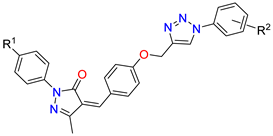
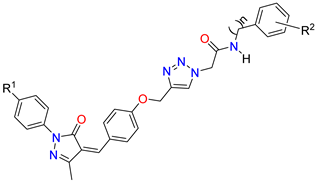
Abstract
COVID-19 infection is now considered one of the leading causes of human death. As an attempt towards the discovery of novel medications for the COVID-19 pandemic, nineteen novel compounds containing 1,2,3-triazole side chains linked to phenylpyrazolone scaffold and terminal lipophilic aryl parts with prominent substituent functionalities were designed and synthesized via a click reaction based on our previous work. The novel compounds were assessed using an in vitro effect on the growth of SARS-CoV-2 virus-infested Vero cells with different compound concentrations: 1 and 10 μM. The data revealed that most of these derivatives showed potent cellular anti-COVID-19 activity and inhibited viral replication by more than 50% with no or weak cytotoxic effect on harboring cells. In addition, in vitro assay employing the SARS-CoV-2-Main protease inhibition assay was done to test the inhibitors’ ability to block the common primary protease of the SARS-CoV-2 virus as a mode of action. The obtained results show that the one non-linker analog 6h and two amide-based linkers 6i and 6q were the most active compounds with IC50 values of 5.08, 3.16, and 7.55 μM, respectively, against the viral protease in comparison to data of the selective antiviral agent GC-376. Molecular modeling studies were done for compound placement within the binding pocket of protease which reveal conserved residues hydrogen bonding and non-hydrogen interactions of 6i analog fragments: triazole scaffold, aryl part, and linker. Moreover, the stability of compounds and their interactions with the target pocket were also studied and analyzed by molecular dynamic simulations. The physicochemical and toxicity profiles were predicted, and the results show that compounds behave as an antiviral activity with low or no cellular or organ toxicity. All research results point to the potential usage of new chemotype potent derivatives as promising leads to be explored in vivo that might open the door to rational drug development of SARS-CoV-2 Main protease potent medicines.
1. Introduction
Single-stranded RNA Coronaviruses have been linked to mild to severe respiratory illnesses [1,2,3]. In 2002, human coronaviruses were recognized as the etiological agents for the global epidemic of the severe acute respiratory syndrome (SARS). At that time, SARS caused the death of more than 800 individuals worldwide. Shortly after, the Middle East Respiratory Syndrome (MERS-CoV) caused an additional wave of lethal respiratory diseases. More recently, the World Health Organization identified COVID-19 as a pandemic disease on 11 March 2020, and it promptly spread worldwide. More than 657,100,000 cases have been verified up to the present moment. This outbreak led to the death of over 6,657,000 individuals, representing a fatality rate of almost more than 1% until the 4 January 2023 [4]. Up till now, no approved effective drugs have been well-recognized to overcome COVID-19, making the discovery of novel powerful antiviral medication an urgent need.
Despite multiple attempts to develop antiviral drugs, especially in response to the rapidly developing pandemic COVID-19, viral diseases remain an increasing global public health concern [5]. Management of patients with COVID-19 still relies on the use of supportive, symptomatic, and repurposed drugs as an indispensable option [6]. Therefore, the exploration of anti-COVID-19 agents is essential to eradicate this pandemic. In the confrontation of this challenge, and over the last few years, numerous studies of ligand and structure-based computational methodologies were applied for the development of a virtual screening approach against libraries of synthetic and natural products that may help in the prediction of potential antiviral agents targeting COVID-19 [7]. Furthermore, several heterocyclic compounds have been developed that mainly target the COVID-19 Main protease [8]. Among these, nitrogen-containing heterocycles including triazoles and pyrazoles have been recognized as promising antivirals against coronaviruses, and their activity was reported to be mediated by targeting the coronavirus’s main protease (Main protease) [9,10,11,12]. This class of nitrogenous heterocycles plays a substantial role in the development of druggable candidates because of its ability to form hydrogen bonds with certain biological targets [13,14]. Moreover, 1,2,3-triazoles resist metabolic degradation relative to other heterocycles.
Two years ago, six 1,2,3-triazole derivatives (Figure 1), have been evaluated in silico against the Main protease of COVID-19. The results show that compound 1 presents a better interaction with the selected biological target than the co-crystallized ligand. Compound 1 with benzofuran and isatin moieties exhibits the highest energy and the best interaction pattern within the active site of the coronavirus main protease. These include four hydrogen-bonding interactions and four electrostatic interactions between the 1,2,3-triazole ring and the essential amino acid residues of the protein target site [9,15]. Moreover, studying the antiviral activity of fused 1,2,3-triazole derivatives 2 and 3 against human coronavirus reveals that the coronavirus’s main protease is the biological target of the antiviral activity of these derivatives [10,16]. One year earlier, the non-nucleoside inhibitory potential of triazole 4 was studied against the hepatitis C virus. Compound 4 demonstrates an EC50 value of 1.163 nM with low cytotoxicity in a subgenomic replicon experiment [17]. On the other hand, certain triazole-dihydropyrimidinone hybrids were synthesized by the application of a copper-catalyzed azide-alkyne cycloaddition reaction, and their antiviral activity was assessed against the human varicella-zoster virus. Without measurable cell growth inhibition, hybrid molecule 5 shows a promising antiviral potential, with an EC50 value of 8.38 μM against the varicella-zosterella zoster virus. Replacement of the triazole-attached phenyl rings in 5 with 4-NO2-phenyl (compound 6) enhances the antiviral potency (EC50 3.62 μM) and reduces the cytotoxicity [18].
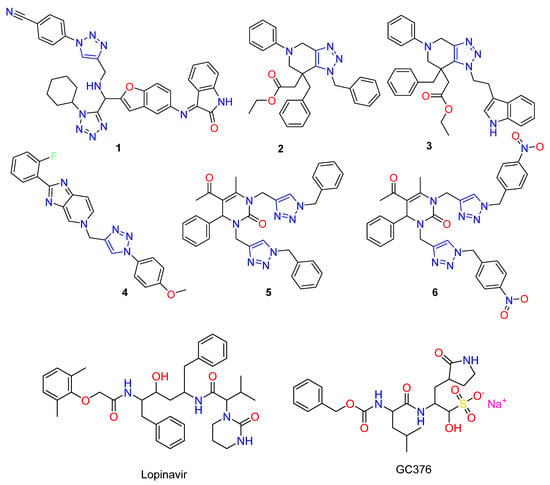
Figure 1.
1,2,3-Triazoles as a pharmacophoric fragment presented in potent antiviral molecules.
On the other hand, pyrazolone is a pharmacophoric nitrogenous ring presented in several molecules with verified antiviral activity, particularly against coronaviruses [19,20,21,22,23,24,25]. It is interesting to note that pyrazolone derivatives 7 and 8 (Figure 2) displayed powerful inhibitory effects against the SARS-CoV Main protease with low micromolar IC50 values of 5.50 and 6.80 μM, respectively [20]. Moreover, pyrazolone analogs and 10 have been evaluated as inhibitors of the Main protease of SARS-CoV and MERS-CoV. Both compounds exert excellent inhibitory effects against SARS and MERS with IC50 values of 6.40 and 5.80 μM (9), 5.80 and 7.40 μM (10), respectively [22]. In our most recent investigations [23,26] a phenylpyrazolone scaffold exhibited good antiviral activity against SARS-CoV infection via a non-covalent inhibition of the main protease, the target protease. That investigation reported the formation of more rigid analogs through benzochromene formation with a terminal aromatic tail and two NH2 and CN functionalities (compound 11). The behavior of these derivatives as antiviral agents was due to the good placement of compounds within the target pocket but showed some steric clashes. This inspired us to explore a chemistry change by combining a phenylpyrazolone scaffold with more flexible moieties that could compensate for the drawbacks in the previous study (Figure 2).
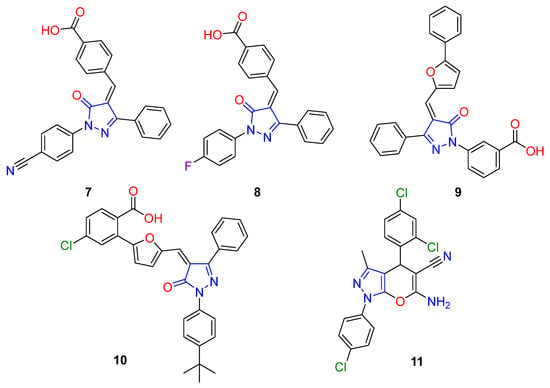
Figure 2.
Pyrazolone-incorporating antiviral agents.
Aim of the Work
Compounds incorporating 1,2,3-triazole, pyrazole, acetamide, and α, β-unsaturated carbonyl fragments (Figure 3) show effective covalent binding potential with key amino acid residues in the protein binding site [27,28,29]. These key amino acid residues include His41, Gly143, Cys145, Glu166, His163, His164, Gln189, and Thr190.
One strategy for the design of a suggested drug candidate is to combine two pharmacophoric fragments of more than one well-known bioactive molecule belonging to a specific therapeutic class in one new scaffold [30,31,32,33]. For example, 1,2,3-triazole-based heterocycles were perfectly applied to develop numerous scaffolds with potent antiviral activity [16,17,18]. On the other hand, the penylpyrazolone scaffold represents a class of nitrogenous compounds with interesting antiviral activity [19,20,21,22]. Hybrids that contain or are linked by a 1,2,3-triazole ring in their structures are considered motivating molecular hybrids that offer a wide array of pharmacophore characteristics. Herein, given the aforementioned features and in continuation of our previous research in the design of suggested bioactive heterocycles [34,35,36,37], we have attempted to synthesize novel 1,2,3-triazoles linked to pyrazole moiety for further investigation as lead compounds in medicinal chemistry (Figure 3). Also, we report the in vitro antiviral evaluation of novel 1,2,3-triazole-pyrazolone hybrid molecules on COVID-19 [38]. It was reported that the complex of COVID-19 Main protease with the co-crystallized ligand, N3, consists of three chains, A, B, and C. Among these, A and C are the only two chains involved in the interaction. These interactions involve hydrogen bindings with the key amino acid residues Thr190, Glu166, Gln189, His164, His163, His41, and Gly143 [28,39]. In our work, the placement of pharmacophoric fragments that contribute to the stability of the scaffold similarly to the bound ligand, including phenoxy and 1,2,3-triazole, was identified within the binding pocket of the main protease target through interactions with the key residues Gln189 and Met49 by hydrogen bonds. In addition, the catalytic Cys145 contributes to the stabilization of the novel structures through stable hydrogen bonding with the NH part of the amide linker, which may help with the activity within the S1 subsite. As appeared from the structure of designed compounds, there is tolerance between all of the components of the target compounds (phenylpyrazolone, arylidene, active 1,2,3-triazole, amide linker, and terminal aromatic part) within the active site and the reflected inhibitory activity against both viral propagation and the Main protease target.
2. Results and Discussion
2.1. Chemistry
As a continuation of our interest in the click chemistry and biological properties of 1,2,3-triazoles [40,41,42,43,44,45], we have attempted to design and synthesize novel 1,2,3-triazoles linked to pyrazolone moiety for further investigation as lead compounds in medicinal chemistry. Thus, the general synthetic protocols adopted for the synthesis of the targeted 1,2,3-triazole-pyrazolone hybrids are represented in Scheme 1, Scheme 2 and Scheme 3. Hybrid molecules that contain or are linked by a 1,2,3-triazole building block are usually referred to as “lead compounds”; these molecular hybrids offer a wide array of pharmacophore characteristics. Given the aforementioned features and researchers’ recent interest in the chemistry of 1,2,3-triazole molecules [13,46,47,48], novel 1,2,3-triazoles linked to pyrazole moiety for further investigation were designed and synthesized as lead compounds in medicinal chemistry as a continuation of our interest on these tunable scaffolds and their biological properties [49,50,51,52,53].
Thus, the protocols of the synthesis of the targeted hybrids are illustrated in Scheme 1, Scheme 2 and Scheme 3. The precursor alkyne derivatives 4a–b used in this study were obtained through the condensation of the commercially available pyrazole-3-one derivatives 3a–b with the synthesized propargylated benzaldehyde 2 using piperidine as a basic reagent (Scheme 1). The propargylated benzaldehyde de 2 was in turn obtained through base-catalyzed (potassium carbonate) alkylation of 4-hydroxybenzaldehyde (1) with propargyl bromide according to the literature [54].
Based on the spectroscopic results, the structures of the pyrazolone-based alkyne side chains 4a–b were revealed. Their IR spectra clearly show the absence of the absorption bands belonging to the C=O and C–H of the aldehyde group, and the presence of characteristic bands at 2140–2150 and 3280–3290 cm−1 confirming the presence of the C≡C and ≡C–H groups in their structures. In addition, no signal was recorded in their 1H NMR for the aldehydic proton which confirms the condensation step. The spectra also revealed distinct singlets at δH 8.97–10.82 ppm and 3.66–3.69 ppm, attributed to new sp2-CH (C=C–H) linker and Sp(≡C–H) protons, respectively. Moreover, the investigation of 13C NMR spectra supported the resulting structures through the recording of signals around δC 79.33–79.39 ppm attributed to the acetylenic carbons (C≡C) and absence of the carbonyl aldehyde carbons. In the Supplementary File, all additional protons and carbons are recorded and discussed.
The aromatic azides 5a–g involved in this study were prepared by diazotization of different substituted aromatic anilines in situ catalyzed by a mixture of sodium nitrite in HCl and afterward treated with sodium azide according to the reported procedure [55]. On the other hand, the functionalized aniline/benzylacetamide azides 5h–o have been successfully prepared through the reaction of an appropriate amine reagent (substituted anilines and benzylamine) with bromoacetyl bromide in basic triethylamine medium using DCM as solvent followed by the addition of sodium azide in a mixture of acetone: water (4:1, v/v) as reported in the literature [56].
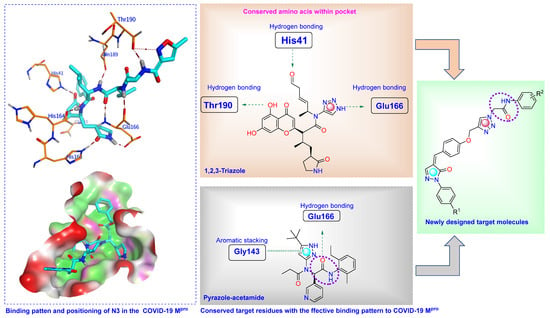
Figure 3.
Strategy design of target molecules.
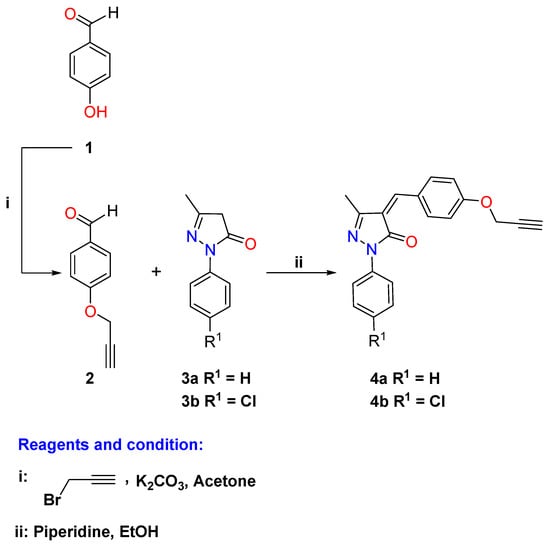
Scheme 1.
Synthesis of the propargylated pyrazolone derivatives 4a–b.
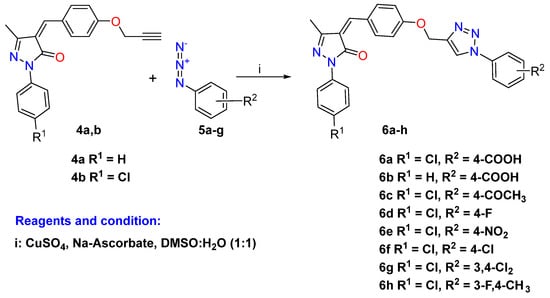
Scheme 2.
Click synthesis of 1,2,3-triazole hybrids carrying aromatic units 6a–h.
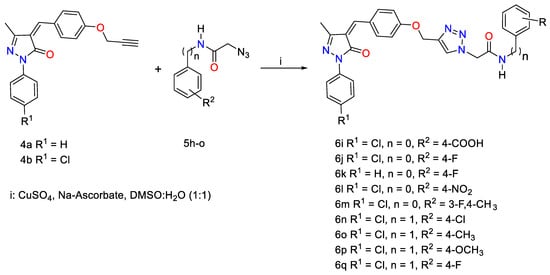
Scheme 3.
Click synthesis of pyrrazole-1,2,3-triazole hybrids carrying phenyl acetamide side chain 6j–q.
Click chemistry introduced green synthetic protocols with higher yields for the synthesis of 1,2,3-triazole through the discovery processes in the medicinal chemistry discipline via azide and alkyne cycloaddition reactions. Thus, the CuAAC 1,3-dipolar cycloaddition reaction of the alkyne-pyrazole derivatives 4a–b with focused aromatic azides 5a–g was carried out in the presence of CuSO4 and Na2CO3 in a solvent mixture of DMSO/H2O at room temperature and afforded the desired pyrazolone-1,2,3-triazole hybrids carrying substituted aromatic units 6a–h in moderate to good yield (86–92%), as described in Scheme 2.
Similarly, novel pyrazolone-1,2,3-triazole hybrids 6j–q carrying phenyl acetamide side chains were designed as a second set and synthesized through the click ligation of the pyrazolone-alkyne building blocks 4a–b with some functionalized phenyl/benzyl acetamide azides 5h–o under optimized Cu(I)-assisted 1,3-dipolar cycloaddition protocol (Scheme 3).
The success of the click reactions was supported by the investigation of the spectroscopic data of the results in the 1,2,3-triazole-pyrazolone hybrids 6a–h and 6i–q, including IR, 1H NMR, 13C NMR, and elemental analyses. As a result, no signals were observed in the alkyne area in their IR spectra, providing strong proof for the cycloaddition reaction’s success. Additionally, their 1H NMR spectra disclosed the absence of the acetylenic protons (≡C–H) and the presence of diagnostic singlet around δH 8.27 to 9.15 ppm attributed to the CH-1,2,3-triazolyl protons, validating the cycloaddition reactions.
The spectra also revealed distinguishable singlets and/or triplets ranging between δH 4.29 to 5.46 ppm related to the protons of one, two, and/or three methylene (–CH2–) bridges constructing the adducts 6j–q. The presence of the acetamide group in the structure of this set of click products 6j–q was also supported by the resonance of characteristic NH-protons recorded at δH 8.79 to 10.82 ppm. Moreover, the click reactions were also validated by the investigation of their 13C NMR spectra. Thus, the absence of signals attributed to the alkyne chain (C≡C) and the presence of extra signals belonging to aromatic carbons of the phenyl rings are good pieces of evidence for the success of the cycloaddition reaction. In addition, the spectra of the click adduct 6i–q revealed new signals at δC 160.4 to 165.9 ppm assigned to the acetamide carbonyl carbons (CONH), supporting the proposed structures. All remaining signals are listed and detailed in the experimental section.
2.2. Pharmacological Testing
2.2.1. Inhibitory Assay of SARS-CoV-2 Main Protease
Because of its critical involvement in the polypeptide of virus catalysis, Main protease (or 3CLpro) is considered a feasible therapeutic target in SARS-CoV-2 [57,58]. Several Main protease inhibitors, including GC-376, PX-12, and carmofur, were identified as covalent inhibitors that alter Cys145’s catalytic function with powerful enzymatic inhibitory effect and cellular antiviral activity, with reactive nitrogen heterocyclic as pyrrolidone, imidazole, or pyrimidine that mimics the glutamine P1 position [59,60]. Furthermore, many non-covalent inhibitors such as ML188 were discovered as SARS-CoV-2 Main protease inhibitors [61]. Moreover, several studies have been conducted on the ML188 series to optimize the enzymatic potency against the SARS-CoV-2 main protease until recently finding the novel non-covalent Main protease inhibitor (23R) with potent cellular antiviral activity [61]. Pyrazolone-triazole hybrids were investigated in vitro for suppression of SARS-CoV-2 Main protease activity and compared to the well-known antiviral GC-376 as a selective Main protease inhibitor. The structure of analogs is distinct according to the terminal part attached to the triazole ring; one ends with lipophilic aryl substituted groups (6a–h), and the other ends with an amide linker and terminal-substituted aryl functionality (6i–q). Accordingly, the results exhibit a better activity profile for the second class with amide linker than the other. As shown in Table 1, it appears that the chlorophenylpyrazolone analogs show a very promising potency, which is better than the unsubstituted phenylpyrazolone. These findings confirm the antiviral activity of such a Cl-phenylpyrazolone scaffold better than plain phenylpyrazolone itself as reported in our previous study [23]. As seen in Table 1, three main classes appear with distinct performances against the studied viral protein. The first one includes the potent analogs 6i, 6h, and 6q that show better inhibition activity on the target enzyme than the reference drug GC-376. The second class, comprising 6d, 6e, 6j, 6m, 6o, and 6p, has relatively lower activity than that of the main protease selective inhibitor with IC50 values range of 25.70–49.39 μM. The third class comprises the remaining phenylpyrazolone-1,2,3-triazole hybrids with much weaker activity compared with the control drug. Among all the new phenylpyrazolone-1,2,3-triazole hybrids, the 4-chlorophenylpyrazolone derivative 6h is the most potent one with ether linkage. Likewise, the 4-chlorophenylpyrazolone derivative with carboxylic acid functionality 6i is the most potent member among all the new amide-linked triazoles. Fluorine substituents on triazole-linked phenyl exert better impact on the activity compared with chlorine and nitro substituents. This can be clearly recognized upon comparing the superior activity of analogs 6h, 6q, and 6m with IC50 values of 5.08, 7.55, and 25.7 μM, respectively, with those of 6a–6d (IC50 values ≥ 76.91 μM). The relatively high micromolar-inhibitory concentration of CG-376 obtained in the current study (12.85 μM) relative to those reported in earlier works [62,63] might be attributed to lab-based handling, sensitivity, and the type of kit used.

Table 1.
SAR effect against SARS-CoV-2 Main protease for the novel compounds.
2.2.2. SAR Analysis of Target Compounds
According to the antiviral results, the target compounds possess powerful antiviral efficiency against SARS-CoV-2 Main protease. The priority structure-activity relationship results were extracted based on the anti-SARS-CoV-2 Main protease activity as indicated in (Table 1). On one hand, the attachment of a para-chlorophenyl fragment to the pyrazolone ring significantly increases main protease the activity. This is obvious in the comparison between the SARS-CoV-2 Main protease inhibitory activity under the effect of 6b and 6k (with unsubstituted aryl) with those of all other derivatives. On the other hand, the attachment of a di-substituted aryl fragment into the triazole ring markedly enhances the activity. For instance, the di-substituted derivative 6m shows a better SARS-CoV-2 main protease inhibitory effect. Moreover, the di-substituted derivative 6h demonstrates better anti-SARS-CoV-2 main protease activity than the control inhibitor. Lastly, the insertion of an acetamide linker has revealed a positive impact on the overall SARS-CoV-2 Main protease inhibitory activity. Accordingly, three N-aryl 1,2,3-triazoles (6a, 6c, and 6n) show comparable inhibitory activity. They inhibit SARS-CoV-2 Main protease with IC50 values of 76.91, 81.14, and 86.61 μM compared with 82.17 lopinavir. Even more, six derivatives (6d, 6e, 6j, 6m, 6o, and 6p) exhibit better SARS-CoV-2 main protease inhibitory activity. They present IC50 values of 42.45, 30.08, 49.39, 25.7, 43.31, and 23.73 μM, respectively. More interestingly, three 1,2,3-triazole-pyrazolone hybrid molecules (6h, 6i, and 6q) inhibit the SARS-CoV-2 Main protease activity more strongly than the specific antiviral agent GC-376 with IC50 values of 5.08, 3.169, and 7.55 μM compared with 12.85 μM. Graphical representations of the SAR analysis are given in Figure 4 and Figure 5.
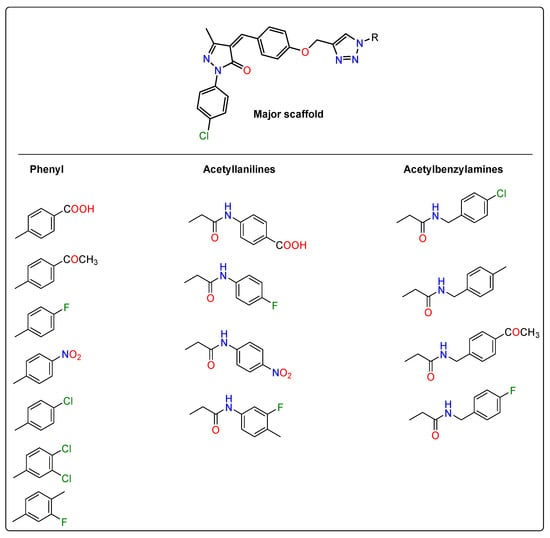
Figure 4.
Structure-activity relationship screen of the anti-SARS-CoV-2 Main protease of target compounds showing the most proper fragments within different scaffolds.
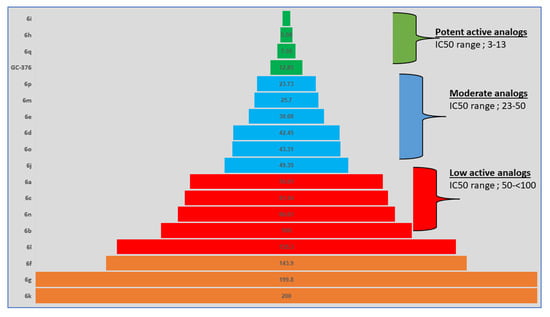
Figure 5.
Pyramid chart showing the distribution of SAR data of compounds. Color-code scheme of green (more actives); blue (moderate actives); red (low actives); yellow (inactives).
2.3. Antiviral Effect against SARS-CoV-2 Activity
Investigation of the newly synthesized compounds for their antiviral capacity to reduce SARS-CoV-2 viral duplication, a cellular antiviral assay for hybrid compounds against SARS-CoV-2 at two doses (1 and 10 μM) on infected Vero E6 cells was done utilizing traditional cell culture with a real-time PCR. The MTT test was used to assess cell viability following analog or remdesivir treatment in Vero E6 cells (CCL-8; ATCC origin donated by the Virology Sector, VACSERA, Giza, Egypt; https://www.vacsera.com (accessed on 21 April 2022)) infected with SARS-CoV-2 at a multiplicity of infection (MOI) of 0.01. At a dose of 60 M, remdesivir was employed as a positive control; it demonstrated no cytotoxicity to Vero E6 cells. Except for five compounds (6a, 6i, 6n, and 6p), all were well-tolerated and have IC50 values of greater than 30 M for the Vero E6 cell lines examined. However, 4-COOH phenyl analog (6i) inhibits Main protease activity well; it is less lethal to Vero E6 cells, with an IC50 value of 22.1 μM and promising inhibition for viral growth at 10 μM by 64% compared to the reference drug. The most potent analogs 4-F and 4-COOH; 6i and 6q show unapparent cytotoxicity with IC50 values above 20 μM for Vero E6 cell lines and better viral growth inhibition by 79 and 64%. They also have the same enzymatic inhibition and cellular antiviral efficacy as the most effective SARS-CoV-2 Main protease inhibitors. Remdesivir and GC-376 were applied as positive controls, and the findings reveal that they effectively suppress viral replication at concentrations of 10 and 1 μM, respectively. Interestingly, 6q, 6h, and 6i were all effective inhibitors of Main protease recombinant SARS-CoV-2 Main protease (IC50: 3.16, 5.08, and 7.55 μM, respectively) and outperform the others in the live viral replication experiment, reaching 79%, 64.2%, and 63 at 10 μM, respectively. In general, none of the pyranopyrazole compounds inhibit viral replication to 50% at the lowest dose (1 μM) (Table 2, Figure 6). Furthermore, several pyranopyrazole compounds do not perform well in the cellular antiviral assay, and their relative ability to suppress viral replication does not always correspond directly with in vitro inhibitory characteristics against main protease [64]. Compound 6a inhibits cellular antiviral activity the least (19.27% at 10 μM), which is consistent with its low Main protease enzymatic inhibition. (IC50 values of 76 μM). Compounds 6p and 6m prove their moderate target Main protease inhibitory effect rather than their viral replication effect with ≈50% at 10 μM. Generally, low matching of SAR data of antiviral effects of target compounds with the above main protease inhibitory data was observed, which might be attributed to the different modes of action of some derivatives, which needs some more extensive biological assays.

Table 2.
Antiviral activity of novel non-covalent SARS-CoV-2 Main protease inhibitors by propagation in Vero E6 cells.
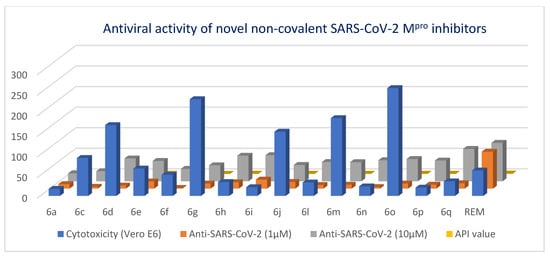
Figure 6.
Pyramid-based chart showing the distribution of antiviral activity of target relative to the reference drug.
2.4. Computational Studies
2.4.1. Molecular Docking Analysis
The active site is situated between the I (amino acids 10–99) and II (amino acids 100–182) barrel domains. Protein dimerization and the construction of a bundle of alpha helices are carried out by the other third domain, which is made up of residues 198 to 306 [55]. The catalytic dyad and dimerization, which complete the active site by bringing Ser161 of the second dimer protomer into proximity with Glu166 and facilitating the development of the substrate specificity pocket and the oxyanion hole, are formed by two major conserved residues, His41 and Cys145 [56]. Two fragments, phenoxy and triazole, were identified which contribute to the stability of the scaffold within the binding pocket through interactions with the key residues Gln189 and Met49 by hydrogen bonds. In addition, the catalytic Cys145 contributes to the stabilization of the compound through stable hydrogen bonding with the NH part of the amide linker which might help in the activity within S1′ subsite. According to reports, the S2 subsite exhibits higher flexibility than the other subsites, accepting smaller substituents in peptide-based inhibitors but favoring leucine or other hydrophobic residues [64]. A common pattern of an aromatic ring formation, stacking interactions with Met49, which drives the terminal phenylpyrazolone component to near to S1’ site, causes many fragments bonded at this point to be referred to as the “aromatic wheel”. The compound fragment phenoxy had an expansion to the residue Gln189 in S3 subsite through aromatic stacking interaction. The interaction analysis of the most prominent active compound 6i revealed non-covalent interactions and the contribution of most chemical moieties in the activity tolerate the pocket with all catalytic sites, as shown in Figure 7.
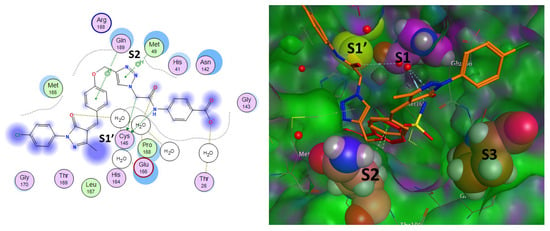
Figure 7.
Docked poses of main protease (PDB ID: 5R80) complexed with Z18197050 and 6i compound with 2D interaction map for 6i (right) and 3D interaction map of both aligned compounds (left). The catalytic sites are presented in bold black as S1, S1′, S2, and S3 subsites. Fill-space models of active site residues were presented. In 2D part, the interactions were presented in dotted-colored lines.
2.4.2. Molecular Dynamic Analysis
Herein, we looked at the protein’s secondary structure of main protease coupled to the 6i new active analog before and after docking to understand the conformational changes and their stability. The main protease secondary structure has 49,540 total atoms, a zero-net charge, 51.040 mM of Na+ ions, 53.956 mM of Cl ions, and 12,468 water molecules when it was put through an MD simulation test.
100 ns MD runs were used to estimate the RMSD and RMSF for the complicated reference and 6i analog forms. The ligand’s binding can prevent the protein from unfolding and stabilize it [65]. To better comprehend the conformational changes brought on by ligand contact, the RMSD record of the main protease response before and after docking of the 6i triazole derivative, which found equilibrium within 20 ns, is shown in Figure 8 (up for the reference drug, down for 6i). In the MD simulation, the target protein was confirmed to be stable at RMSD 20, which is a respectable value for protein structures that demonstrate the complex stability.
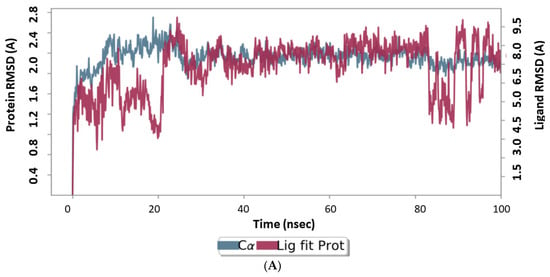
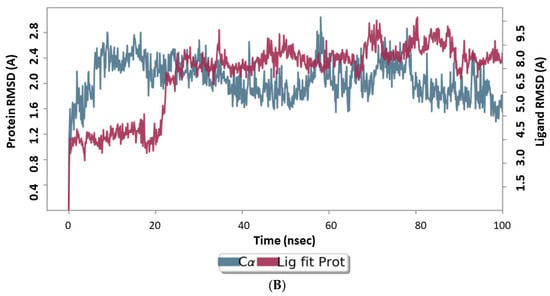
Figure 8.
Results of MD of Main protease target (PDB ID: 5R80). (A) RMSD of the complex main protease (PDB ID: 5R80) complexed with Z18197050; (B) RMSD of the complex Main protease (PDB ID: 5R80) complexed with 6i. RMSD values are calculated from run of the average change in displacement of a selection of atoms for a particular frame with respect to a reference frame trajectory.
The N- and C-terminal residues oscillates above 2.0 for the reference drug and below 2.0 for the 6i derivative, according to the RMSF plot for main protease using the reference and 6i compounds. The predicted RMSF of the protein-ligand complex (Figure 8A) is less than 2.0, indicating conformational stability during the simulation. The other secondary structures remain consistent throughout the trajectory. In contrast to the complex with 6i, which has 25.96% helices and 9.77% strands, the Main protease complex with reference (Figure 8B) includes 25.27% helices and 8.81% strands. The little unfolding of the α-helices throughout the complex’s MD is shown by the minor rise in the percentage of β-strands.
The intermolecular interactions of the Main protease complexes were examined during the MD simulation, and the results are shown in Figure 9A–C. This analysis confirms the molecular docking findings by demonstrating that the studied trajectories contain many potential contacts of both polar and non-polar interactions. Furthermore, Cys106 os the conserved residue among the multiple interactions presented, as seen by the plot of interaction percentages vs. binding site residues (Figure 9B,C) (interaction fraction greater than 1.0). The plot, shown in Figure 10, demonstrates that the binding site residues of the Main protease complex, Cys106, Asp167, Asp109, Lys48, and Glu107, were involved in hydrogen bonding at various simulation times, supporting the conclusion that the docking results are compatible with the MD results. The 6i compound’s hydrophobic interactions with the main protease complex are mediated by the triazole, linker, and terminal pyrazolone fragments.
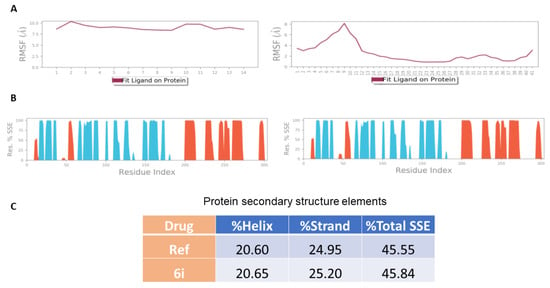
Figure 9.
MD of Main protease (PDB ID: 5R80) data. (A) Secondary structure elements (SSE) of the complex Main protease (PDB ID: 5R80) with Z18197050 and 6i. No significance indicates that conformational stability appears from the absence of any change in the SSE percentage between both the apo and bound proteins. Ligand RMSF shows the ligand’s fluctuations broken down by atom, corresponding to the 2D structure in the top panel. The plot (B) reports SSE distribution by residue index throughout the protein structure. In plot (C), it shows the distribution of all elements of secondary structures appeared from MD runs of reference and 6i compounds.
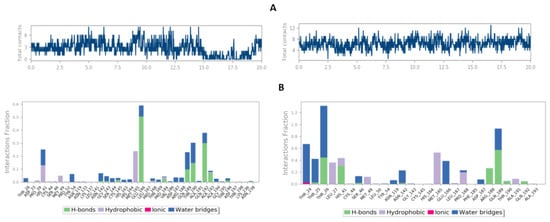
Figure 10.
Results of the timeline of total residues contacts of main protease (PDB ID: 5R80) complexed with two reference (right (A)) and 6i compounds (left (A)). Bar chart of normalized molecular interactions throughout the run. The % of simulation time for the specific interaction is shown in Y-axis that maintained of the complex Main protease (PDB ID: 5R80) complexed with Z18197050 (right (B)) and 6i (left (B)) compounds. The top panel (A) shows the total number of specific contacts the protein makes with the ligand over the course of the trajectory. The (B) plot shows the interaction types with the corresponding residues between the reference ligand and 6i compound.
2.4.3. Prediction of Drug-Likeness and ADME Properties
The adverse effects risk, such as reproductive consequences, irritation, tumorigenicity, and mutagenicity alongside the drug-relevant characteristics, such as cLogP, LogS (solubility), MW, drug-likeness, and overall drug-score, were estimated using OSIRIS Property Explorer. The two potent and low active analogs 6i and 6f were compared to the published drug in in silico physicochemical and toxicological assessments using the OSIRIS Property Explorer tool. cLogP, LogS (solubility), MW, drug-likeness, and total drug-score parameters are scored and color-coded, as shown in Table 3. Properties with a high risk of unfavorable consequences, such as mutagenicity, tumorigenicity, and impacts on reproductive physiology), are also rated and color-coded. Red indicates a high risk of toxicity, and green indicates no risk, hence the data on toxicity risks reflect behavior that is consistent with the substance. It is interesting to note that the potential drug-likeness values of compound 6i are much greater than those of compound 6f and GC-376, which has a negative value of 28.05. Nevertheless, the in silico analysis performed by OSIRIS Property Explorer revealed that adding 4-COOH to the aryl ring can maintain a low risk of tumorigenic and mutagenic toxicity while enhancing. Generally, the values of drug-score of the representative compound 6i (0.24) are less than GC-376 (0.37).

Table 3.
Toxicity risks and physicochemical properties of compounds 6f and 6i in comparison with reported drugs, predicted by OSIRIS Property Explorer.
3. Concluding Remarks
A series of phenylpyrazolone with 1,2,3-triazole and lipophilic aromatic terminals were designed and tested for dual antiviral and Main protease inhibitory action in this work. The majority of the synthesized compounds are equipotent or have greater activity than the reference inhibitors. Most of the compounds exhibit both viral growth inhibition and higher anti-Main protease activity compared with positive control drugs. In addition, amide linker-based compounds reveal more antiviral effects than non-amide ones. Compound 6i has the most potent antiviral effect (64% inhibition) and Main protease inhibitor with IC50 values of 3.1 μM. Based on our results, compound 6i is indicated to have considerable potential as a new pyrazolone-1,2,3-triazole hybrid as the lead compound for discovery of novel antiviral drugs for management of COVID-19 infection. The 6i acid derivative that demonstrates the greatest drug-likeness and drug-score values are considered as a lead for the creation of antiviral medicines in future.
4. Materials and Methods
4.1. Chemistry
None of the reagents or solvents used were further refined and were the best possible quality of analytical reagents. The melting points are uncorrected and were established using Stuart Scientific SMP1, Bibby Scientific, Stone, Staffordshire UK. Spots were seen using a UV light during TLC on UV fluorescent Silica gel Merck 60 F254 plates (254 nm, Sigma-Aldrich Chemie, Taufkirchen, Germany). With a 400–4000 cm−1 range, the SHIMADZU FTIR-Affinity-1S spectrometer (PerkinElmer, Seer Green, Beaconsfield, UK) was employed for identification of the major functional groups. Tetramethyl Silane (TMS) was used as an internal reference in a Bruker spectrometer (400 MHz, PerkinElmer, Seer Green, Beaconsfield, UK) to capture the NMR spectra. A GmbH-Vario EL III Elementar Analyzer (ELTRA GmbH, Haan, Germany) was applied for elemental analyses.
4.1.1. Synthesis and Characterization of 4-(prop-2-yn-1-yloxy)-Benzaldehyde (2)
A mixture of 4-hydroxybenzaldehyde (1) (10 mmol), potassium carbonate (12 mmol) and propargyl bromide (12 mmol) in acetone (25 mL) was heated under reflux for 2 h till the consumption of the start. TLC was used for monitoring (hexane-ethyl acetate). Cooling of the mixture was done by crushed ice water. The resultant precipitate was filtered, water-washed, dried, and crystallized from ethanol to get the desired O-propargylated benzaldehyde 2 as yellowish crystals in 94% yield, mp: 80–81 °C. IR (υ, cm−1): 1550 (C=C), 1710 (C=O), 2150 (C≡C), 2850 (CH-Al), 3080 (CH-Ar), 3240 (≡CH). 1H NMR (400 MHz, CDCl3) δH = 2.59 (t, 1H, J = 4.0 Hz, ≡CH), 4.79 (d, 2H, J = 4.0 Hz, OCH2), 7.08 (d, 2H, J = 8.0 Hz, Ar-H), 7.87 (d, 2H, J = 8.0 Hz, Ar-H), 9.90 (s, 1H, CHO). 13C NMR (100 MHz, CDCl3): δC = 55.94 (OCH2); 76.44 (C≡CH); 77.54 (C≡CH); 115.16, 130.53, 131.94, 162.36 (Ar-C); 190.88 (C=O). Calculated for C10H8O2: C, 74.99; H, 5.03. Found: C, 75.23; H, 5.25.
(Z)-1-(4-Chlorophenyl)-3-Methyl-4-(4-(prop-2-yn-1-yloxy)Benzylidene)-1H-Pyrazol-5(4H)-One (4a)
It was obtained as orange pellets in 88% yield, mp: 134–135 °C. IR (υ, cm−1): 1570 (C=C), 1700 (C=O), 2150 (C≡C), 2930 (CH-Al), 3080 (CH-Ar), 3290 (≡CH). 1H NMR (400 MHz, DMSO-d6) δH = 2.31 (s, 3H, CH3), 3.69 (s, 1H, ≡CH), 4.96 (s, 2H, OCH2), 7.17 (d, 2H, J = 8.0 Hz, Ar-H), 7.48 (d, 2H, J = 12.0 Hz, Ar-H), 7.75 (s, 1H, H–C=C), 7.96 (d, 2H, J = 12.0 Hz, Ar-H), 8.68 (d, 2H, J = 8.0 Hz, Ar-H). 13C NMR (100 MHz, DMSO-d6): δC = 13.60 (CH3), 56.34 (OCH2); 79.37 (C≡CH); 79.43 (C≡CH); 115.54, 120.07, 124.31, 127.09, 128.61, 129.21, 137.13, 137.59, 148.79, 152.72, 161.94, 162.25 (Ar-C, C=O).
Calculated for C20H15ClN2O2: C, 68.48; H, 4.31; N, 7.99. Found: C, 68.65; H, 4.55; N, 8.09.
(Z)-3-methyl-1-Phenyl-4-(4-(prop-2-yn-1-yloxy)Benzylidene)-1H-Pyrazol-5(4H)-One (4b)
It was obtained as orange pellets in 90% yield, mp: 158–160 °C. IR (υ, cm−1): 1580 (C=C), 1690 (C=O), 2140 (C≡C), 2880 (CH-Al), 3060 (CH-Ar), 3280 (≡CH). 1H NMR (400 MHz, DMSO-d6) δH = 2.33 (s, 3H, CH3), 3.66 (s, 1H, ≡CH), 4.94 (s, 2H, OCH2), 7.15 (d, 2H, J = 8.0 Hz, Ar-H), 7.38–7.45 (m, 3H, Ar-H), 7.77 (s, 1H, H–C=C), 7.94 (d, 2H, J = 12.0 Hz, Ar-H), 8.67 (d, 2H, J = 8.0 Hz, Ar-H). 13C NMR (100 MHz, DMSO-d6): δC = 13.67 (CH3), 56.38 (OCH2); 79.33 (C≡CH); 79.41 (C≡CH); 114.79, 115.50, 119.82, 124.28, 127.02, 128.66, 129.24, 137.04, 137.48, 148.67, 152.66, 161.87, 162.18 (Ar-C, C=O). Calculated for C20H16N2O2: C, 75.93; H, 5.10; N, 8.86. Found: C, 75.78; H, 5.31; N, 8.99.
4.1.2. General Click Procedure for the Synthesis of 1,2,3-Triazole-Pyrazolehybrids 6a–q
A solution of propargylated pyrazoles 4a–b (1 mmol) in DMSO was added with stirring to copper sulphate solution (0.10 g) and sodium ascorbate (0.15 g) in water (10 mL) (10 mL). The suitable azide 5a–o (1 mmol) was then added to the reaction mixture, which was agitated at room temperature for 24–40 h. The reaction was monitored by TLC (hexane-ethyl acetate). When the reaction was finished, the liquid was put into chilled water. The resulting precipitate was collected through filtering, washed with ammonium chloride solution, and recrystallized from ethanol/DMF to get the anticipated 1,2,3-triazoles 6a–q. All molecular characterizations are described in the Supplementary File.
4.2. Main Protease Inhibition Assay
The COV2-SARS-CoV-2 protease enzyme assay [66,67,68] was described in the manufacturing protocol (BPS Bioscience). The methodology is described in the Supplementary File.
4.3. SARS-CoV-2 Antiviral Assay
The effect of target chemical treatments on SARS-CoV-2 viral load (SARS-CoV-2 isolate EGY/WAT-2 VACCERA) was assessed by the Real-Time PCR test to detect SARS-CoV-2 viral RNA [69,70]. Total RNA was extracted according to the instructions using the genesig® Coronavirus SARS-CoV-2 Real-Time PCR Assay kit (Primer designTM Ltd., Southampton, UK). All procedures are depicted in the Supplementary File.
4.4. Molecular Docking Study
The simulation was conducted by utilizing the PDB crystal structure of SARS-CoV-19 Main protease for the target molecule 6i in comparison to the reference bound ligand. (PDB: 5R80; https://www.rcsb.org/structure/5R80 (accessed on 21 April 2022)). Autodock 4.0.1.34 tool its graphical interface was utilized for the simulation [71] and (MOE 2014, molecular operating environment software) [72]. The docking protocol was detailed previously [73,74,75]. The pdb target was prepared by applying the default energy minimization protocol after removing the bound ligand and water molecules except the ones needed within the binding pocket. The reproducibility of the docking simulation was validated by docking of the bound ligand as control and the adjustment of method was achieved by calculation of RMSD and analysis of the binding data. Afterward, the 6i compound was docked and output data was analyzed and represented [76].
4.5. Molecular Dynamics Simulation
MD simulations were performed by using templates from the 3D structural crystal of the SARS-CoV 19 main protease (PDB ID: 5R80) combined with the active compound 6i vs. the reference bound drug. Desmond version 3.8 software and the OPLS2005 forcefield were used to execute the simulation [77]; see more details in supporting materials. The production molecular dynamics phase was performed for three independent 100 ns simulations in an isothermal-isobaric (NpT) ensemble at 300 K and 1 bar using Langevin dynamics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph16030463/s1. All materials related to supporting the structures of compounds are deposited. Spectral characterizations and scanned charts of new compounds reported here are included in the Supplementary File.
Author Contributions
Conceptualization, A.M. and M.R.A.; methodology, A.M.; formal analysis, N.R., A.A. and H.S.A.; writing original draft preparation, H.S.A., M.R.A., N.R., K.S. and M.A.A.; writing review and editing, H.S.A., M.R.A., A.H.E.-G., K.S., A.M. and A.A.; supervision, A.M., H.S.A. and M.R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Deanship of Scientific Research at Jouf University under Grant Number (DSR2022-RG-0151).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at Jouf University under Grant Number (DSR2022-RG-0151).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fehr, A.R.; Perlman, S. Coronaviruses: An Overview of Their Replication and Pathogenesis. In Coronaviruses: Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–23. [Google Scholar]
- Pal, M.; Berhanu, G.; Desalegn, C.; Kandi, V. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): An Update. Cureus 2020, 12, e7423. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus Disease 2019–COVID-19. Clin. Microbiol. Rev. 2020, 33, e00028-20. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organization WHO. Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 5 January 2023).
- Mahmoud, A.; Mostafa, A.; Al-Karmalawy, A.A.; Zidan, A.; Abulkhair, H.S.; Mahmoud, S.H.; Shehata, M.; Elhefnawi, M.M.; Ali, M.A. Telaprevir Is a Potential Drug for Repurposing against SARS-CoV-2: Computational and in Vitro Studies. Heliyon 2021, 7, e07962. [Google Scholar] [CrossRef] [PubMed]
- Kutkat, O.; Moatasim, Y.; Al-Karmalawy, A.A.; Abulkhair, H.S.; Gomaa, M.R.; El-Taweel, A.N.; Abo Shama, N.M.; GabAllah, M.; Mahmoud, D.B.; Kayali, G.; et al. Robust Antiviral Activity of Commonly Prescribed Antidepressants against Emerging Coronaviruses: In Vitro and in Silico Drug Repurposing Studies. Sci. Rep. 2022, 12, 12920. [Google Scholar] [CrossRef]
- Abel, R.; Paredes Ramos, M.; Chen, Q.; Pérez-Sánchez, H.; Coluzzi, F.; Rocco, M.; Marchetti, P.; Mura, C.; Simmaco, M.; Bourne, P.E.; et al. Computational Prediction of Potential Inhibitors of the Main Protease of SARS-CoV-2. Front. Chem. 2020, 8, 1162. [Google Scholar] [CrossRef]
- Cui, W.; Yang, K.; Yang, H. Recent Progress in the Drug Development Targeting SARS-CoV-2 Main Protease as Treatment for COVID-19. Front. Mol. Biosci. 2020, 7, 616341. [Google Scholar] [CrossRef]
- Negi, M.; Chawla, P.A.; Faruk, A.; Chawla, V. Role of Heterocyclic Compounds in SARS and SARS CoV-2 Pandemic. Bioorg. Chem. 2020, 104, 104315. [Google Scholar] [CrossRef]
- Karypidou, K.; Ribone, S.R.; Quevedo, M.A.; Persoons, L.; Pannecouque, C.; Helsen, C.; Claessens, F.; Dehaen, W. Synthesis, Biological Evaluation and Molecular Modeling of a Novel Series of Fused 1,2,3-Triazoles as Potential Anti-Coronavirus Agents. Bioorg. Med. Chem. Lett. 2018, 28, 3472–3476. [Google Scholar] [CrossRef]
- Mohamed, M.; Abrigach, F.; El Kadiri, S.; Omar Said Hassane, S.; Abdellattif, M.H.; Touzani, R. Pyrazole, Imidazole and Triazole: In Silico, Docking and ADMET Studies against SARS-CoV-2. Mater. Today Proc. 2023, 72, 3686–3695. [Google Scholar] [CrossRef]
- Seck, I.; Nguemo, F. Triazole, Imidazole, and Thiazole-Based Compounds as Potential Agents against Coronavirus. Results Chem. 2021, 3, 100132. [Google Scholar] [CrossRef]
- Madasu, C.; Karri, S.; Sangaraju, R.; Sistla, R.; Uppuluri, M.V. Synthesis and Biological Evaluation of Some Novel 1,2,3-Triazole Hybrids of Myrrhanone B Isolated from Commiphora Mukul Gum Resin: Identification of Potent Antiproliferative Leads Active against Prostate Cancer Cells (PC-3). Eur. J. Med. Chem. 2020, 188, 111974. [Google Scholar] [CrossRef]
- Chandrashekhar, M.; Nayak, V.L.; Ramakrishna, S.; Mallavadhani, U.V. Novel Triazole Hybrids of Myrrhanone C, a Natural Polypodane Triterpene: Synthesis, Cytotoxic Activity and Cell Based Studies. Eur. J. Med. Chem. 2016, 114, 293–307. [Google Scholar] [CrossRef]
- Cortés-García, C.J.; Chacón-García, L.; Mejía-Benavides, J.E.; Díaz-Cervantes, E. Tackling the SARS-CoV-2 Main Protease Using Hybrid Derivatives of 1,5-Disubstituted Tetrazole-1,2,3-Triazoles: An in Silico Assay. PeerJ Phys. Chem. 2020, 2, e10. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-Containing Hybrids as Leads in Medicinal Chemistry: A Recent Overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Liu, M.; Xu, Q.; Guo, S.; Zuo, R.; Hong, Y.; Luo, Y.; Li, Y.; Gong, P.; Liu, Y. Design, Synthesis, and Structure-Activity Relationships of Novel Imidazo[4,5-c]Pyridine Derivatives as Potent Non-Nucleoside Inhibitors of Hepatitis C Virus NS5B. Bioorg. Med. Chem. 2018, 26, 2621–2631. [Google Scholar] [CrossRef]
- Kaoukabi, H.; Kabri, Y.; Curti, C.; Taourirte, M.; Rodriguez-Ubis, J.C.; Snoeck, R.; Andrei, G.; Vanelle, P.; Lazrek, H.B. Dihydropyrimidinone/1,2,3-Triazole Hybrid Molecules: Synthesis and Anti-Varicella-Zoster Virus (VZV) Evaluation. Eur. J. Med. Chem. 2018, 155, 772–781. [Google Scholar] [CrossRef]
- Sharma, R.; Chawla, P.A.; Chawla, V.; Verma, R.; Nawal, N.; Gupta, V. Therapeutic Journey of 5-Pyrazolones as a Versatile Scaffold: A Review. Mini-Rev. Med. Chem. 2021, 21, 1770–1795. [Google Scholar] [CrossRef]
- Ramajayam, R.; Tan, K.-P.; Liu, H.-G.; Liang, P.-H. Synthesis and Evaluation of Pyrazolone Compounds as SARS-Coronavirus 3C-like Protease Inhibitors. Bioorg. Med. Chem. 2010, 18, 7849–7854. [Google Scholar] [CrossRef]
- Zhao, Z.; Dai, X.; Li, C.; Wang, X.; Tian, J.; Feng, Y.; Xie, J.; Ma, C.; Nie, Z.; Fan, P.; et al. Pyrazolone Structural Motif in Medicinal Chemistry: Retrospect and Prospect. Eur. J. Med. Chem. 2020, 186, 111893. [Google Scholar] [CrossRef]
- Kumar, V.; Tan, K.-P.; Wang, Y.-M.; Lin, S.-W.; Liang, P.-H. Identification, Synthesis and Evaluation of SARS-CoV and MERS-CoV 3C-like Protease Inhibitors. Bioorg. Med. Chem. 2016, 24, 3035–3042. [Google Scholar] [CrossRef]
- Malebari, A.M.; Ahmed, H.E.A.; Ihmaid, S.K.; Omar, A.M.; Muhammad, Y.A.; Althagfan, S.S.; Aljuhani, N.; El-Sayed, A.A.A.A.; Halawa, A.H.; El-Tahir, H.M.; et al. Exploring the Dual Effect of Novel 1,4-Diarylpyranopyrazoles as Antiviral and Anti-Inflammatory for the Management of SARS-CoV-2 and Associated Inflammatory Symptoms. Bioorg. Chem. 2023, 130, 106255. [Google Scholar] [CrossRef] [PubMed]
- Mushtaque, M.; Avecilla, F.; Haque, A.; Perwez, A.; Khan, M.S.; Rizvi, M.M.A. Experimental and Theoretical Studies of a Pyrazole-Thiazolidin-2,4-Di-One Hybrid. J. Mol. Struct. 2017, 1141, 417–427. [Google Scholar] [CrossRef]
- Mushtaque, M.; Ahamad, S.; Jahan, M.; Hussain, K.; Khan, M.S. Azole-Based Compounds as Antiamoebic Agents: A Perspective Using Theoretical Calculations. RSC Adv. 2016, 6, 815–824. [Google Scholar] [CrossRef]
- Musa, A.; Ihmaid, S.K.; Hughes, D.L.; Said, M.A.; Hamada, S.; El-ghorab, A.H.; Abdelgawad, M.A.; Shalaby, K.; Shaker, M.E.; Alharbi, K.S.; et al. The Anticancer and EGFR-TK / CDK-9 Dual Inhibitory Potentials of New Synthetic Pyranopyrazole and Pyrazolone Derivatives: X-Ray Crystallography, in Vitro, and in Silico Mechanistic Investigations. J. Biomol. Struct. Dyn. 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; He, F.; Liu, D.; Fang, M.; Wu, Z.; Xu, D. AI-Aided Design of Novel Targeted Covalent Inhibitors against SARS-CoV-2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Mengist, H.M.; Dilnessa, T.; Jin, T. Structural Basis of Potential Inhibitors Targeting SARS-CoV-2 Main Protease. Front. Chem. 2021, 9, 622898. [Google Scholar] [CrossRef]
- Jacobs, J.; Grum-Tokars, V.; Zhou, Y.; Turlington, M.; Saldanha, S.A.; Chase, P.; Eggler, A.; Dawson, E.S.; Baez-Santos, Y.M.; Tomar, S.; et al. Discovery, Synthesis, And Structure-Based Optimization of a Series of N-(Tert-Butyl)-2-(N-Arylamido)-2-(Pyridin-3-Yl) Acetamides (ML188) as Potent Noncovalent Small Molecule Inhibitors of the Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) 3. J. Med. Chem. 2013, 56, 534–546. [Google Scholar] [CrossRef]
- Viegas-Junior, C.; Barreiro, E.J.; Fraga, C.A.M.; Danuello, A.; da Silva Bolzani, V. Molecular Hybridization: A Useful Tool in the Design of New Drug Prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef]
- Abulkhair, H.S.; Turky, A.; Ghiaty, A.; Ahmed, H.E.A.; Bayoumi, A.H. Novel Triazolophthalazine-Hydrazone Hybrids as Potential PCAF Inhibitors: Design, Synthesis, in Vitro Anticancer Evaluation, Apoptosis, and Molecular Docking Studies. Bioorg. Chem. 2020, 100, 103899. [Google Scholar] [CrossRef]
- Ji, H.; Stanton, B.Z.; Igarashi, J.; Li, H.; Martásek, P.; Roman, L.J.; Poulos, T.L.; Silverman, R.B. Minimal Pharmacophoric Elements and Fragment Hopping, an Approach Directed at Molecular Diversity and Isozyme Selectivity. Design of Selective Neuronal Nitric Oxide Synthase Inhibitors. J. Am. Chem. Soc. 2008, 130, 3900–3914. [Google Scholar] [CrossRef]
- Khayat, M.T.; Ahmed, H.E.A.; Omar, A.M.; Muhammad, Y.A.; Mohammad, K.A.; Malebari, A.M.; Khayyat, A.N.; Halawa, A.H.; Abulkhair, H.S.; Al-Karmalawy, A.A.; et al. A Novel Class of Phenylpyrazolone-Sulphonamides Rigid Synthetic Anticancer Molecules Selectively Inhibit the Isoform IX of Carbonic Anhydrases Guided by Molecular Docking and Orbital Analyses. J. Biomol. Struct. Dyn. 2023, 1–19. [Google Scholar] [CrossRef]
- El-Shershaby, M.H.; Ghiaty, A.; Bayoumi, A.H.; Ahmed, H.E.A.; El-Zoghbi, M.S.; El-Adl, K.; Abulkhair, H.S. 1,2,4-Triazolo[4,3-c]Quinazolines: A Bioisosterism-Guided Approach towards the Development of Novel PCAF Inhibitors with Potential Anticancer Activity. New J. Chem. 2021, 45, 11136–11152. [Google Scholar] [CrossRef]
- Abulkhair, H.S.; Elmeligie, S.; Ghiaty, A.; El-Morsy, A.; Bayoumi, A.H.; Ahmed, H.E.A.; El-Adl, K.; Zayed, M.F.; Hassan, M.H.; Akl, E.N.; et al. In Vivo- and in Silico-Driven Identification of Novel Synthetic Quinoxalines as Anticonvulsants and AMPA Inhibitors. Arch. Pharm. 2021, 354, 2000449. [Google Scholar] [CrossRef]
- Hammoud, M.M.; Khattab, M.; Abdel-Motaal, M.; Van der Eycken, J.; Alnajjar, R.; Abulkhair, H.S.; Al-Karmalawy, A.A. Synthesis, Structural Characterization, DFT Calculations, Molecular Docking, and Molecular Dynamics Simulations of a Novel Ferrocene Derivative to Unravel Its Potential Antitumor Activity. J. Biomol. Struct. Dyn. 2022. ahead of print. [Google Scholar] [CrossRef]
- Aljuhani, A.; Ahmed, H.E.A.; Ihmaid, S.K.; Omar, A.M.; Althagfan, S.S.; Alahmadi, Y.M.; Ahmad, I.; Patel, H.; Ahmed, S.; Almikhlafi, M.A.; et al. In Vitro and Computational Investigations of Novel Synthetic Carboxamide-Linked Pyridopyrrolopyrimidines with Potent Activity as SARS-CoV-2-M Pro Inhibitors. RSC Adv. 2022, 12, 26895–26907. [Google Scholar] [CrossRef]
- Boopathi, S.; Poma, A.B.; Kolandaivel, P. Novel 2019 Coronavirus Structure, Mechanism of Action, Antiviral Drug Promises and Rule out against Its Treatment. J. Biomol. Struct. Dyn. 2020, 39, 3409–3418. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and Discovery of Its Inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Aouad, M.R.; Khan, D.J.O.; Said, M.A.; Al-Kaff, N.S.; Rezki, N.; Ali, A.A.; Bouqellah, N.; Hagar, M. Novel 1,2,3-Triazole Derivatives as Potential Inhibitors against COVID-19 Main Protease: Synthesis, Characterization, Molecular Docking and DFT Studies. ChemistrySelect 2021, 6, 3468–3486. [Google Scholar] [CrossRef]
- Said, M.A.; Khan, D.J.O.; Al-blewi, F.F.; Al-Kaff, N.S.; Ali, A.A.; Rezki, N.; Aouad, M.R.; Hagar, M. New 1,2,3-Triazole Scaffold Schiff Bases as Potential Anti-COVID-19: Design, Synthesis, DFT-Molecular Docking, and Cytotoxicity Aspects. Vaccines 2021, 9, 1012. [Google Scholar] [CrossRef]
- Alzahrani, A.Y.; Shaaban, M.M.; Elwakil, B.H.; Hamed, M.T.; Rezki, N.; Aouad, M.R.; Zakaria, M.A.; Hagar, M. Anti-COVID-19 Activity of Some Benzofused 1,2,3-Triazolesulfonamide Hybrids Using in Silico and in Vitro Analyses. Chemom. Intell. Lab. Syst. 2021, 217, 104421. [Google Scholar] [CrossRef]
- Al-Humaidi, J.Y.; Shaaban, M.M.; Rezki, N.; Aouad, M.R.; Zakaria, M.; Jaremko, M.; Hagar, M.; Elwakil, B.H. 1,2,3-Triazole-Benzofused Molecular Conjugates as Potential Antiviral Agents against SARS-CoV-2 Virus Variants. Life 2022, 12, 1341. [Google Scholar] [CrossRef] [PubMed]
- Ihmaid, S.K.; Aljuhani, A.; Alsehli, M.; Rezki, N.; Alawi, A.; Aldhafiri, A.J.; Salama, S.A.; Ahmed, H.E.A.; Aouad, M.R. Discovery of Triaromatic Flexible Agents Bearing 1,2,3-Triazole with Selective and Potent Anti-Breast Cancer Activity and CDK9 Inhibition Supported by Molecular Dynamics. J. Mol. Struct. 2022, 1249, 131568. [Google Scholar] [CrossRef]
- Albelwi, F.F.; Abdu Mansour, H.M.; Elshatanofy, M.M.; El Kilany, Y.; Kandeel, K.; Elwakil, B.H.; Hagar, M.; Aouad, M.R.; El Ashry, E.S.H.; Rezki, N.; et al. Design, Synthesis and Molecular Docking of Novel Acetophenone-1,2,3-Triazoles Containing Compounds as Potent Enoyl-Acyl Carrier Protein Reductase (InhA) Inhibitors. Pharmaceuticals 2022, 15, 799. [Google Scholar] [CrossRef]
- Othman, E.M.; Fayed, E.A.; Husseiny, E.M.; Abulkhair, H.S. Apoptosis Induction, PARP-1 Inhibition, and Cell Cycle Analysis of Leukemia Cancer Cells Treated with Novel Synthetic 1,2,3-Triazole-Chalcone Conjugates. Bioorg. Chem. 2022, 123, 105762. [Google Scholar] [CrossRef] [PubMed]
- Othman, E.M.; Fayed, E.A.; Husseiny, E.M.; Abulkhair, H.S. Rationale Design, Synthesis, Cytotoxicity Evaluation, and in Silico Mechanistic Studies of Novel 1,2,3-Triazoles with Potential Anticancer Activity. New J. Chem. 2022, 46, 12206–12216. [Google Scholar] [CrossRef]
- Othman, E.M.; Fayed, E.A.; Husseiny, E.M.; Abulkhair, H.S. The Effect of Novel Synthetic Semicarbazone- and Thiosemicarbazone-Linked 1,2,3-Triazoles on the Apoptotic Markers, VEGFR-2, and Cell Cycle of Myeloid Leukemia. Bioorg. Chem. 2022, 127, 105968. [Google Scholar] [CrossRef]
- Reda Aouad, M.; Almehmadi, M.A.; Faleh Albelwi, F.; Teleb, M.; Tageldin, G.N.; Abu-Serie, M.M.; Hagar, M.; Rezki, N. Targeting the Interplay between MMP-2, CA II and VEGFR-2 via New Sulfonamide-Tethered Isomeric Triazole Hybrids; Microwave-Assisted Synthesis, Computational Studies and Evaluation. Bioorg. Chem. 2022, 124, 105816. [Google Scholar] [CrossRef]
- Ihmaid, S.K.; Alraqa, S.Y.; Aouad, M.R.; Aljuhani, A.; Elbadawy, H.M.; Salama, S.A.; Rezki, N.; Ahmed, H.E.A. Design of Molecular Hybrids of Phthalimide-Triazole Agents with Potent Selective MCF-7/HepG2 Cytotoxicity: Synthesis, EGFR Inhibitory Effect, and Metabolic Stability. Bioorg. Chem. 2021, 111, 104835. [Google Scholar] [CrossRef]
- Abul-Khair, H.; Elmeligie, S.; Bayoumi, A.; Ghiaty, A.; El-Morsy, A.; Hassan, M.H. Synthesis and Evaluation of Some New (1,2,4) Triazolo(4,3-a)Quinoxalin- 4(5h)-One Derivatives as AMPA Receptor Antagonists. J. Heterocycl. Chem. 2013, 50, 1202–1208. [Google Scholar] [CrossRef]
- El-Adl, K.; El-Helby, A.G.A.; Sakr, H.; Ayyad, R.R.; Mahdy, H.A.; Nasser, M.; Abulkhair, H.S.; El-Hddad, S.S.A. Design, Synthesis, Molecular Docking, Anticancer Evaluations, and in Silico Pharmacokinetic Studies of Novel 5-[(4-Chloro/2,4-Dichloro)Benzylidene]Thiazolidine-2,4-Dione Derivatives as VEGFR-2 Inhibitors. Arch. Pharm. 2021, 354, e202000279. [Google Scholar] [CrossRef]
- Khedr, F.; Ibrahim, M.K.; Eissa, I.H.; Abulkhair, H.S.; El-Adl, K. Phthalazine-Based VEGFR-2 Inhibitors: Rationale, Design, Synthesis, in Silico, ADMET Profile, Docking, and Anticancer Evaluations. Arch. Pharm. 2021, 354, 202100201. [Google Scholar] [CrossRef]
- Alam, F.; Khan, M.; Ateeq, M. Synthesis of Triazole-Based Nonionic Surfactants for Nanostructured Drug Delivery: Investigation of Their Physicochemical and Biological Aspects. J. Surfactants Deterg. 2019, 22, 1419–1427. [Google Scholar] [CrossRef]
- Minvielle, M.J.; Bunders, C.A.; Melander, C. Indole–Triazole Conjugates Are Selective Inhibitors and Inducers of Bacterial Biofilms. MedChemComm 2013, 4, 916. [Google Scholar] [CrossRef]
- Wang, G.; Peng, Z.; Wang, J.; Li, X.; Li, J. Synthesis, in Vitro Evaluation and Molecular Docking Studies of Novel Triazine-Triazole Derivatives as Potential α-Glucosidase Inhibitors. Eur. J. Med. Chem. 2017, 125, 423–429. [Google Scholar] [CrossRef]
- Roomi, M.W.; Monterrey, J.C.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. In Vitro Modulation of MMP-2 and MMP-9 in Human Cervical and Ovarian Cancer Cell Lines by Cytokines, Inducers and Inhibitors. Oncol. Rep. 2010, 23, 605–614. [Google Scholar] [CrossRef]
- Roomi, M.W.; Monterrey, J.C.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. Patterns of MMP-2 and MMP-9 Expression in Human Cancer Cell Lines. Oncol. Rep. 2009, 21, 1323–1333. [Google Scholar] [CrossRef]
- Vihinen, P.; Kähäri, V.-M. Matrix Metalloproteinases in Cancer: Prognostic Markers and Therapeutic Targets. Int. J. Cancer 2002, 99, 157–166. [Google Scholar] [CrossRef]
- Ii, M.; Yamamoto, H.; Adachi, Y.; Maruyama, Y.; Shinomura, Y. Role of Matrix Metalloproteinase-7 (Matrilysin) in Human Cancer Invasion, Apoptosis, Growth, and Angiogenesis. Exp. Biol. Med. 2006, 231, 20–27. [Google Scholar] [CrossRef]
- Lockbaum, G.J.; Reyes, A.C.; Lee, J.M.; Tilvawala, R.; Nalivaika, E.A.; Ali, A.; Kurt Yilmaz, N.; Thompson, P.R.; Schiffer, C.A. Crystal Structure of SARS-CoV-2 Main Protease in Complex with the Non-Covalent Inhibitor ML188. Viruses 2021, 13, 174. [Google Scholar] [CrossRef]
- Xia, Z.; Sacco, M.; Hu, Y.; Ma, C.; Meng, X.; Zhang, F.; Szeto, T.; Xiang, Y.; Chen, Y.; Wang, J. Rational Design of Hybrid SARS-CoV-2 Main Protease Inhibitors Guided by the Superimposed Cocrystal Structures with the Peptidomimetic Inhibitors GC-376, Telaprevir, and Boceprevir. ACS Pharmacol. Transl. Sci. 2021, 4, 1408–1421. [Google Scholar] [CrossRef]
- Ma, C.; Sacco, M.D.; Hurst, B.; Townsend, J.A.; Hu, Y.; Szeto, T.; Zhang, X.; Tarbet, B.; Marty, M.T.; Chen, Y.; et al. Boceprevir, GC-376, and Calpain Inhibitors II, XII Inhibit SARS-CoV-2 Viral Replication by Targeting the Viral Main Protease. Cell Res. 2020, 30, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Osipiuk, J.; Azizi, S.-A.; Dvorkin, S.; Endres, M.; Jedrzejczak, R.; Jones, K.A.; Kang, S.; Kathayat, R.S.; Kim, Y.; Lisnyak, V.G.; et al. Structure of Papain-like Protease from SARS-CoV-2 and Its Complexes with Non-Covalent Inhibitors. Nat. Commun. 2021, 12, 743. [Google Scholar] [CrossRef] [PubMed]
- Singer, C.F.; Kronsteiner, N.; Marton, E.; Kubista, M.; Cullen, K.J.; Hirtenlehner, K.; Seifert, M.; Kubista, E. MMP-2 and MMP-9 Expression in Breast Cancer-Derived Human Fibroblasts Is Differentially Regulated by Stromal-Epithelial Interactions. Breast Cancer Res. Treat. 2002, 72, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.S.; Lalonde, T.; Xu, S.; Liu, W.R. Learning from the Past: Possible Urgent Prevention and Treatment Options for Severe Acute Respiratory Infections Caused by 2019-nCoV. ChemBioChem 2020, 21, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal Structure of SARS-CoV-2 Main Protease Provides a Basis for Design of Improved α-Ketoamide Inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef]
- Pitsillou, E.; Liang, J.; Ververis, K.; Lim, K.W.; Hung, A.; Karagiannis, T.C. Identification of Small Molecule Inhibitors of the Deubiquitinating Activity of the SARS-CoV-2 Papain-Like Protease: In Silico Molecular Docking Studies and In Vitro Enzymatic Activity Assay. Front. Chem. 2020, 8, 623971. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, M.; Chen, C.Z.; Guo, H.; Shen, M.; Hu, X.; Shinn, P.; Klumpp-Thomas, C.; Michael, S.G.; Zheng, W. Identification of SARS-CoV-2 3CL Protease Inhibitors by a Quantitative High-Throughput Screening. ACS Pharmacol. Transl. Sci. 2020, 3, 1008–1016. [Google Scholar] [CrossRef]
- Chung, Y.-S.; Lee, N.-J.; Woo, S.H.; Kim, J.-M.; Kim, H.M.; Jo, H.J.; Park, Y.E.; Han, M.-G. Validation of Real-Time RT-PCR for Detection of SARS-CoV-2 in the Early Stages of the COVID-19 Outbreak in the Republic of Korea. Sci. Rep. 2021, 11, 14817. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Integrated Computer-Aided Molecular Design Platform. Available online: https://www.chemcomp.com/Products.htm (accessed on 12 May 2022).
- Ahmed, H.E.A.; Ihmaid, S.K.; Omar, A.M.; Shehata, A.M.; Rateb, H.S.; Zayed, M.F.; Ahmed, S.; Elaasser, M.M. Design, Synthesis, Molecular Docking of New Lipophilic Acetamide Derivatives Affording Potential Anticancer and Antimicrobial Agents. Bioorg. Chem. 2018, 76, 332–342. [Google Scholar] [CrossRef]
- Zaki, A.A.; Kaddah, M.M.Y.; Abulkhair, H.S.; Ashour, A. Unravelling the Antifungal and Antiprotozoal Activities and LC-MS/MS Quantification of Steroidal Saponins Isolated from Panicum Turgidum. RSC Adv. 2022, 12, 2980–2991. [Google Scholar] [CrossRef]
- Husseiny, E.M.; Abulkhair, H.S.; El-Dydamony, N.M.; Anwer, K.E. Exploring the Cytotoxic Effect and CDK-9 Inhibition Potential of Novel Sulfaguanidine-Based Azopyrazolidine-3,5-Diones and 3,5-Diaminoazopyrazoles. Bioorg. Chem. 2023, 133, 106397. [Google Scholar] [CrossRef]
- Douangamath, A.; Fearon, D.; Gehrtz, P.; Krojer, T.; Lukacik, P.; Owen, C.D.; Resnick, E.; Strain-Damerell, C.; Aimon, A.; Ábrányi-Balogh, P.; et al. Crystallographic and Electrophilic Fragment Screening of the SARS-CoV-2 Main Protease. Nat. Commun. 2020, 11, 5047. [Google Scholar] [CrossRef]
- Bowers, K.J.; Chow, D.E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. In Proceedings of the ACM/IEEE SC 2006 Conference (SC’06), Tampa, FL, USA, 11–17 November 2006; IEEE: Piscataway, NJ, USA, 2006; p. 43. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).