Positron Annihilation Lifetime Spectroscopy as a Special Technique for the Solid-State Characterization of Pharmaceutical Excipients, Drug Delivery Systems, and Medical Devices—A Systematic Review
Abstract
1. Introduction
2. Results
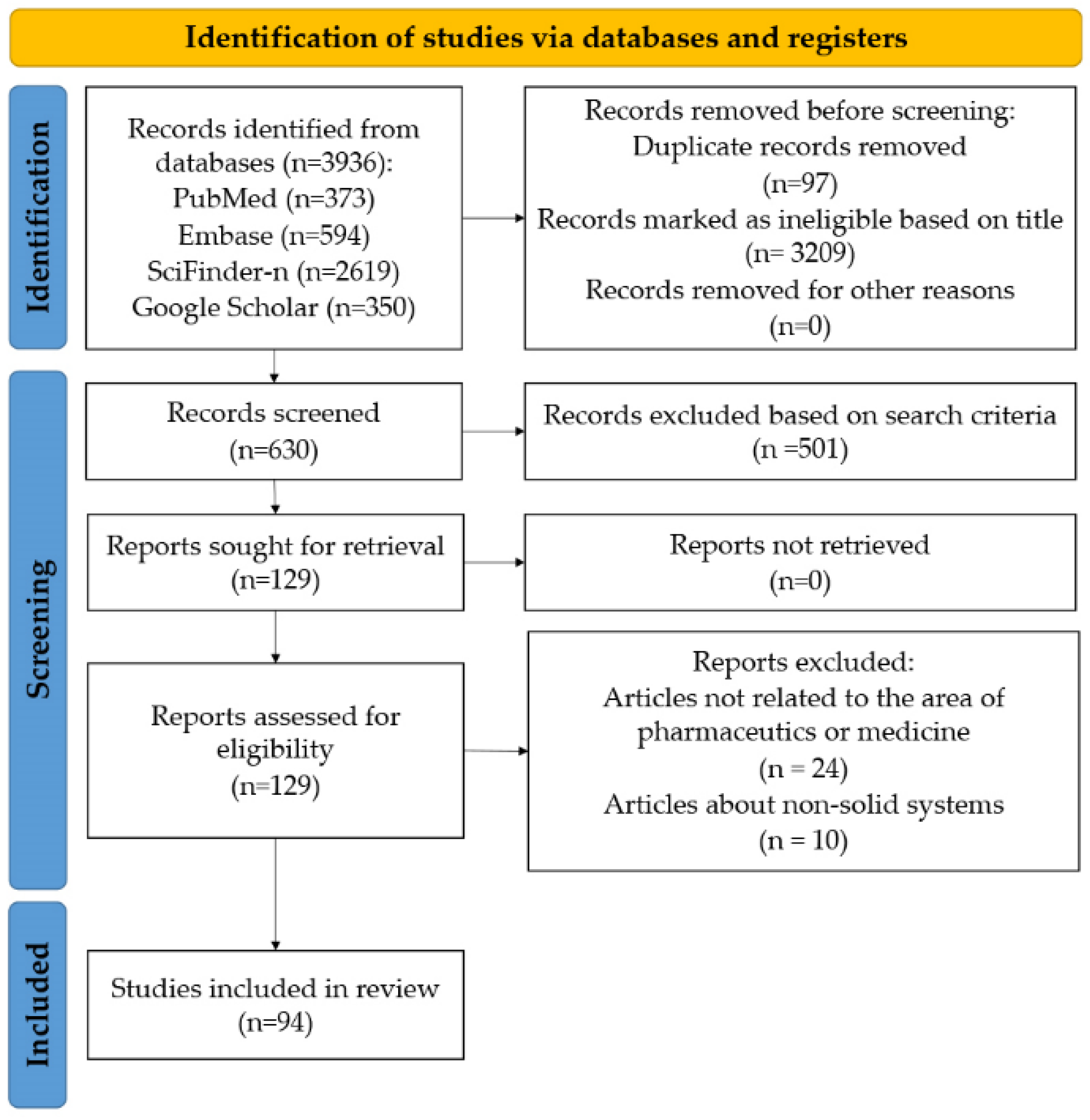
2.1. Database Search and Included Studies
2.2. Results of Studies
2.2.1. Substances
Active Pharmaceutical Ingredients
Excipients
Processed Excipients
2.2.2. Delivery Bases and Drug Delivery Systems
Free and Drug-Loaded Polymer Films
Neat and Loaded Micro- and Nanofibers
Freeze-Dried Formulations and Biologics
Tablets
Transdermal Patches
Intrauterine Delivery Systems
Nanostructured Delivery Bases and Drug Delivery Systems
2.2.3. Medical Devices
Contact Lenses and Intraocular Lenses
Dental Fillers
Conclusions and Discussion of Reviews
3. Discussion
3.1. Validation of the Results of the PALS Method
3.2. Brunauer, Emmett, and Teller (BET) Method
3.3. 129-Xe Magnetic Resonance Spectroscopy (Xe-NMR)
3.3.1. Differential Scanning Calorimetry (DSC)
3.3.2. Imaging Techniques
3.3.3. Complementary Use of PALS with Other Analytical Methods
4. Research Methods
4.1. Eligibility Criteria
4.2. Search Strategy
4.3. Data Analysis
- (a)
- Dosage form and drug;
- (b)
- Carrier system;
- (c)
- PALS as a basic or supportive testing method, in addition to other microstructural methods;
- (d)
- The aim of the study;
- (e)
- Results;
- (f)
- Conclusion.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, M.; Bartholomew, N.; Fulvio, A.D. Positron annihilation lifetime spectroscopy using fast scintillators and digital electronics. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2019, 943, 162507. [Google Scholar] [CrossRef]
- Zelkó, R.; Orbán, A.; Süvegh, K. Tracking of the physical ageing of amorphous pharmaceutical polymeric excipients by positron annihilation spectroscopy. J. Pharm. Biomed. Anal. 2006, 40, 249–254. [Google Scholar] [CrossRef]
- Fong, C.; Dong, A.W.; Hill, A.J.; Boyd, B.J.; Drummond, C.J. Positron annihilation lifetime spectroscopy (PALS): A probe for molecular organisation in self-assembled biomimetic systems. Phys. Chem. Chem. Phys. 2015, 17, 17527–17540. [Google Scholar] [CrossRef]
- Bigg, D.M. A review of positron annihilation lifetime spectroscopy as applied to the physical aging of polymers. Polym. Eng. Sci. 1996, 36, 737–743. [Google Scholar] [CrossRef]
- Parz, P.; Fuchsbichler, B.; Koller, S.; Bitschnau, B.; Mautner, F.A.; Puff, W.; Würschum, R. Charging-induced defect formation in LixCoO2 battery cathodes studied by positron annihilation spectroscopy. Appl. Phys. Lett. 2013, 102, 151901. [Google Scholar] [CrossRef]
- Seidlmayer, S.; Irmgard, B.; Markus, R.; Thomas, G.; Ralph, G.; Hubert, A.G.; Cristoph, H. First-cycle defect evolution of Li1−xNi1/3Mn1/3Co1/3O2 lithium ion battery electrodes investigated by positron annihilation spectroscopy. J. Power Sources 2016, 336, 224–230. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Duan, S.; Yang, X.; Liang, G. Insight into cobalt-doping in Li2FeSiO4 cathode material for lithium-ion battery. J. Power Sources 2015, 274, 194–202. [Google Scholar] [CrossRef]
- Pagot, G.; Toso, V.; Barbiellini, B.; Ferragut, R.; Di Noto, V. Positron Annihilation Spectroscopy as a Diagnostic Tool for the Study of LiCoO2 Cathode of Lithium-Ion Batteries. Condens. Matter 2021, 6, 28. [Google Scholar] [CrossRef]
- Stassin, T.; Cruz, J.A.; Rodríguez-Hermida, S.; Stassen, I.; Marreiros, J.; Krishtab, M.; Dickmann, M.; Egger, W.; Vankelecom, I.F.; Furukawa, S.; et al. Porosimetry for Thin Films of Metal–Organic Frameworks: A Comparison of Positron Annihilation Lifetime Spectroscopy and Adsorption-Based Methods. Adv. Mater. 2021, 33, 2006993. [Google Scholar] [CrossRef]
- Taylor, C.N.; Urban-Klaehn, J.; Le, T.T.; Zaleski, R.; Rimer, J.D.; Gering, K.L. Catalyst Deactivation Probed by Positron Annihilation Spectroscopy. ACS Catal. 2021, 11, 14967–14976. [Google Scholar] [CrossRef]
- Mitchell, S.; Gerchow, L.; Warringham, R.; Crivelli, P.; Pérez-Ramírez, J. Shedding New Light on Nanostructured Catalysts with Positron Annihilation Spectroscopy. Small Methods 2018, 2, 1800268. [Google Scholar] [CrossRef]
- Nuruddin, M.; Chowdhury, R.A.; Lopez-Perez, L.; Montes, F.J.; Youngblood, Y.P.; Howarter, J.A. Influence of Free Volume Determined by Positron Annihilation Lifetime Spectroscopy (PALS) on Gas Permeability of Cellulose Nanocrystal Films. ACS Appl. Mater. Interfaces 2020, 12, 24380–24389. [Google Scholar] [CrossRef]
- Resch, L.; Karner, A.; Sprengel, W.; Würschum, R.; Schennach, R. Water intake of cellulose materials monitored by positron annihilation lifetime spectroscopy. Cellulose 2022, 29, 1357–1363. [Google Scholar] [CrossRef]
- Li, J.; Laptev, R.; Bordulev, I.; Siemek, K.; Horodek, P.; Shen, H.; Lomygin, A.; Cui, L. Positron Annihilation Spectroscopy Study of Metallic Materials after High-Speed Cutting. Materials 2022, 15, 1017. [Google Scholar] [CrossRef] [PubMed]
- Badawi, E.A.; Abdel-rahman, M.A.; Mahmoud, S.A. Investigation of defects (migration and formation) in metals by positron annihilation lifetime. Appl. Surf. Sci. 1999, 149, 211–216. [Google Scholar] [CrossRef]
- Klym, H.; Karbovnyk, I.; Piskunov, S.; Popov, A.I. Positron Annihilation Lifetime Spectroscopy Insight on Free Volume Conversion of Nanostructured MgAl2O4 Ceramics. Nanomaterials 2021, 11, 3373. [Google Scholar] [CrossRef]
- Klym, H.; Ingram, A.; Shpotyuk, O.; Hadzaman, I.; Solntsev, V.; Hotra, O.; Popov, A.I. Positron annihilation characterization of free volume in micro- and macro-modified Cu0.4Co0.4Ni0.4Mn1.8O4 ceramics. Low Temp. Phys. 2016, 42, 601–605. [Google Scholar] [CrossRef]
- Selim, F.A. Positron annihilation spectroscopy of defects in nuclear and irradiated materials—A review. Mater. Charact. 2021, 174, 110952. [Google Scholar] [CrossRef]
- Chikkakuntappa, R. Positron Annihilation Spectroscopy: Polymer Blends and Miscibility. In Characterization of Polymer Blends: Miscibility, Morphology and Interfaces, 1st ed.; Sabu, T., Yves, G., Jyotishkumar, P., Eds.; Wiley-VCH: Weinheim, Germany, 2014; Volume 2, pp. 877–920. [Google Scholar]
- Consolati, G.; Quasso, F. Morphology of Free-Volume Holes in Amorphous Polymers by Means of Positron Annihilation Lifetime Spectroscopy. In Polymer Physics: From Suspensions to Nanocomposites and Beyond; Wiley: Hoboken, NJ, USA, 2010; pp. 391–419. [Google Scholar] [CrossRef]
- Eldrup, M.; Lightbody, D.; Sherwood, J.N. The temperature dependence of positron lifetimes in solid pivalic acid. Chem. Phys. 1981, 63, 51–58. [Google Scholar] [CrossRef]
- Goworek, T.; Bozena, J.; Ciesielski, K.; Wawryszczuk, J. Positronium in large voids. Silicagel. Chem. Phys. Lett. 1997, 272, 91–95. [Google Scholar] [CrossRef]
- Goworek, T.; Ciesielski, K.; Jasinksa, B.; Wawryszczuk, J. Positronium states in the pores of silica gel. Chem. Phys. 1998, 230, 305–315. [Google Scholar] [CrossRef]
- Goworek, T. Mesopore characterization by PALS. Radiat. Phys. Chem. 2003, 68, 331–337. [Google Scholar] [CrossRef]
- Krsjak, V.; Degmova, J.; Noga, P.; Petriska, M.; Sojak, S.; Saro, M.; Neuhold, I.; Slugen, V. Application of Positron Annihilation Spectroscopy in Accelerator-Based Irradiation Experiments. Materials 2021, 14, 6238. [Google Scholar] [CrossRef]
- Viktor, I.G.; Evgenii, P.P. Positron annihilation spectroscopy in materials structure studies. Phys.-Uspekhi 2002, 45, 59. [Google Scholar] [CrossRef]
- Russell, H.; Morton, A.; Keeble, D.J. Determination of positron annihilation lifetime spectroscopy instrument timing resolution function and source terms using standard samples. J. Phys. Conf. Ser. 2019, 1253, 012014. [Google Scholar] [CrossRef]
- Maisey, M.N. Positron Emission Tomography in Clinical Medicine. In Positron Emission Tomography: Basic Sciences; Bailey, D.L., Townsend, D.W., Valk., P.E., Maisey, M.N., Eds.; Springer: London, UK, 2005; pp. 1–12. [Google Scholar]
- Bailey, D.L.; Karp, J.S.; Surti, S. Physics and Instrumentation in PET. In Positron Emission Tomography: Basic Sciences; Bailey, D.L., Townsend, D.W., Valk., P.E., Maisey, M.N., Eds.; Springer: London, UK, 2005; pp. 13–39. [Google Scholar] [CrossRef]
- Chen, H.; Van Horn, J.; Jean, Y. Applications of Positron Annihilation Spectroscopy to Life Science. Defect Diffus. Forum 2012, 331, 275–293. [Google Scholar] [CrossRef]
- Hui, L.; Yundong, S.; Kai, Z.; Jingbiao, P.; Zhu, W. A simplified digital positron lifetime spectrometer based on a fast digital oscilloscope. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2011, 625, 29–34. [Google Scholar] [CrossRef]
- Nyamweya, N. Applications of Polymer Blends in Drug Delivery. Future J. Pharm. Sci. 2021, 7. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef]
- Makarewicz, C.; Safandowska, M.; Idczak, R.; Rozanski, A. Positron Annihilation Lifetime Spectroscopic Analysis of Plastic Deformation of High-Density Polyethylene. Macromolecules 2021, 54, 9649–9662. [Google Scholar] [CrossRef]
- Dlubek, G.; Shaikh, M.Q.; Rätzke, K.; Pionteck, J.; Paluch, M.; Faupel, F. Subnanometre size free volumes in amorphous Verapamil hydrochloride: A positron lifetime and PVT study through T(g) in comparison with dielectric relaxation spectroscopy. Eur. J. Pharm. Sci. 2010, 41, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.M.; Sharshar, T.; Mohamed, M.A.; Ibrahim, O.B.; Refat, M.S. Study of chemical bonding, physical and biological effect of metformin drug as an organized medicine for diabetes patients with chromium(III) and vanadium(IV) ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 323–332. [Google Scholar] [CrossRef]
- Mahmoud, K.R.; Khodair, A.I.; Shaban, S.Y. Positron annihilation lifetime studies of changes in free volume on some biorelevant nitrogen heterocyclic compounds and their S-glycosylation. Appl. Radiat. Isot. 2015, 105, 303–307. [Google Scholar] [CrossRef]
- Jasińska, B.; Kędzior, M.; Śniegocka, M.; Kozioł, A.E.; Wawrzycka-Gorczyca, I. Investigation of the free volume in olanzapine by PALS. Phys. Status Solidi C 2009, 6, 2432–2434. [Google Scholar] [CrossRef]
- Kiss, D.; Süvegh, K.; Marek, T.; Dévényi, L.; Novák, C.; Zelkó, R. Tracking the physical aging of poly(ethylene oxide): A technical note. Aaps Pharmscitech 2006, 7, E95–E98. [Google Scholar] [CrossRef]
- Antal, I.; Kállai, N.; Luhn, O.; Bernard, J.; Nagy, Z.K.; Szabó, B.; Klebovich, I.; Zelkó, R. Supramolecular elucidation of the quality attributes of microcrystalline cellulose and isomalt composite pellet cores. J. Pharm. Biomed. Anal. 2013, 84, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Shpotyuk, O.; Bujňáková, Z.; Baláž, P.; Ingram, A.; Shpotyuk, Y. Positron annihilation lifetime study of polyvinylpyrrolidone for nanoparticle-stabilizing pharmaceuticals. J. Pharm. Biomed. Anal. 2016, 117, 419–425. [Google Scholar] [CrossRef]
- Kállai-Szabó, B.; Sinka, M.; Stiedl, B.; Sebe, I.; Antal, I.; Zelkó, R. Tracking of the solubility enhancement and drug release stability of melt extrudates containing mebendazole. J. Drug Deliv. Sci. Technol. 2014, 24, 514–518. [Google Scholar] [CrossRef]
- Martini, F.; Hughes, D.J.; Badolato Bönisch, G.; Zwick, T.; Schäfer, C.; Geppi, M.; Alam, M.A.; Ubbink, J. Antiplasticization and phase behavior in phase-separated modified starch-sucrose blends: A positron lifetime and solid-state NMR study. Carbohydr. Polym. 2020, 250, 116931. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Chen, H.; Zhang, J.; Suzuki, R.; Ohdaira, T.; Feldstein, M.M.; Jean, Y.C. The Depth Profile of Free Volume in Drug Delivery Polymers Studied by Positron Annihilation Spectroscopy. Mater. Sci. Forum 2004, 445–446, 319–321. [Google Scholar] [CrossRef]
- Szabó, B.; Süvegh, K.; Zelkó, R. Real time positron annihilation lifetime spectroscopy for the detection of the hydrocolloid gel-film transition of polymers. Polym. Test. 2012, 31, 546–549. [Google Scholar] [CrossRef]
- Panzarasa, G.; Osypova, A.; Consolati, G.; Pandini, S. Microsegregating blends of ethyl cellulose and poly(vinyl pyrrolidone): A combined thermo-mechanical and positron annihilation spectroscopy study. Cellulose 2019, 26, 1619–1630. [Google Scholar] [CrossRef]
- Kelemen, A.; Katona, B.; Módra, S.; Aigner, Z.; Sebe, I.; Pintye-Hódi, K.; Zelkó, R.; Regdon, G., Jr.; Kristó, K. Effects of Sucrose Palmitate on the Physico-Chemical and Mucoadhesive Properties of Buccal Films. Molecules 2020, 25, 5248. [Google Scholar] [CrossRef] [PubMed]
- Zelkó, R.; Orbán, Á.; Süvegh, K.; Riedl, Z.; Rácz, I. Effect of plasticizer on the dynamic surface tension and the free volume of Eudragit systems. Int. J. Pharm. 2002, 244, 81–86. [Google Scholar] [CrossRef]
- Díaz-Calderón, P.; MacNaughtan, B.; Hill, S.; Mitchell, J.; Enrione, J. Reduction of enthalpy relaxation in gelatine films by addition of polyols. Int. J. Biol. Macromol. 2018, 109, 634–638. [Google Scholar] [CrossRef]
- Townrow, S.; Kilburn, D.; Alam, A.; Ubbink, J. Molecular packing in amorphous carbohydrate matrixes. J. Phys. Chem. B 2007, 111, 12643–12648. [Google Scholar] [CrossRef]
- Kilburn, D.; Claude, J.; Schweizer, T.; Alam, A.; Ubbink, J. Carbohydrate polymers in amorphous states: An integrated thermodynamic and nanostructural investigation. Biomacromolecules 2005, 6, 864–879. [Google Scholar] [CrossRef]
- Townrow, S.; Roussenova, M.; Giardiello, M.I.; Alam, A.; Ubbink, J. Specific volume-hole volume correlations in amorphous carbohydrates: Effect of temperature, molecular weight, and water content. J. Phys. Chem. B 2010, 114, 1568–1578. [Google Scholar] [CrossRef]
- Marek, T.; Süvegh, K.; Kéry, I.; Zelko, R.; Regdon, G.; Pintye-Hódi, K.; Vértes, A. The effect of plasticizer on the ageing of Metolose films. Radiat. Phys. Chem. 2007, 76, 165–168. [Google Scholar] [CrossRef]
- Pintye-Hódi, K.; Regdon, G.; Erős, I.; Süvegh, K.; Marek, T.; Kéry, I.; Zelkó, R. Metolose–PEG interaction as seen by positron annihilation spectroscopy. Int. J. Pharm. 2006, 313, 66–71. [Google Scholar] [CrossRef]
- Hugenschmidt, C.; Ceeh, H. The free volume in dried and H2O-loaded biopolymers studied by positron lifetime measurements. J. Phys. Chem. B 2014, 118, 9356–9360. [Google Scholar] [CrossRef] [PubMed]
- Pacułt, J.; Rams-Baron, M.; Chrząszcz, B.; Jachowicz, R.; Paluch, M. Effect of Polymer Chain Length on the Physical Stability of Amorphous Drug-Polymer Blends at Ambient Pressure. Mol. Pharm. 2018, 15, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, J.; Tao, L.; Wang, J.; Waknis, V.; Pan, D.; Hubert, M.; Raghavan, K.; Patel, J. The effect of polymeric excipients on the physical properties and performance of amorphous dispersions: Part I, free volume and glass transition. Pharm. Res. 2015, 32, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Gottnek, M.; Süvegh, K.; Pintye-Hódi, K.; Regdon, G. Effects of excipients on the tensile strength, surface properties and free volume of Klucel® free films of pharmaceutical importance. Radiat. Phys. Chem. 2013, 89, 57–63. [Google Scholar] [CrossRef]
- Söylemez, M.A.; Güven, O. Preparation and detailed structural characterization of Penicillin G imprinted polymers by PALS and XPS. Radiat. Phys. Chem. 2019, 159, 174–180. [Google Scholar] [CrossRef]
- Gregorí Valdes, B.S.; Serro, A.P.; Gordo, P.M.; Silva, A.; Gonçalves, L.; Salgado, A.; Marto, J.; Baltazar, D.; Dos Santos, R.G.; Bordado, J.M.; et al. New Polyurethane Nail Lacquers for the Delivery of Terbinafine: Formulation and Antifungal Activity Evaluation. J. Pharm. Sci. 2017, 106, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Szabó, B.; Kállai, N.; Tóth, G.; Hetényi, G.; Zelkó, R. Drug release profiles and microstructural characterization of cast and freeze dried vitamin B12 buccal films by positron annihilation lifetime spectroscopy. J. Pharm. Biomed. Anal. 2014, 89, 83–87. [Google Scholar] [CrossRef]
- Bölcskei, É.; Süvegh, K.; Marek, T.; Regdon, G.; Pintye-Hódi, K. Testing of the structure of macromolecular polymer films containing solid active pharmaceutical ingredient (API) particles. Radiat. Phys. Chem. 2011, 80, 799–802. [Google Scholar] [CrossRef]
- Li, J.; Hubert, M.; Pinnamaneni, S.; Tao, L.; Zhao, J.; Sharif, S.; Ramakrishnan, R.K.; Nazarenko, S. Effect of Moisture Sorption on Free Volume and Relaxation of Spray Dried Dispersions: Relation to Drug Recrystallization. J. Pharm. Sci. 2020, 109, 1050–1058. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, R.; Zhou, J.; Fan, H.; Shi, B. On-demand drug delivery from temperature-responsive polyurethane membrane. React. Funct. Polym. 2011, 71, 525–535. [Google Scholar] [CrossRef]
- Sipos, E.; Szabó, Z.I.; Rédai, E.; Szabó, P.; Sebe, I.; Zelkó, R. Preparation and characterization of nanofibrous sheets for enhanced oral dissolution of nebivolol hydrochloride. J. Pharm. Biomed. Anal. 2016, 129, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Sebe, I.; Szabó, B.; Nagy, Z.K.; Szabó, D.; Zsidai, L.; Kocsis, B.; Zelkó, R. Polymer structure and antimicrobial activity of polyvinylpyrrolidone-based iodine nanofibers prepared with high-speed rotary spinning technique. Int. J. Pharm. 2013, 458, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Kazsoki, A.; Domján, A.; Süvegh, K.; Zelkó, R. Microstructural characterization of papaverine-loaded HPC/PVA gels, films and nanofibers. Eur. J. Pharm. Sci. 2018, 122, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Sebe, I.; Bodai, Z.; Eke, Z.; Kállai-Szabó, B.; Szabó, P.; Zelkó, R. Comparison of directly compressed vitamin B12 tablets prepared from micronized rotary-spun microfibres and cast films. Drug Dev. Ind. Pharm. 2015, 41, 1438–1442. [Google Scholar] [CrossRef] [PubMed]
- Sebe, I.; Kállai-Szabó, B.; Kovács, K.N.; Szabadi, E.; Zelkó, R. Micro- and macrostructural characterization of polyvinylpirrolidone rotary-spun fibres. Drug Dev. Ind. Pharm. 2015, 41, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Sebe, I.; Kállai-Szabó, B.; Oldal, I.; Zsidai, L.; Zelkó, R. Development of laboratory-scale high-speed rotary devices for a potential pharmaceutical microfibre drug delivery platform. Int. J. Pharm. 2020, 588, 119740. [Google Scholar] [CrossRef]
- Sipos, E.; Csatári, T.; Kazsoki, A.; Gergely, A.; Bitay, E.; Szabó, Z.I.; Zelkó, R. Preparation and Characterization of Fenofibrate-Loaded PVP Electrospun Microfibrous Sheets. Pharmaceutics 2020, 12, 612. [Google Scholar] [CrossRef]
- Sebe, I.; Ostorházi, E.; Bodai, Z.; Eke, Z.; Szakács, J.; Kovács, N.K.; Zelkó, R. In vitro and in silico characterization of fibrous scaffolds comprising alternate colistin sulfate-loaded and heat-treated polyvinyl alcohol nanofibrous sheets. Int. J. Pharm. 2017, 523, 151–158. [Google Scholar] [CrossRef]
- Szabó, P.; Sebe, I.; Stiedl, B.; Kállai-Szabó, B.; Zelkó, R. Tracking of crystalline-amorphous transition of carvedilol in rotary spun microfibres and their formulation to orodispersible tablets for in vitro dissolution enhancement. J. Pharm. Biomed. Anal. 2015, 115, 359–367. [Google Scholar] [CrossRef]
- Kazsoki, A.; Szabó, P.; Süvegh, K.; Vörös, T.; Zelkó, R. Macro- and microstructural tracking of ageing-related changes of papaverine hydrochloride-loaded electrospun nanofibrous buccal sheets. J. Pharm. Biomed. Anal. 2017, 143, 62–67. [Google Scholar] [CrossRef]
- Kovács, A.; Kazsoki, A.; Démuth, B.; Szirányi, B.; Madarász, J.; Süvegh, K.; Zelkó, R. Influence of Aqueous Solubility-Enhancing Excipients on the Microstructural Characteristics of Furosemide-Loaded Electrospun Nanofibers. Pharmaceutics 2020, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Kazsoki, A.; Szabó, P.; Domján, A.; Balázs, A.; Bozó, T.; Kellermayer, M.; Farkas, A.; Balogh-Weiser, D.; Pinke, B.; Darcsi, A.; et al. Microstructural Distinction of Electrospun Nanofibrous Drug Delivery Systems Formulated with Different Excipients. Mol. Pharm. 2018, 15, 4214–4225. [Google Scholar] [CrossRef] [PubMed]
- Arany, P.; Róka, E.; Mollet, L.; Coleman, A.W.; Perret, F.; Kim, B.; Kovács, R.; Kazsoki, A.; Zelkó, R.; Gesztelyi, R.; et al. Fused Deposition Modeling 3D Printing: Test Platforms for Evaluating Post-Fabrication Chemical Modifications and In-Vitro Biological Properties. Pharmaceutics 2019, 11, 277. [Google Scholar] [CrossRef]
- Vajdai, A.; Szabó, B.; Süvegh, K.; Zelkó, R.; Ujhelyi, G. Tracking of the viability of Stenotrophomonas maltophilia bacteria population in polyvinylalcohol nanofiber webs by positron annihilation lifetime spectroscopy. Int. J. Pharm. 2012, 429, 135–137. [Google Scholar] [CrossRef]
- Krüger-Szabó, A.; Aigner, Z.; Balogh, E.; Sebe, I.; Zelkó, R.; Antal, I. Microstructural analysis of the fast gelling freeze-dried sodium hyaluronate. J. Pharm. Biomed. Anal. 2015, 104, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Roussenova, M.; Andrieux, J.C.; Alam, M.A.; Ubbink, J. Hydrogen bonding in maltooligomer-glycerol-water matrices: Relation to physical state and molecular free volume. Carbohydr. Polym. 2014, 102, 566–575. [Google Scholar] [CrossRef]
- Roussenova, M.; Murith, M.; Alam, A.; Ubbink, J. Plasticization, antiplasticization, and molecular packing in amorphous carbohydrate-glycerol matrices. Biomacromolecules 2010, 11, 3237–3247. [Google Scholar] [CrossRef]
- Sharma, S.K.; Roudaut, G.; Fabing, I.; Duplâtre, G. Characterization of a sucrose/starch matrix through positron annihilation lifetime spectroscopy: Unravelling the decomposition and glass transition processes. Phys. Chem. Chem. Phys. 2010, 12, 14278–14284. [Google Scholar] [CrossRef]
- Chieng, N.; Cicerone, M.T.; Zhong, Q.; Liu, M.; Pikal, M.J. Characterization of dynamics in complex lyophilized formulations: II. Analysis of density variations in terms of glass dynamics and comparisons with global mobility, fast dynamics, and Positron Annihilation Lifetime Spectroscopy (PALS). Eur. J. Pharm. Biopharm. 2013, 85, 197–206. [Google Scholar] [CrossRef]
- Goshima, H.; Forney-Stevens, K.M.; Liu, M.; Qian, K.K.; Tyagi, M.; Cicerone, M.T.; Pikal, M.J. Addition of Monovalent Electrolytes to Improve Storage Stability of Freeze-Dried Protein Formulations. J. Pharm. Sci. 2016, 105, 530–541. [Google Scholar] [CrossRef]
- Zelkó, R.; Süvegh, K. Correlation between the release characteristics of theophylline and the free volume of polyvinylpyrrolidone. Eur. J. Pharm. Sci. 2005, 24, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Szente, V.; Süvegh, K.; Marek, T.; Zelkó, R. Prediction of the stability of polymeric matrix tablets containing famotidine from the positron annihilation lifetime distributions of their physical mixtures. J. Pharm. Biomed. Anal. 2009, 49, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Papp, J.; Szente, V.; Süvegh, K.; Zelkó, R. Correlation between the free volume and the metoprolol tartrate release of Metolose patches. J. Pharm. Biomed. Anal. 2010, 51, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Liu, C.; Zhang, P.; Quan, P.; Cao, X.; Fang, L. Mechanistic Insights of the Critical Role of Hydrogen Donor in Controlling Drug Release from Acrylate Adhesive. J. Pharm. Sci. 2020, 109, 1096–1104. [Google Scholar] [CrossRef]
- Papp, J.; Marton, S.; Süvegh, K.; Zelkó, R. The influence of Metolose structure on the free volume and the consequent metoprolol tartrate release of patches. Int. J. Biol. Macromol. 2009, 44, 6–8. [Google Scholar] [CrossRef]
- Patai, K.; Szente, V.; Süvegh, K.; Zelkó, R. Tracking of the micro-structural changes of levonorgestrel-releasing intrauterine system by positron annihilation lifetime spectroscopy. J. Pharm. Biomed. Anal. 2010, 53, 902–905. [Google Scholar] [CrossRef]
- Marek, T.; Süvegh, K.; Vértes, A.; Ernst, A.; Bauer, R.; Weil, T.; Wiesler, U.; Klapper, M.; Müllen, K. Positron annihilation study of polyphenylene dendrimers. Radiat. Phys. Chem. 2003, 67, 325–330. [Google Scholar] [CrossRef]
- Rocha, L.S.R.; Simões, A.Z.; Macchi, C.; Somoza, A.; Giulietti, G.; Ponce, M.A.; Longo, E. Synthesis and defect characterization of hybrid ceria nanostructures as a possible novel therapeutic material towards COVID-19 mitigation. Sci. Rep. 2022, 12, 3341. [Google Scholar] [CrossRef]
- Podder, S.; Halder, S.; Roychowdhury, A.; Das, D.; Ghosh, C.K. Superb hydroxyl radical-mediated biocidal effect induced antibacterial activity of tuned ZnO/chitosan type II heterostructure under dark. J. Nanoparticle Res. 2016, 18, 294. [Google Scholar] [CrossRef]
- Mume, E.; Lynch, D.E.; Uedono, A.; Smith, S.V. Investigating the binding properties of porous drug delivery systems using nuclear sensors (radiotracers) and positron annihilation lifetime spectroscopy—Predicting conditions for optimum performance. Dalton Trans. 2011, 40, 6278–6288. [Google Scholar] [CrossRef]
- Filipecki, J.; Sitarz, M.; Kocela, A.; Kotynia, K.; Jelen, P.; Filipecka, K.; Gaweda, M. Studying functional properties of hydrogel and silicone-hydrogel contact lenses with PALS, MIR and Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 131, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Filipecka, K.; Budaj, M.; Miskowiak, B.; Makowska-Janusik, M.; Filipecki, J. Comparison of Occurrence of Free Volumes for Rigid Gas Permeable and Soft Contact Lenses. Polim. Med. 2015, 45, 31–35. [Google Scholar] [PubMed]
- Sane, P.; Tuomisto, F.; Holopainen, J.M. Void volume variations in contact lens polymers. Contact Lens Anterior Eye 2011, 34, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Filipecki, J.; Kocela, A.; Korzekwa, W. Study of free volumes of polymer hydrogel and -silicone-hydrogel contact lenses by means of the positron annihilation lifetime spectroscopy method. Polim. Med. 2014, 44, 255–260. [Google Scholar] [PubMed]
- Filipecki, J.; Kocela, A.; Korzekwa, P.; Miedzinski, R.; Filipecka, K.; Golis, E.; Korzekwa, W. Structural study of polymer hydrogel contact lenses by means of positron annihilation lifetime spectroscopy and UV-vis-NIR methods. J. Mater. Sci. Mater. Med. 2013, 24, 1837–1842. [Google Scholar] [CrossRef]
- Kotynia, K.; Kocela, A.; Filipecki, J.; Filipecka, K.; Korzekwa, P.; Golis, E. Structural studies of polymer hydrogel and silicone hydrogel contact lenses by means of positron lifetime spectroscopy methods. Polim. Med. 2013, 43, 21–28. [Google Scholar]
- Filipecki, J.; Kotynia, K.; Filipecka, K. Investigation of the Degree of Disorder of the Structure of Polymer Soft Contact Lenses Using Positron Annihilation Lifetime Spectroscopy PALS. Polim. Med. 2016, 46, 17–23. [Google Scholar] [CrossRef]
- Deepa Urs, M.V.; Ranganathaiah, C. Glucose and water diffusion kinetics study in a fluorosilicone acrylate contact lens material by positron lifetime spectroscopy. J. Biomater. Sci. Polym. Ed. 2007, 18, 641–654. [Google Scholar] [CrossRef]
- Deepa Urs, M.V.; Ranganathaiah, C. Influence of spoliation in poly(2-hydroxy ethyl methacrylate) soft contact lens on its free volume and optical transparency. J. Mater. Sci. Mater. Med. 2008, 19, 1355–1361. [Google Scholar] [CrossRef]
- Ferreira Marques, M.F.; Gordo, P.M.; Gil, C.L.; Kajcsos, Z.; Gil, M.H.; Mariz, M.J.; de Lima, A.P. Positron lifetime studies in vinyl polymers of medical importance. Radiat. Phys. Chem. 2003, 68, 485–488. [Google Scholar] [CrossRef]
- Chamerski, K.; Korzekwa, W.; Miedzinski, R.; Filipecki, J. Modification influence on the structural parameters of polymer ophthalmic materials. Opt. Appl. 2016, 46, 47–55. [Google Scholar] [CrossRef]
- Chamerski, K.; Stopa, M.; Jelen, P.; Lesniak, M.; Sitarz, M.; Filipecki, J. Spectroscopic studies of the silicone oil impact on the ophthalmic hydrogel based materials conducted in time dependent mode. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 192, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chamerski, K.; Lesniak, M.; Sitarz, M.; Stopa, M.; Filipecki, J. An investigation of the effect of silicone oil on polymer intraocular lenses by means of PALS, FT-IR and Raman spectroscopies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 167, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Filipecki, J.; Chamerski, K.; Boyko, O.; Kotynia, K. Ageing phenomenon in acrylic polymer dental materials detected by means of positron annihilation lifetime spectroscopy. Polim. Med. 2014, 44, 21–28. [Google Scholar] [PubMed]
- Shpotyuk, O.; Ingram, A.; Shpotyuk, O.; Bezvushko, E. Light-cured dimethacrylate dental restorative composites under a prism of annihilating positrons. Polim. Med. 2017, 47, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Shirazinia, M.; Mehmandoost-Khajeh-Dad, A.A.; Dehghani, V.; Mehmandoost-Khajeh-Dad, J.; Khaghani, M. The Effect of Curing Light Intensity on Free Volume Size in Some Dental Composites. Polim. Med. 2016, 46, 129–133. [Google Scholar] [CrossRef]
- Pfeifer, C.S.; Shelton, Z.R.; Braga, R.R.; Windmoller, D.; Machado, J.C.; Stansbury, J.W. Characterization of dimethacrylate polymeric networks: A study of the crosslinked structure formed by monomers used in dental composites. Eur. Polym. J. 2011, 47, 162–170. [Google Scholar] [CrossRef]
- Shpotyuk, O.; Ingram, A.; Shpotyuk, O.; Miskiv, A.; Smolar, N. PALS probing of photopolymerization shrinkage in densely packed acrylate-type dental restorative composites. Polim. Med. 2019, 49, 49–56. [Google Scholar] [CrossRef]
- Shpotyuk, O.; Ingram, A.; Shpotyuk, O. Free Volume Structure of Acrylic-Type Dental Nanocomposites Tested with Annihilating Positrons. Nanoscale Res. Lett. 2016, 11, 528. [Google Scholar] [CrossRef]
- Algers, J.; Maurer, F.H.J.; Eldrup, M.; Wang, J.S. Free volume and mechanical properties of Palacos® R bone cement. J. Mater. Sci. Mater. Med. 2003, 14, 955–960. [Google Scholar] [CrossRef]
- Filipecka, K.; Budaj, M.; Miskowiak, B.; Mandecka, S.; Mandecki, R.; Makowska-Janusik, M.; Filipecki, J. A study of the effect of X-ray irradiation on the structure of Narafilcon A biopolymer soft contact lenses. Polim. Med. 2018, 48, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Moskal, P.; Jasińska, B.; Stępień, E.Ł.; Bass, S.D. Positronium in medicine and biology. Nat. Rev. Phys. 2019, 1, 527–529. [Google Scholar] [CrossRef]
- Kucera, S.A.; Felton, L.A.; McGinity, J.W. Physical aging in pharmaceutical polymers and the effect on solid oral dosage form stability. Int. J. Pharm. 2013, 457, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Vasvári, G.; Kalmár, J.; Veres, P.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Haimhoffer, Á.; Rusznyák, Á.; Fenyvesi, F.; et al. Matrix systems for oral drug delivery: Formulations and drug release. Drug Discov. Today Technol. 2018, 27, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Ubbink, J. Structural and thermodynamic aspects of plasticization and antiplasticization in glassy encapsulation and biostabilization matrices. Adv. Drug. Deliv. Rev. 2016, 100, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Abiad, M.G.; Carvajal, M.T.; Campanella, O.H. A Review on Methods and Theories to Describe the Glass Transition Phenomenon: Applications in Food and Pharmaceutical Products. Food Eng. Rev. 2009, 1, 105–132. [Google Scholar] [CrossRef]
- Szakonyi, G.; Zelkó, R. The effect of water on the solid state characteristics of pharmaceutical excipients: Molecular mechanisms, measurement techniques, and quality aspects of final dosage form. Int. J. Pharm. Investig. 2012, 2, 18–25. [Google Scholar] [CrossRef]
- Szabó, P.; Zelko, R.; Antal, I. The Role of Solid State Characterization in Predicting Stability of Solid Dosage Forms. Curr. Pharm. Des. 2016, 22, 5019–5028. [Google Scholar] [CrossRef]
- Wahl, V.; Khinast, J.; Paudel, A. Lyophilized protein powders: A review of analytical tools for root cause analysis of lot-to-lot variability. TrAC Trends Anal. Chem. 2016, 82, 468–491. [Google Scholar] [CrossRef]
- Cicerone, M.T.; Pikal, M.J.; Qian, K.K. Stabilization of proteins in solid form. Adv. Drug Deliv. Rev. 2015, 93, 14–24. [Google Scholar] [CrossRef]
- Gray, A.; Egan, S.; Bakalis, S.; Zhang, Z. Determination of microcapsule physicochemical, structural, and mechanical properties. Particuology 2016, 24, 32–43. [Google Scholar] [CrossRef]
- Dutta, D.; Chatterjee, S.C.; Pujari, P.K. Pore structure of silica gel: A comparative study through BET and PALS. Chem. Phys. 2005, 312, 319–324. [Google Scholar] [CrossRef]
- Paranhos, C.M.; Soares, B.G.; Windmöller, D. Microstructure and free volume evaluation of poly(vinyl alcohol) nanocomposite hydrogels. Eur. Polym. J. 2007, 43, 4882–4890. [Google Scholar] [CrossRef]
- Emmett, S.; Watson, M.J.O.N. Differential Microcalorimeter. U.S. Patent No. 3,263,484, 1966. [Google Scholar]
- Zhang, J.; Zhang, R.; Chen, H.; Li, Y.; Wu, Y.C.; Suzuki, R.; Sandreckski, T.C.; Ohdaira, T.; Jean, Y.C. Surface and interfacial effect on polymer glass transition temperature studied by positron annihilation. Radiat. Phys. Chem. 2003, 68, 535–539. [Google Scholar] [CrossRef]
- Kim, S.H.; Chung, J.W.; Kang, T.J.; Kwak, S.Y.; Suzuki, T. Determination of the glass transition temperature of polymer/layered silicate nanocomposites from positron annihilation lifetime measurements. Polymer 2007, 48, 4271–4277. [Google Scholar] [CrossRef]
- Vértes, A.; Süvegh, K.; Bokor, M.; Domján, A.; Marek, T.; Iván, B.; Vankó, G. Positronium as a tool to monitor changes of chemical structure. Radiat. Phys. Chem. 1999, 55, 541–548. [Google Scholar] [CrossRef]
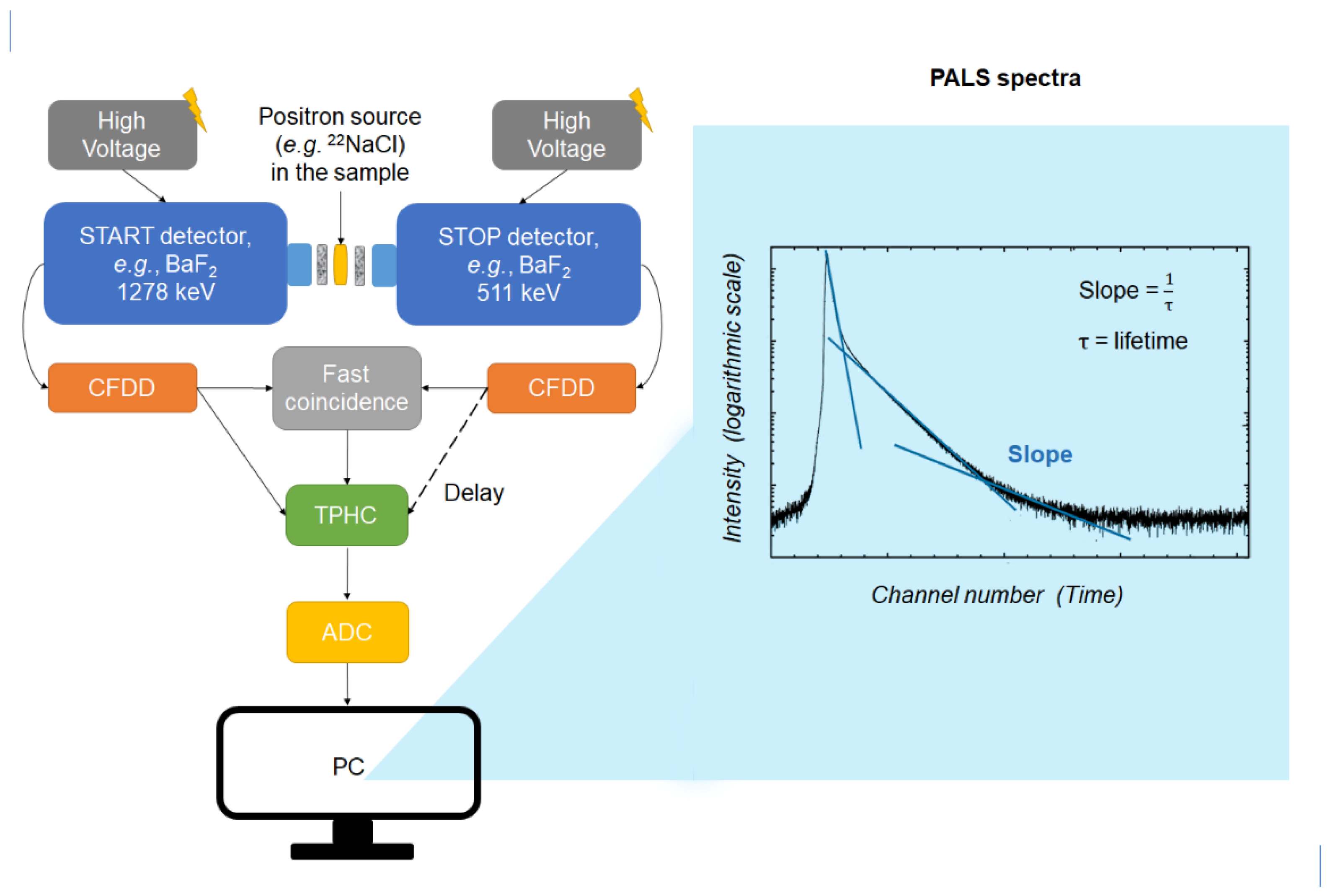
| Positron Annihilation Mode | Characteristic Lifetime in Vacuum | Emitted Photons in Vacuum | Positron Annihilation in Matter | Ref. |
|---|---|---|---|---|
| p-Ps 1 (with antiparallel orientation of the positron and electron spins) | 0.125 ns | Two 0.511 MeV gamma rays | The p-Ps lifetime can be affected by the material because the Coulomb interaction between the p-Ps and material electrons changes the distance between the positron and electron in p-Ps. However, the p-Ps lifetime remains relatively unchanged by the interaction with condensed matter as it undergoes self-annihilation. | [1,3,19] |
| o-Ps 2 (with parallel orientation of the positron and electron spins) | 142 ns | Three 0.511 MeV gamma rays constrained by the conservation of angular momentum | Self-annihilation is forbidden by quantum mechanics in the triplet state of o-Ps, and its lifetime in a material is drastically reduced. o-Ps mainly decays via the “pick-off” process where the positron is annihilated along with an electron with opposed spin in the surrounding material; two 0.511 MeV annihilation photons are created, and the lifetimes are shortened. These lifetimes are still longer than the mean lifetime of p-Ps and also long enough for Ps atoms to “scan” their surroundings and be easily measured. |
| Dosage Form | Drug and Concentration | Carrier Matrix (Chemical and Commercial Name) | Aim of Study | Functionality of the PALS Method | Other Additional Structural Analyses Used Besides PALS 1 | References |
|---|---|---|---|---|---|---|
| (1) Substances | ||||||
| (a) Active pharmaceutical ingredients | ||||||
| Amorphous drug film | Verapamil hydrochloride | - | Characterization of the temperature dependence of the free volume | Microstructural analysis | DSC BDS | [35] |
| Powder | Metformin drug complex with vanadium(III) and chromium(IV) ions | - | Examination and physicochemical characterization of the system | Microstructural analysis | SEM IRS RS | [36] |
| Powder | N-heterocyclic compounds | - | Investigation of the structural characteristics of the system | Microstructural analysis | DBES | [37] |
| Crystalline powder | Olanzapine | - | Examination of temperature and pressure dependence of the drug microstructure | Tracking of microstructural changes upon external stimuli (heat and pressure) | PVT | [38] |
| (b) Excipients | ||||||
| Powder | - | Poly(ethylene oxide) (PEO) | Investigation of poly(ethylene oxide) ageing | Microstructural analysis Tracking of microstructural changes during accelerated stability testing (40 °C, 75% RH, 4 weeks) | SEM DSC | [39] |
| (c) Processed excipients | ||||||
| Pellet cores | - | Microcrystalline cellulose and isomalt | Examination of the relationship between physical attributes and the supramolecular structure of the pellet cores | Microstructural analysis | SM SEM ATR-FTIR NIR | [40] |
| Pellets | - | Polyvinylpirrolidone (PVP) | Investigation of PVP as a stabilizer and binder by the PALS method | Microstructural analysis | XRPD RS SEM EDS | [41] |
| Pellets (melt extrudate) | Mebendazole (20 g/50 g formulation) | Soluplus, Eudragit E PO, povidone (Kollidon K30, PVP30), and Plasdone C17 | Examination and physicochemical characterization of the system | Microstructural analysis | SEM DSC FTIR | [42] |
| Spray-dried polymeric blends | - | Hydrophobically modified starch and sucrose | Investigation of the structure and the effect of sucrose and water in the starch–sucrose phase-separated system | Microstructural analysis | ssNMR | [43] |
| (2) Delivery bases and drug delivery systems | ||||||
| (a) Free and drug-loaded polymer films | ||||||
| Polymeric film | - | Polyvinylpirrolidone (PVP) and poly(ethylene glycol) (PEG), PVP-PEG diacrylate (PVP-PEGDA), PVP-PEG monomethacrylate (PVP-PEGMMA) | Examination of the effect of hydrogen bonding on the depth profile of the free volume in polymeric mixtures and copolymers | Microstructural analysis | DBES | [44] |
| Polymeric film | - | Sodium alginate (SA), Carbopol 71 G | Tracking of the microstructure in real time during film formation | Microstructural analysis | - | [45] |
| Polymeric film | - | Ethylcellulose and PVP blend | Studying phase separation in the polymeric blend | Microstructural analysis | SEM ATR-FTIR EDX DSC | [46] |
| Buccal film | - | Hydroxypropyl-methylcellulose-5 (HPMC-5), Hydroxypropyl-methylcellulose-15 (HPMC-15) | Examination of the effect of the sucrose palmitate permeation enhancer on the physicochemical and mucoadhesive properties of the system | Microstructural analysis | XRD | [47] |
| Solvent cast polymeric film | - | Acrylic polymers (Eudragit L 30D and Eudragit RL 30D) | Examination of the plasticizer effect of dibutyl sebecate on the system | Microstructural analysis | - | [48] |
| Polymeric film | - | Bovine gelatine | Studying the effect of polyol plasticizers on the enthalpy of the system | Microstructural analysis | DSC | [49] |
| Solvent cast polymeric film | - | Maltopolymer–maltose blends | Studying the molecular packing and the effect of water in amorphous carbohydrate matrices | Microstructural analysis | Density measurement | [50] |
| Solvent cast polymeric films | - | Maltodextrin and dextrose | Investigation of the structure of amorphous carbohydrate matrices by combined methods | Microstructural analysis | DSC Density measurement Dilatometry | [51] |
| Solvent cast polymeric film | - | Maltopolymer–maltose blends | Examination of the different effects (temperature, pressure, and water content) on the system | Microstructural analysis | PVT | [52] |
| Polymeric film | - | PVP, Eudragit NE 30 D | Examination of the free volume of polymers under different humidity conditions | Microstructural analysis Tracking of microstructural changes during accelerated stability testing (25 °C; 45, 55, 65, 75% RH, 30 days) | - | [2] |
| Polymeric film | - | Methylcellulose | Investigation of the effect of PEG plasticizer on the aging of the system | Microstructural analysis Tracking of microstructural changes during accelerated stability testing (25 °C; 75% RH, 30 days) | DBES | [53] |
| Solvent cast polymeric film | - | Methylcellulose | Investigation of PEG-Metolose interaction and aging of the system by the PALS method | Microstructural analysis Tracking of microstructural changes during accelerated stability testing (25 °C; 75% RH, 30 days) | DSC DBES | [54] |
| Polymeric film | - | Gelatine and sucrose blend | Examination of the effect of water and drying on the system | Tracking of microstructural changes upon external stimulation (moisture) | - | [55] |
| Polymeric film | Bicalutamide | PVP | Investigation of the effect of the polymer chain length on the recrystallization of amorphous drugs | Microstructural analysis | DSC BDS | [56] |
| Spray-dried dispersions and polymeric films | Indomethacin Ketoconazole | PVP, Polyvinylpirrolidone-vinylacetate (PVP-VA), Hydroxypropylcellulose (HPC), Hydroxymethylcellulose acetate succinate (HPMC-AS) | Investigation of the effect of excipients on the structure of amorphous dispersions | Microstructural analysis | SEM PXRD DSC DMA PVT | [57] |
| Mucoadhesive polymeric film | Lidocaine base (5, 10, 15 w/wt. %) | HPC | Examination of the plasticizer effect of polyols (glycerol and xylitol) on the system | Microstructural analysis | - | [58] |
| Polymeric film | Penicillin G | Polyethylene (PE) and polypropylene (PP) | Examination and characterization of the system | Microstructural analysis | FTIR SEM XPS | [59] |
| Nail lacquers | Terbinafine HCl (1.0% w/w) | Polyurethane (PU) | Development of the system, microstructural analysis, biocompatibility, wettability, and antifungal activity testing | Microstructural analysis | SEM FTIR | [60] |
| Solvent cast and freeze-dried polymeric films | Vitamin B12 (2 mg Vit B12/10 g) | SA and Carbopol 71G | Finding correlations between the drug release profile and supramolecular structure | Microstructural analysis Tracking of microstructural changes during accelerated stability testing (40 °C, 75% RH, 4 weeks) | Digital microscope | [61] |
| Polymeric film | Diclofenac sodium (0, 1, 5 w/wt. %) | Eudragit L 30D-55 | Investigation of the structure of the system with variable drug content and during storage | Microstructural analysis Tracking of microstructural changes during accelerated stability testing (17 °C; 65% RH, 3 weeks) | - | [62] |
| Spray-dried dispersions | Indomethacin Ketoconazole | PVP, PVP-VA copolymer, HPC, HPMC | Examination of drug recrystallization propensity in different carrier systems, moisture sorption, and relaxation | Microstructural analysis Tracking of microstructural changes upon external stimulation (moisture) | Polarized microscopy XRPD | [63] |
| Polymer membrane | Sulfamethoxazole (3.5 wt. %), Paclitaxel (3.0 wt. %) | Polyurethane | Investigation of the structure and drug release of the temperature-modulated drug delivery system | Tracking of microstructural changes upon external stimulation (heat) | DSC | [64] |
| (b) Neat and loaded micro- and nanofibers | ||||||
| Buccal nanofibrous sheets | Nebivolol hydrochloride (0.1 g/20 g water) | PVA | Development of the system for enhanced dissolution of BCS II drugs | Microstructural analysis | ATR-FTIR DSC | [65] |
| Topical nanofibrous sheets | Iodine (2.7–3.1 w/wt. %) | PVP, PVP-VA copolymer | Preparation of the system, physico-chemical characterization | Microstructural analysis | SEM | [66] |
| Buccal nanofibrous sheets | Papaverine hydrochloride (30 mg/g stock solution) | HPC and poly(vinyl alcohol) (PVA) composite | Examination of the microstructure of gels, films, and nanofibers | Microstructural analysis | ssNMR | [67] |
| Rotary spun microfibers—tablets | Vitamin B12 (5 mg/mL) | PVP | Investigation of the structural and mechanical properties of the system | Microstructural analysis | SEM Density measurement | [68] |
| Micro- and nanofibers | - | PVP, PVP-VA copolymer | Studying the influence of parameters for optimum fiber morphology and the mechanical properties of the system | Microstructural analysis | SEM | [69] |
| Nanofibrous sheet | - | PVP, PVP-VA copolymer | Development of a high-speed rotary jet device and examination of the correlation between preparation parameters and fiber morphology | Microstructural analysis | Optical microscopy SEM | [70] |
| Buccal nanofibrous sheets | Fenofibrate (0.2 g fenofibrate/5 mL solution) | PVP | Development and physicochemical characterization of the system | Microstructural analysis | SEM ATR-FTIR | [71] |
| Topical nanofibrous sheets | Colistin-sulfate (15 w/wt. %) | PVA | Preparation of complex, reservoir-type nanofibrous wound dressings | Microstructural analysis Tracking of microstructural changes upon external stimuli (heat) | - | [72] |
| Rotary spun microfibers—orodispersible tablets | Carvedilol (5g/50 mL solution) | HPC | Formulation of the system and tracking the crystalline–amorphous transition of the drug during preparation and stability testing | Microstructural analysis Tracking of the microstructure over accelerated stability testing (40 °C, 75% RH, 4 weeks) | XRPD DSC ATR-FTIR | [73] |
| Buccal nanofibrous sheets | Papaverine hydrochloride (30 mg/g stock solution) | HPC and PVA composite | Monitoring of supramolecular changes of the system under stress conditions | Microstructural analysis Tracking of polymer aging over accelerated stability testing (40 °C, 75% RH, 4 weeks) | SEM FTIR RS | [74] |
| Buccal nanofibrous sheets | Furosemide (1 w/wt. %) | PVP, HPC | Examination of different solubility-enhancing excipients in the prepared systems | Microstructural analysis Tracking of microstructural changes during accelerated stability testing (40 °C, 75% RH, 4 weeks) | SEM ATR-FTIR XRD | [75] |
| Buccal nanofibrous webs | Metoclopramide hydrochloride (3 w/wt. %) | PVA | Examination of the effects of Polysorbate 80 and hydroxypropyl-β-cyclodextrin on the electrospinning process and mechanical properties of the system | Microstructural analysis Tracking of microstructural changes during accelerated stability testing (40 °C, 75% RH, 4 weeks) | SEM AFM PXRD NMR ssNMR | [76] |
| 3D-printed drug delivery base | ||||||
| Polyvalent test plates (PVTP) | - | Oligoamine-modified poly(lactic acid) | Investigation of the connection between the chemical and structural parameters and the cytocompatibility of the chemically modified system | Microstructural analysis | Optical microscopy SEM FTIR | [77] |
| Living organism-loaded system | ||||||
| Nanofibrous web | Stenotrophomas maltophilia | PVA | Tracking of bacterial viability in bacteria-embedded webs | Microstructural analysis Tracking of microstructural changes upon external stimuli (bacterial metabolic product) Tracking bacterial viability over accelerated stability testing (40 °C, 75% RH, 3 weeks) | SEM ATR-FTIR | [78] |
| (c) Freeze-dried formulations and biologics | ||||||
| Lyophilized powder | - | Sodium hyaluronate | Studying the structural and mechanical properties of the system | Microstructural analysis | SEM XRPD | [79] |
| Freeze-dried matrices | - | Maltodextrin | Examination of the effect of glycerol and water excipients on the hydrogen bonding within the system | Microstructural analysis | FTIR DSC | [80] |
| Freeze-dried powder | - | Maltodextrin | Studying the effect of water and glycerol on the structural properties of the system | Microstructural analysis | DSC | [81] |
| Freeze-dried powder | - | Sucrose and starch | Examination of the effect of temperature on the free volume of the system | Microstructural analysis Tracking of microstructural changes upon external stimulation (heat and pressure) | TGA | [82] |
| Lyophilized formulations | Human growth hormone (hGH) | Hydroxyethylstarch (HES)/disaccharide | Evaluation of the effect of disaccharide and polyols on the free volume changes of the system | Microstructural analysis | He pycnometry NS | [83] |
| Freeze-dried formulation | Bovine serum albumin (BSA) and recombinant human serum albumin (rHSA) | Sucrose as a base material and alkali halides (LiCl, NaCl, KCl, RbCl, CsCl) as excipients | Evaluation of the effect on low-level electrolytes on the stability of formulations under stress conditions | Tracking of the microstructure over accelerated stability testing (50 °C, 6 weeks for rHSA and 50 °C, 65 °C, 2 months for BSA samples) | FTIR TAM NS | [84] |
| (d) Tablets | ||||||
| Tablet | Theophylline (100 g/10 mL solution) | PVP | Examination of the drug release properties and free volume of the system under different humidity conditions | Microstructural analysis Tracking of microstructural changes during accelerated stability testing (25 °C; 35, 45, 55, 65, 75% RH, 30 days) | - | [85] |
| Tablet | Famotidine (30 mg/181 mg formulation) | PVP and Carbopol matrix | Investigation of the effect of water on the structure and drug release of the system during storage | Microstructural analysis Tracking of microstructural changes during accelerated stability testing (40 °C, 75% RH, 4 weeks) | - | [86] |
| (e) Transdermal patches | ||||||
| Transdermal patch | Metoprolol tartarate (3 w/wt. %) | Hypromellose and methylcellulose | Examination of drug release | Microstructural analysis | - | [87] |
| Transdermal patch | Zolmitriptan | Pirrolydone adhesive Amide adhesive | Examination of controlled drug release from acrylate adhesives focusing on the role of hydrogen donors | Microstructural analysis | FTIR DSC | [88] |
| Transdermal patch | Metoprolol tartarate | HPMC and Eudragit NE 30D | Investigation of the free volume and drug release from cellulose and acrylate type polymers | Microstructural analysis | - | [89] |
| (f) Intrauterine delivery systems | ||||||
| Intrauterine system | Levonorgestrel (52 mg/intrauterine device) | Polydimethylsiloxane (PDMS) | Tracking the morphology, microstructural changes, and long-term stability of the system | Microstructural analysis Tracking of microstructure over long-term stability testing (in vivo, 5 years) | SEM | [90] |
| (g) Nanostructured delivery bases and drug delivery systems | ||||||
| Dendrimer | - | Polyphenylene | Study of the free volumes of rigid polyphenylene dendrimers | Microstructural analysis | - | [91] |
| Nanoceria | Cerium dioxide | Microcrystalline cellulose | Synthesis of a hybrid nanostructure and structural and spectroscopic characterization of the system | Microstructural analysis Defect characterization | XRD FEG-SEM HR-TEM FTIR RS | [92] |
| Nanocomposite | ZnO | Chitosan | Development and fine-tuning of antibiocidal ZnO/chitosan nanocomposite | Microstructural analysis Defect characterization | XRD HR-TEM FTIR | [93] |
| Nanostructured sensors | ||||||
| Nuclear sensors (radiotracers) | Cu2+ and Co2+ polyazacarboxylate macrocycles, hexa-aza cages | Hollow silica shells | Determination of the binding properties of porous materials and screening by radiotracers | Microstructural analysis | - | [94] |
| (3) Medical devices | ||||||
| (a) Contact lenses and intraocular lenses | ||||||
| Contact lenses | - | Hydrogel, silicone-hydrogel | Determination and comparison of free volumes and the water content in lenses with different materials | Microstructural analysis | MIR RS | [95] |
| Contact lenses | - | Hydrogel, silicone-hydrogel, fluorosilicon-methacrylate-copolymer | Comparison of free volume gaps in contact lenses of different polymer types | Microstructural analysis | - | [96] |
| Contact lenses | - | Hydrogel, silicone-hydrogel | Investigation of the void size and free volumes in the system | Microstructural analysis | - | [97] |
| Contact lenses | - | Hydrogel, silicone-hydrogel | Investigation of the microstructure in different contact lens materials | Microstructural analysis | - | [98] |
| Contact lenses | - | Hydrogel | Examination of the effect of the preparation technology and degree of defect in the structure | Microstructural analysis Defect characterization | UV-vis-NIR | [99] |
| Contact lenses | - | Hydrogel, silicone-hydrogel | Studying the structure of contact lenses and their effect on oxygen permeability | Microstructural analysis Defect characterization | - | [100] |
| Contact lenses | - | Hydrogel, silicone-hydrogel | Comparison of the degree of disorder of lenses with two-state model and Tao–Eldrup model | Microstructural analysis | - | [101] |
| Contact lenses | - | Poly (fluorosilicone acrylate) | Investigation of water and glucose diffusion through the system | Microstructural analysis | - | [102] |
| Contact lenses | - | Poly(2-hydroxy ethyl methacrylate) (PHEMA) | Examination of calcification in soft contact lenses and their effect on free volume holes and optical properties | Microstructural analysis | EDS | [103] |
| Intraocular lenses | - | Polymeric combinations of methyl methacrylate (MMA), buthyl methacrylate (BMA), ethy hexyl acrylate (EHA) monomers | Examination of the free volume in vinyl polymer-based intraocular lenses with different compositions | Microstructural analysis | - | [104] |
| Intraocular lenses | - | 2-phenylethyl acrylate (PEA) and 2-phenylethyl methacrylate (PEMA) copolymer | Examination and comparison of the structure of intraocular implants | Microstructural analysis | - | [105] |
| Intraocular lenses | - | Hydroxyethyl-2-metacrylate (HEMA) | Examination of the time-dependent impact of silicone oil on the system | Microstructural analysis Tracking of microstructural changes during long-term stability testing (37 °C, 6 months) | ATR-FTIR | [106] |
| Intraocular lenses | - | Poly(methyl methacrylate) (PMMA), PHEMA | Investigation of the effect of silicone oil on the internal structure of intraocular lenses | Microstructural analysis | FTIR RS | [107] |
| (b) Dental fillings | ||||||
| Dental filling | - | Bisphenol A-Glycidyl Methacrylate (Bis-GMA) and Tri-ethylene glycol dimethacrylate (TEGMA) | Studying the influence of aging on the dental polymer material | Microstructural analysis Tracking of microstructural changes during long-term stability testing (2 years) | - | [108] |
| Dental filling | - | Bis-GMA and TEGMA | Examination of photopolymerized dimethacrylate-based dental restorative composites | Microstructural analysis | - | [109] |
| Dental filling | - | Coltene, Filtek Z250, DenFil™, Heliomolar2 | Comparison of the photosensitivity and free volumes of four dental restorative materials | Microstructural analysis | - | [110] |
| Dental filling | - | TEGMA and Bis-GMA | Characterization of structural properties in crosslinked dimethacrylate dental composites | Microstructural analysis | NIR | [111] |
| Dental filling | - | ESTA-3® 2 | Examination of volumetric shrinkage during the light-curing polymerization process of the system | Microstructural analysis | - | [112] |
| Dental filling | - | Charisma® 2 | Studying the photopolymerization shrinkage in the system | Microstructural analysis | - | [113] |
| Bone cement | - | Palacos R® bone cement2 | Investigation of the effect of residual monomers on the free volume and mechanical properties of the system | Microstructural analysis | DMA | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokoya, M.M.; Benkő, B.-M.; Süvegh, K.; Zelkó, R.; Sebe, I. Positron Annihilation Lifetime Spectroscopy as a Special Technique for the Solid-State Characterization of Pharmaceutical Excipients, Drug Delivery Systems, and Medical Devices—A Systematic Review. Pharmaceuticals 2023, 16, 252. https://doi.org/10.3390/ph16020252
Shokoya MM, Benkő B-M, Süvegh K, Zelkó R, Sebe I. Positron Annihilation Lifetime Spectroscopy as a Special Technique for the Solid-State Characterization of Pharmaceutical Excipients, Drug Delivery Systems, and Medical Devices—A Systematic Review. Pharmaceuticals. 2023; 16(2):252. https://doi.org/10.3390/ph16020252
Chicago/Turabian StyleShokoya, Mariam Majida, Beáta-Mária Benkő, Károly Süvegh, Romána Zelkó, and István Sebe. 2023. "Positron Annihilation Lifetime Spectroscopy as a Special Technique for the Solid-State Characterization of Pharmaceutical Excipients, Drug Delivery Systems, and Medical Devices—A Systematic Review" Pharmaceuticals 16, no. 2: 252. https://doi.org/10.3390/ph16020252
APA StyleShokoya, M. M., Benkő, B.-M., Süvegh, K., Zelkó, R., & Sebe, I. (2023). Positron Annihilation Lifetime Spectroscopy as a Special Technique for the Solid-State Characterization of Pharmaceutical Excipients, Drug Delivery Systems, and Medical Devices—A Systematic Review. Pharmaceuticals, 16(2), 252. https://doi.org/10.3390/ph16020252






