Targeting Mcl-1 Degradation by Bergenin Inhibits Tumorigenesis of Colorectal Cancer Cells
Abstract
:1. Introduction
2. Results
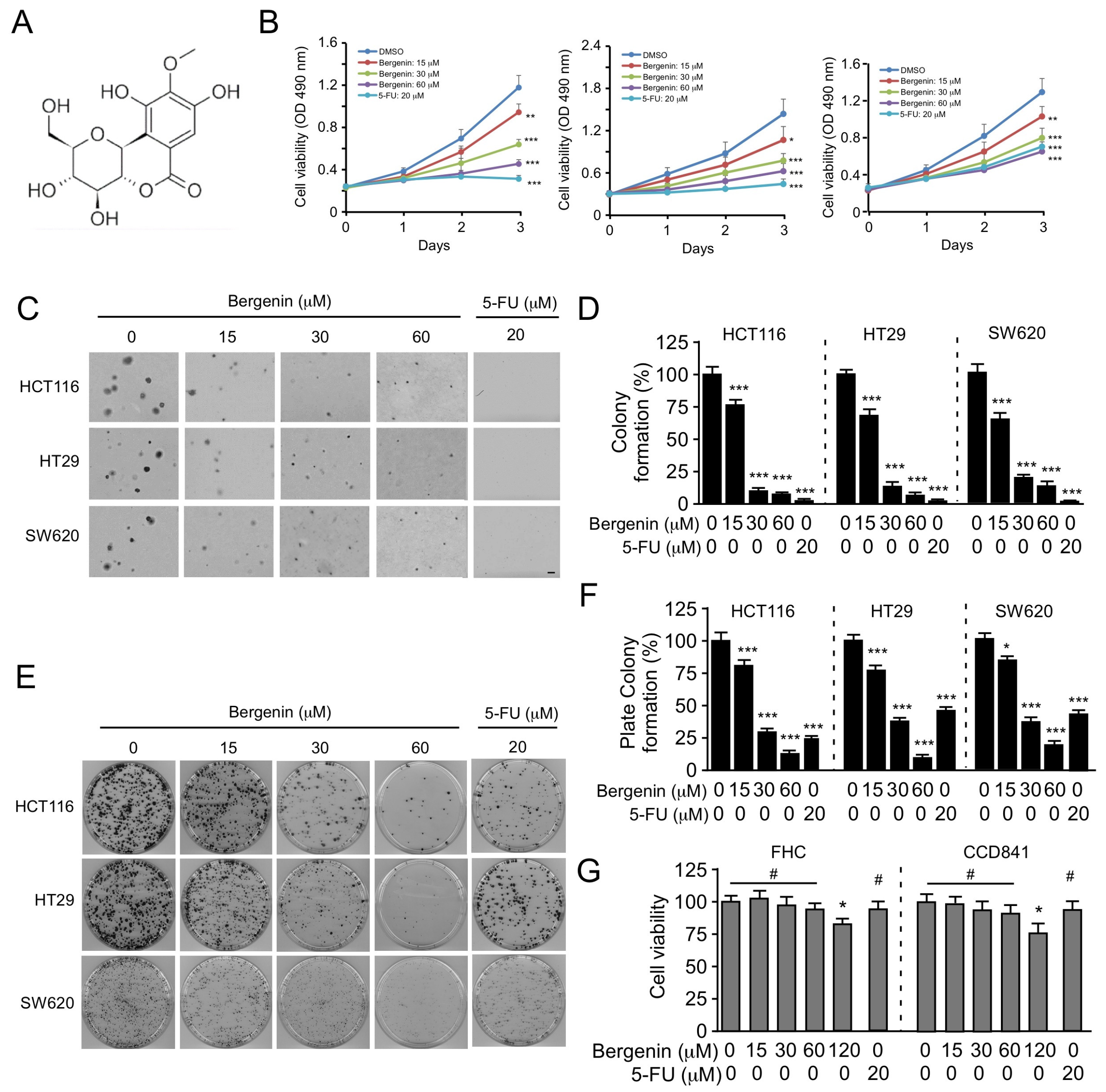
2.1. Bergenin Inhibits the Proliferation of Human CRC Cells
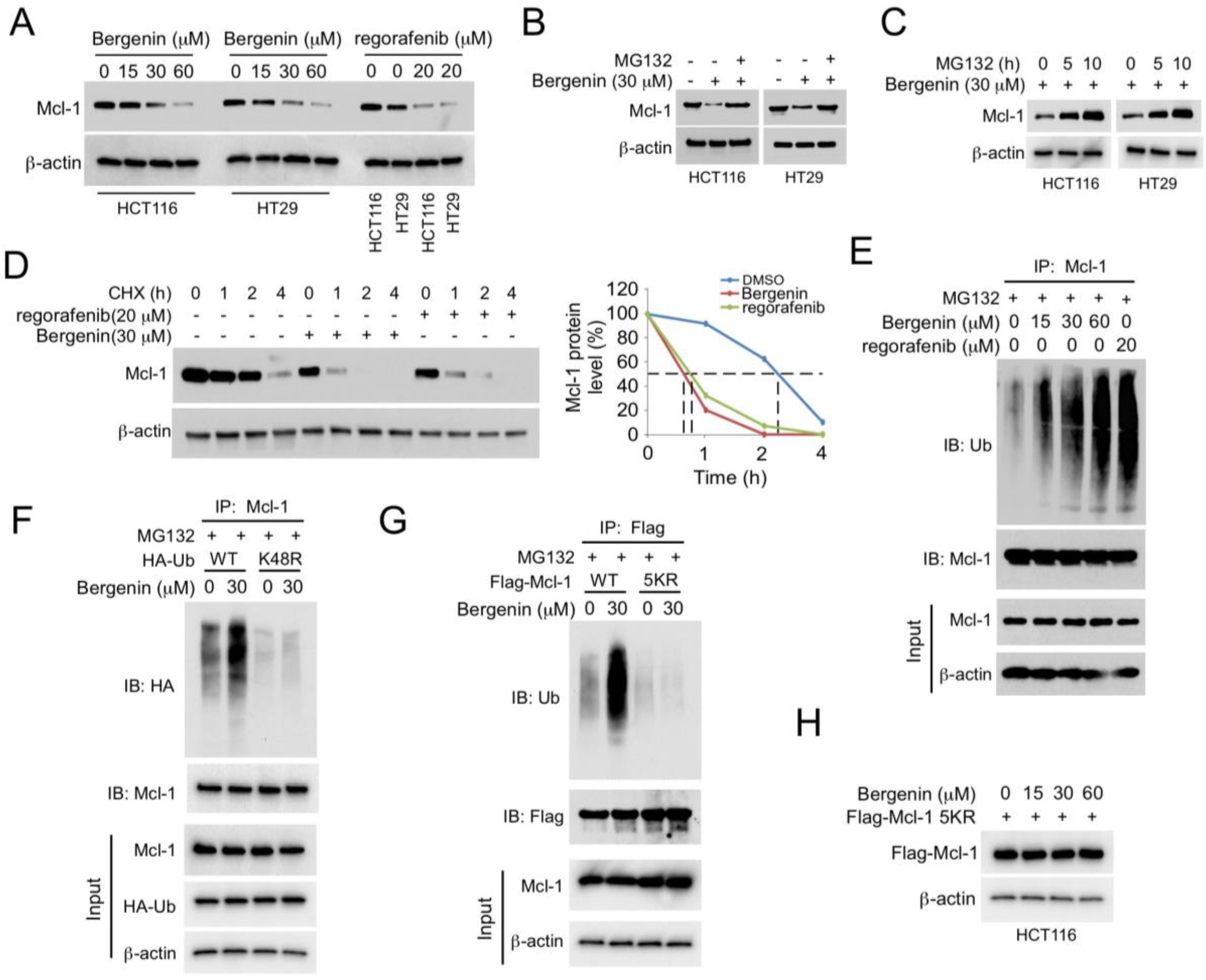
2.2. Bergenin Promotes Mcl-1 Degradation and Ubiquitination
2.3. (FBW7) Is Required for Bergenin-Induced Ubiquitination of Mcl-1
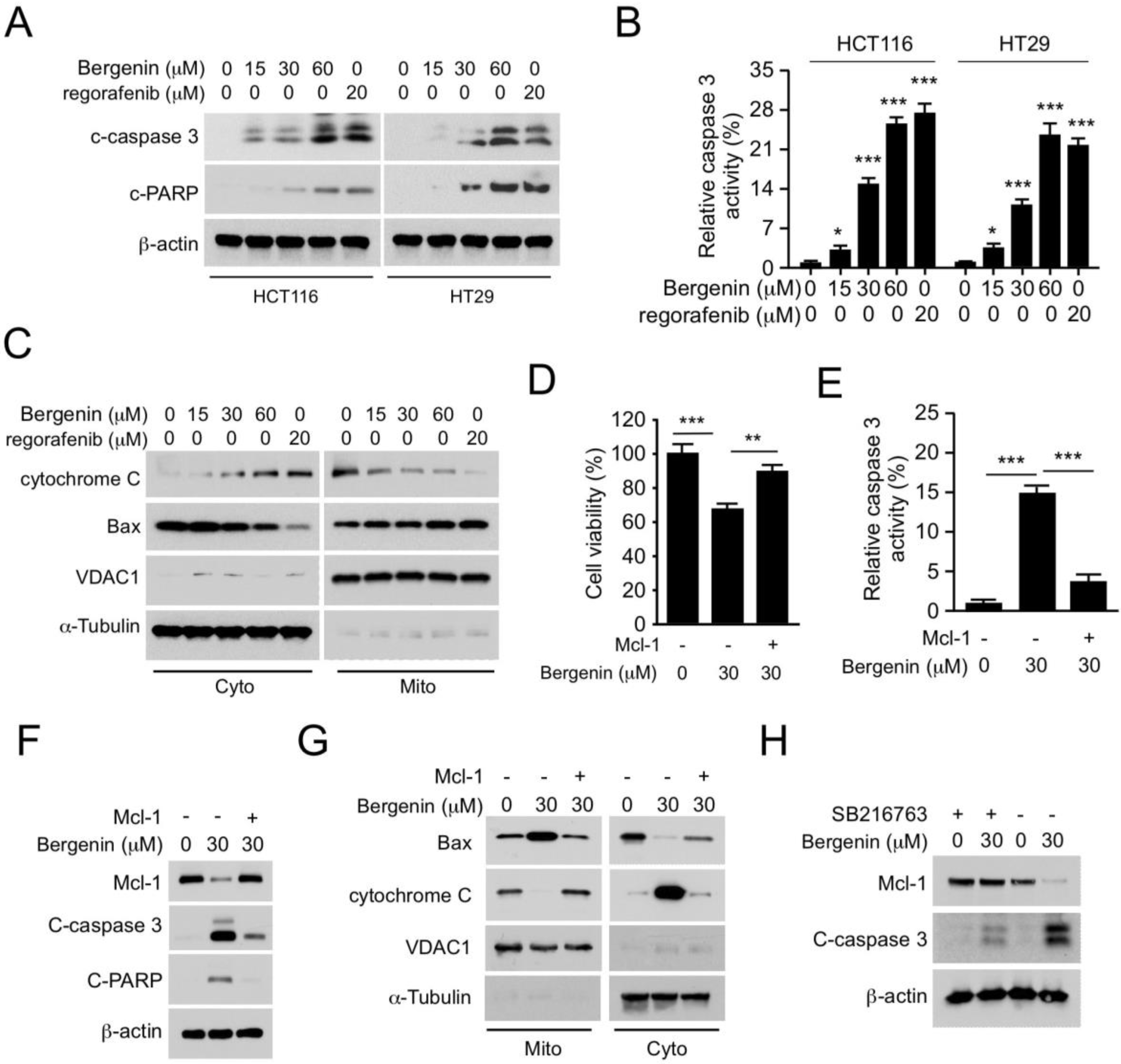
2.4. Bergenin Induces Apoptosis in Human CRC Cells
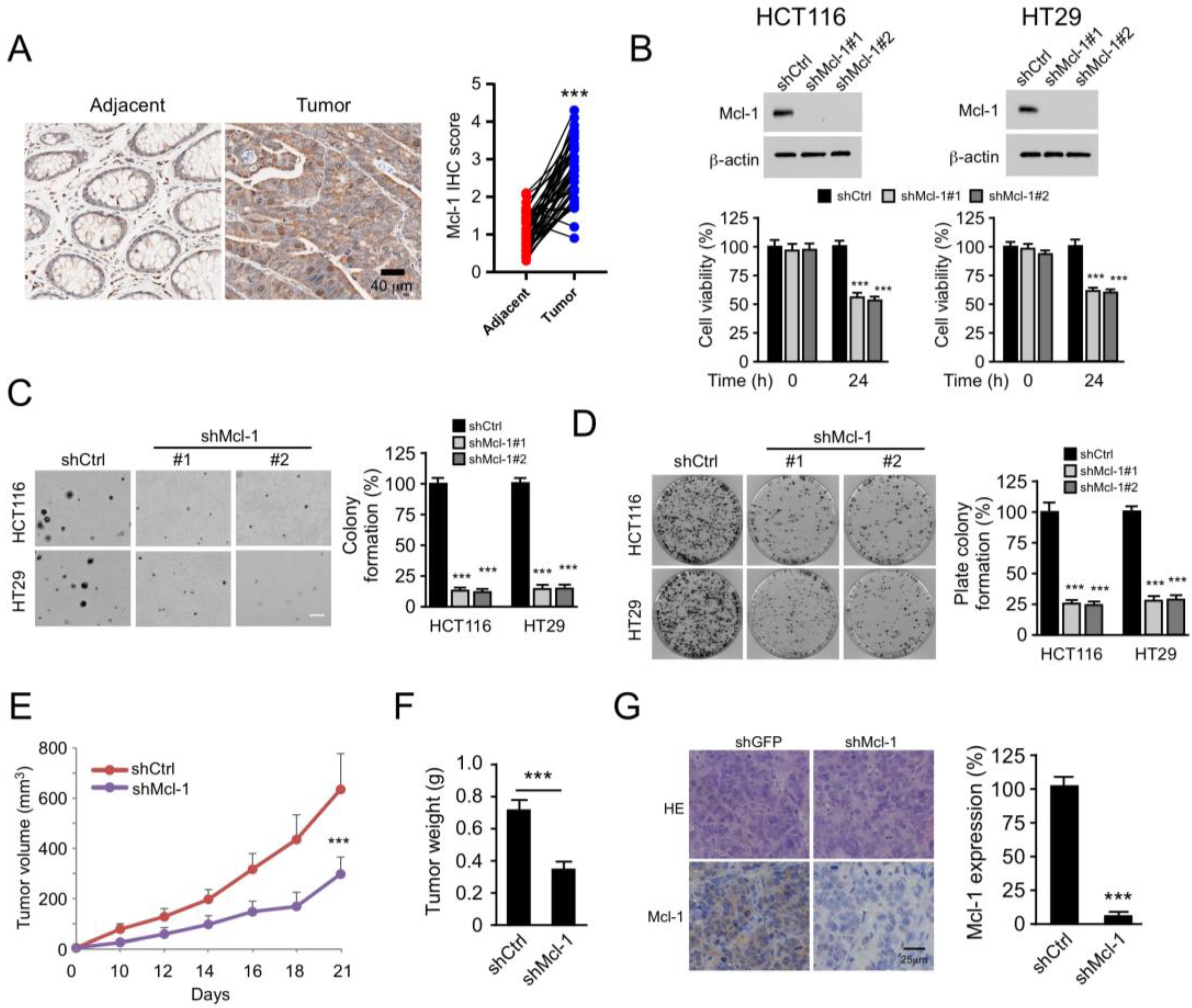
2.5. Mcl-1 Affects the Tumorigenic Properties of Human CRC Cells
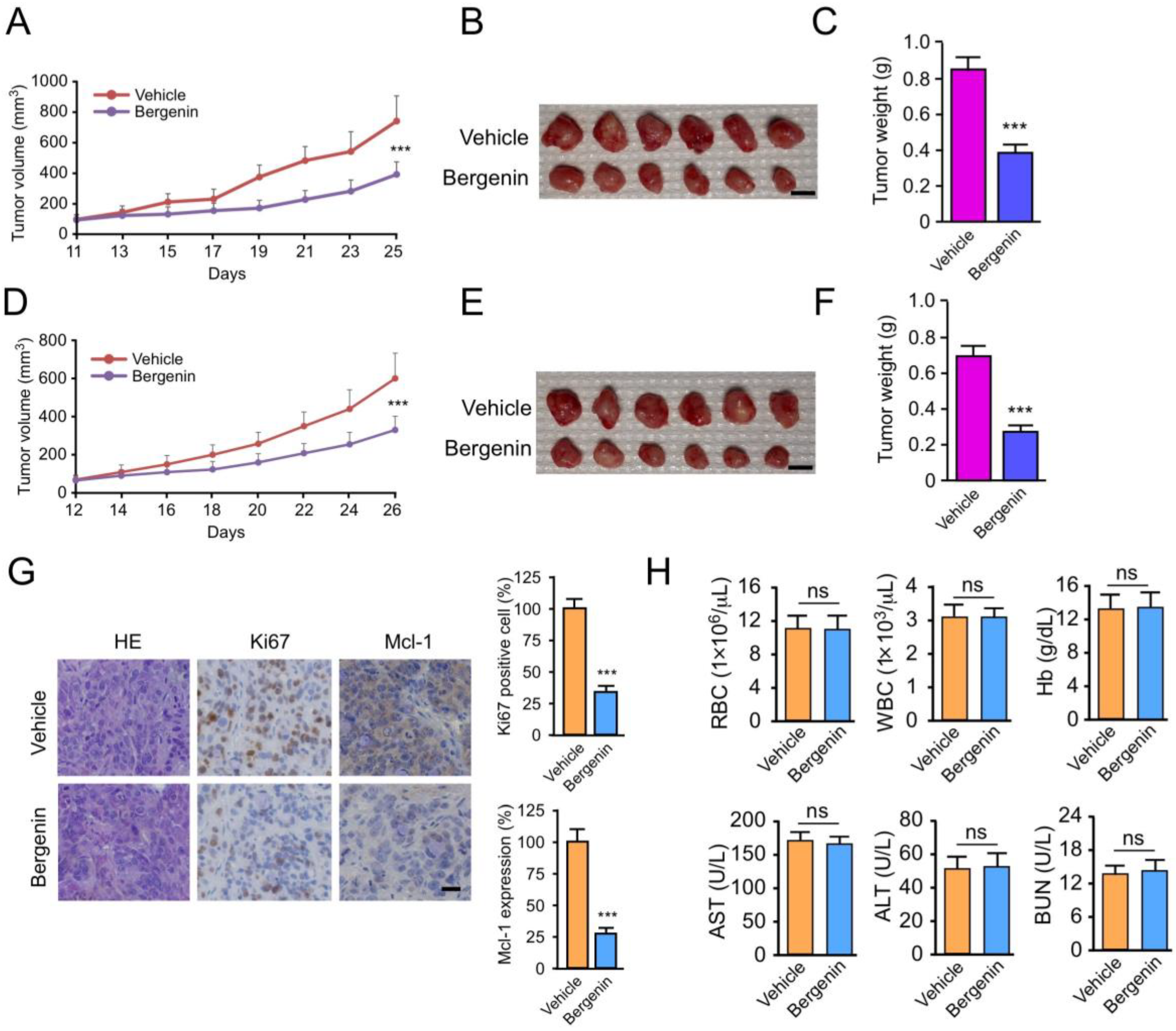
2.6. Bergenin Inhibits Tumor Growth In Vivo
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Cell Lines and Cell Culture
4.3. Clinical Tissue Sample Collections
4.4. Immunohistochemical (IHC) Staining
4.5. Protein Preparation and Western Blotting
4.6. 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) Assay
4.7. Ubiquitination Assay
4.8. In Vivo Tumor Growth Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chen, Z.; Liu, M.; Yang, S.; Song, B.A.; Xu, G.F.; Bhadury, P.S.; Jin, L.H.; Hu, D.H.; Liu, F.; Xue, W.; et al. Studies on the chemical constituents and anticancer activity of Saxifraga stolonifera (L.) Meeb. Bioorg. Med. Chem. 2008, 16, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.A.E.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995, 378, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Daressy, F.; Séguy, L.; Favre, L.; Corvaisier, S.; Apel, C.; Groo, A.-C.; Litaudon, M.; Dumontet, V.; Malzert-Fréon, A.; Desrat, S.; et al. NA1-115-7, from Zygogynum pancheri, is a new selective MCL-1 inhibitor inducing the apoptosis of hematological cancer cells but non-toxic to normal blood cells or cardiomyocytes. Biomed. Pharmacother. 2022, 154, 113546. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.; Eberlein, C.; Yu, J.; Gris-Oliver, A.; Ong, S.H.; Yelland, U.; Cureton, N.; Staniszewska, A.; McEwen, R.; Fox, M.; et al. AKT-mTORC1 reactivation is the dominant resistance driver for PI3Kβ/AKT inhibitors in PTEN-null breast cancer and can be overcome by combining with Mcl-1 inhibitors. Oncogene 2022, 41, 5046–5060. [Google Scholar] [CrossRef]
- Fan, J.; Bellon, M.; Ju, M.; Zhao, L.; Wei, M.; Fu, L.; Nicot, C. Clinical significance of FBXW7 loss of function in human cancers. Mol. Cancer 2022, 21, 87. [Google Scholar] [CrossRef]
- Geismann, C.; Hauser, C.; Grohmann, F.; Schneeweis, C.; Bölter, N.; Gundlach, J.-P.; Schneider, G.; Röcken, C.; Meinhardt, C.; Schäfer, H.; et al. NF-κB/RelA controlled A20 limits TRAIL-induced apoptosis in pancreatic cancer. Cell Death Dis. 2023, 14, 3. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Holt, J.; Schwalb, H.; Elbourne, H.; Te Marvelde, L.; Reid, C. Risk factors for recurrence in colorectal cancer: A retrospective analysis in a regional Australian hospital. ANZ J. Surg. 2021, 91, 2482–2486. [Google Scholar] [CrossRef]
- Inuzuka, H.; Shaik, S.; Onoyama, I.; Gao, D.; Tseng, A.; Maser, R.S.; Zhai, B.; Wan, L.; Gutierrez, A.; Lau, A.W.; et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 2011, 471, 104–109. [Google Scholar] [CrossRef]
- Jayakody, R.S.; Wijewardhane, P.; Herath, C.; Perera, S. Bergenin: A computationally proven promising scaffold for novel galectin-3 inhibitors. J. Mol. Model. 2018, 24, 302. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, D.; Zhang, B.; Lu, H. Bergenin Ameliorates MPTP-Induced Parkinson’s Disease by Activating PI3K/Akt Signaling Pathway. J. Alzheimer’s Dis. 2019, 72, 823–833. [Google Scholar] [CrossRef]
- Kim, G.; Jang, S.-K.; Kim, Y.J.; Jin, H.-O.; Bae, S.; Hong, J.; Park, I.-C.; Lee, J.H. Inhibition of Glutamine Uptake Resensitizes Paclitaxel Resistance in SKOV3-TR Ovarian Cancer Cell via mTORC1/S6K Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 8761. [Google Scholar] [CrossRef]
- Kotschy, A.; Szlavik, Z.; Murray, J.; Davidson, J.; Maragno, A.L.; Le Toumelin-Braizat, G.; Chanrion, M.; Kelly, G.L.; Gong, J.-N.; Moujalled, D.M.; et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 2016, 538, 477–482. [Google Scholar] [CrossRef]
- Koul, B.; Kumar, A.; Yadav, D.; Jin, J.-O. Genus: Traditional Uses, Phytochemistry and Pharmacology. Molecules 2020, 25, 5555. [Google Scholar] [CrossRef]
- Liang, C.; Pei, S.; Ju, W.; Jia, M.; Tian, D.; Tang, Y.; Mao, G. Synthesis and in vitro and in vivo antitumour activity study of 11-hydroxyl esterified bergenin/cinnamic acid hybrids. Eur. J. Med. Chem. 2017, 133, 319–328. [Google Scholar] [CrossRef]
- Liang, Y.-Y.; Niu, F.-Y.; Xu, A.-A.; Jiang, L.-L.; Liu, C.-S.; Liang, H.-P.; Huang, Y.-F.; Shao, X.-F.; Mo, Z.-W.; Yuan, Y.-W. Increased MCL-1 synthesis promotes irradiation-induced nasopharyngeal carcinoma radioresistance via regulation of the ROS/AKT loop. Cell Death Dis. 2022, 13, 131. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Yu, C.; Zhang, P.; Gu, S.; Wang, G.; Xiao, H.; Li, S. Bergenin inhibits bladder cancer progression via activating the PPARγ/PTEN/Akt signal pathway. Drug Dev. Res. 2021, 82, 278–286. [Google Scholar] [CrossRef]
- Liu, S.; Qiao, X.; Wu, S.; Gai, Y.; Su, Y.; Edwards, H.; Wang, Y.; Lin, H.; Taub, J.W.; Wang, G.; et al. c-Myc plays a critical role in the antileukemic activity of the Mcl-1-selective inhibitor AZD5991 in acute myeloid leukemia. Apoptosis Int. J. Program. Cell Death 2022, 27, 913–928. [Google Scholar] [CrossRef]
- Lu, K.-W.; Lu, T.-J.; Chueh, F.-S.; Lai, K.-C.; Hsia, T.-C.; Peng, S.-F.; Cheng, C.-C.; Chou, Y.-C.; Hsu, F.-T. Allyl Isothiocyanate (AITC) Induces Apoptotic Cell Death In Vitro and Exhibits Anti-Tumor Activity in a Human Glioblastoma GBM8401/Model. Int. J. Mol. Sci. 2022, 23, 10411. [Google Scholar] [CrossRef]
- Mamur, S.; Gündüzer, E.; Yaman, M. Toxicological aspect of bioinsecticide pyrethrum extract and expressions of apoptotic gene levels in human hepotacellular carcinoma HepG2 cells. Toxicol. Mech. Methods 2022, 32, 373–384. [Google Scholar] [CrossRef]
- Maurer, U.; Charvet, C.; Wagman, A.S.; Dejardin, E.; Green, D.R. Glycogen Synthase Kinase-3 Regulates Mitochondrial Outer Membrane Permeabilization and Apoptosis by Destabilization of MCL-1. Mol. Cell 2006, 21, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Mojsa, B.; Lassot, I.; Desagher, S. Mcl-1 ubiquitination: Unique regulation of an essential survival protein. Cells 2014, 3, 418–437. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Negi, A.; Murphy, P.V. Development of Mcl-1 inhibitors for cancer therapy. Eur. J. Med. Chem. 2021, 210, 113038. [Google Scholar] [CrossRef]
- Newell, A.M.B.; Yousef, G.G.; Lila, M.A.; Ramírez-Mares, M.V.; de Mejia, E.G. Comparative in vitro bioactivities of tea extracts from six species of Ardisia and their effect on growth inhibition of HepG2 cells. J. Ethnopharmacol. 2010, 130, 536–544. [Google Scholar] [CrossRef]
- Pal, P.; Zhang, P.; Poddar, S.K.; Zheng, G. Patent landscape of inhibitors and PROTACs of the anti-apoptotic BCL-2 family proteins. Expert Opin. Ther. Pat. 2022, 32, 1003–1026. [Google Scholar] [CrossRef]
- Park, K.-R.; Kwon, Y.-J.; Cho, M.; Kwon, I.K.; Hong, J.T.; Yun, H.-M. 11-O-Galloyl Bergenin from Leaves Induces Autophagy and Apoptosis in Human Osteosarcoma. Am. J. Chin. Med. 2021, 49, 2017–2031. [Google Scholar] [CrossRef]
- Prew, M.S.; Adhikary, U.; Choi, D.W.; Portero, E.P.; Paulo, J.A.; Gowda, P.; Budhraja, A.; Opferman, J.T.; Gygi, S.P.; Danial, N.N.; et al. MCL-1 is a master regulator of cancer dependency on fatty acid oxidation. Cell Rep. 2022, 41, 111445. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Xu, Z.; Gao, H.; Feng, W.; Li, W.; Miao, Y.; Xu, Z.; Zong, Y.; Zhao, J.; et al. KLF5 inhibition overcomes oxaliplatin resistance in patient-derived colorectal cancer organoids by restoring apoptotic response. Cell Death Dis. 2022, 13, 303. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, V.; Bharate, S.S.; Vishwakarma, R.A. Synthesis, pH dependent, plasma and enzymatic stability of bergenin prodrugs for potential use against rheumatoid arthritis. Biorg. Med. Chem. 2017, 25, 5513–5521. [Google Scholar] [CrossRef]
- Sinicrope, F.A.; Ruan, S.B.; Cleary, K.R.; Stephens, L.C.; Lee, J.J.; Levin, B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995, 55, 237–241. [Google Scholar]
- Song, X.; Shen, L.; Tong, J.; Kuang, C.; Zeng, S.; Schoen, R.E.; Yu, J.; Pei, H.; Zhang, L. Mcl-1 inhibition overcomes intrinsic and acquired regorafenib resistance in colorectal cancer. Theranostics 2020, 10, 8098–8110. [Google Scholar] [CrossRef]
- Souza, S.d.M.; Santos de Souza, L.; Silva, V.R.; Botelho Pereira Soares, M.; Pereira Bezerra, D.; Wagner da Silva Gois, R.; Carlota da Silva, H.; Pinheiro Santiago, G.M.; Gadelha Militao, G.C. Natural Dibenzo[b,f]oxepines, Pacharin and Bauhiniastatin-1, Isolated from Bauhinia acuruana Induce Apoptosis on Breast Cancer Cells via MCL-1 Protein Reduction. Planta Med. 2022. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Tan, W.; Lu, J.; Huang, M.; Li, Y.; Chen, M.; Wu, G.; Gong, J.; Zhong, Z.; Xu, Z.; Dang, Y.; et al. Anti-cancer natural products isolated from chinese medicinal herbs. Chin. Med. 2011, 6, 27. [Google Scholar] [CrossRef]
- Tsai, H.-L.; Tsai, Y.-C.; Chen, Y.-C.; Huang, C.-W.; Chen, P.-J.; Li, C.-C.; Su, W.-C.; Chang, T.-K.; Yeh, Y.-S.; Yin, T.-C.; et al. induces apoptosis and prevents angiogenesis with bevacizumab in colon cancer through direct inhibition of/via under hypoxia. Aging 2022, 14, 6668–6688. [Google Scholar] [CrossRef]
- Tunio, M.A.; Phillips, K.; Baker, P. Amaurosis Fugax: A Rare Oxaliplatin-Induced Ocular Toxicity—A Report of Three Cases. Case Rep. Oncol. 2022, 15, 133–137. [Google Scholar] [CrossRef]
- Venkateswara Rao, B.; Pavan Kumar, P.; Ramalingam, V.; Karthik, G.; Andugulapati, S.B.; Suresh Babu, K. Piperazine tethered bergenin heterocyclic hybrids: Design, synthesis, anticancer activity, and molecular docking studies. RSC Med. Chem. 2022, 13, 978–985. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting MCL-1 in cancer: Current status and perspectives. J. Hematol. Oncol. 2021, 14, 67. [Google Scholar] [CrossRef]
- Wu, K.; Woo, S.-M.; Seo, S.-U.; Kwon, T.-K. Inhibition of BMI-1 Induces Apoptosis through Downregulation of DUB3-Mediated Mcl-1 Stabilization. Int. J. Mol. Sci. 2021, 22, 10107. [Google Scholar] [CrossRef]
- Wu, X.; Luo, Q.; Liu, Z. Ubiquitination and deubiquitination of MCL1 in cancer: Deciphering chemoresistance mechanisms and providing potential therapeutic options. Cell Death Dis. 2020, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Chen, K.; Xu, L.; Wang, T.; Guo, C. Bergenin Exerts Hepatoprotective Effects by Inhibiting the Release of Inflammatory Factors, Apoptosis and Autophagy via the PPAR-γ Pathway. Drug Des. Devel. Ther. 2020, 14, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xiang, J.-W.; Gao, Q.; Bai, Y.-Y.; Huang, Z.-X.; Hu, X.-H.; Wang, L.; Li, D.W.-C. MAB21L1 promotes survival of lens epithelial cells through control of αB-crystallin and ATR/CHK1/p53 pathway. Aging 2022, 14, 6128–6148. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Yao, M.Y.; Wang, Y.F.; Zhao, Y.; Ling, L.J.; He, Y.; Wen, J.; Zheng, M.Y.; Jiang, H.L.; Xie, C.Y. BCL-2 inhibitor synergizes with PI3Kδ inhibitor and overcomes FLT3 inhibitor resistance in acute myeloid leukaemia. Am. J. Cancer Res. 2022, 12, 3829. [Google Scholar]
- Yu, X.; Zhou, L.; Liu, W.; Liu, L.; Gao, F.; Li, W.; Liu, H. Skp2 stabilizes Mcl-1 and confers radioresistance in colorectal cancer. Cell Death Dis. 2022, 13, 249. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhou, T.; Zhao, F.; Xiong, D.; He, B.; Hua, Q.; Lin, M.; Deng, L.; Sang, X.; Xie, W.; et al. p62-Nrf2 Regulatory Loop Mediates the Anti-Pulmonary Fibrosis Effect of Bergenin. Antioxidants 2022, 11, 307. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Yin, C.; Gong, P.; Zhang, Z.; Zhao, L.; Waxman, S.; Jing, Y. Artesunate improves venetoclax plus cytarabine AML cell targeting by regulating the Noxa/Bim/Mcl-1/p-Chk1 axis. Cell Death Dis. 2022, 13, 379. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, Y.; Liu, J.; Tang, X.; Wang, Y.; Li, X.; Li, H.; Zhou, X.; Tang, S.; Tang, Y.; et al. CircIFNGR2 enhances proliferation and migration of CRC and induces cetuximab resistance by indirectly targeting KRAS via sponging to MiR-30b. Cell Death Dis. 2023, 14, 24. [Google Scholar] [CrossRef]
- Zhu, H.-L.; Dai, L.-M.; Xiong, Y.-W.; Shi, X.-T.; Liu, W.-B.; Fu, Y.-T.; Zhou, G.-X.; Zhang, S.; Gao, L.; Zhang, C.; et al. Gestational exposure to environmental cadmium induces placental apoptosis and fetal growth restriction via Parkin-modulated MCL-1 degradation. J. Hazard. Mater. 2022, 424, 127268. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, Y.; Li, X.; Han, S.; Zhou, L.; Li, W. Targeting Mcl-1 Degradation by Bergenin Inhibits Tumorigenesis of Colorectal Cancer Cells. Pharmaceuticals 2023, 16, 241. https://doi.org/10.3390/ph16020241
Gan Y, Li X, Han S, Zhou L, Li W. Targeting Mcl-1 Degradation by Bergenin Inhibits Tumorigenesis of Colorectal Cancer Cells. Pharmaceuticals. 2023; 16(2):241. https://doi.org/10.3390/ph16020241
Chicago/Turabian StyleGan, Yu, Xiaoying Li, Shuangze Han, Li Zhou, and Wei Li. 2023. "Targeting Mcl-1 Degradation by Bergenin Inhibits Tumorigenesis of Colorectal Cancer Cells" Pharmaceuticals 16, no. 2: 241. https://doi.org/10.3390/ph16020241
APA StyleGan, Y., Li, X., Han, S., Zhou, L., & Li, W. (2023). Targeting Mcl-1 Degradation by Bergenin Inhibits Tumorigenesis of Colorectal Cancer Cells. Pharmaceuticals, 16(2), 241. https://doi.org/10.3390/ph16020241





