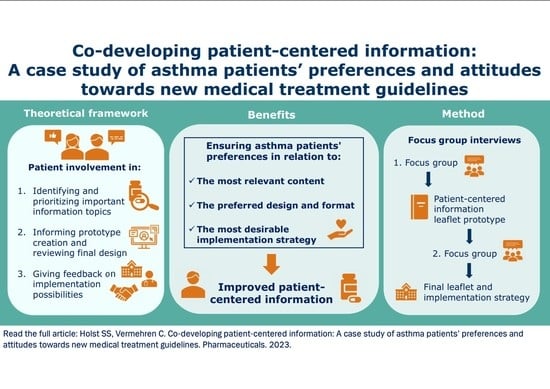
Co-Developing Patient-Centered Information: A Focus Group Study of Asthma Patients’ Preferences and Attitudes towards New Medical Treatment Guidelines
Abstract
1. Introduction
- Asthma patients’ preferences for the design and content of written patient-centered information.
- Asthma patients’ attitude towards how the written patient-centered information could support them in decision-making regarding their pharmacological treatment change.
2. Results
2.1. Identification of Important Topics about the New MART Approach
2.2. Feedback on the Design of Written Patient-Centered Information
2.3. Implementation of Written Patient-Centered Information
3. Discussion
4. Materials and Methods
4.1. Design
4.2. Qualitative Focus Group Interview Method
- Focus group:Focus on patients’ attitudes to information content and implementation before preparing the prototype information leaflet. The information leaflet prototype was developed based on the results of this focus group interview.
- Focus group:Same focus, but the informants were asked to evaluate the information leaflet prototype and provide input for improvements.
- Identification: asthma patients’ suggestions for the most relevant information that a written patient-orientated material should contain.
- Feedback on design: asthma patients’ preferences regarding a written patient-orientated leaflet—including the layout, language, and relevance of information.
- Implementation: the implementation of written patient-orientated material in the meeting with healthcare professionals such as staff at local community pharmacies and general practitioners, and how the material could be used in these meetings from the patients’ perspective.
- An introductory assignment where the interviewees could write down their immediate thoughts before consensus was reached in the group.
- A thorough review of their pharmacological asthma treatment made by a clinical pharmacist, focusing on potential pharmacological treatment changes based on the new MART approach.
- A brainstorm exercise:
- ○
- In focus group one: suggestions for relevant information for an information leaflet and a ranking of the information based on importance.
- ○
- In focus group two: suggestions for changes to the first prototype of the leaflet and a ranking of the changes based on importance.
- ○
- A group discussion of the use of a patient-centered information leaflet in two different cases; at the local community pharmacy and at the general practitioner’s office, identified in our previous study [23].
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poulsen, R.; Petersen, S.B.; Plaschke, P.; Backer, V. National Asthma Guideline in Diagnostics from The Danish Pulmonary Medicine Society. The Danish Pulmonary Medicine Society. Available online: https://lungemedicin.dk/astma-diagnostik/ (accessed on 13 April 2022).
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2022. Available online: https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf (accessed on 28 February 2023).
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2019. Available online: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf (accessed on 28 February 2023).
- O’Byrne, P.M.; FitzGerald, J.M.; Bateman, E.D.; Barnes, P.J.; Zhong, N.; Keen, C.; Jorup, C.; Lamarca, R.; Ivanov, S.; Reddel, H.K. Inhaled Combined Budesonide-Formoterol as Needed in Mild Asthma. N. Engl. J. Med. 2018, 378, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.R.; Bateman, E.D.; Sears, M.R.; O’Byrne, P.M. What have we learnt about asthma control from trials of budesonide/formoterol as maintenance and reliever? Respirology 2020, 25, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.T.; Heaney, L.G. Nonadherence in difficult asthma—Facts, myths, and a time to act. Patient Prefer. Adherence 2013, 7, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Partridge, M.R.; van der Molen, T.; Myrseth, S.-E.; Busse, W.W. Attitudes and actions of asthma patients on regular maintenance therapy: The INSPIRE study. BMC Pulm. Med. 2006, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Price, D.; Fletcher, M.; van der Molen, T. Asthma control and management in 8,000 European patients: The REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim. Care Respir. Med. 2014, 24, 14009. [Google Scholar] [CrossRef]
- Tavakoli, H.; FitzGerald, J.M.; Lynd, L.D.; Sadatsafavi, M. Predictors of inappropriate and excessive use of reliever medications in asthma: A 16-year population-based study. BMC Pulm. Med. 2018, 18, 33. [Google Scholar] [CrossRef]
- Ahmad, A.; Sorensen, K. Enabling and hindering factors influencing adherence to asthma treatment among adolescents: A systematic literature review. J. Asthma 2016, 53, 862–878. [Google Scholar] [CrossRef]
- George, M. Adherence in Asthma and COPD: New Strategies for an Old Problem. Respir. Care 2018, 63, 818–831. [Google Scholar] [CrossRef]
- Miles, C.; Arden-Close, E.; Thomas, M.; Bruton, A.; Yardley, L.; Hankins, M.; Kirby, S.E. Barriers and facilitators of effective self-management in asthma: Systematic review and thematic synthesis of patient and healthcare professional views. NPJ Prim. Care Respir. Med. 2017, 27, 57. [Google Scholar] [CrossRef]
- Van de Hei, S.J.; Dierick, B.J.H.; Aarts, J.E.P.; Kocks, J.W.H.; van Boven, J.F.M. Personalized Medication Adherence Management in Asthma and Chronic Obstructive Pulmonary Disease: A Review of Effective Interventions and Development of a Practical Adherence Toolkit. J. Allergy Clin. Immunol. Pract. 2021, 9, 3979–3994. [Google Scholar] [CrossRef]
- Jia, X.; Zhou, S.; Luo, D.; Zhao, X.; Zhou, Y.; Cui, Y.-M. Effect of pharmacist-led interventions on medication adherence and inhalation technique in adult patients with asthma or COPD: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2020, 45, 904–917. [Google Scholar] [CrossRef]
- Madden, M.; Speed, E. Beware Zombies and Unicorns: Toward Critical Patient and Public Involvement in Health Research in a Neoliberal Context. Front. Sociol. 2017, 2, 7. Available online: https://www.frontiersin.org/articles/10.3389/fsoc.2017.00007 (accessed on 20 January 2023). [CrossRef]
- Esmail, L.; Moore, E.; Rein, A. Evaluating patient and stakeholder engagement in research: Moving from theory to practice. J. Comp. Eff. Res. 2015, 4, 133–145. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Hinton, L.; Finlay, T.; Macfarlane, A.; Fahy, N.; Clyde, B.; Chant, A. Frameworks for supporting patient and public involvement in research: Systematic review and co-design pilot. Health Expect. 2019, 22, 785–801. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Mullins, C.D.; Gronseth, G.S.; Gagliardi, A.R. Recommendations for patient engagement in guideline development panels: A qualitative focus group study of guideline-naïve patients. PLoS ONE 2017, 12, e0174329. [Google Scholar] [CrossRef]
- Gierisch, J.M.; Hughes, J.M.; Williams, J.W.; Gordon, A.M.; Goldstein, K.M. Qualitative Exploration of Engaging Patients as Advisors in a Program of Evidence Synthesis: Cobuilding the Science to Enhance Impact. Med Care 2019, 57, S246–S252. [Google Scholar] [CrossRef]
- Van Beusekom, M.M.; Kerkhoven, A.H.; Bos, M.J.W.; Guchelaar, H.-J.; van den Broek, J.M. The extent and effects of patient involvement in pictogram design for written drug information: A short systematic review. Drug Discov. Today 2018, 23, 1312–1318. [Google Scholar] [CrossRef]
- Woo, B.F.Y.; Bulto, L.N.; Hendriks, J.M.L.; Lim, T.W.; Tam, W.W.S. The information needs of patients with atrial fibrillation: A scoping review. J. Clin. Nurs. 2021. [Google Scholar] [CrossRef]
- Wang, L.Y.T.; Lua, J.Y.H.; Chan, C.X.C.; Ong, R.L.L.; Wee, C.F.; Woo, B.F.Y. Health information needs and dissemination methods for individuals living with ischemic heart disease: A systematic review. Patient Educ. Couns. 2022, 108, 107594. [Google Scholar] [CrossRef]
- Holst, S.S.; Sabedin, E.; Sabedin, E.; Vermehren, C. A Shift in Asthma Treatment According to New Guidelines: An Evaluation of Asthma Patients’ Attitudes towards Treatment Change. Int. J. Environ. Res. Public Health 2023, 20, 3453. [Google Scholar] [CrossRef]
- Andersson, Å.; Vilhelmsson, M.; Fomichov, V.; Lindhoff Larsson, A.; Björnsson, B.; Sandström, P.; Drott, J. Patient involvement in surgical care-Healthcare personnel views and behaviour regarding patient involvement. Scand. J. Caring Sci. 2021, 35, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Snijders, H.S.; Kunneman, M.; Bonsing, B.A.; de Vries, A.C.; Tollenaar, R.a.E.M.; Pieterse, A.H.; Stiggelbout, A.M. Preoperative risk information and patient involvement in surgical treatment for rectal and sigmoid cancer. Colorectal. Dis. 2014, 16, O43–O49. [Google Scholar] [CrossRef] [PubMed]
- Clausen, J.A.; Juhl, M.; Rydahl, E. Quality assessment of patient leaflets on misoprostol-induced labour: Does written information adhere to international standards for patient involvement and informed consent? BMJ Open 2016, 6, e011333. [Google Scholar] [CrossRef] [PubMed]
- Flink, M.; Öhlén, G.; Hansagi, H.; Barach, P.; Olsson, M. Beliefs and experiences can influence patient participation in handover between primary and secondary care--a qualitative study of patient perspectives. BMJ Qual. Saf. 2012, 21 (Suppl. S1), i76–i83. [Google Scholar] [CrossRef]
- Turner, J.P.; Richard, C.; Lussier, M.-T.; Lavoie, M.-E.; Farrell, B.; Roberge, D.; Tannenbaum, C. Deprescribing conversations: A closer look at prescriber–patient communication. Ther. Adv. Drug Saf. 2018, 9, 687–698. [Google Scholar] [CrossRef]
- Richard, C.; Glaser, E.; Lussier, M.-T. Communication and patient participation influencing patient recall of treatment discussions. Health Expect. 2017, 20, 760–770. [Google Scholar] [CrossRef]
- Lockwood, C.; Munn, Z.; Porritt, K. Qualitative Research Synthesis: Methodological Guidance for Systematic Reviewers Utilizing Meta-Aggregation. 2015. Available online: https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fjbi.global%2Fsites%2Fdefault%2Ffiles%2F2021-10%2FChecklist_for_Qualitative_Research.docx&wdOrigin=BROWSELINK (accessed on 28 February 2023).
- Tong, A.; Sainsbury, P.; Craig, J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int. J. Qual. Health Care 2007, 19, 349–357. [Google Scholar] [CrossRef]
- Robson, C.; McCartan, K. Real World Research—A Resource for Users of Social Research Methods in Applied Settings, 4th ed.; John Wiley & Sons Ltd: Chichester, UK, 2016. [Google Scholar]
- Malterud, K. Systematic text condensation: A strategy for qualitative analysis. Scand. J. Public. Health 2012, 40, 795–805. [Google Scholar] [CrossRef]
- Saunders, B.; Sim, J.; Kingstone, T.; Baker, S.; Waterfield, J.; Bartlam, B.; Burroughs, H.; Jinks, C. Saturation in qualitative research: Exploring its conceptualization and operationalization. Qual Quant 2018, 52, 1893–1907. [Google Scholar] [CrossRef]
| GINA Guideline Year | Step 1 | Step 2 | Step 3 | Step 4 |
|---|---|---|---|---|
| 2018 | SABA as needed | SABA as needed + low dose ICS | SABA as needed + low dose ICS/LABA combination | SABA as needed + medium dose ICS/LABA combination |
| 2022 | Low dose ICS + formoterol combination as needed | Low dose ICS + formoterol combination as needed | Low dose ICS + formoterol combination daily and as needed | Medium dose ICS + formoterol combination daily and SABA as needed |
| First Focus Group Interview | ||||
|---|---|---|---|---|
| Interviewee Abbreviation | Sex | Age | Severity of Asthma | Education * |
| F1A | Female | 66 | Mild | Long |
| F1B | Male | 58 | Severe | Long |
| F1C | Female | 31–50 * | Severe | Long |
| F1D | Male | 55 | Severe | Vocational |
| F1E | Female | Over 71 * | Moderate | Vocational |
| Second Focus Group Interview | ||||
| Interviewee abbreviation | Sex | Age | Severity of asthma | Education * |
| F2A | Female | 28 | Severe | Long |
| F2B | Female | 57 | Mild | Vocational |
| F2C | Male | 55 | Severe | Vocational |
| F2D | Male | 73 | Severe | Vocational |
| Themes | Subthemes | Quotes |
|---|---|---|
| Identification of important topics about the new MART approach | Effect and side effects | “It’s a really big step to say goodbye to something that you know works, to something that some others say works, but that I’m not actually sure about.” (F1D)“After all, you get exactly the same side effects [with the new MART approach] as you would always get [with your current treatment].” (F2A) |
| Usage | “When should I take it [the medicine]? And when do I have to see the doctor? When should I react to the fact that this is not controlled well enough?” (F1D)“But I think that if you were schooled in [taking the medicine as needed] from the start […]. In other words, we probably need to educate people more.” (F2A) | |
| Finance and access | “Is it [the new asthma medicine] something they have in stock at the pharmacy?” (F1C)“And then when you say those magic words: “It’s a little bit cheaper”—then you’re always up for sale.” (F2C) | |
| Symptom assessment | “As a new person with asthma [...] I would be very doubtful: “Is this asthma or is it something else?” Do I now have to use it [the medication]?”” (F1A)“I think I would end up using it [the inhaler] too little [if it only were as needed] Because I would tend to think: “Ah, it is not quite bad enough for me to take it”. Whereas the fixed routine of having to take it [the medicine] morning and evening makes me take it.” (F1D)“I think that if you don’t know your own illness very well, you might sometimes be a bit in doubt: “Should I take it [the inhaler] or shouldn’t I take it?” (F2C) | |
| Feedback on the design of written patient-centered information | Format | “Maybe it [the leaflet] is almost a postcard size you get, where the names [of the medicine] are just there, so you can remember. And quite briefly.” (F1B)“I think there is a bit too much text […] and it is some difficult and long words to read, if you are not such a good reader.” (F2B)“F2D: And then it [the leaflet] must be much larger. It must be a real one that you can flip through. F2B: Yes, a booklet with some pictures.” (F2D and F2B) |
| Better recollection | “It would be positive to have something in writing, because otherwise I would have forgotten it 26 s later.” (F1D) | |
| Increased patient autonomy | “But then you [with the leaflet] could say [to the doctor]: “Hello, it’s not just me who thinks this [new MART approach is advantageous], it’s also the Danish Pulmonary Society who thinks this and the Danish Capital Region.” (F2A) | |
| Information overload | “Basically, I don’t really believe in such leaflets here to be honest. Because today we are overwhelmed with information about all sorts of things.” (F2C) | |
| Implementation of written patient-centered information | At the GP clinic | “It would be there [at the GP] that I would seek my primary advice on whether something needs to be changed in my medication and such.” (F1C)“I think that people are very loyal to the fact that they have to actually go to their GP with this problem.” (F2C)“It [the leaflet] is not something you just have handed out. There must be words along the way.” (F2D) |
| At the pharmacy | “No, for me the pharmacy is a commercial business that just happens to have some legal rights to sell some goods that others are not allowed to sell.” (F1D)“I think it’s a good idea to be made aware: “There is something new [the MART approach]. You can just talk to your doctor about it.” I think that’s fine.” (F1E)“I think it depends on whether you trust the person you are talking to. It is very good [...] when the pharmacy asks if you know how to take your medicine. But sometimes I’m like: “I think you are entering an area where it is a relationship between patient and doctor”.” (F2C)“I think it’s a good idea that when a pharmacist dispenses asthma medicine, they can draw attention to this leaflet and say: “I would recommend that you talk to your doctor about it.” (F2B) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holst, S.S.; Vermehren, C. Co-Developing Patient-Centered Information: A Focus Group Study of Asthma Patients’ Preferences and Attitudes towards New Medical Treatment Guidelines. Pharmaceuticals 2023, 16, 456. https://doi.org/10.3390/ph16030456
Holst SS, Vermehren C. Co-Developing Patient-Centered Information: A Focus Group Study of Asthma Patients’ Preferences and Attitudes towards New Medical Treatment Guidelines. Pharmaceuticals. 2023; 16(3):456. https://doi.org/10.3390/ph16030456
Chicago/Turabian StyleHolst, Sara Sommer, and Charlotte Vermehren. 2023. "Co-Developing Patient-Centered Information: A Focus Group Study of Asthma Patients’ Preferences and Attitudes towards New Medical Treatment Guidelines" Pharmaceuticals 16, no. 3: 456. https://doi.org/10.3390/ph16030456
APA StyleHolst, S. S., & Vermehren, C. (2023). Co-Developing Patient-Centered Information: A Focus Group Study of Asthma Patients’ Preferences and Attitudes towards New Medical Treatment Guidelines. Pharmaceuticals, 16(3), 456. https://doi.org/10.3390/ph16030456