Real-World Study on Vedolizumab Serum Concentration, Efficacy, and Safety after the Transition from Intravenous to Subcutaneous Vedolizumab in Inflammatory Bowel Disease Patients: Single-Center Experience
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients and Protocol Description
4.2. Outcomes
4.3. Vedolizumab Serum Trough Concentration
4.4. Statistics
4.5. Ethical Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohns Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohns Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Hanauer, S.; Colombel, J.-F.; Sands, B.E.; Lukas, M.; Fedorak, R.N.; Lee, S.; Bressler, B.; et al. Vedolizumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2013, 369, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.-F.; Sandborn, W.J.; van Assche, G.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Baert, F.; Danese, S.; Krznarić, Ž.; Kobayashi, T.; Yao, X.; Chen, J.; Rosario, M.; Bhatia, S.; Kisfalvi, K.; et al. Efficacy and Safety of Vedolizumab Subcutaneous Formulation in a Randomized Trial of Patients With Ulcerative Colitis. Gastroenterology 2020, 158, 562–572. [Google Scholar] [CrossRef]
- Vermeire, S.; D’Haens, G.; Baert, F.; Danese, S.; Kobayashi, T.; Loftus, E.V.; Bhatia, S.; Agboton, C.; Rosario, M.; Chen, C.; et al. Efficacy and Safety of Subcutaneous Vedolizumab in Patients With Moderately to Severely Active Crohn’s Disease: Results From the VISIBLE 2 Randomised Trial. J. Crohns Colitis 2022, 16, 27–38. [Google Scholar] [CrossRef]
- Restellini, S.; Afif, W. Update on TDM (Therapeutic Drug Monitoring) with Ustekinumab, Vedolizumab and Tofacitinib in Inflammatory Bowel Disease. J. Clin. Med. 2021, 10, 1242. [Google Scholar] [CrossRef]
- Albader, F.; Golovics, P.A.; Gonczi, L.; Bessissow, T.; Afif, W.; Lakatos, P.L. Therapeutic Drug Monitoring in Inflammatory Bowel Disease: The Dawn of Reactive Monitoring. World J. Gastroenterol. 2021, 27, 6231–6247. [Google Scholar] [CrossRef]
- Rosario, M.; Polhamus, D.G.; Chen, C.M.; Sun, W.; Dirks, N.L. P490 A Vedolizumab Population Pharmacokinetic Model Including Intravenous and Subcutaneous Formulations for Patients with Ulcerative Colitis. J. Crohns Colitis 2019, 13, S357. [Google Scholar] [CrossRef]
- Rosario, M.; Polhamus, D.; Dirks, N.; Lock, R.; Yao, X.; Chen, J.; Chen, C.; Sun, W.; Feagan, B.; Sandborn, W.; et al. P529 Exposure–Response Relationship of Vedolizumab Subcutaneous Treatment in Patients with Ulcerative Colitis: VISIBLE 1. J. Crohns Colitis 2019, 13, S377–S378. [Google Scholar] [CrossRef]
- Volkers, A.; Straatmijer, T.; Duijvestein, M.; Sales, A.; Levran, A.; van Schaik, F.; Maljaars, J.; Gecse, K.; Ponsioen, C.; Grootjans, J.; et al. Real-World Experience of Switching from Intravenous to Subcutaneous Vedolizumab Maintenance Treatment for Inflammatory Bowel Diseases. Aliment Pharmacol. Ther. 2022, 56, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Wiken, T.; Høivik, M.; Buer, L.; Bolstad, N.; Moum, B.; Medhus, A. P376 Switching from Intravenous to Subcutaneous Vedolizumab Maintenance Treatment; Feasibility, Safety and Clinical Outcome. J. Crohns Colitis 2022, 16, i378–i379. [Google Scholar] [CrossRef]
- Ventress, E.; Young, D.; Rahmany, S.; Harris, C.; Bettey, M.; Smith, T.; Moyses, H.; Lech, M.; Gwiggner, M.; Felwick, R.; et al. Transitioning from Intravenous to Subcutaneous Vedolizumab in Patients with Inflammatory Bowel Disease [TRAVELESS]. J. Crohns Colitis 2022, 16, 911–921. [Google Scholar] [CrossRef]
- Bergqvist, V.; Holmgren, J.; Klintman, D.; Marsal, J. Real-World Data on Switching from Intravenous to Subcutaneous Vedolizumab Treatment in Patients with Inflammatory Bowel Disease. Aliment Pharmacol. Ther. 2022, 55, 1389–1401. [Google Scholar] [CrossRef]
- Jonaitis, L.; Marković, S.; Farkas, K.; Gheorghe, L.; Krznarić, Ž.; Salupere, R.; Mokricka, V.; Spassova, Z.; Gatev, D.; Grosu, I.; et al. Intravenous versus Subcutaneous Delivery of Biotherapeutics in IBD: An Expert’s and Patient’s Perspective. BMC Proc. 2021, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Remy, C.; Caron, B.; Gouynou, C.; Haghnejad, V.; Jeanbert, E.; Netter, P.; Danese, S.; Peyrin-Biroulet, L. Inflammatory Bowel Disease Patients’ Acceptance for Switching from Intravenous Infliximab or Vedolizumab to Subcutaneous Formulation: The Nancy Experience. J. Clin. Med. 2022, 11, 7296. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.; Hardman, A.; Carbery, I.; Broglio, G.; Greer, D.; Selinger, C.P. Uptake of a Switching Program for Patients Receiving Intravenous Infliximab and Vedolizumab to Subcutaneous Preparations. J. Clin. Med. 2022, 11, 5669. [Google Scholar] [CrossRef]
- Little, R.D.; Ward, M.G.; Wright, E.; Jois, A.J.; Boussioutas, A.; Hold, G.L.; Gibson, P.R.; Sparrow, M.P. Therapeutic Drug Monitoring of Subcutaneous Infliximab in Inflammatory Bowel Disease—Understanding Pharmacokinetics and Exposure Response Relationships in a New Era of Subcutaneous Biologics. J. Clin. Med. 2022, 11, 6173. [Google Scholar] [CrossRef]
- vande Casteele, N.; Sandborn, W.J.; Feagan, B.G.; Vermeire, S.; Dulai, P.S.; Yarur, A.; Roblin, X.; Ben-Horin, S.; Dotan, I.; Osterman, M.T.; et al. Real-world Multicentre Observational Study Including Population Pharmacokinetic Modelling to Evaluate the Exposure–Response Relationship of Vedolizumab in Inflammatory Bowel Disease: ERELATE Study. Aliment Pharmacol. Ther. 2022, 56, 463–476. [Google Scholar] [CrossRef]
- Löwenberg, M.; Vermeire, S.; Mostafavi, N.; Hoentjen, F.; Franchimont, D.; Bossuyt, P.; Hindryckx, P.; Rispens, T.; de Vries, A.; van der Woude, C.J.; et al. Vedolizumab Induces Endoscopic and Histologic Remission in Patients With Crohn’s Disease. Gastroenterology 2019, 157, 997–1006. [Google Scholar] [CrossRef]
- Pouillon, L.; Vermeire, S.; Bossuyt, P. Vedolizumab Trough Level Monitoring in Inflammatory Bowel Disease: A State-of-the-Art Overview. BMC Med. 2019, 17, 89. [Google Scholar] [CrossRef]
- Danese, S.; Subramaniam, K.; van Zyl, J.; Adsul, S.; Lindner, D.; Roth, J.; Vermeire, S. Vedolizumab Treatment Persistence and Safety in a 2-Year Data Analysis of an Extended Access Programme. Aliment Pharmacol. Ther. 2021, 53, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Amiot, A.; Serrero, M.; Peyrin-Biroulet, L.; Filippi, J.; Pariente, B.; Roblin, X.; Buisson, A.; Stefanescu, C.; Trang-Poisson, C.; Altwegg, R.; et al. Three-Year Effectiveness and Safety of Vedolizumab Therapy for Inflammatory Bowel Disease: A Prospective Multi-Centre Cohort Study. Aliment Pharmacol. Ther. 2019, 50, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.F.; Bradshaw, J.M. A SIMPLE INDEX OF CROHN’S-DISEASE ACTIVITY. Lancet 1980, 315, 514. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target Strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
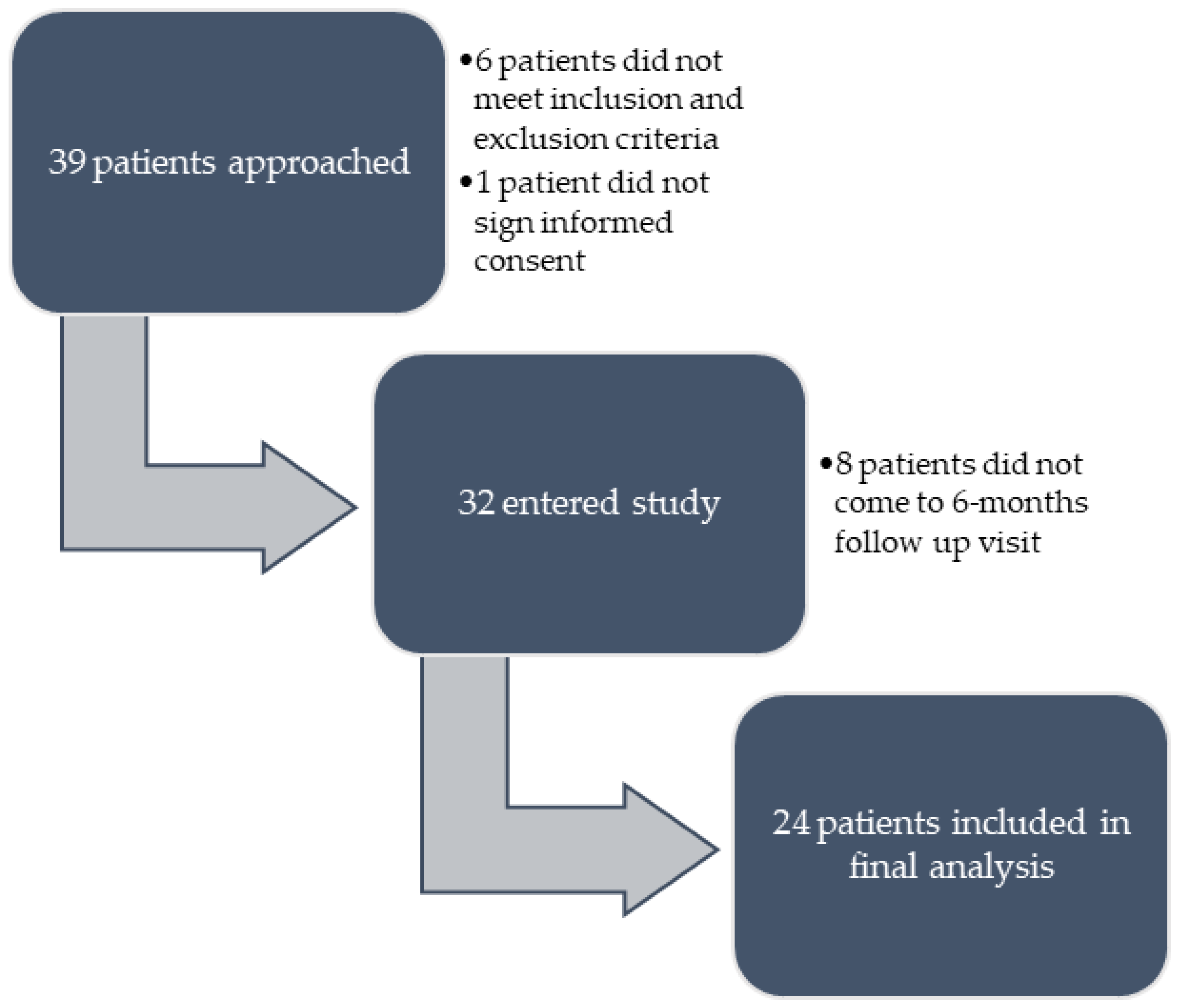

| Patient Characteristics (N = 24) | |
|---|---|
| Female, n (%) | 8 (33.3) |
| Age, years, median (minimum, maximum) | 50 (25, 77) |
| Body mass, kilograms, median (minimum, maximum) | 85 (55, 116) |
| Currently smoking, n (%) | 2 (8.3) |
| Diagnosis, n (%) | |
| CD | 11 (45.8) |
| UC | 13 (54.2) |
| Duration of IV vedolizumab therapy, months, median (minimum, maximum) | 11 (5–58) |
| Age at onset, CD, N = 11 (n, %) | |
| A1: <16 years | 1 (9.1) |
| A2: 17–40 years | 5 (45.45) |
| A3: >40 years | 5 (45.45) |
| Disease location of CD, N = 11 (n, %) | |
| L1: Ileal | 2 (18.2) |
| L2: Ileocolonic | 7 (63.6) |
| L3: Colonic | 2 (18.2) |
| L4: Upper gastrointestinal tract | 1 (9.1) |
| CD behaviour, N = 11 (n, %) | |
| B1: Inflammatory | 7 (63.6) |
| B2: Stricturing | 3 (27.3) |
| B3: Fistulizing | 1 (9.1) |
| P: Perianal disease | 1 (9.1) |
| Age at onset, UC, N = 13 (n, %) | |
| A1: <16 years | 0 (0) |
| A2: 17–40 years | 6 (46.2) |
| A3: >40 years | 7 (53.8) |
| Disease location of UC, N = 13 (n, %) | |
| E1: Proctitis | 0 (0) |
| E2: Left-sided colitis | 8 (61.5) |
| E3: Extensive colitis | 5 (38.5) |
| Therapy prior to beginning of IV vedolizumab, n (%) | |
| Biologic-naïve | 13 (54.2) |
| ASA, corticosteroids | 8 (33.3) |
| AZA, MTX | 5 (20.8) |
| Biologic-experienced | 11 (45.8) |
| 1 | 5 (20.8) |
| 2 or more | 6 (25.0) |
| Prior surgery due to IBD, (n, %) | |
| CD patients | |
| Bowel resection | 5 (20.8) |
| Perianal disease | 1 (4.2) |
| Liver transplantation due to PSC | 1 (4.2) |
| UC patients | 0 (0) |
| Concomitant therapy for IBD | |
| Corticosteroids, n (%) | 1 (4.2) |
| AZA or MTX, n (%) | 1 (4.2) |
| Disease activity | |
| HBI, n (median, minimum–maximum) | 11 (0, 0–3) |
| PMS, n (median, minimum–maximum) | 13 (0, 0–0) |
| Clinical remission, n (%) | 24 (100) |
| CRP, mg/L, n (median, minimum–maximum) | 23 (3.6, 0.4–23.1) |
| FC, µg/g, n (median, minimum–maximum) | 16 (67, 16–772) |
| Biochemical remission, n (%) | 16 (66.7) |
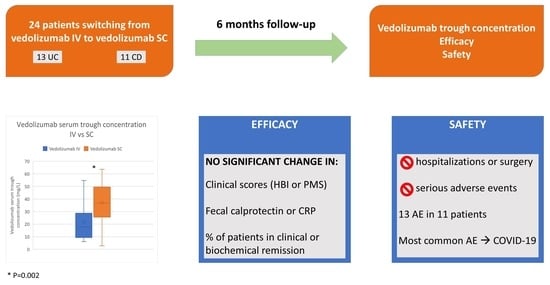
| Serum vedolizumab trough concentration during IV therapy (mg/L), mean (SD) | 22.57 (15.42) |
| Outcome | Baseline | After 6 Months | p Value |
|---|---|---|---|
| Vedolizumab serum trough concentration (mg/L), n = 22, mean (SD) | 22.86 (15.66) | 35.62 (15.46) | 0.002 * |
| Fecal calprotectin (μg/g), n = 15, median (minimum–maximum) | 67 (16–772) | 58.5 (16–1230) | 0.570 ** |
| C-reactive protein (mg/L), n = 19, median (minimum–maximum) | 3.6 (0.4–23.1) | 6.8 (0.6–34.5) | 0.126 ** |
| Harley-Bradshaw index, n = 11, median (minimum–maximum) | 0 (0–3) | 0 (0–3) | 0.317 ** |
| Partial Mayo score, n = 13, median (minimum–maximum) | 0 (0) | 0 (0–4) | 0.102 ** |
| Treatment-Emergent Adverse Events (n, %) | All Patients (N = 24) |
|---|---|
| Infections and infestations | 5 (20.8) |
| COVID-19 | 4 (16.6) |
| Fungal foot infection | 1 (4.2) |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 2 (8.4) |
| Anogenital warts | 1 (4.2) |
| Bowen’s disease | 1 (4.2) |
| General disorders and administration site conditions | 2 (8.4) |
| Pyrexia | 1 (4.2) |
| Injection site erythema | 1 (4.2) |
| Skin and subcutaneous tissue disorders | 2 (8.4) |
| Urticaria | 1 (4.2) |
| Pruritus | 1 (4.2) |
| Blood and lymphatic system disorders | 1 (4.2) |
| Iron deficiency anaemia | 1 (4.2) |
| Musculoskeletal and connective tissue disorders | 1 (4.2) |
| Arthritis | 1 (4.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oršić Frič, V.; Borzan, V.; Šahinović, I.; Borzan, A.; Kurbel, S. Real-World Study on Vedolizumab Serum Concentration, Efficacy, and Safety after the Transition from Intravenous to Subcutaneous Vedolizumab in Inflammatory Bowel Disease Patients: Single-Center Experience. Pharmaceuticals 2023, 16, 239. https://doi.org/10.3390/ph16020239
Oršić Frič V, Borzan V, Šahinović I, Borzan A, Kurbel S. Real-World Study on Vedolizumab Serum Concentration, Efficacy, and Safety after the Transition from Intravenous to Subcutaneous Vedolizumab in Inflammatory Bowel Disease Patients: Single-Center Experience. Pharmaceuticals. 2023; 16(2):239. https://doi.org/10.3390/ph16020239
Chicago/Turabian StyleOršić Frič, Vlasta, Vladimir Borzan, Ines Šahinović, Andrej Borzan, and Sven Kurbel. 2023. "Real-World Study on Vedolizumab Serum Concentration, Efficacy, and Safety after the Transition from Intravenous to Subcutaneous Vedolizumab in Inflammatory Bowel Disease Patients: Single-Center Experience" Pharmaceuticals 16, no. 2: 239. https://doi.org/10.3390/ph16020239
APA StyleOršić Frič, V., Borzan, V., Šahinović, I., Borzan, A., & Kurbel, S. (2023). Real-World Study on Vedolizumab Serum Concentration, Efficacy, and Safety after the Transition from Intravenous to Subcutaneous Vedolizumab in Inflammatory Bowel Disease Patients: Single-Center Experience. Pharmaceuticals, 16(2), 239. https://doi.org/10.3390/ph16020239