Design, Synthesis, and Neuroprotective Activity of Phenoxyindole Derivatives on Antiamyloid Beta (Aβ) Aggregation, Antiacetylcholinesterase, and Antioxidant Activities
Abstract
1. Introduction
2. Results and Discussion
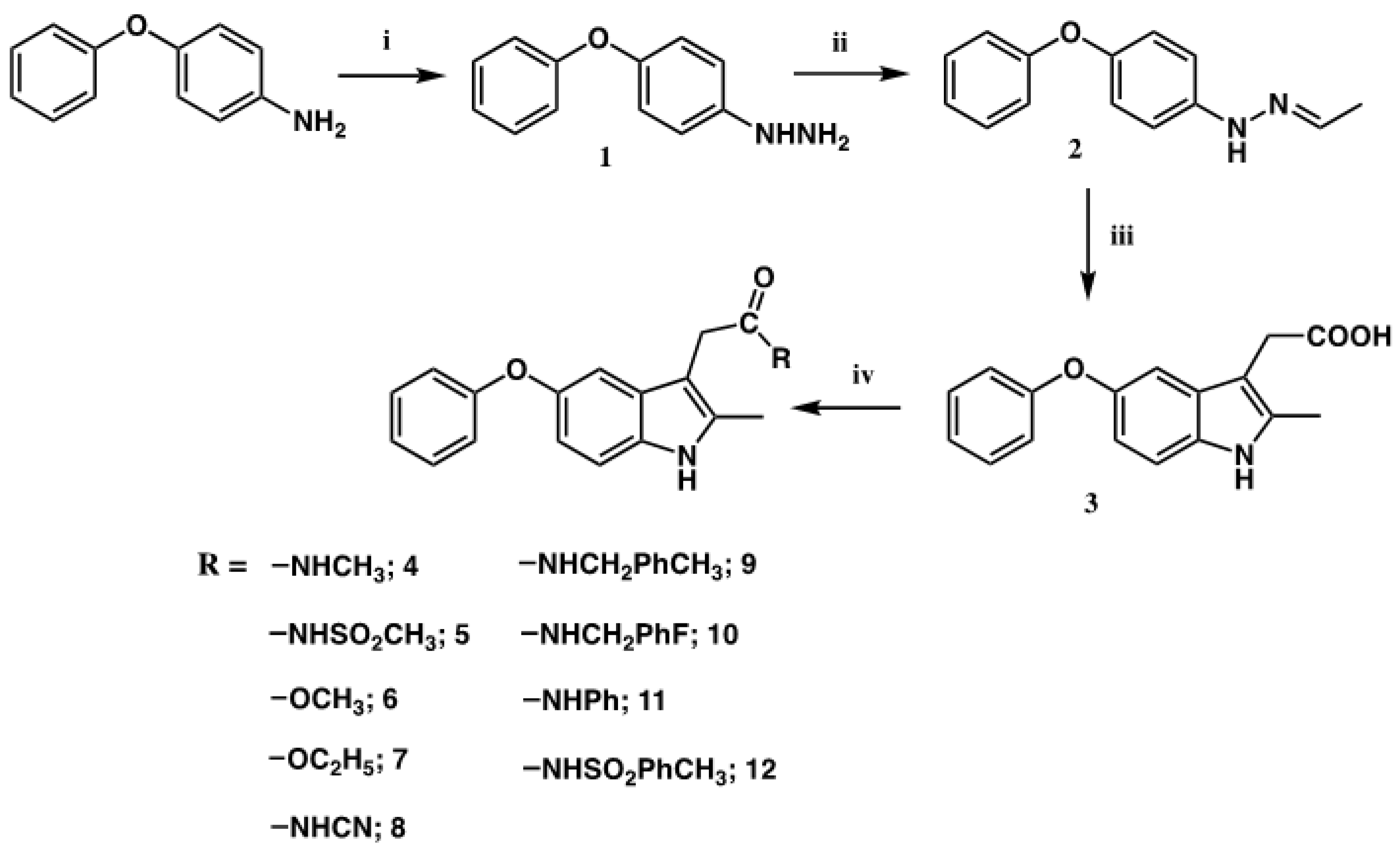
2.1. Synthesis
2.2. Biological Activities
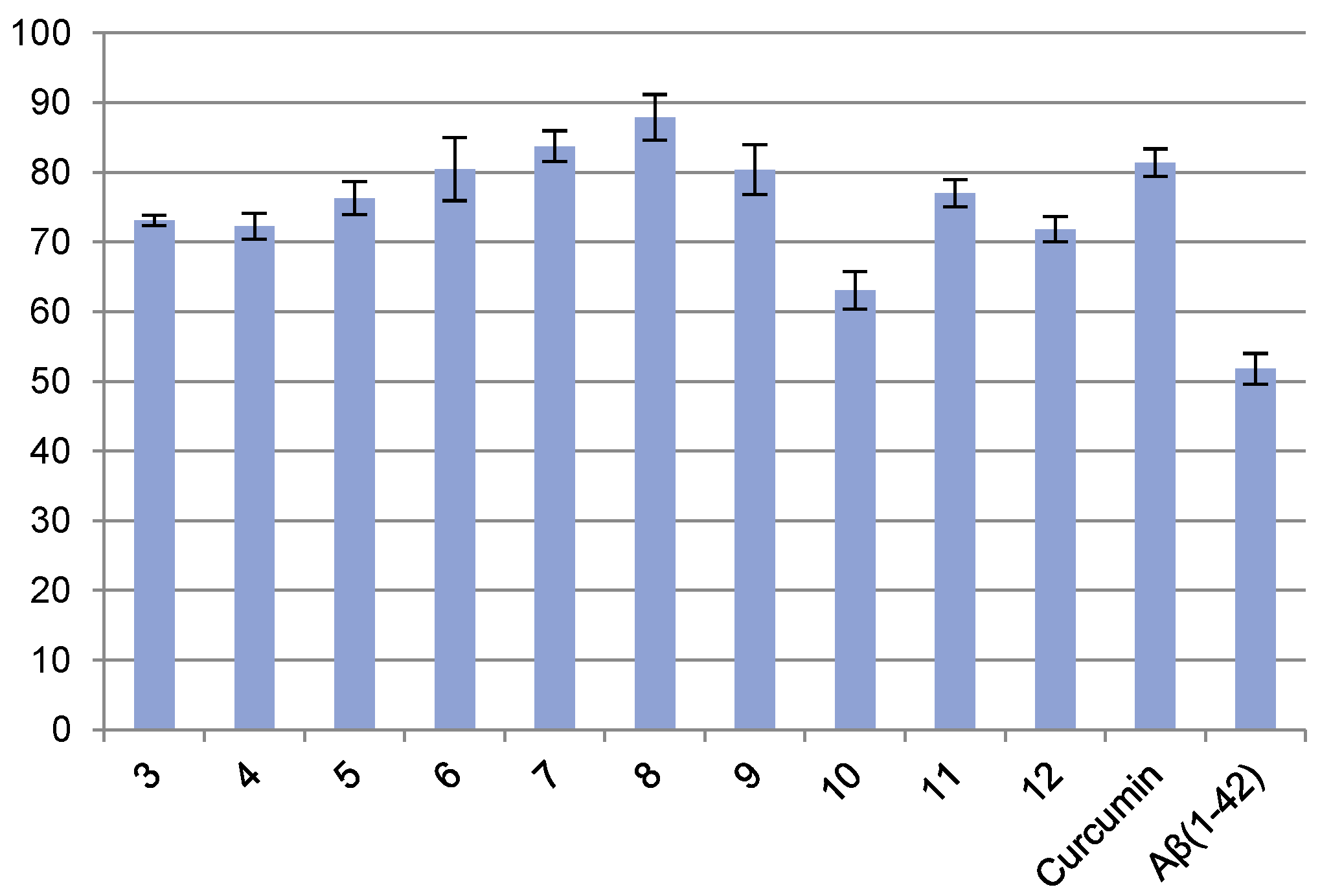
2.2.1. Anti-Aβ Aggregation Activity (Thioflavin T Fluorescence Assay: ThT Assay)
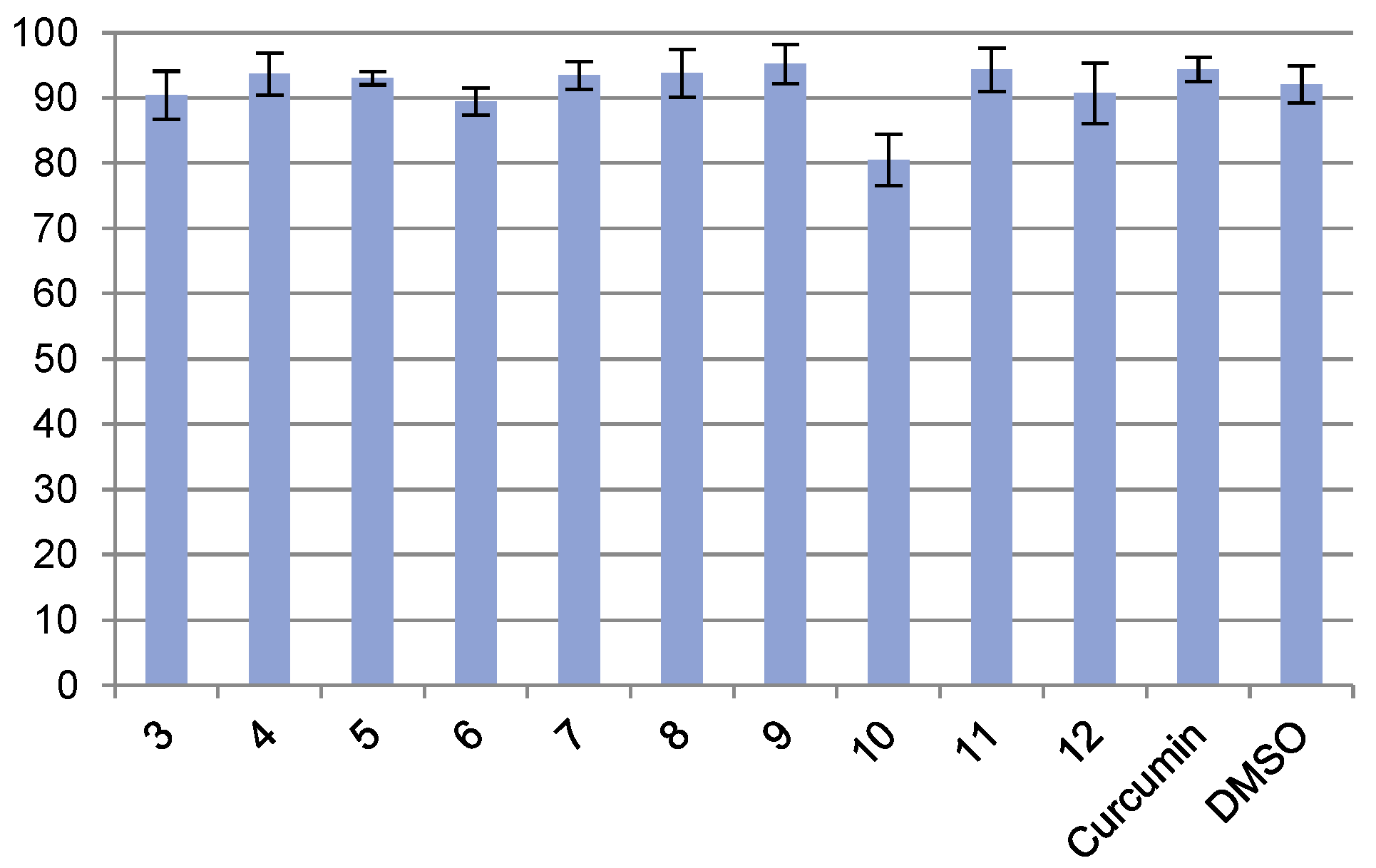
2.2.2. Protection of Neuronal SK-N-SH Neuroblastoma against Aβ42-Induced Cell Death
2.2.3. Antioxidant Activity (DPPH Assay)
2.2.4. Antiacetylcholinesterase Activity (Ellman Assay) [50]
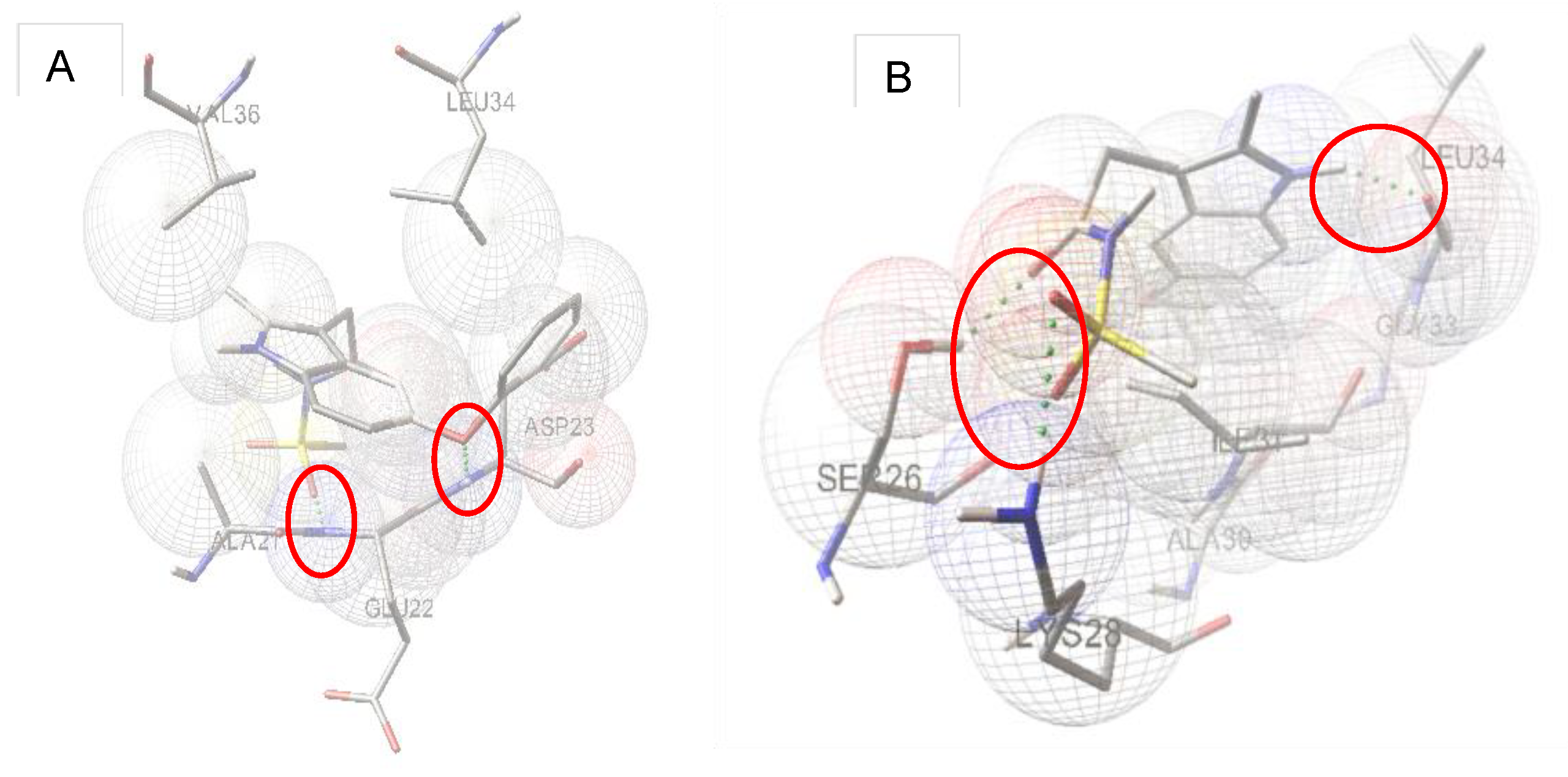
2.3. Molecular Modeling
2.4. In Silico Prediction of CNS Assess
3. Conclusions
4. Material and Methods
4.1. Synthesis
4.1.1. 4-phenoxyhydrazine (1)
4.1.2. 1-Ethylidene-2-(4-phenoxyphenyl) Hydrazine (2)
4.1.3. 2-Methyl-3-(carboxymethyl)-5-phenoxy Indole (3)
4.1.4. General Procedure for the Synthesis of 3-Substituted-5-phenoxyindole Derivatives
4.1.5. 2-Methyl-3-(methyl ethanamide-2-yl)-5-phenoxy Indole (4)
4.1.6. 2-Methyl-3-(methylsulfonyl ethanamide-2-yl)-5-phenoxy Indole (5)
4.1.7. 2-Methyl-3-(ethyl ethanoate-2-yl)-5-phenoxy Indole (6)
4.1.8. 2-Methyl-3-(methyl ethanoate-2-yl)-5-phenoxy Indole (7)
4.1.9. 2-Methyl-3-(cyano ethanamide-2-yl)-5-phenoxy Indole (8)
4.1.10. 2-Methyl-3-(4-methylbenzyl ethanamide-2-yl)-5-phenoxy Indole (9)
4.1.11. 2-Methyl-3-(4-fluorobenzyl ethanamide-2-yl)-5-phenoxy Indole (10)
4.1.12. 2-Methyl-3-(phenyl ethanamide-2-yl)-5-phenoxy Indole (11)
4.1.13. 2-Methyl-3-(4-methylbenzesulfonyl ethanamide-2-yl)-5-phenoxy Indole (12)
4.2. Biological Assay
4.2.1. Evaluation of Antiaggregation Activity (Thioflavin T Fluorescence Assay: ThT Assay)
4.2.2. Protection of Neuronal SK-N-SH Neuroblastoma against Aβ42 Induced Cell Death
4.2.3. Determination of Morphological Cell Changes
4.2.4. Evaluation of Acetylcholinesterase Inhibitory Activity
4.2.5. Evaluation of Antioxidant Assay
4.3. Docking Studies
4.4. In Silico Prediction of CNS Access
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wisniewski, T.; Ghiso, J.; Frangione, B. Biology of Aβ amyloid in Alzheimer’s disease. Neurobiol. Dis. 1997, 4, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. The molecular pathology of Alzheimer’s disease. Neuron 1991, 6, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Froelich-Fabre, S.; Bhat, R.V. Mechanisms of tauopathies. Drug Discov. Today Dis. Mech. 2004, 1, 391–398. [Google Scholar] [CrossRef]
- Schmidt, M.; Sachse, C.; Richter, W.; Xu, C.; Faendrich, M.; Grigorieff, N. Comparison of Alzheimer Aβ(1–40) and Aβ(1–42) amyloid fibrils reveals similar protofilament structures. Proc. Natl. Acad. Sci. USA 2009, 106, 19813–19818. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 29, 353–356. [Google Scholar] [CrossRef]
- Haass, C. Take five—BACE and the γ-secretase quartet conduct Alzheimer’s amyloid β-peptide generation. EMBO J. 2004, 23, 483–488. [Google Scholar] [CrossRef]
- Tarek, M.; Arash, S.; Praveen, P.N.R. Amyloid cascade in Alzheimer’s disease: Recent advances in medicinal chemistry. Eur. J. Med. Chem. 2016, 113, 258–272. [Google Scholar]
- Lichtenthaler, S.F. Alpha-Secretase cleavage of the amyloid precursor protein: Proteolysis regulated by signaling pathways and protein trafficking. Curr. Alzheimer Res. 2012, 9, 165–177. [Google Scholar] [CrossRef]
- Lidia, P.; Celia, F. Therapeutic strategies targeting Amyloid-β in Alzheimer’s disease. Curr. Alzheimer Res. 2019, 16, 418–452. [Google Scholar]
- Haass, C.; Steiner, H. Alzheimer disease gamma-secretase: A complex story of GxGD-type presenilin proteases. Trends Cell Biol. 2002, 12, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Petkova, A.T.; Buntkowsky, G.; Dyda, F.; Leapman, R.D.; Yau, W.M.; Tycko, R. Solid State NMR Reveals a pH-dependent Antiparallel β-Sheet Registry in Fibrils Formed by a β-Amyloid Peptide. J. Mol. Biol. 2004, 335, 247–260. [Google Scholar] [CrossRef]
- Lührs, T.; Ritter, C.; Adrian, M.; Riek-Loher, D.; Bohrmann, B.; Dőbeli, H.; Schubert, D.; Riek, R. 3D structure of Alzheimer’s amyloid-β(1–42) fibrils. Proc. Natl. Acad. Sci. USA 2005, 102, 17342–17347. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, K.L.; Gordon, D.J.; Petkova, A.T.; Tycko, R.; Meredith, S.C. Abeta40-Lactam(D23/K28) models a conformation highly favorable for nucleation of amyloid. Biochemistry 2005, 44, 6003–6014. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kienlen-Campard, P.; Ahmed, M.; Liu, W.; Li, H.; Elliott, J.I.; Aimoto, S.; Constantinescu, S.N.; Octave, J.N.; Smith, S.O. Inhibitors of amyloid toxicity based on β-sheet packing of Aβ40 and Aβ42. Biochemistry 2006, 45, 5503–5516. [Google Scholar] [CrossRef]
- Shewmaker, F.; McGlinchey, R.P.; Wickner, R.B. Structural insights into functional and pathological amyloid. J. Biol. Chem. 2011, 286, 16533–16540. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Vassar, R.; De Strooper, B.; Hardy, J.; Willem, M.; Singh, N.; Zhou, J.; Yan, R.; Vanmechelen, E.; De Vos, A.; et al. The β-secretase BACE1 in Alzheimer’s disease. Biol. Psychiatry 2021, 89, 745–756. [Google Scholar] [CrossRef]
- Moussa, C.E.H. Beta-secretase inhibitors in phase I and phase II clinical trials for Alzheimer’s disease. Expert Opin. Investig. Drug 2017, 26, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Tang, J. Prospects of β-Secretase Inhibitors for the Treatment of Alzheimer’s Disease. ChemMedChem 2015, 10, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Polgar, T.; Keseru, G.M. Structure-based β-secretase (BACE1) inhibitors. Curr. Pharm. Design 2014, 20, 3373–3379. [Google Scholar] [CrossRef] [PubMed]
- Mekala, S.; Nelson, G.; Li, Y.-M. Recent developments of small molecule γ-secretase modulators for Alzheimer’s disease. RSC Med. Chem. 2020, 11, 1003–1022. [Google Scholar] [CrossRef]
- Tang, M.; Taghibiglou, C. The Mechanisms of action of curcumin in Alzheimer’s disease. J. Alzheimers Dis. 2017, 58, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, S.; Franco, V.; Sorrentino, S.; Zucca, S.; Pandini, C.; Rota, S.; Bernuzzi, S.; Costa, A.; Sinforiani, E.; Pansarasa, O.; et al. Curcumin and novel synthetic analogs in cell-based studies of Alzheimer’s disease. Front. Pharmacol. 2018, 9, 1404. [Google Scholar] [CrossRef] [PubMed]
- Voulgaropoulou, S.D.; van Amelsvoort, T.A.M.J.; Prickaerts, J.; Vingerhoets, C. The effect of curcumin on cognition in Alzheimer’s disease and healthy aging: A systematic review of pre-clinical and clinical studies. Brain Res. 2019, 1725, 146476. [Google Scholar] [CrossRef]
- Murakami, K.; Irie, K. Three structural features of functional food components and herbal medicine with amyloid β42 anti-aggregation properties. Molecules 2019, 24, 2125. [Google Scholar] [CrossRef]
- Henriquez, G.; Gomez, A.; Guerrero, E.; Narayan, M. Potential Role of Natural Polyphenols against Protein Aggregation Toxicity: In Vitro, In Vivo, and Clinical Studies. ACS Chem. Neurosci. 2020, 11, 2915–2934. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, S.; Hassani, S.; Khan, F.; Ziaee, M.; Abdollahi, M. Cinnamon, a promising prospect towards Alzheimer’s disease. Pharmacol. Res. 2018, 130, 241–258. [Google Scholar] [CrossRef]
- Rajasekhar, K.; Chakrabarti, M.; Govindaraju, T. Function and toxicity of amyloid beta and recent therapeutic interventions targeting amyloid beta in Alzheimer’s disease. Chem. Commun. 2015, 51, 13434–13450. [Google Scholar] [CrossRef]
- Abbas, B.; Syed, N.; Jantan, I. Synthetic curcumin analogs as inhibitors of β -amyloid peptide aggregation: Potential therapeutic and diagnostic agents for Alzheimer’s disease. Mini-Rev. Med. Chem. 2015, 15, 1110–1121. [Google Scholar] [CrossRef]
- Mao, F.; Yan, J.; Li, J.; Jia, X.; Miao, H.; Sun, Y.; Huang, L.; Li, X. New multi-target-directed small molecules against Alzheimer’s disease: A combination of resveratrol and clioquinol. Org. Biomol. Chem. 2014, 12, 5936–5944. [Google Scholar] [CrossRef]
- Alaya, S.; Genevaux, P.; Hureau, C.; Faller, P. (Bio)chemical Strategies To Modulate Amyloid-β Self-Assembly. ACS Chem. Neurosci. 2019, 10, 3366–3374. [Google Scholar] [CrossRef]
- Doig, A.J.; Derreumaux, P. Inhibition of protein aggregation and amyloid formation by small molecules. Curr. Opin. Struct. Biol. 2015, 30, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Ayyannan, S.R.; Panda, G. Perspectives on Inhibiting β-Amyloid Aggregation through Structure-Based Drug Design. ChemMedChem 2015, 10, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Du, X.-Q.; Geng, M.-Y. Small molecule inhibitors of amyloid β peptide aggregation as a potential therapeutic strategy for Alzheimer’s disease. Acta Pharmacol. Sin. 2011, 32, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Kaur, A.; Goyal, B. Benzofuran and Indole: Promising scaffolds for drug development in Alzheimer’s disease. ChemMedChem 2018, 13, 1275–1299. [Google Scholar] [CrossRef] [PubMed]
- Prade, E.; Bittner, H.J.; Sarkar, R.; Lopez del Amo, J.M.; Althoff-Ospelt, G.; Multhaup, G.; Hildebrand, P.W.; Reif, B. Sulindac sulfide induces the formation of large oligomeric aggregates of the Alzheimer’s disease amyloid-β peptide which exhibit reduced neurotoxicity. Biochemistry 2016, 55, 1839–1849. [Google Scholar] [CrossRef]
- Shukla, M.; Govitrapong, P.; Boontem, P.; Reiter, R.J.; Satayavivad, J. Mechanisms of melatonin in alleviating Alzheimer’s disease. Curr. Neuropharmacol. 2017, 15, 1010–1031. [Google Scholar] [CrossRef]
- Bartolini, M.; Bertucci, C.; Cavrini, V.; Andrisano, V. beta-Amyloid aggregation induced by human acetylcholinesterase: Inhibition studies. Biochem. Pharmacol. 2003, 65, 407–416. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Jomova, K.; Kollar, V.; Rusko, M.; Valko, M. Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch. Toxicol. 2019, 93, 2491–2513. [Google Scholar] [CrossRef]
- Savelieff, M.G.; DeToma, A.S.; Derrick, J.S.; Lim, M.H. The ongoing search for small molecules to study metal-associated amyloid-β species in Alzheimer’s disease. Acc. Chem. Res. 2014, 47, 2475–2482. [Google Scholar] [CrossRef]
- Robinson, B. The Fischer Indole Synthesis. Chem. Rev. 1963, 63, 373–401. [Google Scholar] [CrossRef]
- Przheval’skii, N.M.; Kostromina, L.Y.; Grandberg, I.I. New data on the mechanism of the Fischer indole synthesis (review). Chem. Heterocyc. Compd. 1988, 24, 709–721. [Google Scholar] [CrossRef]
- Bajwa, G.; Brown, R.K. Fischer indole synthesis. The reaction of N′-methyl-2,6-dimethylphenylhydrazine hydrochloride with 2-methylcyclohexanone and 2,6-dimethylcyclohexanone. Can. J. Chem. 1970, 48, 2293–2299. [Google Scholar] [CrossRef]
- Wolfe, L.S.; Calabrese, M.F.; Nath, A.; Blaho, D.V.; Miranker, A.D.; Xiong, Y. Protein-induced photophysical changes to the amyloid indicator dye thioflavin T. Proc. Natl. Acad. Sci. USA 2010, 107, 16863–16868. [Google Scholar] [CrossRef]
- Hudson, S.A.; Ecroyd, H.; Kee, T.W.; Carver, J.A. The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS J. 2009, 276, 5960–5972. [Google Scholar] [CrossRef]
- Mohamed, T.; Shakeri, A.; Tin, G.; Rao, P.N.N. Structure–activity relationship studies of isomeric 2,4-diaminoquinazolines on β-amyloid aggregation kinetics. ACS Med. Chem. Lett. 2016, 7, 502–507. [Google Scholar] [CrossRef]
- Reinke, A.A.; Gestwicki, J.E. Structure–activity Relationships of Amyloid Beta-aggregation Inhibitors Based on Curcumin: Influence of Linker Length and Flexibility. Chem. Biol. Drug Des. 2007, 70, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Vistica, D.T.; Skehan, P.; Scudiero, D.; Monks, A.; Pittman, A.; Boyd, M.R. Tetrazolium-based assays for cellular viability: A critical examination of selected parameters affecting formazan production. Cancer Res. 1991, 51, 2515–2520. [Google Scholar] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M.; Hrabinova, M.; Kuca, K.; Simonato, J.-P. Assessment of Acetylcholinesterase Activity Using Indoxylacetate and Comparison with the Standard Ellman’s Method. Int. J. Mol. Sci. 2011, 12, 2631–2640. [Google Scholar] [CrossRef]
- Delfino, R.T.; Ribeiro, T.S.; Figueroa-Villar, J.D. Organophosphorus Compounds as Chemical Warfare Agents: A Review. J. Brazil. Chem. Soc. 2009, 20, 407–428. [Google Scholar] [CrossRef]
- Crivori, P.; Cruciani, G.; Carrupt, P.A.; Testa, B. Predicting blood-brain barrier permeation from three-dimensional molecular structure. J. Med. Chem. 2000, 43, 2204–2216. [Google Scholar] [CrossRef] [PubMed]
- Richter, L.; Munter, L.-M.; Ness, J.; Hildebrand, P.W.; Dasari, M.; Unterreitmeier, S.; Bulic, B.; Beyermann, M.; Gust, R.; Reif, B.; et al. Amyloid beta 42 peptide (Aβ42)-lowering compounds directly bind to Aβ and interfere with amyloid precursor protein (APP) transmembrane dimerization. Proc. Natl. Acad. Sci. USA 2010, 107, 14597–14602. [Google Scholar] [CrossRef] [PubMed]


| Entry | IC50 (µM) ± RSD |
|---|---|
| 3 | 18.81 ± 1.26 |
| 4 | 3.26 ± 1.11 |
| 5 | 3.18 ± 0.87 |
| 6 | 19.14 ± 2.13 |
| 7 | 13.98 ± 1.55 |
| 8 | 20.62 ± 2.79 |
| 9 | 53.67 ± 1.53 |
| 10 | 72.67 ± 2.34 |
| 11 | 13.08 ± 3.11 |
| 12 | 9.48 ± 1.25 |
| Curcumin | 11.09 ± 1.78 |
| Entry | Concentration (µM) | % Viability ± RSD |
|---|---|---|
| 3 | 31.25 | 90.42 ± 3.69 |
| 4 | 31.25 | 93.69 ± 3.23 |
| 5 | 62.50 | 93.04 ± 1.02 |
| 6 | 31.25. | 89.47 ± 2.11 |
| 7 | 31.25 | 93.43 ± 2.15 |
| 8 | 62.50 | 93.80 ± 3.66 |
| 9 | 31.25 | 95.21 ± 3.00 |
| 10 | 31.25 | 80.51 ± 3.96 |
| 11 | 15.63 | 94.32 ± 3.31 |
| 12 | 15.63 | 90.73 ± 4.64 |
| Curcumin | 15.63 | 94.36 ± 1.83 |
| DMSO | 125 (1.25%) | 92.08 ± 2.86 |
| Entry | Concentration (µM) | % Viability ± RSD |
|---|---|---|
| 3 | 18.81 | 73.10 ± 0.75 |
| 4 | 3.26 | 72.27 ± 1.85 |
| 5 | 3.18 | 76.28 ± 2.39 |
| 6 | 19.14 | 80.44 ± 4.51 |
| 7 | 13.98 | 83.74 ± 2.21 |
| 8 | 20.62 | 87.90 ± 3.26 |
| 9 | 31.25 [a] | 80.38 ± 3.54 |
| 10 | 31.25 [a] | 63.05 ± 2.70 |
| 11 | 13.08 | 76.99 ± 1.97 |
| 12 | 9.48 | 71.85 ± 1.82 |
| Curcumin | 11.09 | 81.37 ± 1.98 |
| Aβ(1–42) | 51.82 ± 2.19 |
| Entry | IC50 (µM) ± RSD |
|---|---|
| 3 | 47.12 ± 0.55 |
| 4 | 175.74 ± 1.23 |
| 5 | 28.18 ± 1.40 |
| 6 | 190.43 ± 0.55 |
| 7 | 144.93 ± 1.22 |
| 8 | 85.51 ± 1.60 |
| 9 | 264.78 ± 0.34 |
| 10 | 85.97 ± 0.80 |
| 11 | 111.07 ± 1.31 |
| 12 | 121.19 ± 1.80 |
| Vitamin C | 13.35 ± 1.52 |
| Entry | % Inhibition ± RSD |
|---|---|
| 3 | 29.30 ± 4.89 |
| 4 | 9.73 ± 4.49 |
| 5 | −5.66 ± 2.79 |
| 6 | 19.85 ± 4.96 |
| 7 | 11.02 ± 4.88 |
| 8 | 6.61 ± 4.95 |
| 9 | 12.5 ± 4.57 |
| 10 | 12.45 ± 4.98 |
| 11 | 30.04 ± 4.63 |
| 12 | 31.52 ± 4.90 |
| Galantamine | 97.74 ± 4.03 |
| Entry | HP [a] | BE [b] (kcal/mole) | HA [c] | HD [d] | Cluster |
|---|---|---|---|---|---|
| 3 | Ala21, Asp23, Leu34, Met35 | −7.37 | CO:Asp23 | No | 13 |
| 4 | Ala21-Asp23, Leu34 | −7.60 | PhO:Asp23 | No | 15 |
| 5 | Ala21-Asp23, Leu34, Val36 | −7.17 | SO2:Glu22 PhO:Asp23 | No | 16 |
| 6 | Ala21-Asp23, Leu34 | −6.99 | CO:Asp23 | No | 20 |
| 7 | Ala21-Asp23, Gly33, Leu34 | −7.63 | CO:Asp23 | No | 20 |
| 8 | Ala21-Asp23, Gly33-Met35 | −7.80 | NH(3):Asp23 | No | 11 |
| 9 | Phe19, Leu34, Ala21-Asp23, Val36-Gly38 | −7.24 | No | No | 15 |
| 10 | Phe19-Asp23, Leu34, Val36 | −6.49 | No | NH(3):Ala21 | 12 |
| 11 | Ala21-Asp23, Leu34, Val36 | −7.50 | CO:Asp23 | No | 13 |
| 12 | Phe19-Asp23, Leu34, Val36, Gly37 | −8.3 | SO2:Ala21 | No | 60 |
| Entry | HP [a] | BE [b] (kcal/mole) | HA [c] | HD [d] | Cluster |
|---|---|---|---|---|---|
| 3 | Ser26, Lys28, Ile31, Leu34 | −7.79 | OH:Lys28 | No | 60 |
| 4 | Ser26, Asn27, Ala30, Ile31, Gly33, Leu34 | −7.35 | CO:Ser26, Asn27 | No | 37 |
| 5 | Ser26, Lys28, Ala30, Ile31, Gly33, Leu34 | −8.47 | CO:Ser26, SO2:Lys28 | NH(1): Leu34 | 54 |
| 6 | Ser26, Asn27, Ala30, Ile31, Gly33, Leu34 | −6.93 | EtO:Asn27 | No | 30 |
| 7 | Ser26, Asn27, Ala30, Ile31, Gly33, Leu34 | −7.29 | CO:Ser26 CH3O:Asn27 | NH(1): Ile31 | 41 |
| 8 | Ser26-Lys28, Ile31, Gly33, Leu34 | −7.95 | CO:Ser26 | NH(1): Ile31 | 74 |
| 9 | Gly25-Asn27, Ala30, Ile31, Gly33, Leu34 | −7.14 | CO:Asn27 | No | 32 |
| 10 | Ser26-Lys28, Ile31, Gly33, Leu34 | −6.85 | CO:Asn27 | NH(1): Ile31 | 36 |
| 11 | Lys28, Ala30, Ile31, Gly33-Met35 | −7.65 | CO:Lys28 | No | 30 |
| 12 | Ser26, Lys28, Ala30, Ile31, Gly33-Met35 | −7.55 | CO:Ser26, SO2:Lys28 | NH(1): Leu34 | 20 |
| Entry | Log BB [Log (Cbrain/Cblood)] |
|---|---|
| 3 | −1.247 |
| 4 | −0.521 |
| 5 | −1.538 |
| 6 | −0.407 |
| 7 | −0.407 |
| 8 | −0.98 |
| 9 | −0.639 |
| 10 | −0.406 |
| 11 | −0.493 |
| 12 | −1.387 |
| Donepezil | 0.59 |
| Rivastigmine | 0.401 |
| Parameters | ||
|---|---|---|
| PDB code | 2BEG | 1QWP |
| Resolution | 2.00 | 2.00 |
| No. Gridpoint in x,y,z | 128, 70, 50 | 48, 48, 48 |
| Spacing (Å) | 0.375 | 0.375 |
| Grid center | Center on macromolecule | Center on macromolecule |
| Smooth | 0.5 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laivut, S.; Moongkarndi, P.; Kitphati, W.; Rukthong, P.; Sathirakul, K.; Sripha, K. Design, Synthesis, and Neuroprotective Activity of Phenoxyindole Derivatives on Antiamyloid Beta (Aβ) Aggregation, Antiacetylcholinesterase, and Antioxidant Activities. Pharmaceuticals 2023, 16, 355. https://doi.org/10.3390/ph16030355
Laivut S, Moongkarndi P, Kitphati W, Rukthong P, Sathirakul K, Sripha K. Design, Synthesis, and Neuroprotective Activity of Phenoxyindole Derivatives on Antiamyloid Beta (Aβ) Aggregation, Antiacetylcholinesterase, and Antioxidant Activities. Pharmaceuticals. 2023; 16(3):355. https://doi.org/10.3390/ph16030355
Chicago/Turabian StyleLaivut, Somjate, Primchanien Moongkarndi, Worawan Kitphati, Pattarawit Rukthong, Korbtham Sathirakul, and Kittisak Sripha. 2023. "Design, Synthesis, and Neuroprotective Activity of Phenoxyindole Derivatives on Antiamyloid Beta (Aβ) Aggregation, Antiacetylcholinesterase, and Antioxidant Activities" Pharmaceuticals 16, no. 3: 355. https://doi.org/10.3390/ph16030355
APA StyleLaivut, S., Moongkarndi, P., Kitphati, W., Rukthong, P., Sathirakul, K., & Sripha, K. (2023). Design, Synthesis, and Neuroprotective Activity of Phenoxyindole Derivatives on Antiamyloid Beta (Aβ) Aggregation, Antiacetylcholinesterase, and Antioxidant Activities. Pharmaceuticals, 16(3), 355. https://doi.org/10.3390/ph16030355







