Abstract
Salvia lavandulifolia Vahl essential oil is becoming more popular as a cognitive enhancer and treatment for memory loss. It is high in natural antioxidants and has spasmolytic, antiseptic, analgesic, sedative, and anti-inflammatory properties. Its aqueous extract has hypoglycemic activity and is used to treat diabetic hyperglycemia, but few studies have focused on it. The objective of this work is to evaluate the various biological and pharmacological powers of Salvia lavandulifolia Vahl leaf aqueous extract. Quality control of the plant material was first carried out. Followed by a phytochemical study on the aqueous extract of S. lavandulifolia leaves, namely phytochemical screening and determination of total polyphenols, flavonoids, and condensed tannins contents. Then, the biological activities were undertaken, in particular the antioxidant activity (total antioxidant activity and trapping of the DPPH° radical) and the antimicrobial activity. The chemical composition of this extract was also determined by HPLC-MS-ESI. Finally, the inhibitory effect of the α-amylase enzyme as well as the antihyperglycaemic effect was evaluated in vivo in normal rats overloaded with starch or D-glucose. The aqueous extract obtained by use of the decoction of leaves of S. lavandulifolia contains 246.51 ± 1.69 mg EQ of gallic acid/g DE, 23.80 ± 0.12 mg EQ quercetin/g DE, and 2.46 ± 0.08 mg EQ catechin /g DE. Its total antioxidant capacity is around 527.03 ± 5.95 mg EQ of ascorbic acid/g DE. At a concentration of 5.81 ± 0.23 µg/mL, our extract was able to inhibit 50% of DPPH° radicals. Moreover, it showed bactericidal effect against Proteus mirabilis, fungicidal against Aspergillus niger, Candida albicans, Candida tropicalis, and Saccharomyces cerevisiae, and fungistatic against Candida krusei. A marked antihyperglycemic activity (AUC = 54.84 ± 4.88 g/L/h), as well as a significant inhibitory effect of α-amylase in vitro (IC50 = 0.99 ± 0.00 mg/mL) and in vivo (AUC = 51.94 ± 1.29 g/L/h), is recorded in our extract. Furthermore, its chemical composition reveals the presence of 37.03% rosmarinic acid, 7.84% quercetin rhamnose, 5.57% diosmetin-rutinoside, 5.51% catechin dimer, and 4.57% gallocatechin as major compounds. The antihyperglycemic and α-amylase inhibitory activities, associated with the antioxidant properties of S. lavandulifolia, justify its use in the treatment of diabetes in traditional medicine and highlight its potential introduction into antidiabetic drugs.
1. Introduction
Aromatic and medicinal plants have played a crucial role in the treatment and prevention of a wide range of diseases for thousands of years [1]. The therapeutic uses of herbs are based on plant chemistry [2]. Having an awareness of the chemical make-up of plants enables one to have a greater comprehension of the therapeutic potential of certain plants. Secondary metabolites, in particular, have been shown to have a variety of biological effects and have been labeled as antioxidants, antibiotics, antifungals, and antivirals and thus are capable of protecting plants from pathogens [3]. Sixty percent to eighty percent of the world’s population uses herbal medicine as its primary source of healthcare [4]. The Lamiaceae family is a plant family with a global distribution that includes approximately 236 genera (6900–7200 species). Salvia is the family’s largest genus, with nearly 900 species [5].
It is widespread in the tropics as well as in the temperate zones of the world in the following order: the Mediterranean basin, central Asia, the American continent, the Pacific islands, and equatorial Africa and China [6]. Species of this genus have shown many biological activities [7]. In traditional medicine, sage is one of the oldest medicinal plants used by man and is considered a panacea. It is used for its antibacterial, antiviral, antioxidant, antimalarial, anti-inflammatory, antidiabetic, cardiovascular, and antitumor effects [8]. In addition, it helps preserve food thanks to its antioxidant properties [9]. S. lavandulifolia is a widely distributed species in the Mediterranean basin, in eastern Spain, extending to the western Mediterranean: southeastern Spain and northwestern Africa (Morocco and Algeria) [10,11]. In traditional medicine, S. lavandulifolia is used to treat and prevent various diseases. It is used as a fungicidal, virucidal, and bactericidal agent [12]. Additionally, the aqueous extract of this herb is used as a popular hypoglycemic remedy for diabetic patients [13]. In Morocco, this plant grows spontaneously in various regions [12]. It is generally used as a spice in the cosmetics industry [14]. Its leaves are used in traditional medicine as an antiseptic, healing, choleretic, astringent, and hypoglycemic remedy [15,16], and its antibacterial and antidiabetic activities had also been confirmed [17]. Diabetes is a group of metabolic diseases in which hyperglycemia is the main characteristic. The high level of glucose in the blood generates, among other consequences, oxygen free radicals by auto-oxidation of glucose, which is associated with the pathogenesis of diabetes complications [18]. The persistence of the high content of glucose in the blood leads to its auto-oxidation. It will also induce the generation of free radicals, thus causing diabetic complications. For this, the control of hyperglycemia by use of natural products is a good way to prevent their appearance in diabetic patients. The main objective of this work is the characterization of phytochemicals and the evaluation of the effects: antioxidant, antimicrobial, anti-hyperglycaemic, and α-amylase enzyme inhibitor in vitro and in vivo of the aqueous extract of S. lavandulifolia, which is highly valued in traditional medicine as an aromatic plant with a medicinal character.
2. Results and Discussion
2.1. Quality Control of Plant Material
Plant material quality control results are shown in Table 1. The moisture content of the leaves of S. lavandulifolia is around 10.5%; this value is significantly lower than the limit value set at 12% [19]. The pH of our plant is acidic (5.26: acidophilic); its titratable acidity is 0.61% and contains 5.47% of mineral matter. The dosage of metallic trace elements present in S. lavandulifolia showed quite low levels of arsenic (0.0055 mg/g), chromium (0.0012 mg/g), antimony (0.001 mg/g), cadmium (0.0001 mg/g), copper (0.004 mg/g), and titanium (0.0049 mg/g) against a slightly high iron content (0.5099 mg/g) and an absence of lead. It is to be noted that these results are below the limit values for each MTE.

Table 1.
Humidity level, pH, ash, and heavy metals present in S. lavandulifolia.
2.2. Phytochemical Screening
Phytochemical screening is a preliminary and of great importance step since it reveals the presence of constituents known for their various biological activities and medicinal properties. The results of the screening of the leaves of S. lavandulifolia are shown in Table 2. The phytochemical characterization tests carried out made it possible to highlight the richness of our plant in primary and secondary metabolites. Indeed, our plant is rich in proteins, lipids (sterols and triterpenes), sugars, and carbohydrates (oses and holosides) as well as polyphenols such as flavonoids (leucoanthocyanins, flavones), tannins, alkaloids, mucilages, and saponosides. These results agree with the literature, indeed, many researchers have confirmed the presence of sugars, polyphenols, and flavonoids in S. lavandulifolia [20,21,22].

Table 2.
Chemical families present in S. lavandulifolia.
2.3. Contents of Polyphenols, Flavonoids, and Condensed Tannins
Polyphenols are secondary metabolites abundantly present in almost all species of aromatic and medicinal plants. These metabolites are essential for human nutrition due to their antioxidant properties and are beneficial to health [23]. The results of the various assays are grouped in Figure 1.
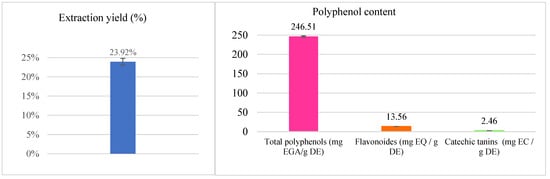
Figure 1.
Extraction yield and contents of polyphenols, flavonoids and catechic tannins in the aqueous extract of S. lavandulifolia.
The extract of S. lavandulifolia is composed of 246.51 ± 1.69 mg EAG/g DE, 13.56 ± 0.08 mg EQ/g DE, and 2.46 ± 0.08 mg EC/g DE of total polyphenols, flavonoids, and condensed tannins, respectively. Our results are almost identical to those found by Boutahiri et al. (2021) in terms of total polyphenol content. They found that the aqueous extract of S. lavandulifolia obtained by decoction contains 252.67 ± 5.40 mg EAG/g DE [17]. Polyphenols are considered the most abundant group of secondary metabolites in plants, through which they defend themselves against predators and intrusions [24]. They are distinguished by the presence of hydroxyl groups, which enable them to be reactive by chelating metal ions and neutralizing free radicals through hydrogen atoms or electrons, thereby reducing their prooxidant activity [25,26]. Thanks to these characteristics, these molecules, endowed with preventive and curative effects of several diseases related to oxidative stress, have received great interest [27,28]. The family of polyphenols is divided into several classes of which flavonoids represent the majority (60%). They are responsible for the attractive colors of flowers, fruits, and leaves. They are more precisely called “nutraceuticals” thanks to their different pharmacological effects on the body. The advantages of being readily absorbed by the intestine, their ability to combat free radicals, and their hypoglycemic effect are what define flavonoids [29] as well as their hypoglycemic effect [30]. Tannins also have antioxidant power. Indeed, they block the formation of superoxide and the peroxidation of lipids [31].
Condensed tannins are oligomeric and polymeric byproducts of the biosynthesis of flavonoids [32]. Additionally, their capacity to scavenge free radicals is well known. They may not be present, but their quantity in the extracts is lower than that of the other bioactive compounds [33].
2.4. Chemical Composition of S. lavandulifolia Extract
The HPLC chromatogram and the chemical composition of the aqueous extract of S. lavandulifolia are shown in Figure 2 and Table 3. The below results show that our extract contains rosmarinic acid (37.03%), followed by quercetin rhamnose (7.84%), diosmetin-rutinoside (5.57%), catechin dimer (5.51%), gallocatechin (4.57%), luteolin (4.12%), caffeic acid (4.02%), carnosol (3.97%), catechin (2.62%), rhamnetin (1.84%), rutin (1.78%), azelaic acid (1.61%), ferulic acid (1.48%), and vanillic acid (1.46%) covering more than 80% of the overall composition of the extract. Several of these compounds are known for their important pharmacological activities. For example, rosmarinic acid, our major compound, is reputed to be antibacterial [34], antiviral [35], anti-inflammatory [36], anti-cancer [37], health-enhancing [38], and antioxidant [34,39]. Luteolin is known for its inhibitory effect on α-glucosidase and α-amylase enzymes [40]; it also has antioxidant, antimicrobial, anti-inflammatory, antidiabetic, neuroprotective, anticancer, and cardioprotective properties [41]. The antiradical activity of caffeic acid has been demonstrated [42]. Carnosol, for its part, is considered antioxidant, anticancer, anti-inflammatory, and antimicrobial [43]. Previous studies compiled by Claudia Musial and her collaborators indicated antitumor, antioxidant, anti-inflammatory, antimicrobial, antiviral, antidiabetic, antiobesity, and hypotensive effects related to catechins [44].
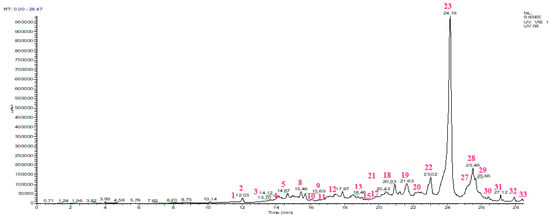
Figure 2.
HPLC chromatogram of the aqueous extract of Salvia lavandulifolia Vahl.

Table 3.
Chemical composition of the aqueous extract of Salvia lavandulifolia Vahl.
Our results agree with those of Salima Boutahiri et al., having identified rosmarinic acid, apigenin, luteolin, myricetin, herniarin, caffeic acid, protocatechuic acid, coumarin, cinnamic acid, vanillic acid, gallic acid, and chlorogenic acid in the aqueous extract of S. lavandulifolia leaves [17]. Salvador Cafligueral et al. revealed the presence of apigenin, luteolin, rosmarinic acid, quercetin-3-O-β-D-glucoside, luteolin-7-O-β-D-glucoside, luteolin-4′-O-glucoronide, luteolin-7-O-rutinoside, and other compounds such as nepetin and 5-Hydroxy-7, 4′-dimethoxyflavone in the soluble fraction of petroleum ether and chloroform extracts from the leaves of S. lavandulifolia [45].
2.5. Antioxidant Properties
The different antioxidant activities of Salvia lavandulifolia are shown in Table 4.

Table 4.
PM and DPPH° antioxidant activities of the aqueous extract of Salvia lavandulifolia Vahl.
2.5.1. Total Antioxidant Capacity
The PM (phosphomolybdate) test, such as CUPRAC (copper (II) ion reducing capacity) and FRAP (iron (III) reducing capacity), was selected to analyze the reduction capacity of S. lavandulifolia extract. This method involves the transfer of a single electron. In this system, the electron from the antioxidant that has been oxidized is transferred to the sub-strate, which prevents the oxidant from being reduced [46]. This assay quantifies the rate of reduction between antioxidant, oxidant, and molybdenum ligands and assesses the degree of reduction of Mo (VI) to Mo (V). It entails thermally generating auto-oxidation over an extended period of time at a high temperature. The advantage of this assay is to give a direct estimate of the antioxidant’s reducing capacity. Our extract showed a total antioxidant capacity of 527.03 ± 5.95 mg EQ AA/g ES as shown in Table 4. The latter is clearly high; this can be explained by the presence of luteolin known for its antioxidant capacity, chelator of transition metals [41].
2.5.2. Free Radical Scavenging DPPH°
DPPH° is a stable free radical that is frequently utilized to evaluate the antioxidant activity of natural compounds in a straightforward, quick, and accurate manner [47]. The results of this test, shown in Figure 3, indicate that S. lavandulifolia leaves have very significant antiradical activity, with an IC50 of 5.81 ± 0.23 µg/mL; however, its effect remains lower than that of acid ascorbic (IC50 = 3.06 ± 0.30 µg/mL). These results are consistent with the previous study by Pop et al., 2016 where the ability to scavenge DPPH° free radicals by the methanolic extract of S. lavandulifolia was demonstrated [48]. However, the antiradical property of this plant is linked to its richness in polyphenols. The latter is composed of hydroxyl groups that can neutralize free radicals [49]. Further UHLPC analysis shows the presence of caffeic acid and rutin potential responsible in minor part for this high capacity. As well as rosmarinic acid in particular, identified with a percentage of 37%, is reputed to have an important antiradical property [38,50].
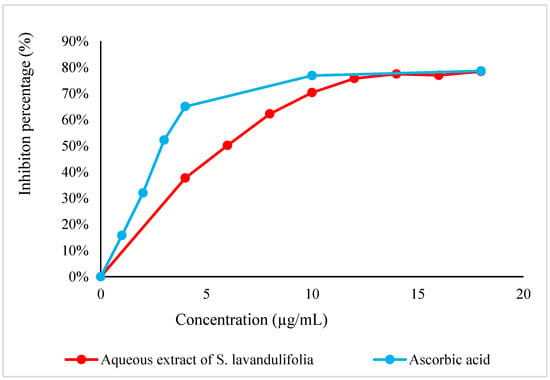
Figure 3.
DPPH° radical scavenging curve by the aqueous extract of S. lavandulifolia.
2.6. Antimicrobial Activity
The antimicrobial activity of S. lavandulifolia extract was evaluated against strains of bacteria and fungi (Table 5). The results of the MIC values obtained show that the extract was more active against the strains tested. The lowest MIC found is 2.34 mg/mL against Staphylococcus aureus. The strains Escherichia coli, Pseudomonas aeruginosa, and Candida krusei are inhibited with an extract concentration of 18.75 mg/mL. In addition, the extract has also inhibited the following strains: Enterobacter cloacae, Klebsiella pneumoniae, Staphylococcus epidermidis, Candida albicans, Candida tropicalis, and Saccharomyces cerevisiae with an MIC = 37.5 mg/mL each. While Escherichia coli ESBL, Proteus mirabilis, Streptococcus agalactiae (B), Aspergillus niger, Candida dubliniensis, Candida kyfer, and Candida parapsilosis strains are inhibited starting from a concentration of 75 mg/mL. According to the MBC/MIC ratio, the extract of S. lavandulifolia reported a bactericidal effect against Proteus mirabilis and fungicidal against strains Aspergillus niger, Candida albicans, Candida tropicalis, Saccharomyces cerevisiae, and Candida krusei. Based on these findings, Salvia lavandulifolia extract could potentially be used as natural preservatives in foods against well-known causative agents of foodborne illnesses such as S. aureus and E. coli [17]. In this work, the inhibitory activities of this extract are probably due mainly to the action of the majority compounds in this extract: rosmarinic acid, quercetin rhamnose, diosmetin-rutinoside, and catechin dimer [21]. The antimicrobial activity of rosmarinic acid against various bacterial and fungi strains has been described by a number of authors. Additionally, a study was carried out by Giner and his associates [51] on the combination of hydroalcoholic extracts of S. lavandulifolia, S. rosmarinus, and T. mastichina. With an MIC value of 12.8 mg/mL, they demonstrated that it is effective against E. coli and E. aerogenes; these data are consistent with our findings.

Table 5.
MIC and MBC/MFC of the microbial strains studied.
2.7. Toxicity
Traditional MAP-based treatments can induce toxicity problems leading to treatment failures. For this, we conducted pharmacological tests in vivo after first assessing the toxicity of the aqueous extract of S. lavandulifolia. This experiment aims to demonstrate that the therapeutic doses of S. lavandulifolia extract (0.5 g/kg, 1 g/kg, or 2 g/kg) do not cause short-term toxicity in healthy mice. According to the test’s findings, the extract is not toxic even at a dose of 2 g/kg. Throughout the entire follow-up period, it did not result in any toxicity symptoms (such as diarrhea, vomiting, abnormal mobility, etc.) or fatalities. According to Perry et al. (2001), long-term use of S. lavandulifolia as a food flavoring agent did not induce adverse effects [52].
2.8. Antihyperglycemic Effect
In normal rats, oral administration of the S. lavandulifolia aqueous extract at 400 mg/Kg 30 min before the glucose overload significantly reduced post-prandial hyperglycemia at 60 min (p < 0.01, 1.13 ± 0.14 g/L) and at 90 min (p < 0.01, 0.89 ± 0.17 g/L). In a similar manner, glibenclamide significantly reduced postprandial hyperglycemia for two hours after glucose overload at 60 min (p < 0.01; 1.08 ± 0.09 g/L) and 90 min (p < 0.05; 1.09 ± 0.10 g/L). In comparison to pretreated distilled water, there was no discernible difference in blood glucose levels between the two groups at 150 min; (0.76 ± 0.07 g/L) at 150 min (1.22 ± 0.11 g/L) at 90 min, and at 60 min (1.37 ± 0.16 g/L) (Figure 4A). In rats treated with the aqueous extract of S. lavandulifolia (54.84 ± 4.88 g/L/h) as opposed to rats treated with distilled water (62.91 ± 4.32 g/L/h), the area under the curve (AUC glucose) is significantly lower in the former group (p < 0.01) (Figure 4B). It is also less than the positive control (glibenclamide), which is significantly less (55.95 ± 1.69 g/L/h; p < 0.01) than the rats given distilled water. Additionally, it is less than the positive control (glibenclamide), which has a significantly lower concentration (55.95 ± 1.69 g/L/h; p < 0.01) than rats given distilled water as a treatment.
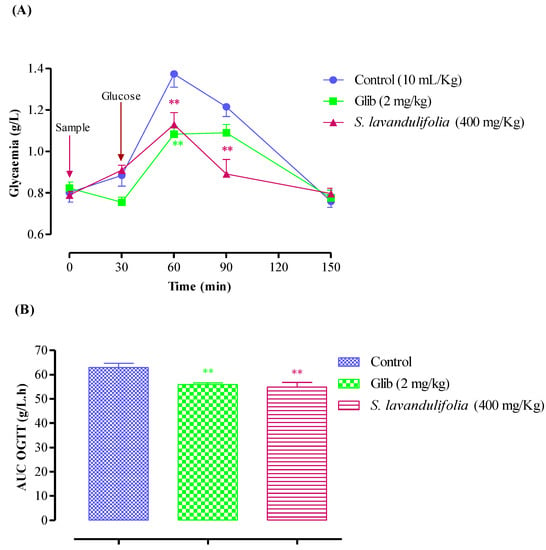
Figure 4.
Postprandial blood glucose (A) and area under the postprandial curve (B) in normal rats after administration of the products tested (S. lavandulifolia extract and glibenclamide), ** p < 0.01; compared to the control.
These results are consistent with those of Jimenez et al. from 1986, who demonstrated that administration of an aqueous extract of S. lavandulifolia 60 min before glucose over-load induced marked antihyperglycemic activity, compared to administration of glucose alone. This finding suggests that intestinal glucose uptake may be a key factor that may explain this activity [53]. The majority of Salvia species used in traditional medicine to treat diabetes frequently work by boosting insulin secretion, boosting adipose tissue and skeletal muscle glucose uptake, and reducing intestinal glucose absorption and hepatic glycogenolysis [54]. In a different study, the hypoglycemic activity of S. lavandulifolia was attributed to its capacity to promote glucose uptake in peripheral tissues (muscle and adipose tissue), which results in the return of blood sugar to normal levels [54]. Studies on this plant’s aqueous extract have similarly demonstrated its hypoglycemic effects by raising pancreatic insulin secretion and peripheral glucose uptake [54,55].
2.9. Pancreatic α-Amylase Inhibitory Effect
2.9.1. In Vitro Test
An enzyme called pancreatic α-amylase breaks down polysaccharides (such as starch and glycogen) into disaccharides. Figure 5 depicts the impact of the S. lavandulifolia aqueous extract on the in vitro activity of this enzyme. In fact, our extract significantly inhibited the activity, with an IC50 of 0.99 ± 0.00 mg/mL compared to IC50 = 0.52 ± 0.01 mg/mL for acarbose. Inhibiting α-amylase activity is one of the most efficient therapeutic strategies to manage postprandial hyperglycemia in diabetic patients [56]. The concentration of fibers in S. lavandulifolia and the presence of inhibitors on these fibers reduce the accessibility of starch to the enzyme, decreasing the activity of α-amylase. These are just a few of the factors that contribute to this inhibition [57].
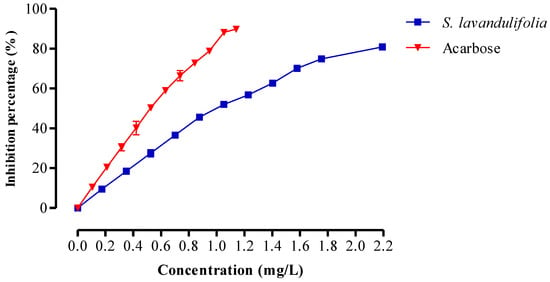
Figure 5.
Inhibitory effect on α-amylase activity by aqueous extract of S. lavandulifolia and acarbose, in vitro.
2.9.2. In Vivo Test
Oral administration of aqueous extract of S. lavandulifolia at C = 400 mg/kg 30 min before starch overload in normal rats significantly reduced postprandial hyperglycemia at 60 min (p < 0.001, 0.90 ± 0.06 g/L), at 90 min (p < 0.001; 0.83 ± 0.03 g/L), and at 150 min (p < 0.001; 0.84 ± 0.02).
Similar to this, acarbose significantly reduced postprandial hyperglycemia during the two hours that followed starch overload at 60 min (p < 0.001, 0.89 ± 0.06 g/L), at 90 min (p < 0.001, 0.85 ± 0.08 g/L), and at 150 min (p < 0.001, 0.78 ± 0.09 g/L) (Figure 6A). Rats pretreated with distilled water only recorded remarkable hyperglycemia, unlike the first and second groups, at 60 min (1.07 ± 0.02 g/L), at 90 min (1.15 ± 0. 07 g/L), and 150 min (1.05 ± 0.04 g/L). In addition, the area under the curve (AUC glucose) was significantly lower (p < 0.001) in rats treated with plant extracts (51.94 ± 1.29 g/L/h) than those treated with acarbose (52.05 ± 4.27 g/L/h) and distilled water (61.82 ± 1.53 g/L/h) (Figure 6B). As mentioned above, S. lavandulifolia is rich in polyphenols and flavonoids. Moreover, phytochemical analysis of this aqueous extract showed that it contains apigenin, rosmarinic acid, and luteolin, responsible for the inhibitory effect of pancreatic α-amylase [40,58,59,60], hence, the drop in blood sugar levels.
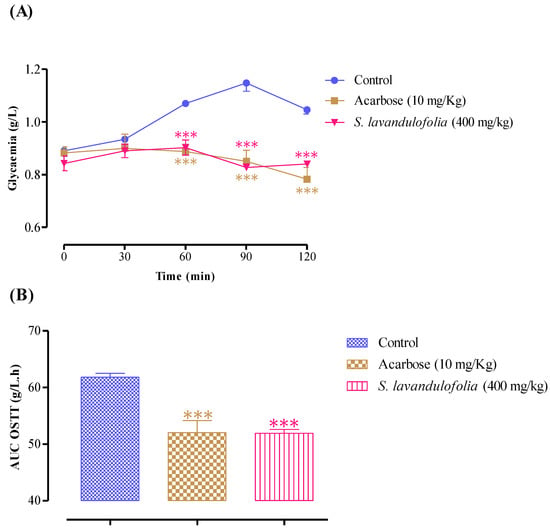
Figure 6.
Effect of S. lavandulifolia extract and acarbose on the variation in postprandial glycaemia in normal rats (A), with a representation in the form of areas under the curves (B), *** p < 0.001: relative to the control.
3. Material and Methods
3.1. Plant Material
S. lavandulifolia is a species of the genus Salvia, family Lamiaceae, and order Lamiales. It is commonly called lavender sage or Spanish sage. Our drug was harvested in the rural town of Ouled Ali in the province of Boulemane-Morocco in May 2020 (Table 6) and was dried in the open air and protected from light for two weeks.

Table 6.
Information about the studied plant.
3.2. Quality Control of Plant Material of S. Lavandulifolia
3.2.1. Humidity Level
A quantity of 5 g of dry plants was put in Petri dishes and left in the oven at a temperature of 100 ± 5 °C for 24 h [61,62].
m1: initial mass of the plant before drying in the oven (g),
m2: final mass of the plant after drying in the oven (g).
3.2.2. pH Determination
A mass (5 g) of the sample was combined with 500 mL of distilled water. A stirrer and magnetic bar were used to stir the mixture for 5 min at room temperature. After that, the mixture was filtered. The pH was determined using an STPURE electrode-equipped benchtop pH meter, the Ohaus Starter 3100 [63]. The pH reading was then taken by placing the electrode of the pH meter into a volume of filtrate.
3.2.3. Determination of Titratable Acidity
Ten grams of herbal drug powder was extracted by use of 100 mL of boiling water for 15 min. Following filtration, the mixture was combined with 20 mL of distilled water from 10 mL of the filtrate. Following the addition of a few drops of phenolphthalein, the titration procedure was continued using a solution of NaOH (0.01 N) until a persistent pink color was achieved. The volume of NaOH poured up to the equivalence point is converted into equivalent citric acid using the following formula [64].
3.2.4. Ash Content
The organic matter content is determined by calculating the difference in weight before and after calcination. The latter consists of passing 2 g of ground sample in a muffle furnace at a temperature of 550 °C, up to the total destruction of any carbonaceous particles (light gray or whitish color) [65]. The organic matter content is calculated using the following formula:
OM%: Organic matter;
m1: Pre-calcination capsule and sample mass;
m2: Post-calcination capsule and sample mass;
TS: Test sample.
The ash content was calculated as follows: MM% = 100 − MO%
3.2.5. Dosage of Metallic Trace Elements (MTE) by ICP-AES
For the determination of MTE contents (As, Cr, Sb, Pb, Cd, Fe, Cu, and Ti), the mineralization protocol with aqua regia (HNO3 + 3HCl) was adopted. The method consists of mixing 0.1 g of crushed plant material with 3 mL of aqua regia and heating it under reflux (200 °C) for two hours. After cooling and settling, the supernatant is recovered and filtered on a membrane (0.45 µm) and then made up to 15 mL with water. Notably, the inductively coupled plasma atomic emission spectrometer (ICP-AES) was used to measure the MTE concentrations. [66].
3.3. Preparation of the Aqueous Extract of Salvia lavandulifolia
Briefly, 30 g of the crushed plant was introduced into a reflux assembly with 750 mL of distilled water. The mixture was heated at 80 °C with stirring for one hour and filtered [62]. The extract was dried in an oven at 70 °C overnight in a silicone mold and then collected in amber glass bottles.
Y: yield; m2: mass of the extract; m1: mass of the crushed plant.
3.4. Phytochemical Screening
Phytochemical screening tests are qualitative tests that consist of detecting the different families of compounds present in plant material. They are based on coloring, precipitation, or complexation reactions [67,68].
3.5. Phenolic Compounds
3.5.1. Determination of Total Polyphenol Content
The Singleton et al. protocol was utilized to determine total phenolic content (TPC), with a few minor modifications [69]. Quantities of 15 µL (C = 25 mg/mL) of extract, 1.5 mL of Folin Ciocalteux’s reagent (10%), and 1.5 mL of Na2CO3 (7.5%) were introduced, respectively, into 50 mL volumetric flasks and supplemented with distilled water. The mixture was blended and incubated at room temperature for 40 min in the dark. The concentration of phenolic compounds in the S. lavandulifolia extract was expressed in equivalents of gallic acid (EGA), and the absorbance at a wavelength of 760 nm was measured.
3.5.2. Determination of Flavonoid Content
To test tubes, 30 µL (C = 25 mg/mL) of extract, 2 mL of distilled water, and 10 µL of aluminum chloride prepared in methanol (10%, m/V) were added. Pure methanol was used to dilute the mixture to a total volume of 5 mL. The solutions were mixed and incubated in the dark for 30 min. At 433 nm, absorbance was measured, and flavonoid concentration was expressed as quercetin equivalents (QE) [70].
3.5.3. Determination of Condensed Tannin Content
100 µL (C = 25 mg/mL) of extract, 3 mL of vanillin methanolic solution (4%, m/V), and 1.5 mL of HCl (37%) were added in test tubes. The contents of the tubes were mixed and incubated at room temperature for 20 min in the dark. At 499 nm, absorbance was measured, and condensed tannin concentration was expressed in CE catechin equivalent [71].
3.6. Identification of the Chemical Composition by HPLC-MS-ESI
The HPLC-MS analysis was conducted by the Dionex UltiMate 3000 ULC/HPLC system coupled to an Exactive mass spectrometer with an ESI ionization source and an orbitrap analyzer. A volume of 10 μL of extract dissolved in distilled water (C = 100 μg/mL) was injected into a C18 column with 100 mm long, 2.1 mm in diameter, and with 1.7 µm pores. The temperature was programmed at 30 °C, while the flow rate was 0.45 mL/min. The mobile phase contained two solvents: solvent A (Water + formic acid (0.1%), v/v) and solvent B (Acetonitrile + formic acid (0.1%), v/v). The established elution gradient was “A+B” [98:2] (0–19 min), “A+B” [70:30] (20–24 min), “A+B” [5:95] (25 min), and “A+B” [98:2] (26–30 min). The detection was carried out using a diode array detector by scanning in the wavelength range of 280–360 nm, as well as by the spectrometer of mass (Exactive) after negative ionization. Data were acquired using MASS LYNX software(Version 4.2). The molecules were identified based on retention time, mass spectrum, molecular weight, and by comparison with standards (injected under the same conditions as the extract): caffeic acid, coumaric acid, ferulic acid, gallic acid, rosmarinic acid, sinapic acid, syringic acid, tannic acid, trans-cinnamic acid, vanillic acid, apigenin, catechin, coumarin, kaempferol, luteolin, myricetin, and rutin.
3.7. Antioxidant Activity Test
3.7.1. Total Antioxidant Capacity
The test sample was mixed with 1 mL of ammonium molybdate (4 mM), 1 mL of sodium phosphate (28 mM), and 1 mL of sulfuric acid (0.6 M) in test tubes. The tubes’ contents were combined and incubated at 95 °C for ninety minutes before being normalized for 20 to 30 min at room temperature. At 695 nm, the measurement was made. The results were expressed in milligram equivalents of ascorbic acid per gram of dry extract (mg EAA/g DE), with ascorbic acid serving as the control [72].
3.7.2. 2,2′-Diphenyl-1-Picryl Hydroxyl Test
An increasing volume of extract was put into test tubes, and ethanol was added to reach a total volume of 200 µL. A quantity of 2.8 mL of ethanolic solution of DPPH° (24 µg/mL, m/V) was added to the mixture and left to incubate for 30 min in the dark. Notably, UV–Vis absorbance was measured at 515 nm [73].
AC: Absorbance of the negative control, AS: Absorbance of the sample, IC50: Inhibiting concentration 50% of DPPH° radicals (mg/mL), CDPPH: Concentration of DPPH° (mg/mL).
EC50 effective concentration was used to calculate the antiradical potency. The higher the ARP, the more effective the antioxidant [74].
EC50: Effective concentration of the sample.
3.8. Antimicrobial Activity
3.8.1. Preparation of Microbial Suspensions
The antimicrobial activity of S. lavandulifolia aqueous extract was tested against nine bacteria and fungi (Table 7). These pathogenic microorganisms are frequently encountered in many infections, causing clinical and therapeutic issues. All strains were first frozen in 20% glycerol stock at −80 °C and then regenerated on Mueller–Hinton or Sabouraud broths and finally subcultured.

Table 7.
List of bacterial and fungal strains tested.
3.8.2. Determination of MIC and MBC/MFC
The minimum inhibitory concentration (MIC) was determined by use of 96-well microplates using the reference method of microdilution [75]. From an extract stock solution prepared in a 30:70 ethanol/distilled water mixture, a series of dilutions were carried out to obtain various concentrations ranging from 75 to 4.6875 mg/mL, with a final volume of 100.00 μL in Mueller–Hinton (MH) broth for the bacterial strain and Sabouraud broth for the fungal strain. Following that, 100 µL of microbial suspension (inoculum) with a final concentration of 106 CFU/mL for bacteria or 104 CFU/mL for fungi was added to the different wells (1 to 11), with the 11th and 12th wells serving as growth and sterility controls, respectively. After incubating for 24 h at 37 °C, 10 μL of resazurin was added to each well as a microbial growth indicator before reincubating for two hours at 37 °C. The change in color from purplish blue to bright pink revealed growth. The MIC is defined as the lowest concentration that prevents resazurin from changing color. To determine the MBC or MFC, 10 µL was taken from each well where no growth could be seen and put on MH agar for bacterial growth or Sabouraud for fungal growth for 24 h at 37 °C. After incubation, MBC/MFC is determined as the lowest concentration inhibiting colony formation on solid agar medium [76]. To evaluate antimicrobial potency, the MBC/MIC or MFC/MIC ratio can be calculated. Indeed, if the ratio is less than 4, the extract is bactericidal/fungicidal; if it is greater than 4, the sample is bacteriostatic/fungistatic [77].
3.9. Animals
Wistar rats (200–250 g) and albino mice (25–35 g) were used in this study and were reared under ideal conditions photoperiods of 12 h light/12 h dark and 22 ± 2 °C) with free access to water and food. This test was carried out in accordance with the Organization for Economic Cooperation and Development’s guidelines (OECD) [78].
3.10. Acute Toxicity
A quantity of 24 albino mice (20–35 g) on an empty stomach (14 h) were randomly distributed into four groups (n = 6; ♂/♀ = 1). The control group received distilled water (10 mL/kg) and the treated groups received the doses: 0.5 g/kg, 1 g/kg, and 2 g/kg. When the test first began, the mice were weighed. Immediately afterwards, they received a single dose of the test extract, orally. Then, they were continuously monitored for 10 h to report any apparent signs of toxicity. For the remaining 14 days, the mice were kept under daily surveillance for further clinical or behavioral signs of toxicity. This test was performed in accordance with the guidelines of the Organization for Economic Co-operation and Development (OECD) [78].
3.11. Antihyperglycemic Effect
The oral glucose tolerance test was performed to evaluate the antihyperglycemic (postprandial glucose) effect in vivo [79]. Normal rats were divided into three groups (n = 6; ♂/♀ = 1): control group: distilled water (10 mL/kg) and test groups: normal rats force-fed with the extract (400 mg/kg) or glibenclamide (2 mg/kg). First, blood glucose was measured at t0 just before administration of the test product (distilled water, aqueous extracts, or glibenclamide). A total of 30 min later, another measurement of glycemia was carried out then the rats were overloaded with D-glucose (2 g/kg). Subsequently, the variation in blood glucose was measured every half hour up to 90 min and then after one hour.
3.12. Pancreatic α-Amylase Inhibitory Effect
3.12.1. In Vitro Test
The Nageswara Rao Thalapaneni et al. [80] method with some modifications was used to examine the inhibitory effect of S. lavandulifolia aqueous extract on pancreatic-amylase enzymatic activity. Increasing volumes of S. lavandulifolia extract/acarbose were added to test tubes, which were then filled to a capacity of 200 µL with distilled water.
The tubes were then filled with 200 µL of pancreatic α-amylase solution (61.33 U/mL) and 200 µL of phosphate buffer (PB) solution (0.02 M; pH = 6.9), with the exception of the correction series, where the PB solution was used in its place. A time of 10 min at 37 °C were spent pre-incubating the tubes.
The tubes were then re-incubated for 15 min at 37 °C with 200 µL of a 0.5% starch solution. After adding 600 µL of DNSA (2.5%) to halt the enzymatic reaction, the tubes were heated in a boiling water bath for 8 min. The tubes were placed in an ice bath to stop the reaction, and then 10 mL of diluted water was added to each tube. In order to compare the absorbance at 540 nm to the correction series, a spectrophotometer was used. The percentage inhibition was calculated using the equation shown below:
with:
AbControl: absorption of enzyme activity without inhibitor;
AbTest: Absorption of enzymatic activity in the presence of the extract or acarbose;
AbBlank/Blank of the corresponding tube: a series of corrections is carried out in parallel with each series where the enzyme is replaced by the PB.
3.12.2. In Vivo Test
The purpose of this test is to confirm the enzymatic activity of pancreatic-amylase-inhibitory effect of S. lavandulifolia aqueous extract in vivo in normal rats by taking into account the effect of intestinal lumen on the extract’s inhibitory effect. Normal fasting rats (180–250 g, 14 h) were divided into three groups (n = 6; ♂/♀ = 1): the control group was given distilled water (10 mL/kg), while the treated groups were given the extract (400 mg/kg) or acarbose (10 mg/kg). After measuring blood glucose, an adequate volume of the extract/distilled water/acarbose was administered (t0) to begin the oral starch tolerance test. A second measurement of glycemia was taken 30 min later (t1), and the rats were then overloaded with starch (3 g/kg). The variation in glycemia was measured at t2 = 60 min, t3 = 90, and t4 = 120 min.
3.13. Statistical Analysis
The results were statistically analyzed using ANOVA (one-way analysis of variance with Tukey’s post hoc test), and they are shown as means and standard deviation. Statistics were considered to be significant at p-values of p < 0.05, p < 0.01, and p < 0.00.
4. Conclusions
Aromatic and medicinal plants are a precious gift of nature for human beings; they have been used since antiquity in food, beverages, traditional medicine, and cosmetics. Salvia lavandulifolia Vahl is a widespread plant in Morocco; its leaves are often used for their high content of essential oil whose virtues are well known. The extracts, on the other hand, are very little studied by researchers, and their properties are found most of the time in articles—ethnopharmacological surveys or some very old ones. The aqueous extract of the leaves of S. lavandulifolia harvested in Ouled Ali-Boulemane-Morocco is very rich in polyphenols; it recorded a remarkable total antioxidant capacity and antiradical power. It is also considered bactericidal against Proteus mirabilis, fungicidal against Aspergillus niger, Candida albicans, Candida tropicalis, and Saccharomyces cerevisiae, and fungistatic against Candida krusei. Administered 30 min before an acute oral administration of glucose, our extract significantly lowered hyperglycemia in vivo in rats. Thanks to its content of rosmarinic acid, apigenin, and luteolin, it essentially could also inhibit the activity of the enzyme α-amylase in vitro and in vivo.
Author Contributions
All authors have contributed to writing the original draft, formal analysis, investigation, funding acquisition, resources, reviewing and editing, data validation, and data curation. T.Z.: Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Researchers Supporting Project number (RSP-2023R437), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project, King Saud University, Riyadh, Saudi Arabia for funding this work through the project number (RSP-2023R437).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hassan, I.-M.; Shehu, A.; Zezi, A.U.; Magaji, M.G.; Yaâ, J. Ethnobotanical Survey of Medicinal Plants Commonly Used in Snakebites in North Western Nigeria. J. Med. Plants Res. 2020, 14, 468–474. [Google Scholar]
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal Plant Analysis: A Historical and Regional Discussion of Emergent Complex Techniques. Front. Pharmacol. 2020, 10, 1480. [Google Scholar] [CrossRef]
- Hussein, R.A.; El-Anssary, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. Herb. Med. 2019, 1, 13. [Google Scholar]
- Wanjohi, B.K.; Sudoi, V.; Njenga, E.W.; Kipkore, W.K. An Ethnobotanical Study of Traditional Knowledge and Uses of Medicinal Wild Plants among the Marakwet Community in Kenya. Evid.-Based Complement. Altern. Med. 2020, 2020, 3208634. [Google Scholar] [CrossRef] [PubMed]
- Tamokou, J.D.D.; Mbaveng, A.T.; Kuete, V. Antimicrobial Activities of African Medicinal Spices and Vegetables. In Medicinal Spices and Vegetables from Africa; Elsevier: Amsterdam, The Netherlands, 2017; pp. 207–237. [Google Scholar]
- Ramamoorthy, T.P. Mexican Lamiaceae: Diversity, Distribution, Endemism, and Evolution; Oxford University Press: New York, NY, USA, 1993; pp. 513–539. [Google Scholar]
- Serrano-Vega, R.; Pérez-González, C.; Alonso-Castro, Á.J.; Zapata-Morales, J.R.; Pérez-Gutiérrez, S. Anti-Inflammatory and Antinociceptive Activities of Salvia Keerlii. Pharmacogn. Mag. 2020, 16, 27. [Google Scholar]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.-D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.-M.; Stefanescu, R.; Bild, V.; Melnic, S. Medicinal Plants of the Family Lamiaceae in Pain Therapy: A Review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef]
- Topçu, G. Bioactive Triterpenoids from Salvia Species. J. Nat. Prod. 2006, 69, 482–487. [Google Scholar] [CrossRef]
- Joran, M.J.; Martínez, C.; Moñino, M.I.; Lax, V.; Quílez, M.; Sotomayor, J.A. Chemical Characterization of Salvia Lavandulifolia Subsp. Vellerea in South-Eastern Spain. Acta Hortic. 2009, 826, 317–324. [Google Scholar] [CrossRef]
- Sáez, L.; Salvia, L. Verbenaceae-Labiatae-Callitrichaceae. In Flora Iberica; Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2010; Volume XII, pp. 298–326. [Google Scholar]
- Zrira, S.; Menut, C.; Bessiere, J.M.; Elamrani, A.; Benjilali, B. A Study of the Essential Oil of Salvia Lavandulifolia Vahl from Morocco. J. Essent. Oil Bear. Plants 2004, 7, 232–238. [Google Scholar] [CrossRef]
- Zarzuelo, A.; Risco, S.; Gamez, M.J.; Jimenez, J.; Camara, M.; Martinez, M.A. Hypoglycemic Action of Salvia Lavandulifolia Vahl. SSP. Oxyodon: A Contribution to Studies of the Mechanism of Action. Life Sci. 1990, 47, 909–915. [Google Scholar] [CrossRef]
- Corbeil, C.; Mehran, A.R.; Mehran, D. Nature in Cosmetics and Skin Care: A Compendium of Ingredients Used in Cosmetic and Skin Care Chemistry; Allured: Carol Stream, IL, USA, 2000; ISBN 0931710790. [Google Scholar]
- Bellakhdar, J. La Pharmacopée Marocaine Traditionnelle: Médecine Arabe Ancienne et Savoirs Populaires; Ibis Press: Lake Worth, FL, USA, 1997; ISBN 978-2-910728-03-8. [Google Scholar]
- Katiri, A.; Barkaoui, M.; Msanda, F.; Boubaker, H. Ethnobotanical Survey of Medicinal Plants Used for the Treatment of Diabetes in the Tizi n’ Test Region (Taroudant Province, Morocco). J. Pharmacogn. Nat. Prod. 2017, 3, 2472-0992. [Google Scholar] [CrossRef]
- Boutahiri, S.; Eto, B.; Bouhrim, M.; Mechchate, H.; Saleh, A.; Al Kamaly, O.; Drioiche, A.; Remok, F.; Samaillie, J.; Neut, C.; et al. Lavandula Pedunculata (Mill.) Cav. Aqueous Extract Antibacterial Activity Improved by the Addition of Salvia Rosmarinus Spenn., Salvia Lavandulifolia Vahl and Origanum Compactum Benth. Life 2022, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Raccah, D. Épidémiologie et physiopathologie des complications dégénératives du diabète sucré. EMC—Endocrinologie 2004, 1, 29–42. [Google Scholar] [CrossRef]
- Claudet, E. Séchage Des Plantes Aromatiques et Médicinales. 2015. Available online: https://interbiocorse.org/wp-content/uploads/2019/12/FT-sechage-PPAM.pdf (accessed on 26 February 2023).
- Johnston, G.A.R.; Beart, P.M. Flavonoids: Some of the Wisdom of Sage?: Commentary. Br. J. Pharmacol. 2004, 142, 809–810. [Google Scholar] [CrossRef]
- Lu, Y.; Yeap Foo, L. Polyphenolics of Salvia—A Review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Zarzuelo, A.; Gámez, J.M.; Utrilla, P.; Jiménez, J.; Jiménez, I. Luteolin 5-Rutinoside from Salvia Lavandulifolia ssp. Oxyodon. Phytochemistry 1995, 40, 1321–1322. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic Compounds in Plants and Agri-Industrial by-Products: Antioxidant Activity, Occurrence, and Potential Uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; Zarrelli, A.; Pinto, G.; Pollio, A. Plant Polyphenols and Their Anti-Cariogenic Properties: A Review. Molecules 2011, 16, 1486–1507. [Google Scholar] [CrossRef]
- Zhu, M.; Phillipson, J.D.; Greengrass, P.M.; Bowery, N.E.; Cai, Y. Plant Polyphenols: Biologically Active Compounds or Non-Selective Binders to Protein? Phytochemistry 1997, 44, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Charlton, A.J.; Baxter, N.J.; Khan, M.L.; Moir, A.J.G.; Haslam, E.; Davies, A.P.; Williamson, M.P. Polyphenol/Peptide Binding and Precipitation. J. Agric. Food Chem. 2002, 50, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Giglio, R.V.; Patti, A.M.; Cicero, A.F.G.; Lippi, G.; Rizzo, M.; Toth, P.P.; Banach, M. Polyphenols: Potential Use in the Prevention and Treatment of Cardiovascular Diseases. Curr. Pharm. Des. 2018, 24, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J. Polyphenols: A Potential New Strategy for the Prevention and Treatment of Anxiety and Depression. Curr. Nutr. Food Sci. 2010, 6, 13–18. [Google Scholar] [CrossRef]
- Hbika, A.; Daoudi, N.E.; Bouyanzer, A.; Bouhrim, M.; Mohti, H.; Loukili, E.H.; Mechchate, H.; Al-Salahi, R.; Nasr, F.A.; Bnouham, M. Artemisia absinthium L. Aqueous and Ethyl Acetate Extracts: Antioxidant Effect and Potential Activity In Vitro and In Vivo against Pancreatic α-Amylase and Intestinal α-Glucosidase. Pharmaceutics 2022, 14, 481. [Google Scholar] [CrossRef]
- Adejoh, I.P.; Agatemor, U.M. Anti-Radical and Inhibitory Effect of Some Common Nigerian Medicinal Plants on Alpha Glucosidase, Aldose Reductase and Angiotensin Converting Enzyme: Potential Protective Mechanisms against Diabetic Complications. Int. J. Adv. Res. Biol. Sci 2018, 5, 188–201. [Google Scholar]
- Lavid, N.; Schwartz, A.; Yarden, O.; Tel-Or, E. The Involvement of Polyphenols and Peroxidase Activities in Heavy-Metal Accumulation by Epidermal Glands of the Waterlily (Nymphaeaceae). Planta 2001, 212, 323–331. [Google Scholar] [CrossRef]
- Lutgen, P. Tannins in Artemisia: The Hidden Treasure of Prophylaxis. Pharm. Pharmacol. Int. J. 2018, 6, 176–181. [Google Scholar] [CrossRef]
- Dhull, S.B.; Kaur, P.; Purewal, S.S. Phytochemical Analysis, Phenolic Compounds, Condensed Tannin Content and Antioxidant Potential in Marwa (Origanum Majorana) Seed Extracts. Resour.-Effic. Technol. 2016, 2, 168–174. [Google Scholar] [CrossRef]
- Jordán, M.J.; Lax, V.; Rota, M.C.; Lorán, S.; Sotomayor, J.A. Relevance of Carnosic Acid, Carnosol, and Rosmarinic Acid Concentrations in the In Vitro Antioxidant and Antimicrobial Activities of Rosmarinus Officinalis (L.) Methanolic Extracts. J. Agric. Food Chem. 2012, 60, 9603–9608. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, Y.; Ikeda, S.; Uwai, K.; Taguchi, R.; Chayama, K.; Sakaguchi, T.; Narita, R.; Yao, W.-L.; Takeuchi, F.; Otakaki, Y.; et al. Rosmarinic Acid Is a Novel Inhibitor for Hepatitis B Virus Replication Targeting Viral Epsilon RNA-Polymerase Interaction. PLoS ONE 2018, 13, e0197664. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Ma, X.; Guo, S.; Zhang, T.; Zhao, G.; Wu, H.; Wang, X.; Deng, G. Anti-Inflammatory Effects of Rosmarinic Acid in Lipopolysaccharide-Induced Mastitis in Mice. Inflammation 2018, 41, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Radziejewska, I.; Supruniuk, K.; Bielawska, A. Anti-Cancer Effect of Combined Action of Anti-MUC1 and Rosmarinic Acid in AGS Gastric Cancer Cells. Eur. J. Pharmacol. 2021, 902, 174119. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Gopi, M.; Karthik, K.; Malik, Y.S.; Dhama, K. Rosmarinic Acid: Modes of Action, Medicinal Values and Health Benefits. Anim. Health Res. Rev. 2017, 18, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Aldoghachi, F.E.H.; Noor Al-Mousawi, U.M.; Shari, F.H. Antioxidant Activity of Rosmarinic Acid Extracted and Purified from Mentha Piperita. Arch. Razi Inst. 2021, 76, 1279–1287. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kwon, C.-S.; Son, K.H. Inhibition of Alpha-Glucosidase and Amylase by Luteolin, a Flavonoid. Biosci. Biotechnol. Biochem. 2000, 64, 2458–2461. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Distribution and Biological Activities of the Flavonoid Luteolin. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Djeddi, S.; Yannakopoulou, E.; Papadopoulos, K. Activités anti-radicalaires de l’huile essentielle et des extraits bruts de Thymus numidicus Poiret., Algérie. Afr. J. Online 2015, 11, 58–65. [Google Scholar]
- O’Neill, E.J.; Den Hartogh, D.J.; Azizi, K.; Tsiani, E. Anticancer Properties of Carnosol: A Summary of In Vitro and In Vivo Evidence. Antioxidants 2020, 9, 961. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Cafligueral, S.; Iglesias’, J.; Hamburger, M.; Hostettmann, K. Phenolic Constituents of Salvia Lavanduljfolia ssp. Lavandulifolia. Planta Med. 1989, 55, 92. [Google Scholar] [CrossRef]
- Phatak, R.S.; Hendre, A.S. Total Antioxidant Capacity (TAC) of Fresh Leaves of Kalanchoe Pinnata. J. Pharmacogn. Phytochem. 2014, 2, 32–35. [Google Scholar]
- Al-Qudah, M.A.; Al-Jaber, H.I.; Abu Zarga, M.H.; Abu Orabi, S.T. Flavonoid and Phenolic Compounds from Salvia Palaestina L. Growing Wild in Jordan and Their Antioxidant Activities. Phytochemistry 2014, 99, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Pop, A.V.; Tofană, M.; Socaci, S.A.; Pop, C.; Rotar, A.M.; Nagy, M.; Salanţă, L. Determination of Antioxidant Capacity and Antimicrobial Activity of Selected Salvia Species. Bull. UASVM Food Sci. Technol. 2016, 73, 14–18. [Google Scholar] [CrossRef]
- Ali, A.M.A.; El-Nour, M.E.M.; Yagi, S.M. Total Phenolic and Flavonoid Contents and Antioxidant Activity of Ginger (Zingiber Officinale Rosc.) Rhizome, Callus and Callus Treated with Some Elicitors. J. Genet. Eng. Biotechnol. 2018, 16, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Tepe, B. Antioxidant Potentials and Rosmarinic Acid Levels of the Methanolic Extracts of Salvia Virgata (Jacq), Salvia Staminea (Montbret & Aucher Ex Bentham) and Salvia Verbenaca (L.) from Turkey. Bioresour. Technol. 2007, 99, 1584–1588. [Google Scholar] [CrossRef]
- Giner, M.J.; Vegara, S.; Funes, L.; Martí, N.; Saura, D.; Micol, V.; Valero, M. Antimicrobial Activity of Food-Compatible Plant Extracts and Chitosan against Naturally Occurring Micro-Organisms in Tomato Juice. J. Sci. Food Agric. 2012, 92, 1917–1923. [Google Scholar] [CrossRef]
- Perry, N.S.L.; Houghton, P.J.; Sampson, J.; Theobald, A.E.; Hart, S.; Lis-Balchin, M.; Hoult, J.R.S.; Evans, P.; Jenner, P.; Milligan, S. In-Vitro Activity of S. Lavandulaefolia (Spanish Sage) Relevant to Treatment of Alzheimer’s Disease. J. Pharm. Pharmacol. 2001, 53, 1347–1356. [Google Scholar] [CrossRef]
- Jimenez, J.; Risco, S.; Ruiz, T.; Zarzuelo, A. Hypoglycemic Activity of Salvia Lavandulifolia. Planta Med. 1986, 52, 260–262. [Google Scholar] [CrossRef]
- Mahdizadeh, R.; Moein, S.; Soltani, N.; Malekzadeh, K.; Mahmoodreza, M. Study the Molecular Mechanism of Salvia Species in Prevention of Diabetes. Int. J. Pharm. Sci. Res. 2018, 9, 4512–4521. [Google Scholar] [CrossRef]
- Bnouham, M.; Ziyyat, A.; Mekhfi, H.; Tahri, A.; Legssyer, A. Medicinal Plants with Potential Antidiabetic Activity-A Review of Ten Years of Herbal Medicine Research (1990–2000). Int. J. Diabetes Metab. 2006, 14, 1–25. [Google Scholar] [CrossRef]
- Benayad, O.; Bouhrim, M.; Tiji, S.; Kharchoufa, L.; Addi, M.; Drouet, S.; Hano, C.; Lorenzo, J.M.; Bendaha, H.; Bnouham, M. Phytochemical Profile, α-Glucosidase, and α-Amylase Inhibition Potential and Toxicity Evaluation of Extracts from Citrus aurantium (L) Peel, a Valuable By-Product from Northeastern Morocco. Biomolecules 2021, 11, 1555. [Google Scholar] [CrossRef]
- Ouassou, H.; Bouhrim, M.; Bencheikh, N.; Addi, M.; Hano, C.; Mekhfi, H.; Ziyyat, A.; Legssyer, A.; Aziz, M.; Bnouham, M. In Vitro Antioxidant Properties, Glucose-Diffusion Effects, α-Amylase Inhibitory Activity, and Antidiabetogenic Effects of C. Europaea Extracts in Experimental Animals. Antioxidants 2021, 10, 1747. [Google Scholar] [CrossRef] [PubMed]
- Hano, C.; Renouard, S.; Molinié, R.; Corbin, C.; Barakzoy, E.; Doussot, J.; Lamblin, F.; Lainé, E. Flaxseed (Linum Usitatissimum L.) Extract as Well as (+)-Secoisolariciresinol Diglucoside and Its Mammalian Derivatives Are Potent Inhibitors of α-Amylase Activity. Bioorganic Med. Chem. Lett. 2013, 23, 3007–3012. [Google Scholar] [CrossRef]
- Proença, C.; Freitas, M.; Ribeiro, D.; Tomé, S.M.; Oliveira, E.F.T.; Viegas, M.F.; Araújo, A.N.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A. Evaluation of a Flavonoids Library for Inhibition of Pancreatic α-Amylase towards a Structure–Activity Relationship. J. Enzym. Inhib. Med. Chem. 2019, 34, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, X.; Sun, W.; Xing, Y.; Xiu, Z.; Zhuang, C.; Dong, Y. Dietary Flavonoids and Acarbose Synergistically Inhibit α-Glucosidase and Lower Postprandial Blood Glucose. J. Agric. Food Chem. 2017, 65, 8319–8330. [Google Scholar] [CrossRef] [PubMed]
- Afnor. NF V03-402. Available online: https://www.boutique.afnor.org/fr-fr/norme/nf-v03402/epices-et-aromates-determination-de-la-teneur-en-eau-methode-par-entraineme/fa032462/325 (accessed on 15 September 2022).
- World Health Organization. Quality Control Methods for Medicinal Plant Materials; World Health Organization: Geneva, Switzerland, 1998; ISBN 978-92-4-154510-5.
- Centre d’Expertise en Analyse Environnementale du Québec Méthode d’Analyse. Détermination Du PH: Méthode Électrométrique; Centre d’Expertise en Analyse Environnementale du Québec Méthode d’Analyse: Québec City, QC, Canada, 2014. [Google Scholar]
- Bergeron, L. Effet de la Teneur en Eau du Sol Sur le Rendement et la Qualité Des Fruits du Bleuet Nain; Université du Québec à Chicoutimi: Chicoutimi, QC, Canada, 1995; ISBN 978-1-4123-0614-0. [Google Scholar]
- Afnor. Norme NF V05-113. Available online: https://www.boutique.afnor.org/fr-fr/norme/nf-v05113/fruits-legumes-et-produits-derives-mineralisation-des-matieres-organiques-m/fa009207/13929 (accessed on 15 September 2022).
- Skujins, S. Handbook for ICP-AES (Varian-Vista): A Short Guide to Vista Series ICP-AES Operation; Varian Medical Systems International AG: Steinhausen, Switzerland, 1998. [Google Scholar]
- Chaouche, T.; Haddouchi, F.; Bekkara, F.A. Phytochemical Study of Roots and Leaves of the Plant Echium Pycnanthum Pomel. Der Pharm. Lett. 2011, 3, 1–4. [Google Scholar]
- Haddouchi, F.; Chaouche, T.M.; Halla, N. Screening phytochimique, activités antioxydantes et pouvoir hémolytique de quatre plantes sahariennes d’Algérie. Phytothérapie 2018, 16, S254–S262. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. ISBN 978-0-12-182200-2. [Google Scholar]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colometric Methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical Factors of Vanillin Assay for Catechins and Proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Molyneux, P. The Use of the Stable Free Radical Diphenylpicryl- Hydrazyl (DPPH) for Estimating Antioxidant Activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Kroyer, G.T. Red Clover Extract as Antioxidant Active and Functional Food Ingredient. Innov. Food Sci. Emerg. Technol. 2004, 5, 101–105. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Prasannabalaji, N.; Muralitharan, G.; Sivanandan, R.; Kumaran, S.; Pugazhvendan, S. Antibacterial Activities of Some Indian Traditional Plant Extracts. Asian Pac. J. Trop. Dis. 2012, 2, S291–S295. [Google Scholar] [CrossRef]
- Bissel, S.J.; Winkler, C.C.; DelTondo, J.; Wang, G.; Williams, K.; Wiley, C.A. Coxsackievirus B4 Myocarditis and Meningoencephalitis in Newborn Twins. Neuropathology 2014, 34, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Tchoumtchoua, J.; Mouchili, O.R.; Ateba, S.B.; Zingue, S.; Halabalaki, M.; Mbanya, J.C.; Skaltsounis, A.-L.; Njamen, D. Safety Assessment of the Methanol Extract of the Stem Bark of Amphimas Pterocarpoides Harms: Acute and Subchronic Oral Toxicity Studies in Wistar Rats. Toxicol. Rep. 2014, 1, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Daoudi, N.E.; Bouhrim, M.; Ouassou, H.; Legssyer, A.; Mekhfi, H.; Ziyyat, A.; Aziz, M.; Bnouham, M. Inhibitory Effect of Roasted/Unroasted Argania Spinosa Seeds Oil on α-Glucosidase, α-Amylase and Intestinal Glucose Absorption Activities. S. Afr. J. Bot. 2020, 135, 413–420. [Google Scholar] [CrossRef]
- Thalapaneni, N.R.; Chidambaram, K.A.; Ellappan, T.; Sabapathi, M.L.; Mandal, S.C. Inhibition of Carbohydrate Digestive Enzymes by Talinum Portulacifolium (Forssk) Leaf Extract. J. Complement. Integr. Med. 2008, 5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).