Ionic Liquids as Tools to Incorporate Pharmaceutical Ingredients into Biopolymer-Based Drug Delivery Systems
Abstract
:1. Introduction
2. Drug Delivery Systems Using Biopolymers as Delivery Matrices and ILs as Formulation Components
2.1. Incorporation of API-ILs into Biopolymeric Materials
2.2. ILs as Preparation Media
2.3. System Based on Biopolymeric Matrices Doped with IL as Adsorption/Desorption Matrices for APIs
2.4. Systems Based on Anchoring ILs onto Polymeric Surfaces as Adsorption/Desorption Matrices for Ionic APIs
3. Conclusions
- -
- ILs can act as solubilizers and stabilizers of drugs;
- -
- Adding ILs into the DDS affects the drug release rate as they participate in both IL-drug and IL-matrix interactions when properly designed. This property of the ILs could be used for slowing drug delivery from biopolymeric matrices;
- -
- Incorporation of the ILs into biopolymeric matrices affects the carrier’s conductivity and its sensitivity to electric stimulation. This anticipates the potential use of these DDSs as stimuli-responsive drug delivery systems for iontophoresis;
- -
- ILs can be used for grafting of the polymers followed by NP coating, to produce a smart magnetic nanocarrier DDS for targeted (multi)drug delivery;
- -
- ILs can be grafted onto polymeric surfaces followed by metathesis, substituting a simple (i.e., Cl−) ion with an ionic API. These materials could be employed as adsorption/desorption matrices for ionic APIs;
- -
- ILs can be used for the formation of ILs-anchored chitosan Schiff bases able to form metal complexes with potent bactericidal effects;
- -
- ILs could be employed for the formation of “hybrid gels” containing both the polymer and the ILs, for drug entrapment and release;
- -
- ILs can be used to solubilize the biopolymer and, at the same time, load the drug while the material is formed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef]
- Wasserscheid, K. Ionic liquids—New “solutions” for transition metal catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Sirieix, J.; Ossberger, M.; Betzemeier, B.; Knochel, P. Palladium catalyzed cross-couplings of organozincs in ionic liquids. Synlett 2000, 11, 1613–1615. [Google Scholar]
- Wasserscheid, P.; Waffenschmidt, H. Ionic liquids in regioselective platinum-catalysed hydroformylation. J. Mol. Catal. A Chem. 2000, 164, 61–67. [Google Scholar] [CrossRef]
- McLachlan, F.; Mathews, C.J.; Smith, P.J.; Welton, T. Palladium-catalyzed Suzuki cross-coupling reactions in ambient temperature ionic liquids: Evidence for the importance of palladium imidazolylidene complexes. Organometallics 2003, 22, 5350–5357. [Google Scholar] [CrossRef]
- Cole, A.C.; Jensen, J.L.; Ntai, I.; Tran, K.L.T.; Weaver, K.J.; Forbes, D.C.; Davis, J.H. Novel Brønsted acidic ionic liquids and their use as dual solvent−catalysts. J. Am. Chem. Soc. 2002, 124, 5962–5963. [Google Scholar] [CrossRef]
- Schulz, P.S.; Müller, N.; Bösmann, A.; Wasserscheid, P. Effective chirality transfer in ionic liquids through ion-pairing effects. Angew. Chem. Int. Ed. 2007, 46, 1293–1295. [Google Scholar] [CrossRef]
- Yokozeki, A.; Shiflett, M.B. Separation of Carbon dioxide and sulfur dioxide gases using room-temperature ionic liquid [Hmim][Tf2N]. Energy Fuels 2009, 23, 4701–4708. [Google Scholar] [CrossRef]
- Hough, W.L.; Smiglak, M.; Rodríguez, H.; Swatloski, R.P.; Spear, S.K.; Daly, D.T.; Pernak, J.; Grisel, J.E.; Carliss, R.D.; Soutullo, M.D.; et al. The third evolution of ionic liquids: Active pharmaceutical ingredients. New J. Chem. 2007, 31, 1429–1436. [Google Scholar] [CrossRef]
- Rogers, R.D.; Daly, D.T.; Swatloski, R.P.; Hough-Troutman, W.L.; Troutman, J.J.H.L.; Marcin, S.; Juliusz, P.; Spear, S.K. Multifunctional Ionic Liquid Compositions for Overcoming Polymorphism and Imparting Improved Properties for Active Pharmaceutical, Biological, Nutritional and Energetic Ingredients. WO2007044693, 19 April 2007. [Google Scholar]
- Ferraz, R.; Branco, L.C.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž. Ionic liquids as active pharmaceutical ingredients. ChemMedChem 2011, 6, 975–985. [Google Scholar] [CrossRef]
- Uddin, M.N.; Basak, D.; Hopefl, R.; Minofar, B. Potential application of ionic liquids in pharmaceutical dosage forms for small molecule drug and vaccine delivery system. J. Pharm. Pharm. Sci. 2020, 23, 158–176. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.; Pereiro, A.B.; Gaspar, M.M.; Reis, C.P. Recent advances in ionic liquids and nanotechnology for drug delivery. Nanomedicine 2021, 16, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Hough, W.L.; Rogers, R.D. Ionic liquids then and now: From solvents to materials to active pharmaceutical ingredients. Bull. Chem. Soc. Jpn. 2007, 80, 2262–2269. [Google Scholar] [CrossRef]
- Shamshina, J.; Kelley, S.; Gurau, G.; Rogers, R.D. Chemistry: Develop ionic liquid drugs. Nature 2015, 528, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J. Polymorphism in Molecular Crystals, IUCR Monographs on Crystallography 14; Oxford Science Publications: Oxford, UK, 2002. [Google Scholar]
- Schuster, D.; Laggner, C.; Langer, T. Why drugs fail—A study on side effects in new chemical entities. Curr. Pharm. Des. 2005, 11, 3545–3559. [Google Scholar] [CrossRef] [PubMed]
- Wojnarowska, Z.; Paluch, M.; Grzybowski, A.; Adrjanowicz, K.; Grzybowska, K.; Kaminski, K.; Wlodarczyk, P.; Pionteck, J. Study of molecular dynamics of pharmaceutically important protic ionic liquid-verapamil hydrochloride. I. Test of thermodynamic scaling. J. Chem. Phys. 2009, 131, 104505. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Morris, K.R. Polymorphism in pharmaceutical solids. In Drugs and the Pharmaceutical Sciences; Brittain, H.G., Ed.; Informa Healthcare USA, Inc.: New York, NY, USA, 1999. [Google Scholar]
- Sarbajna, R.M.; Preetam, A.; Sivalakshmi, A.; Devi, A.S.; Suryanarayana, M.V.; Sethi, M.; Dutta, D. Studies on crystal modifications of Ganciclovir. Mol. Cryst. Liq. Cryst. 2011, 537, 141–154. [Google Scholar] [CrossRef]
- Roque-Flores, R.L.; Guzei, I.A.; Matos, J.D.R.; Yu, L. Polymorphs of the antiviral drug ganciclovir. Acta Cryst. 2017, C73, 1116–1120. [Google Scholar] [CrossRef]
- Bauer, J.; Spanton, S.; Henry, R.; Quick, J.; Dziki, W.; Porter, W.; Morris, J. Ritonavir: An extraordinary example of conformational polymorphism. Pharm. Res. 2001, 18, 859–866. [Google Scholar] [CrossRef]
- Morissette, S.L.; Soukasene, S.; Levinson, D.; Cima, M.J.; Almarsson, O. Elucidation of crystal form diversity of the HIV protease inhibitor ritonavir by high-throughput crystallization. Proc. Natl. Acad. Sci. USA 2003, 100, 2180–2184. [Google Scholar] [CrossRef] [PubMed]
- Frizzo, C.P.; Gindri, I.M.; Tier, A.Z.; Buriol, L.; Moreira, D.N.; Martins, M.A.P. Pharmaceutical Salts: Solids to Liquids by Using Ionic Liquid Design. In Ionic Liquids: New Aspects for the Future; InTech: London, UK, 2013; pp. 557–579. [Google Scholar]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Curreri, A.M.; Mitragotri, S.; Tanner, E.E.L.; Curreri, A.M.; Mitragotri, S.; Tanner, E.E.L. Recent advances in ionic liquids in biomedicine. Adv. Sci. 2021, 8, e2004819. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, B.; Shi, W.; Huang, W.; Qian, H. Ionic liquids for enhanced drug delivery: Recent progress and prevailing challenges. Mol. Pharmaceutics 2022, 19, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Julio, A.; Costa Lima, S.A.; Reis, S.; Santos de Almeida, T.; Fonte, P. Development of ionic liquid-polymer nanoparticle hybrid systems for delivery of poorly soluble drugs. J. Drug Delivery Sci. Technol. 2020, 56, 100915–100921. [Google Scholar] [CrossRef]
- Gamboa, A.; Schüßler, N.; Soto-Bustamante, E.; Romero-Hasler, P.; Meinel, L.; Morales, J.O. Delivery of ionizable hydrophilic drugs based on pharmaceutical formulation of ion pairs and ionic liquids. Eur. J. Pharm. Biopharm. 2020, 156, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Md Moshikur, R.; Chowdhury, M.R.; Moniruzzaman, M.; Goto, M. Biocompatible ionic liquids and their applications in pharmaceutics. Green Chem. 2020, 22, 8116–8139. [Google Scholar] [CrossRef]
- Chowdhury, M.R.; Moshikur, R.M.; Wakabayashi, R.; Tahara, Y.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Development of a novel ionic liquid-curcumin complex to enhance its solubility, stability, and activity. Chem. Commun. 2019, 55, 7737–7740. [Google Scholar] [CrossRef]
- Dobler, D.; Schmidts, T.; Klingenhöfer, I.; Runkel, F. Ionic liquids as ingredients in topical drug delivery systems. Int. J. Pharm. 2013, 441, 620. [Google Scholar] [CrossRef]
- Li, J.; Shen, L.; Yang, X.; Zhang, Y.; Liu, X.; Zhang, Y.; Xu, W. Novel Acid Response Ionic Liquid Micro Capsule and Its Preparation Method and Application. CN 113750074A, 7 December 2021. [Google Scholar]
- Liang, P.; Yu, J.; Xu, J.; Tan, L.; Ren, X.; Meng, X. Targeted Microwave Controlled Release Drug-Loaded Nanospheres for Treating Liver Cancer and Preparation Method Thereof. CN 107982535A, 4 May 2018. [Google Scholar]
- Cui, X.; Wang, C.; Zhang, D.; Yang, X.; Xiong, Y.; Yang, Y.; Qu, Y. Ionic Liquid Microemulsion and Application Thereof. CN 106420610A, 22 February 2017. [Google Scholar]
- Viau, L.; Tourné-Péteilh, C.; Devoisselle, J.M.; Vioux, A. Ionogels as drug delivery system: One-step sol-gel synthesis using imidazolium ibuprofenate ionic liquid. Chem. Commun. 2010, 46, 228–230. [Google Scholar] [CrossRef]
- Nor, S.B.M.; Woi, P.M.; Ng, S.H. Characterisation of ionic liquids nanoemulsion loaded with piroxicam for drug delivery system. J. Mol. Liq. 2017, 234, 30. [Google Scholar]
- Ziółkowski, B.; Diamond, D. Thermoresponsive poly(ionic liquid) hydrogels. Chem. Commun. 2013, 49, 10308. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, J.R. Drug Release from Matrix Devices. In Recent Advances in Drug Delivery Systems; Anderson, J.M., Kim, S.W., Eds.; Springer: Boston, MA, USA, 1984. [Google Scholar]
- Bica, K.; Rodriguez, H.; Gurau, G.; Cojocaru, O.A.; Riisager, A.; Fehrmann, R.; Rogers, R.D. Pharmaceutically active ionic liquids with solids handling, enhanced thermal stability, and fast release. Chem. Commun. 2012, 48, 5422–5424. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Bergström, C.A.S.; Vinarov, Z.; Kuentz, M.; Brouwers, J.; Augustijns, P.; Brandl, M.; Bernkop-Schnürch, A.; Shrestha, N.; Préat, V.; et al. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur. J. Pharm. Sci. 2019, 137, 104967. [Google Scholar] [CrossRef]
- Klemm, D. Cellulose. In Biopolymers in 10 Volumes; De Baets, S., Vandamme, E., Steinbüchel, A.T., Eds.; Polysaccharides II: Polysaccharides from Eukaryotes; Wiley-VCH: Weinheim, Germany, 2004; Volume 6. [Google Scholar]
- Barikani, M.; Oliaei, E.; Seddiqi, H.; Honarkar, H. Preparation and application of chitin and its derivatives: A review. Iran. Polym. J. 2014, 23, 307–326. [Google Scholar] [CrossRef]
- García, M.C. Drug delivery systems based on nonimmunogenic biopolymers. In Engineering of Biomaterials for Drug Delivery Systems; Parambath, A., Ed.; Woodhead Publishing (Elsevier): Cambridge, UK, 2018; pp. 317–344. [Google Scholar]
- Courtenay, J.C.; Sharma, R.I.; Scott, J.L. Recent advances in modified cellulose for tissue culture applications. Molecules 2018, 23, 654. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose biomaterials for tissue engineering. Front. Bioeng. Biotech. 2019, 7, 45. [Google Scholar] [CrossRef]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. Lond. B 2019, 364, 2127–2139. [Google Scholar] [CrossRef]
- Clark, W.R.; Gao, D. Properties of membranes used for hemodialysis therapy. Semin. Dial. 2002, 15, 191–195. [Google Scholar] [CrossRef]
- Deppisch, R.; Storr, M.; Buck, R.; Göhl, H. Blood material interactions at the surfaces of membranes in medical applications. Sep. Purif. Technol. 1998, 14, 241–254. [Google Scholar] [CrossRef]
- Xu, Z.-K.; Huang, X.-J.; Wan, L.-S. 7: Membranes with Glycosylated Surface. In Surface Engineering of Polymer Membranes. Advanced Topics in Science and Technology in China; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Sindhu, K.A.; Prasanth, P. Medical Applications of Cellulose and Its Derivatives: Present and Future. In Nanocellulose Polymer Nanocomposites: Fundamentals and Applications; Thakur, V.K., Ed.; Wiley: Weinheim, Germany, 2014. [Google Scholar]
- Foster, A.B.; Webber, J.M. Chitin. In Advances in Carbohydrate Chemistry; Melville, L., Wolfrom, R., Tipson, S., Eds.; Academic Press: Oxford, UK, 1961; pp. 371–393. [Google Scholar]
- Mi, F.-L.; Shyu, S.-S.; Lin, Y.-M.; Wu, Y.-B.; Peng, C.-K.; Tsai, Y.-H. Chitin/PLGA blend microspheres as a biodegradable drug delivery system: A new delivery system for protein. Biomaterials 2003, 24, 5023–5036. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.C.A.; Tai, B.C.U. Chitin—A promising biomaterial for tissue engineering and stem cell technology. Biotech. Adv. 2013, 31, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, T.; Majima, T.; Suenaga, N.; Iwasaki, N.; Yamane, S.; Minami, A. Rotator cuff regeneration using chitin fabric as an acellular matrix. J. Shoulder Elb. Surg. 2006, 15, 112–118. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellulose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Dissolution and Processing of Cellulose Using Ionic Liquids. WO 03/029329, 10 April 2003. [Google Scholar]
- Remsing, R.C.; Swatloski, R.P.; Rogers, R.D.; Moyna, G. Mechanism of cellulose dissolution in the ionic liquid1-n-butyl-3-methylimidazolium chloride: A 13C and 35/37Cl NMR relaxation study on model systems. Chem. Commun. 2006, 12, 1271–1273. [Google Scholar] [CrossRef]
- Rabideau, B.D.; Ismail, A.E. Mechanisms of hydrogen bond formation between ionic liquids and cellulose and the influence of water content. Phys. Chem. Chem. Phys. 2015, 17, 5767–5775. [Google Scholar] [CrossRef]
- Kostag, M.; Jedvert, K.; Achtel, C.; Heinze, T.; El-Seoud, O.A. Recent advances in solvents for the dissolution, shaping and derivatization of cellulose: Quaternary ammonium electrolytes and their solutions in water and molecular solvents. Molecules 2018, 22, 511. [Google Scholar] [CrossRef]
- Rahman, M.; Rodriguez, H.; Sun, N.; Swatloski, R.P.; Daly, D.T.; Rogers, R.D. Ionic Liquid Systems for the Processing of Biomass, Their Components and/or Derivatives, and Mixture Thereof. U.S. Patent 8,668,807, 11 March 2014. [Google Scholar]
- Qin, Y.; Lu, X.; Sun, N.; Rogers, R.D. Dissolution or extraction of crustacean shells using ionic liquids to obtain high molecular weight purified chitin and direct production of chitin films and fibers. Green Chem. 2010, 12, 968–971. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Dissolution of Biomass Using Ionic Liquids. In Structures and Interactions of Ionic Liquids; Zhang, S., Wang, J., Lu, X., Zhou, Q., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 79–105. [Google Scholar]
- Wu, Y.; Sasaki, T.; Irie, S.; Sakurai, K. A novel biomass-ionic liquid platform for the utilization of native chitin. Polymer 2008, 49, 2321–2327. [Google Scholar] [CrossRef]
- Turner, M.B.; Spear, S.K.; Holbrey, J.D.; Daly, D.T.; Rogers, R.D. Ionic liquid-reconstituted cellulose composites as solid support matrices for biocatalyst immobilization. Biomacromolecules 2005, 6, 2497–2502. [Google Scholar] [CrossRef]
- Turner, M.B.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Production of bioactive cellulose films reconstituted from ionic liquids. Biomacromolecules 2004, 5, 1379–1384. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Rogers, R.D. Chitin nanobeads. Biopolymeric microbeads as alternatives to synthetic plastics. H PC Today 2018, 13, 9–12. [Google Scholar]
- Shamshina, J.L.; Zavgorodnya, O.; Choudhary, H.; Frye, B.; Newbury, N.; Rogers, R.D. In search of stronger/cheaper chitin nanofibers through electrospinning of chitin–cellulose composites using an ionic liquid platform. ACS Sustain. Chem. Eng. 2018, 6, 14713–14722. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Zavgorodnya, O.; Bonner, J.R.; Gurau, G.; DiNardo, T.; Rogers, R.D. “Practical” electrospinning of biopolymers in ionic liquids. ChemSusChem 2016, 10, 106–111. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Abidi, N. Choosing the right strategy: Cryogrinding vs. ball milling—Comparing apples to apples. Green Chem. 2021, 23, 9646–9657. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Gurau, G.; Block, L.E.; Hansen, L.; Dingee, C.; Walters, A.; Rogers, R.D. Chitin–calcium alginate composite fibers for wound care dressings spun from ionic liquid solution. J. Mater. Chem. B 2014, 2, 3924–3936. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Zavgorodnya, O.; Berton, P.; Chhotaray, P.K.; Choudhary, H.; Rogers, R.D. Ionic liquid platform for spinning composite chitin-poly(lactic acid) fibers. ACS Sustain. Chem. Eng. 2018, 6, 10241–10251. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Cellulose composites with graphene for tissue engineering applications. Materials 2020, 13, 5347. [Google Scholar] [CrossRef] [PubMed]
- Hai, T.A.P.; Sugimoto, R. Fluorescence control of chitin and chitosan fabricated via surface functionalization using direct oxidative polymerization. RSC Adv. 2018, 8, 7005–7013. [Google Scholar]
- Morais, E.S.; Silva, N.H.C.S.; Sintra, T.E.; Santos, S.A.O.; Neves, B.M.; Almeida, I.F.; Costa, P.C.; Correia-Sá, I.; Ventura, S.P.M.; Silvestre, A.J.D.; et al. Anti-inflammatory and antioxidant nanostructured cellulose membranes loaded with phenolic-based ionic liquids for cutaneous application. Carbohydr. Polym. 2019, 206, 187–197. [Google Scholar] [CrossRef]
- Chantereau, G.; Sharma, M.; Abednejad, A.; Neves, B.M.; Sèbe, V.C.; Freire, M.G.; Freire, C.S.R.; Silvestre, A.J.D. Design of nonsteroidal anti-inflammatory drug-based ionic liquids with improved water solubility and drug delivery. ACS Sustain. Chem. Eng. 2019, 7, 14126–14134. [Google Scholar] [CrossRef]
- Sastry, N.V.; Singh, D.K.; Trivedi, P.A. Hybrid hydrogel systems of micelles of drug anion containing ionic liquid and biopolymers: Rheological behavior and drug release. Colloids Surf. A 2018, 555, 668–678. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhang, J.; Feng, H.; Yan, J. Biological-Friendly Slow-Release Nanofiber and Preparation Method Thereof (Mechanical Translation). CN113493941A, 12 October 2021. [Google Scholar]
- Viswanathan, G.; Murugesan, S.; Pushparaj, V.; Nalamasu, O.; Ajayan, P.M.; Linhardt, R.J. Preparation of biopolymer fibers by electrospinning from room temperature ionic liquids. Biomacromolecules 2006, 7, 415–418. [Google Scholar] [CrossRef]
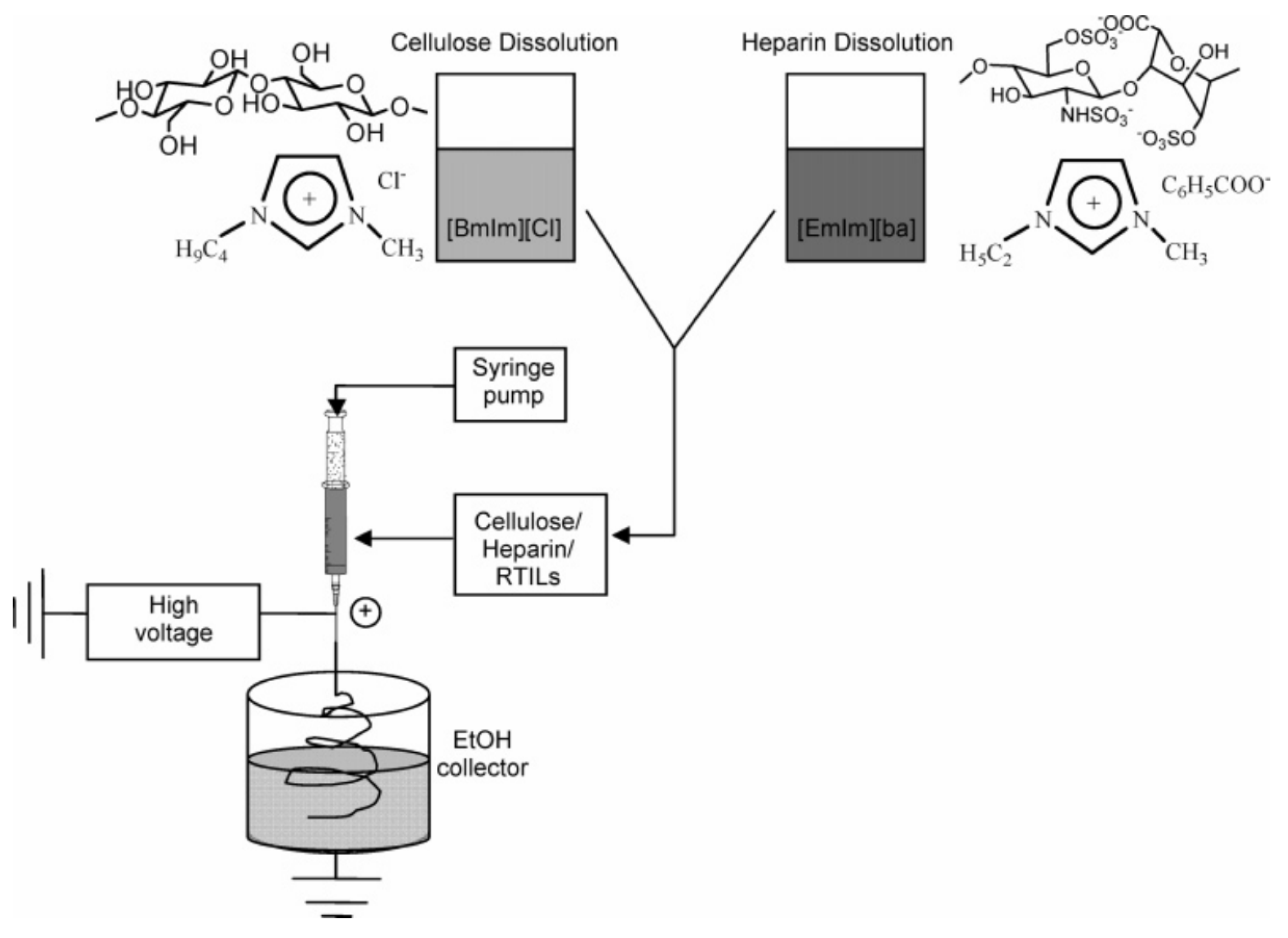
- Park, T.-J.; Lee, S.-H.; Simmons, T.J.; Martin, J.G.; Mousa, S.A.; Snezhkova, E.A.; Sarnatskaya, V.V.; Nikolaev, V.G.; Linhardt, R.J. Heparin–cellulose–charcoal composites for drug detoxification prepared using room temperature ionic liquids. Chem. Commun. 2008, 5022–5024. [Google Scholar] [CrossRef] [PubMed]
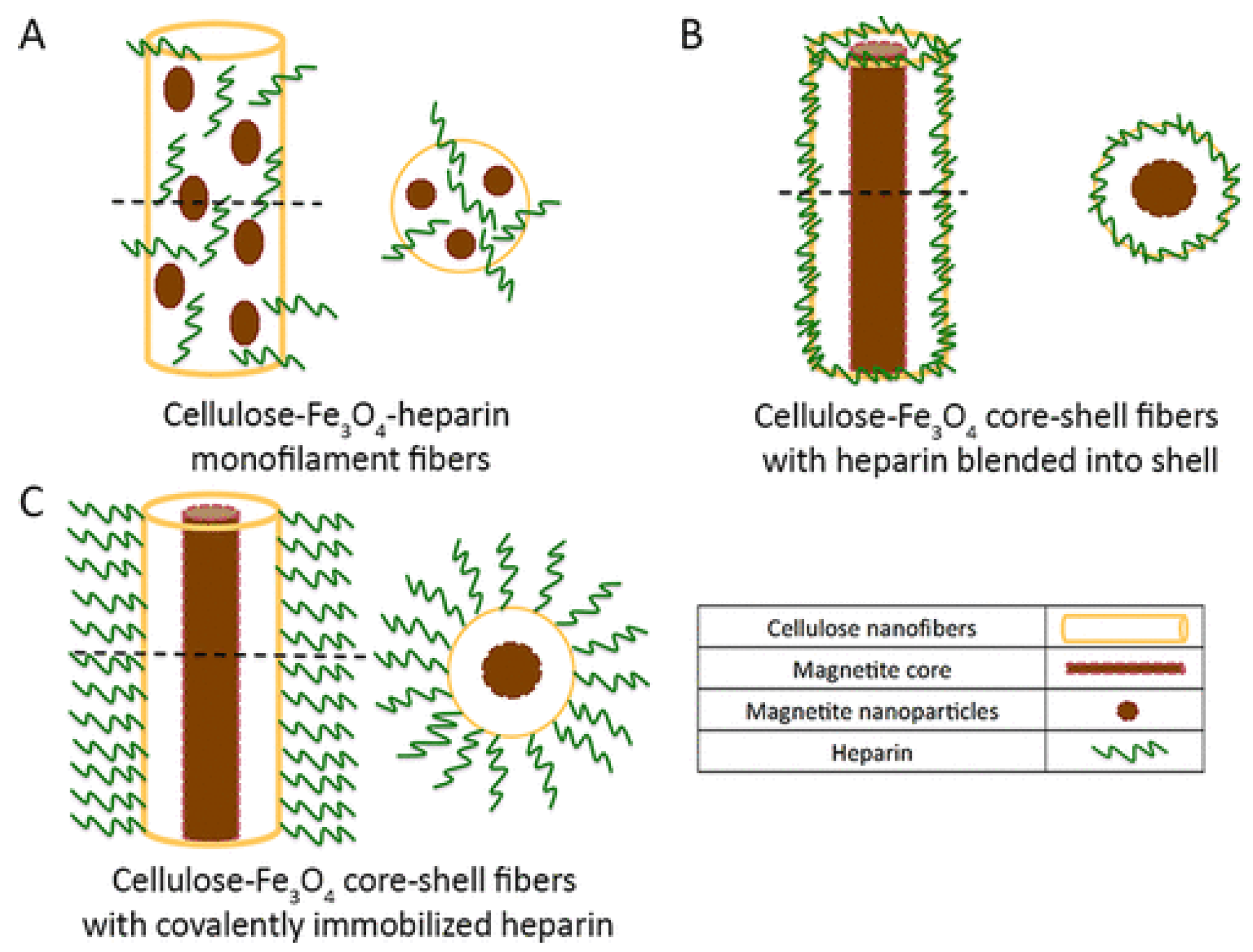
- Hou, L.; Udangawa, W.M.R.N.; Pochiraju, A.; Dong, W.; Zheng, Y.; Linhardt, R.J.; Simmons, T.J. Synthesis of heparin-immobilized, magnetically addressable cellulose nanofibers for biomedical applications. ACS Biomater. Sci. Eng. 2016, 2, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.M.A.; Cortez, A.R.; Barsan, M.M.; Santos, J.B.; Brett, C.M.A.; de Sousa, H.C. Development of greener multi-responsive chitosan biomaterials doped with biocompatible ammonium ionic liquids. ACS Sustain. Chem. Eng. 2013, 1, 1480–1492. [Google Scholar] [CrossRef]
- Safdar, R.; Gnanasundaram, N.; Appusamy, A.; Thanabalan, M. Synthesis, physiochemical properties, colloidal stability evaluation and potential of ionic liquid modified CS-TPP MPs in controlling the release rate of insulin. J. Drug Delivery Sci. Tech. 2021, 64, 102575. [Google Scholar] [CrossRef]
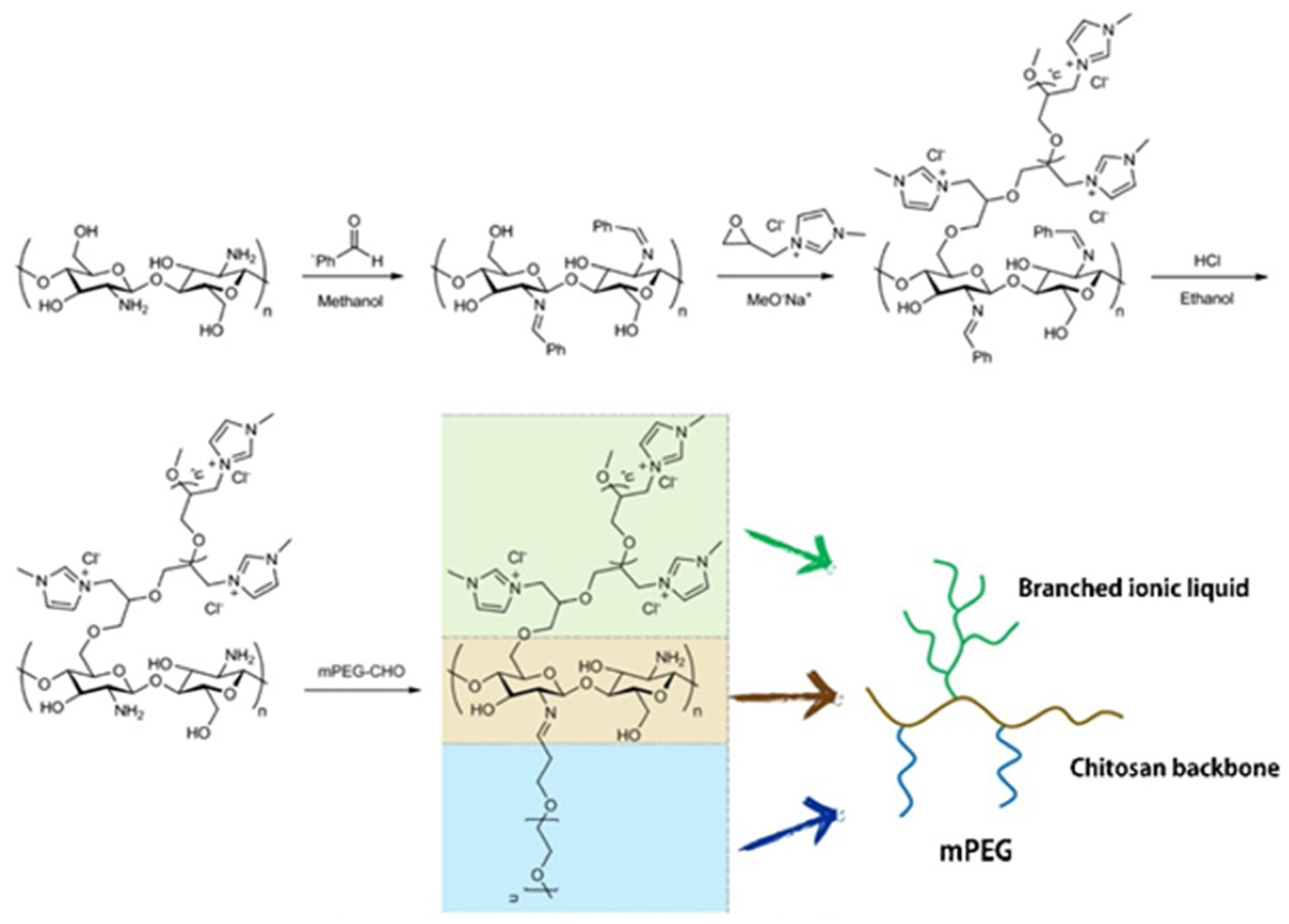
- Rahimi, M.; Shafiei-Irannejad, V.; Safa, K.D.; Salehi, R. Multi-branched ionic liquid-chitosan as a smart and biocompatible nano-vehicle for combination chemotherapy with stealth and targeted properties. Carbohydr. Polym. 2018, 196, 299–312. [Google Scholar] [CrossRef]
- Santos, S.S.O.; Millan, R.D.S.; Speziali, M.G. Versatile grafted microcrystalline cellulose with ionic liquid as new Losartan-controlled release material. Eur. Polym. J. 2020, 124, 109490. [Google Scholar] [CrossRef]
- Santos, S.S.O.; Millan, R.D.S.; Speziali, M.G. Process, for Anchoring Ionic Liquid in Cellulose, Cellulose-Based Products and Use as Adsorption/Desorption Matrix for Anionic Components. BR 102018069690 A2 20200407, 7 April 2020. [Google Scholar]
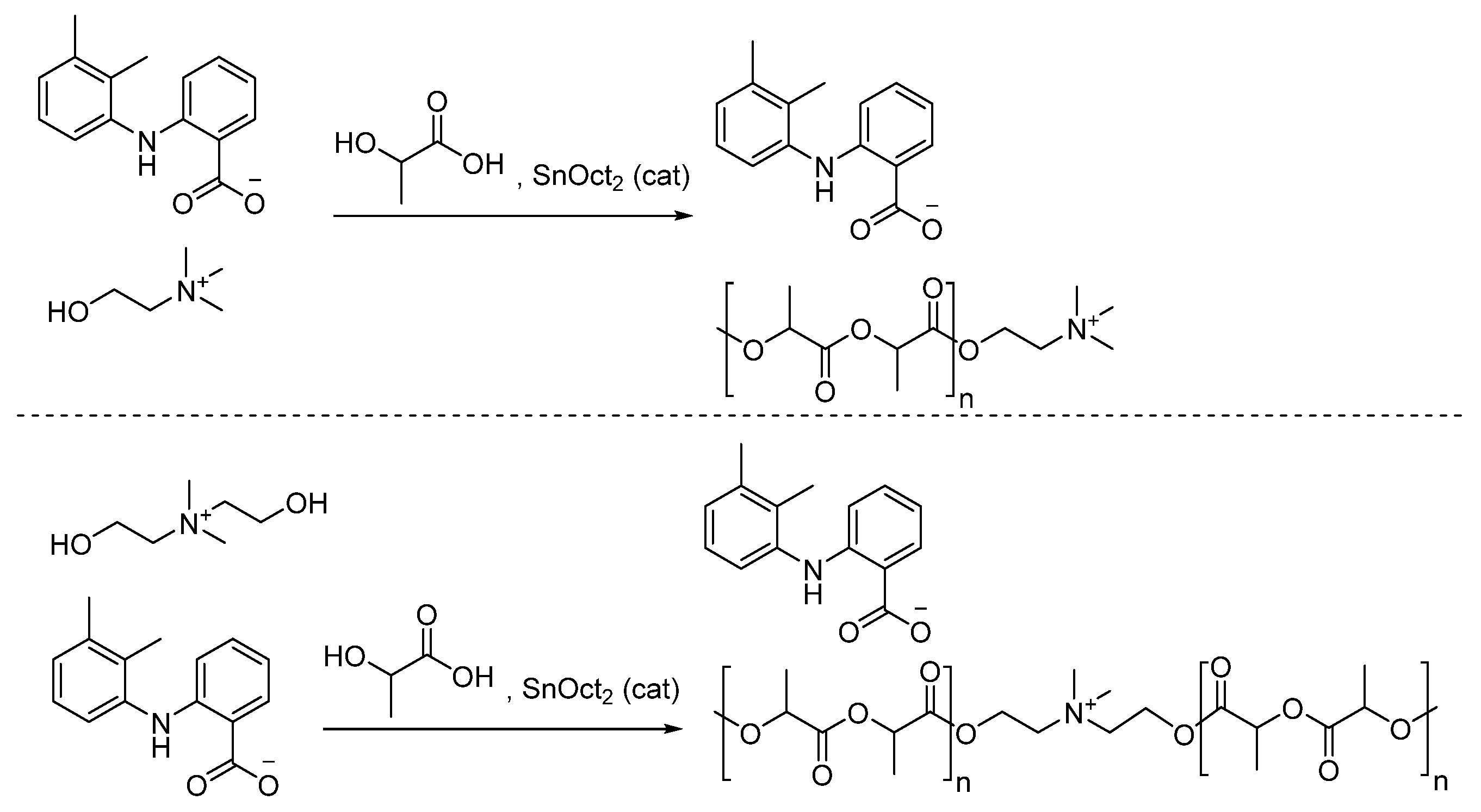
- Halayqa, M.; Zawadzki, M.; Domańska, U.; Plichta, A. API-ammonium ionic liquid—Polymer compounds as a potential tool for delivery systems. J. Molec. Liq. 2017, 248, 972–980. [Google Scholar] [CrossRef]
- Elshaarawya, R.F.M.; Refaeec, A.A.; El-Sawi, E.A. Pharmacological performance of novel poly-(ionic liquid)-grafted chitosan-N-salicylidene Schiff bases and their complexes. Carbohydr. Polym. 2016, 146, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Cherukuvada, S.; Nangia, A. Polymorphism in an API ionic liquid: Ethambutol dibenzoate trimorphs. CrystEngComm 2012, 14, 7840–7843. [Google Scholar] [CrossRef]
- Lu, Y.; Qi, J.; Wu, W. Ionic liquids-based drug delivery: A perspective. Pharm. Res. 2022, 39, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Licart. Available online: https://www.licart.com/ (accessed on 3 February 2023).
- Centene Corporation. Clinical Policy: Diclofenac (Cambia, Flector, Licart, Pennsaid, Solaraze, Zipsor, Zorvolex). Available online: https://www.superiorhealthplan.com/content/dam/centene/Superior/policies/pharmacy-policies/CP.PCH.28-12012021.pdf (accessed on 3 February 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berton, P.; Shamshina, J.L. Ionic Liquids as Tools to Incorporate Pharmaceutical Ingredients into Biopolymer-Based Drug Delivery Systems. Pharmaceuticals 2023, 16, 272. https://doi.org/10.3390/ph16020272
Berton P, Shamshina JL. Ionic Liquids as Tools to Incorporate Pharmaceutical Ingredients into Biopolymer-Based Drug Delivery Systems. Pharmaceuticals. 2023; 16(2):272. https://doi.org/10.3390/ph16020272
Chicago/Turabian StyleBerton, Paula, and Julia L. Shamshina. 2023. "Ionic Liquids as Tools to Incorporate Pharmaceutical Ingredients into Biopolymer-Based Drug Delivery Systems" Pharmaceuticals 16, no. 2: 272. https://doi.org/10.3390/ph16020272
APA StyleBerton, P., & Shamshina, J. L. (2023). Ionic Liquids as Tools to Incorporate Pharmaceutical Ingredients into Biopolymer-Based Drug Delivery Systems. Pharmaceuticals, 16(2), 272. https://doi.org/10.3390/ph16020272







