Abstract
Oral delivery has become the route of choice among all other types of drug administrations. However, typical chronic disease drugs are often poorly water-soluble, have low dissolution rates, and undergo first-pass metabolism, ultimately leading to low bioavailability and lack of efficacy. The lipid-based formulation offers tremendous benefits of using versatile excipients and has great compatibility with all types of dosage forms. Self-microemulsifying drug delivery system (SMEDDS) promotes drug self-emulsification in a combination of oil, surfactant, and co-surfactant, thereby facilitating better drug solubility and absorption. The feasible preparation of SMEDDS creates a promising strategy to improve the drawbacks of lipophilic drugs administered orally. Selecting a decent mixing among these components is, therefore, of importance for successful SMEDDS. Quality by Design (QbD) brings a systematic approach to drug development, and it offers promise to significantly improve the manufacturing quality performance of SMEDDS. Furthermore, it could be benefited efficiently by conducting pre-formulation studies integrated with the statistical design of experiment (DoE). In this review, we highlight the recent findings for the development of microemulsions and SMEDDS by using DoE methods to optimize the formulations for drugs in different excipients with controllable ratios. A brief overview of DoE concepts is discussed, along with its technical benefits in improving SMEDDS formulations.
1. Introduction
Numerous compounds are selected as potential drug candidates by employing high-throughput screening tools. However, >75% of the compounds under current development have poor aqueous solubility [1,2]. In addition, due to the difficulty in disintegrating and dissolving in the gastrointestinal tract, the bioavailability of poorly soluble drugs after oral administration is prone to be low. Physical and chemical modifications of poorly water-soluble drugs have been used to increase their solubility and bioavailability, but there are still some limitations [3,4]. For example, salt form and derivatization may alter the physiochemical properties; however, the change of pH in the physiological environment may lead to drug aggregation or precipitation [5]. Size reduction by micronization could be used to increase the bioavailability of poorly soluble drugs; however, the increased electrostatic interaction between particles may result in difficulties for further compounding and packaging [6]. Recently, lipid-based drug delivery systems, including emulsions [7], microemulsions, self-microemulsifying drug delivery systems (SMEDDS) [8], solid lipid nanoparticle (SLN) [9], nanostructured lipid carrier (NLC) [10,11], and liposome [12], have gained increasing attention for the past decade by virtue of improving the oral bioavailability of poor water-soluble or lipophilic compounds.
2. Lipid-Based Formulation for Oral Administration
2.1. Lipid Formulation Classification System
The concept of the lipid formulation classification system (LFCS) was introduced by Pouton in 2000 [13] and further well-defined in 2006 [14]. The designation of LFCS depends on the amount of oil (triglycerides or mixed glycerides), surfactant (lipophilic or hydrophilic surfactants), and co-solvent phase. Table 1 shows the four types of compositions and properties of LFCS, which could be used to simulate or interpret different lipid formulations in vivo. Briefly, type I formulations have oils requiring further digestion and emulsification by lipase and endogenous surfactant. This system is suitable for drugs with higher solubility in oils, forming coarse dispersions on dilution. To improve the emulsification and solvent capacities, lipophilic surfactants with hydrophilic-hydrophobic balance (HLB) values of less than 12 are included in type II formulations. However, a continuous phase or coarse emulsion might be found once the content of lipophilic surfactants extends beyond the threshold of 25% (w/w). In type III formulations, co-solvents are included to blend with oil and surfactants to form a self-emulsifying system. The water-soluble components tend to separate from the oil during dispersion and further dissolve in the water [13]. Moreover, the size of type III formulations easily reaches the nanoscale level after self-emulsification; therefore, these delivery systems are commonly referred to as SMEDDS. Type III formulations are classified into type IIIa and type IIIb. In type IIIa formulations, more amounts of lipids are blended with lipophilic surfactants (HLB < 12) and co-solvents to stabilize the emulsion. In contrast, less amount of lipids are mixed with hydrophilic surfactants (HLB > 12) and/or co-solvents in type IIIb formulations. It has been reported that a fine dispersion with a small particle size (<100 nm) could be produced in the formulations when the amounts of hydrophilic surfactants are over 40% (w/w) or combined with co-solvents [13]. In this regard, type IIIb can achieve greater dispersion rates with small particle sizes compared to type IIIa formulations. However, drug precipitation might appear in the dispersion process due to the lower lipid content. Type IV formulations do not contain any oil and constitute lipophilic and hydrophilic surfactants. These formulations are suitable for a drug that is hydrophobic but not lipophilic [14]. Since surfactant is mixed with co-solvent in type IV formulations, it provides better solvent capacity on dilution than using co-solvent alone.

Table 1.
The features of four essential lipid formulation types in the lipid formulation classification system [14,15,16,17].
2.2. The Compositions of Lipid-Based Formulations and Their Role in Enhancement of Bioavailability
2.2.1. Triglycerides and Mixed Glycerides Used as Lipid Phase in Lipid-Based Formulations
Triglyceride is an ester in which three molecules of fatty acid are linked to the alcohol glycerol. Since triglyceride can be completely digested and absorbed after oral administration, the safety concerns are minimized for further pharmaceutical development. Common oils used in the preparation of lipid-based formulation for oral administration are shown in Table 2. Current triglycerides approved by the US Food and Drug Administration (FDA) are mainly derived from plants. According to the length of the fatty acid chain, it could be divided into medium-chain triglycerides (MCT) and long-chain triglycerides (LCT). Basically, MCT is the preferred oil phase used for the preparation of lipid formulations [15,18,19] due to their less suspected oxidative damage [20] and greater solvent capacity compared to LCT [21].

Table 2.
The characterizations of different types of glycerides used in lipid-based formulations [22].
2.2.2. Surfactants
Surfactants are included as an emulsifying agent to avoid phase separation, reduce the interfacial tension and protect the droplets from agglomeration [23]. Presently, the choice of surfactants is still limited due to the safety concern for oral administration. Compared to synthetic surfactants, emulsifiers of natural origin, such as lecithin, have priority for use since they are considered to be safer. Nonionic surfactants are widely used due to the advantages of lower toxicity and irritancy to the GI tract [24], a greater degree of mixing compatibility [25], and maintaining the stability of emulsified vesicles over a wide range of pH or electrolyte [22]. The role of surfactants in these systems is to reduce the interfacial tension and provide sufficient interfacial coverage for microemulsifying the entire oil and water phases [26]. As nonionic surfactants are often used in microemulsions, their selection is very critical, considering the undesirable side effects such as allergy, irritation, or potential intoxication. However, limited references discuss the threshold or maximum dose of nonionic surfactants used in clinicals. Table 3 shows the latest FDA-approved nonionic surfactants with recommended threshold values for lipid-based formulation.

Table 3.
Approved nonionic surfactants by the FDA and their descriptions, along with each latest maximum potency per dosage unit per 20 October 2022 [27]. The n/a refers to data not available for the corresponding surfactant.
2.2.3. Co-Surfactants/Co-Solvents
Like co-surfactants, co-solvents could regulate the partition of surfactant between the aqueous and oil phases, thereby stabilizing microemulsions to exclude unbounded structures such as liquid crystals, gels, or precipitates [28]. Although both co-surfactants and co-solvents can affect the partition of surfactants, the main role of co-solvents is to accelerate the process of emulsification [29], while co-surfactants is to enhance the interface flexibility of the emulsified vesicle [30]. In general, short to medium-chain length alcohols (C2–C12), ethylene glycol, glycerol, propylene glycol, and other above derivations are adequate co-solvents [22]. Among them, ethanol has been used traditionally as a co-solvent in oral solutions, but it may not be suitable for pediatric or other patients who cannot tolerate alcohol.
2.3. Macroemulsions, Microemulsions and Nanoemulsions
Macroemulsion is a thermodynamically unstable state; therefore, oil-water separation often occurs after storage. If co-solvents such as short-chain alcohols are added during high-speed homogenization, nanoemulsions with particle sizes ranging from 100 nm to 1000 nm can be obtained. When a large amount of surfactant is presented in the oil and water phases, microemulsions with particle sizes ranging from 10 to 100 nm can be formed spontaneously [31,32]. Microemulsions and nanoemulsions are all prepared by oil, water, and surfactant, having relatively similar structures (Figure 1). Owing to the small light scattering of microemulsions and nanoemulsions, the appearance of both is mostly transparent or translucent. However, these two types of emulsions have different composition ratios and formation mechanisms. Microemulsions are formed due to the saturated state of surfactant micelles after a large amount of oil is introduced into them. The free energy of colloidal dispersion is smaller than the separate phase. Therefore, when oil, surfactant, and co-surfactant are blended all together, the microemulsions occur rather spontaneously and involve almost no external energy, further indicating a favorable or stable thermodynamic state. In contrast, the nanoemulsions themselves are usually produced by applying shear stress to induce the formation of nano-sized droplets, resulting in an increase in the interfacial surface free energy. In this regard, nanoemulsions are regarded as thermodynamically unstable because they might further decompose into separate phases over time. However, this mechanism might also offer the nanodroplets to be kinetically stable, which is beneficial for long-term storage. The greater the energy barrier between the initial phase state and the emulsion state, the longer the nanoemulsions last before reverting to their original phase [33].
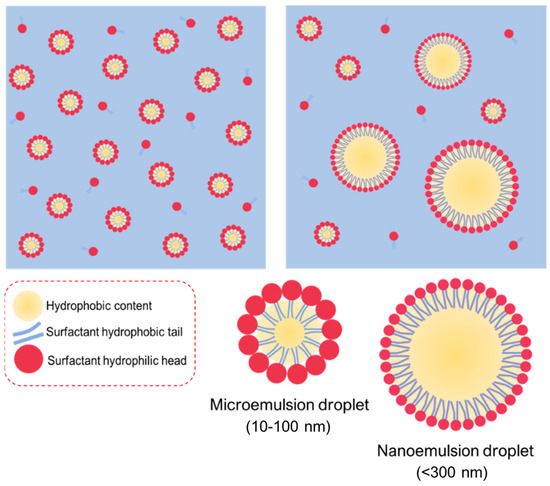
Figure 1.
Illustration of microemulsions and nanoemulsions prepared from the similar elements of oil, water, and surfactant, giving the relatively similar structures with each other.
Particle size is first used to differentiate microemulsions and nanoemulsions. The narrow and sharp peaks refer to microemulsions, whereas the broad or multi-peaks belong to nanoemulsions, suggesting unstable thermodynamics during the nanoemulsion process [33]. Another method for identifying the microemulsions or nanoemulsions is to observe the behavior upon the addition of excess water. In general, a microemulsion is a thermodynamically stable system under a particular range of conditions. However, the system would become unstable during the dilution, and the droplets may break down. Conversely, nanoemulsions are kinetically stable and dilutable with water, which keep the size distribution unchanged with no sign of phase inversion [33].
The advantage of microemulsions and nanoemulsions is their ability to encapsulate lipophilic drugs to enhance their solubility, dissolution (or dispersion) rate, and bioavailability. However, the number of clinical trials related to microemulsions and nanoemulsions decreased over the years [34] due to the large volume and a high proportion of surfactants used in these systems [35]. Table 4 shows the comparison of microemulsions and nanoemulsions.

Table 4.
The comparison of nanoemulsions and microemulsions [22,32].
2.4. Self-Microemulsifying Drug Delivery System (SMEDDS)
Emulsion systems are associated with their own set of complexities, including stability and manufacturing problems associated with their commercial production. SMEDDS belongs to lipid-based self-emulsification systems with isotropic appearance. They are promising formulations for delivering poorly water-soluble lipophilic drugs and can spontaneously generate oil-in-water (w/o) nanosized droplets under gentle blending after dilution in an aqueous medium. Their self-dispersion behavior and small droplet sizes upon dispersion have been shown to improve drug absorption from the large interfacial area. Recently, much attention has been focused on this formulation owing to the ease of manufacture [29], higher drug loading capacity [36,37], and the reduction in food effect [38,39]. Compared to microemulsions and nanoemulsions, SMEDDS can significantly reduce the dose volume, which results in attractive commercial viability and patient compliance. In general, a water-free system not only bears lower solvent effects but also diminishes the dosing volume and increases drug stability. Another advantage of SMEDDS is that drug absorption is less affected by food. Dronedarone is a famous example. Dronedarone is an anti-arrhythmic agent with different bioavailability in fed and fast states [40]. Compared to the fasted state, the AUC0–24 h and Cmax of the fed state were approximately 10-fold and 8-fold higher, respectively, after oral administration of marketed dronedarone product (Multaq®) to beagle dogs [39]. However, SMEDDS formulation significantly mitigated the food effect as AUC0–24 h and Cmax in the fed state were only 2.9-fold and 2.6-fold higher, respectively. In this regard, it is speculated that SMEDDS may reduce the variability of drug absorption between pre- and post-prandial state, thereby improving therapeutic efficacy and patient compliance.
2.4.1. Formulation Design and Factors Affecting SMEDDS Formulations
SMEDDS formulations consist of mixing aqueous and oily phases in the presence of surfactants and/or co-solvents. Except for the excipient’s selection, several factors are known to influence the formation of a stable SMEDD, such as preparation conditions, equipment conditions, and preparation temperature. Therefore, to develop a successful formulation, it is critical to understand the scientific information behind the system compositions and preparation conditions, which will affect the phase behavior in each excipient.
Screening of Excipients
In general, SMEDDS formulations are prepared by mixing different proportions of oil, surfactant, and co-solvent selected by ternary phase diagrams. Construction of ternary phase diagrams is frequently used to determine the types of structures resulting from emulsification and to characterize the behavior of a formulation along a dilution process. After equilibration at atmospheric temperature for a period of time, the drugs are added to the mixture and agitated gently to reach the expected concentration. The appearance of formulations should be transparent and clear without any precipitation. Since external forces are added to accelerate the equilibration during SMEDDS preparation, it is necessary to figure out the sequence of adding the excipients and drugs because it will affect the final appearance. In addition, as the solvent capacity of surfactants in SMEDDS will decrease after solubilizing the drug in co-surfactant [41], the sequence in adding the excipients and drugs not only affect the equilibration of formulation but also the drug solubility.
Active Pharmaceutical Ingredient (API) Dose
The SMEDDS is a suitable template for highly hydrophobic APIs, which could dissolve in the oil of formulation. In general, APIs with log p larger than five are more suitable to encapsulate in the SMEDDS with high-loading doses [29]. Since SMEDDS belongs to type IIIb lipid-based formulation, more drugs can be loaded into the formulation when higher amounts of surfactant are used. However, if water-soluble constituents are present in SMEDDS, formulation development requires further consideration because it can initiate precipitation of the drug from the formulation into the GI tract medium.
Polarity of the Lipid Phase
The digestion of lipid excipients and drug partitioning in SMEDDS begins in the GI tract, involving lipid emulsification and solubilization. During this period, some changes in the properties of the protected APIs in oil droplets could be found [42]. Once the lipase catalyzes the oil droplets, there are differences in the absorption quality and biodistribution of the drug, depending on the lipids sealing it. Then, the drug will be fractionated, dissolved in intestinal fluid, and facilitated by the lipoproteins to transport from the lymphatic system to the blood. Therefore, it is necessary to consider the criterion of lipid selection during SMEDDS formulation.
Caliph and co-workers have compared the triglyceride chains used in SMEDDS. They demonstrated that the combination of medium and long-chain fatty acids improved the droplet formation of microemulsions and increased the bioavailability in 12 h compared to that of using long-chain fatty acid only [43]. Lipids and/or glycerides with longer chains are preferable to act as the oil phase for SMEDDS because they can transform to triglycerides which is more favorable to associate with the chylomicron [44].
2.4.2. Characterization and Evaluation Methods for SMEDDS Formulations
Droplet size is an important parameter in the assessment of SMEDDS since it influences the lipolysis process, drug release, and, consequently, absorption. The droplet size distribution of microemulsion vesicles can be determined by either electron microscopy or light-scattering techniques. The surface charge is determined using a zeta potential analyzer by measuring the zeta potential of the preparations. Zeta potential is the electrical potential in the interfacial double layer of a dispersed particle or droplet versus a point in the continuous phase away from the interface. It is often used as an indicator of droplet stability, where values more positive than +30 mV and more negative than −30 mV indicate good stability against coalescence [45].
The characteristics of SMEDDS not only include droplet size and z-potential but also self-emulsification time, which can generally be evaluated using a USP Type II dissolution apparatus. Briefly, the formulation was added into distilled water maintained at 37 °C, and the time to form a clear solution was recorded with gentle agitation provided at 100 rpm [38]. If the emulsion rapidly forms a clear appearance in less than 1 min, it can be considered as grade I. Grade II indicates the opacity of the emulsion is slightly foggy within 2 min. If a bright white emulsion forms within 3 min, it can be regarded as grade III. Grade IV shows the appearance of dull and grayish-white emulsion with a slightly oily appearance for more than 3 min. In contrast, grade V exhibits poor emulsification with large oil droplets present on the surface [46].
The degree of lipolysis in vitro is also used to evaluate the pre-formulations of SMEDDS. The degradation rate affects the toxicological acceptability and the matrix-controlled release of drugs. In general, lipids digested by lipases to form amphiphilic products are a key process in controlling the utility of most lipid-based formulations. The interaction of these digested products with endogenous amphiphilic components such as bile salts, phospholipids, and cholesterol results in the formation of colloidal structures (e.g., droplet vesicles and micelles). These colloidal structures act as a lipophilic reservoir, enabling the partitioning of drugs into colloidal phases during the gastrointestinal transition. Moreover, exogenous lipids may insert into the bile salts or phospholipid structure, promoting micelle expansion and solubility enhancement. The experimental device consisting of a thermally stable reaction vessel under continuous agitation and a pH-stat with an automated burette to add NaOH solution is used to mimic the in vivo situation of lipolysis. FaSSIF or FeSSIF solution is commonly used as the experimental medium. After lipolysis, the digested mixture is ultra-centrifuged to separate the aqueous phase and sedimentation phase. It is believed that an aqueous phase contains the colloidal structure and dissolved drug, which is imperative for absorption. The sedimentation phase usually contains calcium soap of fatty acid and precipitated drugs, which can be used to evaluate the sedimentation velocity of the lipid-based formulation.
The stability assessment of SMEDDS under different stress can be used to predict their shelf life. As the extra force is included in the SMEDDS manufacturing process, the stability of these formulations depends on the thermodynamic equilibrium. Commonly used experimental tests for stability evaluation include centrifugation tests, freeze-thaw cycle tests, thermal stress tests, and dilution stability [47]. Basically, a SMEDDS pre-formulation is centrifugated for more than 20 min at 3000–13,000 rpm. The appearance of the post-centrifugated suspension was observed and correlated with the size distribution upon self-emulsification in the aqueous. Freeze-thaw cycles are regarded as an experiment to determine the thermal stability of SMEDDS. Some APIs or excipients might be sedimented when SMEDDS is stored at low temperatures. For a stable formulation, the sedimentation should rapidly re-dissolve in SMEDDS as the temperature rises to room temperature. Three freeze-thaw cycles are usually performed on the SMEDDS suspension, including freezing at −20 °C for 48 h and followed by thawing at 40 °C for 48 h. For thermal stress testing, the samples will be placed in a certain temperature range (45 °C to 80 °C) for a period of time to observe whether phase separation occurs. Dilution stability is to evaluate the thermodynamic stability of SMEDDS upon dilution in water. For this purpose, various dilution ratios of the dispersive medium should be tested to determine the consistency of droplet size.
2.4.3. New Strategy for SMEDDS Development
As mentioned above, SMEDDS formulations are used to increase the bioavailability of APIs that are difficult to dissolve and have low bioavailability. Although they are regarded as the most appropriate method to increase drug solubility and bioavailability in oral drug administration, there are still few available products on the pharmaceutical market formulated as SMEDDS. This is associated with the several challenges and difficulties that may be encountered during the SMEDDS preparation and administration process.
API deposition from SMEDDS is one of the most common factors. It is known that drugs encapsulated in SMEDDS must be presented in a dissolved state during transit in the GIT. However, some of the encapsulated drugs are strongly affected by the change of pH values upon contact with GI fluids, resulting in ionization and cancellation of absorption [48,49]. The use of water-soluble solvents or volatile oils may interfere with drug solubility (which increases drug precipitation) when further dilution or high-temperature tests are performed. It is essential for drugs to present in a well-dissolved state in lipid-based delivery. The combined surfactant/co-surfactant and lipid imbalance also increase the possibility of drug precipitation if a greater amount of surfactant/co-surfactant was added than the lipid used in the formulation [50]. The incorporation of polymers to SMEDDS is possible to minimize drug precipitation in vivo [51].
Most of the marketed SMEDDS formulations are in soft gelatin capsules, which causes handling issues and also increases the cost of the product. However, gelatin capsules are associated with few drawbacks. Immature stability can be detrimental from this endeavor as the liquid form is susceptible to possible exposure from hydrolysis, temperature/pH changes, and light, which induce drug/excipient degradation, especially unsaturated lipids as they tend to be oxidized by impurities originating from the gelatin capsule [52,53]. Volatile excipients such as co-solvents in SMEDDS formulations are known to migrate into the shells of soft or hard gelatin capsules, resulting in the precipitation of the lipophilic drugs. Thus, combined polymers and the preparation of solid SMEDDS seems to be a logical solution to address these [54].
The efficiency of oral absorption of the hydrophobic drug from the SMEDDS depends on many formulation-related parameters, such as surfactant concentration, oil/surfactant ratios, the polarity of the emulsion, droplet size, and charge, all of which, in essence, determine the self-emulsification ability. Small changes in material attributes could cause poor product performance in SMEDDS development. The ratio of the oil, surfactant, and co-solvent phases is a key factor in producing a suitable SMEDDS formulation. It has been shown that the formulation efficiency of drugs is affected by the oil/surfactant pairing properties, surfactant concentration, oil/surfactant ratio, and the temperature at which self-emulsification occurs. Therefore, in order to obtain the most efficient self-emulsification zone, the selection of the pharmaceutical excipients is very critical to produce an effective delivery system with better bioavailability. Once a list of suitable excipients is determined, screening of binary drug excipients for solubility, compatibility, and stability will be followed to identify the most appropriate lipid system for the drug in question. According to the LFCS category, SMEDDS can be obtained when the proportions of oil, hydrophilic surfactant, and co-solvent are within <20%, 20–50%, and 20–50%, respectively. However, the range of individual ratios suggested in the LFCS is too wide to find a suitable pre-formulation in a limited time period. Moreover, it can be time-consuming for a formulations scientist to determine the optimal composition of the formulation by a traditional approach.
Quality by design (QbD) is a regulatory-driven approach that adopts a multitude of techniques in product development. This approach can help us to choose the most appropriate component and systemically optimize the formulations. With a controlled and reproducible result, a formulation may meet the specific therapeutic goals. Design of experiment (DoE) and risk assessment techniques based on QbD methodologies are increasingly used in the formulation development of SMEDDS. DoE is a rational and scientific approach for understanding how various formulation/process parameters individually and synergically influence the pivotal product characteristics.
3. Overview of the Quality by Design (QbD) and Design of Experiment (DoE) for Pharmaceutical Development
To achieve consistent formulation effects and better quality control, QbD supports parametric options for strong critical attributes. Since reproducibility is a major concern, it is essential to take into account appropriate experimental factors during the variability processing and control or necessarily eliminate a contradictory factor. In other words, it is preferred to ensure the high quality of a product, even though the greater risks are involved, rather than increasing the run quantities [55,56]. Herein, the experiments are not only statistically evaluated (e.g., t-test) but also have all the studied parameters analyzed, and the outcomes of those are validated.
3.1. Quality by Design (QbD)
QbD in pharmaceuticals involves a systematic methodology incorporated into a series of studies with predetermined objectives, emphasizing the controlled quality of the entire process to produce quality products. Here, risk management is more about how to strategically design and mix inputs and outputs to reduce failure rates. In detail, below are some of the issues in performing pharmaceutical QbD that need to be addressed with reference to the FDA regulations [55,56]: (1) the capability of the selected processes to meet the critical quality attributes, (2) low/minimized product variability amongst the batches, (3) clinical relevance of the developed product specification, (4) efficiency of product manufacture and robustness, and (5) the capability in identifying the problem and management of post-approval change of product.
Several components in QbD include: (1) determining the quality target product profile (QTPP) as critical quality attributes (CQAs) of the developed product, (2) determining the critical material attributes (CMAs) through the design of the product, (3) identifying the critical process parameters (CPPs) through the design of the process and correlating the scale-up principles, CMAs, and CPPs to CQAs, and (4) process capability and continual improvement. By including QbD during the pharmaceutical manufacturing, it is expected that product development could be accelerated with a controlled and measurable risk.
3.2. Design of Experiment (DoE)
As mentioned earlier, product and process understanding are key elements of QbD. To best achieve these objectives, in addition to mechanistic models, DoE is an excellent tool that allows pharmaceutical scientists to systematically manipulate factors according to a prespecified design. Traditionally, common experimentation was designed using OFAT (one-factor-at-a-time), which worked by keeping all other variables constant while varying one variable at the same time [57]. Since each experiment must be performed one at a time, numerous runs would be required to achieve adequate information regarding the condition causing the particular problem. Besides being resource (cost, experiments, time, manpower)-intensive, the OFAT method cannot estimate interactions between the variables. DoE, first coined by Ronald A. Fisher in 1935 [58], however, includes all the factors simultaneously by systematic experiments. It has become increasingly prevalent in the formulation arena over the past few years. DoE is a statistical approach to help establish statistical relationships between a set of input and output variables designated by the system/process being studied. Several terms commonly used to describe the flow of DoE include (1) input/independent variables (, , ,…), (2) output/dependent variables (,…), (3) uncontrollable inputs (, , ,…) [59]. Unlike the trial-error method (OFAT), DoE is more efficient and helps structure experiments rationally. The model built by DoE is not only a mathematical model but rather a formal statistical or correlation model that can be derived between input and output variables, wherein each of which is independent.
3.3. Screening Experiment and Factorial Design
Many experiments contain various types of parameters/factors with different levels that need to be investigated. Therefore, to make use of DoE involved in the experiments, the possible factors are sorted through the screening experiment, leaving only a few factors having a large effect. The screening stage usually occurs in the early stages of the experiment, where all factors are first considered as likely to have little or no effect on the response. Furthermore, it is important to ensure that the selected factors are presented within their upper and lower limits [57].
To determine the limits, researchers usually use a certain background of the factors studied, for example, based on literature studies or empirical data. The studied factors should then meet factor compatibility, where all the selected factors, any combination amongst, or their upper/lower limits are physically recognized by the system. The determined combinations of the selected factors are expressed as zero points and presented as coordinates in a multi-dimensional factorial space, which is referred to as the zero level [57].
The term ‘interval of factor variation’ refers to the number that will become the upper limit when added to the zero level and will become the lower limit when subtracted from the zero level. In a numerical way, this is usually expressed as +1 as the upper limit (high), −1 as the lower limit (low), and 0 as the central/zero level. These terms later would be used in a factorial design, which is one of the screening methods of DoE to study the effects due to a variable or combination of some factors simultaneously on a response being examined. Geometrically, factorial design collects the data at the vertices of a cube in k-dimensions, wherein k is the amount of the studied factors. In full-factorial design (FFD), the data are collected from all the vertices [57,59]. Since this method investigates each factor at 2 levels (i.e., high and low, +1 and −1), therefore it requires 2k experimental runs.
There are circumstances that a particular experiment requires many factors to study. In this case, the fractional factorial design (FrFD) is often used as a strategy against the FFD to deliberately cut the FFD in half [57]. FrFD allows to collect data from specific sub-part of all possible factors, which requires 2k−q runs with –q as the number selected to fractionate the FFD. The most important variable could be identified with this FrFD, allowing for more in-depth tests in the future. FrFD contains several resolutions, and the most important ones are III, IV, and V (regarding the description of each, it has been extensively discussed in another review [60]). FrFD strategy works well in basic designs, such as the most regular fractions, but not in complicated situations, such as some irregular fractions and partial fold designs [59].
In addition, certain fractional factorials have no defining interaction between the factors, such as the Plackett–Burman design (PBD) [59]. PBD is a two-level orthogonal type and is used to develop a proximity fuse [61,62]. The total runs of experiment (N) can be investigated up to N-1 factors with N of multiples of 4 [63,64]. This tool only estimates the main effect of the factors during the process and could not be utilized to obtain surface responses during any optimization [65]. It is recommended to choose a matrix with four or more tests from the selected factors being studied, with three replicates included in the center point of the PBD matrix [64]. Another orthogonal array is the so-called Taguchi method, which is generally similar to fractional factorial experiments. The main objective of this method is to use a standardized method to conduct an experiment and to analyze the results [57,59].
3.4. Response Surface Methodology
Response surface methodology (RSM) is a statistical approach used to generate empirical models that typically correlate responses with multiple input factors. It is possible to study the optimization process using the data gathered in this way from the experiments. The y response is a continuous function of several input variables , , ,… where the screening design is basically used sequentially to obtain the shape of the response surface [59]. Since the goal of RSM is to find the optimal response, some factors are utilized to obtain the process yield. For instance, in order to find the temperature () and pH which has an acceptable particle size of SMEDDS (y), the approximation can be denoted as follow:
is the noise observed in the response y. If the expected response is used herein (i.e., ), so the surface will be known as the surface of the response:
A screening design performed initially is useful herein to quickly identify which input factors affect the response the most [59]. For example, regarding the maximum drug loading response on SMEDDS parameters such as surface morphology, particle size, and zeta potential can be the most influencing input factors. Analysis of variance (ANOVA) is further used to assess the significance of the combined factors or the influence of their individuals on the response [59].
In RSM, the relationship between response and the factors is apparently not identified yet. Therefore, the steps in doing the RSM begin with finding an appropriate approximation to determine the correct relationship between the response y and a set of factors x [57,59]. Most of the time, it starts with the low-order polynomial. The first-order model is attributed to a well-modeled response with the factors by linear correlation of the factors:
The second-order model is a polynomial of higher degree which is defined for the system which has curvature:
The , , , represent the model constant term, coefficient of the linear term, and coefficient of the quadratic term, respectively. Most of the RSM problems use one or both models to construct the relationship [59,64]. The least squares method is usually then used to estimate the parameters in the polynomial equation. The mathematical model can be considered relevant if the regression is statistically significant and DoEs do not have a meaningful error (lack of fit; usually indicated as p > 0.05). Regarding the coefficient of determination (R2), it is indicated to be data representative if the coefficient value is closer to 1 [59,64].
3.5. Optimization Methodology
RSM is normally employed in the optimization stage of formulation development. Two of the mostly used RSM methods are known as central composite design (CCD) and Box-Behnken design (BBD) (Figure 2). Three-level full factorial design is another type of optimization that is used if two or three input factors are investigated. The number of experiments is set using 3k; for example, if there are three input factors, the total runs will be 33, 27 experiments [59]. CCD is one of the examples for fitting a second-order model. It has two level (−1 and +1) factorials with an additional point (axial point or star and center point), which allows for the estimation of the effect of pure squares [59,64]. Mathematically, it consists of 2k factorial with factorial runs of 2k axial or star runs and nC as center runs. The CCD is often used in sequential experimentation, wherein the 2k will be used to fit the first-order model, followed by the axial runs to allow the quadratic terms to be incorporated into the model. Mathematically, it is a selected design to fit the second-order model with the distance α of the axial runs from the design center and the number of center point nC. The difference between this design and the factorial design is the presence of a single factor with a coded value in CCD, ±α, varied from 1 to . The α involves rotatability, which depends on the factorial portion of the design. Rotatability is important in RSM. This is because when the optimal location is unknown during optimization, the rotatability acts as the basis for selecting an appropriate design that has the same precision for estimation in all directions [59].
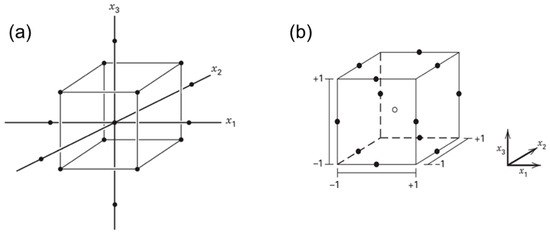
Figure 2.
The central composite design (a) and Box-Behnken design (b) of three factors (k = 3). Adapted from Montgomery [59].
BBD combines 2k factorials with an incomplete block design. It is the three-level design used to fit the response surface. The design is suitable for most of the experiments due to its efficiency and rotatable characteristics. Mathematically, BBD belongs to a spherical design where all the points are on the sphere of radius [59]. The points are not available at the vertices of the cubic area formed by the upper and lower limits for each variable in the BBD. The number of the experiments is usually counted as with as the number of central points [64].
4. Advantages of Applying DoE Techniques for the Development of SMEDDS Formulations
Numerous important parameters need to be involved during the development of SMEDDS formulations, while resources and time are nearly limited. Beyond all that, DoE is one of the effective tools to optimize SMEDDS composition. It offers an efficient experimental formulation that is more rational, ranging from the solubility of the active compound in the combination of excipients, construction of phase diagrams to obtain the most optimal formulation for SMEDDS, all characterizations, and the final responses [66,67,68]. Briefly, an overview of the DoE application in its role in SMEDDS development is presented in Figure 3, wherein it starts from the beginning of the experiments until the evaluation stages.
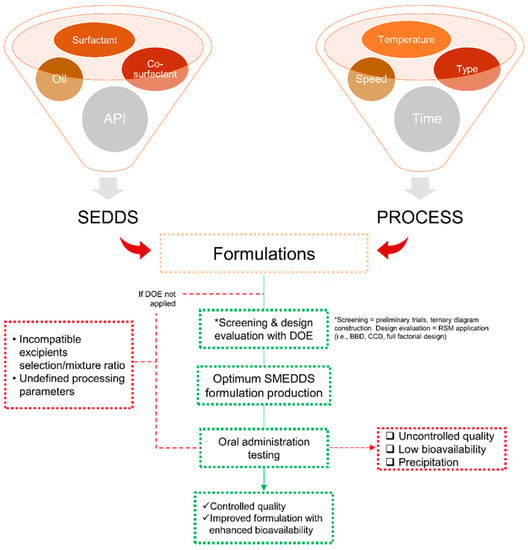
Figure 3.
Scheme of the design of experiment (DoE) application in SMEDDS flow-work starting from the selection of materials and processing attributes in a statistical manner to obtain an optimized SMEDDS formulation/parameter. The mentioned abbreviations include self-emulsifying drug delivery (SEDDS), Box-Behnken design (BBD), and central composite design (CCD).
The following sections are several studies in SMEDDS development using DoE to optimize the variables employed to produce an optimum formulation.
4.1. Box-Behnken Design (BBD)
Marasini and coworkers used BBD to investigate the optimum conditions of spray drying parameters for the solid-SMEDDS flurbiprofen formulation [69]. First, the authors conducted a screening study using a spray drying method with dextran as the solid carrier to obtain a range of independent parameter values, including inlet temperature, feed rate, and carrier concentration. Three levels of three-factors (33) BBD were used thereafter to generate a factorial combination of these independent parameters on responses to evaluate powder characteristics, including %moisture, %yield, drug content, and particle size. All parametric factors contributed to influencing the final product characteristics of SMEDDS with a significance value of p < 0.05. The most critical factor is the concentration of dextran which has a negative effect on the drug content. The authors showed that the optimized parameter validation of the independent variables was close to the predicted value and could reproduce solid SMEDDS with higher yield (58.5%) and drug content (70.1 mg/g) with minimum moisture content (0.72%) and particle size (166.8 nm).
More recently, Dalvadi and coworkers developed zotepine-solid SMEDDS to improve their dissolution rate [68]. Initial screening was performed for the solubility of zotepine in various oil, surfactant, and co-surfactant, which was followed by the construction of pseudo-ternary diagrams to determine the amounts of the selected element. Various solid carriers in different ratios were examined, and Aerosil 200 was chosen as the best one. Three-factor, three-level (33) BBD was then employed to characterize the effect of independent variables (i.e., oleic acid (oil), Tween 80 (surfactant), and PEG400 (co-surfactant)) in the formulation. The % microemulsions transparency and % cumulative drug release were selected based on the principal component analysis as the critical responses used in the BBD. Other variables were also examined, such as the cloud point, emulsification time, and drug content. Irrespective of other variables, the oil content showed an antagonist effect toward both responses significantly, which decreased the % microemulsions transparency and % cumulative drug release (Figure 4a,b). As compared to the conventional zotepine, all optimized parameters produced a higher % transmittance and an improved 30 min-in vitro drug release as final properties of the solid-SMEDDS, which were 98.75% and 86.57%, respectively.
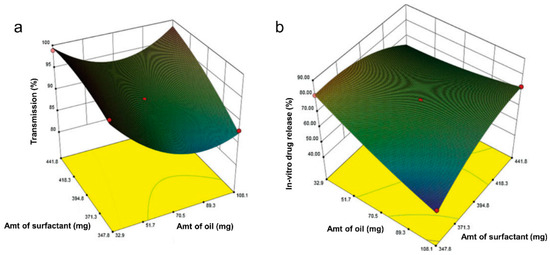
Figure 4.
Response surface plot in three dimensions for BBD showing interaction effects between surfactant and oil on % transmission (a) and % cumulative drug release (b), from the study of zotepine in solid SMEDDS by Dalvadi and coworkers. Adapted from Dalvadi and coworkers [68].
Silva and coworkers developed solid SMEDDS for carvedilol using hot-melt extrusion [70]. Preliminary experiments were done to determine the optimized operating parameters of the extruder with an emphasis on obtaining recirculation time parameters for a homogenous mixing process. BBD was then utilized to evaluate several independent factors, including recirculation time, temperature, and carvedilol concentration, in affecting the cumulative releases in pH 1.2 and pH 6.8. As a result, the increases in recirculation time and temperature significantly lowered the drug release at pH 1.2, while the reduction of both factors increased the release at pH 6.8. In addition, the limited carvedilol solubility significantly affected drug release at pH 6.8, wherein the release was induced if the drug amount decreased. These results underlined another applicability of RSM in constructing efficient and rational variables for system performance used to produce SMEDDS.
Cěrpnjak and coworkers evaluated several solidification methods to produce naproxen solid SMEDDS (tablet), including adsorption, spray-drying, high-shear, and fluid-bed granulation methods [71]. Various carriers were also tested depending on the type of technique to obtain the best solid carrier in transforming the liquid naproxen to a solid state. After obtaining the preliminary results, the spray-drying technique with maltodextrin was selected as the best condition and further used for DoE implementation. The three-factor, two-level (23) factorial design was employed to examine the selected variables, which were inlet temperature, pressure, and pump, on their influences on droplet size, polydispersity index (PDI), and yield. According to the weighted regression coefficients, the antagonistic effects were only indicated in the change of pressure toward the droplet size and the pump speed toward the PDI, whereas the interaction of the three responses had synergistic values. These combined parameters were thus selected to produce the most optimized solid SMEDDS with an inlet temperature of 120 °C, pressure of 50 mmHg, and pump speed of 15 mL/min. Further recent SMEDDS developments governing BBD application are listed in Table 5.

Table 5.
List of the SMEDDS developments along with the applied response surface methodology (RSM) of central composite design (CCD).
4.2. Central Composite Design (CCD)
The central composite design (CCD) is the most commonly used fractional factorial design used in the RSM. It is highly applied in constructing the SMEDDS formulations. The CCD was employed in determining the optimized factors for the osmotic pump capsule developed for SMEDDS [80]. The authors constructed the pseudo-ternary phase diagrams to help examine self-emulsifying regions from various types of oils, surfactants, and co-surfactants, followed by a series of characterizations and analyses. To obtain the optimally controlled release properties, the CCD was done on the elements used in capsule coating, including PEG 400, coating weight, and drug release orifice size. The effect of the independent variables resulted in 81.22% cumulative drug release in 12 with the final formulation of 3% PEG 400, 7.5% coating weight, and 0.5 mm of orifice size. The authors also emphasized the use of lack-of-fit analysis to evaluate critical parameters from the pure error in the replicates (p > 0.05).
Zheng and coworkers demonstrated supersaturable-SMEDDS for ellagic acid to improve its solubility [78]. The screening process was done using ternary phase diagram studies which were then followed by the CCD to find the best formulation. Oil phase and surfactant/co-surfactant mixture masses ratio were investigated as the independent factors toward the responses, including particle size and solubility (Figure 5). The decrease of oil mass has an effect on the decrease of particle size, yet reversely for the surfactant mixture (Km). In contrast, the oil gives an inverse relationship toward the solubility. Further, the optimized conditions of supersaturable SMEDDS were revealed to be 10% ethyl oleate, 67.5% Tween 80, 22.5% PEG 400, 0.5% PVP K30, and 4 mg/g ellagic acid. The presence of PVP K30 incorporated in the optimized excipients inhibited the precipitation of the drug due to the nucleation effect. The in vitro and in vivo results showed an improved antioxidant ability of ellagic acid in supersaturable SMEDDS formulation.
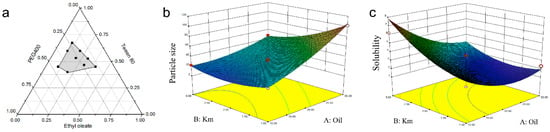
Figure 5.
Tertiary-phase diagram showing emulsion areas (in grey color) of the selected masses of the independent variables containing surfactants (i.e., PEG400 and Tween 80 (Km)) and oil phases (i.e., ethyl oleate) (a). Interaction effects in three-dimensional response surface plots for CCD between Km and oil on particle size (b) as well as on solubility (c). Adapted from Zheng and coworkers [78].
In addition, Tung and coworkers demonstrated DoE on the selection of excipients to produce pellet SMEDDS containing l-tetrahydropalmatine (l-THP) [81]. The pseudo-ternary diagram was made based on water titration to define the optimum range of Capryol 90, Cremophor RH40, and Transcutol HP as excipients in the selected formulations. The solid carrier employed for pellet SMEDDS was Avicel or Aerosil through extrusion and spheronization techniques. After determining Capryol 90 and the Smix (Cremophor RH40 and Transcutol HP; 3:1) in their best ratio, the central composite face (CCF) design was employed to assess the droplet size, PDI, and dissolution efficiency upon them. As a result, the Smix was indicated to be an antagonist affecting the droplet size and PDI significantly, whereas the Capryol 90 showed a synergistic effect. All responses were well defined according to the optimized parameters with dissolution efficiency of 50%, droplet size of <50 nm, and PDI < 0.3 when using 39.5% capryol 90, 59.2% Smix, and 1.3% l-THP to proceed the liquid SMEDDS to the pellet form. Another CCD strategy was used by Yan and coworkers to examine the similar responses (droplet size, PDI, and dissolution efficiency) toward SMEDDS for β-elemene formulation composing poly (acrylic acid) (PAA) entailed on mesoporous silica nanoparticles (MSNPs). The authors emphasized the use of the PAA/MSNPs loaded in SMEDDS to increase the drug loading and to act as the pH triggers in improving a controlled release behavior in an acidic environment [82]. Several reports of CCD applications that have been incorporated in SMEDDS are listed in Table 6.

Table 6.
List of the SMEDDS developments along with the applied response surface methodology (RSM) of Box-Behnken design (BBD).
4.3. The Mixture Design
There are other design methods in DoE apart from RSM, which are also widely used in optimizing parameters to be selected in SMEDDS studies, such as the simplex lattice. In contrast to the previous explanation that the levels of the factors are independent, in the simplex lattice, the factors are seen as mixed elements that are not independent. Thus, the simple lattice is categorized as a mixture experiment. Another type of mixture experiment design is a D-optimal mixture, which belongs to the optimality criterion design of the 2k factorial. D-optimal mixture design is available in many commercial software packages and is normally selected if there are design points that need to be further minimized so as to reduce the total time required to produce an optimal design [59]. The applications of these methods also provide key information throughout their results, such as described in Table 7.

Table 7.
List of the SMEDDS developments along with the applied response surface methodology (RSM) of D-optimal mixture design.
Jain and coworkers developed solid SMEDDS for raltegravir potassium, the first line of HIV treatment, by formulating all the selected components within a tablet excipient to improve better stability and dissolution properties of SMEDDS [93]. The simplex lattice method was then employed to rationally design the optimized amounts of independent variables, Labrasol (as oil), Tween-20 (as surfactant), and PEG400 (as co-surfactant). The cumulative percentage of drug release and globular size were examined afterward as the dependent variables. The optimized formulations of SMEDDS were then proceeded with the selected adsorbents to create solid SMEDDS. As a result, the formation of transparent microemulsions of these variables were 50–60% of Labrasol, 20–30% of Tween-20, and 10–30% of PEG400. The presence of either lipid or lipid with co-surfactant interaction greatly affected the cumulative drug release, as shown by the highest coefficient, suggesting that the greater amount of drug was accordingly increased. Meanwhile, lipids with surfactant or lipids with co-surfactant interaction showed a negative coefficient for the globular size, indicating that the increase of either one proportion decreases the globular size of solid SMEDDS to less than 50 nm.
Another simplex lattice design was also employed by Dhaval and coworkers to investigate seven batches of clofazimine formulations in solid SMEDDS. To do this, the authors used the simplex lattice method to obtain critical parameters toward the responses (particle size and cumulative drug releases in pH 1.2 and pH 6.8) from the screened regions of ternary diagram of the independent variables (Capmul MCM, Tween20, and Labrasol). According to the regression analysis of particle size, the coefficient value of oil was much higher than the other variables, suggesting that the change in Capmul MCM proportion significantly influenced particle size microemulsions. In contrast, the increase of the surfactant (Tween 20) showed a significant decrease of the particle size, as depicted in the 3D response surface and contour plots (Figure 6a). Meanwhile, a lower surfactant level with more oil in pH 1.2 media suggested a decrease in drug release percentage from ~90% to 60% (Figure 6b). A similar trend was found in drug release of pH 6.8 results. Therefore, the authors then concluded to use a high proportion of surfactant to later obtain greater cumulative drug release in the batches studies. The desirability function was then employed to evaluate the closeness of the predicted and actual values obtained from the simplex lattice results. All the designed batches demonstrated acceptable results between the predicted and experimental values (with a bias of <5%), showing the effectiveness of the models. From the optimized parameters, the final cumulative drug release obtained was 85% in less than 60 min at two different dissolution media with a globular size of less than 70 nm.
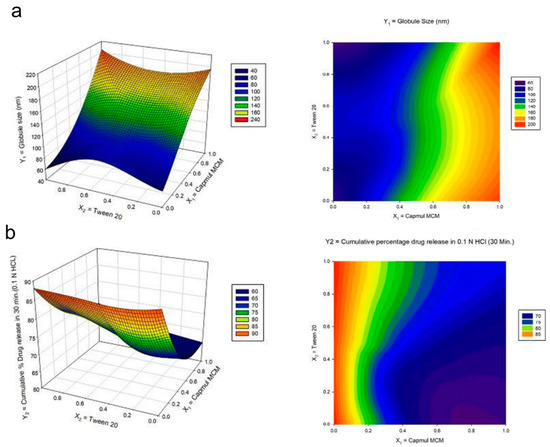
Figure 6.
Response surface plot in three-dimension (3D) (left) and the contour plot (right) for the mixture design of the (a) globule size and (b) cumulative drug release in pH 1.2 media from the study of clofazimine in solid SMEDDS by Dhaval and coworkers. Adapted from Dhaval and coworkers [94].
Lee and coworkers investigated SMEDDS formulations for the BCS IV compound, tolvaptan, through the D-optimal mixture design of DoE [66]. Capryol 90, Tween 20, and Transcutol (or PEG200) were selected for the optimized compositions based on tolvaptan solubility studies. Small particle sizes of <250 nm and an increased cumulative drug release of up to 90% in 60 min were obtained in the formulations involving the oil, surfactant, and co-surfactant with a ratio of 10%, 70%, and 20%, respectively. Their results demonstrate that the successful use of a D-optimal mixture design during the development of tolvaptan-loaded SMEDDS improved the dissolution rate and oral drug bioavailability.
More recently, Na and coworkers carried out SMEDDS to improve the bioavailability of platelet inhibitor, ticagrelor. The authors first performed a preliminary screening to select the optimum excipients from various oils, surfactants, and co-surfactants through solubility and emulsification studies, where the drug in each excipient resulting in greater solubility would be selected. The variables were then selected according to the optimized regions in the pseudo-ternary diagram, including Capmul MCM (oil), Tween 20, or Cremophor EL (surfactant), and Transcutol P (co-surfactant). Scheffe’s mixture design was employed to examine the excipients percentages used with the drug in microemulsions formation toward its solubility, particle size, % transmittance, and % precipitation. As a result, the optimized formulation of ticagrelor in SMEDDS consisting of 10% Capmul MCM, 53.8% Cremophor EL, and 36.2% Transcutol P resulted in maximum values of solubility and % transmittance and minimum values of % precipitation and particle size, along with an exhibited oral bioavailability up to 637.1% as compared to the ticagrelor suspension [95].
There are some reports that only performed the screening process throughout the SMEDDS studies. For instance, Kim and coworkers developed methotreaxate-containing solid SMEDDS [37]. The formulations were done using a spray-drying technique with calcium silicate as the solid carrier. The optimized ratio for castor oil (oil), Tween 80 (surfactant), and Plurol (co-surfactant) were 27:63:10, respectively. The pseudo-ternary diagram was made to assess which formulation could form emulsion simultaneously with a high dissolution rate. As a result, the use of more than 55% surfactant/co-surfactant showed high emulsification efficiency. The methotrexate-containing solid SMEDDS absorption also demonstrated a greater AUC and Cmax of 2.04 and 3.41 fold, respectively, than the free methotrexate [37].
5. Conclusions
SMEDDS have been a popular lipid-based formulation system for the delivery of poorly soluble drugs due to their potential to improve the bioavailability of these active compounds. However, the process of structure formation can be complicated for a complex lipid-based system due to the presence of surfactant, co-surfactant, co-solvent, and carrier that can significantly influence the processing. Using the DoE approach allows formulation scientists to quickly identify interactions between ingredients and reduce the number of experiments required to optimize formulations. As the consequence, scientists have dramatically reduced the time required for formulation development by utilizing this statistical tool. This review illustrates the principles and applications of the most common screening designs applied to SMEDDS development. Furthermore, the use of DoE can be an efficient and fundamental tool to identify and control the variables involved in this scaling-up process to guarantee large-scale production of SMEDDS with the same pharmaceutical activity obtained on the laboratory scale. Finally, the development of SMEDDS by the application of the DoE concept could be a desirable approach to attaining therapeutic and formulary goals.
Author Contributions
C.-M.H. participated in the conceptualization of this manuscript, writing and editing the manuscript. T.-L.Y. participated in preparing the review structure and writing the original draft. A.D.P. participated in preparing the review structure and writing the original draft. C.-T.C. conceived and conceptualized the manuscript writing, editing, and finalization. All authors have read and agreed to the published version of the manuscript.
Funding
This work was mainly supported by the Ministry of Education, Taiwan, and the Ministry of Science and Technology, Taiwan (MOST-111-2314-B-002-152-M3 & MOST-109-2221-E-038-001-MY3).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ghadi, R.; Dand, N. BCS class IV drugs: Highly notorious candidates for formulation development. J. Control. Release 2017, 248, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Singh Chandel, A.K. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef]
- Sharma, V.K.; Koka, A.; Yadav, J.; Sharma, A.K.; Keservani, R.K. Self-micro emulsifying drug delivery systems: A strategy to improve oral bioavailability. Ars Pharm. 2016, 57, 97–109. [Google Scholar] [CrossRef]
- Desai, P.M.; Tan, B.M.; Liew, C.V.; Chan, L.W.; Heng, P.W. Impact of Electrostatics on Processing and Product Performance of Pharmaceutical Solids. Curr. Pharm. Des. 2015, 21, 5923–5929. [Google Scholar] [CrossRef]
- Pedrosa, V.M.; Sanches, A.G.; da Silva, M.B.; Gratao, P.L.; Isaac, V.L.; Gindri, M.; Teixeira, G.H. Production of mycosporine-like amino acid (MAA)-loaded emulsions as chemical barriers to control sunscald in fruits and vegetables. J. Sci. Food Agric. 2022, 102, 801–812. [Google Scholar] [CrossRef]
- Koli, A.R.; Ranch, K.M.; Patel, H.P.; Parikh, R.K.; Shah, D.O.; Maulvi, F.A. Oral bioavailability improvement of felodipine using tailored microemulsion: Surface science, ex vivo and in vivo studies. Int. J. Pharm. 2021, 596, 120202. [Google Scholar] [CrossRef]
- Baek, J.S.; Cho, C.W. Surface modification of solid lipid nanoparticles for oral delivery of curcumin: Improvement of bioavailability through enhanced cellular uptake, and lymphatic uptake. Eur. J. Pharm. Biopharm. 2017, 117, 132–140. [Google Scholar] [CrossRef]
- Murthy, A.; Ravi, P.R.; Kathuria, H.; Malekar, S. Oral Bioavailability Enhancement of Raloxifene with Nanostructured Lipid Carriers. Nanomaterials 2020, 10, 1085. [Google Scholar] [CrossRef]
- Sharma, A.; Streets, J.; Bhatt, P.; Patel, P.; Sutariya, V.; Varghese Gupta, S. Formulation and Characterization of Raloxifene Nanostructured Lipid Carriers for Permeability and Uptake Enhancement Applications. Assay Drug. Dev. Technol. 2022, 20, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, E.; Fang, J.Y.; Tahara, K. Oral mucus-penetrating PEGylated liposomes to improve drug absorption: Differences in the interaction mechanisms of a mucoadhesive liposome. Int. J. Pharm. 2021, 593, 120148. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W. Lipid formulations for oral administration of drugs: Non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur. J. Pharm. Sci. 2000, 11 (Suppl. 2), S93–S98. [Google Scholar] [CrossRef]
- Pouton, C.W. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, Q.; Chen, Z.; Wu, W.; Zhu, Q.; Lu, Y. In vitro and in vivo correlation for lipid-based formulations: Current status and future perspectives. Acta Pharm. Sin. B 2021, 11, 2469–2487. [Google Scholar] [CrossRef] [PubMed]
- Tay, E.; Nguyen, T.H.; Ford, L.; Williams, H.D.; Benameur, H.; Scammells, P.J.; Porter, C.J.H. Ionic Liquid Forms of the Antimalarial Lumefantrine in Combination with LFCS Type IIIB Lipid-Based Formulations Preferentially Increase Lipid Solubility, In Vitro Solubilization Behavior and In Vivo Exposure. Pharmaceutics 2019, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Harwansh, R.; Mirza, M.A.; Hussain, S.; Hussain, A. Oral lipid based drug delivery system (LBDDS): Formulation, characterization and application: A review. Curr. Drug Deliv. 2011, 8, 330–345. [Google Scholar] [CrossRef]
- Feng, W.; Qin, C.; Cipolla, E.; Lee, J.B.; Zgair, A.; Chu, Y.; Ortori, C.A.; Stocks, M.J.; Constantinescu, C.S.; Barrett, D.A.; et al. Inclusion of Medium-Chain Triglyceride in Lipid-Based Formulation of Cannabidiol Facilitates Micellar Solubilization In Vitro, but In Vivo Performance Remains Superior with Pure Sesame Oil Vehicle. Pharmaceutics 2021, 13, 1349. [Google Scholar] [CrossRef]
- Gao, H.; Jia, H.; Dong, J.; Yang, X.; Li, H.; Ouyang, D. Integrated in silico formulation design of self-emulsifying drug delivery systems. Acta Pharm. Sin. B 2021, 11, 3585–3594. [Google Scholar] [CrossRef]
- Jadhav, H.; Waghmare, J.; Annapure, U. Study on oxidative stability of deep fat fried food in Canola oil blended with medium chain triglyceride. Indian J. Chem. Technol. 2022, 29, 95–98. [Google Scholar]
- Kaukonen, A.M.; Boyd, B.J.; Porter, C.J.; Charman, W.N. Drug solubilization behavior during in vitro digestion of simple triglyceride lipid solution formulations. Pharm. Res. 2004, 21, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Cerpnjak, K.; Zvonar, A.; Gasperlin, M.; Vrecer, F. Lipid-based systems as a promising approach for enhancing the bioavailability of poorly water-soluble drugs. Acta Pharm. 2013, 63, 427–445. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Wilson, C.G.; Halbert, G.W.; Mains, J. The gut in the beaker: Missing the surfactants? Int. J. Pharm. 2016, 514, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Gelardi, G.; Mantellato, S.; Marchon, D.; Palacios, M.; Eberhardt, A.B.; Flatt, R.J. admixtures. In Science and Technology of Concrete Admixtures; Aïtcin, P.-C., Flatt, R.J., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 149–218. [Google Scholar] [CrossRef]
- Zhu, Y.; Ye, J.; Zhang, Q. Self-emulsifying Drug Delivery System Improve Oral Bioavailability: Role of Excipients and Physico-chemical Characterization. Pharm. Nanotechnol. 2020, 8, 290–301. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Cook, W.G.; Fenton, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; APhA/Pharmaceutical Press: London, UK, 2012; p. 1033. [Google Scholar]
- Lalanne-Cassou, C.; Carmona, I.; Fortney, L.; Samii, A.; Schechter, R.; Wade, W.; Weerasooriya, U.; Weerasooriya, V.; Yiv, S. Minimizing cosolvent requirements for microemulsion formed with binary surfactant mixtures. J. Dispers. Sci. Technol. 1987, 8, 137–156. [Google Scholar] [CrossRef]
- Dokania, S.; Joshi, A.K. Self-microemulsifying drug delivery system (SMEDDS)—Challenges and road ahead. Drug Deliv. 2015, 22, 675–690. [Google Scholar] [CrossRef]
- Jorgensen, A.M.; Friedl, J.D.; Wibel, R.; Chamieh, J.; Cottet, H.; Bernkop-Schnurch, A. Cosolvents in Self-Emulsifying Drug Delivery Systems (SEDDS): Do They Really Solve Our Solubility Problems? Mol. Pharm. 2020, 17, 3236–3245. [Google Scholar] [CrossRef]
- Prince, L.M. Microemulsions: Theory and Practice; Academic Press: New York, NY, USA, 1977; p. 179. [Google Scholar]
- Anton, N.; Vandamme, T.F. Nano-emulsions and micro-emulsions: Clarifications of the critical differences. Pharm Res. 2011, 28, 978–985. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter. 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Tiwari, P.; Ranjan Sinha, V.; Kaur, R. Chapter 4—Clinical considerations on micro- and nanodrug delivery systems. In Drug Delivery Trends; Shegokar, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 77–101. [Google Scholar] [CrossRef]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef]
- Yang, T.L.; Hsieh, C.M.; Meng, L.J.; Tsai, T.; Chen, C.T. Oleic Acid-Based Self Micro-Emulsifying Delivery System for Enhancing Antifungal Activities of Clotrimazole. Pharmaceutics 2022, 14, 478. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Cho, J.H.; Park, J.H.; Kim, J.S.; Song, E.S.; Kwon, J.; Giri, B.R.; Jin, S.G.; Kim, K.S.; Choi, H.G.; et al. Self-microemulsifying drug delivery system (SMEDDS) for improved oral delivery and photostability of methotrexate. Int. J. Nanomed. 2019, 14, 4949–4960. [Google Scholar] [CrossRef]
- Kamboj, S.; Rana, V. Quality-by-design based development of a self-microemulsifying drug delivery system to reduce the effect of food on Nelfinavir mesylate. Int. J. Pharm. 2016, 501, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Han, S.D.; Jung, S.W.; Jang, S.W.; Son, M.; Kim, B.M.; Kang, M.J. Reduced Food-Effect on Intestinal Absorption of Dronedarone by Self-microemulsifying Drug Delivery System (SMEDDS). Biol. Pharm. Bull. 2015, 38, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Naccarelli, G.V.; Wolbrette, D.L.; Levin, V.; Samii, S.; Banchs, J.E.; Penny-Peterson, E.; Gonzalez, M.D. Safety and efficacy of dronedarone in the treatment of atrial fibrillation/flutter. Clin. Med. Insights Cardiol. 2011, 5, 103–119. [Google Scholar] [CrossRef]
- Renugopal, P.; Sangeetha, S.; Damodharan, N. An Emerging Trend in Solid Self Micro Emulsifying Drug Delivery System. Res. J. Pharm. Technol. 2020, 13, 3028–3034. [Google Scholar] [CrossRef]
- Salawi, A. Self-emulsifying drug delivery systems: A novel approach to deliver drugs. Drug Deliv. 2022, 29, 1811–1823. [Google Scholar] [CrossRef]
- Caliph, S.M.; Charman, W.N.; Porter, C.J.H. Effect of Short-, Medium-, and Long-Chain Fatty Acid-Based Vehicles on the Absolute Oral Bioavailability and Intestinal Lymphatic Transport of Halofantrine and Assessment of Mass Balance in Lymph-Cannulated and Non-cannulated Rats. J. Pharm. Sci. 2000, 89, 1073–1084. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hussain, A.; Hussain, M.S.; Mirza, M.A.; Iqbal, Z. Role of excipients in successful development of self-emulsifying/microemulsifying drug delivery system (SEDDS/SMEDDS). Drug Dev. Ind. Pharm. 2013, 39, 1–19. [Google Scholar] [CrossRef]
- Kadu, P.J.; Kushare, S.S.; Thacker, D.D.; Gattani, S.G. Enhancement of oral bioavailability of atorvastatin calcium by self-emulsifying drug delivery systems (SEDDS). Pharm. Dev. Technol. 2011, 16, 65–74. [Google Scholar] [CrossRef]
- Heshmati, N.; Cheng, X.; Eisenbrand, G.; Fricker, G. Enhancement of oral bioavailability of E804 by self-nanoemulsifying drug delivery system (SNEDDS) in rats. J. Pharm. Sci. 2013, 102, 3792–3799. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hui Zhou, C.; Ping Xu, Z. Chapter 14—Self-Nanoemulsifying Drug-Delivery System. In Nanocarriers for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 421–449. [Google Scholar] [CrossRef]
- Hamed, R.; Awadallah, A.; Sunoqrot, S.; Tarawneh, O.; Nazzal, S.; AlBaraghthi, T.; Al Sayyad, J.; Abbas, A. pH-Dependent Solubility and Dissolution Behavior of Carvedilol--Case Example of a Weakly Basic BCS Class II Drug. AAPS PharmSciTech 2016, 17, 418–426. [Google Scholar] [CrossRef]
- Abuhelwa, A.Y.; Williams, D.B.; Upton, R.N.; Foster, D.J. Food, gastrointestinal pH, and models of oral drug absorption. Eur. J. Pharm. Biopharm 2017, 112, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Akula, S.; Gurram, A.K.; Devireddy, S.R. Self-Microemulsifying Drug Delivery Systems: An Attractive Strategy for Enhanced Therapeutic Profile. Int. Sch. Res. Not. 2014, 2014, 964051. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; Cheng, J.; Shan, B.; Wang, Y.; Wang, R.; Hou, L. Solid self-microemulsifying drug delivery system of Sophoraflavanone G: Prescription optimization and pharmacokinetic evaluation. Eur. J. Pharm. Sci 2019, 136, 104953. [Google Scholar] [CrossRef]
- Musakhanian, J.; Rodier, J.D.; Dave, M. Oxidative Stability in Lipid Formulations: A Review of the Mechanisms, Drivers, and Inhibitors of Oxidation. AAPS PharmSciTech 2022, 23, 151. [Google Scholar] [CrossRef]
- Gabric, A.; Hodnik, Z.; Pajk, S. Oxidation of Drugs during Drug Product Development: Problems and Solutions. Pharmaceutics 2022, 14, 325. [Google Scholar] [CrossRef]
- Yeom, D.W.; Chae, B.R.; Son, H.Y.; Kim, J.H.; Chae, J.S.; Song, S.H.; Oh, D.; Choi, Y.W. Enhanced oral bioavailability of valsartan using a polymer-based supersaturable self-microemulsifying drug delivery system. Int. J. Nanomed. 2017, 12, 3533–3545. [Google Scholar] [CrossRef] [PubMed]
- ICH. ICH Harmonised Tripartite Guideline; Q8 (R2) Pharmaceutical Development; ICH: London, UK, 2009. [Google Scholar]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.; Woodcock, J.J.T.A.j. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef]
- Astakhov, V.P. Design of experiment methods in manufacturing: Basics and practical applications. In Statistical and Computational Techniques in Manufacturing; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–54. [Google Scholar]
- Fisher, R.A. Design of experiments. Br. Med. J. 1936, 1, 554. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Beg, S.; Raza, K. Full Factorial and Fractional Factorial Design Applications in Pharmaceutical Product Development. In Design of Experiments for Pharmaceutical Product Development: Volume I: Basics and Fundamental Principles; Beg, S., Ed.; Springer: Singapore, 2021; pp. 43–53. [Google Scholar] [CrossRef]
- Box, G.E.; Hunter, W.H.; Hunter, S. Statistics for Experimenters; John Wiley and Sons: New York, NY, USA, 1978; Volume 664. [Google Scholar]
- Plackett, R.L.; Burman, J.P. The design of optimum multifactorial experiments. Biometrika 1946, 33, 305–325. [Google Scholar] [CrossRef]
- Mousavi, L.; Tamiji, Z.; Khoshayand, M.R. Applications and opportunities of experimental design for the dispersive liquid–liquid microextraction method—A review. Talanta 2018, 190, 335–356. [Google Scholar] [CrossRef]
- Luiz, M.T.; Viegas, J.S.R.; Abriata, J.P.; Viegas, F.; de Carvalho Vicentini, F.T.M.; Bentley, M.V.L.B.; Chorilli, M.; Marchetti, J.M.; Tapia-Blacido, D.R. Design of experiments (DoE) to develop and to optimize nanoparticles as drug delivery systems. Eur. J. Pharm. Biopharm. 2021, 165, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Sy Mohamad, S.F.; Mohd Said, F.; Abdul Munaim, M.S.; Mohamad, S.; Azizi Wan Sulaiman, W.M.J.C.r.i.b. Application of experimental designs and response surface methods in screening and optimization of reverse micellar extraction. Crit. Rev. Biotechnol. 2020, 40, 341–356. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, G.W. Formulation Approaches for Improving the Dissolution Behavior and Bioavailability of Tolvaptan Using SMEDDS. Pharmaceutics 2022, 14, 415. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.G.; Byeon, J.J.; Wang, M.; Huh, H.W.; Kim, M.K.; Bang, K.H.; Han, M.G.; Lee, H.K.; Cho, C.W. Statistical approach for solidifying ticagrelor loaded self-microemulsifying drug delivery system with enhanced dissolution and oral bioavailability. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109980. [Google Scholar] [CrossRef]
- Dalvadi, H.; Patel, N.; Parmar, K. Systematic development of design of experiments (DoE) optimised self-microemulsifying drug delivery system of Zotepine. J. Microencapsul. 2017, 34, 308–318. [Google Scholar] [CrossRef]
- Marasini, N.; Tran, T.H.; Poudel, B.K.; Choi, H.-G.; Yong, C.S.; Kim, J.O.J.C.; Bulletin, P. Statistical modeling, optimization and characterization of spray-dried solid self-microemulsifying drug delivery system using design of experiments. Chem. Pharm. Bull. 2013, 61, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.D.; Almeida, S.L.; Alonso, E.C.P.; Rocha, P.B.R.; Martins, F.T.; Freitas, L.A.P.; Taveira, S.F.; Cunha-Filho, M.S.S.; Marreto, R.N. Preparation of a solid self-microemulsifying drug delivery system by hot-melt extrusion. Int. J. Pharm. 2018, 541, 1–10. [Google Scholar] [CrossRef]
- Čerpnjak, K.; Pobirk, A.Z.; Vrečer, F.; Gašperlin, M. Tablets and minitablets prepared from spray-dried SMEDDS containing naproxen. Int. J. Pharm. 2015, 495, 336–346. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, S.; Shen, H.; Li, J.; Gao, C.J.A.P. Controlled release of the Nimodipine-loaded self-microemulsion osmotic pump capsules: Development and characterization. AAPS PharmSciTech 2018, 19, 1308–1319. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Lv, C.; Sun, X.; Wang, J.; Zhao, Z. Preparation of a supersaturatable self-microemulsion as drug delivery system for ellagic acid and evaluation of its antioxidant activities. J. Drug Deliv. Sci. Technol. 2019, 53, 101209. [Google Scholar] [CrossRef]
- Tung, N.-T.; Tran, C.-S.; Nguyen, H.-A.; Nguyen, T.-L.; Chi, S.-C.; Nguyen, D.-D.J.I.j.o.p. Development of solidified self-microemulsifying drug delivery systems containing l-tetrahydropalmatine: Design of experiment approach and bioavailability comparison. Int. J. Pharm. 2018, 537, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Wang, Y.; Ma, Y.; Zhao, J.; Liu, Y.; Wang, L. In vitro and in vivo evaluation of poly (acrylic acid) modified mesoporous silica nanoparticles as pH response carrier for β-elemene self-micro emulsifying. Int. J. Pharm. 2019, 572, 118768. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Dudhat, K.; Soniwala, M.; Kotadiya, N.; Mori, D.J.J.o.P.I. DoE-Based Solid Self-microemulsifying Drug Delivery System (S-SMEDDS) Approach for Improving the Dissolution Properties of Raltegravir Potassium. J. Pharm. Innov. 2022. [Google Scholar] [CrossRef]
- Dhaval, M.; Panjwani, M.; Parmar, R.; Soniwala, M.M.; Dudhat, K.; Chavda, J. Application of Simple Lattice Design and Desirability Function for Formulating and Optimizing SMEDDS of Clofazimine. J. Pharm. Innov. 2021, 16, 504–515. [Google Scholar] [CrossRef]
- Na, Y.G.; Byeon, J.J.; Wang, M.; Huh, H.W.; Son, G.H.; Jeon, S.H.; Bang, K.H.; Kim, S.J.; Lee, H.J.; Lee, H.K.; et al. Strategic approach to developing a self-microemulsifying drug delivery system to enhance antiplatelet activity and bioavailability of ticagrelor. Int. J. Nanomed. 2019, 14, 1193–1212. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Q.; Feng, Y.; Wei, Q.; Sun, C.; Firempong, C.K.; Adu-Frimpong, M.; Li, R.; Bao, R.; Toreniyazov, E.; et al. Anti-hyperuricemic property of 6-shogaol via self-micro emulsifying drug delivery system in model rats: Formulation design, in vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2019, 45, 1265–1276. [Google Scholar] [CrossRef]
- Li, F.; Song, S.; Guo, Y.; Zhao, Q.; Zhang, X.; Pan, W.; Yang, X. Preparation and pharmacokinetics evaluation of oral self-emulsifying system for poorly water-soluble drug Lornoxicam. Drug Deliv. 2015, 22, 487–498. [Google Scholar] [CrossRef]
- Qu, Y.; Mu, S.; Song, C.; Zheng, G. Preparation and in vitro/in vivo evaluation of a self-microemulsifying drug delivery system containing chrysin. Drug Dev. Ind. Pharm. 2021, 47, 1127–1139. [Google Scholar] [CrossRef]
- Wang, L.; Yan, W.; Tian, Y.; Xue, H.; Tang, J.; Zhang, L. Self-microemulsifying drug delivery system of phillygenin: Formulation development, characterization and pharmacokinetic evaluation. Pharmaceutics 2020, 12, 130. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, F.; Xu, S.; Yun, K.; Wu, W.; Pan, W. Formulation and evaluation of luteolin supersaturatable self-nanoemulsifying drug delivery system (S-SNEDDS) for enhanced oral bioavailability. J. Drug Deliv. Sci. Technol. 2020, 58, 101783. [Google Scholar] [CrossRef]
- Xie, M.; Wu, J.; Ji, L.; Jiang, X.; Zhang, J.; Ge, M.; Cai, X. Development of triptolide self-microemulsifying drug delivery system and its anti-tumor effect on gastric cancer xenografts. Front. Oncol. 2019, 9, 978. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.e.; Xu, Z.; Li, X.; Wang, Z.; Li, J.; Yang, Z.; Khattak, S.U.; Liu, Y.; Shi, Y. Development and evaluation of rhubarb free anthraquinones loaded self-nanoemulsifying tablets. J. Drug Deliv. Sci. Technol. 2020, 57, 101737. [Google Scholar] [CrossRef]
- Mahore, J.; Shelar, A.; Deshkar, S.; More, G.J.J.R.P. Conceptual design and optimization of self microemulsifying drug delivery systems for dapsone by using Box-Behnken design. J. Res. Pharm. 2021, 25, 179–195. [Google Scholar]
- Lee, D.W.; Marasini, N.; Poudel, B.K.; Kim, J.H.; Cho, H.J.; Moon, B.K.; Choi, H.-G.; Yong, C.S.; Kim, J.O.J.J.o.m. Application of Box–Behnken design in the preparation and optimization of fenofibrate-loaded self-microemulsifying drug delivery system (SMEDDS). J. Microencapsul. 2014, 31, 31–40. [Google Scholar] [CrossRef]
- Yadav, P.; Rastogi, V.; Verma, A. Application of Box–Behnken design and desirability function in the development and optimization of self-nanoemulsifying drug delivery system for enhanced dissolution of ezetimibe. Future J. Pharm. Sci. 2020, 6, 7. [Google Scholar] [CrossRef]
- Visetvichaporn, V.; Kim, K.-H.; Jung, K.; Cho, Y.-S.; Kim, D.-D. Formulation of self-microemulsifying drug delivery system (SMEDDS) by D-optimal mixture design to enhance the oral bioavailability of a new cathepsin K inhibitor (HL235). Int. J. Pharm. 2020, 573, 118772. [Google Scholar] [CrossRef] [PubMed]
- Vaghela, S.; Chaudhary, S.; Chaudhary, A. Formulation Development and Optimization of Blonanserin Liquid SMEDDS using D-Optimal Mixture Design. Curr. Drug Ther. 2022, 17, 266–280. [Google Scholar] [CrossRef]
- Gahlawat, N.; Verma, R.; Kaushik, D. Application of D-optimal Mixture Design for Development and Optimization of Olmesartan Medoxomil Loaded SMEDDS. Curr. Drug Ther. 2020, 15, 548–560. [Google Scholar] [CrossRef]
- Son, H.Y.; Chae, B.R.; Choi, J.Y.; Shin, D.J.; Goo, Y.T.; Lee, E.S.; Kang, T.H.; Kim, C.H.; Yoon, H.Y.; Choi, Y.W. Optimization of self-microemulsifying drug delivery system for phospholipid complex of telmisartan using D-optimal mixture design. PLoS ONE 2018, 13, e0208339. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Thakkar, V.; Gohel, M.; Baldaniya, L.; Gandhi, T. Optimization of Self Micro Emulsifying Drug Delivery System Containing Curcumin and Artemisinin Using D-Optimal Mixture Design. J Saudi J. Med. Pharm. Sci 2017, 3, 388–398. [Google Scholar]
- Xiong, Y.; Zou, Y.; Chen, L.; Xu, Y.; Wang, S.J.A.P. Development and in vivo evaluation of ziyuglycoside i–loaded self-microemulsifying formulation for activity of increasing leukocyte. AAPS PharmSciTech 2019, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Goo, Y.T.; Lee, S.; Choi, J.Y.; Kim, M.S.; Sin, G.H.; Hong, S.H.; Kim, C.H.; Song, S.H.; Choi, Y.W. Enhanced oral absorption of insulin: Hydrophobic ion pairing and a self-microemulsifying drug delivery system using a D-optimal mixture design. Drug Deliv. 2022, 29, 2831–2845. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).