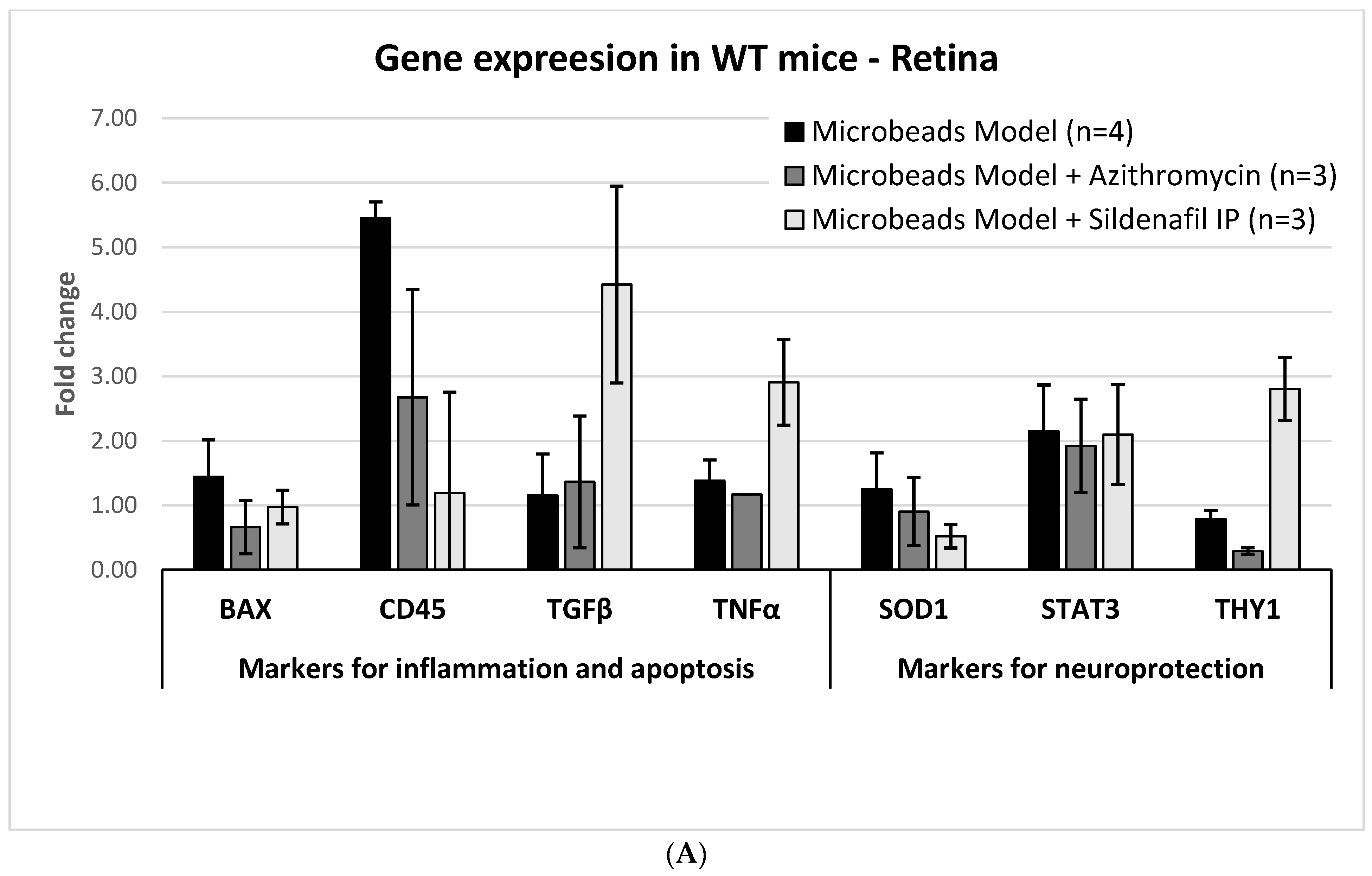
2.3. Molecular Analysis
2.3.1. Glaucoma Microbead Model: WT Mice
2.3.2. AZ Treatment: WT Mice
2.3.3. Sildenafil Treatment: WT Mice
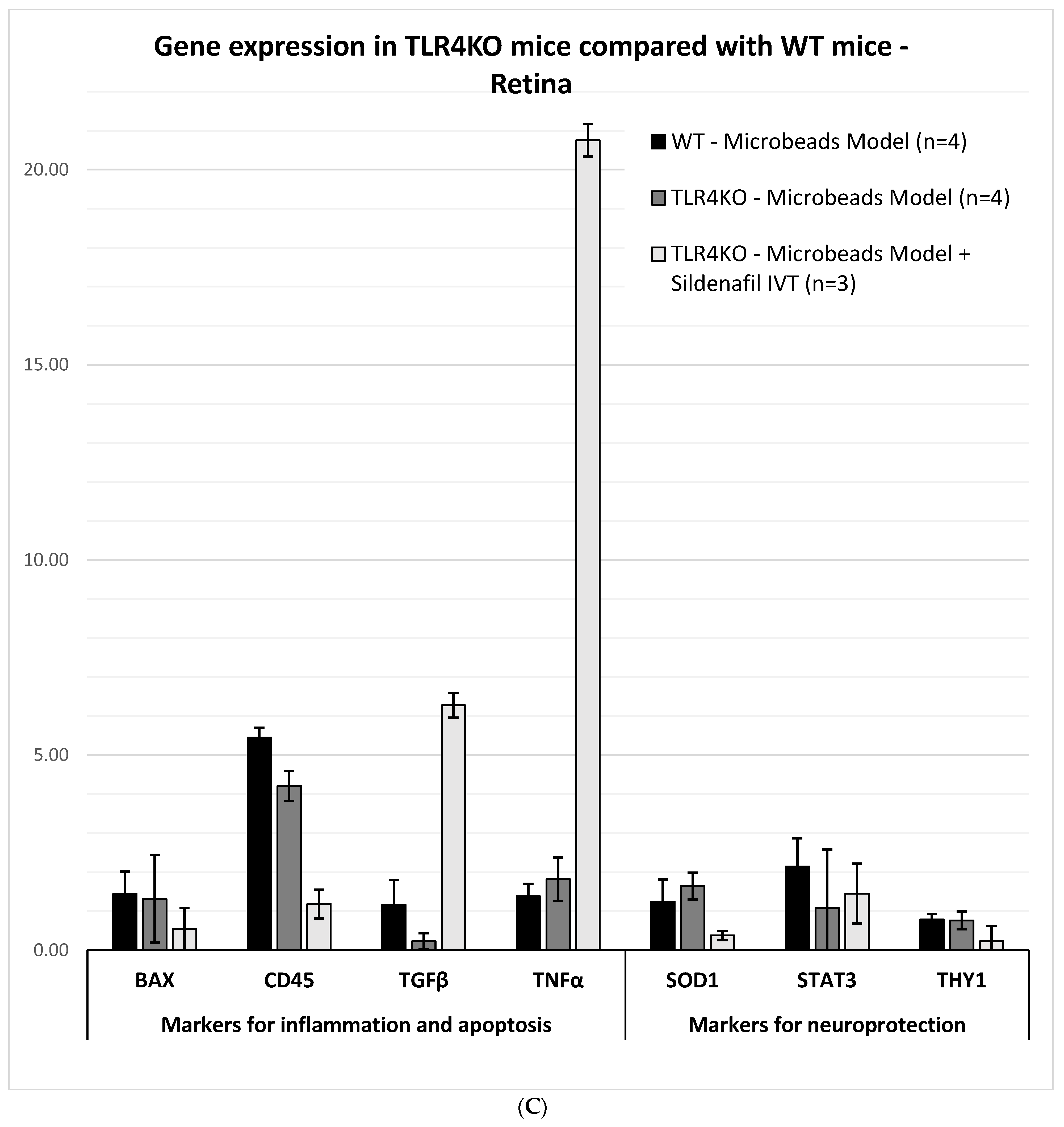
2.3.4. Glaucoma Microbead Model: TLR4KO Mice
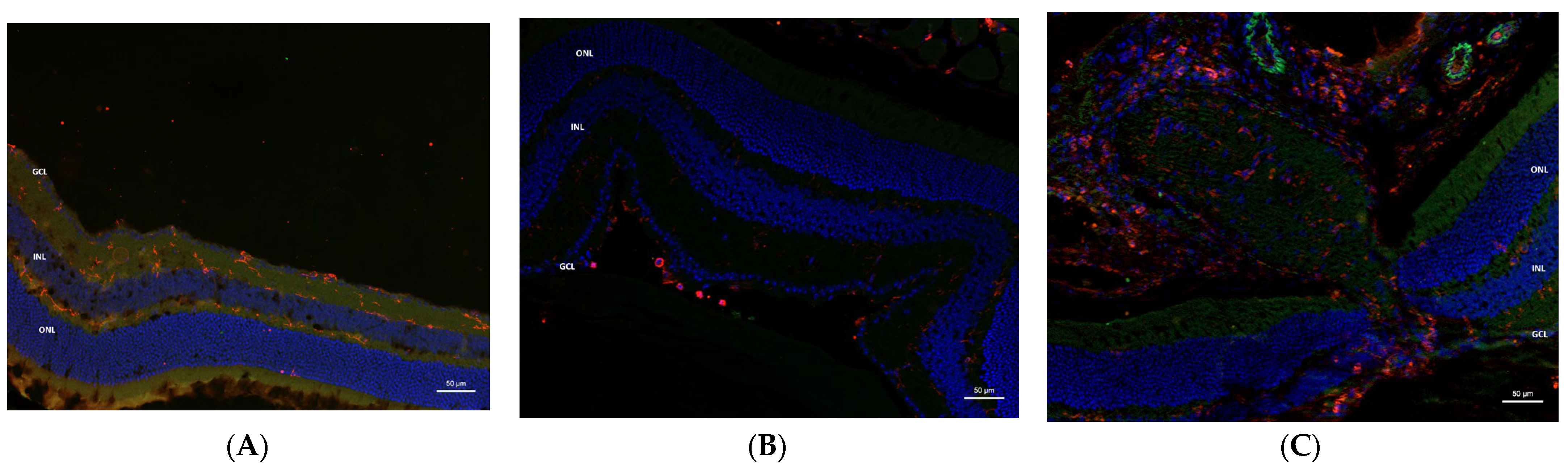
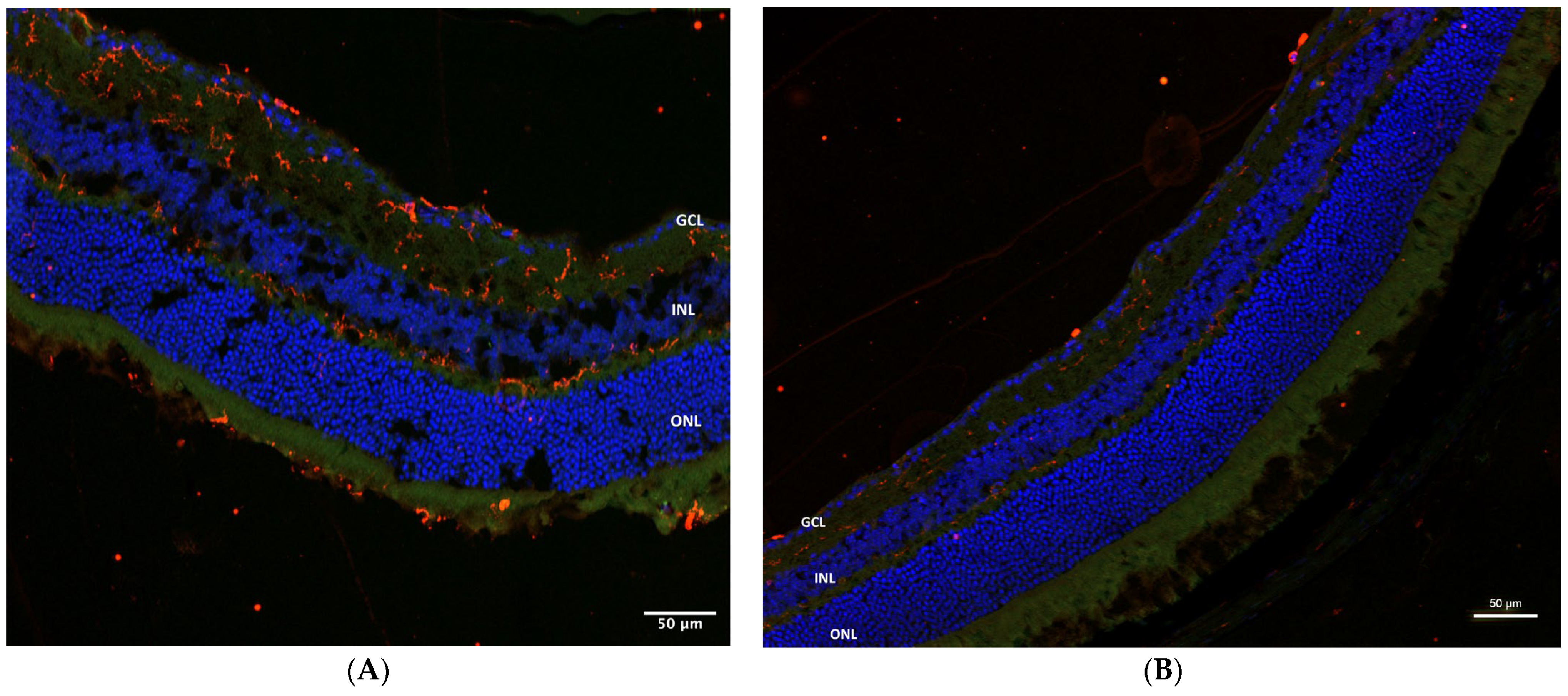
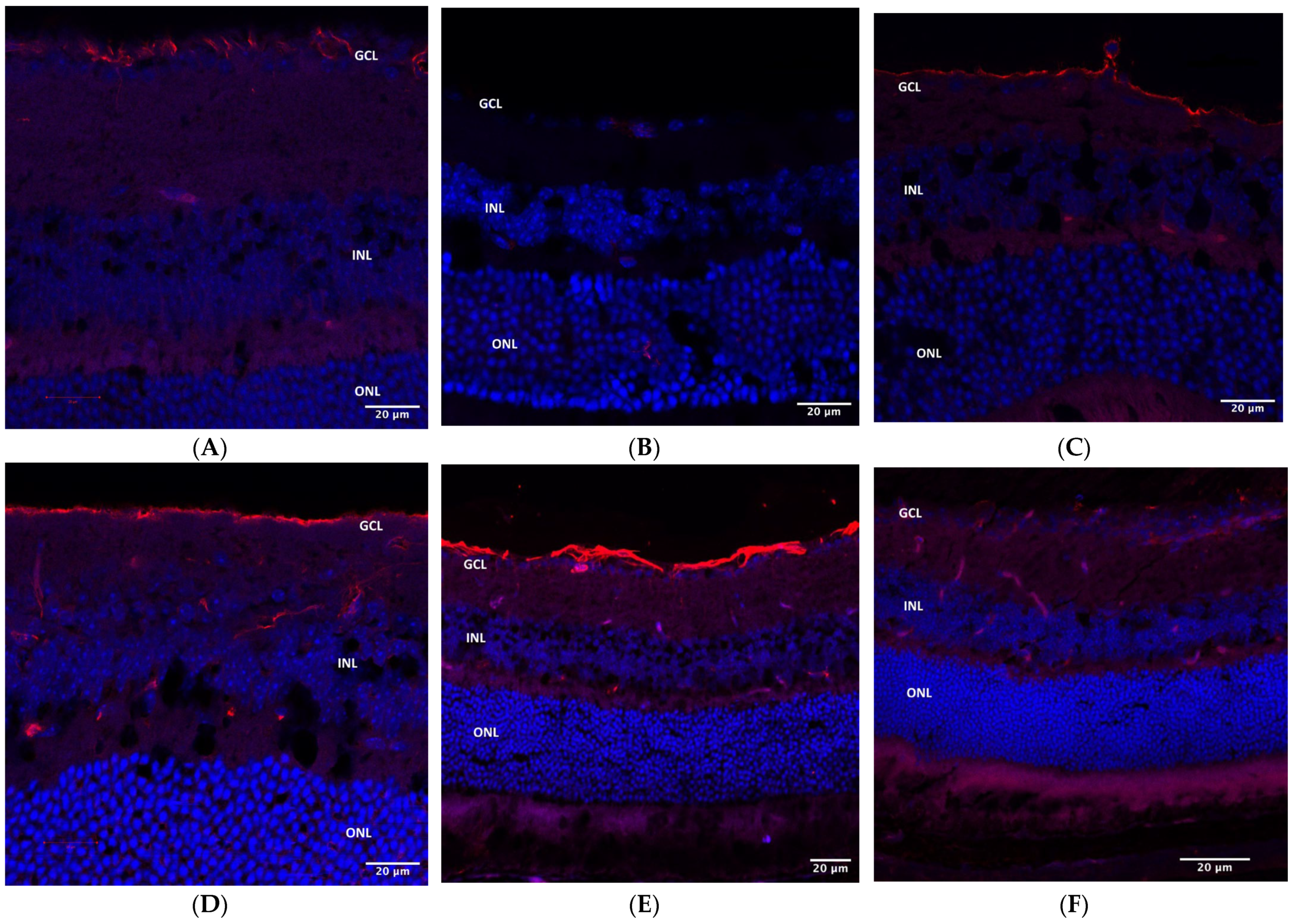
2.4. Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Microbead-Induced Glaucoma Model
4.3. Measurement of Intraocular Pressure
4.4. Dosages
4.5. Treatment with AZ and Sildenafil
4.6. Molecular Analysis
RNA Extraction, Conversion to cDNA, and Analysis by RT-PCR
4.7. Histological Analysis
4.7.1. Cryosection
4.7.2. H&E Staining, RGC Count, and Retinal Thickness Measurement
4.7.3. Immunofluorescent Assays
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, S.; Stankowska, D.L.; Ellis, D.Z.; Krishnamoorthy, R.R.; Yorio, T. Targets of Neuroprotection in Glaucoma. J. Ocul. Pharmacol. Ther. 2018, 34, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.D.; Khawaja, A.P.; Weizer, J.S. Glaucoma in Adults—Screening, Diagnosis, and Management: A Review. JAMA J. Am. Med. Assoc. 2021, 325, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, D.S.; Da Silva, G.A.; Ezra-Elia, R.; Carvalho, M.; Quitzan, J.G.; Ofri, R.; Laus, J.L.; Laufer-Amorim, R. Histological, Morphometric, Protein and Gene Expression Analyses of Rat Retinas with Ischaemia-Reperfusion Injury Model Treated with Sildenafil Citrate. Int. J. Exp. Pathol. 2017, 98, 147–157. [Google Scholar] [CrossRef]
- Arora, S.; Surakiatchanukul, T.; Arora, T.; Cagini, C.; Lupidi, M.; Chhablani, J. Sildenafil in Ophthalmology: An Update. Surv. Ophthalmol. 2022, 67, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Wang, N.N.; Zhang, T.Y.; Wang, F.; Wu, C.F.; Yang, J.Y. Neuroprotection by Sildenafil: Neuronal Networks Potentiation in Acute Experimental Stroke. CNS Neurosci. Ther. 2014, 20, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Charriaut-Marlangue, C.; Nguyen, T.; Bonnin, P.; Duy, A.P.; Leger, P.L.; Csaba, Z.; Pansiot, J.; Bourgeois, T.; Renolleau, S.; Baud, O. Sildenafil Mediates Blood-Flow Redistribution and Neuroprotection after Neonatal Hypoxia-Ischemia. Stroke 2014, 45, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, A.; Weiss, S.; Vieyra, M.; Nicholson, J.D.; Muhsinoglu, O.; Barinfeld, O.; Zadok, D.; Goldenberg-Cohen, N. Ocular Effects of Sildenafil in Naïve Mice and a Mouse Model of Optic Nerve Crush. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1987–1995. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, P.; Zhou, Y.; Chiang, C.-W.; Tan, J.; Hou, Y.; Stauffer, S.; Li, L.; Pieper, A.A.; Cummings, J.; et al. Endophenotype-Based in Silico Network Medicine Discovery Combined with Insurance Record Data Mining Identifies Sildenafil as a Candidate Drug for Alzheimer’s Disease. Nat. Aging 2021, 1, 1175–1188. [Google Scholar] [CrossRef]
- Xiong, Y.; Wintermark, P. The Role of Sildenafil in Treating Brain Injuries in Adults and Neonates. Front. Cell. Neurosci. 2022, 16. [Google Scholar] [CrossRef]
- Etminan, M.; Sodhi, M.; Mikelberg, F.S.; Maberley, D. Risk of Ocular Adverse Events Associated With Use of Phosphodiesterase 5 Inhibitors in Men in the US. JAMA Ophthalmol. 2022, 140, 480–484. [Google Scholar] [CrossRef]
- Campbell, U.B.; Walker, A.M.; Gaffney, M.; Petronis, K.R.; Creanga, D.; Quinn, S.; Klein, B.E.K.; Laties, A.M.; Lewis, M.; Sharlip, I.D.; et al. Acute Nonarteritic Anterior Ischemic Optic Neuropathy and Exposure to Phosphodiesterase Type 5 Inhibitors. J. Sex. Med. 2015, 12, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Cho, K.S.; Thee, E.F.; Jager, M.J.; Chen, D.F. Neuroinflammation and Microglia in Glaucoma: Time for a Paradigm Shift. J. Neurosci. Res. 2019, 97, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, A.; Friedman Gohas, M.; Sternfeld, A.; Daoud Zreiq, N.; Muhsinoglu, O.; Ofri, R.; BarKana, Y.; Goldenberg-Cohen, N. Histological and Molecular Characterization of Glaucoma Model Induced by One or Two Injections of Microbeads to the Anterior Chamber of Mice. Int. Ophthalmol. 2022, 42, 3763–3775. [Google Scholar] [CrossRef]
- Parnham, M.J.; Haber, V.E.; Giamarellos-Bourboulis, E.J.; Perletti, G.; Verleden, G.M.; Vos, R. Azithromycin: Mechanisms of Action and Their Relevance for Clinical Applications. Pharmacol. Ther. 2014, 143, 225–245. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Papanas, N.; Kioumis, I.; Chatzaki, E.; Maltezos, E.; Zarogoulidis, K. Macrolides: From in Vitro Anti-Inflammatory and Immunomodulatory Properties to Clinical Practice in Respiratory Diseases. Eur. J. Clin. Pharmacol. 2012, 68, 479–503. [Google Scholar] [CrossRef]
- Barks, J.D.E.; Liu, Y.; Wang, L.; Pai, M.P.; Silverstein, F.S. Repurposing Azithromycin for Neonatal Neuroprotection. Pediatr. Res. 2019, 86, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Varano, G.P.; Parisi, V.; Adornetto, A.; Cavaliere, F.; Amantea, D.; Nucci, C.; Corasaniti, M.T.; Morrone, L.A.; Bagetta, G.; Russo, R. Post-Ischemic Treatment with Azithromycin Protects Ganglion Cells against Retinal Ischemia/Reperfusion Injury in the Rat. Mol. Vis. 2017, 23, 911. [Google Scholar]
- Amantea, D.; Petrelli, F.; Greco, R.; Tassorelli, C.; Corasaniti, M.T.; Tonin, P.; Bagetta, G. Azithromycin Affords Neuroprotection in Rat Undergone Transient Focal Cerebral Ischemia. Front. Neurosci. 2019, 13, 1256. [Google Scholar] [CrossRef]
- Sappington, R.M.; Carlson, B.J.; Crish, S.D.; Calkins, D.J. The Microbead Occlusion Model: A Paradigm for Induced Ocular Hypertension in Rats and Mice. Investig. Ophthalmol. Vis. Sci. 2010, 51, 207–216. [Google Scholar] [CrossRef]
- Dai, C.; Khaw, P.T.; Yin, Z.Q.; Li, D.; Raisman, G.; Li, Y. Structural Basis of Glaucoma: The Fortified Astrocytes of the Optic Nerve Head Are the Target of Raised Intraocular Pressure. Glia 2012, 60, 13–28. [Google Scholar] [CrossRef]
- Foxton, R.H.; Finkelstein, A.; Vijay, S.; Dahlmann-Noor, A.; Khaw, P.T.; Morgan, J.E.; Shima, D.T.; Ng, Y.S. VEGF-A Is Necessary and Sufficient for Retinal Neuroprotection in Models of Experimental Glaucoma. Am. J. Pathol. 2013, 182, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, J.; Coleman, A.L. Intraocular Pressure Fluctuation a Risk Factor for Visual Field Progression at Low Intraocular Pressures in the Advanced Glaucoma Intervention Study. Ophthalmology 2008, 115, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Matlach, J.; Bender, S.; König, J.; Binder, H.; Pfeiffer, N.; Hoffmann, E.M. Investigation of Intraocular Pressure Fluctuation as a Risk Factor of Glaucoma Progression. Clin. Ophthalmol. 2019, 13, 9. [Google Scholar] [CrossRef]
- Lee, P.P.; Walt, J.W.; Rosenblatt, L.C.; Siegartel, L.R.; Stern, L.S. Association between Intraocular Pressure Variation and Glaucoma Progression: Data from a United States Chart Review. Am. J. Ophthalmol. 2007, 144, 901–907. [Google Scholar] [CrossRef]
- Barks, J.D.E.; Liu, Y.; Dopp, I.A.; Silverstein, F.S. Azithromycin Reduces Inflammation-Amplified Hypoxic–Ischemic Brain Injury in Neonatal Rats. Pediatr. Res. 2022, 92, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Mac Nair, C.E.; Fernandes, K.A.; Schlamp, C.L.; Libby, R.T.; Nickells, R.W. Tumor Necrosis Factor Alpha Has an Early Protective Effect on Retinal Ganglion Cells after Optic Nerve Crush. J. Neuroinflammation 2014, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Ray, A.; Rodgers, K.; Ergorul, C.; Hyman, B.T.; Huang, W.; Grosskreutz, C.L. Global Gene Expression Changes in Rat Retinal Ganglion Cells in Experimental Glaucoma. Invest. Ophthalmol. Vis. Sci. 2010, 51, 4084–4095. [Google Scholar] [CrossRef]
- Usui-Ouchi, A.; Eade, K.; Giles, S.; Ideguchi, Y.; Ouchi, Y.; Aguilar, E.; Wei, G.; Marra, K.V.; Berlow, R.B.; Friedlander, M. Deletion of Tgfβ Signal in Activated Microglia Prolongs Hypoxia-Induced Retinal Neovascularization Enhancing Igf1 Expression and Retinal Leukostasis. Glia 2022, 70, 1762–1776. [Google Scholar] [CrossRef]
- Ramarao, S.; Pang, Y.; Carter, K.; Bhatt, A. Azithromycin Protects Oligodendrocyte Progenitor Cells against Lipopolysaccharide-Activated Microglia-Induced Damage. Dev. Neurosci. 2022, 44, 1–12. [Google Scholar] [CrossRef]
- Ausó, E.; Gómez-vicente, V.; Esquiva, G. Visual Side Effects Linked to Sildenafil Consumption: An Update. Biomedicines 2021, 9, 291. [Google Scholar] [CrossRef]
- Duarte-Silva, E.; da Rocha Araújo, S.M.; Oliveira, W.H.; de Lós, D.B.; de França, M.E.R.; Bonfanti, A.P.; Peron, G.; de Lima Thomaz, L.; Verinaud, L.; de Santana Nunes, A.K.; et al. Sildenafil Ameliorates EAE by Decreasing Apoptosis in the Spinal Cord of C57BL/6 Mice. J. Neuroimmunol. 2018, 321, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, R.; Jiang, L.; Wang, X.; Feng, Z.; He, J.; He, X.; Du, G. Regulatory Effects of Toll-like Receptor 4 Knockout on CD4(+) and CD8(+) T Lymphocytes and Interleukin-17 During Myocardial Ischemia. Ann. Clin. Lab. Sci. 2020, 50, 761–768. [Google Scholar] [PubMed]
- Fu, H.; Liu, H. Deletion of Toll-like Receptor 4 Ameliorates Diabetic Retinopathy in Mice. Arch. Physiol. Biochem. 2023, 129, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Hyakkoku, K.; Hamanaka, J.; Tsuruma, K.; Shimazawa, M.; Tanaka, H.; Uematsu, S.; Akira, S.; Inagaki, N.; Nagai, H.; Hara, H. Toll-like Receptor 4 (TLR4), but Not TLR3 or TLR9, Knock-out Mice Have Neuroprotective Effects against Focal Cerebral Ischemia. Neuroscience 2010, 171, 258–267. [Google Scholar] [CrossRef]
- Morzaev, D.; Nicholson, J.D.; Caspi, T.; Weiss, S.; Hochhauser, E.; Goldenberg-Cohen, N. Toll-like Receptor-4 Knockout Mice Are More Resistant to Optic Nerve Crush Damage than Wild-Type Mice. Clin. Exp. Ophthalmol. 2015, 43, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Li, F.; Chen, H.; Wang, Y.; Zhu, Y.; Yang, X.; Zhu, J.; Wu, F.; Ouyang, H.; Ge, J.; et al. Caspase-8 Promotes NLRP1/NLRP3 Inflammasome Activation and IL-1β Production in Acute Glaucoma. Proc. Natl. Acad. Sci. USA 2014, 111, 11181–11186. [Google Scholar] [CrossRef]
- Morgan, J.E.; Tribble, J.R. Microbead Models in Glaucoma. Exp. Eye Res. 2015, 141, 9–14. [Google Scholar] [CrossRef]
- Calkins, D.J.; Lambert, W.S.; Formichella, C.R.; McLaughlin, W.M.; Sappington, R.M. The Microbead Occlusion Model of Ocular Hypertension in Mice. Glaucoma: Methods Protoc. 2018, 1695, 23–39. [Google Scholar]
- Liu, H.; Ding, C. Establishment of an Experimental Glaucoma Animal Model: A Comparison of Microbead Injection with or without Hydroxypropyl Methylcellulose. Exp. Ther. Med. 2017, 14, 1953–1960. [Google Scholar] [CrossRef]
- Walker, D.K.; Ackland, M.J.; James, G.C.; Muirhead, G.J.; Rance, D.J.; Wastall, P.; Wright, P.A. Pharmacokinetics and Metabolism of Sildenafil in Mouse, Rat, Rabbit, Dog and Man. Xenobiotica 1999, 29, 297–310. [Google Scholar] [CrossRef]
- Jiao, H.; Provis, J.M.; Natoli, R.; Rutar, M. Ablation of C3 Modulates Macrophage Reactivity in the Outer Retina during Photo-Oxidative Damage. Mol. Vis. 2020, 26, 679. [Google Scholar] [PubMed]

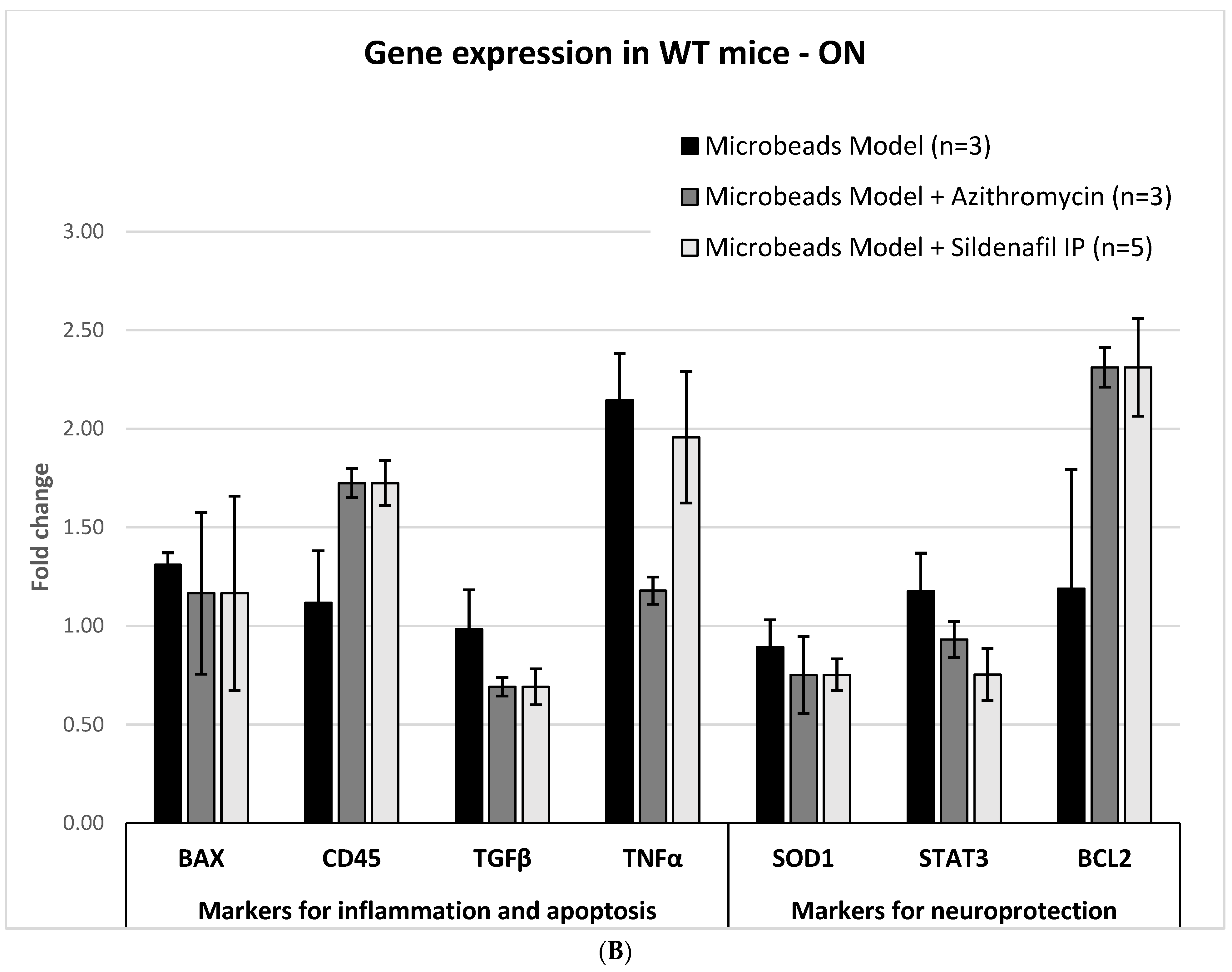
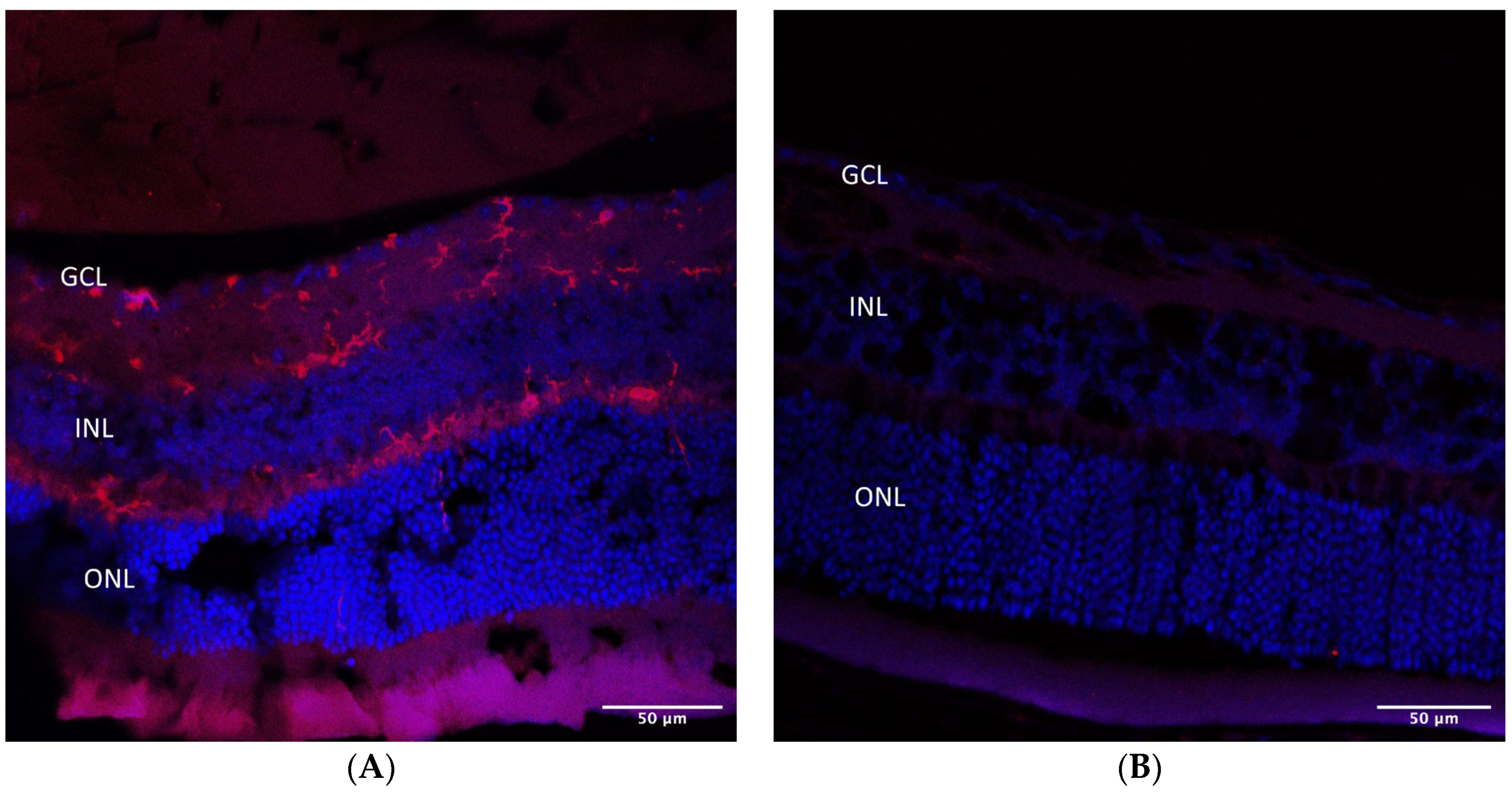

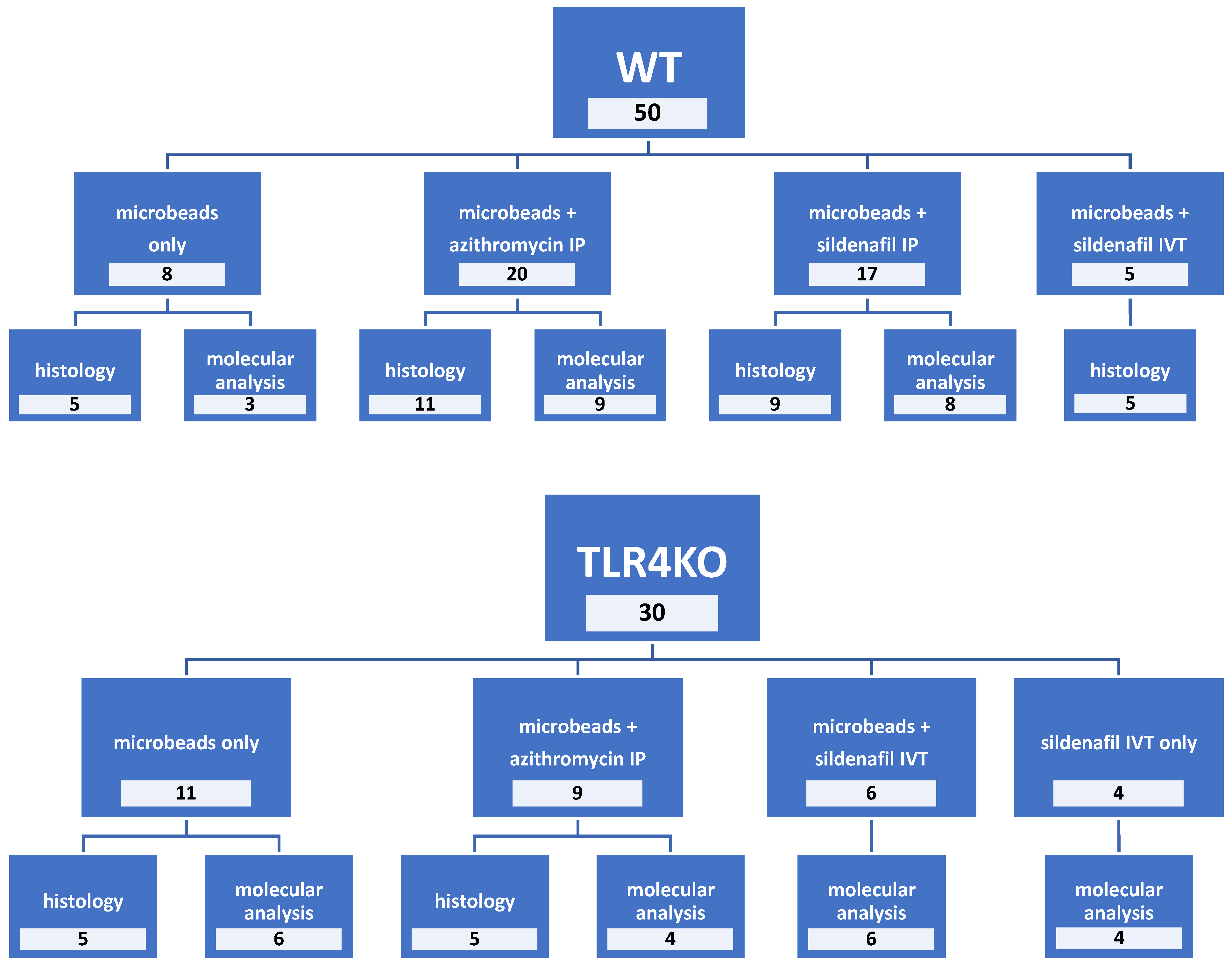
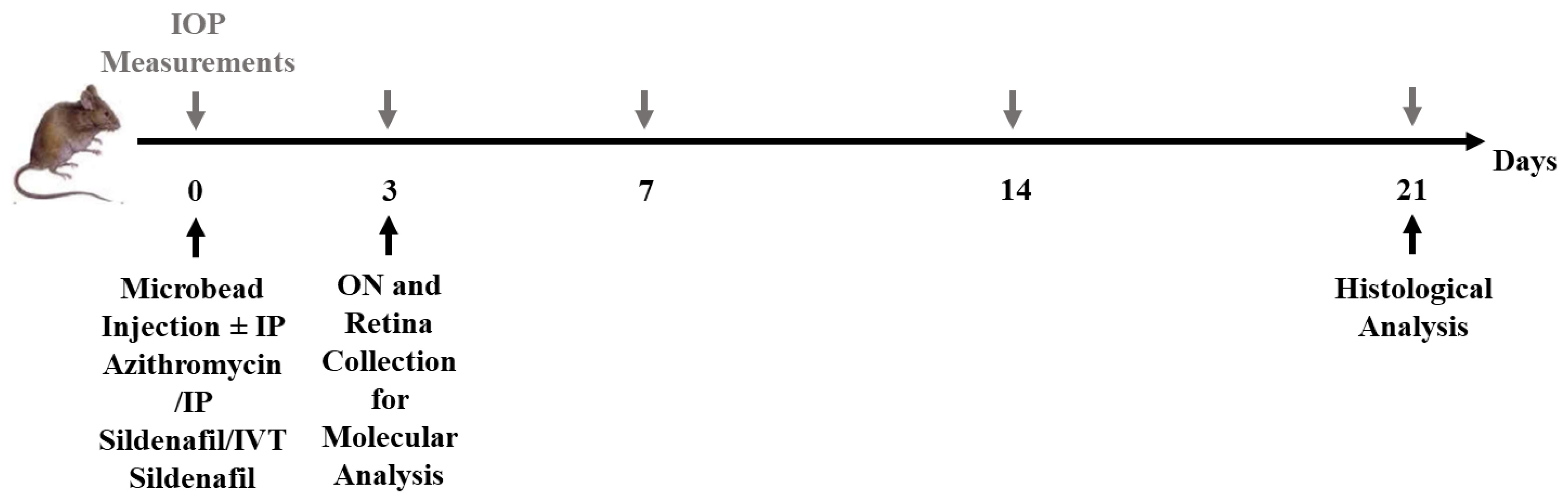
| (A) | |||||
|---|---|---|---|---|---|
| Group | Eye | Retinal Thickness (µm) | Retinal Ganglion Cell Count (Number of Cells in 200 µm Section) | ||
| Mean (SD) | p-Value | Mean (SD) | p-Value | ||
| Microbeads only | right | 183.27 (30.93) | 0.009 | 20.37 (3.82) | 0.003 |
| left | 198.34 (28.94) | 22.41 (4.35) | |||
| Microbeads + IP azithromycin | right | 189.72 (23.71) | 0.002 | 21.87 (4.92) | <0.001 |
| left | 200.79 (27.98) | 25.13 (5.02) | |||
| Microbeads + IP or IVT sildenafil | right | 192.55 (17.68) | 0.565 | 21.12 (4.71) | 0.798 |
| left | 193.79 (19.42) | 21.24 (3.9) | |||
| Microbeads + IP sildenafil | right | 193.44 (17.21) | 0.363 | 20.68 (4.56) | 0.145 |
| left | 195.73 (18.91) | 21.42 (3.67) | |||
| Microbeads + IVT sildenafil | right | 186.99 (19.97) | 0.405 | 23.96 (4.8) | 0.003 |
| left | 191.3 (19.92) | 21.06 (4.12) | |||
| (B) | |||||
| Group | Retinal Thickness (µm) | Retinal Ganglion Cell Count (Number of Cells in 200µm Section) | |||
| Mean (SD) | p-Value * | Mean (SD) | p-Value * | ||
| Microbeads only | 183.27 (30.93) | 20.37 (3.82) | |||
| Microbeads + IP azithromycin | 189.62 (23.71) | 0.188 | 21.87 (4.92) | 0.017 | |
| Microbeads + IP or IVT sildenafil | 192.55 (17.68) | 0.037 | 21.12 [4.71) | 0.226 | |
|
|
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sela, T.C.; Zahavi, A.; Friedman-Gohas, M.; Weiss, S.; Sternfeld, A.; Ilguisonis, A.; Badash, D.; Geffen, N.; Ofri, R.; BarKana, Y.; et al. Azithromycin and Sildenafil May Have Protective Effects on Retinal Ganglion Cells via Different Pathways: Study in a Rodent Microbead Model. Pharmaceuticals 2023, 16, 486. https://doi.org/10.3390/ph16040486
Sela TC, Zahavi A, Friedman-Gohas M, Weiss S, Sternfeld A, Ilguisonis A, Badash D, Geffen N, Ofri R, BarKana Y, et al. Azithromycin and Sildenafil May Have Protective Effects on Retinal Ganglion Cells via Different Pathways: Study in a Rodent Microbead Model. Pharmaceuticals. 2023; 16(4):486. https://doi.org/10.3390/ph16040486
Chicago/Turabian StyleSela, Tal Corina, Alon Zahavi, Moran Friedman-Gohas, Shirel Weiss, Amir Sternfeld, Astrid Ilguisonis, Danielle Badash, Noa Geffen, Ron Ofri, Yaniv BarKana, and et al. 2023. "Azithromycin and Sildenafil May Have Protective Effects on Retinal Ganglion Cells via Different Pathways: Study in a Rodent Microbead Model" Pharmaceuticals 16, no. 4: 486. https://doi.org/10.3390/ph16040486
APA StyleSela, T. C., Zahavi, A., Friedman-Gohas, M., Weiss, S., Sternfeld, A., Ilguisonis, A., Badash, D., Geffen, N., Ofri, R., BarKana, Y., & Goldenberg-Cohen, N. (2023). Azithromycin and Sildenafil May Have Protective Effects on Retinal Ganglion Cells via Different Pathways: Study in a Rodent Microbead Model. Pharmaceuticals, 16(4), 486. https://doi.org/10.3390/ph16040486







