Phenolic Acids-Mediated Regulation of Molecular Targets in Ovarian Cancer: Current Understanding and Future Perspectives
Abstract
1. Introduction
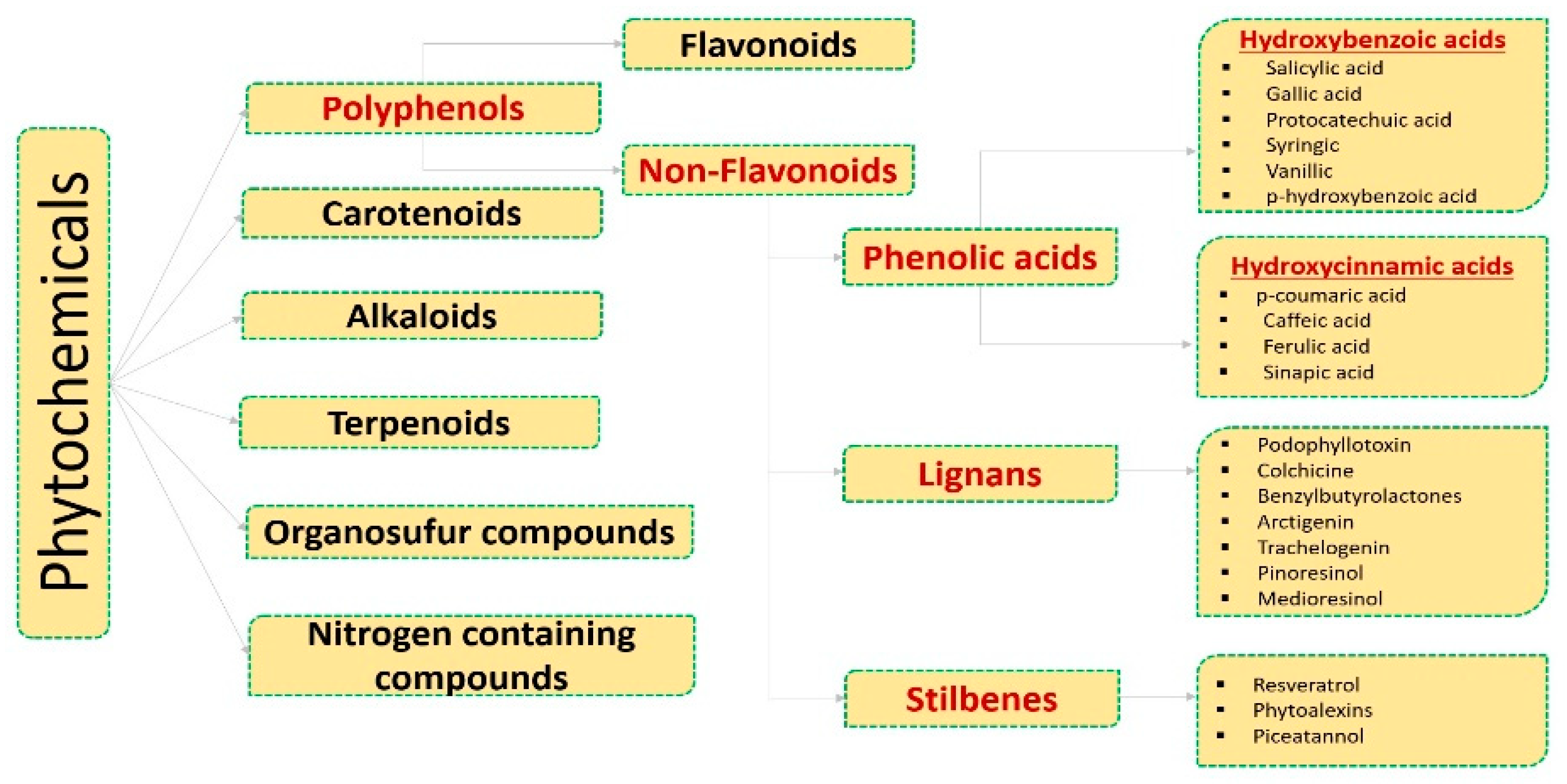
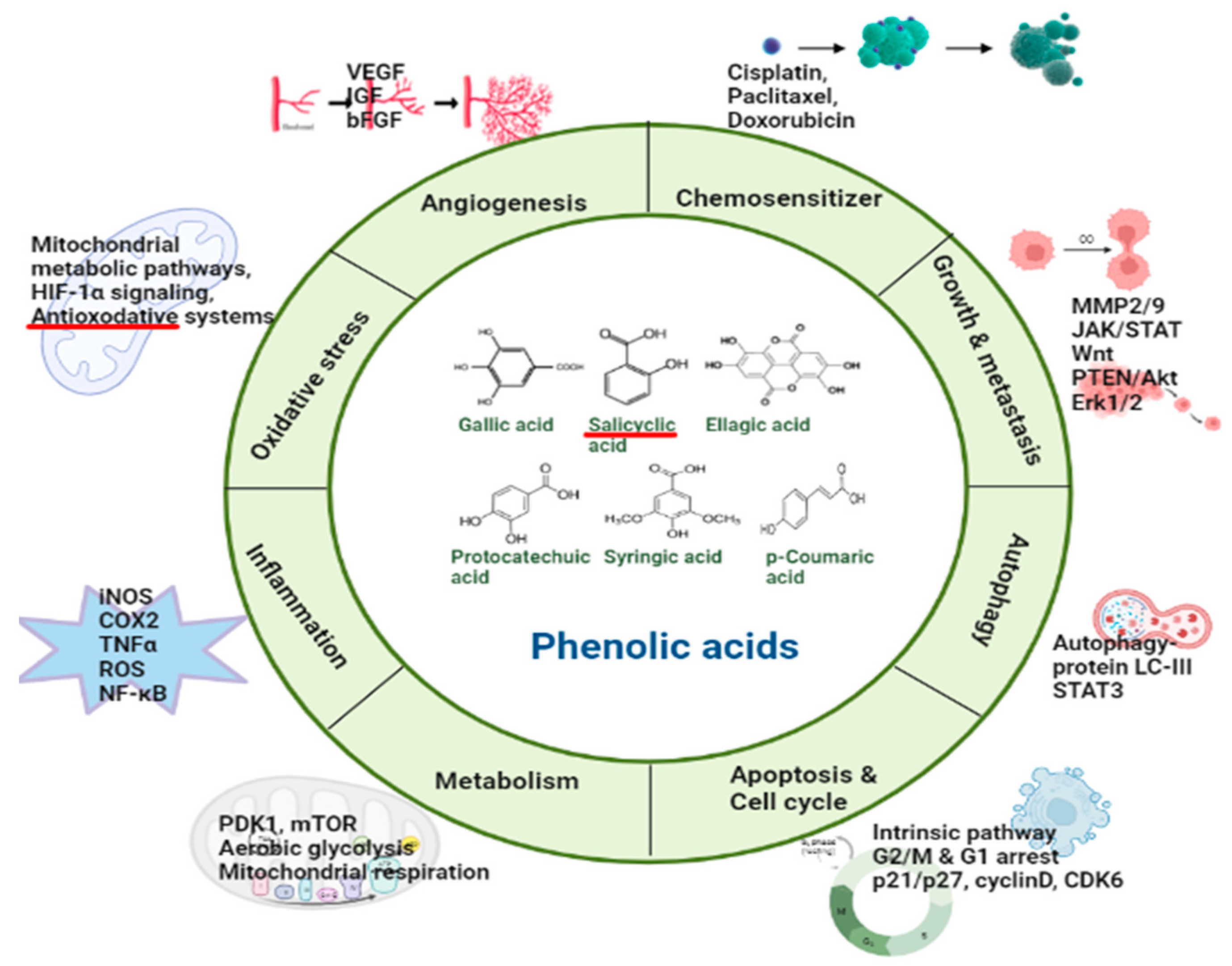
2. Phenolic Acids Involved in the Regulation of Molecular Targets
2.1. Hydroxybenzoic Acids
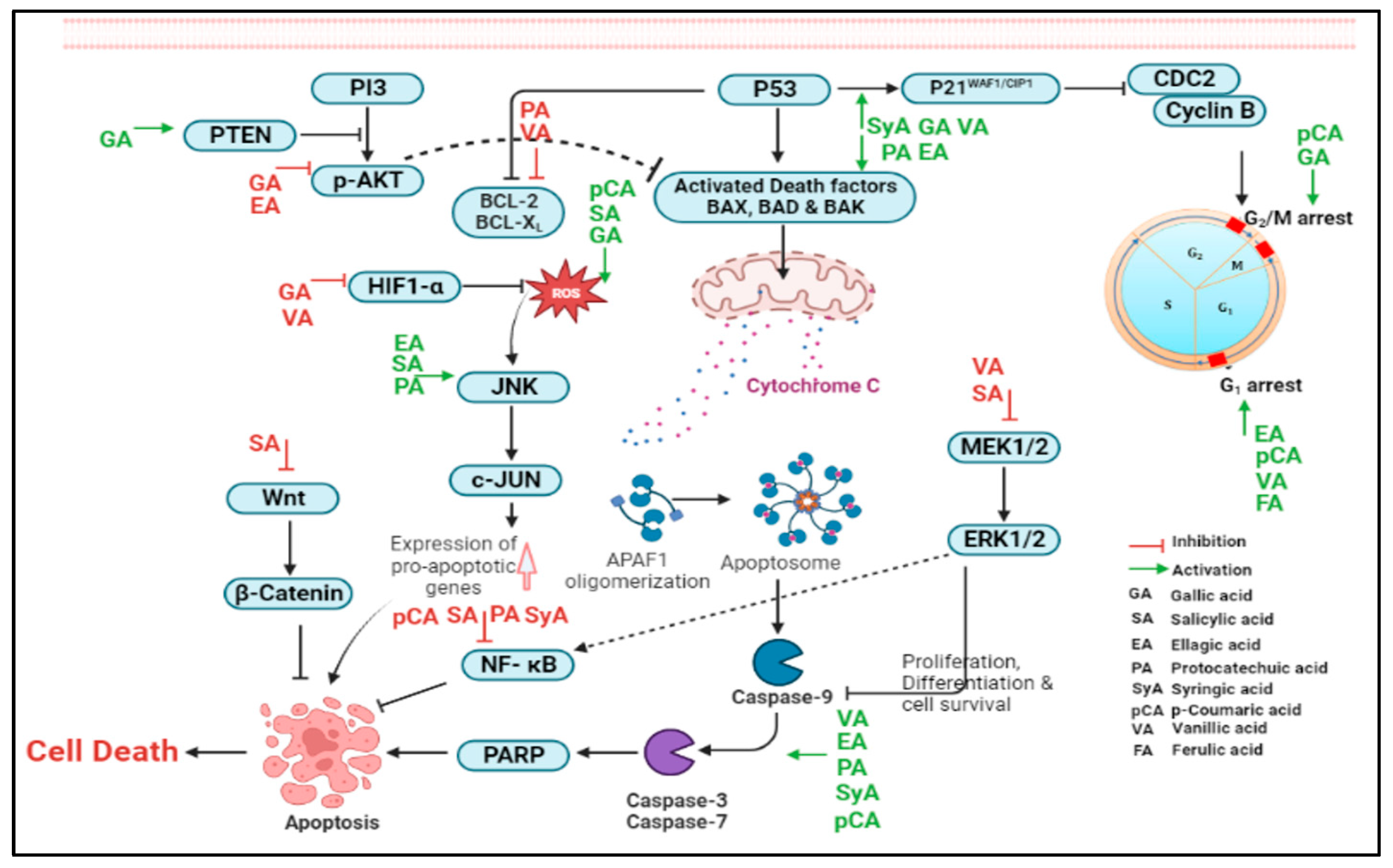
2.1.1. Gallic Acid
2.1.2. Salicylic Acid
2.1.3. Ellagic Acid
2.1.4. Protocatechuic Acid
2.1.5. Syringic Acid
2.1.6. Vanillic Acid
2.2. Hydroxy Cinnamic Acids
2.2.1. Caffeic Acid
2.2.2. p-Coumaric Acid
2.2.3. Ferulic Acid
2.2.4. Sinapic Acid
3. Summary and Future Perspectives
4. Conclusions
5. Limitation of Phenolic Acids
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sebastian, A.M.; Peter, D. Artificial Intelligence in Cancer Research: Trends, Challenges and Future Directions. Life 2022, 12, 1991. [Google Scholar] [CrossRef] [PubMed]
- Jabir, N.R.; Firoz, C.K.; Zughaibi, T.A.; Alsaadi, M.A.; Abuzenadah, A.M.; Al-Asmari, A.I.; Alsaieedi, A.; Ahmed, B.A.; Ramu, A.K.; Tabrez, S. A literature perspective on the pharmacological applications of yohimbine. Ann. Med. 2022, 54, 2861–2875. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Coburn, S.b.; Bray, F.; Sherman, M.e.; Trabert, B. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int. J. Cancer 2017, 140, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Roett, M.A.; Evans, P. Ovarian cancer: An overview. Am. Fam. Physician 2009, 80, 609–616. [Google Scholar] [PubMed]
- Klotz, D.M.; Wimberger, P. Cells of origin of ovarian cancer: Ovarian surface epithelium or fallopian tube? Arch. Gynecol. Obstet. 2017, 296, 1055–1062. [Google Scholar] [CrossRef]
- Doubeni, C.A.; Doubeni, A.R.; Myers, A.E. Diagnosis and Management of Ovarian Cancer. Am. Fam. Physician 2016, 93, 937–944. [Google Scholar]
- Marulanda, K.; Maduekwe, U.N. Disparities in the Management of Peritoneal Surface Malignancies. Surg. Oncol. Clin. N. Am. 2022, 31, 29–41. [Google Scholar] [CrossRef]
- Akter, S.; Rahman, M.A.; Hasan, M.N.; Akhter, H.; Noor, P.; Islam, R.; Shin, Y.; Rahman, M.D.H.; Gazi, M.S.; Huda, M.N.; et al. Recent Advances in Ovarian Cancer: Therapeutic Strategies, Potential Biomarkers, and Technological Improvements. Cells 2022, 11, 650. [Google Scholar] [CrossRef]
- Brasseur, K.; Gévry, N.; Asselin, E. Chemoresistance and targeted therapies in ovarian and endometrial cancers. Oncotarget 2016, 8, 4008–4042. [Google Scholar] [CrossRef] [PubMed]
- Jaaback, K.; Johnson, N.; Lawrie, T.A. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst. Rev. 2016, 2016, CD005340. [Google Scholar] [CrossRef]
- Mammadova, J.; Redden, A.; Cruz, R.; Ujhazi, B.; Ellison, M.; Gatewood, T.; Duff, C.; Canella, A.; Somboonwit, C.; Sriaroon, C.; et al. Case Report: Initial Treatment Adjustments and Complications in Ovarian Cancer Patient With Inborn Error of Immunity. Front. Oncol. 2022, 12, 843741. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M.; Krajewski, R.; Makuch, S.; Agrawal, S. Phytochemicals in Gynecological Cancer Prevention. Int. J. Mol. Sci. 2021, 22, 1219. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Usmani, D.; Tarique, M.; Naz, H.; Ashraf, M.; Raliya, R.; Tabrez, S.; Zughaibi, T.A.; Alsaieedi, A.; Hakeem, I.J.; et al. The Role of Natural Products and Their Multitargeted Approach to Treat Solid Cancer. Cells 2022, 11, 2209. [Google Scholar] [CrossRef] [PubMed]
- Tabrez, S.; Hoque, M.; Suhail, M.; Khan, M.I.; Zughaibi, T.A.; Khan, A.U. Identification of anticancer bioactive compounds derived from Ficus sp. by targeting Poly[ADP-ribose]polymerase 1 (PARP-1). J. King Saud Univ. Sci. 2022, 34, 102079. [Google Scholar] [CrossRef]
- Abuzenadah, A.M.; Al-Sayes, F.; Mahafujul Alam, S.S.; Hoque, M.; Karim, S.; Hussain, I.M.R.; Tabrez, S. Identification of Potential Poly (ADP-Ribose) Polymerase-1 Inhibitors Derived from Rauwolfia serpentina: Possible Implication in Cancer Therapy. Evid. Based Complement. Altern. Med. 2022, 2022, 3787162. [Google Scholar] [CrossRef]
- Abuzenadah, A.M.; Al-Sayes, F.; Mahafujul Alam, S.S.; Hoque, M.; Karim, S.; Hussain, I.M.R.; Tabrez, S. Elucidating Antiangiogenic Potential of Rauwolfia serpentina: VEGFR-2 Targeting-Based Molecular Docking Study. Evid. Based Complement. Altern. Med. 2022, 2022, 6224666. [Google Scholar] [CrossRef] [PubMed]
- Tabrez, S.; Zughaibi, T.A.; Hoque, M.; Suhail, M.; Khan, M.I.; Khan, A.U. Targeting Glutaminase by Natural Compounds: Structure-Based Virtual Screening and Molecular Dynamics Simulation Approach to Suppress Cancer Progression. Molecules 2022, 27, 5042. [Google Scholar] [CrossRef] [PubMed]
- Jabir, N.R.; Khan, M.S.; Alafaleq, N.O.; Naz, H.; Ahmed, B.A. Anticancer potential of yohimbine in drug-resistant oral cancer KB-ChR-8-5 cells. Mol. Biol. Rep. 2022, 49, 9565–9573. [Google Scholar] [CrossRef]
- George, B.P.; Chandran, R.; Abrahamse, H. Role of Phytochemicals in Cancer Chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef]
- Farvid, M.S.; Chen, W.Y.; Michels, K.B.; Cho, E.; Willett, W.C.; Eliassen, A.H. Fruit and vegetable consumption in adolescence and early adulthood and risk of breast cancer: Population based cohort study. BMJ 2016, 353, i2343. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Peng, Y.; Qiao, Y.; Huang, Y.; Song, F.; Zhang, M.; Song, F. Consumption of flavonoids and risk of hormone-related cancers: A systematic review and meta-analysis of observational studies. Nutr. J. 2022, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Badar Ul Islam, n.; Khan, M.S.; Husain, F.M.; Rehman, M.T.; Zughaibi, T.A.; Abuzenadah, A.M.; Urooj, M.; Kamal, M.A.; Tabrez, S. mTOR Targeted Cancer Chemoprevention by Flavonoids. Curr. Med. Chem. 2021, 28, 8068–8082. [Google Scholar] [CrossRef]
- Ul Islam, B.; Suhail, M.; Khan, M.S.; Ahmad, A.; Zughaibi, T.A.; Husain, F.M.; Rehman, M.T.; Tabrez, S. Flavonoids and PI3K/Akt/mTOR Signaling Cascade: A Potential Crosstalk in Anticancer Treatment. Curr. Med. Chem. 2021, 28, 8083–8097. [Google Scholar] [CrossRef]
- Zughaibi, T.A.; Suhail, M.; Tarique, M.; Tabrez, S. Targeting PI3K/Akt/mTOR Pathway by Different Flavonoids: A Cancer Chemopreventive Approach. Int. J. Mol. Sci. 2021, 22, 12455. [Google Scholar] [CrossRef]
- Pundir, M.; Sharma, A.; Kumar, J. Phytochemicals used as inhibitors in the treatment of ovarian cancer: A Mini-review. Mater. Today Proc. 2022, 48, 1620–1625. [Google Scholar] [CrossRef]
- Hua, X.; Yu, L.; You, R.; Yang, Y.; Liao, J.; Chen, D.; Yu, L. Association among Dietary Flavonoids, Flavonoid Subclasses and Ovarian Cancer Risk: A Meta-Analysis. PLoS ONE 2016, 11, e0151134. [Google Scholar] [CrossRef]
- Gates, M.A.; Tworoger, S.S.; Hecht, J.L.; De Vivo, I.; Rosner, B.; Hankinson, S.E. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int. J. Cancer 2007, 121, 2225–2232. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Libro, R.; Giacoppo, S.; Soundara Rajan, T.; Bramanti, P.; Mazzon, E. Natural Phytochemicals in the Treatment and Prevention of Dementia: An Overview. Molecules 2016, 21, 518. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Arora, S.; Singh, S.; Singh, A.P. Phytochemicals, microRNAs, and Cancer: Implications for Cancer Prevention and Therapy. In Mitochondria as Targets for Phytochemicals in Cancer Prevention and Therapy; Chandra, D., Ed.; Springer: New York, NY, USA, 2013; pp. 187–206. [Google Scholar]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol. 2020, 11, 580208. [Google Scholar] [CrossRef]
- Doğan, D.; Meydan, İ.; Kömüroğlu, A.U. Protective Effect of Silymarin and Gallic Acid against Cisplatin-Induced Nephrotoxicity and Hepatotoxicity. Int. J. Clin. Pract. 2022, 2022, e6541026. [Google Scholar] [CrossRef] [PubMed]
- Aborehab, N.M.; Osama, N. Effect of Gallic acid in potentiating chemotherapeutic effect of Paclitaxel in HeLa cervical cancer cells. Cancer Cell Int. 2019, 19, 154. [Google Scholar] [CrossRef]
- Aborehab, N.M.; Elnagar, M.R.; Waly, N.E. Gallic acid potentiates the apoptotic effect of paclitaxel and carboplatin via overexpression of Bax and P53 on the MCF-7 human breast cancer cell line. J. Biochem. Mol. Toxicol. 2021, 35, e22638. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, M. Gallic acid reduces cell viability, proliferation, invasion and angiogenesis in human cervical cancer cells. Oncol. Lett. 2013, 6, 1749–1755. [Google Scholar] [CrossRef]
- Rosman, R.; Saifullah, B.; Maniam, S.; Dorniani, D.; Hussein, M.Z.; Fakurazi, S. Improved Anticancer Effect of Magnetite Nanocomposite Formulation of GALLIC Acid (Fe₃O₄-PEG-GA) Against Lung, Breast and Colon Cancer Cells. Nanomaterials 2018, 8, 83. [Google Scholar] [CrossRef]
- Pham, H.N.T.; Sakoff, J.A.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. Comparative cytotoxic activity between kaempferol and gallic acid against various cancer cell lines. Data Brief 2018, 21, 1033–1036. [Google Scholar] [CrossRef]
- He, Z.; Li, B.; Rankin, G.O.; Rojanasakul, Y.; Chen, Y.C. Selecting bioactive phenolic compounds as potential agents to inhibit proliferation and VEGF expression in human ovarian cancer cells. Oncol. Lett. 2015, 9, 1444–1450. [Google Scholar] [CrossRef]
- He, Z.; Chen, A.Y.; Rojanasakul, Y.; Rankin, G.O.; Chen, Y.C. Gallic acid, a phenolic compound, exerts anti-angiogenic effects via the PTEN/AKT/HIF-1α/VEGF signaling pathway in ovarian cancer cells. Oncol. Rep. 2016, 35, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Varela-Rodríguez, L.; Sánchez-Ramírez, B.; Hernández-Ramírez, V.I.; Varela-Rodríguez, H.; Castellanos-Mijangos, R.D.; González-Horta, C.; Chávez-Munguía, B.; Talamás-Rohana, P. Effect of Gallic acid and Myricetin on ovarian cancer models: A possible alternative antitumoral treatment. BMC Complement. Med. Ther. 2020, 20, 110. [Google Scholar] [CrossRef] [PubMed]
- Frost, S.C. Physiological functions of the alpha class of carbonic anhydrases. Subcell. Biochem. 2014, 75, 9–30. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- He, Z.; Liu, X.; Wu, F.; Wu, S.; Rankin, G.O.N.; Martinez, I.; Rojanasakul, Y.; Chen, Y.C. Gallic Acid Induces S and G2 Phase Arrest and Apoptosis in Human Ovarian Cancer Cells In Vitro. Appl. Sci. 2021, 11, 3807. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Carranza, J.N.; Díaz, J.F.; Redondo-Horcajo, M.; Barasoain, I.; Alvarez, L.; Lastres, P.; Romero-Estrada, A.; Aller, P.; González-Maya, L. Gallic acid sensitizes paclitaxel-resistant human ovarian carcinoma cells through an increase in reactive oxygen species and subsequent downregulation of ERK activation. Oncol. Rep. 2018, 39, 3007–3014. [Google Scholar] [CrossRef]
- YouGuo, C.; ZongJi, S.; XiaoPing, C. Modulatory effect of Ganoderma lucidum polysaccharides on serum antioxidant enzymes activities in ovarian cancer rats. Carbohydr. Polym. 2009, 78, 258–262. [Google Scholar] [CrossRef]
- Hawley, S.A.; Fullerton, M.D.; Ross, F.A.; Schertzer, J.D.; Chevtzoff, C.; Walker, K.J.; Peggie, M.W.; Zibrova, D.; Green, K.A.; Mustard, K.J.; et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science 2012, 336, 918–922. [Google Scholar] [CrossRef]
- Duthie, G.G.; Wood, A.D. Natural salicylates: Foods, functions and disease prevention. Food Funct. 2011, 2, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Higgs, G.A.; Salmon, J.A.; Henderson, B.; Vane, J.R. Pharmacokinetics of aspirin and salicylate in relation to inhibition of arachidonate cyclooxygenase and antiinflammatory activity. Proc. Natl. Acad. Sci. USA 1987, 84, 1417–1420. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.-L.; Huang, P.-H.; Kao, C.-L.; Leu, H.-B.; Cheng, Y.-H.; Liao, Y.-W.; Yang, Y.-P.; Chien, Y.; Wang, C.-Y.; Hsiao, C.-Y.; et al. Aspirin attenuates vinorelbine-induced endothelial inflammation via modulating SIRT1/AMPK axis. Biochem. Pharmacol. 2014, 88, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Coudray, C.; Favier, A. Determination of salicylate hydroxylation products as an in vivo oxidative stress marker. Free. Radic. Biol. Med. 2000, 29, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.A.; Cao, Y.; Chan, A.T. Aspirin and colorectal cancer: The promise of precision chemoprevention. Nat. Rev. Cancer 2016, 16, 173–186. [Google Scholar] [CrossRef]
- Chen, W.Y.; Holmes, M.D. Role of Aspirin in Breast Cancer Survival. Curr. Oncol. Rep. 2017, 19, 48. [Google Scholar] [CrossRef]
- Ausina, P.; Branco, J.R.; Demaria, T.M.; Esteves, A.M.; Leandro, J.G.B.; Ochioni, A.C.; Mendonça, A.P.M.; Palhano, F.L.; Oliveira, M.F.; Abou-Kheir, W.; et al. Acetylsalicylic acid and salicylic acid present anticancer properties against melanoma by promoting nitric oxide-dependent endoplasmic reticulum stress and apoptosis. Sci. Rep. 2020, 10, 19617. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Z.; Zhang, F.; Qiao, M.; Yan, Z.; Wei, Q.; Wang, J.; Liu, H.; Fan, J.; Zou, Y.; et al. A Blockade of IGF Signaling Sensitizes Human Ovarian Cancer Cells to the Anthelmintic Niclosamide-Induced Anti-Proliferative and Anticancer Activities. Cell. Physiol. Biochem. 2016, 39, 871–888. [Google Scholar] [CrossRef]
- Sekulovski, N.; MacLean, J.A., II; Bheemireddy, S.R.; Yu, Z.; Okuda, H.; Pru, C.; Plunkett, K.N.; Matzuk, M.; Hayashi, K. Niclosamide’s potential direct targets in ovarian cancer†. Biol. Reprod. 2021, 105, 403–412. [Google Scholar] [CrossRef]
- Yo, Y.-T.; Lin, Y.-W.; Wang, Y.-C.; Balch, C.; Huang, R.-L.; Chan, M.W.Y.; Sytwu, H.-K.; Chen, C.-K.; Chang, C.-C.; Nephew, K.P.; et al. Growth inhibition of ovarian tumor-initiating cells by niclosamide. Mol. Cancer Ther. 2012, 11, 1703–1712. [Google Scholar] [CrossRef]
- Arend, R.C.; Londoño-Joshi, A.I.; Samant, R.S.; Li, Y.; Conner, M.; Hidalgo, B.; Alvarez, R.D.; Landen, C.N.; Straughn, J.M.; Buchsbaum, D.J. Inhibition of Wnt/β-catenin pathway by niclosamide: A therapeutic target for ovarian cancer. Gynecol. Oncol. 2014, 134, 112–120. [Google Scholar] [CrossRef] [PubMed]
- King, M.L.; Lindberg, M.E.; Stodden, G.R.; Okuda, H.; Ebers, S.D.; Johnson, A.; Montag, A.; Lengyel, E.; MacLean Ii, J.A.; Hayashi, K. WNT7A/β-catenin signaling induces FGF1 and influences sensitivity to niclosamide in ovarian cancer. Oncogene 2015, 34, 3452–3462. [Google Scholar] [CrossRef] [PubMed]
- Walters Haygood, C.L.; Arend, R.C.; Gangrade, A.; Chettiar, S.; Regan, N.; Hassmann, C.J.; Li, P.-K.; Hidalgo, B.; Straughn, J.M.; Buchsbaum, D.J. Niclosamide Analogs for Treatment of Ovarian Cancer. Int. J. Gynecol. Cancer 2015, 25, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Arend, R.C.; Londoño-Joshi, A.I.; Gangrade, A.; Katre, A.A.; Kurpad, C.; Li, Y.; Samant, R.S.; Li, P.-K.; Landen, C.N.; Yang, E.S.; et al. Niclosamide and its analogs are potent inhibitors of Wnt/β-catenin, mTOR and STAT3 signaling in ovarian cancer. Oncotarget 2016, 7, 86803–86815. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, F.; Liu, Y.; Ma, L.; Qu, G.; Lv, Q.; An, J.; Yang, S.; Lu, B.; Cao, Q. Niclosamide inhibits ovarian carcinoma growth by interrupting cellular bioenergetics. J. Cancer 2020, 11, 3454–3466. [Google Scholar] [CrossRef]
- Satoh, K.; Zhang, L.; Zhang, Y.; Chelluri, R.; Boufraqech, M.; Nilubol, N.; Patel, D.; Shen, M.; Kebebew, E. Identification of Niclosamide as a Novel Anticancer Agent for Adrenocortical Carcinoma. Clin. Cancer Res. 2016, 22, 3458–3466. [Google Scholar] [CrossRef]
- Lin, C.-K.; Bai, M.-Y.; Hu, T.-M.; Wang, Y.-C.; Chao, T.-K.; Weng, S.-J.; Huang, R.-L.; Su, P.-H.; Lai, H.-C. Preclinical evaluation of a nanoformulated antihelminthic, niclosamide, in ovarian cancer. Oncotarget 2016, 7, 8993–9006. [Google Scholar] [CrossRef]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Ceci, C.; Lacal, P.M.; Tentori, L.; De Martino, M.G.; Miano, R.; Graziani, G. Experimental Evidence of the Antitumor, Antimetastatic and Antiangiogenic Activity of Ellagic Acid. Nutrients 2018, 10, 1756. [Google Scholar] [CrossRef]
- Block, K.I.; Koch, A.C.; Mead, M.N.; Tothy, P.K.; Newman, R.A.; Gyllenhaal, C. Impact of antioxidant supplementation on chemotherapeutic toxicity: A systematic review of the evidence from randomized controlled trials. Int. J. Cancer 2008, 123, 1227–1239. [Google Scholar] [CrossRef]
- Nuñez-Sánchez, M.A.; González-Sarrías, A.; García-Villalba, R.; Monedero-Saiz, T.; García-Talavera, N.V.; Gómez-Sánchez, M.B.; Sánchez-Álvarez, C.; García-Albert, A.M.; Rodríguez-Gil, F.J.; Ruiz-Marín, M.; et al. Gene expression changes in colon tissues from colorectal cancer patients following the intake of an ellagitannin-containing pomegranate extract: A randomized clinical trial. J. Nutr. Biochem. 2017, 42, 126–133. [Google Scholar] [CrossRef]
- Nuñez-Sánchez, M.A.; Dávalos, A.; González-Sarrías, A.; Casas-Agustench, P.; Visioli, F.; Monedero-Saiz, T.; García-Talavera, N.V.; Gómez-Sánchez, M.B.; Sánchez-Álvarez, C.; García-Albert, A.M.; et al. MicroRNAs expression in normal and malignant colon tissues as biomarkers of colorectal cancer and in response to pomegranate extracts consumption: Critical issues to discern between modulatory effects and potential artefacts. Mol. Nutr. Food Res. 2015, 59, 1973–1986. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Lu, L.-C.; Tsai, M.-H.; Chen, Y.-J.; Chen, Y.-Y.; Yao, S.-P.; Hsu, C.-P. The inhibitory effect of ellagic Acid on cell growth of ovarian carcinoma cells. Evid. Based Complement. Altern. Med. 2013, 2013, 306705. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zeng, Z.; Wang, S.; Li, T.; Mastriani, E.; Li, Q.-H.; Bao, H.-X.; Zhou, Y.-J.; Wang, X.; Liu, Y.; et al. Main components of pomegranate, ellagic acid and luteolin, inhibit metastasis of ovarian cancer by down-regulating MMP2 and MMP9. Cancer Biol. Ther. 2017, 18, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Engelke, L.H.; Hamacher, A.; Proksch, P.; Kassack, M.U. Ellagic Acid and Resveratrol Prevent the Development of Cisplatin Resistance in the Epithelial Ovarian Cancer Cell Line A2780. J. Cancer 2016, 7, 353–363. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, H.S.; Cho, S.-G.; Shin, Y.C.; Ko, S.-G. Rubus coreanus Miquel extract causes apoptosis of doxorubicin-resistant NCI/ADR-RES ovarian cancer cells via JNK phosphorylation. Mol. Med. Rep. 2016, 13, 4065–4072. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, F.G.; Alshehri, M.A.; Shati, A.A.; Al-Kahtani, M.A.; Alsheri, A.S.; Massoud, E.E.; El-Kott, A.F.; El-Mekkawy, H.I.; Al-Ramlawy, A.M.; Abdraboh, M.E. The anti-tumourigenic effect of ellagic acid in SKOV-3 ovarian cancer cells entails activation of autophagy mediated by inhibiting Akt and activating AMPK. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1611–1621. [Google Scholar] [CrossRef]
- Buskaran, K.; Bullo, S.; Hussein, M.Z.; Masarudin, M.J.; Mohd Moklas, M.A.; Fakurazi, S. Anticancer Molecular Mechanism of Protocatechuic Acid Loaded on Folate Coated Functionalized Graphene Oxide Nanocomposite Delivery System in Human Hepatocellular Carcinoma. Materials 2021, 14, 817. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Zhang, S.; Gai, Z.; Gui, T.; Chen, J.; Chen, Q.; Li, Y. Antioxidant Effects of Protocatechuic Acid and Protocatechuic Aldehyde: Old Wine in a New Bottle. Evid. Based Complement. Altern. Med. 2021, 2021, 6139308. [Google Scholar] [CrossRef]
- Okpara, E.S.; Adedara, I.A.; Guo, X.; Klos, M.L.; Farombi, E.O.; Han, S. Molecular mechanisms associated with the chemoprotective role of protocatechuic acid and its potential benefits in the amelioration of doxorubicin-induced cardiotoxicity: A review. Toxicol. Rep. 2022, 9, 1713–1724. [Google Scholar] [CrossRef]
- Stanga, S.; Caretto, A.; Boido, M.; Vercelli, A. Mitochondrial Dysfunctions: A Red Thread across Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 3719. [Google Scholar] [CrossRef]
- Peiffer, D.S.; Zimmerman, N.P.; Wang, L.-S.; Ransom, B.W.S.; Carmella, S.G.; Kuo, C.-T.; Siddiqui, J.; Chen, J.-H.; Oshima, K.; Huang, Y.-W.; et al. Chemoprevention of esophageal cancer with black raspberries, their component anthocyanins, and a major anthocyanin metabolite, protocatechuic acid. Cancer Prev. Res. 2014, 7, 574–584. [Google Scholar] [CrossRef]
- Guttenplan, J.B.; Chen, K.-M.; Sun, Y.-W.; Kosinska, W.; Zhou, Y.; Kim, S.A.; Sung, Y.; Gowda, K.; Amin, S.; Stoner, G.D.; et al. Effects of Black Raspberry Extract and Protocatechuic Acid on Carcinogen-DNA Adducts and Mutagenesis, and Oxidative Stress in Rat and Human Oral Cells. Cancer Prev. Res. 2016, 9, 704–712. [Google Scholar] [CrossRef]
- Xie, Z.; Guo, Z.; Wang, Y.; Lei, J.; Yu, J. Protocatechuic acid inhibits the growth of ovarian cancer cells by inducing apoptosis and autophagy. Phytother. Res. 2018, 32, 2256–2263. [Google Scholar] [CrossRef]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological properties of protocatechuic Acid and its potential roles as complementary medicine. Evid. Based Complement. Altern. Med. 2015, 2015, 593902. [Google Scholar] [CrossRef]
- Crespo, I.; San-Miguel, B.; Mauriz, J.L.; Ortiz de Urbina, J.J.; Almar, M.; Tuñón, M.J.; González-Gallego, J. Protective Effect of Protocatechuic Acid on TNBS-Induced Colitis in Mice Is Associated with Modulation of the SphK/S1P Signaling Pathway. Nutrients 2017, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-N.; Xie, L.-Z.; Shen, Y.; Li, J.; Guo, Y.; Fu, Y.; Liu, F.-Y.; Han, F.-J. Insights into the Role of Oxidative Stress in Ovarian Cancer. Oxidative Med. Cell. Longev. 2021, 2021, 8388258. [Google Scholar] [CrossRef] [PubMed]
- Tesaro, I. A Phase 1B/2 Multicohort Umbrella Study to Evaluate the Safety and Efficacy of Novel Treatments and/or Combinations of Treatments in Participants with Ovarian Cancer (OPAL); NCT03574779. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03574779/ (accessed on 9 December 2022).
- Drisko, J. Antioxidant Effects on the Outcome of Ovarian Cancer; NCT00228319. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT00228319 (accessed on 16 June 2018).
- Akim, A.M.; Ling, L.C.; Rahmat, A.; Zakaria, Z.A. Antioxidant and anti-proliferative activities of Roselle juice on Caov-3, MCF-7, MDA-MB-231 and HeLa cancer cell lines. AJPP Afr. J. Pharm. Pharmacol. 2011, 5, 957–965. [Google Scholar]
- Gouveia, B.B.; Barberino, R.d.S.; Dos Santos Silva, R.L.; Lins, T.L.B.G.; da Silva Guimarães, V.; do Monte, A.P.O.; Palheta, R.C.; de Matos, M.H.T. Involvement of PTEN and FOXO3a Proteins in the Protective Activity of Protocatechuic Acid Against Cisplatin-Induced Ovarian Toxicity in Mice. Reprod. Sci. 2021, 28, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.C.; Panchal, S.S.; Allam, A.A.; Othman, S.I.; Satia, M.; Mandhane, S.N. Syringic Acid Ameliorates Cardiac, Hepatic, Renal and Neuronal Damage Induced by Chronic Hyperglycaemia in Wistar Rats: A Behavioural, Biochemical and Histological Analysis. Molecules 2022, 27, 6722. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Xiao, J. Protective effects of syringic acid on inflammation, apoptosis and intestinal barrier function in Caco-2 cells following oxygen-glucose deprivation/reoxygenation-induced injury. Exp. Ther. Med. 2022, 23, 66. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Srivastava, A.; Srivastava, S.; Rawat, A.K.S.; Khan, A.R. HPTLC-densitometric determination and kinetic studies on antioxidant potential of monomeric phenolic acids (MPAs) from Bergenia species. RSC Adv. 2014, 4, 52647–52657. [Google Scholar] [CrossRef]
- Cikman, O.; Soylemez, O.; Ozkan, O.F.; Kiraz, H.A.; Sayar, I.; Ademoglu, S.; Taysi, S.; Karaayvaz, M. Antioxidant Activity of Syringic Acid Prevents Oxidative Stress in l-arginine-Induced Acute Pancreatitis: An Experimental Study on Rats. Int. Surg. 2015, 100, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.J.; Lee, J.; Park, J.; Kim, Y.H.; Lee, N.H.; Kim, Y.E.; Song, K.-M.; Chang, P.-S.; Jeong, C.-H.; Jung, S.K. Syringic acid prevents skin carcinogenesis via regulation of NoX and EGFR signaling. Biochem. Pharmacol. 2018, 154, 435–445. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Sitarek, P.; Skała, E.; Toma, M.; Wielanek, M.; Pytel, D.; Wieczfińska, J.; Szemraj, J.; Śliwiński, T. Induction of apoptosis by in vitro and in vivo plant extracts derived from Menyanthes trifoliata L. in human cancer cells. Cytotechnology 2019, 71, 165–180. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Amici, A.; Quiles, J.L.; Battino, M. The inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cell growth. Part 1: The suppression of cell proliferation, promotion of apoptosis and arrest of the cell cycle. Food Funct. 2018, 9, 2145–2157. [Google Scholar] [CrossRef]
- Gheena, S.; Ezhilarasan, D. Syringic acid triggers reactive oxygen species-mediated cytotoxicity in HepG2 cells. Hum. Exp. Toxicol. 2019, 38, 694–702. [Google Scholar] [CrossRef]
- Yang, L.; Qu, C.; Jin, J.; Yang, H.; Pei, L. Syringic acid regulates suppression of the STAT3/JNK/AKT pathway via inhibition of human ovarian teratoma cancer cell (PA-1) growth—In vitro study. J. Biochem. Mol. Toxicol. 2021, 35, e22776. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Chen, L.; Li, Y.; Zhao, S.; Liang, Y. Chemoprotective Effect of Syringic Acid on Cyclophosphamide Induced Ovarian Damage via Inflammatory Pathway. J. Oleo Sci. 2021, 70, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Gulati, M.; Singh, S.K.; Kuppusamy, G.; Kapoor, B.; Mishra, V.; Gupta, S.; Arshad, M.F.; Porwal, O.; Jha, N.K.; et al. Discovering multifaceted role of vanillic acid beyond flavours: Nutraceutical and therapeutic potential. Trends Food Sci. Technol. 2022, 122, 187–200. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.S.; Victorelli, F.D.; Fonseca-Santos, B.; Chorilli, M. A Review of Analytical Methods for p-Coumaric Acid in Plant-Based Products, Beverages, and Biological Matrices. Crit. Rev. Anal. Chem. 2019, 49, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Singh Tuli, H.; Kumar, A.; Ramniwas, S.; Coudhary, R.; Aggarwal, D.; Kumar, M.; Sharma, U.; Chaturvedi Parashar, N.; Haque, S.; Sak, K. Ferulic Acid: A Natural Phenol That Inhibits Neoplastic Events through Modulation of Oncogenic Signaling. Molecules 2022, 27, 7653. [Google Scholar] [CrossRef]
- Pandi, A.; Kalappan, V.M. Mechanisms involved in the anticancer effects of sinapic acid. Bull. Natl. Res. Cent. 2022, 46, 259. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Nam, S.-Y.; Kim, H.-Y.; Jin, M.H.; Kim, M.H.; Roh, S.S.; Kim, H.-M. Anti-allergic inflammatory effect of vanillic acid through regulating thymic stromal lymphopoietin secretion from activated mast cells. Nat. Prod. Res. 2018, 32, 2945–2949. [Google Scholar] [CrossRef]
- Park, J.; Cho, S.Y.; Kang, J.; Park, W.Y.; Lee, S.; Jung, Y.; Kang, M.-W.; Kwak, H.J.; Um, J.-Y. Vanillic Acid Improves Comorbidity of Cancer and Obesity through STAT3 Regulation in High-Fat-Diet-Induced Obese and B16BL6 Melanoma-Injected Mice. Biomolecules 2020, 10, 1098. [Google Scholar] [CrossRef]
- Ingole, A.; Kadam, M.P.; Dalu, A.P.; Kute, S.M.; Mange, P.R.; Theng, V.D.; Lahane, O.R.; Nikas, A.P.; Kawal, Y.V.; Nagrik, S.U.; et al. A Review of the Pharmacological Characteristics of Vanillic Acid. J. Drug Deliv. Ther. 2021, 11, 200–204. [Google Scholar] [CrossRef]
- Gong, J.; Zhou, S.; Yang, S. Vanillic Acid Suppresses HIF-1α Expression via Inhibition of mTOR/p70S6K/4E-BP1 and Raf/MEK/ERK Pathways in Human Colon Cancer HCT116 Cells. Int. J. Mol. Sci. 2019, 20, 465. [Google Scholar] [CrossRef]
- Bhavani, P.; Subramanian, P.; Kanimozhi, S. Preventive Efficacy of Vanillic Acid on Regulation of Redox Homeostasis, Matrix Metalloproteinases and Cyclin D1 in Rats Bearing Endometrial Carcinoma. Indian J. Clin. Biochem. 2017, 32, 429–436. [Google Scholar] [CrossRef]
- Sitarek, P.; Skała, E.; Toma, M.; Wielanek, M.; Szemraj, J.; Skorski, T.; Białas, A.J.; Sakowicz, T.; Kowalczyk, T.; Radek, M.; et al. Transformed Root Extract of Leonurus sibiricus Induces Apoptosis through Intrinsic and Extrinsic Pathways in Various Grades of Human Glioma Cells. Pathol. Oncol. Res. 2017, 23, 679–687. [Google Scholar] [CrossRef]
- Kumar, P.P.B.S.; Ammani, K.; Mahammad, A.; Gosala, J. Vanillic acid induces oxidative stress and apoptosis in non-small lung cancer cell line. Int. J. Recent Sci. Res. 2013, 3, 1077–1083. [Google Scholar]
- Yuan, J.; Lan, H.; Jiang, X.; Zeng, D.; Xiao, S. Bcl-2 family: Novel insight into individualized therapy for ovarian cancer (Review). Int. J. Mol. Med. 2020, 46, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Si, L.; Jia, Y.; Jian, W.; Yu, Q.; Wang, M.; Lin, R. Kaempferol exerts anti-proliferative effects on human ovarian cancer cells by inducing apoptosis, G0/G1 cell cycle arrest and modulation of MEK/ERK and STAT3 pathways. J. Balk. Union Oncol. 2019, 24, 975–981. [Google Scholar]
- Maity, S.; Kinra, M.; Nampoothiri, M.; Arora, D.; Pai, K.S.R.; Mudgal, J. Caffeic acid, a dietary polyphenol, as a promising candidate for combination therapy. Chem. Pap. 2022, 76, 1271–1283. [Google Scholar] [CrossRef]
- Jabir, N.R.; Islam, M.T.; Tabrez, S.; Shakil, S.; Zaidi, S.K.; Khan, F.R.; Araújo, L.d.S.; de Meneses, A.-A.P.M.; Santos, J.V.d.O.; Melo-Cavalcante, A.A.d.C. An insight towards anticancer potential of major coffee constituents. Biofactors 2018, 44, 315–326. [Google Scholar] [CrossRef]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef]
- Silva, H.; Lopes, N.M.F. Cardiovascular Effects of Caffeic Acid and Its Derivatives: A Comprehensive Review. Front. Physiol. 2020, 11, 595516. [Google Scholar] [CrossRef]
- Genaro-Mattos, T.C.; Maurício, Â.Q.; Rettori, D.; Alonso, A.; Hermes-Lima, M. Antioxidant Activity of Caffeic Acid against Iron-Induced Free Radical Generation--A Chemical Approach. PLoS ONE 2015, 10, e0129963. [Google Scholar] [CrossRef]
- Damasceno, S.S.; Dantas, B.B.; Ribeiro-Filho, J.; Antônio M Araújo, D.; Galberto M da Costa, J. Chemical Properties of Caffeic and Ferulic Acids in Biological System: Implications in Cancer Therapy. A Review. Curr. Pharm. Des. 2017, 23, 3015–3023. [Google Scholar] [CrossRef] [PubMed]
- Sidoryk, K.; Jaromin, A.; Filipczak, N.; Cmoch, P.; Cybulski, M. Synthesis and Antioxidant Activity of Caffeic Acid Derivatives. Molecules 2018, 23, 2199. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yang, F.; Zhang, M.; Lam, C.; Qiao, Y.; Xiao, J.; Zhang, D.; Ge, Y.; Fu, L.; Xie, D. Antiproliferative activity and SARs of caffeic acid esters with mono-substituted phenylethanols moiety. Bioorganic Med. Chem. Lett. 2017, 27, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.-N.; Wang, C.C.N.; Liao, W.-C.; Lan, Y.-H.; Hung, C.-C. Caffeic Acid Attenuates Multi-Drug Resistance in Cancer Cells by Inhibiting Efflux Function of Human P-Glycoprotein. Molecules 2020, 25, 247. [Google Scholar] [CrossRef]
- Alam, M.; Ashraf, G.M.; Sheikh, K.; Khan, A.; Ali, S.; Ansari, M.M.; Adnan, M.; Pasupuleti, V.R.; Hassan, M.I. Potential Therapeutic Implications of Caffeic Acid in Cancer Signaling: Past, Present, and Future. Front. Pharmacol. 2022, 13, 845871. [Google Scholar] [CrossRef] [PubMed]
- Birková, A.; Hubková, B.; Bolerázska, B.; Mareková, M.; Čižmárová, B. Caffeic acid: A brief overview of its presence, metabolism, and bioactivity. Bioact. Compd. Health Dis. 2020, 3, 74–81. [Google Scholar] [CrossRef]
- Yang, W.S.; Jeong, D.; Yi, Y.-S.; Park, J.G.; Seo, H.; Moh, S.H.; Hong, S.; Cho, J.Y. IRAK1/4-targeted anti-inflammatory action of caffeic acid. Mediat. Inflamm. 2013, 2013, 518183. [Google Scholar] [CrossRef]
- Sun, L.-L.; Holowatyj, A.; Xu, X.-E.; Wu, J.-Y.; Wu, Z.-Y.; Shen, J.-H.; Wang, S.-H.; Li, E.-M.; Yang, Z.-Q.; Xu, L.-Y. Histone demethylase GASC1, a potential prognostic and predictive marker in esophageal squamous cell carcinoma. Am. J. Cancer Res. 2013, 3, 509–517. [Google Scholar]
- Jia, R.; Mi, Y.; Yuan, X.; Kong, D.; Li, W.; Li, R.; Wang, B.; Zhu, Y.; Kong, J.; Ma, Z.; et al. GASC1-Adapted Neoadjuvant Chemotherapy for Resectable Esophageal Squamous Cell Carcinoma: A Prospective Clinical Biomarker Trial. J. Oncol. 2020, 2020, 1607860. [Google Scholar] [CrossRef]
- Pelinson, L.P.; Assmann, C.E.; Palma, T.V.; da Cruz, I.B.M.; Pillat, M.M.; Mânica, A.; Stefanello, N.; Weis, G.C.C.; de Oliveira Alves, A.; de Andrade, C.M.; et al. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol. Biol. Rep. 2019, 46, 2085–2092. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, F.; Chen, L.; Yang, Y.; Cao, S.; Ye, Y.; Wang, X.; Mu, J.; Li, Z.; Li, L. Blockage of TGFβ-SMAD2 by demethylation-activated miR-148a is involved in caffeic acid-induced inhibition of cancer stem cell-like properties in vitro and in vivo. FEBS Open Bio 2015, 5, 466–475. [Google Scholar] [CrossRef]
- Sirota, R.; Gibson, D.; Kohen, R. The timing of caffeic acid treatment with cisplatin determines sensitization or resistance of ovarian carcinoma cell lines. Redox Biol. 2017, 11, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Gherman, C.; Braicu, O.L.; Zanoaga, O.; Jurj, A.; Pileczki, V.; Maralani, M.; Drigla, F.; Braicu, C.; Budisan, L.; Achimas-Cadariu, P.; et al. Caffeic acid phenethyl ester activates pro-apoptotic and epithelial–mesenchymal transition-related genes in ovarian cancer cells A2780 and A2780cis. Mol. Cell. Biochem. 2016, 413, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-L.; Han, N.-Z.; Liu, S.-S. Caffeic acid phenethyl ester inhibits the progression of ovarian cancer by regulating NF-?B signaling. Biomed. Pharmacother. 2018, 99, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Kleczka, A.; Kubina, R.; Dzik, R.; Jasik, K.; Stojko, J.; Cholewa, K.; Kabała-Dzik, A. Caffeic Acid Phenethyl Ester (CAPE) Induced Apoptosis in Serous Ovarian Cancer OV7 Cells by Deregulation of BCL2/BAX Genes. Molecules 2020, 25, 3514. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. p-Coumaric Acid as An Active Ingredient in Cosmetics: A Review Focusing on its Antimelanogenic Effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Song, X.; Li, L.; Sun, J.; Jaiswal, Y.; Huang, J.; Liu, C.; Yang, W.; Williams, L.; Zhang, H.; et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 2019, 111, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, S.; Sinha, B.; Choudhury, B.P.; Jha, N.K.; Palit, P.; Kundu, S.; Mandal, S.C.; Kolesarova, A.; Yousef, M.I.; Ruokolainen, J.; et al. Scavenging Properties of Plant-Derived Natural Biomolecule Para-Coumaric Acid in the Prevention of Oxidative Stress-Induced Diseases. Antioxidants 2021, 10, 1205. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J. Anti-Inflammatory Effects of p-coumaric Acid in LPS-Stimulated RAW264.7 Cells: Involvement of NF-κB and MAPKs Pathways. Med. Chem. 2016, 6, 327–330. [Google Scholar] [CrossRef]
- Radziejewska, I.; Supruniuk, K.; Tomczyk, M.; Izdebska, W.; Borzym-Kluczyk, M.; Bielawska, A.; Bielawski, K.; Galicka, A. p-Coumaric acid, Kaempferol, Astragalin and Tiliroside Influence the Expression of Glycoforms in AGS Gastric Cancer Cells. Int. J. Mol. Sci. 2022, 23, 8602. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, G.; Xiao, Q.; Zhou, Y.; Wei, Y.; Gong, Z. Antiangiogenic effects of AA-PMe on HUVECs in vitro and zebrafish in vivo. Oncotargets Ther. 2018, 11, 1871–1884. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.H.; Rajamanickam, V.; Nagarajan, S. Antiproliferative effect of p-Coumaric acid targets UPR activation by downregulating Grp78 in colon cancer. Chem. Biol. Interact. 2018, 291, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Lange, T.S.; Kim, K.K.; Brard, L. A coumarin derivative (RKS262) inhibits cell-cycle progression, causes pro-apoptotic signaling and cytotoxicity in ovarian cancer cells. Investig. New Drugs 2011, 29, 63–72. [Google Scholar] [CrossRef]
- Alrehaili, A.A.; AlMourgi, M.; Gharib, A.F.; Elsawy, W.H.; Ismail, K.A.; Hagag, H.M.; Anjum, F.; Raafat, N. Clinical significance of p27 Kip1 expression in advanced ovarian cancer. Appl. Cancer Res. 2020, 40, 6. [Google Scholar] [CrossRef]
- Ekinci Akdemir, F.N.; Albayrak, M.; Çalik, M.; Bayir, Y.; Gülçin, İ. The Protective Effects of p-Coumaric Acid on Acute Liver and Kidney Damages Induced by Cisplatin. Biomedicines 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Su, J.; Xu, H.; Yu, S.; Liu, Y.; Zhang, Y.; Sun, L.; Yue, Y.; Zhou, X. Dicumarol inhibits PDK1 and targets multiple malignant behaviors of ovarian cancer cells. PLoS ONE 2017, 12, e0179672. [Google Scholar] [CrossRef]
- Karataş, M.O.; Tekin, S.; Alici, B.; Sandal, S. Cytotoxic effects of coumarin substituted benzimidazolium salts against human prostate and ovarian cancer cells. J. Chem. Sci. 2019, 131, 69. [Google Scholar] [CrossRef]
- Ayazoglu Demir, E.; Mentese, A.; Kucuk, H.; Turkmen Alemdar, N.; Demir, S. p-Coumaric acid alleviates cisplatin-induced ovarian toxicity in rats. J. Obstet. Gynaecol. Res. 2022, 48, 411–419. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; Xu, F.; Chu, C.; Li, X.; Shi, X.; Zheng, W.; Wang, Z.; Jia, Y.; Xiao, W. Use of Ferulic Acid in the Management of Diabetes Mellitus and Its Complications. Molecules 2022, 27, 6010. [Google Scholar] [CrossRef]
- Pandi, A.; Raghu, M.H.; Chandrashekar, N.; Kalappan, V.M. Cardioprotective effects of Ferulic acid against various drugs and toxic agents. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 92. [Google Scholar] [CrossRef]
- Raj, N.D.; Singh, D. A critical appraisal on ferulic acid: Biological profile, biopharmaceutical challenges and nano formulations. Health Sci. Rev. 2022, 5, 100063. [Google Scholar] [CrossRef]
- Karimvand, M.N.; Kalantar, H.; Khodayar, M.J. Cytotoxic and Apoptotic Effects of Ferulic Acid on Renal Carcinoma Cell Line (ACHN). Jundishapur J. Nat. Pharm. Prod. 2020, 15, e81969. [Google Scholar] [CrossRef]
- Cai, M.; Hu, Z.; Liu, J.; Gao, J.; Liu, C.; Liu, D.; Tan, M.; Zhang, D.; Lin, B. Beclin 1 expression in ovarian tissues and its effects on ovarian cancer prognosis. Int. J. Mol. Sci. 2014, 15, 5292–5303. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yu, H.; Guo, W.; Kong, Y.; Gu, l.; Li, Q.; Yang, S.; Zhang, Y.; Wang, Y. The anticancer effects of ferulic acid is associated with induction of cell cycle arrest and autophagy in cervical cancer cells. Cancer Cell Int. 2018, 18, 102. [Google Scholar] [CrossRef] [PubMed]
- Pang, G.; Yi, T.; Luo, H.; Jiang, L. Preclinical findings: The pharmacological targets and molecular mechanisms of ferulic acid treatment for COVID-19 and osteosarcoma via targeting autophagy. Front. Endocrinol. 2022, 13, 971687. [Google Scholar] [CrossRef]
- Shin, D.-S.; Kim, K.W.; Chung, H.Y.; Yoon, S.; Moon, J.-O. Effect of sinapic acid against carbon tetrachloride-induced acute hepatic injury in rats. Arch. Pharmacal Res. 2013, 36, 626–633. [Google Scholar] [CrossRef]
- Ansari, M.A. Sinapic acid modulates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Biomed. Pharmacother. 2017, 93, 646–653. [Google Scholar] [CrossRef]
- Lee, H.E.; Kim, D.H.; Park, S.J.; Kim, J.M.; Lee, Y.W.; Jung, J.M.; Lee, C.H.; Hong, J.G.; Liu, X.; Cai, M.; et al. Neuroprotective effect of sinapic acid in a mouse model of amyloid β(1-42) protein-induced Alzheimer’s disease. Pharmacol. Biochem. Behav. 2012, 103, 260–266. [Google Scholar] [CrossRef]
- Hu, X.; Geetha, R.V.; Surapaneni, K.M.; Veeraraghavan, V.P.; Chinnathambi, A.; Alahmadi, T.A.; Manikandan, V.; Manokaran, K. Lung cancer induced by Benzo(A)Pyrene: ChemoProtective effect of sinapic acid in swiss albino mice. Saudi J. Biol. Sci. 2021, 28, 7125–7133. [Google Scholar] [CrossRef]
- Balaji, C.; Muthukumaran, J.; Nalini, N. Effect of sinapic acid on 1,2 dimethylhydrazine induced aberrant crypt foci, biotransforming bacterial enzymes and circulatory oxidative stress status in experimental rat colon carcinogenesis. Bratisl. Med. J. 2015, 116, 560–566. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, H.; Tan, P.; Huang, M.; Shi, H.; Sun, B.; Cheng, Y.; Li, T.; Mou, Z.; Li, Q.; et al. Sinapic acid inhibits pancreatic cancer proliferation, migration, and invasion via downregulation of the AKT/Gsk-3β signal pathway. Drug Dev. Res. 2022, 83, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Zhang, Y.-Y.; Zhu, B.-L.; Feng, F.-Z.; Zhang, H.-T.; Yan, H.; Zhou, B. MiR-203a-3p regulates the biological behaviors of ovarian cancer cells through mediating the Akt/GSK-3β/Snail signaling pathway by targeting ATM. J. Ovarian Res. 2019, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, H.; Li, W.; Wang, Z.; Dong, Z.; Lan, H.; Wang, C.; Song, J.-L. Effects of Sinapic Acid Combined with Cisplatin on the Apoptosis and Autophagy of the Hepatoma Cells HepG2 and SMMC-7721. Evid. Based Complement. Altern. Med. 2021, 2021, e6095963. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Selection and Use of Essential Medicines (2007)—TRS 946; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Chen, M.; Wang, J.; Lu, J.; Bond, M.C.; Ren, X.-R.; Lyerly, H.K.; Barak, L.S.; Chen, W. The anti-helminthic niclosamide inhibits Wnt/Frizzled1 signaling. Biochemistry 2009, 48, 10267–10274. [Google Scholar] [CrossRef]
- Fonseca, B.D.; Diering, G.H.; Bidinosti, M.A.; Dalal, K.; Alain, T.; Balgi, A.D.; Forestieri, R.; Nodwell, M.; Rajadurai, C.V.; Gunaratnam, C.; et al. Structure-activity analysis of niclosamide reveals potential role for cytoplasmic pH in control of mammalian target of rapamycin complex 1 (mTORC1) signaling. J. Biol. Chem. 2012, 287, 17530–17545. [Google Scholar] [CrossRef]
| Phenolic Compounds | Plant Sources | References |
|---|---|---|
| Gallic acid (GA) | Processed beverages—red wine, green tea berry/tea leaves, pomegranate root bark, gallnuts, oak bark | [34] |
| Salicylic acid (SA)/Salicylate derivatives | Berries—blue, boysen, logan, Nuts and dry fruits—apricot, dates, raisins Fruit—orange Beverage—black tea condiments—aniseed, cumin, hot paprika, thyme | [52] |
| Ellagic acid (EA) | Fruit—pomegranate, grape Berries—blackberry, raspberry strawberry, cranberry, and blueberry Nuts and dry fruits—walnuts, chestnuts, almonds | [69] |
| Protocatechuic acid (PCA) | Black rice, green tea, olive oil, honey, Fruits and nuts, plums, gooseberries, grapes, almonds, soybean, star anise, medical rosemary, and cinnamon As a bioactive constituent of medicinal plants, such as Hibiscus sabdariffa, Ginkgo biloba, Hypericum perforatum, Cibotium barometz, Stenoloma chusanum, Ching, and Ilex chinensis Sims | [80,81] |
| Syringic acid (SyA) | Dates, olives, pumpkin, grapes, spices, acai, red wine, palm, honey | [31] |
| Vanillic acid (VA) | Green tea | [105] |
| Caffeic acid (CA) | coffee beans, olives, berries, potatoes, carrots | [106] |
| p-Coumaric acid (p-CA) | Fruits—apples, pears, strawberries Vegetables—tomatoes, carrots, onions, garlic Cereals—maize, wheat | [107] |
| Ferulic acid (FA) | Fruits—pineapple, bananas Vegetables—spinach, beetroot Whole grain—oat | [108] |
| Sinapic acid (SA) | Fruits—oranges, grapefruits, cranberries Herbs—canola, mustard seed, rapeseed | [109] |
| Phenolic Acids | Anticancer Mechanisms | Study Model | Dose/IC50 | References |
|---|---|---|---|---|
| A. Hydroxybenzoic Acids | ||||
| Gallic acid | Increased cytotoxicity and increased inhibition in cellular growth | A2780 cells | >50 µM | [42] |
| Gallic acid | Inhibition of VEGF, increased PTEN expression, downregulation of Akt phosphorylation and HIF-1α expression, and antiangiogenic effects | OVCAR-3 and A2780/CP70 | OVCAR-3 = 66.86% A2780/CP70 = 30.10% at 40 μM | [44] |
| Gallic acid | ROS generation, decreased cell viability, affects cytoskeleton, cell cycle arrest, and induction of apoptosis | SKOV-3 and OVCAR-3 | SKOV-3 = 50 μg/mL OVCAR-3 = 43 μg/mL | [45] |
| Gallic acid | Carbonic anhydrase IX protein, PI3K, and caspase-3-mediated mechanism of action, upregulation of proapoptotic proteins (Bax and Bad), p53 protein activation, and induction of apoptosis | OVCAR-3 and A2780/CP70 | OVCAR-3 = 22.14 μM A2780/CP70 = 33.53 μM | [48] |
| Gallic acid | ROS-mediated inactivation of ERK | A2780 doxorubicin-sensitive and -resistant A2780AD | A2780 = 25% at 50 μM A2780AD = 20% at 100 μM | [49] |
| Salicylic acid | ELK1/SRF, AP-1, YC/MAX, and NF-кB Inhibited proliferation, migration, cell cycle progression, and induction of apoptosis | SKOV3 and HeyA8 | SKOV3 = 65 HeyA8 = 53.4% at 1µM | [59] |
| Salicylic acid | RBPs—FXR1 and IGF2BP2, high expression of RBPs associated with reduced survival of OC patients | ES2, SKOV3, A2780, IP1, OV90, OVCAR3/4/5/8, KURAMOCHI, and OVSAHO cancer cells | 0.54–3.09 μM | [60] |
| Salicylic acid | WNT7A/β-catenin signaling, increased E-cadherin and SLUG levels, inhibited tumor growth and progression | SKOV3.ip1 cells | 0–10 μM | [63] |
| Salicylic acid | MEK1/2-ERK1/2; ROS-dependent JNK signaling, inhibited cell growth and induced apoptosis; reduced mitochondrial respiration as well as aerobic glycolysis | SKOV3 and HO8910 | SKOV3 = 4.82 µM HO8910 = 7.12 µM | [66] |
| Ellagic acid | Inhibited cellular proliferation, induced G1-arrest, elevated p53 and Cip1/p21 levels, decreased cyclin D1 and E levels, induced apoptosis, increased Bax: Bcl-2 ratio, apoptotic induction, and autophagy inhibition | ES-2 and PA-1 | ES-2 = 60% PA-1 = 90% at 25 μM | [74] |
| Ellagic acid | Inhibited tumor growth, inhibit metastasis by downregulating MMP-2 and MMP-9 expression | A2780 | 5, 10, and 15 μg/mL | [75] |
| Ellagic acid | Prevented cisplatin resistance | A2780 | 17.0 µM | [76] |
| Ellagic acid | JNK and Akt phosphorylation and induction of apoptosis | NCI/ADR RES ovarian cancer cells | Plant extract 100 and 200 µg/mL | [77] |
| Ellagic acid | Proliferation suppression and moderate inhibition of VEGF secretion | OVCAR-3 and A2780/CP70 cells | 40 μM | [43] |
| Ellagic acid | Cytotoxic activity, autophagy activation mediated by Akt inhibition and AMPK activation, decreased mTORC1 and p-Akt | SKOV-3 | 36.6 μM | [78] |
| Protocatechuic acid | Antiproliferative effect | Caov-3 | [92] | |
| Protocatechuic acid | Decreased cell viability and capacity to form colonies | OVCAR3, SKOV3, and A2780 cells | [86] | |
| Protocatechuic acid | G2/M phase cell cycle arrest, activated PARP and caspase 3, and upregulated and downregulated Bax and Bcl-2, respectively | OVCAR3 | [31] | |
| Syringic acid | Suppression of STAT3/JNK/Akt pathway, growth inhibition, modified apoptosis-related protein expression level, viz., caspase-3, 8, 9, reduced levels of proinflammatory cytokines, viz., TNF, IL-2, IL-6, and IL-10 | PA-1 | 25 μM/mL | [103] |
| Vanillic acid | G1 phase arrest, inhibited proliferation, suppression of HIF-1α, and inhibition of mTOR/p70S6K/eIF4E-binding protein 1 and Raf/MEK/ERK pathways | HCT-116 | 30 µM | [113] |
| Vanillic acid | Prevent angiogenesis, suppress cellular proliferation through phospho-p70S6K, phospho-mTOR, phospho-4E-BP1, p-eIF4E, phospho-c-Raf, phospho- MEK1/2, and phospho-ERK1/2 signaling pathways | LNCaP and DU145 | ~25 µM | [105] |
| L. sibiricus root extract (Vanillic acid) | Enhanced caspases 3, 8, and 9 mRNA levels and reduced the mRNA levels of Bcl-2 | Glioma cells | 0.1–1.5 mg/mL | [115] |
| B. Hydroxy Cinnamic Acids | ||||
| Caffeic acid | Enhances cisplatin cytotoxicity and increases the amount of platinum bound to nuclear DNA, increases in the apoptotic cascade by increased caspase activity | A2780 and A2780cisR | 5–20 µM | [135] |
| Caffeic acid | Activates proapoptotic and epithelial–mesenchymal transition-related genes in ovarian cancer | A2780 and A2780cis | A2780 = 34.98 µM A2780cis = 58.01 µM | [136] |
| Restrains the progression of ovarian cancer via inactivation of NF-κB signaling, decreased cell viability, migration, and invasion accompanied by an obstructed Ki67 and PCNA expression, nuclear translocation of p65, inhibition of IκB phosphorylation, and NF-κB p65 DNA binding | SKOV-3 | 50 μM | [137] | |
| Caffeic acid | Increased cytotoxicity, decreased lysosomal activity, and the total synthesis of cellular proteins, induced apoptosis via dysregulation of Bax/Bcl-2 ratio | OV7 serum ovarian cancer cells | XTT-142.58 µM NR-81.43 µM SRB-80.08 µM | [138] |
| p-Coumaric acid | Inhibits cell cycle progression, induces proapoptotic signaling, increased cytotoxicity, downregulation of cyclin D and p21-independent inhibition of CDK-6 | OVCAR-3 | 75% cytotoxicity with 5 µM | [146] |
| p-Coumaric acid | Increased cytotoxicity | A2780 | ~10 µM | [150] |
| Ferulic acid | Increased apoptosis index, decreased Bcl-2 expression and higher Bax expression | ACHN | 39.5 μM | [155] |
| Ferulic acid | Reduced MMP-9 mRNA expression and autophagy-related protein Beclin1 levels | Hela and Caski | Hela = 88.3% Caski = 85.4% with 2.0 mM | [157] |
| Sinapic acid | Increased cytotoxicity, apoptotic activity via elevation of ROS and caspases activity (caspase-3 and caspase-9) | A549 | 50 µM | [162] |
| Sinapic acid | Downregulation of Akt/Gsk-3β signal pathway | Pancreatic cancer cells | [164] | |
| Sinapic acid | Inhibits cancer cell proliferation and migration and induces apoptosis | HepG2 | 1795 μM | [166] |
| Phenolic Acids | Anticancer Mechanisms | Study Model | Dose/IC50 | References |
|---|---|---|---|---|
| A. Hydroxybenzoic Acids | ||||
| Gallic acid | ATM/Chk2/p53 activation, COX-2/NF-kB, GSH inhibition and inhibition of tumor lesions development | Mice | 50 mg/kg of body weight | [45] |
| Gallic acid | Reduced MDA production and oxidative stress (SOD, CAT, GSH-Px, TAOC activity) and increased serum antioxidant enzymes activity | Rats | Plant extract 150 mg/kg body weigh | [50] |
| Salicylic acid | Wnt/β-catenin, increased cytotoxicity in combination with carboplatin | 34 patients’ ascites with primary ovarian cancer | [62] | |
| Salicylic acid | Wnt, mTOR, and STAT3, antiproliferative, cell cycle arrest, induced apoptosis, and platinum resistance reversal | Tumor spheres from ascites of all OC patients who were suspected to have ovarian cancer and scheduled to undergo surgery and cells from a chemo-resistant, patient-derived xenograft | 0.1 to 5 µM | [64] |
| Salicylic acid | Decreased expression of p-MSK1, p-MEK1/2, and p-ERK1/2, and suppressed tumor growth | Xenograft tumor model | 20 mg/kg body weight | [66] |
| Ellagic acid | Inhibited tumor growth, inhibited metastasis by downregulating MMP-2 and MMP-9 expression | Nude mice | 50 mg/kg body weight | [75] |
| Salicylic acid | Decreased expression of p-MSK1, p-MEK1/2, and p-ERK1/2, and suppressed tumor growth | Xenograft tumor model | [66] | |
| Salicylic acid | WNT7A/β-catenin signaling, increased E-cadherin and SLUG levels, inhibited tumor growth and progression | SKOV3.ip1 cells and xenograft mouse model | 200 mg/kg body weight | [63] |
| Protocatechuic acid | Induced apoptosis, maintained cell proliferation and mitochondrial function, reduced ROS production, and increased GSH expression through PTEN and FOXO3a proteins | Mice | 20 and 50 mg/kg body weight | [93] |
| Syringic acid | Chemoprotective action, decreased nitric oxide, myeloperoxidase, catalase, glutathione, glutathione peroxidase, superoxide dismutase, and malondialdehyde levels in both serum and ovarian tissue. Suppressed luteinizing hormones, antimullerian hormone, estradiol, follicle-stimulating hormone, and ovarian follicles. downregulated cytokines, inflammatory mediators, and caspase-3 | Swiss albino Wistarrats | 5–20 mg/kg body weight | [104] |
| B. Hydroxy Cinnamic Acids | ||||
| p-Coumaric acid | Prevent cisplatin-induced hepatotoxicity and nephrotoxicity | Wistar rats | 100 mg/kg body weight | [148] |
| p-Coumaric acid | Inhibit oxidative stress, inflammation, and apoptosis | Rat | 4 mg/kg | [151] |
| Sinapic acid | Improve oxidative burden and other abnormalities | Rats | 80 mg/kg body weight | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazam, N.; Jabir, N.R.; Ahmad, I.; Alharthy, S.A.; Khan, M.S.; Ayub, R.; Tabrez, S. Phenolic Acids-Mediated Regulation of Molecular Targets in Ovarian Cancer: Current Understanding and Future Perspectives. Pharmaceuticals 2023, 16, 274. https://doi.org/10.3390/ph16020274
Nazam N, Jabir NR, Ahmad I, Alharthy SA, Khan MS, Ayub R, Tabrez S. Phenolic Acids-Mediated Regulation of Molecular Targets in Ovarian Cancer: Current Understanding and Future Perspectives. Pharmaceuticals. 2023; 16(2):274. https://doi.org/10.3390/ph16020274
Chicago/Turabian StyleNazam, Nazia, Nasimudeen R. Jabir, Iftikhar Ahmad, Saif A. Alharthy, Mohd Shahnawaz Khan, Rashid Ayub, and Shams Tabrez. 2023. "Phenolic Acids-Mediated Regulation of Molecular Targets in Ovarian Cancer: Current Understanding and Future Perspectives" Pharmaceuticals 16, no. 2: 274. https://doi.org/10.3390/ph16020274
APA StyleNazam, N., Jabir, N. R., Ahmad, I., Alharthy, S. A., Khan, M. S., Ayub, R., & Tabrez, S. (2023). Phenolic Acids-Mediated Regulation of Molecular Targets in Ovarian Cancer: Current Understanding and Future Perspectives. Pharmaceuticals, 16(2), 274. https://doi.org/10.3390/ph16020274







