In Silico Evaluation of Quercetin Methylated Derivatives on the Interaction with Secretory Phospholipases A2 from Crotalus durissus terrificus and Bothrops jararacussu
Abstract
1. Introduction
2. Results
2.1. Properties of Compounds
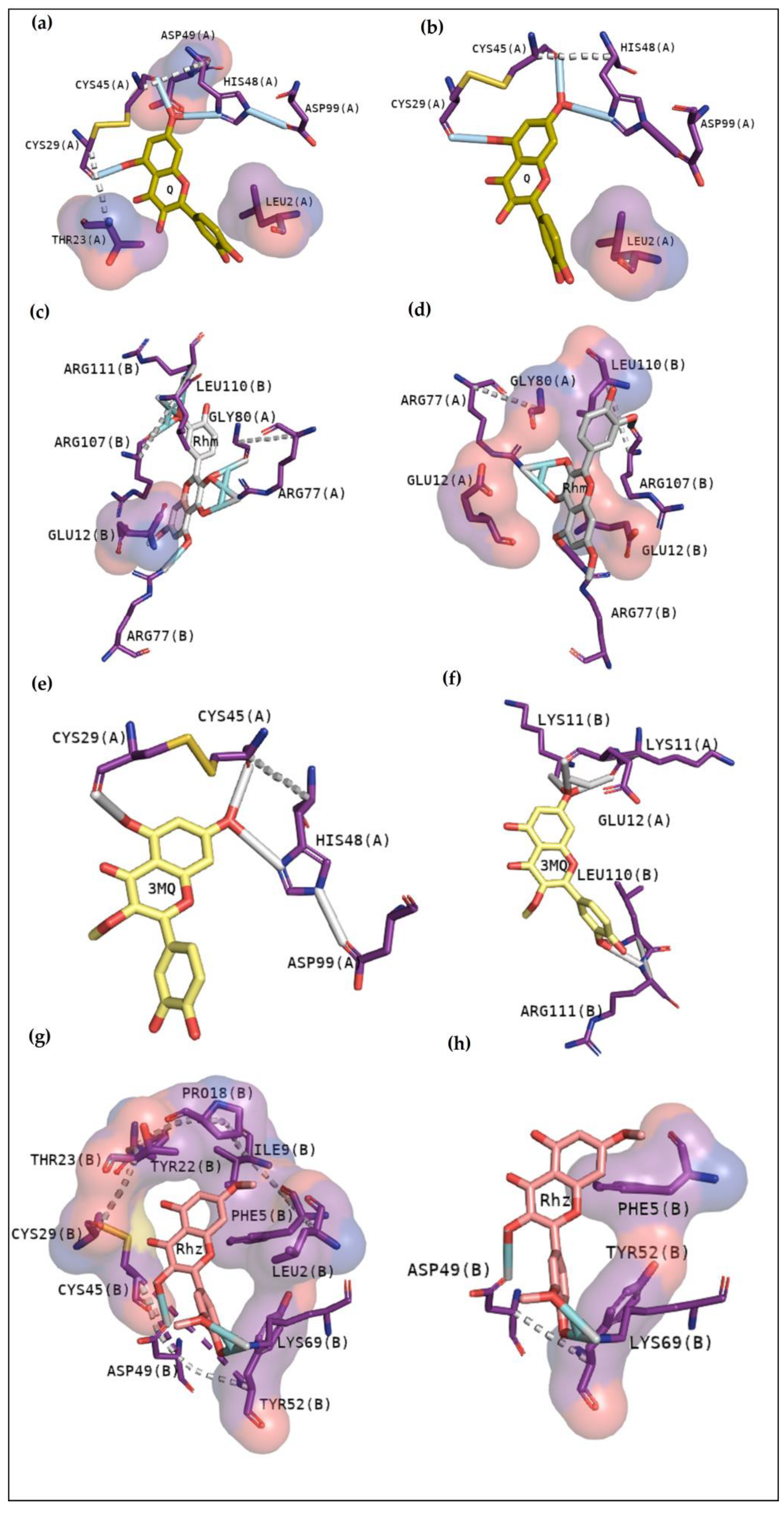
2.2. Molecular Docking
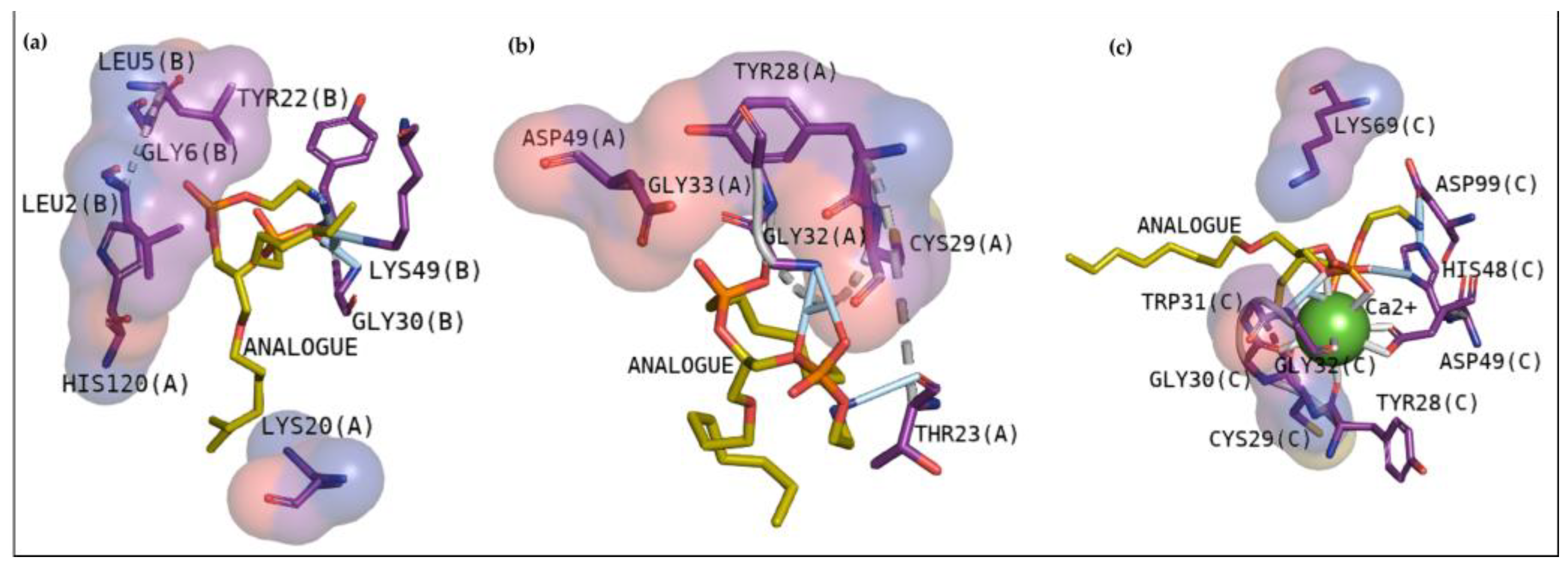
2.2.1. Cavity Analysis
2.2.2. Docking Analysis with a Transitional Analogous
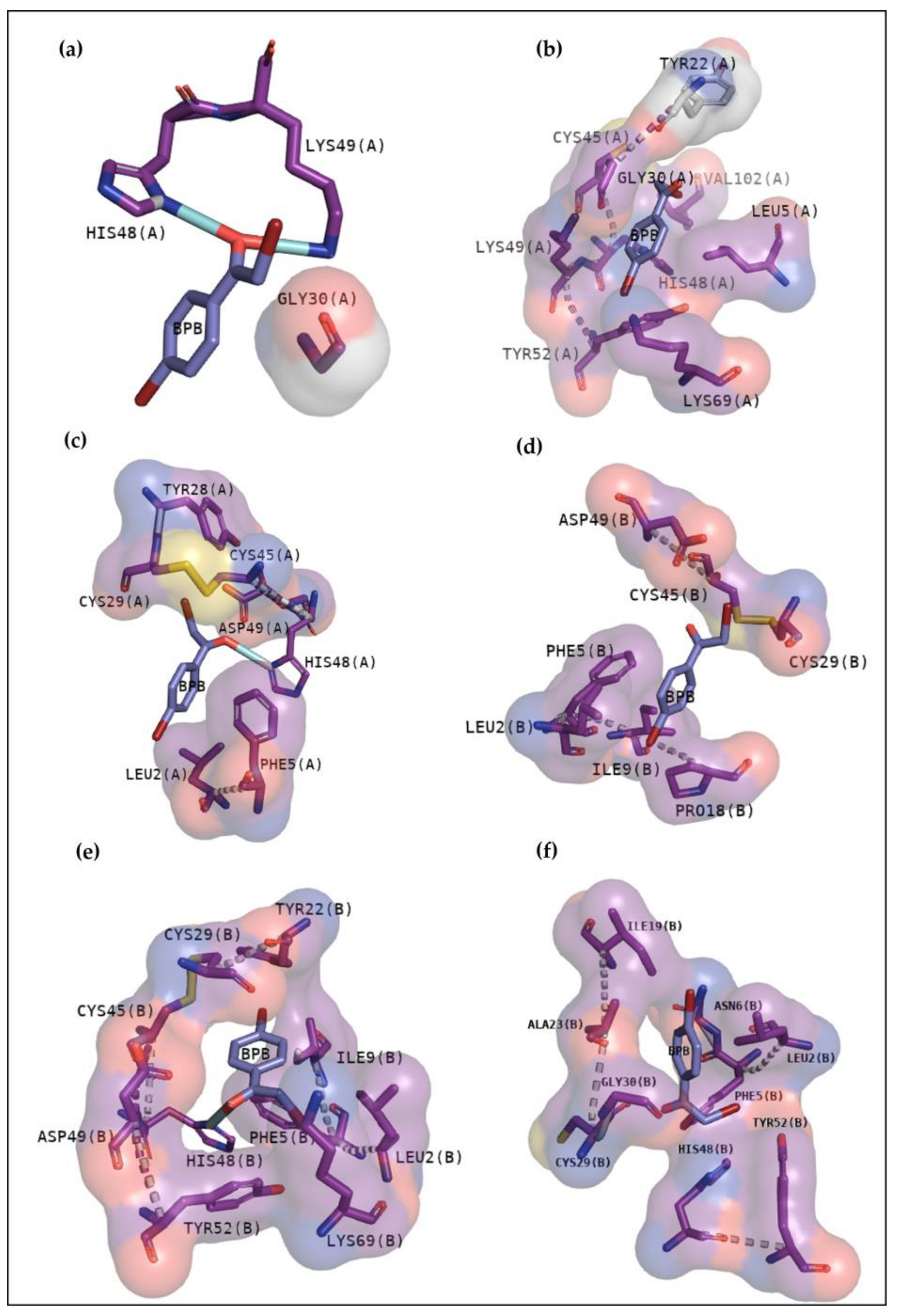
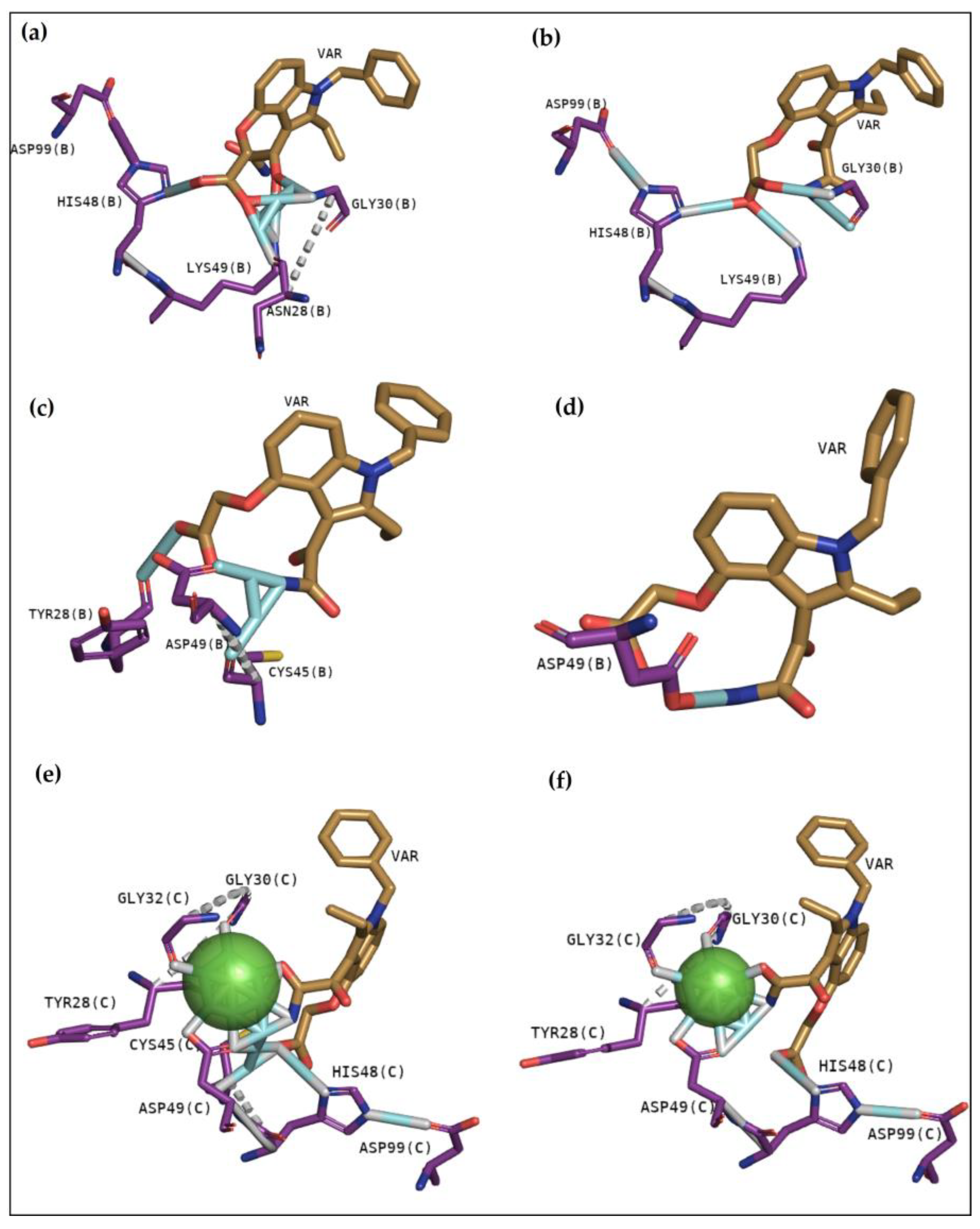
2.2.3. Classical Inhibitors BPB and Var
2.2.4. BthTX-I
2.2.5. BthTX-II
2.2.6. CdtsPLA2 Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Evaluation of Compounds’ Properties and Preparation
4.2. Proteins Preparation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Li, Y.; Xu, L.; Shi, J.; Yu, X.; Wang, X.; Li, X.; Jiang, H.; Yang, T.; Yin, X.; et al. Quercetin Attenuates Podocyte Apoptosis of Diabetic Nephropathy Through Targeting EGFR Signaling. Front. Pharmacol. 2022, 12, 792777. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Jiang, Y.; Yang, J.; Zhao, Y.; Tian, M.; Yang, B. Structure, bioactivity, and synthesis of methylated flavonoids. Ann. N. Y. Acad. Sci. 2017, 1398, 120–129. [Google Scholar] [CrossRef]
- Magar, R.T.; Sohng, J.K. A Review on Structure, Modifications and Structure-Activity Relation of Quercetin and Its Derivatives. J. Microbiol. Biotechnol. 2020, 30, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Karancsi, Z.; Kovács, D.; Pézsa, N.P.; Gálfi, P.; Jerzsele, Á.; Farkas, O. The Impact of Quercetin and Its Methylated Derivatives 3-o-Methylquercetin and Rhamnazin in Lipopolysaccharide-Induced Inflammation in Porcine Intestinal Cells. Antioxidants 2022, 11, 1265. [Google Scholar] [CrossRef] [PubMed]
- Belchor, M.N.; Gaeta, H.H.; Rodrigues, C.F.B.; Costa, C.R.D.C.; Toyama, D.D.O.; Passero, L.F.D.; Laurenti, M.D.; Toyama, M.H. Evaluation of Rhamnetin as an Inhibitor of the Pharmacological Effect of Secretory Phospholipase A2. Molecules 2017, 22, 1441. [Google Scholar] [CrossRef]
- Devi, A.; Namsa, N.D.; Doley, R. In silico and in vitro neutralization of PLA2 activity of Daboxin P by butein, mimosine and bakuchiol. Int. J. Biol. Macromol. 2020, 165, 1066–1078. [Google Scholar] [CrossRef]
- Batsika, C.S.; Gerogiannopoulou, A.-D.D.; Mantzourani, C.; Vasilakaki, S.; Kokotos, G. The design and discovery of phospholipase A2 inhibitors for the treatment of inflammatory diseases. Expert Opin. Drug Discov. 2021, 16, 1287–1305. [Google Scholar] [CrossRef]
- Fernandes, C.A.H.; Borges, R.J.; Lomonte, B.; Fontes, M.R.M. A structure-based proposal for a comprehensive myotoxic mechanism of phospholipase A2-like proteins from viperid snake venoms. Biochim. Biophys. Acta 2014, 1844, 2265–2276. [Google Scholar] [CrossRef]
- Azevedo, F.V.P.d.V.; Lopes, D.S.; Zóia, M.A.P.; Correia, L.I.V.; Saito, N.; Fonseca, B.B.; Polloni, L.; Teixeira, S.C.; Goulart, L.R.; Ávila, V.d.M.R. A New Approach to Inhibiting Triple-Negative Breast Cancer: In Vitro, Ex Vivo and In Vivo Antiangiogenic Effect of BthTx-II, a PLA2-Asp-49 from Bothrops jararacussu Venom. Biomolecules 2022, 12, 258. [Google Scholar]
- Salvador, G.H.M.; Pinto, Ê.K.R.; Ortolani, P.L.; Fortes-Dias, C.L.; Cavalcante, W.L.G.; Soares, A.M.; Lomonte, B.; Lewin, M.R.; Fontes, M.R.M. Structural basis of the myotoxic inhibition of the Bothrops pirajai PrTX-I by the synthetic varespladib. Biochimie 2023, 207, 1–10. [Google Scholar] [CrossRef]
- Faure, G.; Xu, H.; Saul, F.A. Crystal Structure of Crotoxin Reveals Key Residues Involved in the Stability and Toxicity of This Potent Heterodimeric β-Neurotoxin. J. Mol. Biol. 2011, 412, 176–191. [Google Scholar] [CrossRef]
- Nemecz, D.; Ostrowski, M.; Ravatin, M.; Saul, F.; Faure, G. Crystal Structure of Isoform CBd of the Basic Phospholipase A2 Subunit of Crotoxin: Description of the Structural Framework of CB for Interaction with Protein Targets. Molecules 2020, 25, 5290. [Google Scholar] [CrossRef]
- Marchi-Salvador, D.P.; Fernandes, C.A.; Silveira, L.B.; Soares, A.M.; Fontes, M.R. Crystal structure of a phospholipase A2 homolog complexed with p-bromophenacyl bromide reveals important structural changes associated with the inhibition of myotoxic activity. Biochim. Biophys. Acta 2009, 1794, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Youngman, N.; Walker, A.; Naude, A.; Coster, K.; Sundman, E.; Fry, B.G. Varespladib (LY315920) neutralises phospholipase A2 mediated prothrombinase-inhibition induced by Bitis snake venoms. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 236, 108818. [Google Scholar] [CrossRef]
- Salvador, G.H.M.; Cardoso, F.F.; Gomes, A.A.; Cavalcante, W.L.G.; Gallacci, M.; Fontes, M.R.M. Search for efficient inhibitors of myotoxic activity induced by ophidian phospholipase A2-like proteins using functional, structural and bioinformatics approaches. Sci. Rep. 2019, 9, 510. [Google Scholar] [CrossRef]
- Souza, J.D.; Oliveira, I.C.F.; Yoshida, E.H.; Cantuaria, N.M.; Cogo, J.C.; Torres-Bonilla, K.A.; Hyslop, S.; Junior, N.J.S.; Floriano, R.S.; Gutiérrez, J.M.; et al. Effect of the phospholipase A2 inhibitor Varespladib, and its synergism with crotalic antivenom, on the neuromuscular blockade induced by Crotalus durissus terrificus venom (with and without crotamine) in mouse neuromuscular preparations. Toxicon 2022, 214, 54–61. [Google Scholar] [CrossRef] [PubMed]
- White, S.P.; Scott, D.L.; Otwinowski, Z.; Gelb, M.H.; Sigler, P.B. Crystal Structure of Cobra-Venom Phospholipase A2 in a Complex with a Transition-State Analogue. Science 1990, 250, 1560–1563. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Sudharshan, S.; Dhananjaya, B.L. Antibacterial potential of a basic phospholipase A2 (VRV-PL-VIIIa) from Daboia russelii pulchella (Russell’s viper) venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 17. [Google Scholar] [CrossRef]
- Rifaioglu, A.S.; Atas, H.; Martin, M.J.; Cetin-Atalay, R.; Atalay, V.; Doğan, T. Recent applications of deep learning and machine intelligence on in silico drug discovery: Methods, tools and databases. Brief. Bioinform. 2019, 20, 1878–1912. [Google Scholar] [CrossRef] [PubMed]
- Cotrim, C.A.; de Oliveira, S.C.B.; Filho, E.B.S.D.; Fonseca, F.V.; Baldissera, L., Jr.; Antunes, E.; Ximenes, R.M.; Monteiro, H.S.A.; Rabello, M.M.; Hernandes, M.Z.; et al. Quercetin as an inhibitor of snake venom secretory phospholipase A2. Chemico-Biological Interactions. Chem. Biol. Interact. 2011, 189, 9–16. [Google Scholar] [CrossRef]
- Zhao, H.; Tang, L.; Wang, X.; Zhou, Y.; Lin, Z. Structure of a snake venom phospholipase A2 modified by p-bromo-phenacyl-bromide. Toxicon 1998, 36, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.C.; de Castro, R.C.; Toyama, M.; Giglio, J.R.; Marangoni, S.; De Nucci, G.; Antunes, E. Inflammatory oedema induced by the Lys-49 phospholipase A2 homologue piratoxin-i in the rat and rabbit: Effect of polyanions and p-bromophenacyl bromide. Biochem. Pharmacol. 2000, 59, 1289–1294. [Google Scholar] [CrossRef]
- Takeda, A.A.S.; Santos, J.I.D.; Marcussi, S.; Silveira, L.B.; Soares, A.M.; Fontes, M.R.M. Crystallization and preliminary X-ray diffraction analysis of an acidic phospholipase A2 complexed with p-bromophenacyl bromide and alpha-tocopherol inhibitors at 1.9- and 1.45-A resolution. Biochim. Biophys Acta 2004, 1699, 281–284. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Hu, Q.; Gao, S.; Ma, X.; Zhang, W.; Shen, Y.; Chen, F.; Lai, L.; Pei, J. CavityPlus: A web server for protein cavity detection with pharmacophore modelling, allosteric site identification and covalent ligand binding ability prediction. Nucleic Acids Res. 2018, 46, W374–W379. [Google Scholar] [CrossRef]
- Kumar, R.; Vijayalakshmi, S.; Nadanasabapathi, S. Health Benefits of Quercetin. Def. Life Sci. J. 2017, 2, 142–151. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Li, W. Rhamnetin ameliorates macrophage-mediated inflammation and pro-atherosclerosis pathways in apolipoprotein E-deficient mice. J. Physiol. Pharmacol. 2021, 72, 249–258. [Google Scholar]
- Marchi-Salvador, D.P.; Corrêa, L.C.; Magro, A.J.; Oliveira, C.Z.; Soares, A.M.; Fontes, M.R.M. Insights into the role of oligomeric state on the biological activities of crotoxin: Crystal structure of a tetrameric phospholipase A2 formed by two isoforms of crotoxin B from Crotalus durissus terrificus venom. Proteins 2008, 72, 883–891. [Google Scholar] [CrossRef]
- Borges, R.J.; Salvador, G.H.M.; Campanelli, H.B.; Pimenta, D.C.; de Oliveira Neto, M.; Usón, I.; Fontes, M.R.M. BthTX-II from Bothrops jararacussu venom has variants with different oligomeric assemblies: An example of snake venom phospholipases A2 versatility. Int. J. Biol. Macromol. 2021, 191, 255–266. [Google Scholar] [CrossRef]
- Cedro, R.C.A.; Menaldo, D.L.; Costa, T.R.; Zoccal, K.F.; Sartim, M.A.; Santos-Filho, N.A.; Faccioli, L.H.; Sampaio, S.V. Cytotoxic and inflammatory potential of a phospholipase A2 from Bothrops jararaca snake venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 33. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.P.; Silva, V.A.O.; Silvestrini, A.V.P.; de Macedo, L.H.; Caetano, G.F.; Reis, R.M.; Mazzi, M.V. Crotoxin from Crotalus durissus terrificus venom: In vitro cytotoxic activity of a heterodimeric phospholipase A2 on human cancer-derived cell lines. Toxicon 2018, 156, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
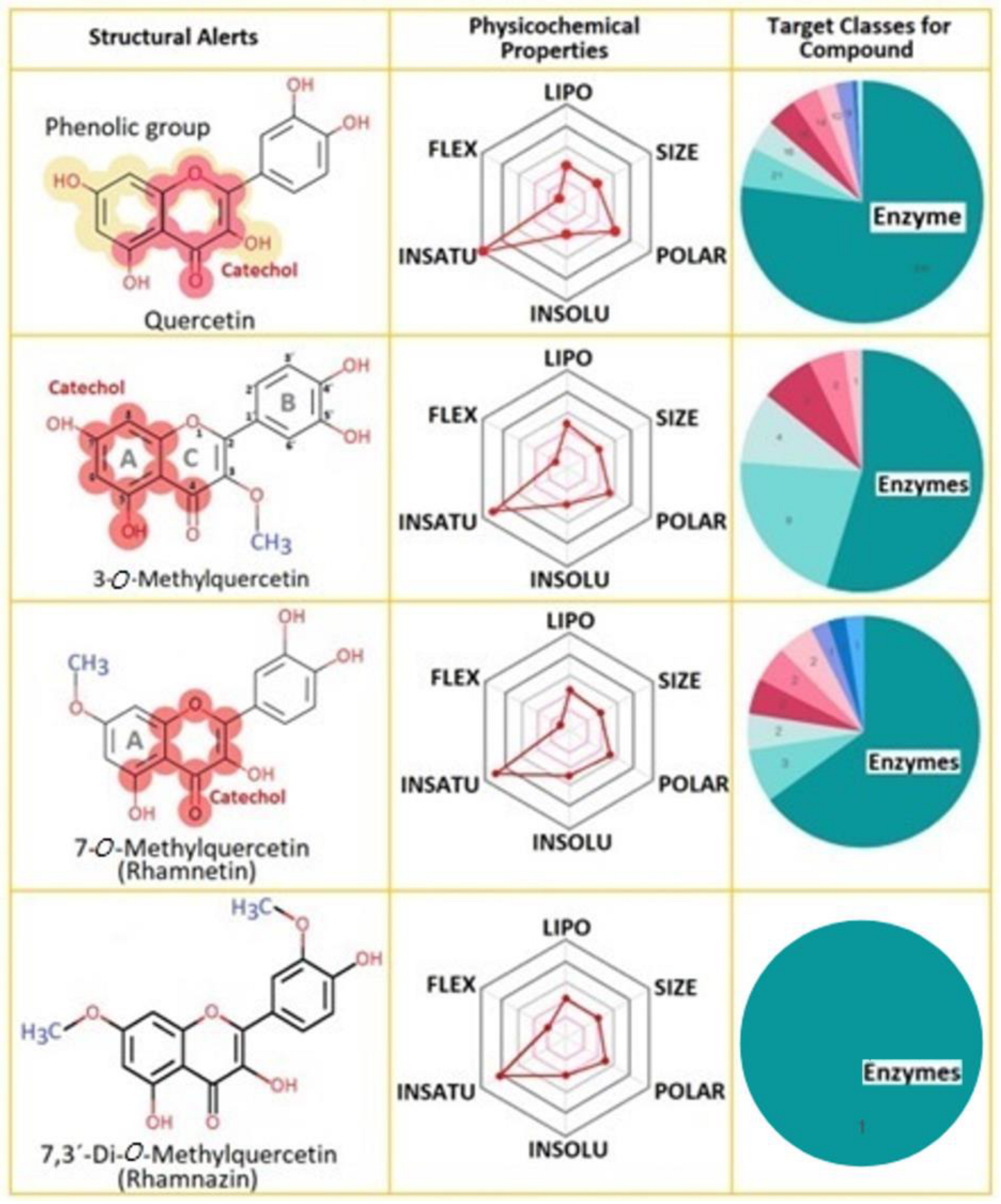



| Q | Rhm | 3MQ | Rhz | |
|---|---|---|---|---|
| MW (g/mol) | 302.24 | 316.26 | 316.26 | 330.29 |
| Num. rotatable bonds | 1 | 2 | 2 | 3 |
| Num. H-bond acceptors | 7 | 7 | 7 | 7 |
| Num. H-bond donors | 5 | 4 | 4 | 3 |
| TPSA | 131.36 Å2 | 120.36 Å2 | Å2 | 109.36 Å2 |
| Lipophilicity (Consensus Log Po/w) | 1.23 | 1.63 | 1.75 | 2.02 |
| 1º Cluster | 2º Cluster | |||
|---|---|---|---|---|
| Affinity (kcal/mol) | Rmsd (l.b.; u.b.) | Affinity (kcal/mol) | Rmsd (l.b.; u.b.) | |
| BthTX-I:BPB | −5.6 | 0; 0 | −5.4 | 2.086; 4.796 |
| BthTX-II:BPB | −5 | 0; 0 | −5 | 0.102; 1.460 |
| CdtsPLA2:BPB | −5.5 | 0; 0 | −5.4 | 1.129; 2.875 |
| 1º Cluster | 2º Cluster | |||
|---|---|---|---|---|
| Affinity (kcal/mol) | Rmsd (l.b.; u.b.) | Affinity (kcal/mol) | Rmsd (l.b.; u.b.) | |
| BthTX-I:Var | −8.7 | 0; 0 | −8.5 | 1.871; 2.797 |
| BthTX-II:Var | −7.1 | 0; 0 | −6.7 | 2.444; 3.928 |
| CdtsPLA2:Var | −8.3 | 0; 0 | −8.2 | 1.942; 3.649 |
| 1º Cluster | 2º Cluster | |||
|---|---|---|---|---|
| Affinity (kcal/mol) | Rmsd (l.b.; u.b.) | Affinity (kcal/mol) | Rmsd (l.b.; u.b.) | |
| Q | −8.2 | 0; 0 | −7.9 | 2.655; 3.657 |
| Rhm | −8.2 | 0; 0 | −8.1 | 1.625; 2.395 |
| 3MQ | −7.9 | 0; 0 | −7.9 | 2.817; 4.116 |
| Rhz | −8.2 | 0; 0 | −8 | 2.465; 3.041 |
| 1º Cluster | 2º Cluster | |||
|---|---|---|---|---|
| Affinity (kcal/mol) | Rmsd (l.b.; u.b.) | Affinity (kcal/mol) | Rmsd (l.b.; u.b.) | |
| Q | −7.4 | 0; 0 | −7.3 | 1.688; 2.723 |
| Rhm | −7.7 | 0; 0 | −7.5 | 0.629; 1.395 |
| 3MQ | −6.9 | 0; 0 | −6.7 | 1.908; 2.325 |
| Rhz | −7.3 | 0; 0 | −6.9 | 1.826; 6.889 |
| 1º Cluster | 2º Cluster | |||
|---|---|---|---|---|
| Affinity (kcal/mol) | Rmsd (l.b.; u.b.) | Affinity (kcal/mol) | Rmsd (l.b.; u.b.) | |
| Q | −9.7 | 0; 0 | −8 | 1.878; 2.823 |
| Rhm | −9.4 | 0; 0 | −8.2 | 0.530; 1.426 |
| 3MQ | −9.4 | 0; 0 | −8.5 | 1.908; 2.325 |
| Rhz | −8.9 | 0; 0 | −8.8 | 1.807; 4.896 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belchor, M.N.; Costa, C.R.d.C.; Roggero, A.; Moraes, L.L.F.; Samelo, R.; Annunciato, I.; de Oliveira, M.A.; Sousa, S.F.; Toyama, M.H. In Silico Evaluation of Quercetin Methylated Derivatives on the Interaction with Secretory Phospholipases A2 from Crotalus durissus terrificus and Bothrops jararacussu. Pharmaceuticals 2023, 16, 597. https://doi.org/10.3390/ph16040597
Belchor MN, Costa CRdC, Roggero A, Moraes LLF, Samelo R, Annunciato I, de Oliveira MA, Sousa SF, Toyama MH. In Silico Evaluation of Quercetin Methylated Derivatives on the Interaction with Secretory Phospholipases A2 from Crotalus durissus terrificus and Bothrops jararacussu. Pharmaceuticals. 2023; 16(4):597. https://doi.org/10.3390/ph16040597
Chicago/Turabian StyleBelchor, Mariana Novo, Caroline Ramos da Cruz Costa, Airam Roggero, Laila L. F. Moraes, Ricardo Samelo, Isabelly Annunciato, Marcos Antonio de Oliveira, Sergio F. Sousa, and Marcos Hikari Toyama. 2023. "In Silico Evaluation of Quercetin Methylated Derivatives on the Interaction with Secretory Phospholipases A2 from Crotalus durissus terrificus and Bothrops jararacussu" Pharmaceuticals 16, no. 4: 597. https://doi.org/10.3390/ph16040597
APA StyleBelchor, M. N., Costa, C. R. d. C., Roggero, A., Moraes, L. L. F., Samelo, R., Annunciato, I., de Oliveira, M. A., Sousa, S. F., & Toyama, M. H. (2023). In Silico Evaluation of Quercetin Methylated Derivatives on the Interaction with Secretory Phospholipases A2 from Crotalus durissus terrificus and Bothrops jararacussu. Pharmaceuticals, 16(4), 597. https://doi.org/10.3390/ph16040597







