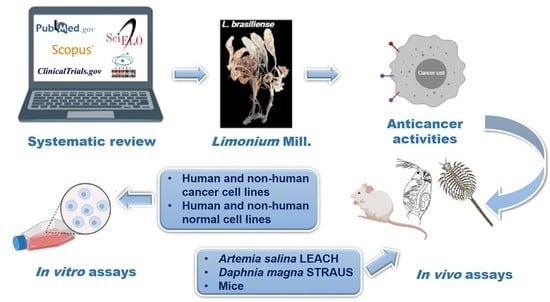
Chemical Constituents, Anticancer and Anti-Proliferative Potential of Limonium Species: A Systematic Review
Abstract
1. Introduction
2. Results
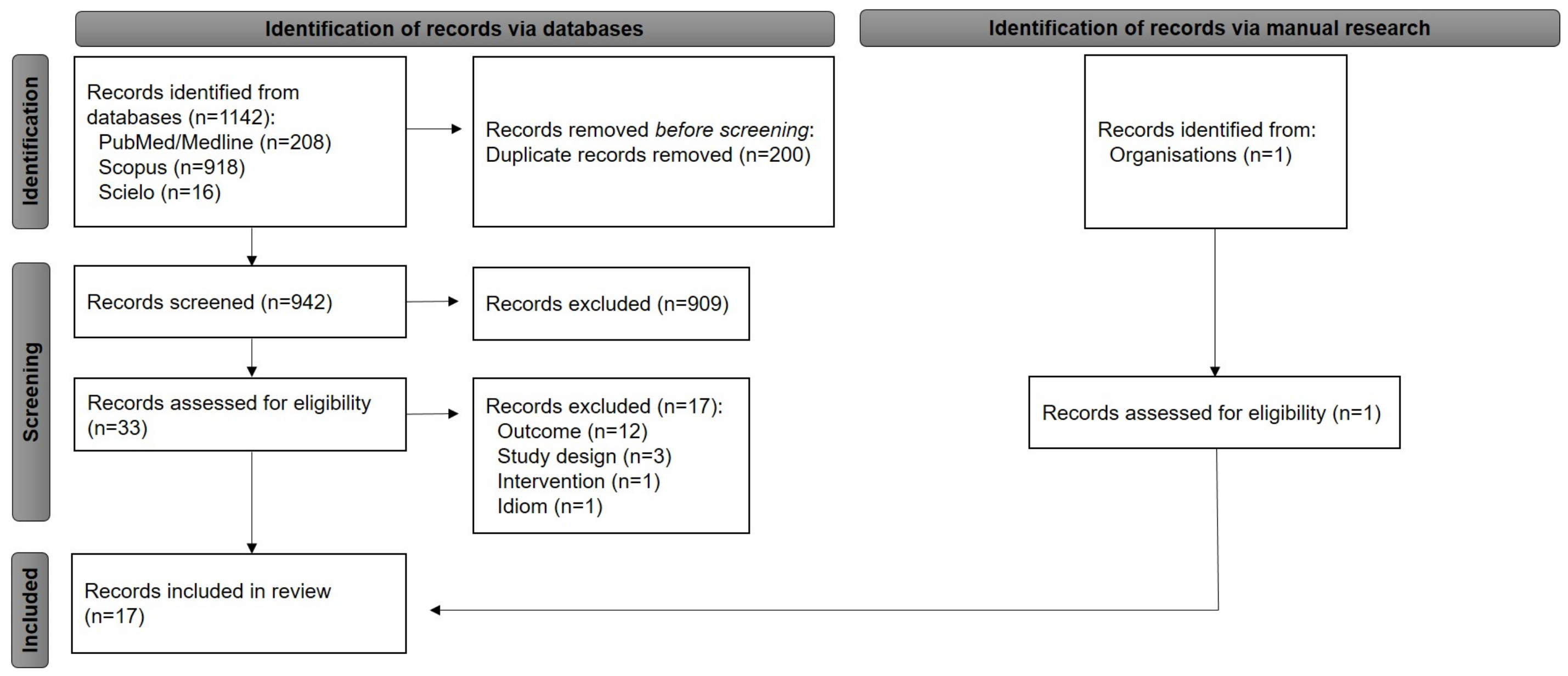
2.1. Literature Search Results and Main Characteristics of Included Studies
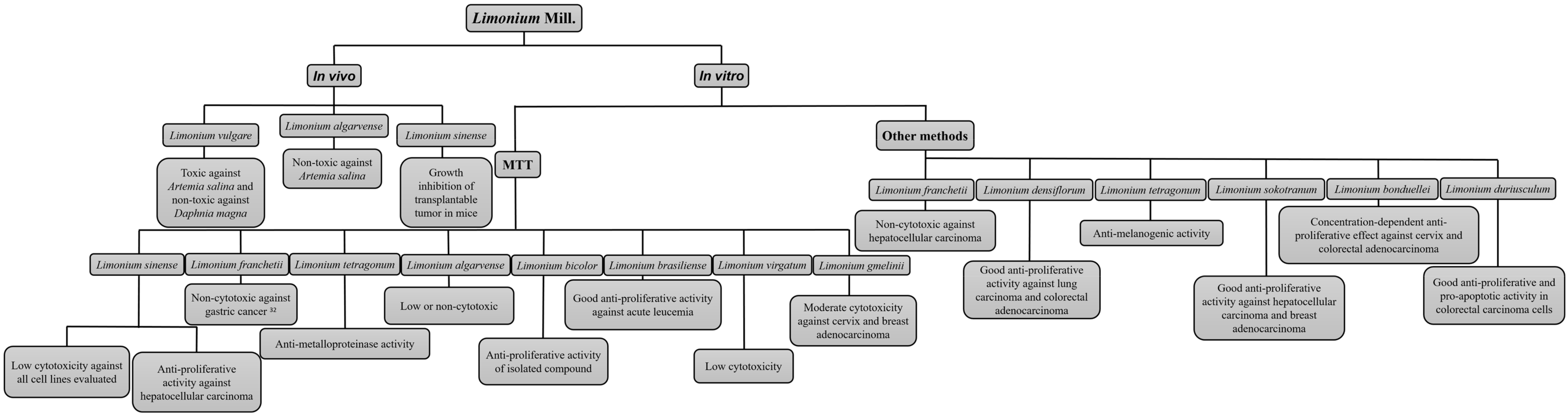
2.2. Anticancer and Anti-Proliferative Activities of Limonium Species
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Systematic Literature Search and Eligibility Criteria
4.3. Data Extraction and Reporting Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal plants and cancer chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Sreekala, C.; Zhang, Z.; Budhraja, A.; Ding, S.; Son, Y.O.; Wang, X.; Hitron, A.; Hyun-Jung, K.; Wang, L.; et al. Cancer prevention with promising natural products: Mechanisms of action and molecular targets. Anti-Cancer Agents Med. Chem. 2012, 12, 1159–1184. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Khor, T.O.; Shu, L.; Su, Z.-Y.; Fuentes, F.; Lee, J.-H.; Kong, A.-N.T. Plants vs. cancer: A review on natural phytochemicals in preventing and treating cancers and their druggability. Anti-Cancer Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef]
- Tariq, A.; Sadia, S.; Pan, K.; Ullah, I.; Mussarat, S.; Sun, F.; Abiodun, O.O.; Batbaatar, A.; Li, Z.; Song, D.; et al. A systematic review on ethnomedicines of anti-cancer plants. Phytother. Res. 2017, 31, 173–344. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef]
- Khan, Y.H.; Uttra, A.M.; Qasim, S.; Mallhi, T.H.; Alotaibi, N.H.; Rasheed, M.; Alzarea, A.I.; Iqbal, M.S.; Alruwaili, N.K.; Khan, S.-U.-D.; et al. Potential role of phytochemicals against matrix metalloproteinase induced breast cancer; an explanatory review. Front. Chem. 2021, 8, 592152. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Munir, N.; Mahmood, Z.; Riaz, M.; Akram, M.; Rebezov, M.; Kuderinova, N.; Moldabayeva, Z.; Shariati, M.A.; Rauf, A.; et al. Molecular targets for the management of cancer using Curcuma longa Linn. phytoconstituents: A review. Biomed. Pharmacother. 2021, 135, 111078. [Google Scholar] [CrossRef]
- Khan, H. Medicinal plants in light of history: Recognized therapeutic modality. J. Evid. Based Complement. Altern. Med. 2014, 19, 216–219. [Google Scholar] [CrossRef]
- Ijaz, S.; Akhtar, N.; Khan, M.S.; Hameed, A.; Irfan, M.; Arshad, M.D.; Ali, S.; Asrar, M. Plant derived anticancer agents: A green approach towards skin cancers. Biomed. Pharmacother. 2018, 103, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Plants of the World Online (POWO). Plumbaginaceae Juss. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30000293-2#children (accessed on 21 March 2022).
- Plants of the World Online (POWO). Limonium Mill. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:331722-2#children (accessed on 21 March 2022).
- Murray, A.P.; Rodriguez, S.; Frontera, M.A.; Tomas, M.A.; Mulet, M.C. Antioxidant metabolites from Limonium brasiliense (Boiss.) Kuntze. Z. Nat. C 2004, 59, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Medini, F.; Fellah, H.; Ksouri, R.; Abdelly, C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J. Taibah Univ. Sci. 2014, 8, 216–224. [Google Scholar] [CrossRef]
- Benmeddour, T.; Laouer, H.; Flamini, G.; Akkal, S. Chemical composition of essential oil of Limonium bonduellei. Chem. Nat. Compd. 2018, 54, 188–190. [Google Scholar] [CrossRef]
- Gadetskaya, A.V.; Tarawneh, A.H.; Zhusupova, G.E.; Gemejiyeva, N.G.; Cantrell, C.L.; Cutler, S.J.; Ross, S.A. Sulfated phenolic compounds from Limonium caspium: Isolation, structural elucidation, and biological evaluation. Fitoterapia 2015, 104, 80–85. [Google Scholar] [CrossRef]
- Geng, D.; Chi, X.; Dong, Q.; Hu, F. Antioxidants screening in Limonium aureum by optimized on-line HPLC-DPPH assay. Ind. Crops Prod. 2015, 67, 492–497. [Google Scholar] [CrossRef]
- Medini, F.; Bourgou, S.; Lalancette, K.; Snoussi, M.; Mkadmini, K.; Coté, I.; Abdelly, C.; Legault, J.; Ksouri, R. Phytochemical analysis, antioxidant, anti-inflammatory, and anticancer activities of the halophyte Limonium densiflorum extracts on human cell lines and murine macrophages. S. Afr. J. Bot. 2015, 99, 158–164. [Google Scholar] [CrossRef]
- Blainski, A.; Gionco, B.; Oliveira, A.G.; Andrade, G.; Scarminio, I.S.; Silva, B.D.; Lopes, N.P.; Mello, J.C.P. Antibacterial activity of Limonium brasiliense (Baicuru) against multidrugresistant bacteria using a statistical mixture design. J. Ethnopharmacol. 2017, 198, 313–323. [Google Scholar] [CrossRef]
- Caleare, A.O.; Hensel, A.; Mello, J.C.P.; Pinha, A.B.; Panizzon, G.P.; Lechtenberg, M.; Petereit, F.; Nakamura, C.V. Flavan-3-ols and proanthocyanidins from Limonium brasiliense inhibit the adhesion of Porphyromonas gingivalis to epithelial host cells by interaction with gingipains. Fitoterapia 2017, 118, 87–93. [Google Scholar] [CrossRef]
- Sereia, A.L.; Oliveira, M.T.; Baranoski, A.; Marques, L.L.M.; Ribeiro, F.M.; Isolani, R.G.; Medeiros, D.C.; Chierrito, D.; Lazarin-Bidóia, D.; Zielinski, F.; et al. In vitro evaluation of the protective effects of plant extracts against amyloid-beta peptide-induced toxicity in human neuroblastoma SH-SY5Y cells. PLoS ONE 2019, 14, e0212089. [Google Scholar] [CrossRef]
- Al-madhagi, W.M.; Hashim, N.M.; Ali, N.A.A.; Othman, R. Phytochemical screening, cytotoxic and antimicrobial activities of Limonium socotranum and Peperomia blanda extracts. Trop Biomed. 2019, 36, 11–21. [Google Scholar]
- Souid, A.; Bellani, L.; Gabriele, M.; Pucci, L.; Smaoui, A.; Abdelly, C.; Hamed, K.B.; Longo, V. Phytochemical and biological activities in Limonium species collected in different biotopes of Tunisia. Chem. Biodivers. 2019, 16, e1900216. [Google Scholar] [CrossRef] [PubMed]
- Tuasha, N.; Petros, B.; Asfaw, Z. Plants used as anticancer agents in the Ethiopian traditional medical practices: A systematic review. Evid. Based Complement. Altern. Med. 2018, 18, 6274021. [Google Scholar] [CrossRef]
- Lellau, T.F.; Liebezeit, G. Cytotoxic and antitumor activities of ethanolic extracts of salt marsh plants from the lower Saxonian Wadden Sea, Southern North Sea. Pharm. Biol. 2003, 41, 293–300. [Google Scholar] [CrossRef]
- Tang, X.-H.; Yan, L.-F.; Gao, J.; Yang, X.-L.; Xu, Y.-X.; Ge, H.-Y.; Yang, H.-D. Antitumor and immunomodulatory activity of polysaccharides from the root of Limonium sinense Kuntze. Int. J. Biol. Macromol. 2012, 51, 1134–1139. [Google Scholar] [CrossRef]
- Kong, N.-N.; Fang, S.-T.; Wang, J.-H.; Wang, Z.-H.; Xia, C.-H. Two new flavonoid glycosides from the halophyte Limonium franchetii. J. Asian Nat. Prod. Res. 2014, 16, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-H.; Yu, F.; Liu, J.; Gao, J.; Yan, L.-F.; Dong, M.-M. Isolation and identification of anti-tumor polysaccharide LSP21 from Limonium sinense (Girard) Kuntze. Int. J. Biol. Macromol. 2014, 70, 138–142. [Google Scholar] [CrossRef]
- Bae, M.-J.; Karadeniz, F.; Lee, S.-G.; Seo, Y.; Kong, C.-S. Inhibition of MMP-2 and MMP-9 activities by Limonium tetragonum extract. Prev. Nutr. Food Sci. 2016, 21, 38–43. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Neves, V.; Martins, A.; Rauter, A.P.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. In vitro antioxidant and anti-inflammatory properties of Limonium algarvense flowers’ infusions and decoctions: A comparison with green tea (Camellia sinensis). Food Chem. 2016, 200, 322–329. [Google Scholar] [CrossRef]
- Bae, M.J.; Karadeniz, F.; Oh, J.H.; Yu, G.H.; Jang, M.; Nam, K.; Seo, Y.; Kong, C. MMP-Inhibitory effects of flavonoid glycosides from edible medicinal halophyte Limonium tetragonum. Evid. Based Complement. Altern. Med. 2017, 2017, 6750274. [Google Scholar] [CrossRef]
- Chen, J.; Teng, J.; Ma, L.; Tong, H.; Ren, B.; Wang, L.; Li, W. Flavonoids isolated from the flowers of Limonium bicolor and their in vitro antitumor evaluation. Pharm. Mag. 2017, 13, 222–225. [Google Scholar] [CrossRef]
- Cordeiro, M.F. Avaliação das atividades imunomoduladora, antineoplásica e antibacteriana de rizomas de Limonium brasiliense, sementes de Paullinia cupana e cascas do caule de Trichilia catigua. Ph.D. Thesis, Universidade Federal de Pernambuco, Centro de Biociências, Programa de Pós-Graduação em Inovação Terapêutica, Recife, Brazil, 2017; pp. 1–142. [Google Scholar]
- Lee, S.-G.; Karadeniz, F.; Seo, Y.; Kong, C.-S. Anti-melanogenic effects of flavonoid glycosides from Limonium tetragonum (Thunb.) Bullock via inhibition of tyrosinase and tyrosinase-related proteins. Molecules 2017, 22, 1480. [Google Scholar] [CrossRef] [PubMed]
- Sahli, R.; Rivière, C.; Neut, C.; Bero, J.; Sahuc, M.-E.; Smaoui, A.; Beaufay, C.; Roumy, V.; Hennebelle, T.; Rouillé, Y.; et al. An ecological approach to discover new bioactive extracts and products: The case of extremophile plants. J. Pharm. Pharmacol. 2017, 69, 1041–1055. [Google Scholar] [CrossRef]
- Amrani, A.; Lahneche, A.M.; Benaissa, O.; Boubekri, N.; Demirtas, I.; Benayache, F.; Benayache, S.; Zama, D. In vitro antiproliferative and inhibition of oxidative DNA damage activities of n-butanol extract of Limonium bonduelli from Algeria. Braz. Arch. Biol. Technol. 2019, 62, e19170779. [Google Scholar] [CrossRef]
- Hamadou, M.H.; Kerkatou, M.; Gatto, P.; Pancher, M.; Bisio, A.; Inga, A.; Menad, A.; Benayache, S.; Benayache, F.; Ameddah, S. Apigenin rich Limonium duriusculum (de Girard) Kuntze promotes apoptosis in HCT116 cancer cells. Nat. Prod. Res. 2019, 35, 2910–2914. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.J.; Monteiro, I.; Castañeda-Loaiza, V.; Placines, C.; Oliveira, M.C.; Reis, C.; Caperta, A.D.; Soares, F.; Pousão-Ferreira, P.; Pereira, C.; et al. Growth performance, in vitro antioxidant properties and chemical composition of the halophyte Limonium algarvense Erben are strongly influenced by the irrigation salinity. Ind. Crops Products. 2020, 143, 111930. [Google Scholar] [CrossRef]
- Tuohongerbieke, A.; Li, J.; Sabir, G.; Xin, X.; Hu, M.; Duan, X.; Liu, L.; Tang, D.; Zhu, J.; Aisa, H.A. Lignanamides from the roots of Limonium gmelinii (Willd.) Kuntze and their anti-diabetic, cytotoxic and anti-inflammatory activities. Phytochemistry 2021, 184, 112648. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Hassan, M.A.; Estrelles, E.; Soriano, P.; López-Gresa, M.P.; Bellés, J.M.; Boscaiu, M.; Vicente, O. Unraveling salt tolerance mechanisms in halophytes: A comparative study on four mediterranean Limonium species with different geographic distribution patterns. Front. Plant Sci. 2017, 8, 1438. [Google Scholar] [CrossRef]
- González-Orenga, S.; Grigore, M.N.; Boscaiu, M.; Vicente, O. Constitutive and induced salt tolerance mechanisms and potencial uses of Limonium Mill. Species. Agronomy 2021, 11, 413. [Google Scholar] [CrossRef]
- Dholwani, K.K.; Saluja, A.K.; Gupta, A.R.; Shah, D.R. A review on plant-derived natural products and their analogs with anti-tumor activity. Indian J. Pharmacol. 2008, 40, 49–58. [Google Scholar] [CrossRef]
- Kuete, V.; Efferth, T. African flora has the potential to fight multidrug resistance of cancer. Biomed. Res. Int. 2015, 2015, 914813. [Google Scholar] [CrossRef]
- American Cancer Society (ACS). How Chemotherapy Drugs Work. Available online: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/chemotherapy/how-chemotherapy-drugs-work.html (accessed on 21 March 2022).
- Jain, C.K.; Majumder, H.K.; Roychoudhury, S. Natural compounds as anticancer agents targeting DNA topoisomerases. Curr. Genomics. 2017, 18, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef] [PubMed]
- Vardanyan, R.S.; Hruby, V.J. Synthesis of Essential Drugs, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2006; pp. 389–418. [Google Scholar]
- Ina, K.; Kataoka, T.; Ando, T. The use of Lentinan for treating gastric cancer. Anticancer. Agents Med. Chem. 2013, 13, 681–688. [Google Scholar] [CrossRef]
- Masuda, M.; Itoh, K.; Murata, K.; Naruto, S.; Uwaya, A.; Isami, F.; Matsuda, H. Inhibitory effects of Morinda citrifolia extract and its constituents on melanogenesis in murine B16 melanoma cells. Biol. Pharm. Bull. 2012, 35, 78–83. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- McCauley, J.; Zivanovic, A.; Skropeta, D. Bioassays for anticancer activities. In Metabolomics Tools for Natural Product Discovery. Methods in Molecular Biology (Methods and Protocols); Roessner, U., Dias, D., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 191–205. [Google Scholar]
- Cox, P.A. Pharmacology, Biodiversity. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 703–715. [Google Scholar]
- Aslantürk, Ö.S. In vitro cytotoxicity and cell viability assays: Principles, advantages, and disadvantages. In Genotoxicity—A Predictable Risk to Our Actual World; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: London, UK, 2017; pp. 1–17. [Google Scholar] [CrossRef]
- Michael, A.S.; Thompson, C.G.; Abramovitz, M. Artemia salina as a test organism for bioassay. Science 1956, 123, 464. [Google Scholar] [CrossRef]
- Waart, J.; Van Aken, F.; Pouw, H. Detection of orally toxic microbial metabolites in foods with bioassay systems. Zent. Bakteriol. Orig. A 1972, 222, 96–114. [Google Scholar]
- Adema, D.M.M. Daphnia magna as a test animal in acute and chronic toxicity tests. Hydrobiologia 1978, 59, 125–134. [Google Scholar] [CrossRef]
- Canton, J.H.; Adema, D.M.M. Reproducibility of short-term and reproduction toxicity experiments with Daphnia magna and comparison of the sensitivity of Daphnia magna with Daphnia pulex and Daphnia cucullata in short-term experiments. Hydrobiologia 1978, 59, 135–140. [Google Scholar] [CrossRef]
- Sandbacka, M.; Christianson, I.; Isomaa, B. The acute toxicity of surfactants on fish cells, Daphnia magna and fish—A comparative study. Toxicol. Vitr. 2000, 14, 61–68. [Google Scholar] [CrossRef]
- Arcanjo, D.D.R.; Albuquerque, A.C.M.; Melo-Neto, B.; Santana, L.C.L.R.; Medeiros, M.G.F.; Citó, A.M.G.L. Bioactivity evaluation against Artemia salina Leach of medicinal plants used in Brazilian Northeastern folk medicine. Braz. J. Biol. 2012, 72, 505–509. [Google Scholar] [CrossRef]
- Visconti, R.; Grieco, D. New insights on oxidative stress in cancer. Curr. Opin. Drug Discov. Devel. 2009, 12, 240–245. [Google Scholar] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of antioxidants and natural products in inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Mohamed, S.I.A.; Jantan, I.; Haque, M.A. Naturally occurring immunomodulators with antitumor activity: An insight on their mechanisms of action. Int. Immunopharmacol. 2017, 50, 291–304. [Google Scholar] [CrossRef]
- Nuzzo, G.; Senese, G.; Gallo, C.; Albiani, F.; Romano, L.; d’Ippolito, G.; Manzo, E.; Fontana, A. Antitumor potential of immunomodulatory natural products. Mar. Drugs. 2022, 20, 386. [Google Scholar] [CrossRef]
- Ruiz-Riaguas, A.; Zengin, G.; Sinan, K.I.; Salazar-Mendías, C.; Llorent-Martínez, E.J. Phenolic profile, antioxidant activity, and enzyme inhibitory properties of Limonium delicatulum (Girard) Kuntze and Limonium quesadense Erben. J. Chem. 2020, 2020, 1016208. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Hamed, K.B.; Chibani, F.; Abdelly, C.; Magne, C. Growth, sodium uptake and antioxidant responses of coastal plants differing in their ecological status under increasing salinity. Biologia 2014, 69, 193–201. [Google Scholar] [CrossRef]
- Bakhshi, S.; Abbaspour, H.; Saeidisar, S. Study of phytochemical changes, enzymatic and antioxidant activity of two halophyte plants: Salsola dendroides Pall and Limonium reniforme (Girard) Lincz in different seasons. Bulg. Chem. Communications. 2018, 50, 374–382. [Google Scholar]
- Jafari, S.; Saeidnia, S.; Abdollahi, M. Role of natural phenolic compounds in cancer chemoprevention via regulation of the cell cycle. Curr. Pharm. Biotechnol. 2014, 15, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Chierrito, D.; Villas-Boas, C.B.; Tonin, F.S.; Fernandez-Llimos, F.; Sanches, A.C.C.; de Mello, J.C.P. Using cell cultures for the investigation of treatments for attention deficit hyperactivity disorder: A systematic review. Curr. Neuropharmacol. 2019, 17, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2. 2021. Available online: https://www.training.cochrane.org/handbook (accessed on 24 October 2021).
- Joanna Briggs Institute (JBI). Methodology for JBI Scoping Reviews Joanna Briggs Institute. Joanna Briggs Institute Reviewers’ Manual. 2015 ed./Supplement. Australia: Joanna Briggs Institute. 2015. Available online: https://nursing.lsuhsc.edu/jbi/docs/reviewersmanuals/scoping-.pdf (accessed on 24 October 2021).
- Hooijmans, C.R.; Rovers, M.M.; Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]

| Reference Number | Country | Plant Species | Part of Plant Used | Cell Line | Bioassay/ Model Used | Compound Tested | Positive Control | Time of Treatment (h) | Other Biological Activities |
|---|---|---|---|---|---|---|---|---|---|
| In vivo | |||||||||
| [25] | Germany | L. vulgare | NR | NR | Artemia salina Daphnia magna | EtOH extract | HgCl2 solution (1%) | 6 24 48 | NR |
| [26] | China | L. sinense | Roots | HepG2 | Mice | Crude LSP | Cyclophosphamide Lentinan with 5-fluoracil 5-Fluoracil | 312 | Immunomodulatory effects |
| [30] | Portugal | L. algarvense | Flowers | NR | Artemia salina | Aq extract | NR | 48 | Antioxidant and anti-inflammatory activities |
| In vitro | |||||||||
| [26] | China | L. sinense | Roots | HeLa HepG2 K562 | MTT | Crude LSP | NR | 24 | Immunomodulatory effects |
| [28] | China | L. sinense | Roots | HepG2 | MTT | LSP11 LSP21 LSP31 | 5-Fluorouracil | 24 | NR |
| [27] | China | L. franchetii | Whole | BGC-823 | MTT | 12 isolated compounds | NR | NR | NR |
| C6 HepG2 | Sulforhodamine B | ||||||||
| [18] | Tunisia | L. densiflorum | Leaves | A549 DLD-1 WS1 | Resazurin reduction test | DCM extract EtOH extract MeOH extract Hex extract | Etoposide | 48 | Antioxidant and anti-inflammatory activities |
| [29] | Korea | L. tetragonum | NR | HT-1080 | MTT | DCM fraction | NR | 48 | Determination of enzymatic activities of MMPs, mRNA expression of MMPs and TIMPs via RT-PCR, and detection of immunoreactive proteins via Western blotting |
| [31] | Korea | L. tetragonum | NR | HT-1080 | MTT | DCM extract (Hex fraction and 85% MeOH fraction) Aq extract (BuOH fraction and Aq fraction) | NR | 48 | Determination of enzymatic activities of MMPs, mRNA expression of MMPs and TIMPs via RT-PCR, and detection of immunoreactive proteins via Western blotting |
| [34] | Korea | L. tetragonum | NR | B16-F10 | Spectrophotometric method | Hex fraction 85% MeOH fraction BuOH fraction Aq fraction | Kojic acid | 0.5 | DOPA oxidase activity, cellular tyrosinase activity, melanin content, melanogenesis-related mRNA expression via RT-PCR, and detection of TRP via Western blotting |
| [30] | Portugal | L. algarvense | Flowers | HepG2 N9 S17 RAW-264.7 | MTT | Aq extract | NR | 72 | Antioxidant and anti-inflammatory activities |
| [38] | Portugal | L. algarvense | Flowers Leaves Peduncles | HEK-293 HepG2 RAW-264.7 | MTT | EtOH extract | NR | 72 | Antioxidant activity |
| [32] | China | L. bicolor | Flowers | LoVo MCF-7 U-2OS | MTT | 15 isolated compounds | 5-Fluorouracil | 48 | NR |
| [33] | Brazil | L. brasiliense | Rhizome | HepG2 HL-60 K562 MOLT-4 PANC-1 PBMC SK-MEL-28 T-47D Toledo Vero | MTT | CE Aq fraction EAF Subfractions (A-K) Isolated compounds (SA, SB, EGCG) | Amsacrine | 72 | Selectivity index, anti-migration and anti-clonogenic potential, and immunomodulatory activity |
| [35] | France | L. virgatum | Leaves Stems | J774 WI-38 | MTT | MeOH extract | Camptothecin | 72 | Antiradical, antimicrobial, and antiviral activity |
| [22] | Yemen | L. sokotranum | Flowers Leaves Stem | HepG2 MCF-7 | Sulforhodamine B | PE extract DCM extract MeOH extract | Doxorubicin | 48 | Antibacterial and antifungal activity |
| [36] | Algeria | L. bonduellei | Flowers Leaves | HeLa HT-29 | xCELLigence RTCA | BuOH extract | NR | 48 72 | DNA damage inhibition efficiency |
| [37] | Algeria | L. duriusculum | Flowers Leaves | HCT116 | Calcein-AM Hoechst 33342 | BuOH extract Apigenin | NR | 48 | Measures of relative levels of p53, MDM2, p21, total and p-ERK proteins, and PARP cleavage via western blotting |
| [39] | China | L. gmelinii | Roots | A549 HeLa MCF-7 | MTT | EtOAc extract 19 isolated compounds | Doxorubicin | 48 | Anti-diabetic and anti-inflammatory activities |
| Reference Number | Plant Species | Class of Metabolite | Compounds | Number of Isolated Compounds Tested In Vitro * |
|---|---|---|---|---|
| Primary metabolites | ||||
| [28] | L. sinense | Polysaccharide | LSP21 (glucose, galactose and mannose) | |
| [38] | L. algarvense | Amino acid | N-acetyl-tryptophan | |
| Fatty acids | Oxo-tridecanoic acid sulphate Trihydroxy-10-octadecenoic acid Trihydroxy-10,15-octadecadienoic acid | |||
| Polysaccharide | Hex-3-en-olxylopyranosyl-(1-6)-glicopyranoside Sucrose or isomeric structures | |||
| Secondary metabolites | ||||
| [27] | L. franchetii | Flavonoids | Apigenin Dihydrokaempferol Kaempferol-3-O-α-L-rhamnopyranoside Luteolin Myricetin Myricetin-3-O-(2″-O-galloyl)-α-L-rhamnopyranoside Myricetin-3-O-(3″-O-galloyl)-α-L-rhamnopyranoside Myricetin-3-O-α-L-rhamnopyranoside Quercetin Quercetin-3-O-(2″-O-tigloyl)-α-L-rhamnopyranoside Quercetin-3-O-(3″-O-tigloyl)-α-L-rhamnopyranoside Quercetin-3-O- α-L-rhamnopyranoside | (1) (2) (3) (4) (5) (6) (7) (8) (9) (10) (11) (12) |
| [18] | L. densiflorum | Flavonoids | Catechin hydrate Isorhamnetin Myricetin | |
| Phenolic acids | Ellagic acid Gallic acid Sinapic acid trans 3-hydroxycinnamic acid | |||
| [30] | L. algarvense | Flavonoid | Apigenin | |
| Phenolic acids | Caffeic acid Coumaric acid Ferulic acid Gallic acid p-Hydroxybenzoic acid Salicylic acid Syringic acid | |||
| [38] | Lignin | Pinoresinol sulphate | ||
| Flavonoids | 2′-C-methyl myricetin-3-O-rhamnoside-galloyl 4′-methyl eriodictyol-galloyl-rhamnose Apigenin Apigenin derivative Apigenin-O-glucoside Apigenin-O-glucuronide Dihydrokaempferol Epigallocatechin gallate Eriodictyol Eriodyctiol-O-glucoside Isorhamnetin-3-O-rutinoside Licoagroside B Luteolin Luteolin-7-O-glucoside Luteolin-7-O-rhamnoside Methyl licoagroside B Myricetin Myricetin-3-O-(2″-O-galloyl)-glucoside Myricetin-3-O-acetyl-deoxyhexose Myricetin-3-O-acetyl-hexoside Myricetin-3-O-pentoside Myricetin-ethyl acetoacetate-galloyl Myricetin-galloyl-acetyl deoxyhexose Myricetin-O-(galloyl)-deoxyhexose Myricitin-3-O-glucoside Myricitin-3-O-rhamnose Myricitin-3-O-rutinoside Naringenin Naringenin derivative Quercetin Quercetin derivative Quercetin-3-O-rhamnoside Quercetin-hexoside derivative Quercetin-O-galloy-glucoside Quercetin-O-hexoside Quercetin-tetramethyl ether- -dihydroxyethylfructopyranose Rutin | |||
| Phenolic acids | Feruloyltyramine Glucosyringic acid Syringic acid | |||
| Tannins | Digalloyl-hexoside Galloylglucoside derivative Galloyl-hexoside Galloylhexoside derivative | |||
| Phenylpropanoid | Sinapyl alcohol sulphate | |||
| [31] | L. tetragonum | Flavonoids | Myricetin 3-galactoside Quercetin 3-O-β-galactopyranoside | (13) (14) |
| [34] | ||||
| [32] | L. bicolor | Flavonoids | Acacetin Eriodictyol Hesperidin Isorhamnetin Kaempferol Kaempferol-3-O-(6″-O-galloyl)-β-D-glucoside Kaempferol-3-O-α-L-rhamnoside Kaempferol-3-O-β-D-glucoside Luteolin Myricetin-3-O-α-L-rhamnoside Quercetin Quercetin-3-O-α-L-rhamnoside Quercetin-3-O-β-D-galactoside Quercetin-3-O-β-D-glucoside Rutin | (15) (16) (17) (18) (19) (20) (21) (22) (4) (23) (9) (24) (25) (26) (27) |
| [33] | L. brasiliense | Tannins | Epigallocatequin-3-O-gallate Samarangenin A Samarangenin B | (28) (29) (30) |
| [35] | L. virgatum | Phenolic amide | N-trans-feruloyl tyramine | |
| [37] | L. duriusculum | Flavonoids | Apigenin Apigenin 7-O-β-D-(6”-methylglucuronide) | (31) (32) |
| [39] | L. gmelinii | Lignanamides | (2,3-trans)-3-(3-hydroxy-5-methoxyphenyl)-N-(4-hydroxyphenethyl)-7-{(E)-3-[(4-hydroxyphenethyl)amino]-3-oxoprop-1-en-1-yl}-2,3-dihydrobenzo[b][1,4]dioxine-2-carboxamide Limoniumin F 3,3′ -demethyl-heliotropamide Limoniumin A Limoniumin B Limoniumin C Limoniumin D 6-hydroxy-4-(4-hydroxy-3-methoxyphenyl)-2-(4-hydroxyphenethyl)-7-methoxy-1H-benzo(f)isoindole-1,3(2H)-dione Cannabisin I Limoniumin E Limoniumin G Limoniumin H Limoniumin I Cannabisin D Cannabisin B Cannabisin C Cannabisin A Cannabisin F Thoreliamide B | (33) (34) (35) (36) (37) (38) (39) (40) (41) (42) (43) (44) (45) (46) (47) (48) (49) (50) (51) |
| Phenolic amide | N-cis-feruloyl tyramine N-trans-feruloyl tyramine | |||
| Reference Number | Cell Line | IC50 (µg/mL)/Compound Tested | Selectivity Index (SI)/ Compound Tested |
|---|---|---|---|
| [18] | A549 | 29 (DCM extract), >200 (EtOH extract), 110 (MeOH extract), >200 (Hex extract), 10 (Etoposide PC) | NR |
| DLD-1 | 85 (DCM extract), >200 (EtOH extract), >200 (MeOH extract), >200 (Hex extract), 80 (Etoposide PC) | ||
| WS1 | >200 (DCM extract), >200 (EtOH extract), 140 (MeOH extract), >200 (Hex extract), 26 (Etoposide PC) | ||
| [33] | HepG2 | >200 (CE), 67.97 (Aq fraction), 59.47 (EAF) | 0.48 (CE) 2.94 (AF) 1.27 (EAF) |
| HL-60 | 61.69 (CE), 49.68 (Aq fraction), 17.26 (EAF) | 1.56 (CE) 4.02 (AF) 4.39 (EAF) | |
| PBMC | 96.78 (CE), >200 (Aq fraction), 75.82 (EAF) | NR | |
| T-47D | 90.68 (CE), >200 (Aq fraction), 77.70 (EAF) | 1.08 (CE) 1.00 (AF) 0.48 (EAF) | |
| HL-60 | 53.27 (SFa), 35.48 (SFb), 44.28 (SFc), 41.63 (SFd), 43.62 (SFe), 8.21 (SFf), 7.35 (SFg), 45.58 (SFh), 55.60 (SFi), 54.06 (SFj), 53.32 (SFk), 1.0 (Amsacrine PC) | NR | |
| K562 | 43.72 (SFa), 52.21 (SFb), 52.75 (SFc), 43.95 (SFd), 47.79 (SFe), 36.13 (SFf), 40.88 (SFg), 49.91 (SFh), 51.85 (SFi), 50.16 (SFj), 37.77 (SFk), 0.9 (Amsacrine PC) | ||
| MOLT-4 | 37.43 (SFa), 34.34 (SFb), 35.99 (SFc), 46.47 (SFd), 45.25 (SFe), 40.42 (SFf), 7.92 (SFg), 20.36 (SFh), 54.92 (SFi), 52.76 (SFj), 9.62 (SFk), NR (Amsacrine PC) | ||
| PANC-1 | >100 (SFa), >100 (SFb), 76.81 (SFc), 45.54 (SFd), >100 (SFe), 58.65 (SFf), >100 (SFg), >100 (SFh), >100 (SFi), >100 (SFj), >100 (SFk), >100(Amsacrine PC) | ||
| SK-MEL-28 | NA | ||
| Toledo | 57.46 (SFa), 57.02 (SFb), 61.29 (SFc), 54.09 (SFd), 55.29 (SFe), 55.29 (SFf), 54.32 (SFg), 57.36(SFh), 60.65 (SFi), 58.68 (SFj), 59.38 (SFk), 0.5 (amsacrine PC) | ||
| K562 | 37.04 (28), 29.24 (29), 51.17 (30) | 2.69 (28) 3.41 (29) 1.95 (30) | |
| Vero | >100 (28), >100 (29), >100 (30) | NR | |
| [37] | HCT116 | 7.60 (BuOH extract), 25.74 * (31), NA (32) | NR |
| [22] | MCF-7 | 19.65 and 14.57 (PE extracts), 17.60 and 21.8 (DCM extracts), 8.70 and 17.18 (MeOH extracts), 3.39 (Doxorubicin PC) | NR |
| HepG2 | 9.97 and 16.97 (PE extracts), 20.62 and 11.15 (DCM extracts), 13.90 and 24.86 (MeOH extracts), 7.38 (Doxorubicin PC) | NR | |
| [39] | HeLa | 25.25 (EtOAc extract), NA ((2), (7) and (13)), 19.24 * (17), 12.85 * (18), 31.57 * (19), 0.23 * (Doxorubicin PC) | NR |
| MCF-7 | NA (EtOAc extract), 20.08 (2), 21.58 (7), 43.28 (13), 28.85 (17), 14.14 (18), NA (19) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gancedo, N.C.; Isolani, R.; de Oliveira, N.C.; Nakamura, C.V.; de Medeiros Araújo, D.C.; Sanches, A.C.C.; Tonin, F.S.; Fernandez-Llimos, F.; Chierrito, D.; de Mello, J.C.P. Chemical Constituents, Anticancer and Anti-Proliferative Potential of Limonium Species: A Systematic Review. Pharmaceuticals 2023, 16, 293. https://doi.org/10.3390/ph16020293
Gancedo NC, Isolani R, de Oliveira NC, Nakamura CV, de Medeiros Araújo DC, Sanches ACC, Tonin FS, Fernandez-Llimos F, Chierrito D, de Mello JCP. Chemical Constituents, Anticancer and Anti-Proliferative Potential of Limonium Species: A Systematic Review. Pharmaceuticals. 2023; 16(2):293. https://doi.org/10.3390/ph16020293
Chicago/Turabian StyleGancedo, Naiara Cássia, Raquel Isolani, Natalia Castelhano de Oliveira, Celso Vataru Nakamura, Daniela Cristina de Medeiros Araújo, Andreia Cristina Conegero Sanches, Fernanda Stumpf Tonin, Fernando Fernandez-Llimos, Danielly Chierrito, and João Carlos Palazzo de Mello. 2023. "Chemical Constituents, Anticancer and Anti-Proliferative Potential of Limonium Species: A Systematic Review" Pharmaceuticals 16, no. 2: 293. https://doi.org/10.3390/ph16020293
APA StyleGancedo, N. C., Isolani, R., de Oliveira, N. C., Nakamura, C. V., de Medeiros Araújo, D. C., Sanches, A. C. C., Tonin, F. S., Fernandez-Llimos, F., Chierrito, D., & de Mello, J. C. P. (2023). Chemical Constituents, Anticancer and Anti-Proliferative Potential of Limonium Species: A Systematic Review. Pharmaceuticals, 16(2), 293. https://doi.org/10.3390/ph16020293