A Descriptive Analysis of Direct Oral Anticoagulant Drugs Dosing Errors Based on Spontaneous Reports from the EudraVigilance Database
Abstract
1. Introduction
2. Results
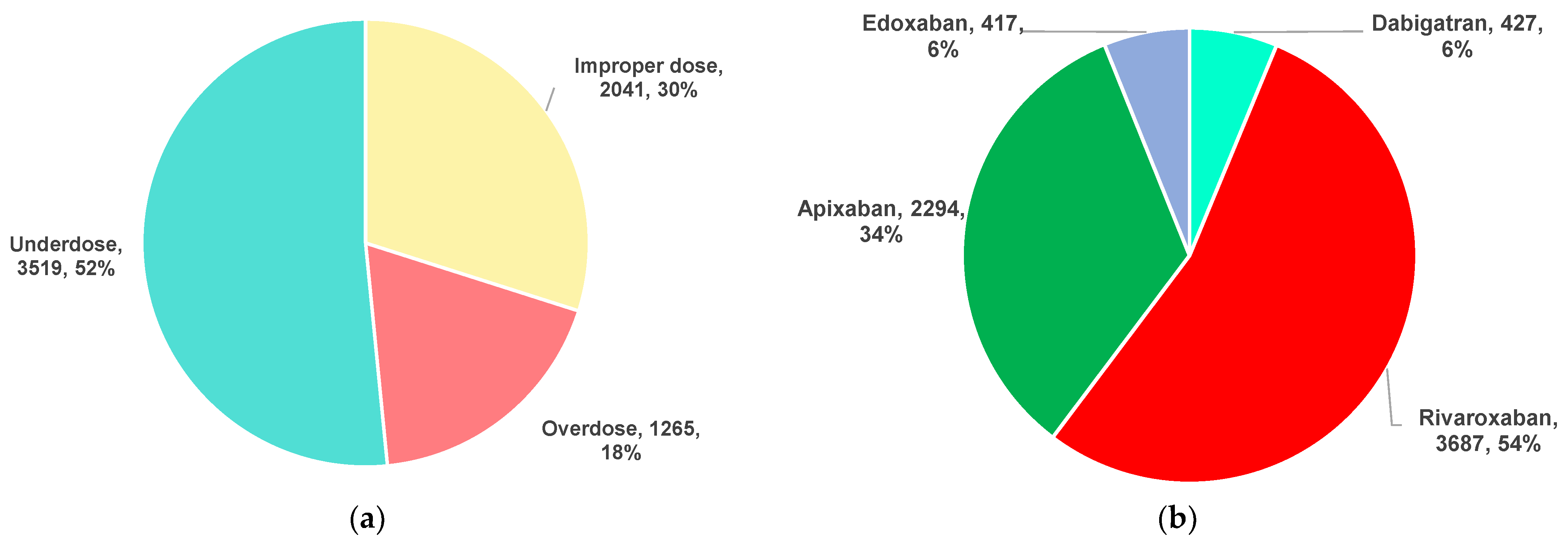
2.1. Descriptive Analysis
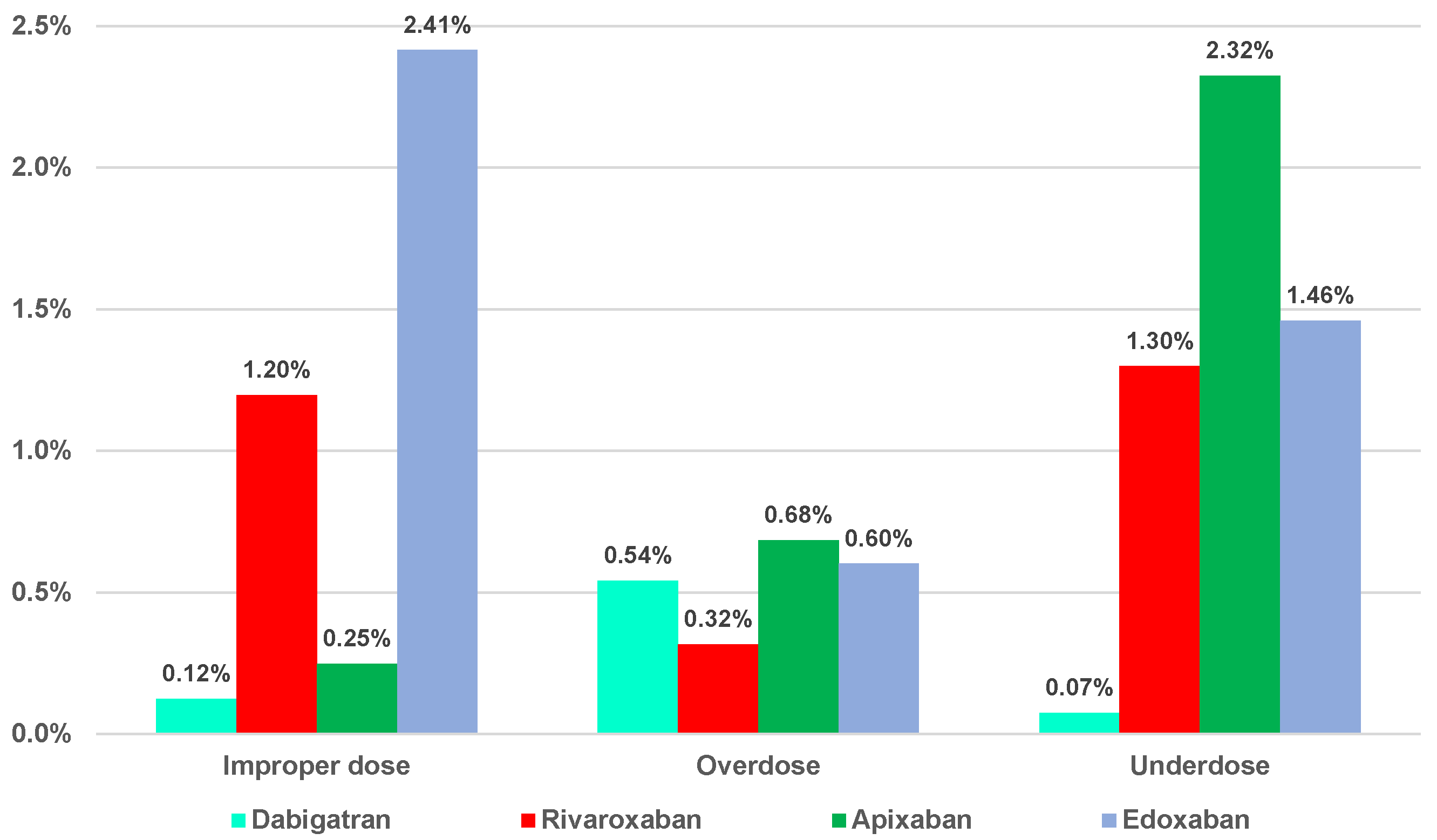
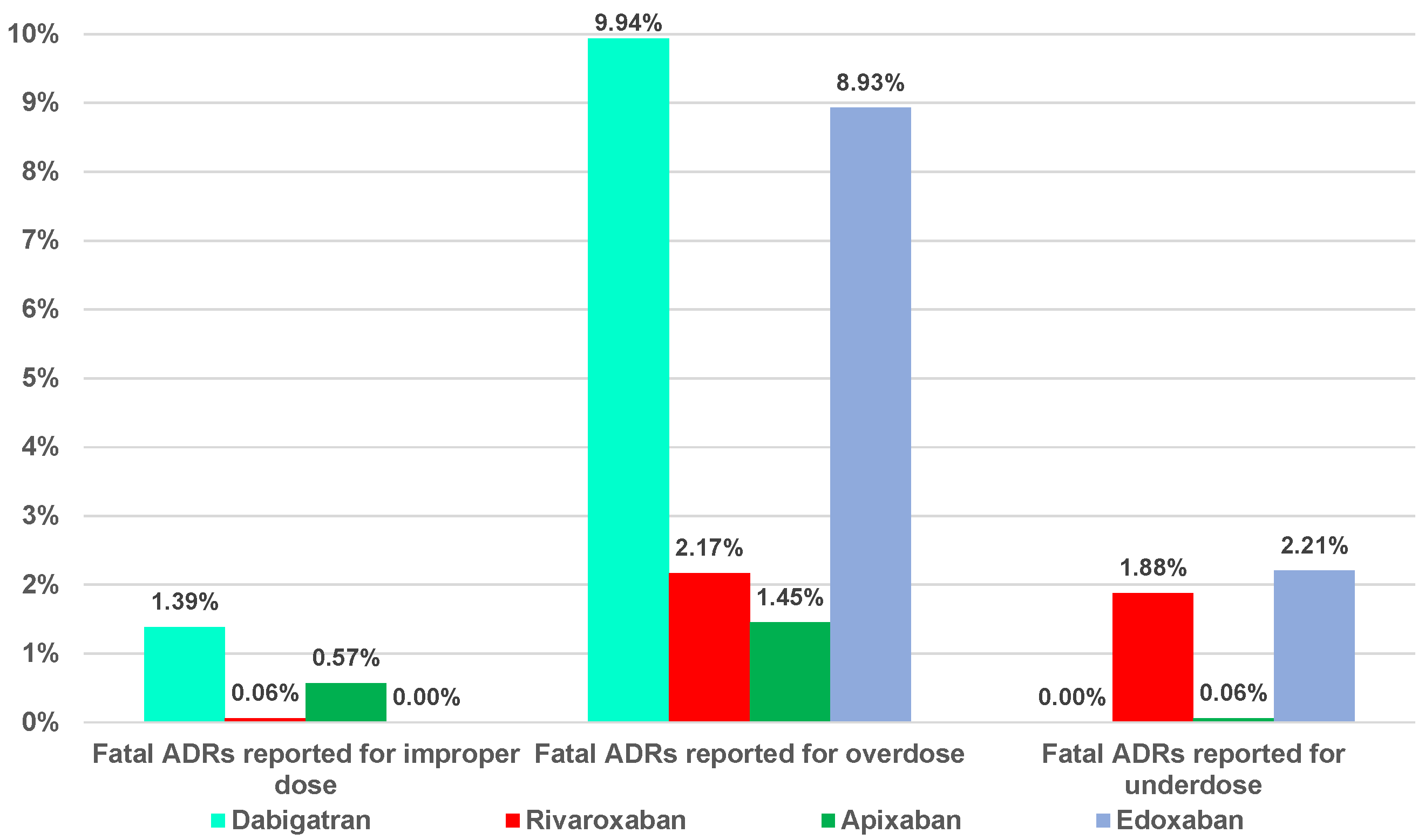
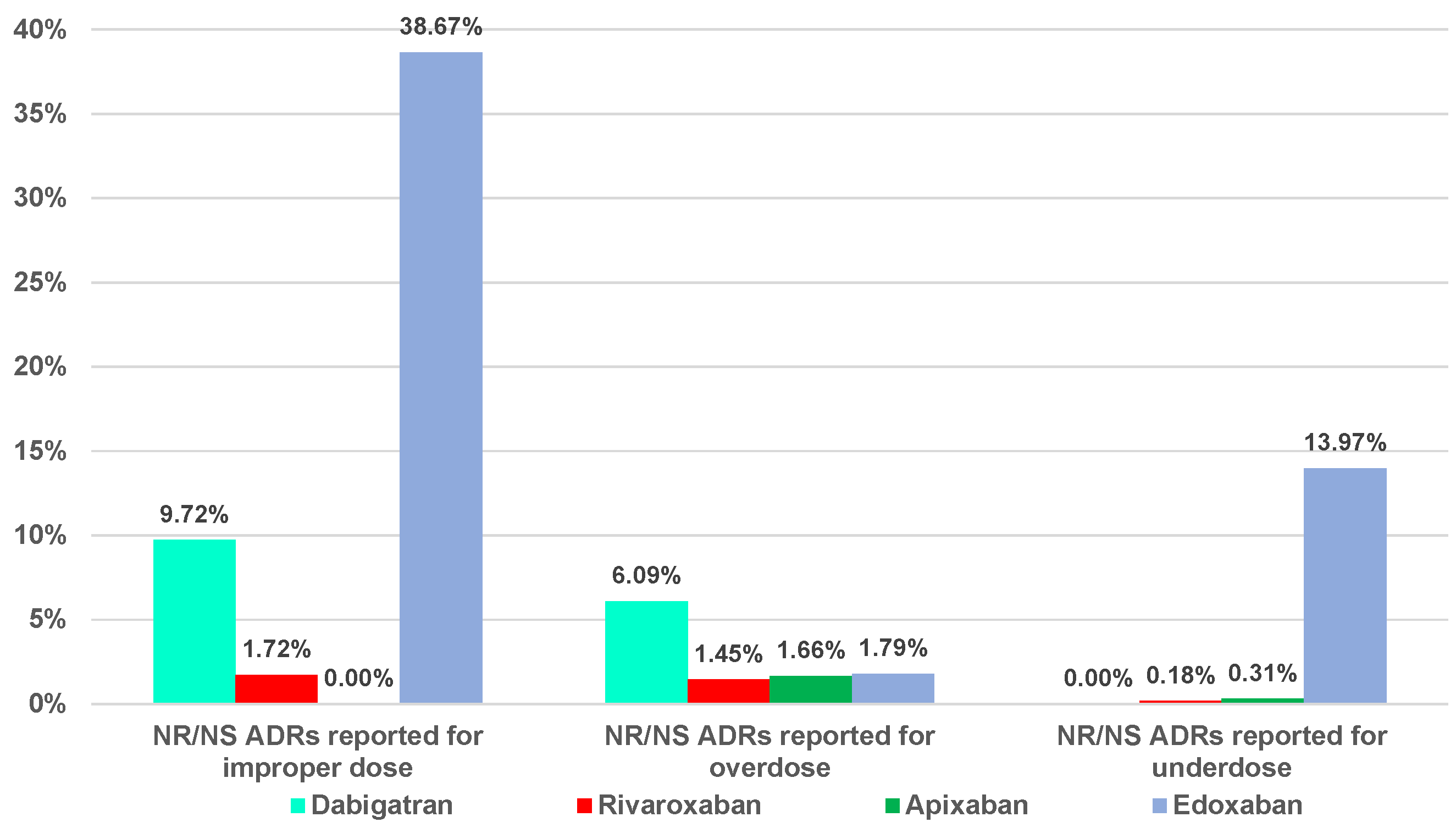
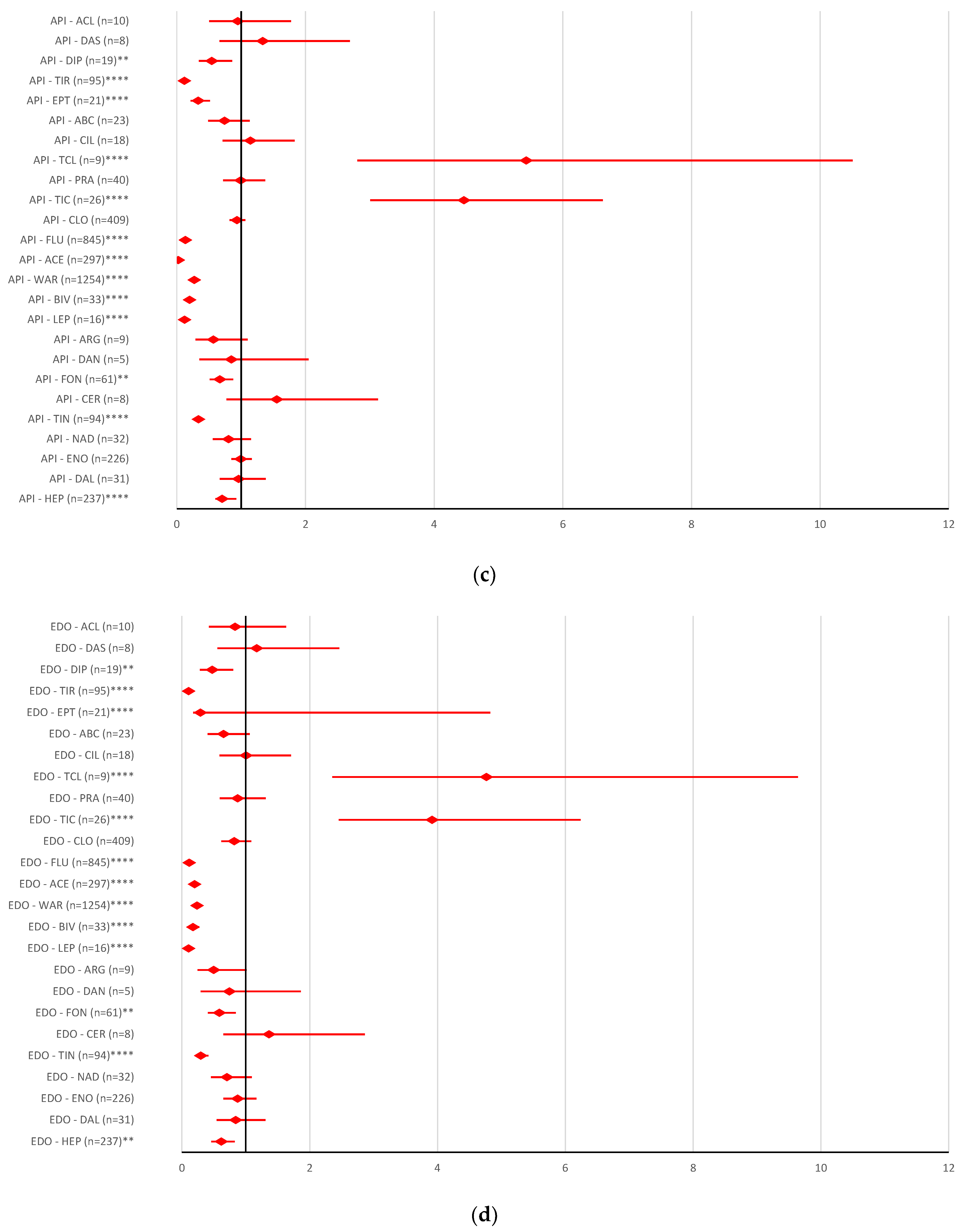
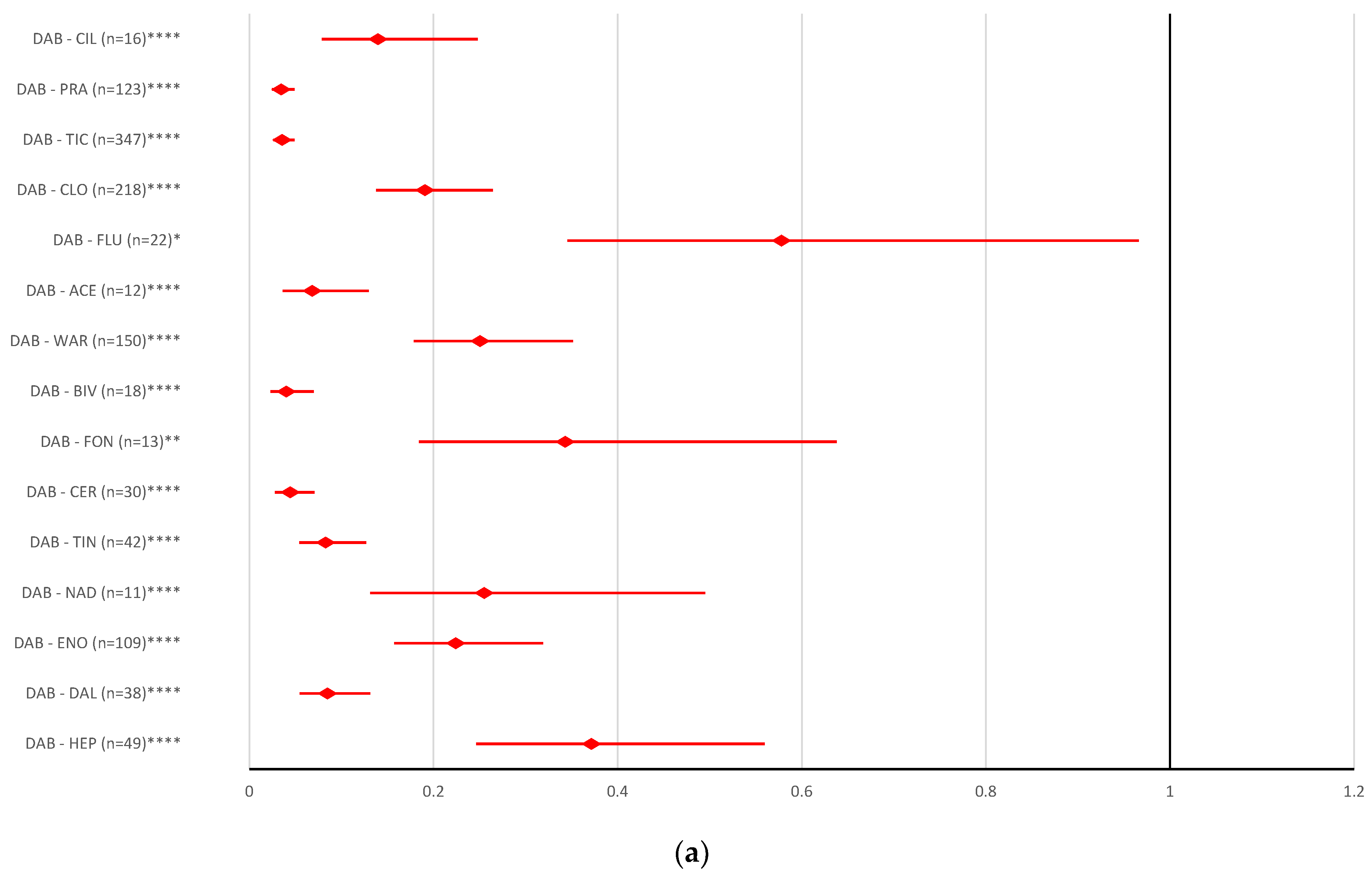
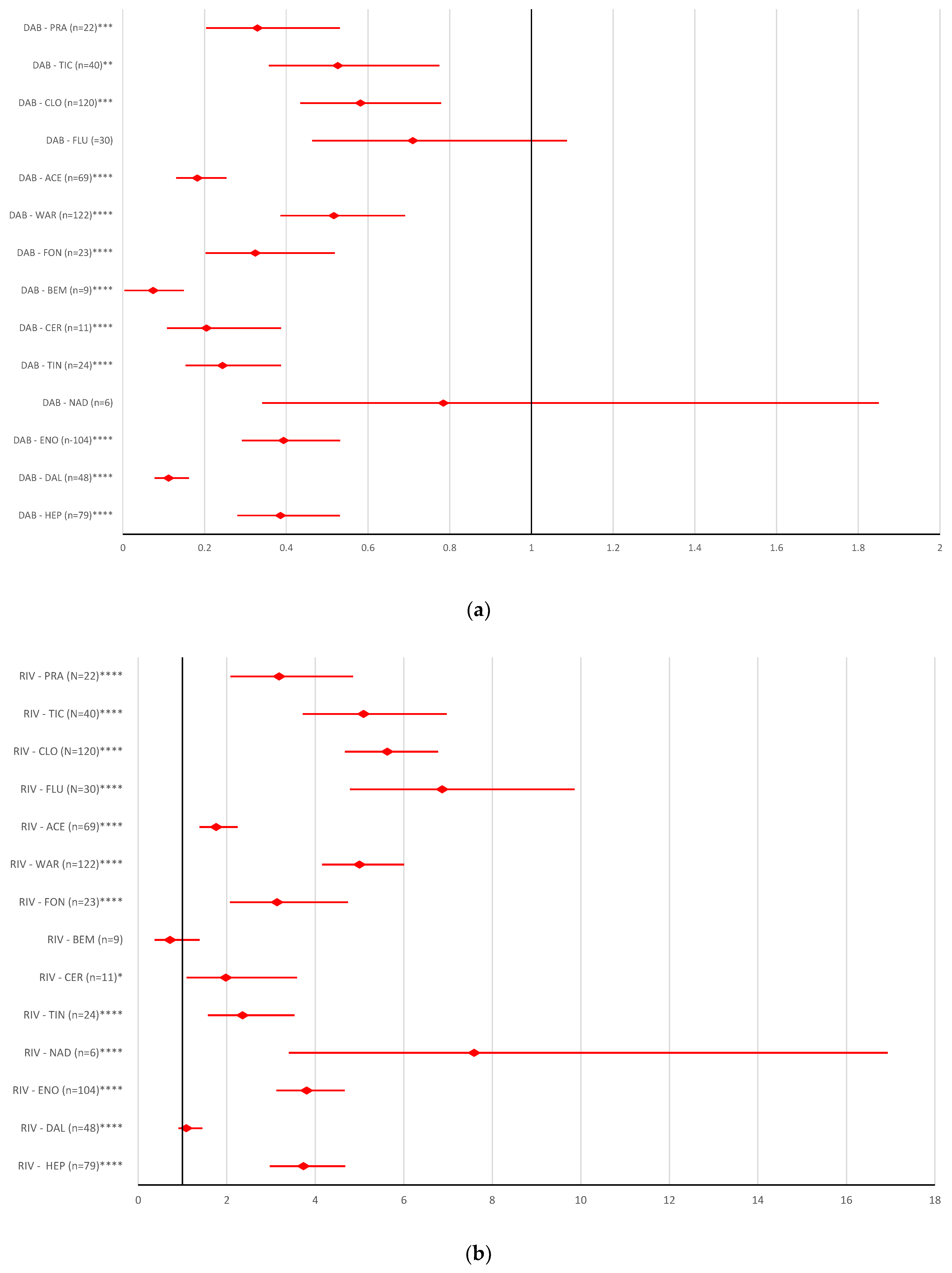
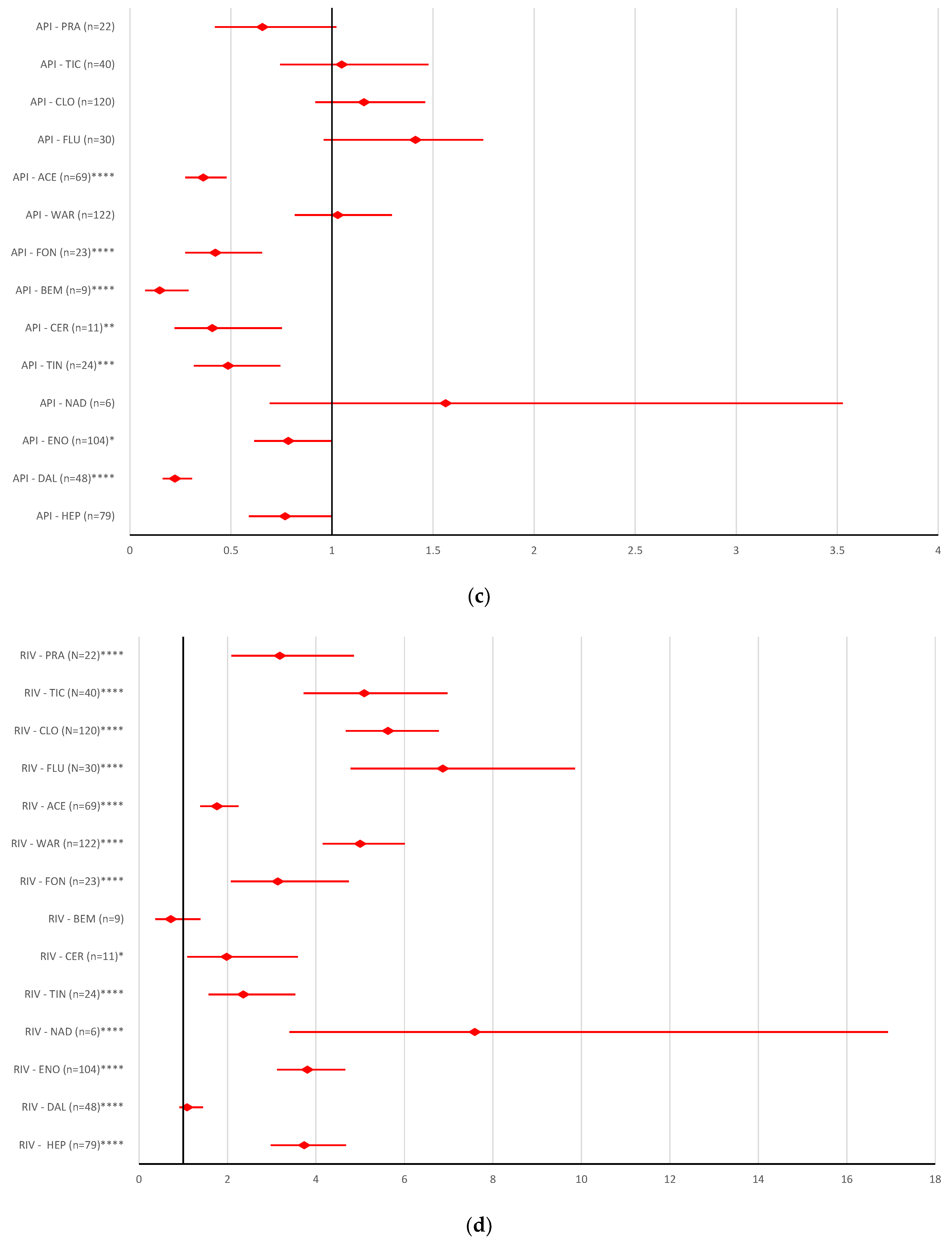
2.2. Disproportionality Analysis
2.2.1. Overdosing Errors
2.2.2. Underdosing Errors
2.2.3. Improper Dose Errors
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Study Design
4.2. Material
4.3. Disproportionality Analysis
4.4. Ethics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | abciximab |
| AC | anticoagulant drugs |
| ACE | acenocoumarol |
| ACL | combination of acetylsalicylic acid and clopidogrel |
| ADRs | adverse reactions |
| AF | atrial fibrillation |
| API | apixaban |
| ARG | argatroban |
| BEM | bemiparin |
| BIV | bivalirudin |
| CER | certoparin |
| CI | confidence interval |
| CIL | cilostazol |
| CLO | clopidogrel |
| DAB | dabigatran |
| DAL | dalteparin |
| DAN | danaparoid |
| DAS | combination of dipyridamole and acetylsalicylic acid |
| DIP | dipyridamole |
| DOACs | direct oral anticoagulant drugs |
| EDO | edoxaban |
| EEA | European Economic Area |
| EMA | European Medicines Agency |
| ENO | enoxaparin |
| EPT | eptifibatide |
| EV | EudraVigilance |
| FLU | fluindione |
| FON | fondaparinux |
| HEP | unfractionated heparin |
| ICSR | Individual Case Safety Report |
| LEP | lepirudin |
| LMWHs | low molecular weight heparins |
| ME | medication errors |
| MedDRA | Medical Dictionary for Regulatory Activities |
| NAD | nadroparin |
| NR/NS | not recovered/not resolved |
| PRA | prasugrel |
| PT | preferred term |
| RIV | rivaroxaban |
| ROR | reporting odds ratio |
| SOC | system organ classes |
| TCL | ticagrelor |
| TIC | ticlopidine |
| TIN | tinzaparin |
| TIR | tirofiban |
| VKAs | vitamin K antagonists |
| WAR | warfarin |
References
- Weisel, J.W.; Litvinov, R.I. Fibrin Formation, Structure and Properties. Subcell. Biochem. 2017, 82, 405. [Google Scholar] [CrossRef] [PubMed]
- Heestermans, M.; Poenou, G.; Hamzeh-Cognasse, H.; Cognasse, F.; Bertoletti, L. Anticoagulants: A Short History, Their Mechanism of Action, Pharmacology, and Indications. Cells 2022, 11, 3214. [Google Scholar] [CrossRef]
- Esmon, C.T. Basic Mechanisms and Pathogenesis of Venous Thrombosis. Blood Rev. 2009, 23, 225. [Google Scholar] [CrossRef]
- Wakefield, T.W.; Myers, D.D.; Henke, P.K. Mechanisms of Venous Thrombosis and Resolution. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 387–391. [Google Scholar] [CrossRef]
- Mackman, N. New Insights into the Mechanisms of Venous Thrombosis. J. Clin. Investig. 2012, 122, 2331. [Google Scholar] [CrossRef]
- Violi, F.; Pastori, D.; Pignatelli, P. Mechanisms and Management Of Thrombo-Embolism In Atrial Fibrillation. J. Atr. Fibrillation 2014, 7, 71–76. [Google Scholar] [CrossRef]
- Roudaut, R.; Serri, K.; Lafitte, S. Thrombosis of Prosthetic Heart Valves: Diagnosis and Therapeutic Considerations. Heart 2007, 93, 137. [Google Scholar] [CrossRef] [PubMed]
- Ghibu, S.; Ilie, I.; Mureșan, A.; Mogoșan, C. Perspectives in the experimental study of the metabolic syndrome. Farmacia 2015, 63, 4. [Google Scholar]
- Kahn, S.R. The Post-Thrombotic Syndrome. Hematol. Am. Soc. Hematol. Educ. Progr. 2016, 2016, 413. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M. Epidemiology and Risk Factors for Venous Thrombosis. Semin. Hematol. 2007, 44, 62. [Google Scholar] [CrossRef] [PubMed]
- Haiey, P.M. Overview of Venous Thromboembolism. Am. J. Manag. Care 2017, 23, S376–S382. [Google Scholar]
- Cuc Ioana, H.; Crina Claudia, T.; Olah, N.; Dehelean, C.; Motoc, A.; Ardelean, S.; Conea, S.; Morgovan, C. Study of the Oral Contraceptives’ Use by Women from Western Romania. Farmacia 2015, 63, 4. [Google Scholar]
- Deep Venous Thrombosis Prophylaxis—StatPearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534865/#!po=31.2500 (accessed on 30 June 2022).
- Brouwers, J.R.B.J.; Roeters van Lennep, J.E.; Beinema, M.J. Biosimilars of Low Molecular Weight Heparins: Relevant Background Information for Your Drug Formulary. Br. J. Clin. Pharmacol. 2019, 85, 2479. [Google Scholar] [CrossRef]
- Enoxaparin—StatPearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539865/ (accessed on 29 June 2022).
- Imberti, D.; Marietta, M.; Polo Friz, H.; Cimminiello, C. The Introduction of Biosimilars of Low Molecular Weight Heparins in Europe: A Critical Review and Reappraisal Endorsed by the Italian Society for Haemostasis and Thrombosis (SISET) and the Italian Society for Angiology and Vascular Medicine (SIAPAV). Thromb. J. 2017, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Gao, Y.; Zheng, M.; Xu, T.; Schoenhagen, P.; Jin, Z. Recent Progress and Market Analysis of Anticoagulant Drugs. J. Thorac. Dis. 2018, 10, 2011–2025. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.A. Andexanet Alfa: First Global Approval. Drugs 2018, 78, 1049. [Google Scholar] [CrossRef]
- Burness, C.B. Idarucizumab: First Global Approval. Drugs 2015, 75, 2155–2161. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Greinacher, A.; Koster, A. Bivalirudin. Thromb. Haemost. 2008, 99, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Stecker, E.; Warden, B.A. Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges. J. Am. Heart Assoc. 2020, 9, 17559. [Google Scholar] [CrossRef]
- McRae, H.L.; Militello, L.; Refaai, M.A. Updates in Anticoagulation Therapy Monitoring. Biomedicines 2021, 9, 262. [Google Scholar] [CrossRef]
- Schulman, S. Advantages and Limitations of the New Anticoagulants. J. Intern. Med. 2014, 275, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zirlik, A.; Bode, C. Vitamin K Antagonists: Relative Strengths and Weaknesses vs. Direct Oral Anticoagulants for Stroke Prevention in Patients with Atrial Fibrillation. J. Thromb. Thrombolysis 2017, 43, 365. [Google Scholar] [CrossRef]
- Fernández, C.S.; Gullón, A.; Formiga, F. The Problem of Underdosing with Direct-Acting Oral Anticoagulants in Elderly Patients with Nonvalvular Atrial Fibrillation. J. Comp. Eff. Res. 2020, 9, 509–523. [Google Scholar] [CrossRef]
- MHRA DOAC Safety Reminder. Drug Ther. Bull. 2020, 58, 150. [CrossRef] [PubMed]
- Kustos, S.A.; Fasinu, P.S. Direct-Acting Oral Anticoagulants and Their Reversal Agents—An Update. Medicines 2019, 6, 103. [Google Scholar] [CrossRef] [PubMed]
- Joel, M.; Gore, M. Overdosing and Underdosing of Novel Direct-Acting Oral Anticoagulants. NEJM J. Watch 2017, 2017. [Google Scholar] [CrossRef]
- Miyazaki, M.; Matsuo, K.; Uchiyama, M.; Nakamura, Y.; Sakamoto, Y.; Misaki, M.; Tokura, K.; Jimi, S.; Okamura, K.; Adachi, S.; et al. Inappropriate Direct Oral Anticoagulant Dosing in Atrial Fibrillation Patients Is Associated with Prescriptions for Outpatients Rather than Inpatients: A Single-Center Retrospective Cohort Study. J. Pharm. Health Care Sci. 2020, 6, 2. [Google Scholar] [CrossRef]
- Shen, N.N.; Zhang, C.; Hang, Y.; Li, Z.; Kong, L.C.; Wang, N.; Wang, J.L.; Gu, Z.C. Real-World Prevalence of Direct Oral Anticoagulant Off-Label Doses in Atrial Fibrillation: An Epidemiological Meta-Analysis. Front. Pharmacol. 2021, 12, 1212. [Google Scholar] [CrossRef]
- Pereira, M.Q.; David, C.; Almeida, A.G.; Brito, D.; Pinto, F.J.; Caldeira, D. Clinical Effects of Off-Label Reduced Doses of Direct Oral Anticoagulants: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2022, 362, 76–82. [Google Scholar] [CrossRef]
- Santos, J.; António, N.; Rocha, M.; Fortuna, A. Impact of Direct Oral Anticoagulant Off-label Doses on Clinical Outcomes of Atrial Fibrillation Patients: A Systematic Review. Br. J. Clin. Pharmacol. 2020, 86, 533. [Google Scholar] [CrossRef]
- Ashraf, H.; Agasthi, P.; Shanbhag, A.; Mehta, R.A.; Rattanawong, P.; Allam, M.; Pujari, S.H.; Mookadam, F.; Freeman, W.K.; Srivathsan, K.; et al. Long-Term Clinical Outcomes of Underdosed Direct Oral Anticoagulants in Patients with Atrial Fibrillation and Atrial Flutter. Am. J. Med. 2021, 134, 788–796. [Google Scholar] [CrossRef]
- Kirchhof, P.; Radaideh, G.; Kim, Y.H.; Lanas, F.; Haas, S.; Amarenco, P.; Turpie, A.G.G.; Bach, M.; Lambelet, M.; Hess, S.; et al. Global Prospective Safety Analysis of Rivaroxaban. J. Am. Coll. Cardiol. 2018, 72, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.W.; Renner, E.; Mouland, E.; Barnes, G.D.; Kuo, L.; Ha, N.B. Clinical Safety Outcomes in Patients With Nonvalvular Atrial Fibrillation on Rivaroxaban and Diltiazem. Ann. Pharmacother. 2019, 53, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Shah, N.D.; Sangaralingham, L.R.; Gersh, B.J.; Noseworthy, P.A. Non-Vitamin K Antagonist Oral Anticoagulant Dosing in Patients with Atrial Fibrillation and Renal Dysfunction. J. Am. Coll. Cardiol. 2017, 69, 2779–2790. [Google Scholar] [CrossRef]
- European Medicines Agency. Good Practice Guide Medication Error Recording Coding Reporting Assessment. Available online: www.ema.europa.eu/contact (accessed on 2 January 2023).
- European Medicines Agency. Good Practice Guide Medication Error Risk Minimisation and Prevention. Available online: www.ema.europa.eu/contact (accessed on 2 January 2023).
- Medication Errors|European Medicines Agency. Available online: https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/medication-errors (accessed on 2 January 2023).
- Pozsgai, K.; Szűcs, G.; Kőnig-Péter, A.; Balázs, O.; Vajda, P.; Botz, L.; Vida, R.G. Analysis of Pharmacovigilance Databases for Spontaneous Reports of Adverse Drug Reactions Related to Substandard and Falsified Medical Products: A Descriptive Study. Front. Pharmacol. 2022, 13, 964399. [Google Scholar] [CrossRef]
- Postigo, R.; Brosch, S.; Slattery, J.; van Haren, A.; Dogné, J.M.; Kurz, X.; Candore, G.; Domergue, F.; Arlett, P. EudraVigilance Medicines Safety Database: Publicly Accessible Data for Research and Public Health Protection. Drug Saf. 2018, 41, 665. [Google Scholar] [CrossRef]
- EudraVigilance|European Medicines Agency. Available online: https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance (accessed on 16 February 2023).
- Dexxience—European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/dexxience (accessed on 1 February 2023).
- Camm, A.J.; Cools, F.; Virdone, S.; Bassand, J.P.; Fitzmaurice, D.A.; Arthur Fox, K.A.; Goldhaber, S.Z.; Goto, S.; Haas, S.; Mantovani, L.G.; et al. Mortality in Patients with Atrial Fibrillation Receiving Nonrecommended Doses of Direct Oral Anticoagulants. J. Am. Coll. Cardiol. 2020, 76, 1425–1436. [Google Scholar] [CrossRef]
- Franchini, M.; Liumbruno, G.M.; Bonfanti, C.; Lippi, G. The Evolution of Anticoagulant Therapy. Blood Transfus. 2016, 14, 175. [Google Scholar] [CrossRef] [PubMed]
- Bielecki, S.; Lee, D.; Hamad, B. The Market for Oral Anticoagulants. Nat. Rev. Drug Discov. 2018, 17, 617–618. [Google Scholar] [CrossRef]
- Bayer Capital Markets Day 2021. Available online: https://www.bayer.com/sites/default/files/BayerCMD2021_CEO_Transcript.pdf (accessed on 2 March 2023).
- Top Selling Pharmaceutical Products Sales Forecast Europe 2022|Statista. Available online: https://www.statista.com/statistics/815185/top-selling-pharmaceutical-products-europe-forecast-sales-trade-name/ (accessed on 2 March 2023).
- García Rodríguez, L.A.; Cea Soriano, L.; de Abajo, F.J.; Valent, F.; Hallas, J.; Gil, M.; Cattaruzzi, C.; Rodriguez-Martin, S.; Vora, P.; Soriano-Gabarró, M.; et al. Trends in the Use of Oral Anticoagulants, Antiplatelets and Statins in Four European Countries: A Population-Based Study. Eur. J. Clin. Pharmacol. 2022, 78, 497–504. [Google Scholar] [CrossRef]
- Harskamp, R.E.; Himmelreich, J.C.L.; Wong, G.W.M.; Teichert, M. Prescription Patterns of Direct Oral Anticoagulants and Concomitant Use of Interacting Medications in the Netherlands. Neth. Heart J. 2021, 29, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Vora, P.; Morgan Stewart, H.; Russell, B.; Asiimwe, A.; Brobert, G. Time Trends and Treatment Pathways in Prescribing Individual Oral Anticoagulants in Patients with Nonvalvular Atrial Fibrillation: An Observational Study of More than Three Million Patients from Europe and the United States. Int. J. Clin. Pract. 2022, 2022, 6707985. [Google Scholar] [CrossRef] [PubMed]
- Joy, M.; Williams, J.; Emanuel, S.; Kar, D.; Fan, X.; Delanerolle, G.; Field, B.C.T.; Heiss, C.; Pollock, K.G.; Sandler, B.; et al. Trends in Direct Oral Anticoagulant (DOAC) Prescribing in English Primary Care (2014–2019). Heart 2022, 109, 195–201. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report. Procedure under Article 5(3) of Regulation (EC) No 726/2004 INN/Active Substance: Dire+ct Oral Anticoagulants (DOACs) Procedure Number: EMEA/H/A-5(3)/1487. Available online: https://www.ema.europa.eu/en/documents/referral/assessment-report-article-53-procedure-direct-oral-anticoagulants-doacs_en.pdf (accessed on 20 February 2023).
- Raccah, B.H.; Erlichman, Y.; Pollak, A.; Matok, I.; Muszkat, M. Prescribing Errors with Direct Oral Anticoagulants and Their Impact on the Risk of Bleeding in Patients with Atrial Fibrillation. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 601. [Google Scholar] [CrossRef]
- Brook, R.; Aswapanyawongse, O.; Tacey, M.; Kitipornchai, T.; Ho, P.; Lim, H.Y. Real-World Direct Oral Anticoagulant Experience in Atrial Fibrillation: Falls Risk and Low Dose Anticoagulation Are Predictive of Both Bleeding and Stroke Risk. Intern. Med. J. 2020, 50, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Arantes, F.B.B.; Furtado, R.H.M. The Great Discovery of DOACs and Why Physicians Insist on Misusing It: A Paradox of the 21 Century. Int. J. Cardiol. 2022, 364, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Moudallel, S.; Cornu, P.; Dupont, A.; Steurbaut, S. Determinants for Under- and Overdosing of Direct Oral Anticoagulants and Physicians’ Implementation of Clinical Pharmacists’ Recommendations. Br. J. Clin. Pharmacol. 2022, 88, 753–763. [Google Scholar] [CrossRef]
- Pirozzi, E.J.; Wills, B.K. Antiplatelet Drug Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Anchidin, O.-I.; Rosianu, S.H.; Nemes, A.; Aldica, M.; Blendea, D.; Molnar, A.; Moldovan, H.; Pop, D. The Effectiveness of Antiplatelet Therapy and the Factors Influencing It in Patients with Acute Coronary Syndrome before and during the COVID-19 Pandemic. Medicina 2022, 59, 84. [Google Scholar] [CrossRef]
- Rymer, J.A.; Webb, L.; McCall, D.; Hills, M.T.; Wang, T.Y. Differences in Preferences between Clinicians and Patients for the Use and Dosing of Direct Oral Anticoagulants for Atrial Fibrillation. J. Am. Heart Assoc. 2021, 10, 20697. [Google Scholar] [CrossRef]
- Ogawa, H.; Hokimoto, S.; Kaikita, K.; Yamamoto, K.; Chitose, T.; Ono, T.; Tsujita, K. Current Status and Prospects of Antiplatelet Therapy in Percutaneous Coronary Intervention in Japan: Focus on Adenosine Diphosphate Receptor Inhibitors. J. Cardiol. 2011, 58, 6–17. [Google Scholar] [CrossRef]
- Cosma, S.A.; Bota, M.; Fleşeriu, C.; Morgovan, C.; Văleanu, M.; Cosma, D. Measuring Patients’ Perception and Satisfaction with the Romanian Healthcare System. Sustainability 2020, 12, 1612. [Google Scholar] [CrossRef]
- Capiau, A.; Mehuys, E.; Dhondt, E.; De Backer, T.; Boussery, K. Physicians’ and Pharmacists’ Views and Experiences Regarding Use of Direct Oral Anticoagulants in Clinical Practice. Br. J. Clin. Pharmacol. 2022, 88, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Patel, P.; Mbusa, D.; Kapoor, A.; Crawford, S.; Sadiq, H.; Rampam, S.; Wagner, J.; Gurwitz, J.H.; Mazor, K.M. Impact of a Pharmacist Intervention on DOAC Knowledge and Satisfaction in Ambulatory Patients. J. Thromb. Thrombolysis 2022, 55, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Pop, G.; Farcaș, A.; Butucă, A.; Morgovan, C.; Arseniu, A.M.; Pumnea, M.; Teodoru, M.; Gligor, F.G. Post-Marketing Surveillance of Statins - A Descriptive Analysis of Psychiatric Adverse Reactions in EudraVigilance. Pharmaceuticals 2022, 15, 1536. [Google Scholar] [CrossRef]
- European Medicines Agency. Screening for Adverse Reactions in EudraVigilance. Available online: www.ema.europa.eu/contact (accessed on 2 January 2023).
- Grundmark, B.; Holmberg, L.; Garmo, H.; Zethelius, B. Reducing the Noise in Signal Detection of Adverse Drug Reactions by Standardizing the Background: A Pilot Study on Analyses of Proportional Reporting Ratios-by-Therapeutic Area. Eur. J. Clin. Pharmacol. 2014, 70, 627–635. [Google Scholar] [CrossRef]



| Drug | Total ADRs | % of Total |
|---|---|---|
| Dabigatran | 57,627 | 13.49% |
| Rivaroxaban | 131,182 | 30.70% |
| Apixaban | 70,424 | 16.48% |
| Edoxaban | 9318 | 2.18% |
| Other ACs | 158,767 | 37.15% |
| 427,318 |
| Improper Dose Errors—Total ADRs | Overdosing Errors—Total ADRs | Underdosing Errors—Total ADRs | Fatal Dosing Errors— Total ADRs | NR/NS Dosing Errors—Total ADRs |
|---|---|---|---|---|
| 0.8193 | 0.7801 | 0.7723 | 0.7652 | −0.6455 |
| 0.1807 | 0.2199 | 0.2277 | 0.2348 | 0.355 |
| Category | ADR | PT |
|---|---|---|
| a | Improper dose | Dose calculation error |
| Dose calculation error associated with device | ||
| Drug dose titration not performed | ||
| Drug titration error | ||
| Incorrect dosage administered | ||
| Incorrect dose administered | ||
| Incorrect dose administered by device | ||
| Incorrect dose administered by product | ||
| Incorrect product dosage form administered | ||
| Product dosage form confusion | ||
| Wrong dosage formulation | ||
| Wrong dose | ||
| b | Overdose | Accidental overdose |
| Extra dose administered | ||
| Overdose | ||
| Prescribed overdose | ||
| c | Underdose | Accidental underdose |
| Drug dose omission by device | ||
| Incomplete dose administered | ||
| Prescribed underdose | ||
| Product dose omission | ||
| Product dose omission in error | ||
| Product dose omission issue | ||
| Underdose |
| Drug Category | Drug | Pharmacologic Class |
|---|---|---|
| Anticoagulant drugs | Heparin | Unfractionated heparin |
| Dalteparin | Low molecular weight heparin | |
| Enoxaparin | Low molecular weight heparin | |
| Nadroprin | Low molecular weight heparin | |
| Tinzaparin | Low molecular weight heparin | |
| Reviparin | Low molecular weight heparin | |
| Parnaparin | Low molecular weight heparin | |
| Certoparin | Low molecular weight heparin | |
| Bemiparin | Low molecular weight heparin | |
| Semuloparin | Low molecular weight heparin | |
| Deligoparin | Low molecular weight heparin | |
| Fondaparinux | Parenteral direct factor Xa inhibitor | |
| Danaparoid | Heparinoid | |
| Pentosan polysulphate | Heparinoid | |
| Argatroban | Parenteral direct thrombin inhibitor | |
| Desirudin | Parenteral direct thrombin inhibitor | |
| Lepirudin | Parenteral direct thrombin inhibitor | |
| Bivalirudin | Parenteral direct thrombin inhibitor | |
| Warfarin | VKA | |
| Acenocoumarol | VKA | |
| Dicumarol | VKA | |
| Phenindione | VKA | |
| Anisindione | VKA | |
| Fluindione | VKA | |
| Antiplatelet drugs | Clopidogrel | Platelet aggregation inhibitors |
| Ticagrelor | Platelet aggregation inhibitors | |
| Prasugrel | Platelet aggregation inhibitors | |
| Ticlopidine | Platelet aggregation inhibitors | |
| Cilostazol | Phosphodiesterase type 3 inhibitor | |
| Triflusal | Platelet aggregation inhibitors | |
| Abciximab | Glycoprotein platelet inhibitors | |
| Eptifibatide | Glycoprotein platelet inhibitors | |
| Tirofiban | Glycoprotein platelet inhibitors | |
| Vorapaxar | Protease-activated receptor-1 antagonists | |
| Dipyridamole | Nucleoside transport and phosphodiesterase type 3 inhibitor | |
| Dipyridamole and Acetylsalicylic acid | Combination | |
| Acetylsalicylic acid and Clopidogrel | Combination |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgovan, C.; Dobrea, C.M.; Chis, A.A.; Juncan, A.M.; Arseniu, A.M.; Rus, L.L.; Gligor, F.G.; Ardelean, S.A.; Stoicescu, L.; Ghibu, S.; et al. A Descriptive Analysis of Direct Oral Anticoagulant Drugs Dosing Errors Based on Spontaneous Reports from the EudraVigilance Database. Pharmaceuticals 2023, 16, 455. https://doi.org/10.3390/ph16030455
Morgovan C, Dobrea CM, Chis AA, Juncan AM, Arseniu AM, Rus LL, Gligor FG, Ardelean SA, Stoicescu L, Ghibu S, et al. A Descriptive Analysis of Direct Oral Anticoagulant Drugs Dosing Errors Based on Spontaneous Reports from the EudraVigilance Database. Pharmaceuticals. 2023; 16(3):455. https://doi.org/10.3390/ph16030455
Chicago/Turabian StyleMorgovan, Claudiu, Carmen Maximiliana Dobrea, Adriana Aurelia Chis, Anca Maria Juncan, Anca Maria Arseniu, Luca Liviu Rus, Felicia Gabriela Gligor, Simona Alexandrina Ardelean, Laurentiu Stoicescu, Steliana Ghibu, and et al. 2023. "A Descriptive Analysis of Direct Oral Anticoagulant Drugs Dosing Errors Based on Spontaneous Reports from the EudraVigilance Database" Pharmaceuticals 16, no. 3: 455. https://doi.org/10.3390/ph16030455
APA StyleMorgovan, C., Dobrea, C. M., Chis, A. A., Juncan, A. M., Arseniu, A. M., Rus, L. L., Gligor, F. G., Ardelean, S. A., Stoicescu, L., Ghibu, S., & Frum, A. (2023). A Descriptive Analysis of Direct Oral Anticoagulant Drugs Dosing Errors Based on Spontaneous Reports from the EudraVigilance Database. Pharmaceuticals, 16(3), 455. https://doi.org/10.3390/ph16030455






