Antioxidants in Traditional Mexican Medicine and Their Applications as Antitumor Treatments
Abstract
1. Introduction
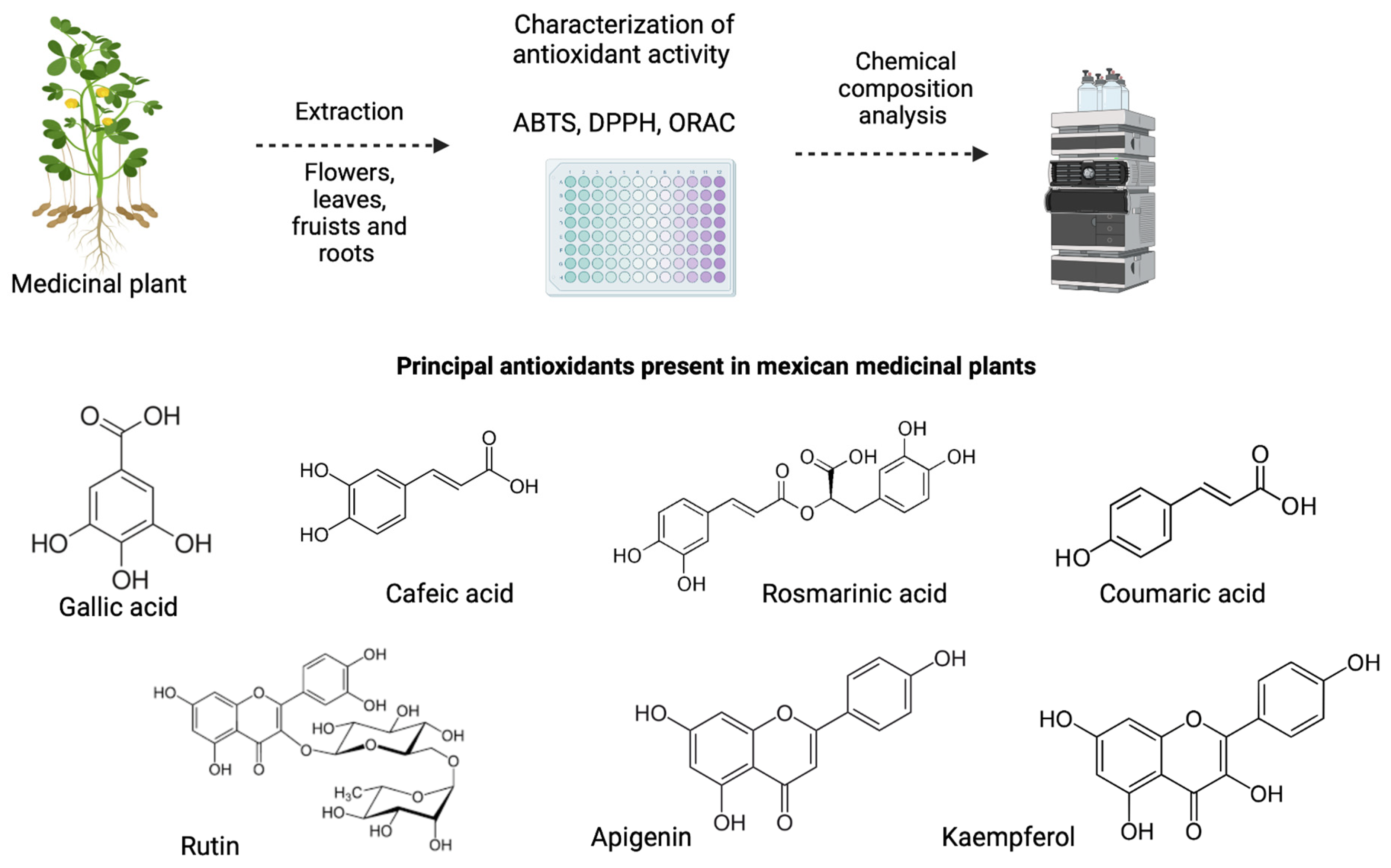
2. Antioxidants
2.1. Antioxidant Activity and Capacity
2.1.1. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Assay
2.1.2. 2,2′-Azinobis (3-Ethylbenzothiazoline-6-Sulphonic Acid (ABTS•+)
2.1.3. Ferric Reducing Antioxidant Power Assay (FRAP)
2.1.4. Oxygen Radical Absorbance Capacity ORAC Assay
2.1.5. Electrochemical Methods
2.2. Mexican Plants with Antioxidant Activity
| Plant | Common Name | Part | DPPH (mg TE/g) | ABTS (mg TE/g) | TPC (mg GAE/g) | Reference |
|---|---|---|---|---|---|---|
| A. muricata | Guanabana | Leaves | 28.1 ± 4.4 | -- | 79.4 ± 6.4 | [22] |
| Castilleja tenuiflora | Cola de borrego | Aerial parts | 49.91 ± 1.80 | 112.85 ± 7.03 | 30.58 ± 2.39 | [23] |
| L. graveolens | Oregano | Leaves | 81.00 ± 5.0 | -- | 270.25 ± 4.1 | [25] |
| Tithonia diversifolia | Sunflower | Leaves | 217.2 ± 8.70 | -- | 14.6 ± 0.64 | [26] |
| Bougainvillea buttiana (Var. Orange and Rose) | Bugambilia | Flowers | 1683.6 ± 143.3 | -- | 29.5 ± 0.05 | [30] |
| Piper auritum | Yerba santa | Leaves | -- | 14.82 ± 2.88 | 6.79 | [31] |
| Justicia spicigera | Muicle | Leaves | 880 ± 94 | 8480 ± 378 | 8520 ± 497 | [32] |
| Tribulus terrestris | Abrojo | Aerial | ~35 | -- | 250 ± 1.17 | [33] |
| Parthenium argentatum A. Gray | Guayule | Leaves | ~21.3–27.4 | -- | ~16–27 | [34] |
| Parmentiera aculeata Kunth | Cuajilote | Fruit | ~160 | -- | ~1980 | [35] |
| Arctostaphylos pungens | Pingüica | Fruit | 6214 ± 132 | 8465 ± 124 | 323.4 ±5.6 | [36] |
| Thymus vulgaris | Tomillo | Leaves | IC50 13.4 μg/mL | IC50 40.03 μg/mL | ~256 | [37] |
| Eryngium carlinae | Hierba del sapo | Inflorescence | 45.02 ± 0.31 | 197.2 ± 75 | 4.32 ± 0.02 | [38,39] |
| Bixa orellana L. | Achiote | Seed | 17.42 ± 0.45 | -- | 62.08 ± 2.21 | [40] |
| Acacia farnesiana | Huizache | Aerial | 89 μmol TE/g ORAC | 3.4 g TE/g FRAP | 565 | [41] |
| Taraxacum officinale | Diente de Leon | Leaves and flowers | 0.950 ± 0.002 | 1.132 ± 0.012 | 0.535 ± 0.033 | [42] |
| Tagetes erecta L. | Cempasuchil | Flowers | 401.47 ± 3.35 | 843.92 ± 4.44 | 108.71 ± 1.13 | [8,43] |
| Arnica montana | Arnica | Roots | -- | -- | 116.9 ± 1.0 | [44] |
| Ruta graveolens L. | Ruda | Leaves | 67.59 ± 0.98 | -- | 30.19 ± 0.16 | [45] |
| Tagetes lucida Cav | Pericon | Flower | --- | -- | 12.7 ± 0.1 | [46] |
| Anchusa officinalis L. | Lengua de Buey | Flower | 57.04 ± 1.08 | -- | 104.03 ± 0.63 | [47] |
| Passiflora incarnata | Pasiflora | Flower | IC50 31.92 μg/mL | -- | -- | [48] |
| Acalypha wilkesiana | Chirrite | Leaves | IC50 53.49 μg/mL | -- | ~50 | [49] |
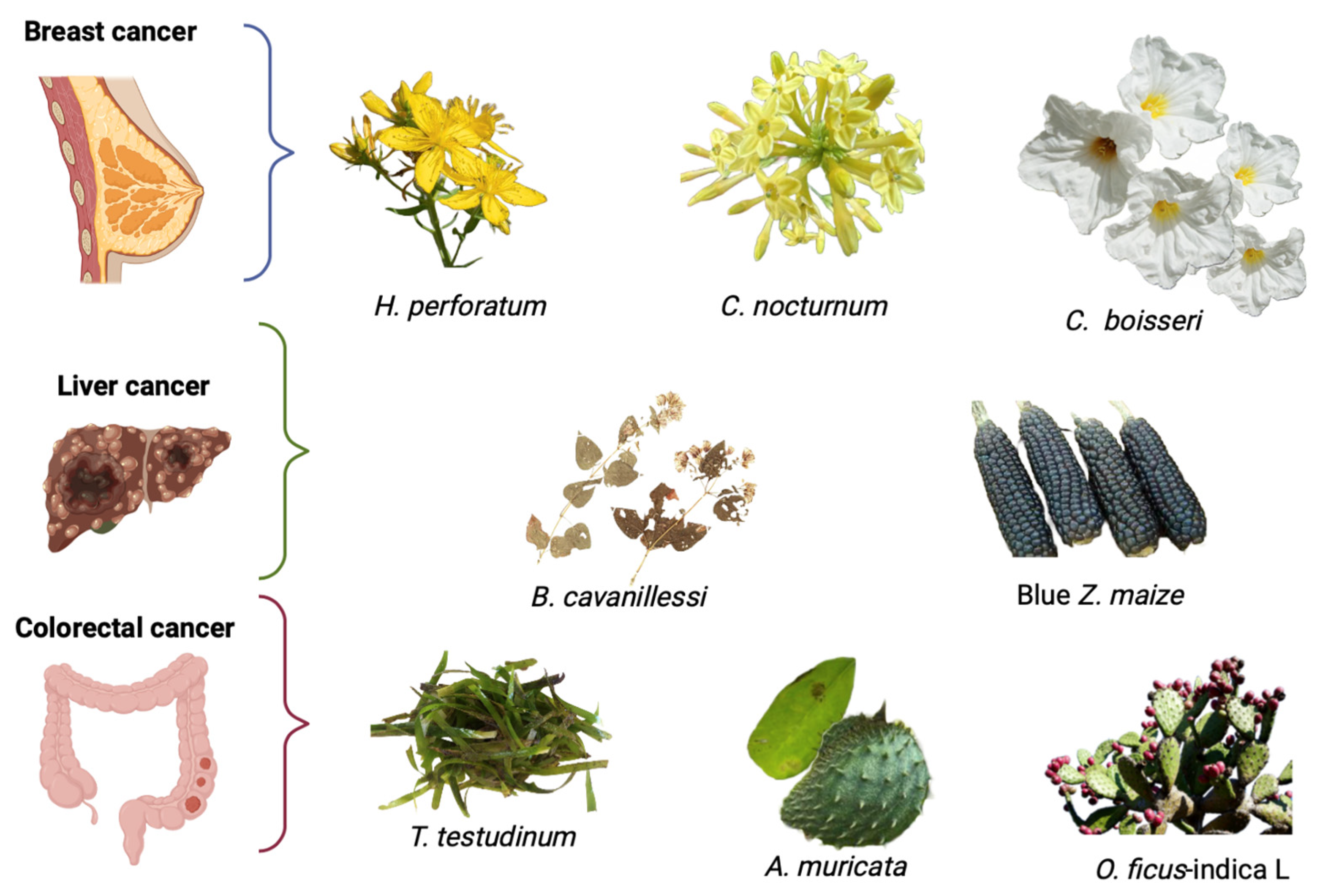
3. Plants in the Treatment of Breast Cancer
4. Plants in the Treatment of Liver Cancer
| Plant | Part of the Plant | Solvent | Cellular Line | IC50 μg/mL | Reference |
|---|---|---|---|---|---|
| Rhoeo discolor | Leaves | Methanol | HeLa | 70 ± 3.2 | [70] |
| Roots | 67 ± 1.4 | ||||
| Lophocereus schottii | Stem | Ethanol | L5178Y | 7.8 | [71] |
| Annona muricata Linn | Leaves | Ethanol | 4T1 | 79.2 ± 0.2 | [72] |
| Annona squoamosa | Seed | Ethanol | PC-3 | 13.08 | [73] |
| SiHa | 16 | ||||
| Barringtonia racemosa | Fruit | Methanol | MCF-7 | 57.61 ± 2.24 | [74] |
| Hibiscus sabdariffa | Flower | Methanol | MCF-7 | 112.10 ± 3.97 | [74,75] |
| Justicia spicigera Schltdl | Leaves | Ethanol | HeLa | 17 | [76] |
| Tagetes lucida Cav. | Flowers | Water | Calu-1 | 100 | [46] |
| HepG2 | 270 | ||||
| Dioon spinulosum | Leaves | Ethanol | MCF-7 | 22.5 | [77] |
| HeLa | 21.8 | ||||
| Amphipterygium adstringens | Leaves | Methanol | UACC-62 | 7.3 | [78] |
| OVCAR-3 | 4.4 | ||||
| NCI-H460 | 28 | ||||
| Lophophora williamsii | Cacti | Methanol | C6 | 1.92 | [55] |
| Amphipterygium | Bark | Methanol | HepG2 | 41.77 ± 6.18 | [79] |
| Vero | 197.98 ± 4.71 | ||||
| Cissus incisa | Leaves | CHCl3/MeOH | HeLa | 63 ± 7 | [80] |
| PC3 | 43 ± 4 | ||||
| Cnidoscolus multilobus (Pax) | Leaves | Ethanol/water | HeLa | 62 | [81] |
| Capsicum chinense | Leaves | Methanol | MCF-7 | 0.38 ± 0.01 | [82] |
| Stems | MCF-7 | 2.01 ± 0.33 | |||
| Peduncles | MCF-7 | 0.46 ± 0.02 | |||
| Semialarium mexicanum (Miers) Mennega | Root bark | Petroleum ether | MDA-MB-231 | 55.5 | [83] |
| MCF10A | 66.8 | ||||
| Asclepias subulata | Aerial | Ethanol | HCT-116 | 0.4 | [84] |
| Agave lechuguilla Torr | Leaves | Ethanol | MCF-7 | >150 | [85] |
| HeLa | 89 | ||||
| Vero | 126 | ||||
| R. communis | Aerial | Methanol | Vero | 34.8 | [86] |
| Ether | 326.8 | ||||
| Hippocratea celastroides | Leaves | Ethanol | MCF-7 | 2.29 | [87] |
| Stem | 2.57 | ||||
| Root | 2.81 | ||||
| Carica papaya L. | Flower | Ethanol | Vero | 62.5 | [88] |
| Smilax aspera L | Roots | Acetone | MDA-MB-231 | 695 | [89] |
| A549 | 535 | ||||
| OVCAR3 | 117 | ||||
| Argemone mexicana Linn. | Whole | Methanol | MCF-7 | 95.50 ± 3.69 | [90] |
| HeLa | 38.01 ± 1.77 | ||||
| Phaseolus vulgaris L. | Bean | Water/methanol | Caco2 | 81.2 | [91] |
5. Plants in the Treatment of Colorectal Cancer
6. Future Perspectives of Medicinal Plant Antioxidants in Cancer Therapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, J.; Jiang, L.; James Kang, Y. Cancer and Stem Cells. Exp. Biol. Med. 2021, 246, 1791–1801. [Google Scholar] [CrossRef]
- Berger, M.F.; Mardis, E.R. The Emerging Clinical Relevance of Genomics in Cancer Medicine. Nat. Rev. Clin. Oncol. 2018, 15, 353–365. [Google Scholar] [CrossRef]
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The Trends Projection Analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- Soto, K.M.; Mendoza, S.; López-Romero, J.M.; Gasca-Tirado, J.R.; Manzano-Ramírez, A. Gold Nanoparticles: Synthesis, Application in Colon Cancer Therapy and New Approaches—Review. Green Chem. Lett. Rev. 2021, 14, 663–676. [Google Scholar] [CrossRef]
- Greenwell, M.; Rahman, P.K.S.M. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef] [PubMed]
- Laha, D.; Nilubol, N.; Boufraqech, M. New Therapies for Advanced Thyroid Cancer. Front. Endocrinol. 2020, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Soto, K.M.; López-Romero, J.M.; Mendoza, S.; Peza-Ledesma, C.; Rivera-Muñoz, E.M.; Velazquez-Castillo, R.R.; Pineda-Piñón, J.; Méndez-Lozano, N.; Manzano-Ramírez, A. Rapid and Facile Synthesis of Gold Nanoparticles with Two Mexican Medicinal Plants and a Comparison with Traditional Chemical Synthesis. Mater. Chem. Phys. 2023, 295, 127109. [Google Scholar] [CrossRef]
- Martíınez, C.C.; Gómez, M.D.; Oh, M.S. Use of Traditional Herbal Medicine as an Alternative in Dental Treatment in Mexican Dentistry: A Review. Pharm. Biol. 2017, 55, 1992–1998. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Salazar, R.; Pozos, M.E.; Cordero, P.; Perez, J.; Salinas, M.C.; Waksman, N. Determination of the Antioxidant Activity of Plants from Northeast Mexico. Pharm. Biol. 2008, 46, 166–170. [Google Scholar] [CrossRef]
- Mateos-Maces, L.; Chávez-Servia, J.L.; Vera-Guzmán, A.M.; Aquino-Bolaños, E.N.; Alba-Jiménez, J.E.; Villagómez-González, B.B. Edible Leafy Plants from Mexico as Sources of Antioxidant Compounds, and Their Nutritional, Nutraceutical and Antimicrobial Potential: A Review. Antioxidants 2020, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of Methods to Determine Antioxidant Capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Gruszycki, M.R.; Valenzuela, G.M.; Báez, M.; Leguiza, P.D.; Gruszycki, A.E.; Alba, D.A. Evaluación de La Actividad Antioxidante En Extractos Hidroalcohólicos de Portulaca Oleracea L. Rev. Colomb. Cienc. Quím.-Farm. 2019, 48, 425–435. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Reiter, J.; Ries, M.; Gruhlke, M.C.H. Screening Procedures and Tests for Antioxidants. In Pathology: Oxidative Stress and Dietary Antioxidants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 389–395. ISBN 9780128159729. [Google Scholar]
- Hoyos-Arbeláez, J.; Vázquez, M.; Contreras-Calderón, J. Electrochemical Methods as a Tool for Determining the Antioxidant Capacity of Food and Beverages: A Review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef]
- Haque, M.A.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Electrochemical Methods to Evaluate the Antioxidant Activity and Capacity of Foods: A Review. Electroanalysis 2021, 33, 1419–1435. [Google Scholar] [CrossRef]
- Secretaría de Medio Ambiente y Recursos Naturales. Available online: https://Www.Gob.Mx/Semarnat/Articulos/Plantas-Medicinales-de-Mexico?Idiom=es (accessed on 23 February 2023).
- Pérez-González, M.Z.; Jiménez-Arellanes, M.A. Biotechnological Processes to Obtain Bioactive Secondary Metabolites from Some Mexican Medicinal Plants. Appl. Microbiol. Biotechnol. 2021, 105, 6257–6274. [Google Scholar] [CrossRef]
- Chamorro-Cevallos, G.; Mojica-Villegas, M.A.; García-Martínez, Y.; Pérez-Gutiérrez, S.; Madrigal-Santillán, E.; Vargas-Mendoza, N.; Morales-González, J.A.; Cristóbal-Luna, J.M. A Complete Review of Mexican Plants with Teratogenic Effects. Plants 2022, 11, 1675. [Google Scholar] [CrossRef]
- Justino, A.B.; Miranda, N.C.; Franco, R.R.; Martins, M.M.; da Silva, N.M.; Espindola, F.S. Annona Muricata Linn. Leaf as a Source of Antioxidant Compounds with in Vitro Antidiabetic and Inhibitory Potential against α-Amylase, α-Glucosidase, Lipase, Non-Enzymatic Glycation and Lipid Peroxidation. Biomed. Pharmacother. 2018, 100, 83–92. [Google Scholar] [CrossRef]
- Valdez-Tapia, R.; Capataz-Tafur, J.; López-Laredo, A.R.; Trejo-Espino, J.L.; Trejo-Tapia, G. Effect of Immersion Cycles on Growth, Phenolics Content, and Antioxidant Properties of Castilleja Tenuiflora Shoots. In Vitr. Cell. Dev. Biol.-Plant 2014, 50, 471–477. [Google Scholar] [CrossRef]
- Bautista-Hernández, I.; Aguilar, C.N.; Martínez-ávila, G.C.G.; Torres-León, C.; Ilina, A.; Flores-Gallegos, A.C.; Kumar Verma, D.; Chávez-González, M.L. Mexican Oregano (Lippia Graveolens Kunth) as Source of Bioactive Compounds: A Review. Molecules 2021, 26, 5156. [Google Scholar] [CrossRef]
- Martínez-Rocha, A.; Puga, R.; Hernández-Sandoval, L.; Loarca-Piña, G.; Mendoza, S. Antioxidant and Antimutagenic Activities of Mexican Oregano (Lippia Graveolens Kunth). Plant Foods Hum. Nutr. 2008, 63, 1–5. [Google Scholar] [CrossRef]
- Pretti, I.R.; da Luz, A.C.; Jamal, C.M.; Batitucci, M.D.C.P. Variation of Biochemical and Antioxidant Activity with Respect to the Phenological Stage of Tithonia Diversifolia Hemsl. (Asteraceae) Populations. Ind. Crops Prod. 2018, 121, 241–249. [Google Scholar] [CrossRef]
- Pantoja Pulido, K.D.; Colmenares Dulcey, A.J.; Isaza Martínez, J.H. New Caffeic Acid Derivative from Tithonia Diversifolia (Hemsl.) A. Gray Butanolic Extract and Its Antioxidant Activity. Food Chem. Toxicol. 2017, 109, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Aquino, R.; Cáceres, A.; Morelli, S.; Rastrelli, L. An Extract of Tagetes Lucida and Its Phenolic Constituents as Antioxidants. J. Nat. Prod. 2002, 65, 1773–1776. [Google Scholar] [CrossRef] [PubMed]
- Céspedes, C.L.; Avila, J.G.; Martínez, A.; Serrato, B.; Calderón-Mugica, J.C.; Salgado-Garciglia, R. Antifungal and Antibacterial Activities of Mexican Tarragon (Tagetes Lucida). J. Agric. Food Chem. 2006, 54, 3521–3527. [Google Scholar] [CrossRef]
- Petricevich, V.L.; Cedillo-Cortezano, M.; Abarca-Vargas, R. Chemical Composition, Antioxidant Activity, Cytoprotective and In Silico Study of Ethanolic Extracts of Bougainvillea × Buttiana (Var. Orange and Rose). Molecules 2022, 27, 6555. [Google Scholar] [CrossRef] [PubMed]
- Conde-Hernández, L.A.; Guerrero-Beltrán, J.Á. Total Phenolics and Antioxidant Activity of Piper Auritum and Porophyllum Ruderale. Food Chem. 2014, 142, 455–460. [Google Scholar] [CrossRef]
- Baqueiro-Peña, I.; Guerrero-Beltrán, J. Physicochemical and Antioxidant Characterization of Justicia Spicigera. Food Chem. 2017, 218, 305–312. [Google Scholar] [CrossRef]
- Hammoda, H.M.; Ghazy, N.M.; Harraz, F.M.; Radwan, M.M.; ElSohly, M.A.; Abdallah, I.I. Chemical Constituents from Tribulus Terrestris and Screening of Their Antioxidant Activity. Phytochemistry 2013, 92, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Jara, F.M.; Carrión, M.E.; Angulo, J.L.; Latorre, G.; López-Córcoles, H.; Zalacain, A.; Hurtado de Mendoza, J.; García-Martínez, M.M.; Carmona, M. Chemical Characterization, Antioxidant Activity and Morphological Traits in the Leaves of Guayule (Parthenium Argentatum A. Gray) and Its Hybrids. Ind. Crops Prod. 2022, 182, 114927. [Google Scholar] [CrossRef]
- Cristina, S.R.; Noemi, N.L.V.; Gabriela, C.B.M.; Gilber, V.G.; Alberto, V.L.A. Antimicrobial Activity, Phenolic and Antioxidant Content of Extracts from Cuajilote (Parmentiera Aculeata Kunth) Fruits at Different Degrees of Ripening. J. Mex. Chem. Soc. 2021, 65, 161–169. [Google Scholar] [CrossRef]
- Navarro-Cortez, R.; Tovar-Jímenez, X.; Mora-Rochín, S.; Rochín-Medina, J.; Aguayo-Rojas, J.C.F. Minerales, Capacidad Antioxidante y Antidepresiva de Pingüica (Arctostaphylos Pungens). Acta Univ. Multidiscip. Sci. J. 2022, 32, 1–15. [Google Scholar]
- Köksal, E.; Bursal, E.; Gülçin, İ.; Korkmaz, M.; Çağlayan, C.; Gören, A.C.; Alwasel, S.H. Antioxidant Activity and Polyphenol Content of Turkish Thyme (Thymus Vulgaris) Monitored by Liquid Chromatography and Tandem Mass Spectrometry. Int. J. Food Prop. 2017, 20, 514–525. [Google Scholar] [CrossRef]
- García-Cerrillo, D.; Noriega-Cisneros, R.; Peña-Montes, D.; Huerta-Cervantes, M.; Ríos-Silva, M.; Salgado-Garciglia, R.; Montoya-Pérez, R.; Saavedra-Molina, A. Antioxidant Effects of Eryngium Carlinae in Diabetic Rats. Asian J. Appl. Sci. 2018, 6. [Google Scholar] [CrossRef]
- Alvarado, T.D.; Mariezcurrena Berasain, M.D.; Salem, A.Z.M.; Pinzón Martínez, D.L. Antimicrobial and Antioxidant Activities of Two Medicinal Plants Cuphea Aequipetala Var. Hispida (Cav.) Koehne and Eryngium Comosum Delaroche F Against Bacteria Related to Equine Infections. J. Equine Vet. Sci. 2020, 94, 103269. [Google Scholar] [CrossRef]
- Van Cuong, T.; Chin, K.B. Effects of Annatto (Bixa Orellana L.) Seeds Powder on Physicochemical Properties, Antioxidant and Antimicrobial Activities of Pork Patties during Refrigerated Storage. Korean J. Food Sci. Anim. Resour. 2016, 36, 476–486. [Google Scholar] [CrossRef]
- Claudia, D.P.; Mario, C.H.; Arturo, N.O.; Noel, M.C.O.; Antonio, N.C.; Teresa, R.A.; Gerardo, L.T.Z.; Margarita, D.M.; Alejandra, Á.I.M.; Rosalina, C.M.Y.; et al. Phenolic Compounds in Organic and Aqueous Extracts from Acacia Farnesiana Pods Analyzed by ULPS-ESI-Q-Oa/TOF-MS. In Vitro Antioxidant Activity and Anti-Inflammatory Response in CD-1 Mice. Molecules 2018, 23, 2386. [Google Scholar] [CrossRef]
- Miłek, M.; Marcinčáková, D.; Legáth, J. Polyphenols Content, Antioxidant Activity, and Cytotoxicity Assessment of Taraxacum Officinale Extracts Prepared through the Micelle-Mediated Extraction Method. Molecules 2019, 24, 1025. [Google Scholar] [CrossRef]
- Burlec, A.F.; Pecio, Ł.; Kozachok, S.; Mircea, C.; Corciovă, A.; Vereştiuc, L.; Cioancă, O.; Oleszek, W.; Hăncianu, M. Phytochemical Profile, Antioxidant Activity, and Cytotoxicity Assessment of Tagetes Erecta L. Flowers. Molecules 2021, 26, 1201. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Świeca, M.; Sugier, D.; Cichocka, J. Comparison of in vitro lipoxygenase, xanthine oxidase inhibitory and antioxidant activity of Arnica Montana and Arnica Chamissonis tinctures. Acta Sci. Pol. Hortorum Cultus 2011, 10, 15–27. [Google Scholar]
- Pavić, V.; Flačer, D.; Jakovljević, M.; Molnar, M.; Jokić, S. Assessment of Total Phenolic Content, in Vitro Antioxidant and Antibacterial Activity of Ruta Graveolens L. Extracts Obtained by Choline Chloride Based Natural Deep Eutectic Solvents. Plants 2019, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Gallardo, K.; Quintero-Rincón, P.; Stashenko, E.E.; Olivero-Verbel, J. Photoprotective Agents Obtained from Aromatic Plants Grown in Colombia: Total Phenolic Content, Antioxidant Activity, and Assessment of Cytotoxic Potential in Cancer Cell Lines of Cymbopogon Flexuosus L. and Tagetes Lucida Cav. Essential Oils. Plants 2022, 11, 1693. [Google Scholar] [CrossRef]
- Boskovic, I.; Đukić, D.A.; Maskovic, P.; Mandić, L.; Perovic, S. Phytochemical Composition and Antimicrobial, Antioxidant and Cytotoxic Activities of Anchusa Officinalis L. Extracts. Biologia 2018, 73, 1035–1041. [Google Scholar] [CrossRef]
- Ingale, S.P.; Kasture, S.B. Antioxidant and Antiparkinsonian Activity of Passiflora Incarnata Leaves. Orient. Pharm. Exp. Med. 2014, 14, 231–236. [Google Scholar] [CrossRef]
- Omotayo, M.; Akoro, S.; Avungbeto, M.; Uwakwe, H. Evaluation of Free Radical Scavenging and Antibacterial Activity of Acalypha Wilkesiana and Terminalia Catappa Methanolic Leaf Extracts. Microbiol. Res. J. Int. 2017, 19, 1–9. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Almalki, W.H.; Makeen, H.A.; Albratty, M.; Meraya, A.M.; Nagraik, R.; Sharma, A.; Kumar, D.; Chellappan, D.K.; Singh, S.K.; et al. Role of Medicinal Plant-Derived Nutraceuticals as a Potential Target for the Treatment of Breast Cancer. J. Food Biochem. 2022, 46, e14387. [Google Scholar] [CrossRef]
- Yap, K.M.; Sekar, M.; Seow, L.J.; Gan, S.H.; Bonam, S.R.; Mat Rani, N.N.I.; Lum, P.T.; Subramaniyan, V.; Wu, Y.S.; Fuloria, N.K.; et al. Mangifera Indica (Mango): A Promising Medicinal Plant for Breast Cancer Therapy and Understanding Its Potential Mechanisms of Action. Breast Cancer Targets Ther. 2021, 13, 471–503. [Google Scholar] [CrossRef]
- Alahmad, A.; Alghoraibi, I.; Zein, R.; Kraft, S.; Dräger, G.; Walter, J.G.; Scheper, T. Identification of Major Constituents of Hypericum Perforatum L. Extracts in Syria by Development of a Rapid, Simple, and Reproducible HPLC-ESI-Q-TOF MS Analysis and Their Antioxidant Activities. ACS Omega 2022, 7, 13475–13493. [Google Scholar] [CrossRef]
- Mirmalek, S.A.; Azizi, M.A.; Jangholi, E.; Yadollah-Damavandi, S.; Javidi, M.A.; Parsa, Y.; Parsa, T.; Salimi-Tabatabaee, S.A.; Ghasemzadeh Kolagar, H.; Alizadeh-Navaei, R. Cytotoxic and Apoptogenic Effect of Hypericin, the Bioactive Component of Hypericum Perforatum on the MCF-7 Human Breast Cancer Cell Line. Cancer Cell Int. 2016, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Rashed, K.N.; Ćirič, A.; Glamočlija, J.; Calhelha, R.C.; Ferreira, I.C.F.R.; Soković, M. Identification of the Bioactive Constituents and the Antibacterial, Antifungal and Cytotoxic Activities of Different Fractions from Cestrum Nocturnum L. Jordan J. Biol. Sci. 2018, 11, 273–279. [Google Scholar]
- Franco-Molina, M.A.; Santana-Krímskaya, S.E.; Madrigal-De-león, L.M.; Coronado-Cerda, E.E.; Zárate-Triviño, D.G.; Hernández-Martínez, S.P.; García-Coronado, P.L.; Rodríguez-Padilla, C. Evaluation of the Cytotoxic and Immunogenic Potential of Temozolamide, Panobinostat, and Lophophora Williamsii Extract against C6 Glioma Cells. EXCLI J. 2021, 20, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Franco-Molina, M.; Gomez-Flores, R.; Tamez-Guerra, P.; Tamez-Guerra, R.; Castillo-Leon, L.; Rodríguez-Padilla, C. In Vitro Immunopotentiating Properties and Tumour Cell Toxicity Induced by Lophophora Williamsii (Peyote) Cactus Methanolic Extract. Phytother. Res. 2003, 17, 1076–1081. [Google Scholar] [CrossRef]
- Owis, A.I.; Abo-Youssef, A.M.; Osman, A.H. Leaves of Cordia Boissieri A. DC. As a Potential Source of Bioactive Secondary Metabolites for Protection against Metabolic Syndrome-Induced in Rats. Z. Nat. 2017, 72, 107–118. [Google Scholar] [CrossRef]
- Viveros-Valdez, E.; Jaramillo-Mora, C.; Oranday-Cárdenas, A.; Morán-Martínez, J.; Carranza-Rosales, P. Antioxidant, Cytotoxic and Alpha-Glucosidase Inhibition Activities from the Mexican Berry “Anacahuita” (Cordia boissieri). Arch. Latinoam. Nutr. 2016, 66, 211–218. [Google Scholar]
- Peña-Morán, O.A.; Villarreal, M.L.; Álvarez-Berber, L.; Meneses-Acosta, A.; Rodríguez-López, V. Cytotoxicity, Post-Treatment Recovery, and Selectivity Analysis of Naturally Occurring Podophyllotoxins from Bursera Fagaroides Var. Fagaroides on Breast Cancer Cell Lines. Molecules 2016, 21, 1013. [Google Scholar] [CrossRef]
- Rojas-Sepúlveda, A.M.; Mendieta-Serrano, M.; Mojica, M.Y.A.; Salas-Vidal, E.; Marquina, S.; Villarreal, M.L.; Puebla, A.M.; Delgado, J.I.; Alvarez, L. Cytotoxic Podophyllotoxin Type-Lignans from the Steam Bark of Bursera Fagaroides Var. Fagaroides. Molecules 2012, 17, 9506–9519. [Google Scholar] [CrossRef]
- Xu, F.; Jin, T.; Zhu, Y.; Dai, C. Immune Checkpoint Therapy in Liver Cancer. J. Exp. Clin. Cancer Res. 2018, 37, 110. [Google Scholar] [CrossRef]
- Li, X.; Ramadori, P.; Pfister, D.; Seehawer, M.; Zender, L.; Heikenwalder, M. The Immunological and Metabolic Landscape in Primary and Metastatic Liver Cancer. Nat. Rev. Cancer 2021, 21, 541–557. [Google Scholar] [CrossRef]
- Yang, W.S.; Zeng, X.F.; Liu, Z.N.; Zhao, Q.H.; Tan, Y.T.; Gao, J.; Li, H.L.; Xiang, Y.B. Diet and Liver Cancer Risk: A Narrative Review of Epidemiological Evidence. Br. J. Nutr. 2020, 124, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Daher, S.; Massarwa, M.; Benson, A.A.; Khoury, T. Current and Future Treatment of Hepatocellular Carcinoma: An Updated Comprehensive Review. J. Clin. Transl. Hepatol. 2018, 6, 69–78. [Google Scholar] [CrossRef]
- Eshiet, E.R.; Zhu, J.; Anderson, T.A.; Smith, E.E. Chemical Characterization of B Rickellia Cavanillesii (A Steraceae) Using Gas Chromatographic Methods. Food Sci. Nutr. 2014, 2, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Cilia-López, V.G.; Cariño-Cortés, R.; Zurita-Salinas, L.R. Ethnopharmacology of the Asteraceae Family in Mexico. Bot. Sci. 2021, 99, 455–486. [Google Scholar] [CrossRef]
- Viñas, R.; Smith, E.E. Preliminary Evaluation of Prodigiosa Herbal Tea: Cytotoxicity and GLUT2 Expression in HepG2 Cells. Toxicol. Environ. Chem. 2013, 95, 669–678. [Google Scholar] [CrossRef]
- Urias-Lugo, D.A.; Heredia, J.B.; Serna-Saldivar, S.O.; Muy-Rangel, M.D.; Valdez-Torres, J.B. Total Phenolics, Total Anthocyanins and Antioxidant Capacity of Native and Elite Blue Maize Hybrids (Zea Mays L.). CYTA—J. Food 2015, 13, 336–339. [Google Scholar] [CrossRef]
- Urias-Lugo, D.A.; Heredia, J.B.; Muy-Rangel, M.D.; Valdez-Torres, J.B.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Anthocyanins and Phenolic Acids of Hybrid and Native Blue Maize (Zea Mays L.) Extracts and Their Antiproliferative Activity in Mammary (MCF7), Liver (HepG2), Colon (Caco2 and HT29) and Prostate (PC3) Cancer Cells. Plant Foods Hum. Nutr. 2015, 70, 193–199. [Google Scholar] [CrossRef]
- Mena-Rejon, G.; Caamal-Fuentes, E.; Cantillo-Ciau, Z.; Cedillo-Rivera, R.; Flores-Guido, J.; Moo-Puc, R. In Vitro Cytotoxic Activity of Nine Plants Used in Mayan Traditional Medicine. J. Ethnopharmacol. 2009, 121, 462–465. [Google Scholar] [CrossRef]
- Orozco-Barocio, A.; Lizbeth Paniagua-Domínguez, B.; Alberto Benítez-Saldaña, P.; Flores-Torales, E.; Velázquez-Magaña, S.; Julieta, H.; Nava, A. Cytotoxic effect of the ethanolic extract of lophocereus schottii: A mexican medicinal plant. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 33–3777. [Google Scholar] [CrossRef]
- Merlín-Lucas, V.; Ordoñez-Razo, R.M.; Calzada, F.; Solís, A.; García-Hernández, N.; Barbosa, E.; Valdés, M. Antitumor Potential of Annona Muricata Linn. An Edible and Medicinal Plant in Mexico: In Vitro, in Vivo, and Toxicological Studies. Molecules 2021, 26, 7675. [Google Scholar] [CrossRef]
- Carrillo Mónica, G.; Callejas Gina Marcela, M.; Zambrano Crispin Astolfo, C.; Bravo Ricardo, V. Antiproliferative activity of total extracts from annona squamosa, petiveria alliacea and punica granatum on cancer cell lines. Pharmacologyonline 2020, 3, 7–18. [Google Scholar]
- Amran, N.; Rani, A.; Mahmud, R.; Yin, K. Antioxidant and Cytotoxic Effect of Barringtonia Racemosa and Hibiscus Sabdariffa Fruit Extracts in MCF-7 Human Breast Cancer Cell Line. Pharmacogn. Res. 2016, 8, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.T.; Chen, J.H.; Chou, F.P.; Lin, H.H. Hibiscus Sabdariffa Leaf Extract Inhibits Human Prostate Cancer Cell Invasion via Down-Regulation of Akt/NF- ΚB/MMP-9 Pathway. Nutrients 2015, 7, 5065–5087. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Ortiz-Sánchez, E.; Domínguez, F.; Arana-Argáez, V.; Juárez-Vázquez, M.D.C.; Chávez, M.; Carranza-Álvarez, C.; Gaspar-Ramírez, O.; Espinosa-Reyes, G.; López-Toledo, G.; et al. Antitumor and Immunomodulatory Effects of Justicia Spicigera Schltdl (Acanthaceae). J. Ethnopharmacol. 2012, 141, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Elghondakly, M.; Moawad, A.; Hetta, M. Cytotoxicity and Chromatographic Analysis of Dioon Spinulosum, Family Zamiaceae. J. Appl. Pharm. Sci. 2020, 10, 75–82. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, A.; Peixoto, I.T.A.; Verde-Star, M.J.; de La Torre-Zavala, S.; Aviles-Arnaut, H.; Ruiz, A.L.T.G. In Vitro Antimicrobial and Antiproliferative Activity of Amphipterygium Adstringens. Evid.-Based Complement. Altern. Med. 2015, 2015, 175497. [Google Scholar] [CrossRef]
- Elizondo-Luévano, J.H.; Gomez-Flores, R.; Verde-Star, M.J.; Tamez-Guerra, P.; Romo-Sáenz, C.I.; Chávez-Montes, A.; Rodríguez-Garza, N.E.; Quintanilla-Licea, R. In Vitro Cytotoxic Activity of Methanol Extracts of Selected Medicinal Plants Traditionally Used in Mexico against Human Hepatocellular Carcinoma. Plants 2022, 11, 2862. [Google Scholar] [CrossRef]
- Nocedo-Mena, D.; Rivas-Galindo, V.M.; Navarro, P.; Garza-González, E.; González-Maya, L.; Ríos, M.Y.; García, A.; Ávalos-Alanís, F.G.; Rodríguez-Rodríguez, J.; del Rayo Camacho-Corona, M. Antibacterial and Cytotoxic Activities of New Sphingolipids and Other Constituents Isolated from Cissus Incisa Leaves. Heliyon 2020, 6, e04671. [Google Scholar] [CrossRef]
- Sánchez-Aguirre, O.A.; Juárez-Aguilar, E.; Montoya-Hernández, E.L.; Vázquez-Hernández, M.; Colorado-Peralta, R.; Sánchez-Medina, A.; Márquez-López, M.E.; Hernández-Romero, D. Antioxidant Potential of Cnidoscolus Multilobus (Pax) I.M. Johnst and Its Antiproliferative and Cytotoxic Effect on Cervical Cancer Cells. Eur. J. Integr. Med. 2022, 53, 102134. [Google Scholar] [CrossRef]
- Chel-Guerrero, L.D.; Scampicchio, M.; Ferrentino, G.; Rodríguez-Buenfil, I.M.; Fragoso-Serrano, M. In Vitro Assessment of Antiproliferative Activity and Cytotoxicity Modulation of Capsicum Chinense By-Product Extracts. Appl. Sci. 2022, 12, 5818. [Google Scholar] [CrossRef]
- Maldonado-Cubas, J.; San Martin-Martínez, E.; Quiroz-Reyes, C.N.; Casañas-Pimentel, R.G. Cytotoxic Effect of Semialarium Mexicanum (Miers) Mennega Root Bark Extracts and Fractions against Breast Cancer Cells. Physiol. Mol. Biol. Plants 2018, 24, 1185–1201. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Alvarez, J.I.; Encarnación, D.R.; Franzblau, S.G. Antimicrobial and Cytotoxic Activity of Some Medicinal Plants from Baja California Sur (Mexico). Pharm. Biol. 2001, 39, 445–449. [Google Scholar] [CrossRef]
- Casillas, F.R.; Cardenas, A.O.; Rivas Morales, C.; Verde Star, M.J.; Cruz-Vega, D.E. Cytotoxic Activity of Agave Lechuguilla Torr. Afr. J. Biotechnol. 2012, 11, 12229–12231. [Google Scholar] [CrossRef]
- De La Torre Rodriguez, Y.C.; Estrada, F.R.M.; Suarez, A.E.F.; de Torres, N.W.; Aranda, R.S. Larvicidal and Cytotoxic Activities of Extracts from 11 Native Plants from Northeastern Mexico. J. Med. Entomol. 2013, 50, 310–313. [Google Scholar] [CrossRef]
- Escobedo Hinojosa, W.I.; Acevedo Quiróz, M.; Romero Álvarez, I.; Escobar Castañeda, P.; Villarreal, M.L.; Taketa, A.C. Anti-Helicobacter Pylori, Gastroprotective, Anti-Inflammatory, and Cytotoxic Activities of Methanolic Extracts of Five Different Populations of Hippocratea Celastroides Collected in Mexico. J. Ethnopharmacol. 2014, 155, 1156–1163. [Google Scholar] [CrossRef]
- Sianipar, M.P.; Suwarso, E.; Rosidah, R. Antioxidant and Anticancer Activities of Hexane Fraction from Carica Papaya l. Male Flower. Asian J. Pharm. Clin. Res. 2018, 11, 81–83. [Google Scholar] [CrossRef]
- Dalkılıç, S.; Korkmaz, İ.; Dalkılıç, L.K.; Akay, G.; Fidan, S. In Vitro Cytotoxic Effects of Smilax Aspera L. Roots on Cancer Cell Lines. Food Biosci. 2022, 46, 101501. [Google Scholar] [CrossRef]
- Datkhile, K.D.; Patil, S.R.; Patil, M.N.; Durgawale, P.P.; Jagdale, N.J.; Deshmukh, V.N. Studies on Phytoconstituents, in Vitro Antioxidant, Antibacterial, and Cytotoxicity Potential of Argemone Mexicana Linn. (Family: Papaveraceae). J. Nat. Sci. Biol. Med. 2020, 11, 198–205. [Google Scholar] [CrossRef]
- Guajardo-Flores, D.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Evaluation of the Antioxidant and Antiproliferative Activities of Extracted Saponins and Flavonols from Germinated Black Beans (Phaseolus Vulgaris L.). Food Chem. 2013, 141, 1497–1503. [Google Scholar] [CrossRef]
- Fan, A.; Wang, B.; Wang, X.; Nie, Y.; Fan, D.; Zhao, X.; Lu, Y. Immunotherapy in Colorectal Cancer: Current Achievements and Future Perspective. Int. J. Biol. Sci. 2021, 17, 3837–3849. [Google Scholar] [CrossRef]
- Eng, C.; Jácome, A.A.; Agarwal, R.; Hayat, M.H.; Byndloss, M.X.; Holowatyj, A.N.; Bailey, C.; Lieu, C.H. A Comprehensive Framework for Early-Onset Colorectal Cancer Research. Lancet Oncol. 2022, 23, e116–e128. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Meza, A.; Arias-Romero, L.E.; Armas-López, L.; Ávila-Moreno, F.; Chirino, Y.I.; Delgado-Buenrostro, N.L.; García-Castillo, V.; Gutiérrez-Cirlos, E.B.; Juárez-Avelar, I.; Leon-Cabrera, S.; et al. Mexican Colorectal Cancer Research Consortium (MEX-CCRC): Etiology, Diagnosis/Prognosis, and Innovative Therapies. Int. J. Mol. Sci. 2023, 24, 2115. [Google Scholar] [CrossRef] [PubMed]
- Zygulska, A.L.; Pierzchalski, P. Novel Diagnostic Biomarkers in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 852. [Google Scholar] [CrossRef] [PubMed]
- Moran, K.L.; Bjorndal, K.A. Simulated Green Turtle Grazing Affects Nutrient Composition of the Seagrass Thalassia Testudinum. Mar. Biol. 2007, 150, 1083–1092. [Google Scholar] [CrossRef]
- Delgado-Roche, L.; González, K.; Mesta, F.; Couder, B.; Tavarez, Z.; Zavala, R.; Hernandez, I.; Garrido, G.; Rodeiro, I.; Vanden Berghe, W. Polyphenolic Fraction Obtained from Thalassia Testudinum Marine Plant and Thalassiolin B Exert Cytotoxic Effects in Colorectal Cancer Cells and Arrest Tumor Progression in a Xenograft Mouse Model. Front. Pharmacol. 2020, 11, 1939. [Google Scholar] [CrossRef]
- Rady, I.; Bloch, M.B.; Chamcheu, R.C.N.; Banang Mbeumi, S.; Anwar, M.R.; Mohamed, H.; Babatunde, A.S.; Kuiate, J.R.; Noubissi, F.K.; el Sayed, K.A.; et al. Anticancer Properties of Graviola (Annona Muricata): A Comprehensive Mechanistic Review. Oxid. Med. Cell. Longev. 2018, 2018, 1826170. [Google Scholar] [CrossRef]
- Daddiouaissa, D.; Amid, A.; Abdullah Sani, M.S.; Elnour, A.A.M. Evaluation of Metabolomics Behavior of Human Colon Cancer HT29 Cell Lines Treated with Ionic Liquid Graviola Fruit Pulp Extract. J. Ethnopharmacol. 2021, 270, 113813. [Google Scholar] [CrossRef]
- Zorofchian Moghadamtousi, S.; Karimian, H.; Rouhollahi, E.; Paydar, M.; Fadaeinasab, M.; Abdul Kadir, H. Annona Muricata Leaves Induce G1 Cell Cycle Arrest and Apoptosis through Mitochondria-Mediated Pathway in Human HCT-116 and HT-29 Colon Cancer Cells. J. Ethnopharmacol. 2014, 156, 277–289. [Google Scholar] [CrossRef]
- Abbas, E.Y.; Ezzat, M.I.; el Hefnawy, H.M.; Abdel-Sattar, E. An Overview and Update on the Chemical Composition and Potential Health Benefits of Opuntia Ficus-Indica (L.) Miller. J. Food Biochem. 2022, 46, e14310. [Google Scholar] [CrossRef]
- Silva, M.A.; Albuquerque, T.G.; Pereira, P.; Ramalho, R.; Vicente, F.; Oliveira, M.B.P.P.; Costa, H.S. Opuntia Ficus-Indica (L.) Mill.: A Multi-Benefit Potential to Be Exploited. Molecules 2021, 26, 951. [Google Scholar] [CrossRef]
- Antunes-Ricardo, M.; Hernández-Reyes, A.; Uscanga-Palomeque, A.C.; Rodríguez-Padilla, C.; Martínez-Torres, A.C.; Gutiérrez-Uribe, J.A. Isorhamnetin Glycoside Isolated from Opuntia Ficus-Indica (L.) MilI Induces Apoptosis in Human Colon Cancer Cells through Mitochondrial Damage. Chem. Biol. Interact. 2019, 310, 108734. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Ricardo, M.; Moreno-García, B.E.; Gutiérrez-Uribe, J.A.; Aráiz-Hernández, D.; Alvarez, M.M.; Serna-Saldivar, S.O. Induction of Apoptosis in Colon Cancer Cells Treated with Isorhamnetin Glycosides from Opuntia Ficus-Indica Pads. Plant Foods Hum. Nutr. 2014, 69, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Ricardo, M.; Guardado-Félix, D.; Rocha-Pizaña, M.R.; Garza-Martínez, J.; Acevedo-Pacheco, L.; Gutiérrez-Uribe, J.A.; Villela-Castrejón, J.; López-Pacheco, F.; Serna-Saldívar, S.O. Opuntia Ficus-Indica Extract and Isorhamnetin-3-O-Glucosyl-Rhamnoside Diminish Tumor Growth of Colon Cancer Cells Xenografted in Immune-Suppressed Mice through the Activation of Apoptosis Intrinsic Pathway. Plant Foods Hum. Nutr. 2021, 76, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Moga, A.; Dimienescu, O.G.; Balan, A.; Dima, L.; Toma, S.I.; Bîgiu, N.F.; Blidaru, A. Pharmacological and Therapeutic Properties of Punica Granatum Phytochemicals: Possible Roles in Breast Cancer Marius. Molecules 2021, 26, 1054. [Google Scholar] [CrossRef]
- Cortez-Trejo, M.C.; Olivas-Aguirre, F.J.; Dufoo-Hurtado, E.; Castañeda-Moreno, R.; Villegas-Quintero, H.; Medina-Franco, J.L.; Mendoza, S.; Wall-Medrano, A. Potential Anticancer Activity of Pomegranate (Punica Granatum L.) Fruits of Different Color: In Vitro and In Silico Evidence. Biomolecules 2022, 12, 1649. [Google Scholar] [CrossRef]
- Rodrigues, C.A.; Nicácio, A.E.; Boeing, J.S.; Garcia, F.P.; Nakamura, C.V.; Visentainer, J.V.; Maldaner, L. Rapid Extraction Method Followed by a D-SPE Clean-up Step for Determination of Phenolic Composition and Antioxidant and Antiproliferative Activities from Berry Fruits. Food Chem. 2020, 309, 125694. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Macri, R.; et al. Nutraceuticals and Cancer: Potential for Natural Polyphenols. Nutrients 2021, 13, 3834. [Google Scholar] [CrossRef]
- Annaji, M.; Poudel, I.; Boddu, S.H.S.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. Resveratrol-Loaded Nanomedicines for Cancer Applications. Cancer Rep. 2021, 4, e1353. [Google Scholar] [CrossRef]
- Merlin, J.P.J.; Rupasinghe, H.P.V.; Dellaire, G.; Murphy, K. Role of Dietary Antioxidants in P53-Mediated Cancer Chemoprevention and Tumor Suppression. Oxid. Med. Cell. Longev. 2021, 2021, 9924328. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto, K.M.; Pérez Bueno, J.d.J.; Mendoza López, M.L.; Apátiga-Castro, M.; López-Romero, J.M.; Mendoza, S.; Manzano-Ramírez, A. Antioxidants in Traditional Mexican Medicine and Their Applications as Antitumor Treatments. Pharmaceuticals 2023, 16, 482. https://doi.org/10.3390/ph16040482
Soto KM, Pérez Bueno JdJ, Mendoza López ML, Apátiga-Castro M, López-Romero JM, Mendoza S, Manzano-Ramírez A. Antioxidants in Traditional Mexican Medicine and Their Applications as Antitumor Treatments. Pharmaceuticals. 2023; 16(4):482. https://doi.org/10.3390/ph16040482
Chicago/Turabian StyleSoto, Karen M., José de Jesús Pérez Bueno, Maria Luisa Mendoza López, Miguel Apátiga-Castro, José M. López-Romero, Sandra Mendoza, and Alejandro Manzano-Ramírez. 2023. "Antioxidants in Traditional Mexican Medicine and Their Applications as Antitumor Treatments" Pharmaceuticals 16, no. 4: 482. https://doi.org/10.3390/ph16040482
APA StyleSoto, K. M., Pérez Bueno, J. d. J., Mendoza López, M. L., Apátiga-Castro, M., López-Romero, J. M., Mendoza, S., & Manzano-Ramírez, A. (2023). Antioxidants in Traditional Mexican Medicine and Their Applications as Antitumor Treatments. Pharmaceuticals, 16(4), 482. https://doi.org/10.3390/ph16040482






