The In Vitro Anti-Parasitic Activities of Emodin toward Toxoplasma gondii
Abstract
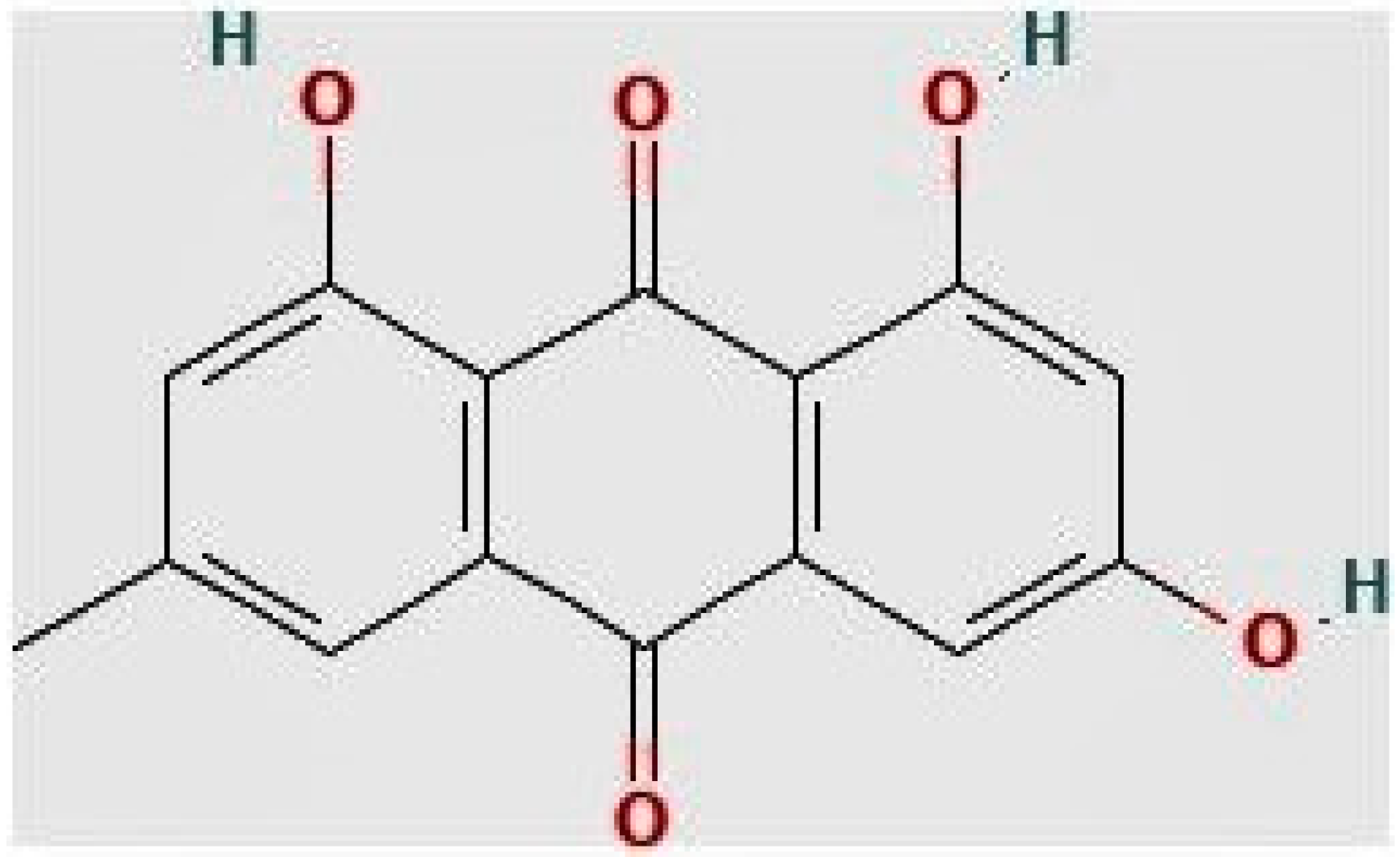
1. Introduction
2. Results
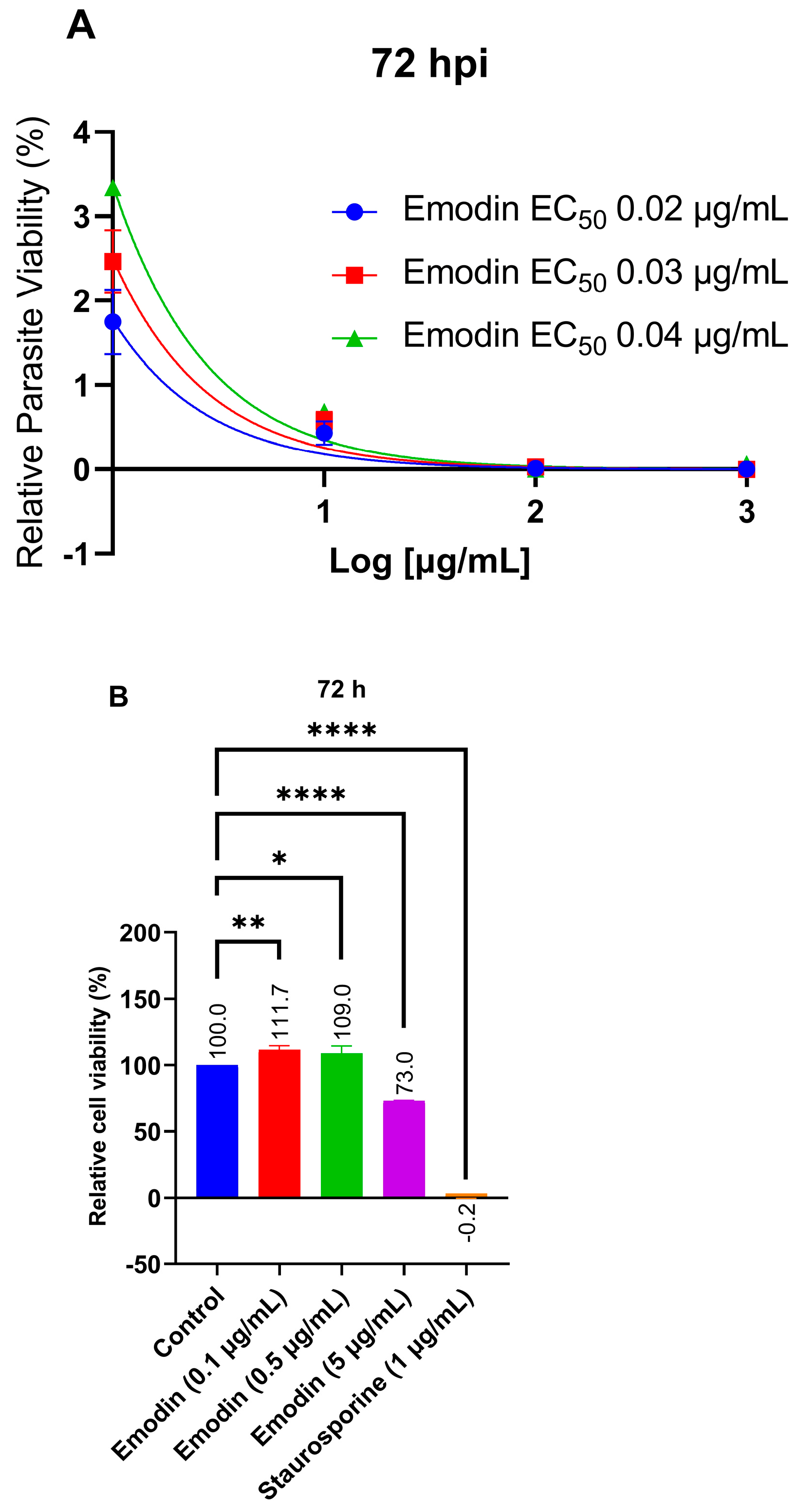
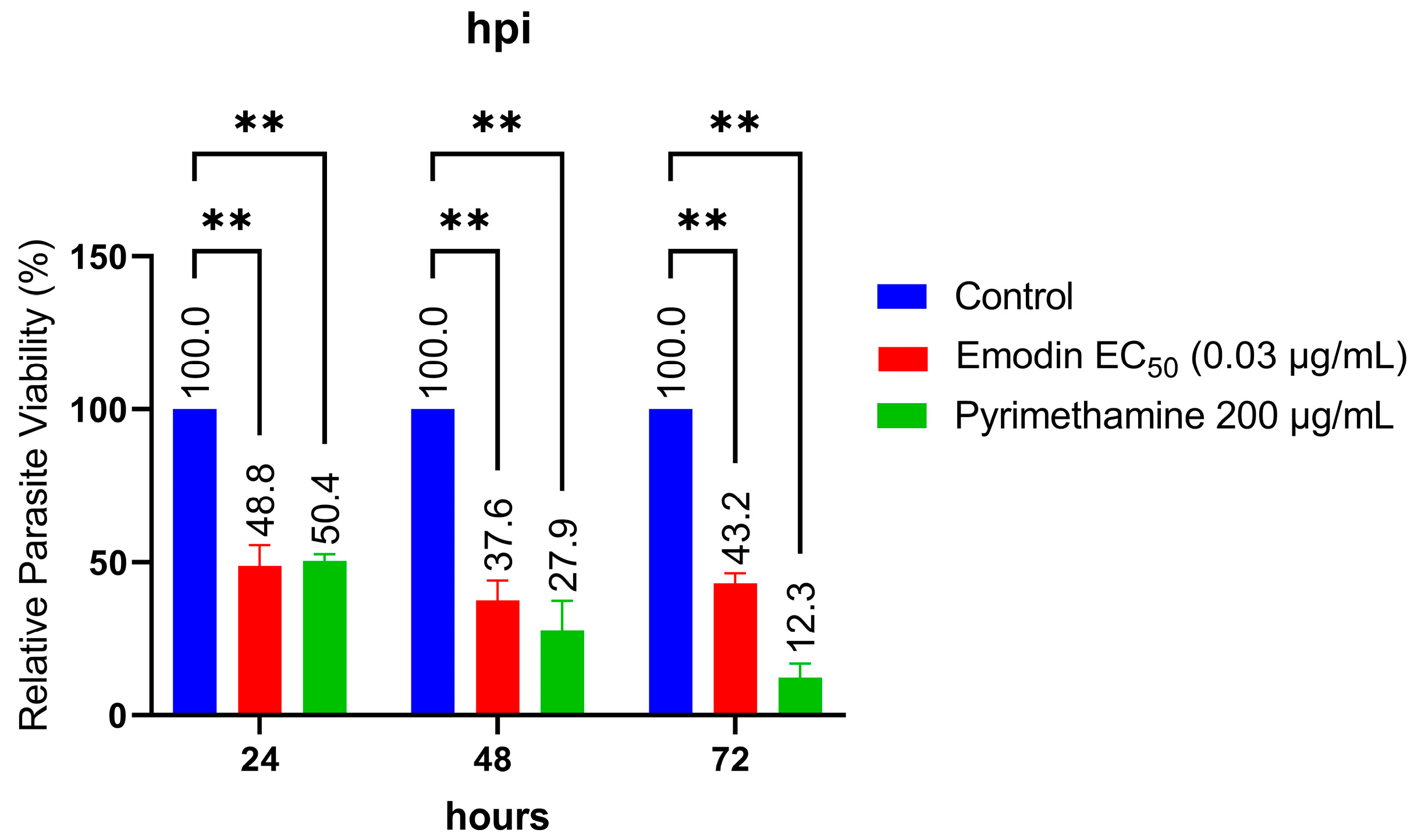
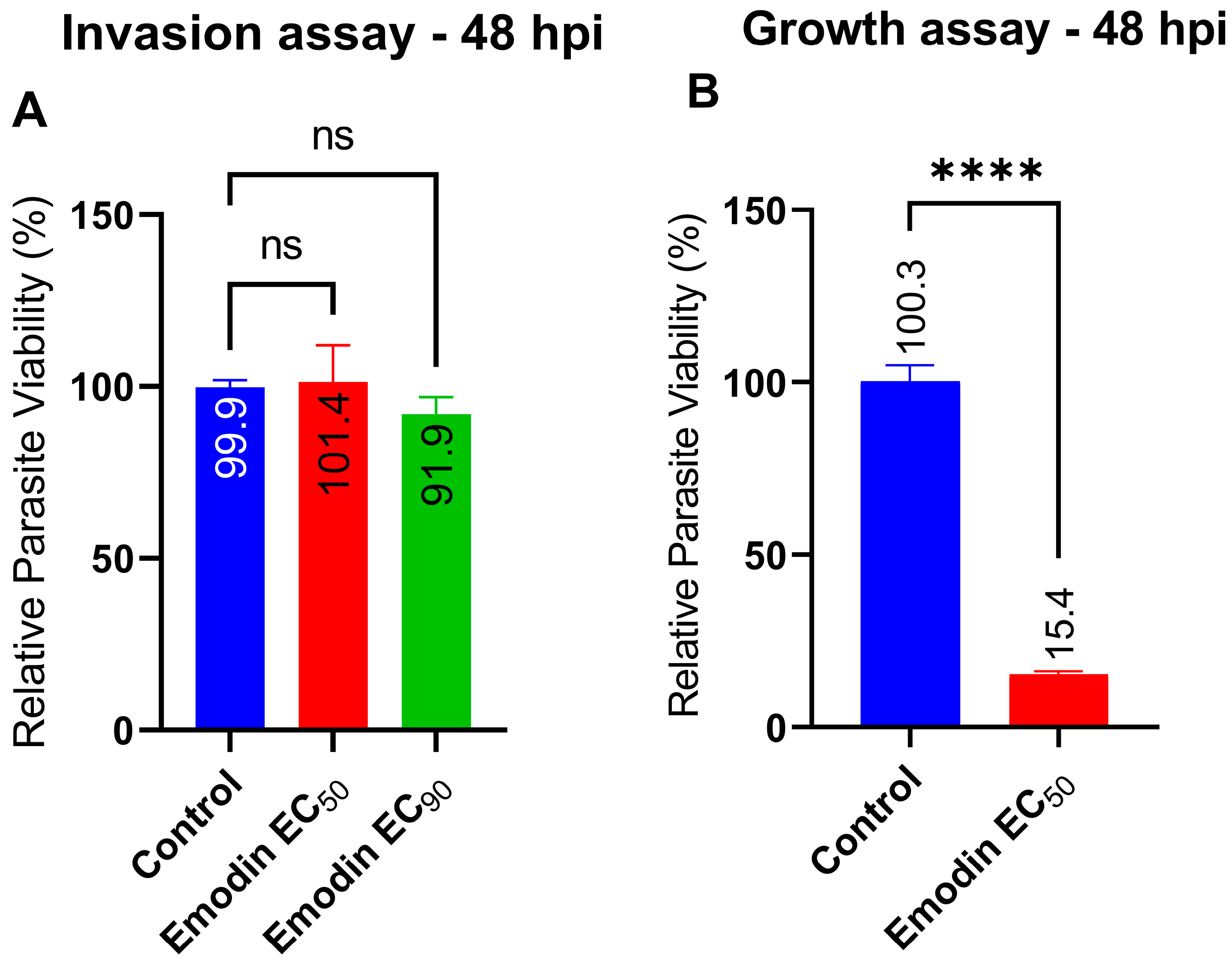
2.1. Emodin Significantly Restricts Parasite Growth
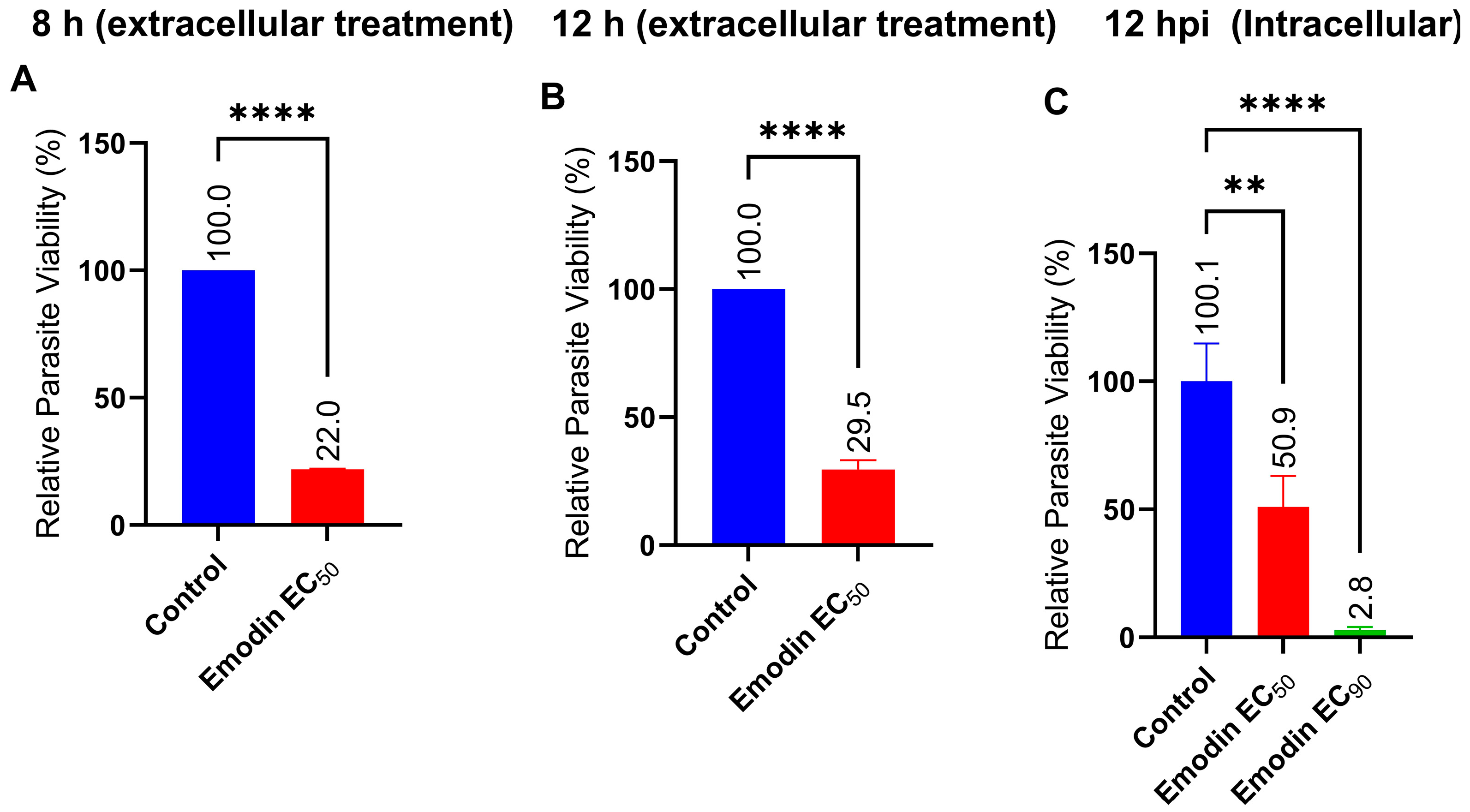
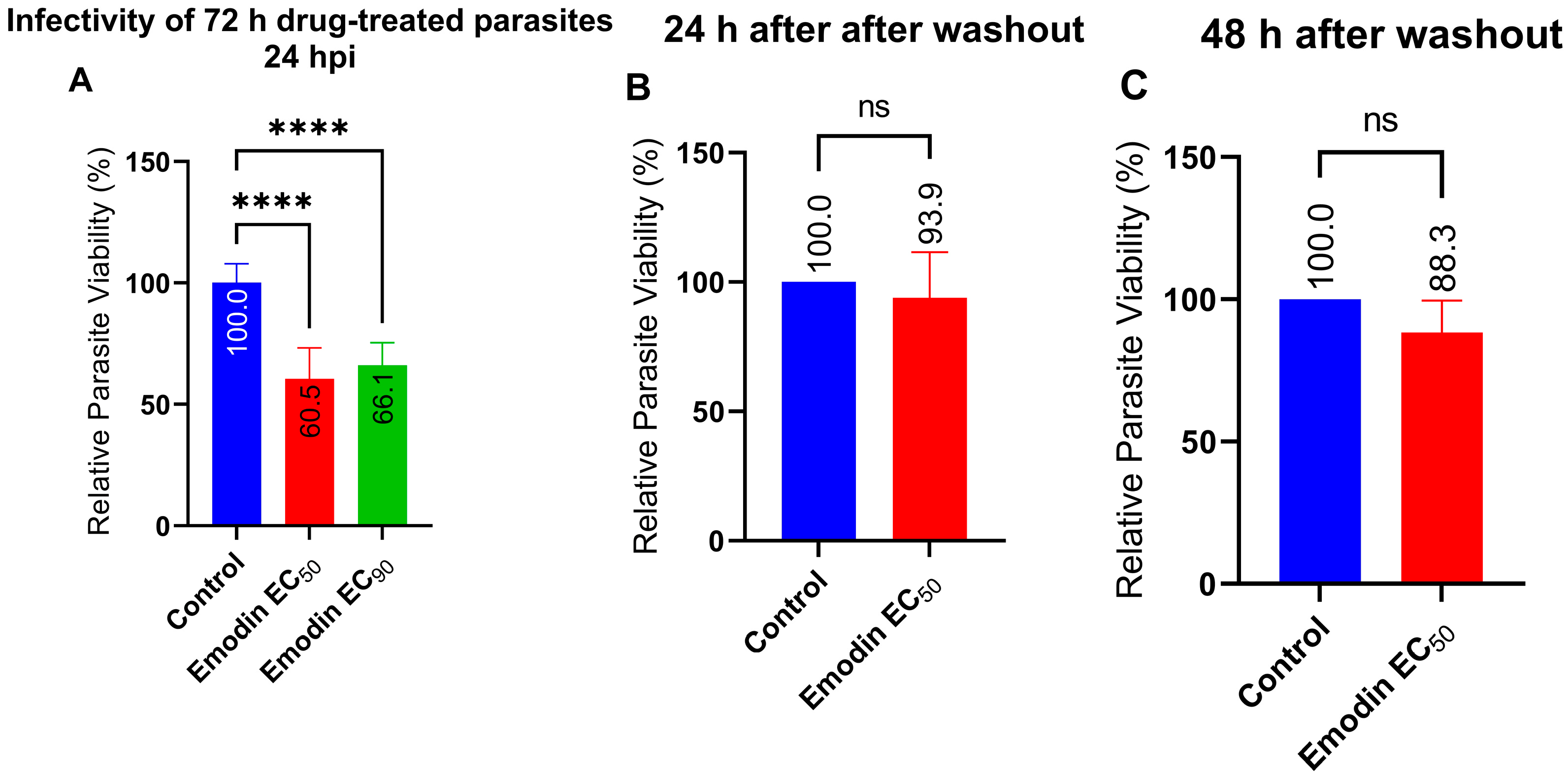
2.2. Emodin Mildly Affects Parasite Infectivity but Has no Detectable Effect on Recovery
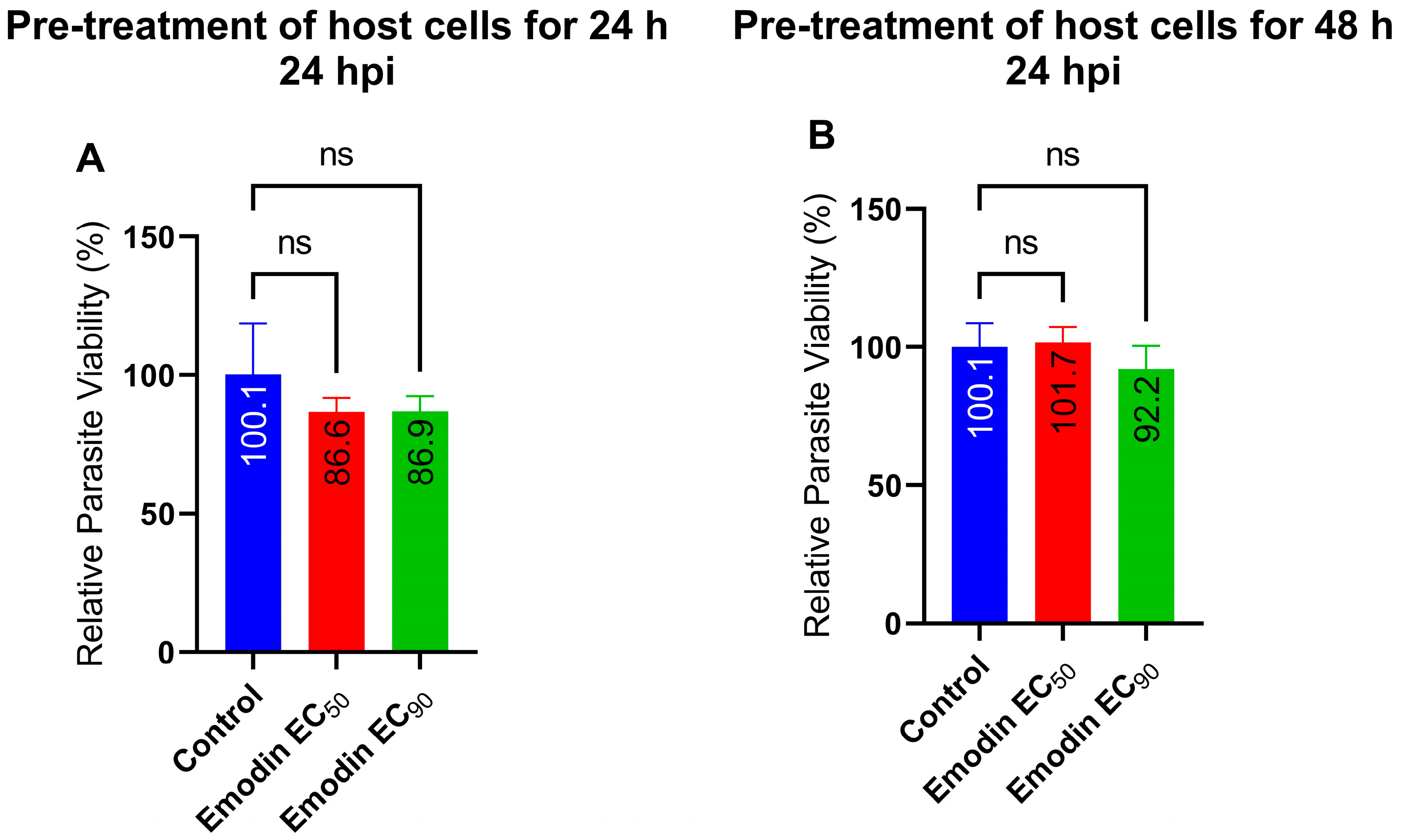
2.3. Parasite Growth Is Unaffected following Pre-Treatment of Host Cells with Emodin
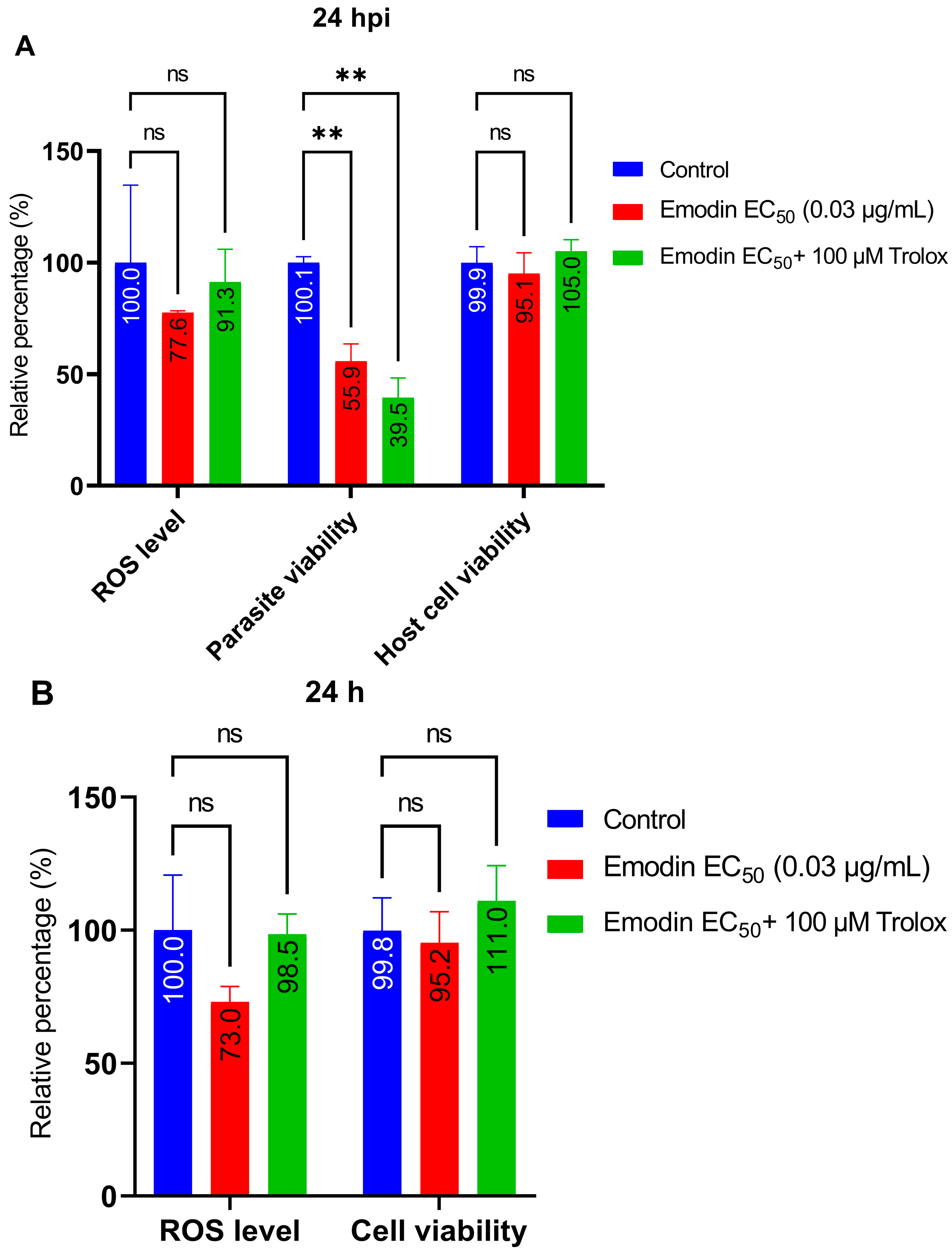
2.4. Trolox and L-Tryptophan Do Not Relieve Emodin-Induced Parasite Growth Suppression
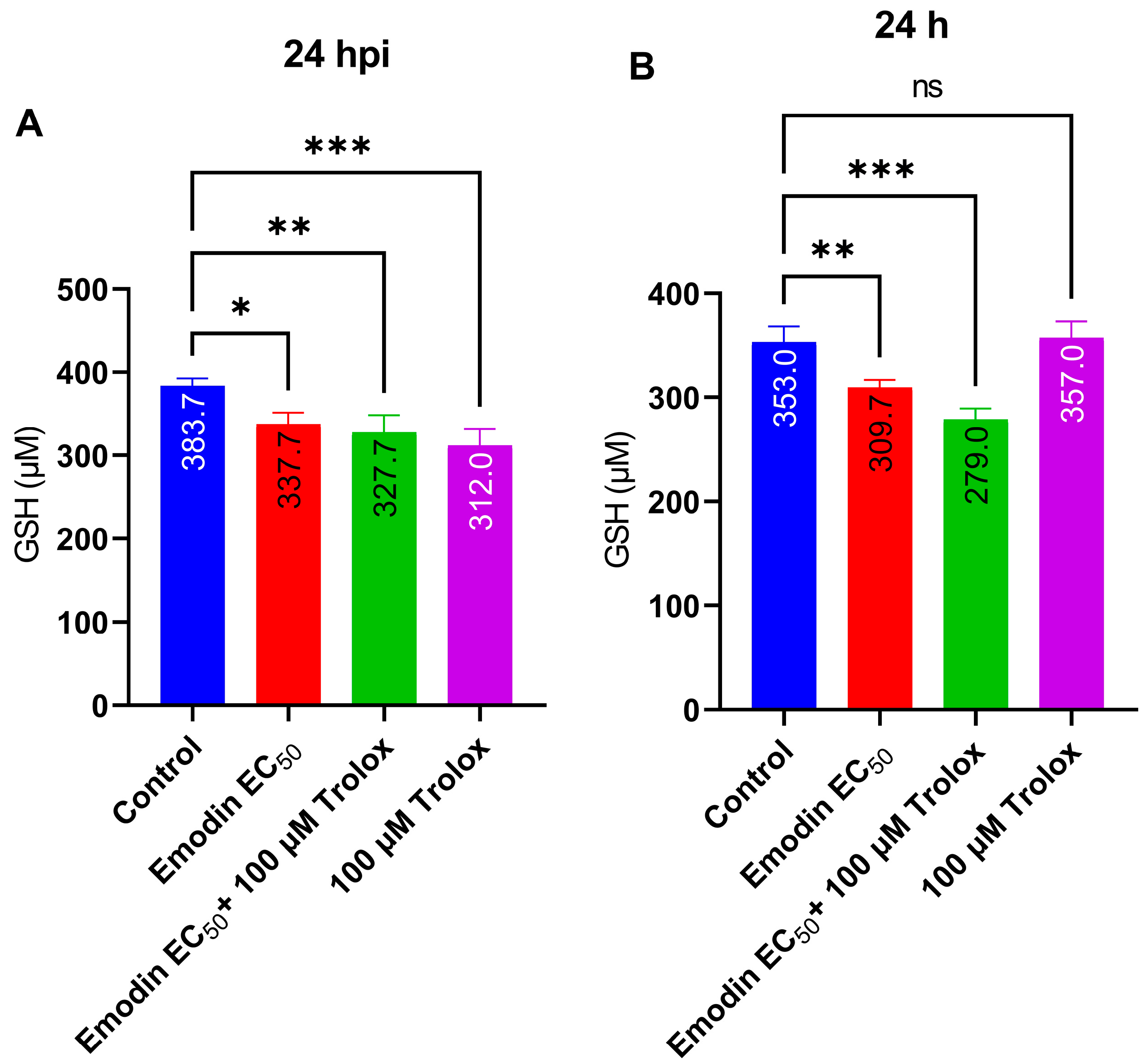
2.5. Emodin Does Not Modify Redox Balance in the Absence or Presence of Toxoplasma Infection
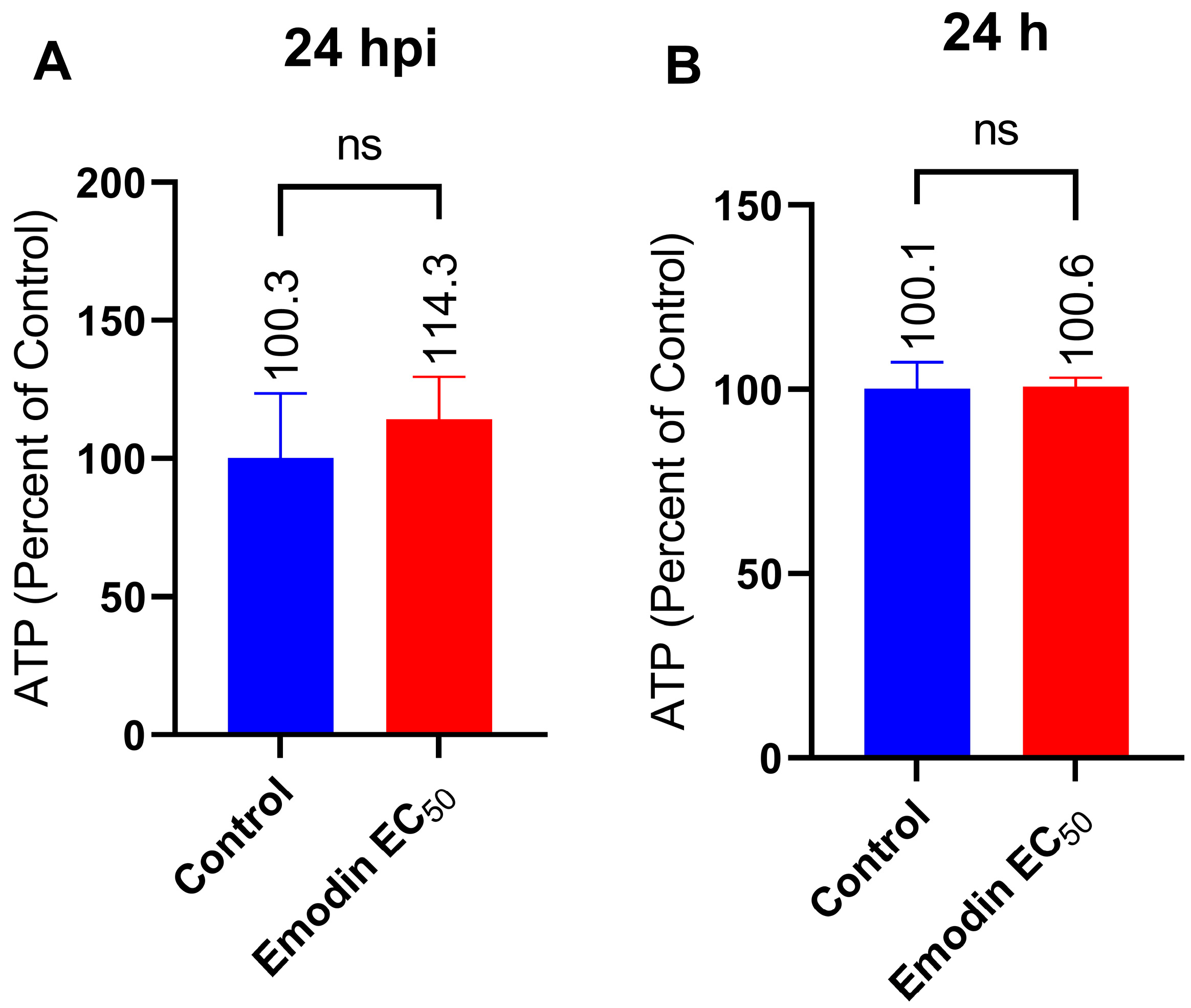
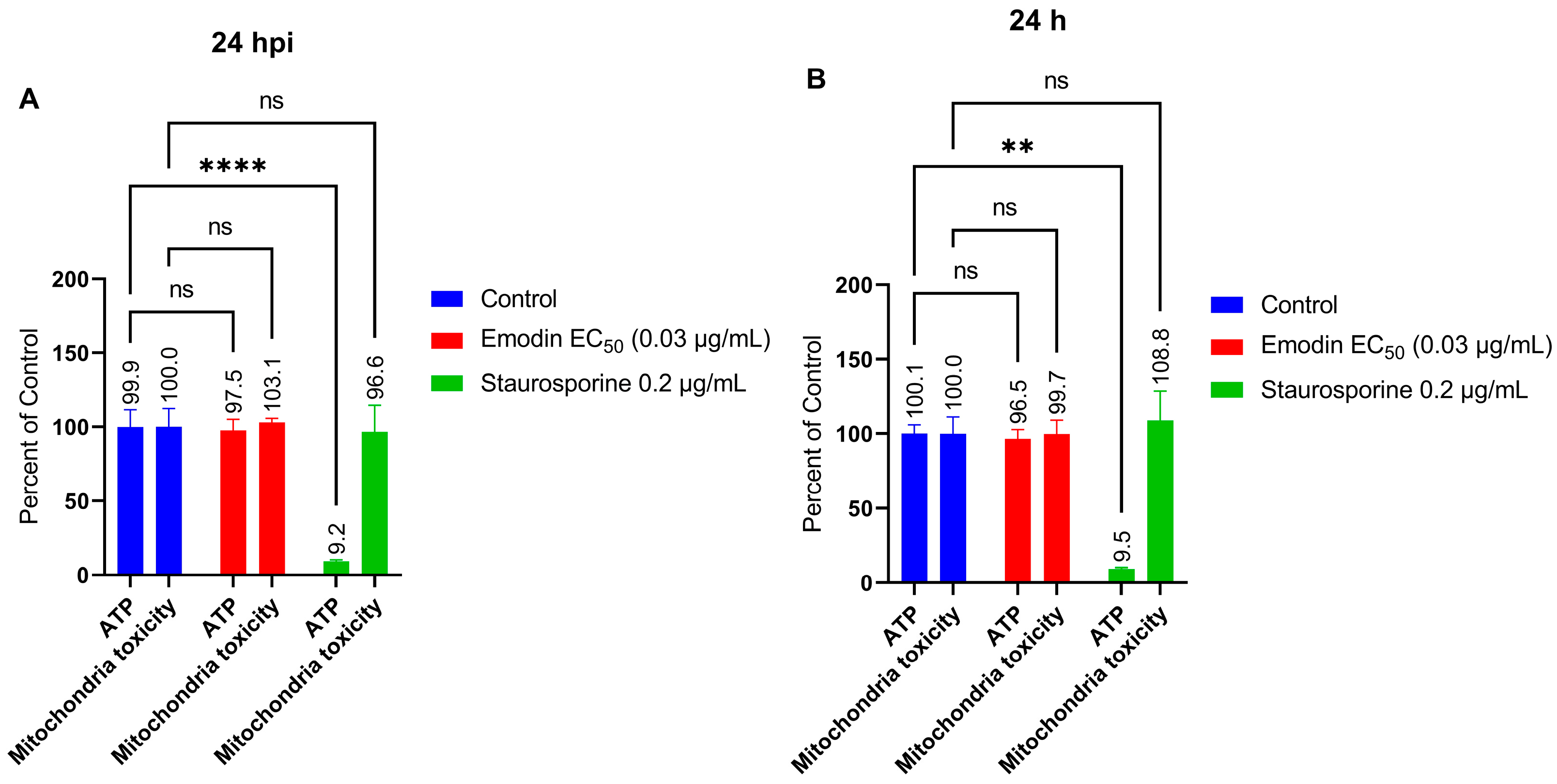
2.6. Mitochondria Membrane Integrity and ATP Production in the Absence or Presence of Toxoplasma Infection Are Not Affected by Emodin
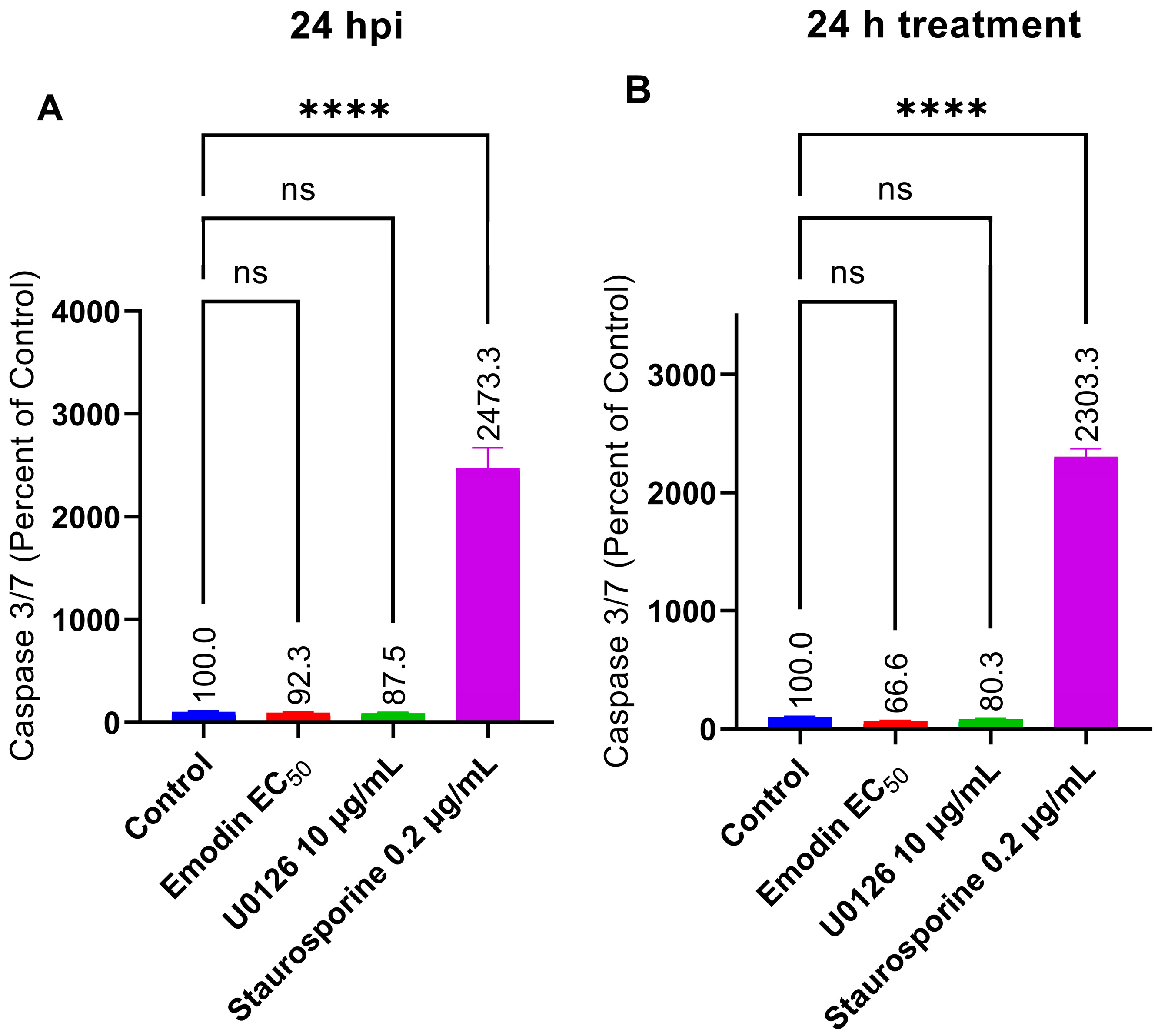
2.7. Caspase 3/7 Activity Is Not Affected by Emodin in the Absence or Presence of Toxoplasma Infection
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Evaluation of Emodin for T. gondii Growth Suppressive Activity In Vitro
4.3. Assays for Parasite Invasion and Growth Inhibition
4.4. Cytotoxicity Assay to Assess Host Cell Viability
4.5. Reversibility Assay
4.6. Assays for Infectivity and Likely Host Cellular Target
4.7. Measurement of Redox Balance
4.8. Measurement of Mitochondrial Toxicity
4.9. Measurement of Caspase 3/7 Activity
4.10. Statistical Analysis and Data Presentation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murata, Y.; Sugi, T.; Weiss, L.M.; Kato, K. Identification of compounds that suppress Toxoplasma gondii tachyzoites and bradyzoites. PLoS ONE 2017, 12, e0178203. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Atolani, O.; Awakan, O.J.; Olaolu, T.D.; Nwonuma, C.O.; Alejolowo, O.; Otohinoyi, D.A.; Rotimi, D.; Owolabi, A.; Batiha, G.E.-S. Focus: Organelles: In vitro screening to identify anti-Toxoplasma compounds and in silico modeling for bioactivities and toxicity. Yale J. Biol. Med. 2019, 92, 369. [Google Scholar] [PubMed]
- Attias, M.; Teixeira, D.E.; Benchimol, M.; Vommaro, R.C.; Crepaldi, P.H.; De Souza, W. The life-cycle of Toxoplasma gondii reviewed using animations. Parasites Vectors 2020, 13, 588. [Google Scholar] [CrossRef]
- Schlüter, D.; Däubener, W.; Schares, G.; Groß, U.; Pleyer, U.; Lüder, C. Animals are key to human toxoplasmosis. Int. J. Med. Microbiol. 2014, 304, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Zhang, N.Z.; Yao, Y.; Wang, T.; Hua, Q.; Zheng, X.; Cong, W.; Tan, F. Investigation of Antiparasitic Activity of Two Marine Natural Products, Estradiol Benzoate, and Octyl Gallate, on Toxoplasma gondii In Vitro. Front. Pharmacol. 2022, 13, 841941. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.S.; Sugi, T.; Han, Y.; Kato, K. Screening of chemical compound libraries identified new anti-Toxoplasma gondii agents. Parasitol. Res. 2018, 117, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Dittmar, A.J.; Drozda, A.A.; Blader, I.J. Drug repurposing screening identifies novel compounds that effectively inhibit Toxoplasma gondii growth. Msphere 2016, 1, e00042-15. [Google Scholar] [CrossRef]
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Huyiligeqi; Ni, J. Emodin: A review of its pharmacology, toxicity and pharmacokinetics. Phytother. Res. 2016, 30, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Xin, G.; Duan, F.; Huang, W.; Tan, T. Study on the antibacterial activities of emodin derivatives against clinical drug-resistant bacterial strains and their interaction with proteins. Ann. Transl. Med. 2020, 8, 92. [Google Scholar] [CrossRef]
- Chen, H.; Huang, R.S.; Yu, X.X.; Ye, Q.; Pan, L.L.; Shao, G.J.; Pan, J. Emodin protects against oxidative stress and apoptosis in HK-2 renal tubular epithelial cells after hypoxia/reoxygenation. Exp. Ther. Med. 2017, 14, 447–452. [Google Scholar] [CrossRef]
- Yang, L.; Lin, S.; Kang, Y.; Xiang, Y.; Xu, L.; Li, J.; Dai, X.; Liang, G.; Huang, X.; Zhao, C. Rhein sensitizes human pancreatic cancer cells to EGFR inhibitors by inhibiting STAT3 pathway. J. Exp. Clin. Cancer Res. 2019, 38, 31. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, M.; Masłyk, M.; Kubiński, K.; Golczyk, H. Emodin, a natural inhibitor of protein kinase CK2, suppresses growth, hyphal development, and biofilm formation of Candida albicans. Yeast 2017, 34, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Nam, W.; Kim, S.P.; Nam, S.H.; Friedman, M. Structure-antioxidative and anti-inflammatory activity relationships of purpurin and related anthraquinones in chemical and cell assays. Molecules 2017, 22, 265. [Google Scholar] [CrossRef] [PubMed]
- Marković, Z.; Jeremić, S.; Marković, J.D.; Pirković, M.S.; Amić, D. Influence of structural characteristics of substituents on the antioxidant activity of some anthraquinone derivatives. Comput. Theor. Chem. 2016, 1077, 25–31. [Google Scholar] [CrossRef]
- Rossi, M.; Wen, K.; Caruso, F.; Belli, S. Emodin scavenging of superoxide radical includes π–π interaction. X-ray crystal structure, hydrodynamic voltammetry and theoretical studies. Antioxidants 2020, 9, 194. [Google Scholar] [CrossRef]
- Dalimi, A.; Delavari, M.; Ghaffarifar, F.; Sadraei, J. In vitro and in vivo antileishmanial effects of aloe-emodin on Leishmania major. J. Tradit. Complement. Med. 2015, 5, 96–99. [Google Scholar] [CrossRef]
- Friedman, M.; Xu, A.; Lee, R.; Nguyen, D.N.; Phan, T.A.; Hamada, S.M.; Panchel, R.; Tam, C.C.; Kim, J.H.; Cheng, L.W. The inhibitory activity of anthraquinones against pathogenic protozoa, bacteria, and fungi and the relationship to structure. Molecules 2020, 25, 3101. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Jung, C.-W.; Lee, W.S.; Jeong, H.-J.; Park, M.-J.; Jang, W.I.; Kim, E.H. Emodin coupled with high LET neutron beam—A novel approach to treat on glioblastoma. J. Radiat. Res. 2022, 63, 817–827. [Google Scholar] [CrossRef]
- Li, X.; Chu, S.; Liu, Y.; Chen, N. Neuroprotective effects of anthraquinones from rhubarb in central nervous system diseases. Evid.-Based Complement. Altern. Med. 2019, 2019, 3790728. [Google Scholar] [CrossRef]
- Meier, N.; Meier, B.; Peter, S.; Wolfram, E. In-silico UHPLC method optimization for aglycones in the herbal laxatives Aloe barbadensis Mill., Cassia angustifolia vahl pods, Rhamnus frangula L. Bark, Rhamnus purshianus DC. bark, and Rheum palmatum L. roots. Molecules 2017, 22, 1838. [Google Scholar] [CrossRef]
- Ponder, K.G.; Boise, L.H. The prodomain of caspase-3 regulates its own removal and caspase activation. Cell Death Discov. 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Cartuche, L.; Sifaoui, I.; Cruz, D.; Reyes-Batlle, M.; López-Arencibia, A.; Javier Fernández, J.; Díaz-Marrero, A.R.; Piñero, J.E.; Lorenzo-Morales, J. Staurosporine from Streptomyces sanyensis activates programmed cell death in Acanthamoeba via the mitochondrial pathway and presents low in vitro cytotoxicity levels in a macrophage cell line. Sci. Rep. 2019, 9, 11651. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Niu, Y.; Zhang, J.; Huang, S.; Ding, P.; Sun, F.; Wang, X. U0126: Not only a MAPK kinase inhibitor. Front. Pharmacol. 2022, 13, 927083. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.S.; Murata, Y.; Sugi, T.; Kato, K. Inorganic nanoparticles kill Toxoplasma gondii via changes in redox status and mitochondrial membrane potential. Int. J. Nanomed. 2017, 12, 1647. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Murata, Y.; Sugi, T.; Han, Y.; Kato, K. Modulation of host HIF-1α activity and the tryptophan pathway contributes to the anti-Toxoplasma gondii potential of nanoparticles. Biochem. Biophys. Rep. 2017, 11, 84–92. [Google Scholar] [CrossRef]
- Ahmad, A.; Syed, F.; Shah, A.; Khan, Z.; Tahir, K.; Khan, A.U.; Yuan, Q. Silver and gold nanoparticles from Sargentodoxa cuneata: Synthesis, characterization and antileishmanial activity. RSC Adv. 2015, 5, 73793–73806. [Google Scholar] [CrossRef]
- Saini, P.; Saha, S.K.; Roy, P.; Chowdhury, P.; Babu, S.P.S. Evidence of reactive oxygen species (ROS) mediated apoptosis in Setaria cervi induced by green silver nanoparticles from Acacia auriculiformis at a very low dose. Exp. Parasitol. 2016, 160, 39–48. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Eseola, A.O.; Plass, W.; Kato, K.; Otuechere, C.A.; Awakan, O.J.; Atolani, O.; Otohinoyi, D.A.; Elebiyo, T.C.; Evbuomwan, I.O. The anti-parasite action of imidazole derivatives likely involves oxidative stress but not HIF-1α signaling. Chem.-Biol. Interact. 2021, 349, 109676. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.S.; Shittu, E.O.; Akpor, O.B.; Rotimi, D.; Batiha, G.E.-S. Silver nanoparticles restrict microbial growth by promoting oxidative stress and DNA damage. EXCLI J. 2020, 19, 492. [Google Scholar]
- Charvat, R.A.; Arrizabalaga, G. Oxidative stress generated during monensin treatment contributes to altered Toxoplasma gondii mitochondrial function. Sci. Rep. 2016, 6, 22997. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, H.; Zhou, J.; Li, F.; Wang, J.; Chen, M.; Liu, Q. Cytotoxicity, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in human non-small cell lung cancer A549 cells. Environ. Sci. Pollut. Res. 2015, 22, 5519–5530. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Ni, B.; Fu, J.; Yin, X.; You, L.; Leng, X.; Liang, X.; Ni, J. Emodin induces apoptosis in human hepatocellular carcinoma HepaRG cells via the mitochondrial caspase-dependent pathway. Oncol. Rep. 2018, 40, 1985–1993. [Google Scholar] [CrossRef]
- Zhang, F.-Y.; Li, R.-Z.; Xu, C.; Fan, X.-X.; Li, J.-X.; Meng, W.-Y.; Wang, X.-R.; Liang, T.-L.; Guan, X.-X.; Pan, H.-D. Emodin induces apoptosis and suppresses non-small-cell lung cancer growth via downregulation of sPLA2-IIa. Phytomedicine 2022, 95, 153786. [Google Scholar] [CrossRef]
- Duan, F.; Xin, G.; Niu, H.; Huang, W. Chlorinated emodin as a natural antibacterial agent against drug-resistant bacteria through dual influence on bacterial cell membranes and DNA. Sci. Rep. 2017, 7, 12721. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Song, X.; Yin, Z.; Jia, R.; Li, Z.; Zhou, X.; Zou, Y.; Li, L.; Yin, L.; Yue, G. The antibacterial activity and action mechanism of emodin from Polygonum cuspidatum against Haemophilus parasuis in vitro. Microbiol. Res. 2016, 186, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar]
- Sharma, R.; Tiku, A.; Giri, A. Pharmacological properties of emodin—Anthraquinone derivatives. J. Nat. Prod. Resour. 2017, 3, 97–101. [Google Scholar]
- Bayat, F.; Haghi, A.M.; Nateghpour, M.; Rahimi-Esboei, B.; Foroushani, A.R.; Amani, A.; Farivar, L.; Talaee, Z.S.; Faryabi, A. Cytotoxicity and Anti-Plasmodium berghei Activity of Emodin Loaded Nanoemulsion. Iran. J. Parasitol. 2022, 17, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Ishiwa, A.; Kobayashi, K.; Takemae, H.; Sugi, T.; Gong, H.; Recuenco, F.C.; Murakoshi, F.; Inomata, A.; Horimoto, T.; Kato, K. Effects of dextran sulfates on the acute infection and growth stages of Toxoplasma gondii. Parasitol. Res. 2013, 112, 4169–4176. [Google Scholar] [CrossRef]

| Treatment Group | EC50 (RH-2F Toxoplasma Tachyzoites) µg/mL | IC50 (Human Foreskin Fibroblast—HFF) µg/mL | Selectivity Index (SI)—Ratio of IC50 to EC50 |
|---|---|---|---|
| Emodin | 0.03 ± 0.01 | 8.3 ± 1.3 | 276 |
| Pyrimethamine | 24.0 ± 2.0 | 62.4 ± 6.0 | 2.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeyemi, O.S.; Ishii, K.; Kato, K. The In Vitro Anti-Parasitic Activities of Emodin toward Toxoplasma gondii. Pharmaceuticals 2023, 16, 447. https://doi.org/10.3390/ph16030447
Adeyemi OS, Ishii K, Kato K. The In Vitro Anti-Parasitic Activities of Emodin toward Toxoplasma gondii. Pharmaceuticals. 2023; 16(3):447. https://doi.org/10.3390/ph16030447
Chicago/Turabian StyleAdeyemi, Oluyomi Stephen, Kosei Ishii, and Kentaro Kato. 2023. "The In Vitro Anti-Parasitic Activities of Emodin toward Toxoplasma gondii" Pharmaceuticals 16, no. 3: 447. https://doi.org/10.3390/ph16030447
APA StyleAdeyemi, O. S., Ishii, K., & Kato, K. (2023). The In Vitro Anti-Parasitic Activities of Emodin toward Toxoplasma gondii. Pharmaceuticals, 16(3), 447. https://doi.org/10.3390/ph16030447







