Exploring the Application of Micellar Drug Delivery Systems in Cancer Nanomedicine
Abstract
1. Introduction
1.1. Targeting TME with a Low pH
1.2. Targeting TME with High Level of GSH
1.3. Targeting Hypoxia TME
1.4. Targeting TME with High Level of ROS
1.5. Targeting Specific Enzymes in TME
1.6. The Enhanced Permeability and Retention (EPR) Effect and Its Application in Nanomedicine Delivery
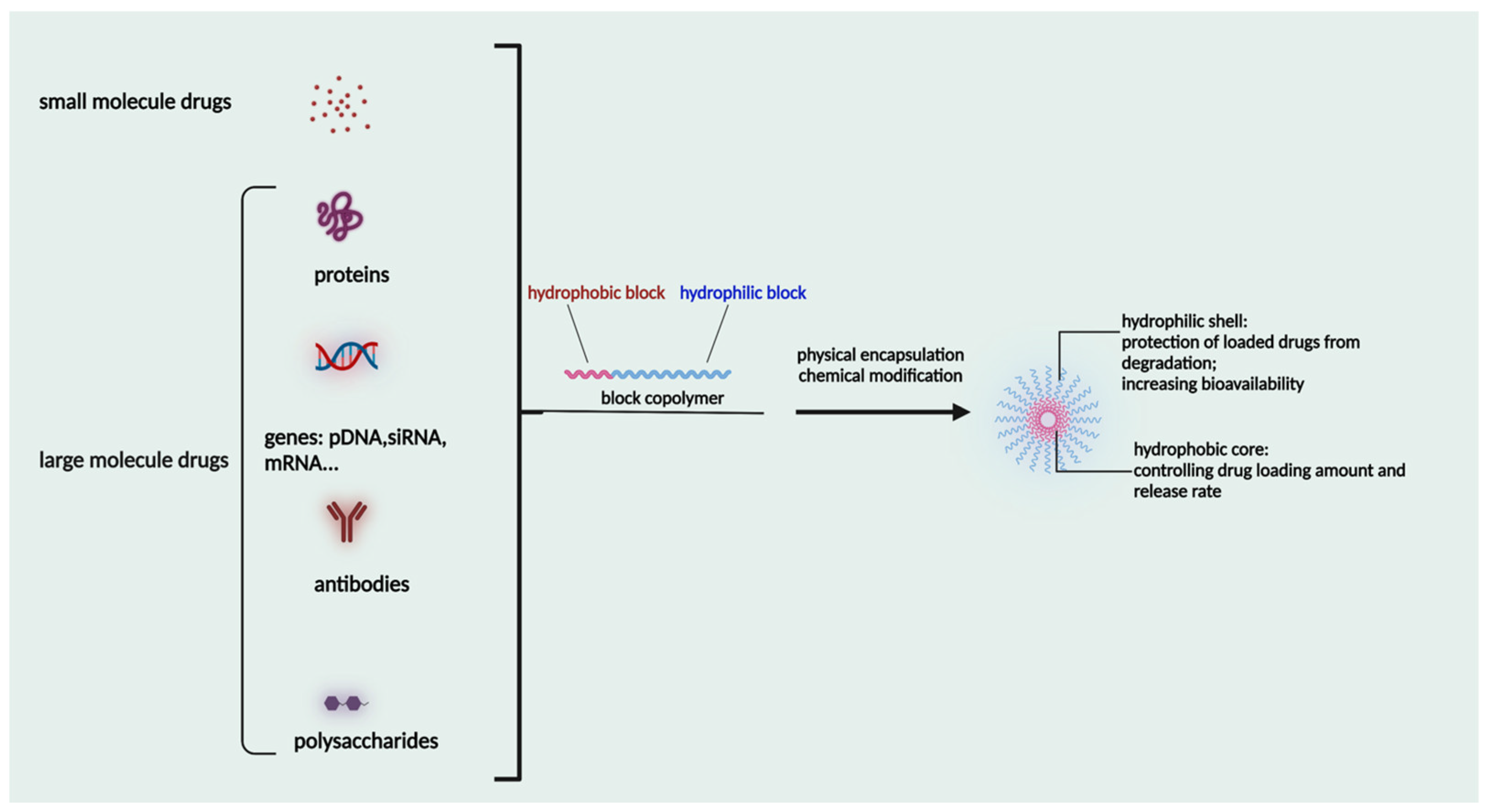
1.7. Micelles as Nanomedicine Delivery Systems
2. Characteristic Features of Micelles
3. Polymers Used for Micelle Formation
4. Micelles in Tumor Targeted Drug Release
4.1. pH Sensitive Micelles
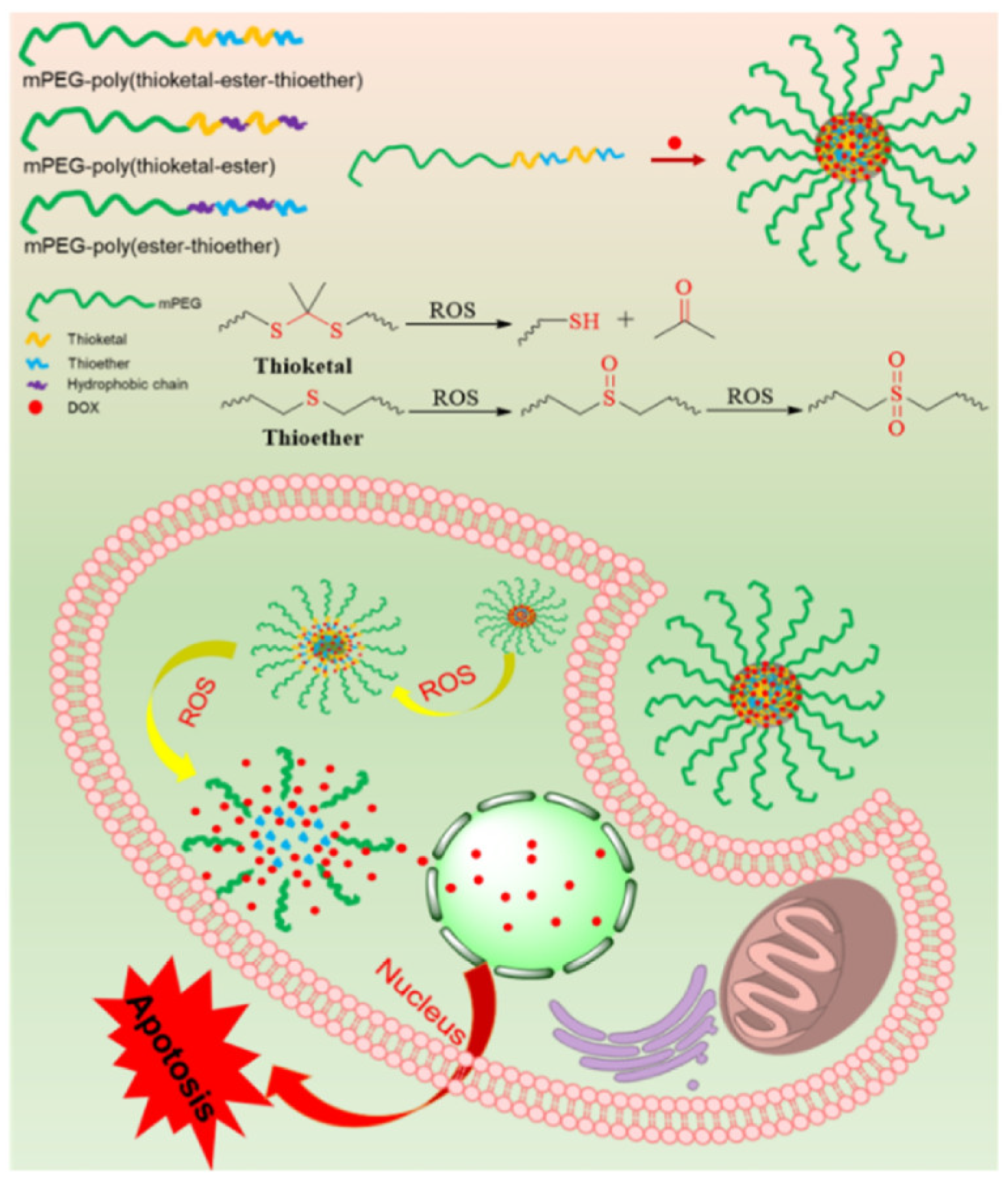
4.2. ROS Sensitive Micelles
4.3. Hypoxia Sensitive Micelles
4.4. Enzyme Sensitive Micelles
4.5. Thermo Sensitive Micelles
4.6. Magnetic Sensitive Micelles
5. Fate of Micelles Post Administration
6. Clinical Trials on Micellar Drug Delivery Systems
6.1. Paclitaxel Micellar Formulations
6.2. SN38 (Irinotecan Metabolite (NK012) Micelles
6.3. Anthracycline Class Drugs—Micellar Formulations
6.4. NC-4016/Oxaliplatin Micelles
6.5. NC-6004/Cisplatin Micelles
6.6. BIND-014/Docetaxel Micelles
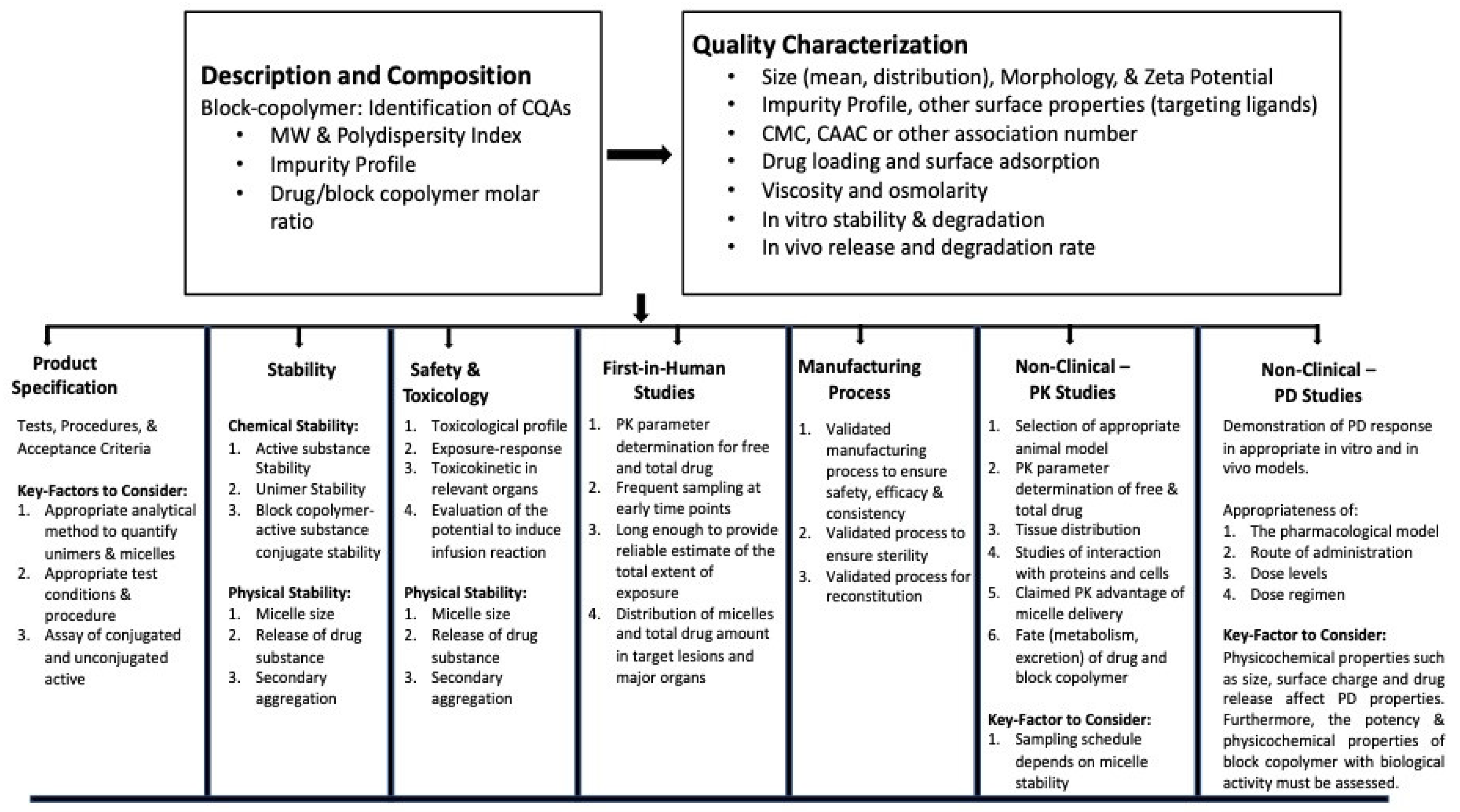
7. Regulatory Submissions
8. Conclusions and Future Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huai, Y.; Hossen, M.N.; Wilhelm, S.; Bhattacharya, R.; Mukherjee, P. Nanoparticle Interactions with the Tumor Microenvironment. Bioconjug. Chem. 2019, 30, 2247–2263. [Google Scholar] [CrossRef] [PubMed]
- Domingues, C.; Santos, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Jarak, I.; Veiga, F.; Barbosa, I.; Dourado, M.; Figueiras, A. Where Is Nano Today and Where Is It Headed? A Review of Nanomedicine and the Dilemma of Nanotoxicology. ACS Nano 2022, 16, 9994–10041. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Jia, Y.; Liu, Y.; Chen, Y.; Zhao, P. Tumor Microenvironment-Based Stimuli-Responsive Nanoparticles for Controlled Release of Drugs in Cancer Therapy. Pharmaceutics 2022, 14, 2346. [Google Scholar] [CrossRef]
- Mbugua, S.N. Targeting Tumor Microenvironment by Metal Peroxide Nanoparticles in Cancer Therapy. Bioinorg. Chem. Appl. 2022, 2022, 5041399. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Sun, G.; Sun, X.; Zhao, L.; Zhong, R.; Peng, Y. Tumor Energy Metabolism and Potential of 3-Bromopyruvate as an Inhibitor of Aerobic Glycolysis: Implications in Tumor Treatment. Cancers 2019, 11, 317. [Google Scholar] [CrossRef]
- Peng, S.; Xiao, F.; Chen, M.; Gao, H. Tumor-Microenvironment-Responsive Nanomedicine for Enhanced Cancer Immunotherapy. Adv. Sci. 2022, 9, e2103836. [Google Scholar] [CrossRef]
- Dharmaratne, N.U.; Kaplan, A.R.; Glazer, P.M. Targeting the Hypoxic and Acidic Tumor Microenvironment with pH-Sensitive Peptides. Cells 2021, 10, 541. [Google Scholar] [CrossRef]
- Qiu, N.; Du, X.; Ji, J.; Zhai, G. A review of stimuli-responsive polymeric micelles for tumor-targeted delivery of curcumin. Drug Dev. Ind. Pharm. 2021, 47, 839–856. [Google Scholar] [CrossRef]
- Du, J.-Z.; Sun, T.-M.; Song, W.-J.; Wu, J.; Wang, J. A tumor-acidity-activated charge-conversional nanogel as an intelligent vehicle for promoted tumoral-cell uptake and drug delivery. Angew. Chem. Int. Ed. Engl. 2010, 49, 3621–3626. [Google Scholar] [CrossRef] [PubMed]
- Du, J.-Z.; Mao, C.-Q.; Yuan, Y.-Y.; Yang, X.-Z.; Wang, J. Tumor extracellular acidity-activated nanoparticles as drug delivery systems for enhanced cancer therapy. Biotechnol. Adv. 2014, 32, 789–803. [Google Scholar] [CrossRef]
- Miao, P.; Sheng, S.; Sun, X.; Liu, J.; Huang, G. Lactate dehydrogenase a in cancer: A promising target for diagnosis and therapy. IUBMB Life 2013, 65, 904–910. [Google Scholar] [CrossRef]
- Cheng, R.; Feng, F.; Meng, F.; Deng, C.; Feijen, J.; Zhong, Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Control. Release Off. J. Control. Release Soc. 2011, 152, 2–12. [Google Scholar] [CrossRef]
- Kurtoglu, Y.E.; Navath, R.S.; Wang, B.; Kannan, S.; Romero, R.; Kannan, R.M. Poly(amidoamine) dendrimer–drug conjugates with disulfide linkages for intracellular drug delivery. Biomaterials 2009, 30, 2112–2121. [Google Scholar] [CrossRef]
- Peng, S.; Men, Y.; Xie, R.; Tian, Y.; Yang, W. Biodegradable phosphorylcholine-based zwitterionic polymer nanogels with smart charge-conversion ability for efficient inhibition of tumor cells. J. Colloid Interface Sci. 2019, 539, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Peng, S.; Yang, P.; Jiang, Q.; Zhang, Y.; Shen, B.; Dong, P.; Pang, Z.; Yang, W. Biodegradable Zwitterionic Nanogels with Long Circulation for Antitumor Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 23509–23521. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Deepagan, V.G.; Yoon, H.Y.; Han, H.S.; Kim, S.H.; Son, S.; Jo, D.G.; Ahn, C.H.; Suh, Y.D.; Kim, K.; et al. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials 2014, 35, 1735–1743. [Google Scholar] [CrossRef]
- Okuda, K.; Okabe, Y.; Kadonosono, T.; Ueno, T.; Youssif, B.G.; Kizaka-Kondoh, S.; Nagasawa, H. 2-Nitroimidazole-tricarbocyanine conjugate as a near-infrared fluorescent probe for In Vivo imaging of tumor hypoxia. Bioconjug. Chem. 2012, 23, 324–329. [Google Scholar] [CrossRef]
- Moyer, M.W. Targeting hypoxia brings breath of fresh air to cancer therapy. Nat. Med. 2012, 18, 636–637. [Google Scholar] [CrossRef]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Frezza, C.; Zheng, L.; Tennant, D.A.; Papkovsky, D.B.; Hedley, B.A.; Kalna, G.; Watson, D.G.; Gottlieb, E. Metabolic profiling of hypoxic cells revealed a catabolic signature required for cell survival. PLoS ONE 2011, 6, e24411. [Google Scholar] [CrossRef]
- Mosesson, Y.; Mills, G.B.; Yarden, Y. Derailed endocytosis: An emerging feature of cancer. Nat. Rev. Cancer 2008, 8, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Roche, O.; Yan, M.S.; Finak, G.; Evans, A.J.; Metcalf, J.L.; Hast, B.E.; Hanna, S.C.; Wondergem, B.; Furge, K.A.; et al. Regulation of endocytosis via the oxygen-sensing pathway. Nat. Med. 2009, 15, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Peng, X.; Gandhi, V. ROS-activated anticancer prodrugs: A new strategy for tumor-specific damage. Ther. Deliv. 2012, 3, 823–833. [Google Scholar] [CrossRef]
- Bai, J.; Jia, X.; Zhen, W.; Cheng, W.; Jiang, X. A Facile Ion-Doping Strategy to Regulate Tumor Microenvironments for Enhanced Multimodal Tumor Theranostics. J. Am. Chem. Soc. 2018, 140, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.S.; Xia, Y. A Reactive Oxygen Species (ROS)-Responsive Polymer for Safe, Efficient, and Targeted Gene Delivery in Cancer Cells. Angew. Chem. Int. Ed. 2013, 52, 6926–6929. [Google Scholar] [CrossRef] [PubMed]
- Qiu, N.; Gao, J.; Liu, Q.; Wang, J.; Shen, Y. Enzyme-Responsive Charge-Reversal Polymer-Mediated Effective Gene Therapy for Intraperitoneal Tumors. Biomacromolecules 2018, 19, 2308–2319. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, J.; Zhang, C.; Cao, Z.; Cheng, D.; Liu, J.; Shuai, X. Stimuli-Responsive Polymeric Nanocarriers for Efficient Gene Delivery. Top. Curr. Chem. 2017, 375, 27. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612. [Google Scholar] [CrossRef]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of Nanoparticles in Medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yang, X.; Yin, Q.; Cai, K.; Wang, H.; Chaudhury, I.; Yao, C.; Zhou, Q.; Kwon, M.; Hartman, J.A.; et al. Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. USA 2014, 111, 15344–15349. [Google Scholar] [CrossRef]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Marzbali, M.Y.; Khosroushahi, A.Y. Polymeric micelles as mighty nanocarriers for cancer gene therapy: A review. Cancer Chemother. Pharmacol. 2017, 79, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Morshed, R.A.; Cheng, Y.; Auffinger, B.; Wegscheid, M.L.; Lesniak, M.S. The potential of polymeric micelles in the context of glioblastoma therapy. Front. Pharmacol. 2013, 4, 157. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.; Batrakova, E.; Alakhov, V.Y. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Release Off. J. Control. Release Soc. 2002, 82, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Perumal, S.-A.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef]
- Topel, Ö.; Çakır, B.A.; Budama, L.; Hoda, N. Determination of critical micelle concentration of polybutadiene-block-poly(ethyleneoxide) diblock copolymer by fluorescence spectroscopy and dynamic light scattering. J. Mol. Liq. 2013, 177, 40–43. [Google Scholar] [CrossRef]
- Amos, D.A.; Markels, J.H.; Lynn, S.; Radke, C.J. Osmotic Pressure and Interparticle Interactions in Ionic Micellar Surfactant Solutions. J. Phys. Chem. B 1998, 102, 2739–2753. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, M.; Prieto, G.; Rega, C.; Varela, L.M.; Sarmiento, F.; Mosquera, V. A Comparative Study of the Determination of the Critical Micelle Concentration by Conductivity and Dielectric Constant Measurements. Langmuir 1998, 14, 4422–4426. [Google Scholar] [CrossRef]
- Scholz, N.; Behnke, T.; Resch-Genger, U. Determination of the Critical Micelle Concentration of Neutral and Ionic Surfactants with Fluorometry, Conductometry, and Surface Tension—A Method Comparison. J. Fluoresc. 2018, 28, 465–476. [Google Scholar] [CrossRef]
- Heakal, F.E.-T.; Elkholy, A.E. Gemini surfactants as corrosion inhibitors for carbon steel. J. Mol. Liq. 2017, 230, 395–407. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Fan, Y.; Zhou, Y.; Wang, X.; Fan, C.; Liu, Y.; Zhang, Q. Preparation and evaluation of novel mixed micelles as nanocarriers for intravenous delivery of propofol. Nanoscale Res. Lett. 2011, 6, 275. [Google Scholar] [CrossRef]
- Bae, Y.; Nishiyama, N.; Fukushima, S.; Koyama, H.; Yasuhiro, M.; Kataoka, K. Preparation and Biological Characterization of Polymeric Micelle Drug Carriers with Intracellular pH-Triggered Drug Release Property: Tumor Permeability, Controlled Subcellular Drug Distribution, and Enhanced In Vivo Antitumor Efficacy. Bioconjug. Chem. 2005, 16, 122–130. [Google Scholar] [CrossRef]
- Nagarajan, R.; Barry, M.; Ruckenstein, E. Unusual selectivity in solubilization by block copolymer micelles. Langmuir 1986, 2, 210–215. [Google Scholar] [CrossRef]
- Xiong, X.-B.; Falamarzian, A.; Garg, S.M.; Lavasanifar, A. Engineering of amphiphilic block copolymers for polymeric micellar drug and gene delivery. J. Control. Release 2011, 155, 248–261. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef]
- Lavasanifar, A.; Samuel, J.; Sattari, S.; Kwon, G.S. Block copolymer micelles for the encapsulation and delivery of amphotericin B. Pharm. Res. 2002, 19, 418–422. [Google Scholar] [CrossRef]
- Markman, J.L.; Rekechenetskiy, A.; Holler, E.; Ljubimova, J.Y. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv. Drug Deliv. Rev. 2013, 65, 1866–1879. [Google Scholar] [CrossRef] [PubMed]
- Minko, T. Soluble polymer conjugates for drug delivery. Drug Discov. Today Technol. 2005, 2, 15–20. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, M.; Wan, H. Discussion about several potential drawbacks of PEGylated therapeutic proteins. Biol. Pharm. Bull. 2014, 37, 335–339. [Google Scholar] [CrossRef]
- Zhang, N.; Wardwell, P.R.; Bader, R.A. Polysaccharide-based micelles for drug delivery. Pharmaceutics 2013, 5, 329–352. [Google Scholar] [CrossRef]
- Thanou, M.; Verhoef, J.C.; Junginger, H.E. Chitosan and its derivatives as intestinal absorption enhancers. Adv. Drug Deliv. Rev. 2001, 50 (Suppl. 1), S91–S101. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Pertici, G. 1—Introduction to bioresorbable polymers for biomedical applications. In Bioresorbable Polymers for Biomedical Applications; Perale, G., Hilborn, J., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2017; pp. 3–29. [Google Scholar] [CrossRef]
- Poole-Warren, L.A.; Patton, A.J. 1—Introduction to biomedical polymers and biocompatibility. In Woodhead Publishing Series in Biomaterials; Poole-Warren, L., Martens, P., Green, R., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2016; pp. 3–31. [Google Scholar] [CrossRef]
- Wen, Y.; Oh, J.K. Recent Strategies to Develop Polysaccharide-Based Nanomaterials for Biomedical Applications. Macromol. Rapid Commun. 2014, 35, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Yang, J.; Kopečková, P.; Kopeček, J. Biodegradable Multiblock Poly[N-(2-hydroxypropyl)methacrylamide] via Reversible Addition-Fragmentation Chain Transfer Polymerization and Click Chemistry. Macromolecules 2011, 44, 2481–2488. [Google Scholar] [CrossRef] [PubMed]
- Alfurhood, J.A.; Sun, H.; Kabb, C.P.; Tucker, B.S.; Matthews, J.H.; Luesch, H.; Sumerlin, B.S. Poly(N-(2-Hydroxypropyl) Methacrylamide)-Valproic Acid Conjugates as Block Copolymer Nanocarriers. Polym. Chem. 2017, 8, 4983–4987. [Google Scholar] [CrossRef]
- Kopecek, J.; Kopecková, P.; Minko, T.; Lu, Z. HPMA copolymer-anticancer drug conjugates: Design, activity, and mechanism of action. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. e.V. 2000, 50, 61–81. [Google Scholar] [CrossRef]
- Tucker, B.S.; Sumerlin, B.S. Poly(N-(2-hydroxypropyl) methacrylamide)-based nanotherapeutics. Polym. Chem. 2014, 5, 1566–1572. [Google Scholar] [CrossRef]
- Soni, V.; Pandey, V.; Tiwari, R.; Asati, S.; Tekade, R.K. Chapter 13—Design and Evaluation of Ophthalmic Delivery Formulations. In Advances in Pharmaceutical Product Development and Research; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 473–538. [Google Scholar] [CrossRef]
- Liu, H.; Chen, H.; Cao, F.; Peng, D.; Chen, W.; Zhang, C. Amphiphilic Block Copolymer Poly (Acrylic Acid)-B-Polycaprolactone as a Novel pH-sensitive Nanocarrier for Anti-Cancer Drugs Delivery: In-Vitro and In-Vivo Evaluation. Polymers 2019, 11, 820. [Google Scholar] [CrossRef]
- Xu, H.; Yao, Q.; Cai, C.; Gou, J.; Zhang, Y.; Zhong, H.; Tang, X. Amphiphilic poly(amino acid) based micelles applied to drug delivery: The In Vitro and In Vivo challenges and the corresponding potential strategies. J. Control. Release Off. J. Control. Release Soc. 2015, 199, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; FitzGerald, P.A.; Kobayashi, Y.; Ueno, K.; Watanabe, M.; Warr, G.G.; Atkin, R. Micelle Structure of Novel Diblock Polyethers in Water and Two Protic Ionic Liquids (EAN and PAN). Macromolecules 2015, 48, 1843–1851. [Google Scholar] [CrossRef]
- Urbánek, T.; Jäger, E.; Jäger, A.; Hrubý, M. Selectively Biodegradable Polyesters: Nature-Inspired Construction Materials for Future Biomedical Applications. Polymers 2019, 11, 1061. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Dehbari, N.; Tavakoli, J.; Khatrao, S.S.; Tang, Y. In Situ polymerized hyperbranched polymer reinforced poly (acrylic acid) hydrogels. Mater. Chem. Front. 2017, 1, 1995–2004. [Google Scholar] [CrossRef]
- Balogun-Agbaje, O.A.; Odeniyi, O.A.; Odeniyi, M.A. Drug delivery applications of poly-γ-glutamic acid. Futur. J. Pharm. Sci. 2021, 7, 125. [Google Scholar] [CrossRef]
- Delbecq, F.; Kawakami, K. Self-assembly study and formation of hydrophobized PVA dense and stable nanoparticles loaded with cholesterol or a steroid-type drug. J. Colloid Interface Sci. 2014, 428, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Chen, J.; Kulkarni, A.; Thompson, D.H. Poly(ethylene glycol)-poly(vinyl alcohol)-adamantanate: Synthesis and stimuli-responsive micelle properties. Soft Matter 2012, 8, 5843–5846. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Trubetskoy, V.S. Which polymers can make nanoparticulate drug carriers long-circulating? Adv. Drug Deliv. Rev. 1995, 16, 141–155. [Google Scholar] [CrossRef]
- Takeuchi, H.; Kojima, H.; Yamamoto, H.; Kawashima, Y. Evaluation of circulation profiles of liposomes coated with hydrophilic polymers having different molecular weights in rats. J. Control. Release Off. J. Control. Release Soc. 2001, 75, 83–91. [Google Scholar] [CrossRef]
- Kuang, C.; Yusa, S.; Sato, T. Micellization and Phase Separation in Aqueous Solutions of Thermosensitive Block Copolymer Poly(N-isopropylacrylamide)-b-poly(N-vinyl-2-pyrrolidone) upon Heating. Macromolecules 2019, 52, 4812–4819. [Google Scholar] [CrossRef]
- Wei, H.; Cheng, S.-X.; Zhang, X.-Z.; Zhuo, R.-X. Thermo-sensitive polymeric micelles based on poly(N-isopropylacrylamide) as drug carriers. Prog. Polym. Sci. 2009, 34, 893–910. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Yang, J.; Hao, X.; Ren, X.; Feng, Y.; Zhang, W. Biodegradable PEI modified complex micelles as gene carriers with tunable gene transfection efficiency for ECs. J. Mater. Chem. B 2016, 4, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Liu, R.; Chen, X.; He, M.; Zhang, Y.; Chen, S. Micelles Based on Lysine, Histidine, or Arginine: Designing Structures for Enhanced Drug Delivery. Front. Bioeng. Biotechnol. 2021, 9, 744657. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, H.M.; Abdelmoneem, M.A.; Abdelsalam, K.; Freag, M.S.; Elkhodairy, K.A.; Elzoghby, A.O. Chapter 9—Poly(Amino Acid) Nanoparticles as a Promising Tool for Anticancer Therapeutics; Kesharwani, P., Paknikar, K., Gajbhiye, V., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 167–204. [Google Scholar] [CrossRef]
- Ghasemi, R.; Abdollahi, M.; Emamgholi Zadeh, E.; Khodabakhshi, K.; Badeli, A.; Bagheri, H.; Hosseinkhani, S. mPEG-PLA and PLA-PEG-PLA nanoparticles as new carriers for delivery of recombinant human Growth Hormone (rhGH). Sci. Rep. 2018, 8, 9854. [Google Scholar] [CrossRef]
- Nair, N.R.; Sekhar, V.C.; Nampoothiri, K.M.; Pandey, A. 32—Biodegradation of Biopolymers. In Production, Isolation and Purification of Industrial Products; Pandey, A., Negi, S., Singh-Nee Nigam, P., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 739–755. [Google Scholar] [CrossRef]
- De Oliveira, J.; Vandenberghe, L.P.S.; Zawadzki, S.F.; Rodrigues, C.; de Carvalho, J.C.; Soccol, C.R. 28—Production and Application of Polylactides. In Production, Isolation and Purification of Industrial Products; Pandey, A., Negi, S., Singh-Nee Nigam, P., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 633–653. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Lu, X.; Yang, X.; Meng, Y.; Li, S. Temperature and pH dually-responsive poly(β-amino ester) nanoparticles for drug delivery. Chin. J. Polym. Sci. 2017, 35, 534–546. [Google Scholar] [CrossRef]
- Hari, S.K.; Gauba, A.; Shrivastava, N.; Tripathi, R.M.; Jain, S.K.; Pandey, A.K. Polymeric micelles and cancer therapy: An ingenious multimodal tumor-targeted drug delivery system. Drug Deliv. Transl. Res. 2022, 13, 135–163. [Google Scholar] [CrossRef]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, X.; Zhang, X. Recent advances in stimuli-responsive polymeric micelles via click chemistry. Polym. Chem. 2019, 10, 34–44. [Google Scholar] [CrossRef]
- Kim, D.; Lee, E.S.; Park, K.; Kwon, I.C.; Bae, Y.H. Doxorubicin Loaded pH-sensitive Micelle: Antitumoral Efficacy against Ovarian A2780/DOXR Tumor. Pharm. Res. 2008, 25, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Meng, Q.; Sun, H.; Su, J.; Yin, Q.; Zhang, Z.; Yu, H.; Chen, L.; Gu, W.; Li, Y. Dual pH-sensitive micelles with charge-switch for controlling cellular uptake and drug release to treat metastatic breast cancer. Biomaterials 2017, 114, 44–53. [Google Scholar] [CrossRef]
- Yang, C.; Xue, Z.; Liu, Y.; Xiao, J.; Chen, J.; Zhang, L.; Guo, J.; Lin, W. Delivery of anticancer drug using pH-sensitive micelles from triblock copolymer MPEG-b-PBAE-b-PLA. Mater. Sci. Eng. C 2018, 84, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, D.; He, B.; Xu, X.; Sheng, M.; Lai, Y.; Wang, G.; Gu, Z. Anti-tumor drug delivery of pH-sensitive poly(ethylene glycol)-poly(L-histidine-)-poly(L-lactide) nanoparticles. J. Control. Release 2011, 152, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Leng, M.; Cai, M.; Huang, L.; Chen, Y.; Luo, X. pH responsive micelles based on copolymers mPEG-PCL-PDEA: The relationship between composition and properties. Colloids Surf. B Biointerfaces 2017, 154, 397–407. [Google Scholar] [CrossRef]
- Liu, P.; Huang, P.; Kang, E.-T. pH-Sensitive Dextran-Based Micelles from Copper-Free Click Reaction for Antitumor Drug Delivery. Langmuir 2021, 37, 12990–12999. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, J.-H.; Bae, S.M.; Shin, H.; Kim, M.S.; Park, S.; Lee, H.; Park, R.-W.; Kim, I.-S.; Kim, K.; et al. Tumoral acidic pH-responsive MPEG-poly(β-amino ester) polymeric micelles for cancer targeting therapy. J. Control. Release 2010, 144, 259–266. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Yang, Y.Q.; Huang, T.X.; Zhao, B.; Guo, X.D.; Wang, J.F.; Zhang, L.J. Self-assembled pH-responsive MPEG-b-(PLA-co-PAE) block copolymer micelles for anticancer drug delivery. Biomaterials 2012, 33, 6273–6283. [Google Scholar] [CrossRef]
- Gebrie, H.T.; Addisu, K.D.; Darge, H.F.; Birhan, Y.S.; Thankachan, D.; Tsai, H.-C.; Wu, S.-Y. pH/redox-responsive core cross-linked based prodrug micelle for enhancing micellar stability and controlling delivery of chemo drugs: An effective combination drug delivery platform for cancer therapy. Biomater. Adv. 2022, 139, 213015. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Y.; Sun, J.; Chen, H.; Liu, Z.; Lin, K.; Ma, P.; Zhang, W.; Zhen, Y.; Zhang, S.; et al. pH/reduction dual-responsive hyaluronic acid-podophyllotoxin prodrug micelles for tumor targeted delivery. Carbohydr. Polym. 2022, 288, 119402. [Google Scholar] [CrossRef]
- Isik, G.; Kiziltay, A.; Hasirci, N.; Tezcaner, A. Lithocholic acid conjugated mPEG-b-PCL micelles for pH responsive delivery to breast cancer cells. Int. J. Pharm. 2022, 621, 121779. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Lu, Z.; Jiang, T.; Han, W.; Chen, X.; Zhao, X. Chitosan coated pH/redox-responsive hyaluronic acid micelles for enhanced tumor targeted co-delivery of doxorubicin and siPD-L1. Int. J. Biol. Macromol. 2022, 222, 1078–1091. [Google Scholar] [CrossRef]
- Wang, L.; Tian, B.; Zhang, J.; Li, K.; Liang, Y.; Sun, Y.; Ding, Y.; Han, J. Coordinated pH/redox dual-sensitive and hepatoma-targeted multifunctional polymeric micelle system for stimuli-triggered doxorubicin release: Synthesis, characterization and In Vitro evaluation. Int. J. Pharm. 2016, 501, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Li, X.; Duan, X.; Li, M.; Niu, P.; Xu, H.; Cai, K.; Yang, H. A pH/ROS Cascade-Responsive Charge-Reversal Nanosystem with Self-Amplified Drug Release for Synergistic Oxidation-Chemotherapy. Adv. Sci. 2019, 6, 1801807. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Deng, M.; Yang, H.; Chen, L.; Xiao, C.; Zhuang, X.; Chen, X. Multi-responsive core-crosslinked poly (thiolether ester) micelles for smart drug delivery. Polymer 2017, 110, 235–241. [Google Scholar] [CrossRef]
- Sun, C.; Liang, Y.; Hao, N.; Xu, L.; Cheng, F.; Su, T.; Cao, J.; Gao, W.; Pu, Y.; He, B. A ROS-responsive polymeric micelle with a π-conjugated thioketal moiety for enhanced drug loading and efficient drug delivery. Org. Biomol. Chem. 2017, 15, 9176–9185. [Google Scholar] [CrossRef]
- Yoo, J.; Rejinold, N.S.; Lee, D.; Jon, S.; Kim, Y.-C. Protease-activatable cell-penetrating peptide possessing ROS-triggered phase transition for enhanced cancer therapy. J. Control. Release 2017, 264, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Li, J.; Zhou, Y.; Yang, S.; Chen, W.; Li, F.; You, B.; Liu, Y.; Zhang, X. Targeted Delivery of Doxorubicin via CD147-Mediated ROS/pH Dual-Sensitive Nanomicelles for the Efficient Therapy of Hepatocellular Carcinoma. AAPS J. 2018, 20, 34. [Google Scholar] [CrossRef]
- Xin, X.; Lin, F.; Wang, Q.; Yin, L.; Mahato, R.I. ROS-Responsive Polymeric Micelles for Triggered Simultaneous Delivery of PLK1 Inhibitor/miR-34a and Effective Synergistic Therapy in Pancreatic Cancer. ACS Appl. Mater. Interfaces 2019, 11, 14647–14659. [Google Scholar] [CrossRef]
- Tang, M.; Hu, P.; Zheng, Q.; Tirelli, N.; Yang, X.; Wang, Z.; Wang, Y.; Tang, Q.; He, Y. Polymeric micelles with dual thermal and reactive oxygen species (ROS)-responsiveness for inflammatory cancer cell delivery. J. Nanobiotechnol. 2017, 15, 39. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, M.; Gao, W.; Yang, Y.; Zhang, J.; Pu, Y.; He, B. Polymeric nanoparticles responsive to intracellular ROS for anticancer drug delivery. Colloids Surf. B Biointerfaces 2019, 181, 252–260. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Q.; An, S.; Lu, Y.; Li, J.; He, X.; Liu, L.; Zhang, Y.; Sun, T.; Jiang, C. ROS-Switchable Polymeric Nanoplatform with Stimuli-Responsive Release for Active Targeted Drug Delivery to Breast Cancer. ACS Appl. Mater. Interfaces 2017, 9, 12227–12240. [Google Scholar] [CrossRef]
- Xu, H.; Yang, M.; Du, Y.; Gao, T.; Liu, Y.; Xiong, L.; Peng, N. Self-assembly of micelles with pH/ROS dual-responsiveness and mitochondrial targeting for potential anti-tumor applications. New J. Chem. 2022, 46, 21235–21244. [Google Scholar] [CrossRef]
- Deng, Y.; Yuan, H.; Yuan, W. Hypoxia-responsive micelles self-assembled from amphiphilic block copolymers for the controlled release of anticancer drugs. J. Mater. Chem. B 2019, 7, 286–295. [Google Scholar] [CrossRef]
- Kang, L.; Fan, B.; Sun, P.; Huang, W.; Jin, M.; Wang, Q.; Gao, Z. An effective tumor-targeting strategy utilizing hypoxia-sensitive siRNA delivery system for improved anti-tumor outcome. Acta Biomater. 2016, 44, 341–354. [Google Scholar] [CrossRef]
- Thambi, T.; Son, S.; Lee, D.S.; Park, J.H. Poly(ethylene glycol)-b-poly(lysine) copolymer bearing nitroaromatics for hypoxia-sensitive drug delivery. Acta Biomater. 2016, 29, 261–270. [Google Scholar] [CrossRef]
- Filipczak, N.; Joshi, U.; Attia, S.A.; Berger Fridman, I.; Cohen, S.; Konry, T.; Torchilin, V. Hypoxia-sensitive drug delivery to tumors. J. Control. Release 2022, 341, 431–442. [Google Scholar] [CrossRef]
- Lu, M.; Huang, X.; Cai, X.; Sun, J.; Liu, X.; Weng, L.; Zhu, L.; Luo, Q.; Chen, Z. Hypoxia-Responsive Stereocomplex Polymeric Micelles with Improved Drug Loading Inhibit Breast Cancer Metastasis in an Orthotopic Murine Model. ACS Appl. Mater. Interfaces 2022, 14, 20551–20565. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Li, Y.; Tian, Y.; Ma, M.; Shi, K.; Shang, X.; Yuan, H.; Hu, F. A Hypoxia-Sensitive Drug Delivery System Constructed by Nitroimidazole and its Application in the Treatment of Hepatocellular Carcinoma. AAPS PharmSciTech 2022, 23, 167. [Google Scholar] [CrossRef]
- Long, M.; Liu, X.; Huang, X.; Lu, M.; Wu, X.; Weng, L.; Chen, Q.; Wang, X.; Zhu, L.; Chen, Z. Alendronate-functionalized hypoxia-responsive polymeric micelles for targeted therapy of bone metastatic prostate cancer. J. Control. Release 2021, 334, 303–317. [Google Scholar] [CrossRef] [PubMed]
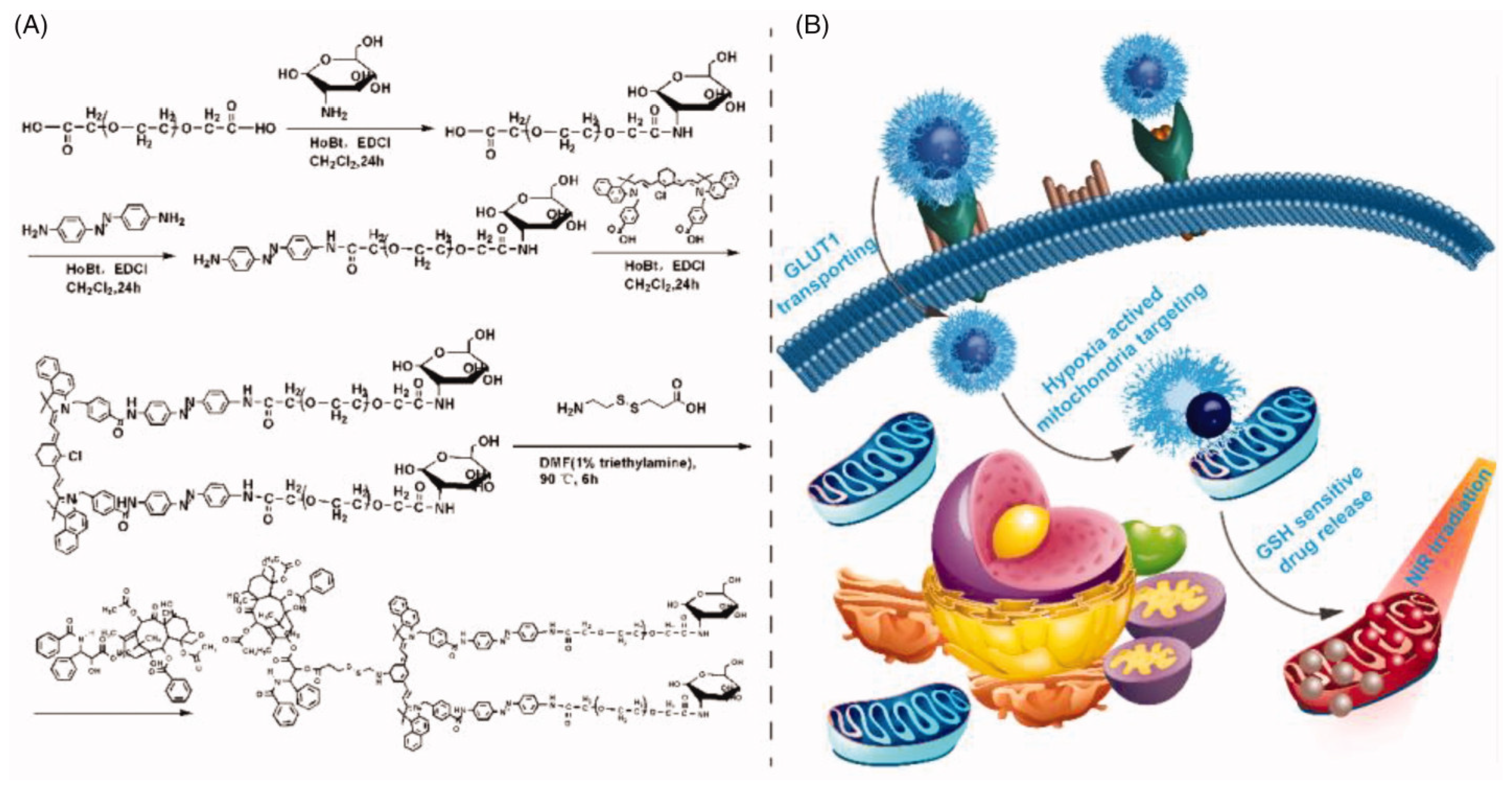
- Ma, P.; Wei, G.; Chen, J.; Jing, Z.; Wang, X.; Wang, Z. GLUT1 targeting and hypoxia-activating polymer-drug conjugate-based micelle for tumor chemo-thermal therapy. Drug Deliv. 2021, 28, 2256–2267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, S.; Zhang, K.; Zhu, Z.; Miao, Y.; Qiu, Y.; Zhang, P.; Zhao, X. Self-assembly of polymer-doxorubicin conjugates to form polyprodrug micelles for pH/enzyme dual-responsive drug delivery. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126669. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Zhang, X.; Wei, X.; Xiong, X.; Zhou, S. Enzyme and Redox Dual-Triggered Intracellular Release from Actively Targeted Polymeric Micelles. ACS Appl. Mater. Interfaces 2017, 9, 3388–3399. [Google Scholar] [CrossRef] [PubMed]
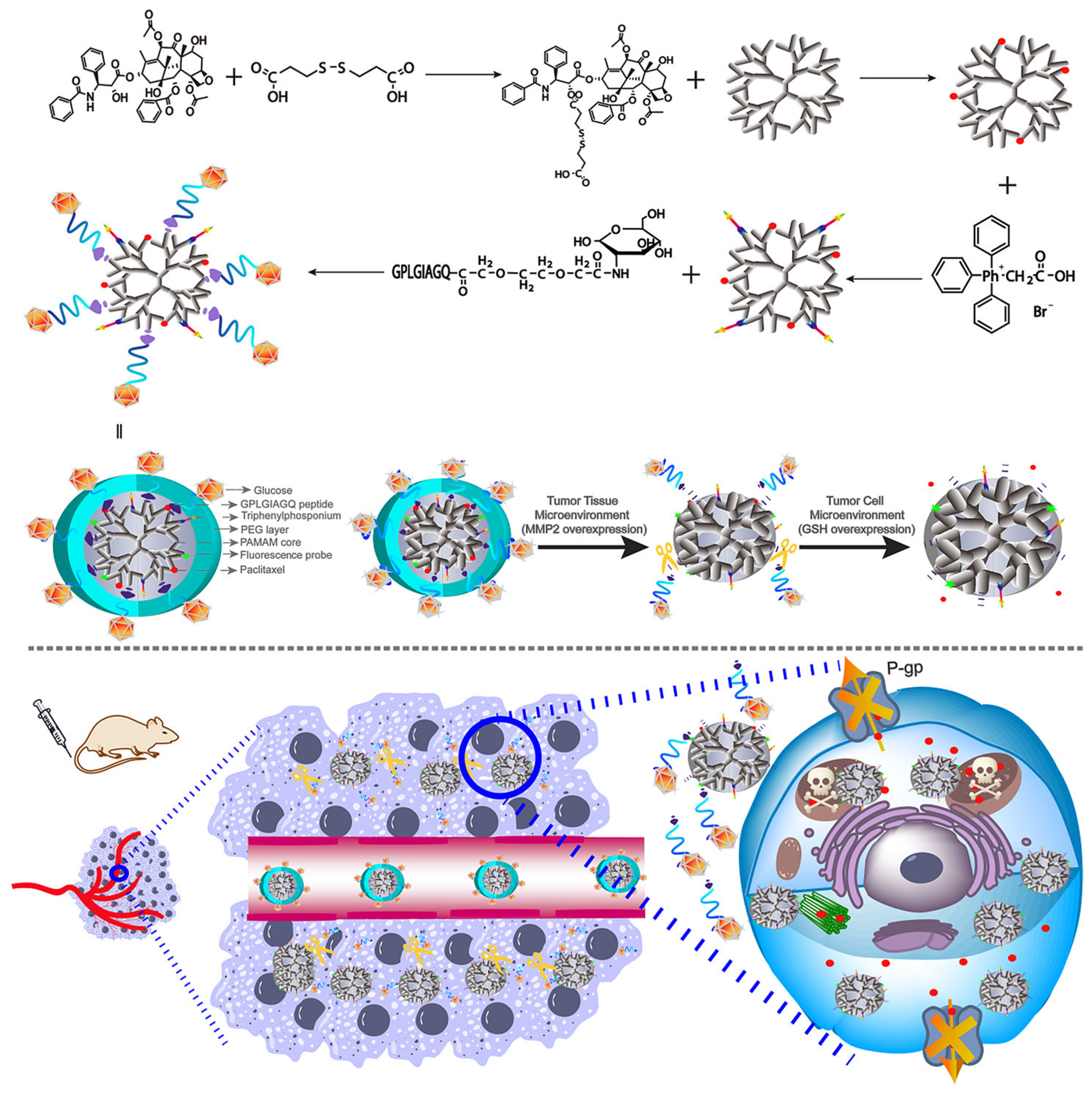
- Zhu, L.; Perche, F.; Wang, T.; Torchilin, V.P. Matrix metalloproteinase 2-sensitive multifunctional polymeric micelles for tumor-specific co-delivery of siRNA and hydrophobic drugs. Biomaterials 2014, 35, 4213–4222. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.C.; Reis, R.L.; Ferreira, H.; Neves, N.M. Glutathione Reductase-Sensitive Polymeric Micelles for Controlled Drug Delivery on Arthritic Diseases. ACS Biomater. Sci. Eng. 2021, 7, 3229–3241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.; Xie, J.; Zhong, Z.; Deng, C. Enzyme-responsive micellar JQ1 induces enhanced BET protein inhibition and immunotherapy of malignant tumors. Biomater. Sci. 2021, 9, 6915–6926. [Google Scholar] [CrossRef]
- Wan, D.; Zhu, Q.; Zhang, J.; Chen, X.; Li, F.; Liu, Y.; Pan, J. Intracellular and extracellular enzymatic responsive micelle for intelligent therapy of cancer. Nano Res. 2022, 16, 2851–2858. [Google Scholar] [CrossRef]
- Han, J.O.; Lee, H.J.; Jeong, B. Thermosensitive core-rigid micelles of monomethoxy poly(ethylene glycol)-deoxy cholic acid. Biomater. Res. 2022, 26, 16. [Google Scholar] [CrossRef]
- Li, G.; Guo, L.; Meng, Y.; Zhang, T. Self-assembled nanoparticles from thermo-sensitive polyion complex micelles for controlled drug release. Chem. Eng. J. 2011, 174, 199–205. [Google Scholar] [CrossRef]
- Chung, J.E.; Yokoyama, M.; Yamato, M.; Aoyagi, T.; Sakurai, Y.; Okano, T. Thermo-responsive drug delivery from polymeric micelles constructed using block copolymers of poly (N-isopropylacrylamide) and poly (butylmethacrylate). J. Control. Release 1999, 62, 115–127. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Wan, Z.; Quan, Z.; Tan, Q. Investigation of thermo-sensitive amphiphilic micelles as drug carriers for chemotherapy in cholangiocarcinoma In Vitro and In Vivo. Int. J. Pharm. 2014, 463, 81–88. [Google Scholar] [CrossRef]
- Lin, M.M.; Kang, Y.J.; Sohn, Y.; Kim, D.K. Dual targeting strategy of magnetic nanoparticle-loaded and RGD peptide-activated stimuli-sensitive polymeric micelles for delivery of paclitaxel. J. Nanoparticle Res. 2015, 17, 248. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, J.; Su, Y.; Huang, J.; Li, J.; Qiu, L.; Zhan, M.; He, X.; Yuan, W.; Li, Y. Fabrication of thermoresponsive magnetic micelles from amphiphilic poly(phenyl isocyanide) and Fe3O4 nanoparticles for controlled drug release and synergistic thermochemotherapy. Polym. Chem. 2021, 12, 2132–2140. [Google Scholar] [CrossRef]
- Lei, B.; Sun, M.; Chen, M.; Xu, S.; Liu, H. pH and Temperature Double-Switch Hybrid Micelles for Controllable Drug Release. Langmuir 2021, 37, 14628–14637. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-C.; Sun, Y.T.; Lee, A.W.; Huang, S.Y.; Fan, W.L.; Chiao, Y.H.; Tsai, H.C.; Lai, J.Y. Self-Assembled Supramolecular Micelles with pH-Responsive Properties for More Effective Cancer Chemotherapy. ACS Biomater. Sci. Eng. 2020, 6, 4096–4105. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, V.; Janakiraman, K.; Krishnaswami, V.; Kandasamy, R. Recent Progress in Stimuli-Responsive Intelligent Nano Scale Drug Delivery Systems: A Special Focus Towards pH-Sensitive Systems. Curr. Drug Targets 2021, 22, 947–966. [Google Scholar] [CrossRef]
- Suarato, G.; Li, W.; Meng, Y. Role of pH-responsiveness in the design of chitosan-based cancer nanotherapeutics: A review. Biointerphases 2016, 11, 04B201. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Xu, X.; Chen, Z.; Ma, L.; Wang, Y.; Guo, X.; Li, J.; Wang, X. Construction of pH-sensitive targeted micelle system co-delivery with curcumin and dasatinib and evaluation of anti-liver cancer. Drug Deliv. 2022, 29, 792–806. [Google Scholar] [CrossRef]
- Lei, J.; Song, Y.; Li, D.; Lei, M.; Tan, R.; Liu, Y.; Zheng, H. pH-sensitive and charge-reversal Daunorubicin-conjugated polymeric micelles for enhanced cancer therapy. J. Appl. Polym. Sci. 2022, 139, 51535. [Google Scholar] [CrossRef]
- Jo, M.J.; Shin, H.J.; Yoon, M.S.; Kim, S.Y.; Jin, C.E.; Park, C.-W.; Kim, J.-S.; Shin, D.H. Evaluation of pH-Sensitive Polymeric Micelles Using Citraconic Amide Bonds for the Co-Delivery of Paclitaxel, Etoposide, and Rapamycin. Pharmaceutics 2023, 15, 154. [Google Scholar] [CrossRef]
- Lin, W.; Kim, D. pH-Sensitive Micelles with Cross-Linked Cores Formed from Polyaspartamide Derivatives for Drug Delivery. Langmuir 2011, 27, 12090–12097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Xiong, D.; Sun, Y.; Zhao, B.; Lin, W.J.; Zhang, L.J. Self-assembled micelles based on pH-sensitive PAE-g-MPEG-cholesterol block copolymer for anticancer drug delivery. Int. J. Nanomed. 2014, 9, 4923–4933. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Su, K.; Zhao, D.; Lu, A.; Zhong, C. Nanomaterials for Tumor Hypoxia Relief to Improve the Efficacy of ROS-Generated Cancer Therapy. Front. Chem. 2021, 9, 649158. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Tabei, Y. Role of oxidative stress in nanoparticles toxicity. Free Radic. Res. 2021, 55, 331–342. [Google Scholar] [CrossRef]
- Zuberek, M.; Grzelak, A. Nanoparticles-Caused Oxidative Imbalance. Adv. Exp. Med. Biol. 2018, 1048, 85–98. [Google Scholar] [CrossRef]
- Chen, H.; He, W.; Guo, Z. An H2O2-responsive nanocarrier for dual-release of platinum anticancer drugs and O2: Controlled release and enhanced cytotoxicity against cisplatin resistant cancer cells. Chem. Commun. 2014, 50, 9714–9717. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Du, G.; Zhang, Z.; Gong, G.; Cai, W.; Wu, L.; Zhao, G. Fabrication of Redox- and pH-Sensitive Self-Assembled Nano-Micelles with Pegylated β-Cyclodextrin for Codelivery of Doxorubicin and Cyclopalladated Ferrocene. Eur. J. Inorg. Chem. 2022, 2022, e202101061. [Google Scholar] [CrossRef]
- Liang, R.; Wong, K.H.; Yang, Y.; Duan, Y.; Chen, M. ROS-responsive dexamethasone micelles normalize the tumor microenvironment enhancing hypericin in cancer photodynamic therapy. Biomater. Sci. 2022, 10, 1018–1025. [Google Scholar] [CrossRef]
- Ge, C.; Zhu, J.; Wu, G.; Ye, H.; Lu, H.; Yin, L. ROS-Responsive Selenopolypeptide Micelles: Preparation, Characterization, and Controlled Drug Release. Biomacromolecules 2022, 23, 2647–2654. [Google Scholar] [CrossRef]
- Goyal, K.; Konar, A.; Kumar, B.S.H.; Koul, V. Lactoferrin-conjugated pH and redox-sensitive polymersomes based on PEG-S-S-PLA-PCL-OH boost delivery of bacosides to the brain. Nanoscale 2018, 10, 17781–17798. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Song, H.; Zhang, H.; Yang, P.; Zhang, C.; Huang, P.; Kong, D.; Wang, W. Engineering biodegradable guanidyl-decorated PEG-PCL nanoparticles as robust exogenous activators of DCs and antigen cross-presentation. Nanoscale 2017, 9, 13413–13418. [Google Scholar] [CrossRef]
- Liang, Y.; Deng, X.; Zhang, L.; Peng, X.; Gao, W.; Cao, J.; Gu, Z.; He, B. Terminal modification of polymeric micelles with π-conjugated moieties for efficient anticancer drug delivery. Biomaterials 2015, 71, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.V.; Ko, H.; Lee, J.; Park, J.H. Recent Progress and Advances in Stimuli-Responsive Polymers for Cancer Therapy. Front. Bioeng. Biotechnol. 2018, 6, 110. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Tian, S.; Liu, Q.; Zheng, C.; Zhang, Z.; Ding, Y.; An, Y.; Liu, Y.; Shi, L. Nanocarriers responsive to a hypoxia gradient facilitate enhanced tumor penetration and improved anti-tumor efficacy. Biomater. Sci. 2019, 7, 2986–2995. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Li, J.; Su, Q.; Yuan, W.; Dai, Y.; Han, C.; Wang, Q.; Feng, W.; Li, F. Ultrasensitive Near-Infrared Fluorescence-Enhanced Probe for In Vivo Nitroreductase Imaging. J. Am. Chem. Soc. 2015, 137, 6407–6416. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Z.; Guo, C.; Guo, H.; Su, Y.; Chen, Q.; Sun, C.; Liu, Q.; Chen, D.; Mu, H. Hypoxia responsive nano-drug delivery system based on angelica polysaccharide for liver cancer therapy. Drug Deliv. 2022, 29, 138–148. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, P.; Tang, L.; Zhang, X.; Shi, F.; Ning, X.; Bi, J.; Qu, Y.; Liu, H. Hypoxia responsive and tumor-targeted mixed micelles for enhanced cancer therapy and real-time imaging. Colloids Surf. B Biointerfaces 2022, 215, 112526. [Google Scholar] [CrossRef]
- Wang, Z.; Mu, X.; Yang, Q.; Luo, J.; Zhao, Y. Hypoxia-responsive nanocarriers for chemotherapy sensitization via dual-mode inhibition of hypoxia-inducible factor-1 alpha. J. Colloid Interface Sci. 2022, 628, 106–115. [Google Scholar] [CrossRef]
- Slor, G.; Olea, A.R.; Pujals, S.; Tigrine, A.; De La Rosa, V.R.; Hoogenboom, R.; Albertazzi, L.; Amir, R.J. Judging Enzyme-Responsive Micelles by Their Covers: Direct Comparison of Dendritic Amphiphiles with Different Hydrophilic Blocks. Biomacromolecules 2021, 22, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Sethuraman, V.T.; Bae, Y.H. Stimuli-sensitive polymeric micelles as anticancer drug carriers. Anticancer Agents Med. Chem. 2006, 6, 525–535. [Google Scholar] [CrossRef]
- Reddy, B.P.K.; Yadav, H.K.S.; Nagesha, D.K.; Raizaday, A.; Karim, A. Polymeric Micelles as Novel Carriers for Poorly Soluble Drugs—A Review. J. Nanosci. Nanotechnol. 2015, 15, 4009–4018. [Google Scholar] [CrossRef]
- Han, Z.; Gong, C.; Li, J.; Guo, H.; Chen, X.; Jin, Y.; Gao, S.; Tai, Z. Immunologically modified enzyme-responsive micelles regulate the tumor microenvironment for cancer immunotherapy. Mater. Today Bio 2022, 13, 100170. [Google Scholar] [CrossRef]
- Ma, P.; Chen, J.; Bi, X.; Li, Z.; Gao, X.; Li, H.; Zhu, H.; Huang, Y.; Qi, J.; Zhang, Y. Overcoming Multidrug Resistance through the GLUT1-Mediated and Enzyme-Triggered Mitochondrial Targeting Conjugate with Redox-Sensitive Paclitaxel Release. ACS Appl. Mater. Interfaces 2018, 10, 12351–12363. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.-J.; Lai, J.-Y. Amination degree of gelatin is critical for establishing structure-property-function relationships of biodegradable thermogels as intracameral drug delivery systems. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Chung, J.E.; Miyazaki, T.; Yokoyama, M.; Sakai, K.; Okano, T. Thermal modulation of intracellular drug distribution using thermoresponsive polymeric micelles. React. Funct. Polym. 2007, 67, 1398–1407. [Google Scholar] [CrossRef]
- Qi, M.; Li, G.; Yu, N.; Meng, Y.; Liu, X. Synthesis of thermo-sensitive polyelectrolyte complex nanoparticles from CS-g-PNIPAM and SA-g-PNIPAM for controlled drug release. Macromol. Res. 2014, 22, 1004–1011. [Google Scholar] [CrossRef]
- Kim, J.; Francis, D.M.; Sestito, L.F.; Archer, P.A.; Manspeaker, M.P.; O’Melia, M.J.; Thomas, S.N. Thermosensitive hydrogel releasing nitric oxide donor and anti-CTLA-4 micelles for anti-tumor immunotherapy. Nat. Commun. 2022, 13, 1479. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Li, G.; Gao, Y.; Jiang, H.; Tao, Q. Thermo-sensitive complex micelles from sodium alginate-graft-poly(N-isopropylacrylamide) for drug release. Int. J. Biol. Macromol. 2016, 86, 296–301. [Google Scholar] [CrossRef]
- Li, G.; Meng, Y.; Guo, L.; Zhang, T.; Liu, J. Formation of thermo-sensitive polyelectrolyte complex micelles from two biocompatible graft copolymers for drug delivery. J. Biomed. Mater. Res. A 2014, 102, 2163–2172. [Google Scholar] [CrossRef]
- Guo, X.; Li, D.; Yang, G.; Shi, C.; Tang, Z.; Wang, J.; Zhou, S. Thermo-triggered Drug Release from Actively Targeting Polymer Micelles. ACS Appl. Mater. Interfaces 2014, 6, 8549–8559. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, S.H.; Kim, S.H.; Park, T.G. Thermally sensitive cationic polymer nanocapsules for specific cytosolic delivery and efficient gene silencing of siRNA: Swelling induced physical disruption of endosome by cold shock. J. Control. Release Off. J. Control. Release Soc. 2008, 125, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, Y.; Guo, C.; Wang, J.; Ma, J.; Liang, X.; Yang, L.-R.; Liu, H.-Z. Temperature-responsive magnetite/PEO-PPO-PEO block copolymer nanoparticles for controlled drug targeting delivery. Langmuir 2007, 23, 12669–12676. [Google Scholar] [CrossRef]
- Gupta, A.K.; Naregalkar, R.R.; Vaidya, V.D.; Gupta, M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine 2007, 2, 23–39. [Google Scholar] [CrossRef]
- Wang, Y.; Kohane, D.S. External triggering and triggered targeting strategies for drug delivery. Nat. Rev. Mater. 2017, 2, 17020. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Yuan, R.; Chen, G.; Blanco, E.; Gao, J.; Shuai, X. Folate-encoded and Fe3O4-loaded polymeric micelles for dual targeting of cancer cells. Polymer 2008, 49, 3477–3485. [Google Scholar] [CrossRef]
- Francis, M.F.; Cristea, M.; Winnik, F.M. Exploiting the vitamin B12 pathway to enhance oral drug delivery via polymeric micelles. Biomacromolecules 2005, 6, 2462–2467. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. In Vitro and cell uptake studies for targeting of ligand anchored nanoparticles for colon tumors. Eur. J. Pharm. Sci. 2008, 35, 404–416. [Google Scholar] [CrossRef]
- Roger, E.; Lagarce, F.; Garcion, E.; Benoit, J.-P. Biopharmaceutical parameters to consider in order to alter the fate of nanocarriers after oral delivery. Nanomedicine 2010, 5, 287–306. [Google Scholar] [CrossRef]
- Vega-Villa, K.R.; Takemoto, J.K.; Yáñez, J.A.; Remsberg, C.M.; Forrest, M.L.; Davies, N.M. Clinical toxicities of nanocarrier systems. Adv. Drug Deliv. Rev. 2008, 60, 929–938. [Google Scholar] [CrossRef]
- Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.-J.; Préat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release Off. J. Control. Release Soc. 2006, 116, 1–27. [Google Scholar] [CrossRef]
- Onoue, S.; Yamada, S.; Chan, H.-K. Nanodrugs: Pharmacokinetics and safety. Int. J. Nanomed. 2014, 9, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shen, R.; Meng, T.; Hu, F.; Yuan, H. Nano-Drug Delivery Systems Based on Different Targeting Mechanisms in the Targeted Therapy of Colorectal Cancer. Molecules 2022, 27, 2981. [Google Scholar] [CrossRef]
- Kesharwani, P.; Gupta, U. Nanotechnology-Based Targeted Drug Delivery Systems for Brain Tumors; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Baranyai, Z.; Baranyai, Z.; Soria-Carrera, H.; Alleva, M.; Millán-Placer, A.C.; Lucía, A.; Martín-Rapún, R.; Aínsa, J.A.; de la Fuente, J.M. Nanotechnology-Based Targeted Drug Delivery: An Emerging Tool to Overcome Tuberculosis. Adv. Ther. 2021, 4, 2000113. [Google Scholar] [CrossRef]
- Kirch, J.; Guenther, M.; Doshi, N.; Schaefer, U.F.; Schneider, M.; Mitragotri, S.; Lehr, C.M. Mucociliary clearance of micro- and nanoparticles is independent of size, shape and charge—An Ex Vivo and In Silico approach. J. Control. Release Off. J. Control. Release Soc. 2012, 159, 128–134. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Perin, F.; Motta, A.; Maniglio, D. Amphiphilic copolymers in biomedical applications: Synthesis routes and property control. Mater. Sci. Eng. C 2021, 123, 111952. [Google Scholar] [CrossRef] [PubMed]
- Vonarbourg, A.; Passirani, C.; Saulnier, P.; Benoit, J.-P. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials 2006, 27, 4356–4373. [Google Scholar] [CrossRef] [PubMed]
- Naksuriya, O.; Shi, Y.; van Nostrum, C.F.; Anuchapreeda, S.; Hennink, W.E.; Okonogi, S. HPMA-based polymeric micelles for curcumin solubilization and inhibition of cancer cell growth. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. e.V 2015, 94, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nagasaki, Y.; Kato, Y.; Sugiyama, Y.; Kataoka, K. Long-circulating poly(ethylene glycol)-poly(D,L-lactide) block copolymer micelles with modulated surface charge. J. Control. Release Off. J. Control. Release Soc. 2001, 77, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Zhang, L.; Fang, X. Pharmacokinetics and biodistribution of paclitaxel-loaded pluronic P105 polymeric micelles. Arch. Pharm. Res. 2008, 31, 530–538. [Google Scholar] [CrossRef]
- Onoue, S.; Kojo, Y.; Suzuki, H.; Yuminoki, K.; Kou, K.; Kawabata, Y.; Yamauchi, Y.; Hashimoto, N.; Yamada, S. Development of novel solid dispersion of tranilast using amphiphilic block copolymer for improved oral bioavailability. Int. J. Pharm. 2013, 452, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Ramsey, J.D.; Kabanov, A. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release Off. J. Control. Release Soc. 2014, 190, 465–476. [Google Scholar] [CrossRef]
- Gong, J.; Chen, M.; Zheng, Y.; Wang, S.; Wang, Y. Polymeric micelles drug delivery system in oncology. J. Control. Release Off. J. Control. Release Soc. 2012, 159, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-P.H.; Horwitz, S.B. Taxol(®): The First Microtubule Stabilizing Agent. Int. J. Mol. Sci. 2017, 18, 1733. [Google Scholar] [CrossRef]
- Kim, S.C.; Kim, D.W.; Shim, Y.H.; Bang, J.S.; Oh, H.S.; Wan Kim, S.; Seo, M.H. In Vivo evaluation of polymeric micellar paclitaxel formulation: Toxicity and efficacy. J. Control. Release Off. J. Control. Release Soc. 2001, 72, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Chen, C.; Pang, X.; Yu, Z.; Qi, Y.; Chen, X.; Liang, H.; Fang, X.; Sha, X. Adding vitamin E-TPGS to the formulation of Genexol-PM: Specially mixed micelles improve drug-loading ability and cytotoxicity against multidrug-resistant tumors significantly. PLoS ONE 2015, 10, e0120129. [Google Scholar] [CrossRef]
- Zheng, X.; Xie, J.; Zhang, X.; Sun, W.; Zhao, H.; Li, Y.; Wang, C. An overview of polymeric nanomicelles in clinical trials and on the market. Chin. Chem. Lett. 2021, 32, 243–257. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Matsumura, Y.; Suzuki, M.; Shimizu, K.; Goda, R.; Nakamura, I.; Nakatomi, I.; Yokoyama, M.; Kataoka, K.; Kakizoe, T. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend In Vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br. J. Cancer 2005, 92, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Sofias, A.M.; Dunne, M.; Storm, G.; Allen, C. The battle of ‘nano’ paclitaxel. Adv. Drug Deliv. Rev. 2017, 122, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Mukai, H.; Kato, K.; Esaki, T.; Ohsumi, S.; Hozomi, Y.; Matsubara, N.; Hamaguchi, T.; Matsumura, Y.; Goda, R.; Hirai, T.; et al. Phase I study of NK105, a nanomicellar paclitaxel formulation, administered on a weekly schedule in patients with solid tumors. Invest New Drugs 2016, 34, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Chin, K.; Yoshikawa, T.; Yamaguchi, K.; Tsuji, Y.; Esaki, T.; Sakai, K.; Kimura, M.; Hamaguchi, T.; Shimada, Y. Phase II study of NK105, a paclitaxel-incorporating micellar nanoparticle, for previously treated advanced or recurrent gastric cancer. Invest New Drugs 2012, 30, 1621–1627. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Mukai, H.; Saeki, T.; Ro, J.; Lin, Y.C.; Nagai, S.E.; Lee, K.S.; Watanabe, J.; Ohtani, S.; Kim, S.B.; et al. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br. J. Cancer 2019, 120, 475–480. [Google Scholar] [CrossRef]
- Lee, S.-W.; Yun, M.H.; Jeong, S.W.; In, C.H.; Kim, J.Y.; Seo, M.H.; Pai, C.M.; Kim, S.O. Development of docetaxel-loaded intravenous formulation, Nanoxel-PMTM using polymer-based delivery system. J. Control. Release 2011, 155, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef]
- Triolimus, C.-D. Therapeutics. Available online: https://co-drx.com/pipeline/triolimus/2023 (accessed on 17 February 2023).
- Hasenstein, J.R.; Shin, H.-C.; Kasmerchak, K.; Buehler, D.; Kwon, G.S.; Kozak, K.R. Antitumor Activity of Triolimus: A Novel Multidrug-Loaded Micelle Containing Paclitaxel, Rapamycin, and 17-AAGMolecular Multitargeting with Triolimus. Mol. Cancer Ther. 2012, 11, 2233–2242. [Google Scholar] [CrossRef]
- Kubiak, T. Polymeric capsules and micelles as promising carriers of anticancer drugs. Polym. Med. 2022, 52, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Zhao, X.; Gao, S.; Huang, D.; Sui, M. Advances in delivery of Irinotecan (CPT-11) active metabolite 7-ethyl-10-hydroxycamptothecin. Int. J. Pharm. 2019, 568, 118499. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Tsuji, A.; Yamaguchi, K.; Takeda, K.; Uetake, H.; Esaki, T.; Amagai, K.; Sakai, D.; Baba, H.; Kimura, M.; et al. A phase II study of NK012, a polymeric micelle formulation of SN-38, in unresectable, metastatic or recurrent colorectal cancer patients. Cancer Chemother. Pharmacol. 2018, 82, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Fukushima, S.; Okamoto, K.; Suzuki, M.; Matsumura, Y.; Yokoyama, M.; Okano, T.; Sakurai, Y.; Kataoka, K. Development of the polymer micelle carrier system for doxorubicin. J. Control. Release Off. J. Control. Release Soc. 2001, 74, 295–302. [Google Scholar] [CrossRef]
- Matsumura, Y.; Hamaguchi, T.; Ura, T.; Muro, K.; Yamada, Y.; Shimada, Y.; Shirao, K.; Okusaka, T.; Ueno, H.; Ikeda, M.; et al. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Br. J. Cancer 2004, 91, 1775–1781. [Google Scholar] [CrossRef]
- Kabanov, A.; Batrakova, E.; Alakhov, V.Y. Pluronic® block copolymers for overcoming drug resistance in cancer. Adv. Drug Deliv. Rev. 2002, 54, 759–779. [Google Scholar] [CrossRef]
- Danson, S.; Ferry, D.; Alakhov, V.; Margison, J.; Kerr, D.; Jowle, D.; Brampton, M.; Halbert, G.; Ranson, M. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br. J. Cancer 2004, 90, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Armstrong, A.; Newman, C.; Alakhov, V.; Pietrzynski, G.; Brewer, J.; Campbell, S.; Corrie, P.; Rowinsky, E.K.; Ranson, M. A phase 2 study of SP1049C, doxorubicin in P-glycoprotein-targeting pluronics, in patients with advanced adenocarcinoma of the esophagus and gastroesophageal junction. Invest New Drugs. 2011, 29, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Yamamoto, Y.; Yasunaga, M.; Koga, Y.; Kuroda, J.; Takigahira, M.; Harada, M.; Saito, H.; Hayashi, T.; Kato, Y.; et al. NC-6300, an epirubicin-incorporating micelle, extends the antitumor effect and reduces the cardiotoxicity of epirubicin. Cancer Sci. 2013, 104, 920–925. [Google Scholar] [CrossRef]
- Mukai, H.; Kogawa, T.; Matsubara, N.; Naito, Y.; Sasaki, M.; Hosono, A. A first-in-human Phase 1 study of epirubicin-conjugated polymer micelles (K-912/NC-6300) in patients with advanced or recurrent solid tumors. Invest New Drugs 2017, 35, 307–314. [Google Scholar] [CrossRef]
- Ueno, T.; Ueno, T.; Endo, K.; Hori, K.; Ozaki, N.; Tsuji, A.; Kondo, S.; Wakisaka, N.; Murono, S.; Kataoka, K.; et al. Assessment of antitumor activity and acute peripheral neuropathy of 1, 2-diaminocyclohexane platinum (II)-incorporating micelles (NC-4016). Int. J. Nanomed. 2014, 9, 3005. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, L.; Zhang, T.; Zhu, Y.L.; Qiu, F.; Wu, X.G.; Wang, X.L.; Hu, F.Q.; Huang, J. Oxaliplatin-incorporated micelles eliminate both cancer stem-like and bulk cell populations in colorectal cancer. Int. J. Nanomed. 2011, 6, 3207. [Google Scholar]
- Osada, A. NC-6004, a novel cisplatin nanoparticle, in combination with pembrolizumab for head and neck cancer. Integr. Clin. Med. 2019, 3. [Google Scholar] [CrossRef]
- Uchino, H.; Matsumura, Y.; Negishi, T.; Koizumi, F.; Hayashi, T.; Honda, T.; Nishiyama, N.; Kataoka, K.; Naito, S.; Kakizoe, T. Cisplatin-incorporating polymeric micelles (NC-6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in rats. Br. J. Cancer 2005, 93, 678–687. [Google Scholar] [CrossRef]
- Plummer, R.; Wilson, R.H.; Calvert, H.; Boddy, A.V.; Griffin, M.; Sludden, J.; Tilby, M.J.; Eatock, M.; Pearson, D.G.; Ottley, C.J.; et al. A Phase I clinical study of cisplatin-incorporated polymeric micelles (NC-6004) in patients with solid tumours. Br. J. Cancer 2011, 104, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Volovat, S.R.; Ciuleanu, T.E.; Koralewski, P.; Olson, J.E.G.; Croitoru, A.; Koynov, K.; Stabile, S.; Cerea, G.; Osada, A.; Bobe, I.; et al. A multicenter, single-arm, basket design, phase II study of NC-6004 plus gemcitabine in patients with advanced unresectable lung, biliary tract, or bladder cancer. Oncotarget 2020, 11, 3105. [Google Scholar] [CrossRef]
- NCT02240238. Available online: https://beta.clinicaltrials.gov/study/NCT02240238 (accessed on 31 January 2023).
- van Oosterom, A.T.; Schriivers, D. Docetaxel (Taxotere®), a review of preclinical and clinical experience. Part II: Clinical experience. Anticancer Drugs 1995, 6, 356–368. [Google Scholar] [CrossRef]
- Zhao, P.; Astruc, D. Docetaxel nanotechnology in anticancer therapy. ChemMedChem 2012, 7, 952–972. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Mita, M.M.; Ramanathan, R.K.; Weiss, G.J.; Mita, A.C.; LoRusso, P.M.; Burris, H.A.; Hart, L.L., 3rd; Low, S.C.; Parsons, D.M.; et al. Phase I study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin. Cancer Res. 2016, 22, 3157–3163. [Google Scholar] [CrossRef]
- Autio, K.A.; Dreicer, R.; Anderson, J.; Garcia, J.A.; Alva, A.; Hart, L.L.; Milowsky, M.I.; Posadas, E.M.; Ryan, C.J.; Graf, R.P.; et al. Safety and efficacy of BIND-014, a docetaxel nanoparticle targeting prostate-specific membrane antigen for patients with metastatic castration-resistant prostate cancer: A phase 2 clinical trial. JAMA Oncol. 2018, 4, 1344–1351. [Google Scholar] [CrossRef]
- Natale, R.; Socinski, M.A.; Hart, L.L.; Lipatov, O.N.; Spigel, D.R.; Gershenhorn, B.; Weiss, G.J.; Kazmi, S.; Karaseva, N.A.; Gladkov, O.A.; et al. 41 Clinical activity of BIND-014 (docetaxel nanoparticles for injectable suspension) as second-line therapy in patients (pts) with Stage III/IV non-small cell lung cancer. Eur. J. Cancer 2014, 50, 19. [Google Scholar] [CrossRef]
- Ledford, H. Bankruptcy filing worries developers of nanoparticle cancer drugs. Nature 2016, 533, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Kumar, A.; Ma, J.; Kuang, Y.; Luo, L.; Sun, X. Density gradient ultracentrifugation for colloidal nanostructures separation and investigation. Sci. Bull. 2018, 63, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Han, H.-K. Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Investig. 2018, 48, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Committee for Medicinal Products for Human Use; European Medicine Agency Joint MHLW/EMA Reflection Paper on the Development of Block Copolymer Micelle Medicinal Products. 2013. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-joint-ministry-health-labour-welfare/european-medicines-agency-reflection-paper-development-block-copolymer-micelle-medicinal-products_en.pdf (accessed on 17 February 2023).
- Zhang, H.; Li, H.; Cao, Z.; Du, J.; Yan, L.; Wang, J. Investigation of the In Vivo integrity of polymeric micelles via large Stokes shift fluorophore-based FRET. J. Control. Release 2020, 324, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zeng, F.; Allen, C. Influence of serum protein on polycarbonate-based copolymer micelles as a delivery system for a hydrophobic anti-cancer agent. J. Control. Release 2005, 103, 481–497. [Google Scholar] [CrossRef]

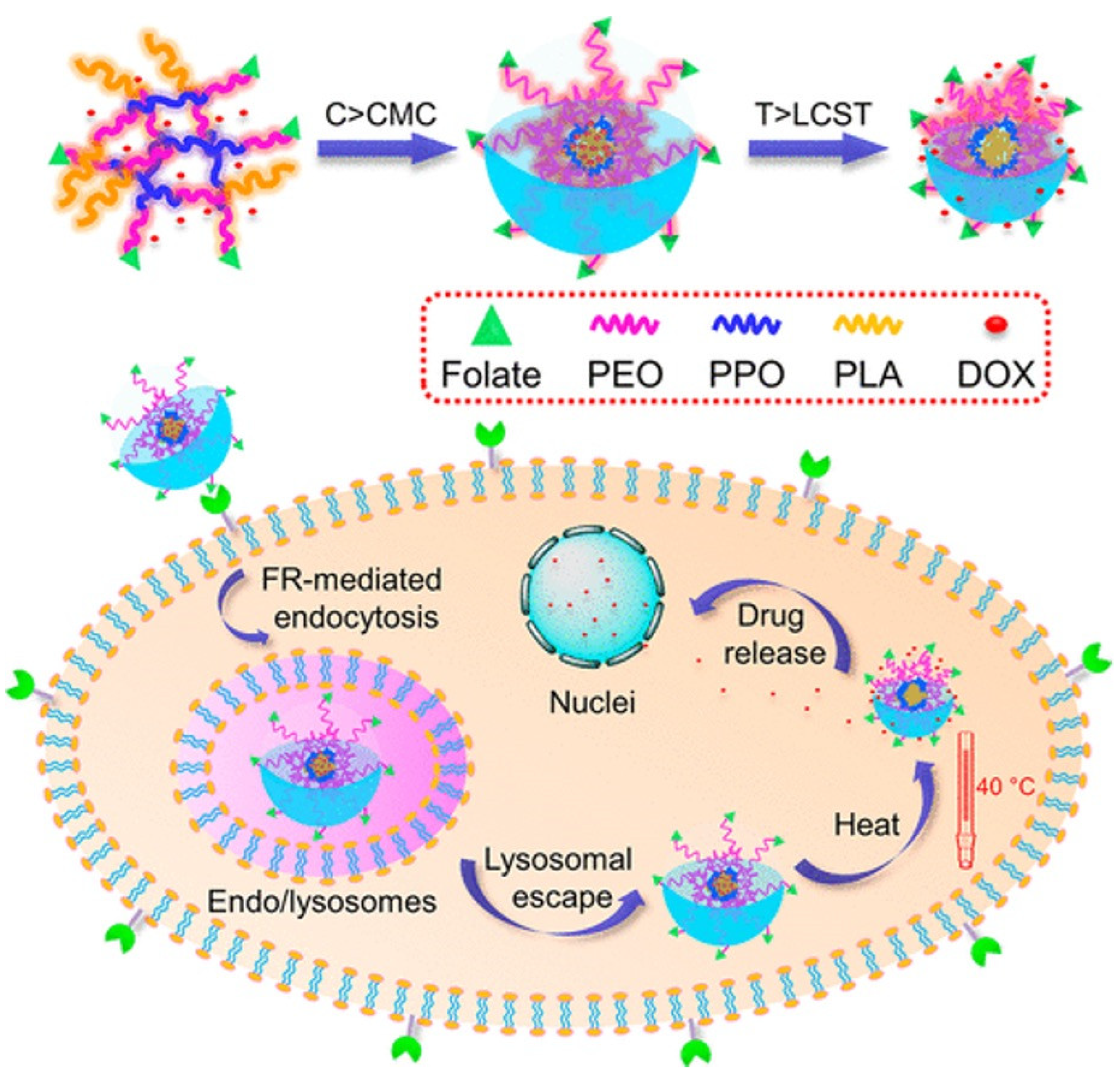

| Polymer | Structure | Advantages | Disadvantages | Ref |
|---|---|---|---|---|
| PEG | 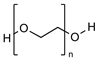 | Clinically approved Stealth behavior Prolonged blood circulation Diminished RES uptake Enhanced permeability and retention effect | Unexpected changes in PK behavior Non-biodegradable | [57,58] |
| Polysaccharides | 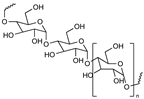 | Non-toxic Biodegradable Stealth behavior Facilitating mucoadhesion Enhanced targeting of specific tissues Enhanced a reduction in the inflammatory response Easy for modification | Its degradation (oxidation) characteristics at high temperatures (above their melting point), which are often required in industrial processes Toxicity due to impurities | [59,60,61,62,63] |
| pHPMA | 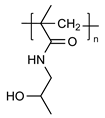 | Non-toxic Non-immunogenic Biocompatible Pendant groups readily engineered | Only a few soluble drug conjugates have entered clinical trials Complicated synthesis The unsatisfactory characteristics of the conjugate molecules The tendency for such conjugates to perform differently in preclinical animal models than in the human body | [65,66,67,68,75] |
| Poly(acrylic acid) |  | pH sensitive, mucoadhesive Biodegradable Biocompatible | Poor mechanical properties, its structures need to be modified for use | [69,70,71,76] |
| Poly(glutamic acid) |  | pH sensitive Biodegradable Biocompatible Easy for chemical modification | α- poly(glutamic acid) synthetically produced has a lower molecular mass which limits its application High cost of production | [71,77] |
| Polyvinyl alcohol | 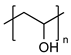 | Widely used for cross-linking synthesis Biocompatible Non-immunogenic Non-toxic | Under wet conditions, its properties are diminished because of the plasticizing action of water molecules | [78,79,80] |
| Poly(N-vinyl-2-pyrrolidone) | 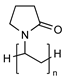 | Containing cationic groups for modification | Non-biodegradable Hygroscopic | [81,82,83] |
| Poly(N-isopropyl acrylamide) (PNIAAm) | 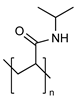 | Thermo sensitive controlling payload release | In vivo studies are still pending for most copolymers, grafted polymers, and biopolymer-conjugates investigated up to today due to high cost and ethical restrictions for in vivo analysis to test their viability | [84,85] |
| Poly(ethylene imine) |  | Facilitating to escape from endosome and payload release in cytoplasm Facilitating cellular uptake | Positively charged with toxicity Difficult to release negatively charged payloads due to strong electro attraction | [86] |
| Polymer | Structure | Advantage | Disadvantage | Ref |
|---|---|---|---|---|
| Poly(histidine) (PHIS) |  | hydrophilic at acidic pH condition; hydrophobic at pH around 7.4, pH sensitive Biocompatible Biodegradable Facilitating to escape from endosome and payload release in cytoplasm | Poly(histidine) is too sensitive to environmental pH, which could affect the stability of the core The chain length also affects the anticancer efficacy and the pH responsive drug release rate | [87,88] |
| Polyethers (i.e., poly(propylene oxide; block copolymers such as Pluronics) | Pluronics: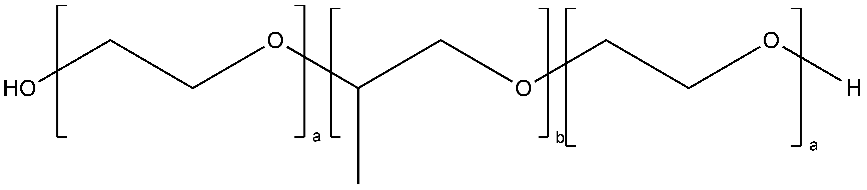 | Widely used, commercially available Non-expensive Pluronics are thermoresponsive. | Low affinity with drug molecules | [34,72] |
| Polyesters (i.e., poly(lactide), poly(lactide-co-glycolide), poly(ε-caprolactone), poly(β-amino ester)), poly(glycolic acid), poly(lactide-co-caprolactone)) | Poly(lactide) (PLA): 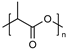 Poly(lactide-co-glycolide) (PLGA): 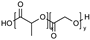 Poly(ε-caprolactone) (PCL): 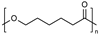 Poly(glycolic acid) (PGA):  Poly(lactide-co-caprolactone) (PLCA): 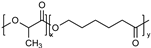 | Biodegradable and biocompatible. These are the most used polymers for drug delivery. They show excellent control on the drug release rates. Poly(β-amino ester) is pH sensitive, providing stimuli-responsive drug release, and is used for gene carriers and increased cell uptake due to positive charges. Poly(glycolic acid) is thermoplastic, enabling a stimuli response. | High hydrophobicity and subsequently entrapment by macrophages through the opsonization process, long-term degradation time, and low loading for hydrophilic drugs. PLA induces the production of lactic acid due to polymer degradation, which leads to the formation of an acidic microenvironment. PLA shows initial burst release with significant drug loss and drug-related toxicity. PCL has a slow degradation time. Poly(β-amino ester): cationic charges may be toxic to cells. Poly(glycolic acid) has a fast degradation time with fast drug release. | [34,73,89,90,91,92,93] |
| Stimuli | Polymeric Carrier | Payload | Release Mechanism | Application | Ref |
|---|---|---|---|---|---|
| pH | Poly(L-histidine)-b-poly(ethylene glycol)/poly(L-lactic acid)-b-poly(ethylene glycol-folate | Doxorubicin | Protonation of PHIS | PHIS destabilizes micelles and triggers doxorubicin release | [97] |
| Poly(ethylene glycol-block-poly[(1,4-butanediol)-diacrylate-β-5-amino-1-pentanol]/2,3-dimethylmaleic anhydride-polyethyleneimine-b-poly[(1,4-butanediol)-diacrylate-β-5-amino-1-pentanol] | Paclitaxel | Protonation of poly[(1,4-butanediol)-diacrylate-β-5-amino-1-pentanol] | 2,3-dimethylmaleic anhydride enhances micelles internalization; poly[(1,4-butanediol)-diacrylate-β-5- amino-1-pentanol] dissociates micelles and triggers paclitaxel release | [98] | |
| Methyl poly(ethylene glycol) ether-b-poly(β-amino esters)-b-poly lactic acid | Doxorubicin | Protonation of poly(β-amino esters) | Poly(β-amino esters decreases hydrophobicity of micelles at acidic condition and triggers doxorubicin release | [99] | |
| Poly(ethylene glycol)-poly(L-histidine)-poly(L-lactide) | Doxorubicin | Protonation of PHIS | PHIS swells and relocates to the surface of the micelles to trigger doxorubicin release | [100] | |
| Methoxy-poly (ethylene glycol)-b-poly (ε-caprolactone)-b-poly (diethylaminoethyl methacrylate) | Curcumin | Protonation of poly (diethylaminoethyl methacrylate) | Poly (diethylaminoethyl methacrylate) switch from hydrophobic to hydrophilic to change micelles structure and triggers release Curcumin | [101] | |
| Poly(2-(diisopropylamino)ethyl methacrylate-co-2-(2′,3′,5′-triiodobenzoyl)ethyl methacrylate) | Dextran/Doxorubicin | Protonation of poly(2-(diisopropylamino) | Poly(2-(diisopropylamino) switch from a hydrophobic to a hydrophilic state under acidic conditions upon protonation, which deceases the stability of micelles and triggers drug release | [102] | |
| Methyl ether poly(ethylene glycol)-poly(β-amino ester) | Camptothecin | Protonation of poly(β-amino ester | Poly(β-amino ester facilitates a pH-dependant micellization/demicellization transition and triggers camptothecin | [103] | |
| Poly (ethylene glycol) methyl ether-b-(poly lactic acid-co-poly (β-amino esters)) | Doxorubicin | Protonation of poly(β-amino ester) | Poly(β-amino ester) destabilizes micelles and triggers doxorubicin release | [104] | |
| Methoxy poly (ethylene oxide)-b-poly (aspartate-hydrazide) | Doxorubicin/SN-38 | Hydrolisis of Hydrazone bond | Se-Se bond exerting redox responsiveness and Hydrazone bond hydrolyzing decrease micelles stability and trigger Doxorubicin/SN-38 release | [105] | |
| Hyaluronic acid-S-S-Podophyllotoxin | Podophyllotoxin | Cleavage of acid-sensitive ester bonds | Ester bonds and disulfide bonds cleave to decrease micelle stability and podophyllotoxin releases from micelles | [106] | |
| Hydrazide functionalized methoxy poly(ethylene glycol)-block-poly(ε-caprolactone) | LCA | Electrorepulsion between LCA and the copolymer | Loss of ionic interaction between LCA and micelles triggers LCA release | [107] | |
| Chitosan coated hyaluronic acid-oleic acid | Doxorubicin/siPD-L1 | Protonation of the amino group of COS | Decomposition of copolymer shell, the swelling of COS, and disulfide bond cleavage trigger drug release | [108] | |
| Methoxypolyethylene glycols-b-poly (6-O-methacryloyl-d-galactopyranose)-disulfide bond-doxorubicin | Doxorubicin | Destability of hydrazone bonds | The destability of hydrazone bonds decrease micelles stability; the break of disulfide bonds causes decreased hydrophobicity in the micellar inner cores and dissociates the conjugates to release doxorubicin | [109] | |
| ROS | Polyethylene glycol-p(2-aminoethyl methacrylate hydrochloride-camptothecin conjugated hydroxyethyl methacrylate-oxalyl chloride | β-Lapachone/camptothecin | Breaking the H2O2-cleavable linkage from camptothecin | The removal of camptothecin enhances the disassembly of the micelles and drug release | [110] |
| Poly(β-thioether ester)-poly (ethylene glycol)-lipoic acid | Doxorubicin | Thioether group and disulfide bond cleavage | The cleavage of disulfide bonds and β-thiopropionate linkers decrease in core crosslinking density and trigger doxorubicin release | [111] | |
| Methoxy poly(ethylene glycol)-thioketal-poly(ε-caprolactone) | Doxorubicin | Thioketal bond cleavage | π–π interactions increase drug loading; thioketal bond cleavage increases doxorubicin release | [112] | |
| Poly(l-methionine-block-l-lysine)-PLGLAG-methoxy poly(ethylene glycol) | Doxorubicin | MMP-sensitive linkers (PLGLAG) cleavage | Poly-l-lysine chains assist the cellular penetration by electrostatic interactions; thioether converts to a sulfoxide moiety to cause a phase transitions and micelle structure break to release Doxorubicin | [113] | |
| CD147-Carboxymethyl chitosan-phenylboronic acid pinacol ester | Doxorubicin/CD147 | Oxidation of phenylboronic acid pinacol ester | The micelles exert CD147 targeting effect; ROS depolymerizes micelles and triggers doxorubicin release | [114] | |
| Poly(ethylene glycol)–poly[aspartamidoethyl(p-boronobenzyl)diethylammonium bromide] | miR-34a mimic/volasertib (BI6727) | Boronic acid reaction | Boronic acid produces tertiary amines and p-quinone methide to enhance micelle degradation and release drugs | [115] | |
| Poly(propylene sulfide)-poly(N-isopropylacrylamide) | Doxorubicin | Hydrophobic (thioether)-to-hydrophilic (sulfoxide, sulfone) transition of thioether | Poly(propylene sulfide) decreases micelles stability and triggers doxorubicin release | [116] | |
| Methyl ether poly(ethylene glycol)-poly(ester-thioether) | Doxorubicin | Oxidation of thioether | Enhance drug loading content via the π-π interaction | [117] | |
| Poly(ethylene glycol)-poly(N6-carbobenzyloxy-l-lysine)-poly(β-benzyl-l-aspartate) | Doxorubicin | Thioketal bond cleavage | The primary-amine-rich pLys block would provide interlace sites for the ROS cleavable cross-linker and then increases doxorubicin release | [118] | |
| Imidazole groups conjugate polyethylene glycol-conjugated triphenylphosphonium | Doxorubicin | TK bonds cleavage | Imidazole groups protonation and TK bonds cleavage release doxorubicin | [119] | |
| Hypoxia | Poly(ethylene glycol)-block-poly(methacrylic acid-co-2-nitroimidazole methacrylate) | Doxorubicin | 2-nitroimidazole converting to hydrophilic 2-aminoimidazole | 2-nitroimidazole groups enhances expansion and self-disassembly of micelles, then triggers doxorubicin release | [120] |
| Polyethyleneimine-C6-2-nitroimidazole | siRNA | 2-nitroimidazole converting to hydrophilic 2-aminoimidazole | 2-nitroimidazole elicits a loose structure to facilitate the siRNA dissociation in the cytoplasm | [121] | |
| Poly(ethylene glycol-poly(ε-(4-nitro)benzyloxycarbonyl-l-lysine) | Doxorubicin | Degradation of poly(ε-(4-nitro)benzyloxycarbonyl-l-lysine) | Self-immolation of poly(ε-(4-nitro)benzyloxycarbonyl-l-lysine) derivative triggers doxorubicin release | [122] | |
| Poly(ethylene glycol)-azobenzene-polyethyleneimine-DOPE | siRNA/ Doxorubicin | Cleavage of azobenzene | Cleavage of azobenzene triggers PEG shedding and leads to drug release | [123] | |
| Methoxy poly(ethylene glycol)-azobenzene-4,4-diamino-poly(d,l-lactide) | Docetaxel | Reductive cleavage of azobenzene | Reductive cleavage leads to structural change of self-assembled micelles and triggers docetaxel release | [124] | |
| Folic acid-poly(ethylene glycol)-2-nitroimidazole | Sorafenib | Hydrophobic-to-hydrophilic transition of nitro of nitroimidazole | Cohesion of the hydrophobic core of the micelles is weakened; hydrophobic inner core weakens the binding force of the hydrophobic drug, which is more prone to drug leakage and promotes sorafenib release | [125] | |
| Alendronate-poly(ethylene glycol)-azobenzene-poly-l-lysine | Doxorubicin | Reductive cleavage of azobenzene | Azobenzene cleavage for micelle disassembly triggers doxorubicin release | [126] | |
| Glucose-poly(ethylene glycol)-azobenzene-IR808-S-S-Paclitaxel | Paclitaxel | Reductive cleavage of azobenzene | Glucose modification promotes cellular uptake; azobenzene cleavage triggers IR808-S-S-PTX release; disulfide bond cleavage triggers paclitaxel release | [127] | |
| Enzyme | Polyethylene glycol-block-poly(acrylic acid) | Doxorubicin | Amidase cleavaging the covalent linked doxorubicin from the micelles | Amidase causes the breakage of amide bond between doxorubicin molecules and polymers, and then triggers disassembly of the micelles to facilitate the doxorubicin release | [128] |
| Monomethyl poly(ethylene glycol)-ss-camptothecin/phenylboronic acid-poly(ethylene glycol)-4,4′-(diazene-1,2-diyl)benzoyl-poly(ε-caprolactone) | Camptothecin | Azoreductase | Azoreductase and NADPH facilitates the azobenzene bonds cleavage and GSH facilitate disulfide bond cleavage, which trigger camptothecin release | [129] | |
| Poly(ethylene glycol)-peptide- polyethyleneimine-1,2-dioleoyl-sn-glycero-3-phosphoethanolamine | Paclitaxel/siRNA | Metalloproteinase 2 cleavage | Polyethyleneimine increases cellular uptake and delivers siRNA and facilitates endosome escape; MMP2 decreases micelles stability and release drugs | [130] | |
| Methoxypolyethylene glycol amine-glutathione-palmitic acid | Dexamethasone | Glutathione reductase | Glutathione reductase breaks micelles structure and triggers dexamethasone release | [131] | |
| Poly(ethylene glycol)-b-poly(l-tyrosine) | JQ1 | Proteinase K | π–π stacking for efficient and stable encapsulation of JQ1; PTyr degradation by proteinase K triggers JQ1 release | [132] | |
| D-α-tocopherol polyethylene glycol 3350 succinate-Gly-Pro-Leu-Gly-Val-Arg-doxorubicin /FA-Asp-Glu-Val-Asp-doxorubicin | Doxorubicin | Matrix metalloproteinase (MMP-9); caspase-3 | MMP-9 increases micelles endocytosis; caspase-3 increases doxorubicin release | [133] | |
| Thermo | Monomethoxy poly(ethylene glycol)-deoxycholic acid | Estradiol | Lower critical solution temperature (LCST) transition of the micelles facilitating dehydration of the PEG shell | Thermosensitive micelles with a rigid core minimizes the initial burst release of estradiol encapsulated by coating the shell at a temperature above its LCST through the thermal transition | [134] |
| Poly(t-butyl acrylate-co-acrylic acid)-b-poly(N-isopropylacrylamide)/chitosan-g-poly(N-isopropylacrylamide) | Doxorubicin | Poly(N-isopropylacrylamide) exerting temperature responsiveness | The pH-sensitive poly(t-butyl acrylate-co-acrylic acid) encapsulates doxorubicin by electrostatic interactions; and the poly(N-isopropylacrylamide) plays the role of aqueous solubilization and responses to temperature changes, and triggers doxorubicin release | [135] | |
| Poly(N-isopropylacrylamide-b- butylmethacrylat | Adriamycin | Poly(N-isopropylacrylamide) phase transistion | Poly(N-isopropylacrylamide) reverses micelle structure to trigger Adriamycin release | [136] | |
| P-(N,N-isopropylacrylamide-co-N-hydroxymethylacrylamide)-b-caprolactone | Doxorubicin | Poly(N-isopropylacrylamide) phase transistion | Poly(N-isopropylacrylamide) reverses micelle structure to trigger doxorubicin release | [137] | |
| Magnetic | RGD-poly[(N-isopropylacrylamide-r-acrylamide)-b-L-lactic acid]/oleic acid -SPIONs | Paclitaxel | Magnetic hyperthermia | Hydrophobic PLA segments incorporates SPIONs and paclitaxel, RGD serves as a targeting moiety, and SPIONs concentrate paclitaxel to targeted sites | [138] |
| Poly(phenyl isocyanide)s | Doxorubicin/Fe3O4 nanoparticles | Magnetic hyperthermia | The loading of magnetic Fe3O4 nanoparticles contributes to the hyperthermia performance; effective drug release due to the morphology change of thermoresponsive poly(phenyl isocyanide)s | [139] |
| Clinical Trial/Drug | Polymeric Carrier | Condition | Status | Phase | Participants | Clinical Trials ID |
|---|---|---|---|---|---|---|
| Pm-Pac/Paclitaxel | PEG-PLA | Non-Small Cell Lung Cancer | Unknown | Phase 3 | 454 | NCT02667743 |
| Genexol-PM/Paclitaxel | PEG-PLA | Taxane-Pretreated Recurrent Breast Cancer | Unknown | Phase 4 | 90 | NCT00912639 |
| PEG-PLA | Advanced Non-Small Cell Lung Cancer | Completed | Phase 2 | 276 | NCT01023347 | |
| PEG-PLA | Advanced Ovarian Cancer | Unknown | Phase1/2 | 74 | NCT00886717 | |
| PEG-PLA | Advanced Urothelial Cancer Previously Treated with Gemcitabine and Platinum | Completed | Phase 2 | 37 | NCT01426126 | |
| PEG-PLA | Advanced Pancreatic Cancer | Completed | Phase 2 | 43 | NCT00111904 | |
| PEG-PLA | Advanced Hepatocelluar Carcinoma After Failure of Sorafenib | Terminated | Phase 2 | 5 | NCT03008512 | |
| PEG-PLA | Advanced Non-small-cell Lung Cancer | Completed | Phase 2 | 45 | NCT01770795 | |
| PEG-PLA | Gynecologic Cancer (Adult Solid Tumor) | Unknown | Phase 1 | 18 | NCT02739529 | |
| PEG-PLA | Pancreatic Cancer | Completed | Phase 1 | 18 | NCT00882973 | |
| PEG-PLA | Metastatic or Locally Recurrent Breast Cancer | Completed | N/A | 111 | NCT02064829 | |
| NANOXEL-M/Docetaxel | PEG-PLA | Esophageal Squamous Cell Carcinoma | Unknown | Phase 2 | 38 | NCT03585673 |
| PEG-PLA | Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma | Unknown | Phase 2 | 31 | NCT02639858 | |
| NC-6004/Cisplatin | PEG-Poly (glutamic acid) | Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck | Terminated | Phase 1 | 4 | NCT02817113 |
| PEG-Poly (glutamic acid) | Locally Advanced or Metastatic Pancreatic Cancer | Completed | Phase 3 | 310 | NCT02043288 | |
| NK105/Paclitaxel | PEG-Polyaspartate | Metastatic or Recurrent Breast Cancer | Completed | Phase 3 | 436 | NCT01644890 |
| NC-4016/Oxaliplatin | PEG-Poly (glutamic acid) | Advanced Solid Tumors or Lymphoma | Completed | Phase 1 | 34 | NCT03168035 |
| NC 6300/Epirubicin | PEG-Polyaspartate | Advanced Solid Tumors or Advanced, Metastatic, or Unresectable Soft Tissue Sarcoma | Unknown | Phase1b/2 | 150 | NCT03168061 |
| NK012/ SN-38 | PEG-Poly (glutamic acid) | Advanced Solid Tumors Followed by a Dose Expansion Phase in Patients With Metastatic Colorectal Cancer | Completed | Phase 1 | 35 | NCT01238939 |
| PEG-Poly (glutamic acid) | Sensitive Relapsed and Refractory Relapsed Small-Cell Lung Cancer | Completed | Phase 2 | 72 | NCT00951613 | |
| PEG-Poly (glutamic acid) | Locally Advanced Non-Resectable and Metastatic Breast Cancer Patients With Triple Negative Phenotype | Completed | Phase 2 | 61 | NCT00951054 | |
| PEG-Poly (glutamic acid) | Refractory Solid Tumors | Completed | Phase 1 | 39 | NCT00542958 | |
| PEG-Poly (glutamic acid) | Advanced Solid Tumors Followed by a Dose Expansion Phase in Patients With Triple Negative Metastatic Breast Cancer | Completed | Phase 1 | 4 | NCT01238952 | |
| BIND-014/Docetaxel | PEG-PLA | Metastatic Castration-Resistant Prostate Cancer | Completed | Phase 2 | 42 | NCT01812746 |
| PEG-PLA | Non-Small Cell Lung Cancer | Completed | Phase 2 | 64 | NCT01792479 | |
| PEG-PLA | Advanced or Metastatic Cancer | Completed | Phase 1 | 58 | NCT01300533 | |
| PEG-PLA | KRAS Mutation Positive or Squamous Cell Non-Small Cell Lung Cancer | Completed | Phase 2 | 69 | NCT02283320 | |
| PEG-PLA | Urothelial Carcinoma, Cholangiocarcinoma, Cervical Cancer and Squamous Cell Carcinoma of the Head and Neck | Terminated | Phase 2 | 73 | NCT02479178 | |
| Paclitaxel Micelles | Micelles (polymer unknown) | Advanced Solid Tumor | Not yet recruiting | Phase 1 | 98 | NCT04778839 |
| Docetaxel Polymeric Micelles | Micelles (polymer unknown) | Advanced Malignant Solid Tumors | Not yet recruiting | Phase 2 | 110 | NCT05254665 |
| Cisplatin Micelles (HA132) | Micelles (polymer unknown) | Advanced Malignant Solid Tumors | Not yet recruiting | Phase 1/2 | 126 | NCT05478785 |
| PLZ4-coated paclitaxel micelles (PPM) | PEG-Cholic acid | Non-myoinvasive Bladder Cancer | Recruiting | Phase 1 | 29 | NCT05519241 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Atluri, K.; Tiwari, A.K.; Babu, R.J. Exploring the Application of Micellar Drug Delivery Systems in Cancer Nanomedicine. Pharmaceuticals 2023, 16, 433. https://doi.org/10.3390/ph16030433
Wang Q, Atluri K, Tiwari AK, Babu RJ. Exploring the Application of Micellar Drug Delivery Systems in Cancer Nanomedicine. Pharmaceuticals. 2023; 16(3):433. https://doi.org/10.3390/ph16030433
Chicago/Turabian StyleWang, Qi, Keerthi Atluri, Amit K. Tiwari, and R. Jayachandra Babu. 2023. "Exploring the Application of Micellar Drug Delivery Systems in Cancer Nanomedicine" Pharmaceuticals 16, no. 3: 433. https://doi.org/10.3390/ph16030433
APA StyleWang, Q., Atluri, K., Tiwari, A. K., & Babu, R. J. (2023). Exploring the Application of Micellar Drug Delivery Systems in Cancer Nanomedicine. Pharmaceuticals, 16(3), 433. https://doi.org/10.3390/ph16030433








