Abstract
Despite recent advances in multimodality therapy for glioblastoma (GB) incorporating surgery, radiotherapy, chemotherapy and targeted therapy, the overall prognosis remains poor. One of the interesting targets for GB therapy is the histone deacetylase family (HDAC). Due to their pleiotropic effects on, e.g., DNA repair, cell proliferation, differentiation, apoptosis and cell cycle, HDAC inhibitors have gained a lot of attention in the last decade as anti-cancer agents. Despite their known underlying mechanism, their therapeutic activity is not well-defined. In this review, an extensive overview is given of the current status of HDAC inhibitors for GB therapy, followed by an overview of current HDAC-targeting radiopharmaceuticals. Imaging HDAC expression or activity could provide key insights regarding the role of HDAC enzymes in gliomagenesis, thus identifying patients likely to benefit from HDACi-targeted therapy.
1. Introduction
Glioblastoma multiforme (GB) is the most malignant tumor in the central nervous system (CNS). Despite recent advances in multimodality therapy for GB incorporating surgery, radiotherapy (RT), chemotherapy and targeted therapy, the overall prognosis remains poor. Almost all tumors recur with a more aggressive form, and there is no standard of care for recurrent GB. The survival rate at 5 years postdiagnosis remains at only 5.8% [1,2,3]. Novel molecular markers were identified improving GB classification and providing powerful prognostic information [4]. However, therapy resistance remains a hurdle. Precision oncology incorporating personalized targeted therapy holds much promise in developing more efficacious and tolerable therapies [3]. One of the interesting targets for GB-targeted therapy is the histone deacetylase family (HDAC). Due to their pleiotropic effects on, e.g., DNA repair, cell proliferation, differentiation, apoptosis and senescence, they have gained a lot of attention in the last decade as anti-cancer agents. In addition, HDAC inhibitors (HDACi) have been applied for the treatment of metabolic disorders and psychiatric or neurodegenerative diseases [5]. The HDAC family contains 18 family members, categorized as following: class I (HDAC1,2,3,8), IIa (HDAC 4,5,7,9), IIb (HDAC 6,10), III (nicotinamide adenine dinucleotide (NAD+)-dependent sirtuins (SIRTs) and IV (HDAC11) [6,7]. Two groups of enzymes control the acetylation and deacetylation of histones: histone acetyltransferase (HAT) and HDACs. The transfer or removal of acetyl groups by HATs and HDACs induce a more open and accessible chromatin structure or chromatin condensation and transcriptional repression [8,9]. Interestingly, the histone acetylation status is reversible and can be targeted by drugs [10]. HDACi have the ability to increase the level of protein acetylation in the cancerous cell, restarting the expression of silenced tumor suppressor genes [11]. However, despite their known underlying mechanism, their therapeutic activity is not well-defined.
The goal of this review is to highlight the current status of HDACi- and HDAC-targeting radiopharmaceuticals for the imaging and therapy of GB.
2. Role of HDAC in GB Pathology
Epigenetic mechanisms, particularly those involving enzymatic modifications of DNA and the associated histone proteins that regulate gene expression are recognized as a major factor contributing to the pathogenesis of GB [10]. The effects of acetylation on gene expression and tumor phenotype and the antitumor mechanism of HDACi in GB has recently been reviewed [11,12]. In gliomas, epigenetic enzymes, such as HDAC, are aberrantly expressed causing the deregulation of processes, featuring growth arrest, cell differentiation, cytotoxicity and apoptosis induction. RNA-sequencing data from the public TCGA (The Cancer Genome Atlas) showed that HDAC4, HDAC5, HDAC6, HDAC8 and HDAC11 expression was significantly lowered in glioma (WHO grade II–IV) when compared to normal brain tissue [9]. Expression levels of HDAC1-3 and HDAC7 appeared to increase with higher malignancy grades [9,13]. Additionally, HDAC3 and HDAC9 overexpression in GB are both correlated with a poor prognosis. The role of SIRT in GB is currently under debate [13].
In vitro, HDAC2 expression is significantly upregulated in GB [14]. The silencing of HDAC4 reactivated p21 (WAF1/Cip1) and inhibited tumor growth in an in vivo human GB model [15]. In addition, HDAC5 is upregulated in U87MG, U251MG, T98G and LN-229 glioma cell lines and promoted their proliferation by the upregulation of Notch 1 [16]. HDAC6 has been shown to promote the proliferation of glioma cells through the primary cilia, MKK7/JNK/c-Jun signaling pathway and attenuating transforming growth factor β (TGFβ) receptor signaling [17,18,19]. HDAC6 activity also plays a role in temozolomide (TMZ) resistance through the regulation of DNA mismatch repair [20].
HDACi are epigenome-targeting molecules divided into different categories based on their target and chemical structure: short-chain fatty acids (e.g., valproic acid (VPA)), hydroxamic acid derivatives (e.g., trichostatin A (TSA), vorinostat (SAHA), belinostat, panobinostat (LBH-589), pracinostat, quisinostat (JNJ-16241199)), carboxylic acid derivatives, cyclic peptides (romidepsin), and benzamides entinostat (MS-275), tacedinaline (CI-994) and mocetinostat (MG-0103)). Other categories include electrophilic ketones, hydro-examines, sirtuin inhibitors and miscellaneous [11,21,22]. FDA approval was granted for vorinostat (Zolinza® Rahway, NJ, USA), belinostat (Beleodaq®, PXD101 East Windsor, NJ, USA), panobinostat (Farydak® Barcelona, Spain) and romidepsin (Istodax® Hayes, UK) for the treatment of hematological malignancies, represented by T-cell lymphomas and multiple myeloma (MM). Tucidinostat (Epidaza®, Chedamide, Shenzhen, China) was approved by China’s National Medical Products Administration [22]. Combined use of panobinostat with the proteasome inhibitor bortezomib has been approved for the treatment of refractory MM [6,7,21,23]. Advances in the abovementioned malignancies prompted HDACi-based anti-cancer research to expand to solid tumors, although the HDACi mechanisms of action in tumors are still sparsely understood. Figure 1 gives an overview of the confirmed mechanism of actions in GB, as recently reviewed [11,24]. As RT and TMZ therapy are standard in GB, the radiosensitizing and chemosensitizing effects of HDACi are of major interest. Presumably, HDACi promote a more open chromatin formation in tumor cells, thereby permitting DNA alkylating agents (e.g., TMZ) to access genomic DNA. Other mechanisms to reverse TMZ resistance have been suggested, e.g., blocking NF-κB-dependent transcription [13,14,25]. In particular, HDAC6 has been identified as a potential target for the treatment of TMZ-resistant GB [20,26,27]. The radiosensitization mechanism in GB could be induced by multiple mechanisms but eventually leads to a decrease in DNA repair [28,29,30,31,32,33,34,35]. Post-irradiation, HDACi have been shown to induce a prolonged expression of phosphorylated H2AX (γH2AX), a marker for DNA double strand breaks (DSBs) [33]. HDACi have also been shown to induce alterations in DNA replication, causing DNA damage [36,37]. Finally, HDACi may be able to assist in reversing abnormal genetic silencing, therefore leading to enhanced cell-cycle arrest and apoptosis from the action of DNA-damaging agents [13].
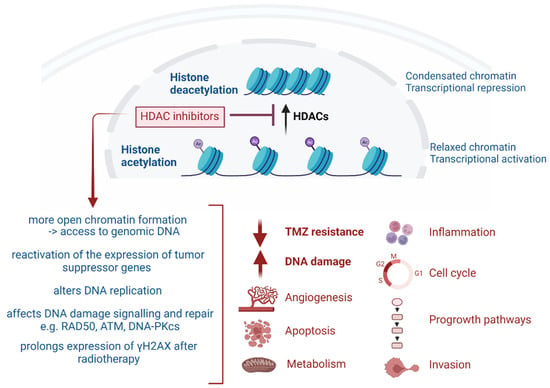
Figure 1.
Overview of the broad effects of HDAC inhibitors.
3. Current Status of HDACi for GB Therapy
An overview of the successful clinical trials investigating HDACi in high-grade glioma is given in Table 1. Studies in pediatric glioma patients were excluded. The previous reviews have focused on the mechanisms of HDACi in GB [11,38].

Table 1.
Overview of clinical trials on HDAC inhibitors (HDACi) in high-grade glioma.
Most research reports on suberoylanilide hydroxamic acid (SAHA, vorinostat), a pan-HDACi, upregulating cancer suppressor genes (p21 (CDKN1A), PTEN, p27) and downregulating Akt-mTOR signaling, CDK2, CDK4 and cyclin D1/E. SAHA therapy triggered GB cell death and promoted hyper-radiosensitivity in wild-type p53 GB cells [10,34,39]. In GB patients, high doses of SAHA monotherapy appeared to be well-tolerated with modest single-agent activity. SAHA combination regimens with TMZ/RT and/or bevacizumab (BEV) have proven to be tolerable, but no statistical improvement in overall survival (OS) and/or progression-free survival (PFS) was noted [40,41,42,43,44,45]. Interestingly, phospholipase D1 (PLD1) has been identified as a target of resistance to vorinostat, and combined therapy with a PLD1 inhibitor might improve efficacy [46].
The hydroxymate-based pan-HDACi belinostat (PXD101, Beleodaq®) is structurally similar to SAHA but shows a greater blood brain barrier (BBB) passage [47]. In 2019, the potential of PXD101 was confirmed in an orthotopic rat glioma model. In a pilot study, PXD101 combined with TMZ/RT-delayed GB recurrence [47,48].
Depsipeptide romidepsin (Istodax®, FR901228, FK228) is a stable prodrug isolated from Chromobacterium violaceum and a class I HDACi [49,50]. In a phase I/II clinical trial in recurrent GB, romidepsin was found to be ineffective as a single agent [49].
Panobinostat (LBH589), a pan-deacetylase inhibitor of class I/II HDAC, is an antineoplastic and antiangiogenic drug that may work synergistically with BEV [51]. However, although this combined treatment strategy was well-tolerated, PFS and OS did not significantly improve compared to BEV monotherapy in recurrent GB [52]. A phase II trial is warranted to assess the combination with fractionated stereotactic re-irradiation therapy [53]. Panobinostat does not cross the BBB, and hence intratumoral or convection-enhanced delivery (CED) administration could be necessary [54].
HDACi valproic acid (valproate, VPA, Depakene), an anticonvulsive drug, has been shown to directly or synergistically exert inhibitory effects on glioma in vitro and in vivo [55]. VPA combined with TMZ/RT showed improvement in survival, but this might be limited to GB patients with wild-type p53 [56,57]. However, a phase III trial is warranted [58].
In the last 2 decades, an extensive amount of preclinical research on HDACi and multi-drug combinations in GB has been performed (see Supplementary Table S1) [19,33,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127]. These studies provided new insights on HDACi-associated signaling processes.
HDACi appear to have a vital role in DNA damage response, and a radiosensitizing effect of HDACi (vorinostat, panobinostat, VPA, entinostat, scriptaid) has been shown in GB in vitro, with support for vorinostat for GB therapy in combination with heavy ion therapy [31,33,35,128]. HDACi have been shown to inhibit GB cell growth mediated by cell cycle arrest and apoptosis, as highlighted in Supplementary Table S1 [129,130,131,132,133,134,135]. The class I/II HDACi trichostatin A (TSA) increased GB apoptosis induction through the p38MAPK-p53 cascade [136]. When combined with the proteasome inhibitor (MG132), 2-deoxy-d-glucose or lomustine (CCNU), synergistic apoptosis induction was shown [137,138,139]. The GB chemosensitization effects of HDACi therapy have also been noted, such as enhancement of TMZ-induced apoptosis [118,140,141,142,143]. However, vorinostat favored the evolution of TMZ resistance through O6-methylguanine DNA methyltransferase (MGMT) overexpression in GB in vivo [144]. HDACi SAHA and MC1568 blocked vascular mimicry in GB, and the inhibiting effects of HDACi on the invasiveness or migration of GB cells have been noted [143,145,146,147,148,149]. Multiple HDACi (vorinostat, romidepsin, MPT0B291, CDK4) have shown to increase the survival time of GB in vivo models [130,131,134,150,151].
Post-HDACi therapy, multiple genes that play a role in complex signaling pathways are up- or down-regulated, as recently summarized [11]. As expected based on preclinical data, the affected genes are involved in cell cycle progression, apoptosis, invasion and progrowth or include oncogenes and GSC markers [11].
Targeted drug combinations may beneficially affect the outcome of GB therapy, with the possible induction of synthetic lethality. Preclinically, promising combinations include a mix of epigenetic modifiers [152], HDACi combined with imipridones (activation of the mitochondrial ClpP protease) or proteasome inhibitors [153,154], panobinostat combined with a dual PI3K/mTOR inhibitor BEZ235 [155] and combining HDACi with MEK inhibitors or RTKi [156,157]. A triple combination therapy, involving panobinostat, OTX015 and sorafenib also showed potential in vitro [158]. Interestingly, the R132H mutation in isocitrate dehydrogenase 1 (IDH1R132H), commonly observed and associated with better survival in GB, has been linked to resistance to the anti-cancer effect of HDACi, such as TSA, vorinostat (SAHA) and valproic acid [159].
4. HDAC-Targeting Radiopharmaceuticals
The association of epigenetic dysfunction with disease and the development of diagnostic or therapeutic agents for treatment are challenging [160]. Most HDACi target a relatively wide spectrum of HDACs that, on their turn, inhibit various biological pathways. Their mechanisms of action as tumor suppressors have not yet been fully elucidated [10]. HDAC-targeting radiopharmaceuticals could provide better insights regarding HDAC tissue expression, HDACi biodistribution and pharmacokinetics and therapeutic efficacy and thereby unravel new insights into the function or behavior of HDACi in vivo [161,162]. Nuclear imaging of HDAC expression in GB may improve the understanding and roleplay of HDAC enzymes within gliomagenesis, identify patients likely to benefit from HDACi-targeted therapy and aid in optimizing therapeutic doses of novel HDACi for glioma treatment [163]. Importantly, there are two main strategies to consider when imaging an epigenetic target in the brain: 1) by direct observation (protein target information independent of its activity) and 2) functional observation (representative visualization of the impact of a protein or enzyme) [160]. Alternative methods to determine HDAC expression include invasive tumor biopsies and the use of peripheral lymphocytes as surrogate biomarkers for global acetylation after HDACi treatment.
An overview of HDACi-based radiopharmaceuticals is given in Figure 2, and Table 2 summarizes the preclinical development of HDAC radiopharmaceuticals. To visualize or treat GB with radiopharmaceuticals, it is particularly important to only consider those HDACi that sufficiently pass the BBB (even at sub-nanomolar concentration) and are of a small enough structure to allow their penetration into the bulky, heterogeneous tumor tissue [29]. In addition, the cellular location of the targeted HDAC needs to be considered, e.g., HDAC class I proteins are found predominantly in the nucleus, while class II proteins are primarily localized in the cytoplasm but can be shuttled between the cytoplasm and nucleus depending on their phosphorylation status [6].
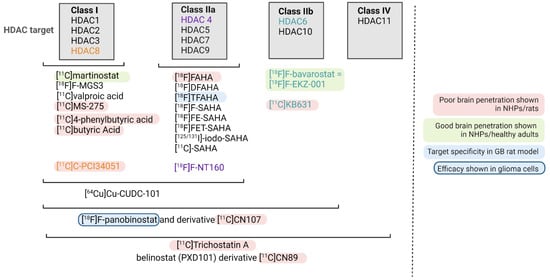
Figure 2.
Overview of current radiopharmaceuticals targeting HDAC. The HDAC class targeted is shown. If only one HDAC enzyme is targeted, this was highlighted in color (orange, purple and green).
To our knowledge, the potential of therapeutic HDAC radiopharmaceuticals for targeted radionuclide therapy (TRT) has not yet been explored. Importantly, possible brain toxicity may be a limiting aspect for this kind of application. HDACs play distinct physiological roles in the brain, and HDACi have pleiotropic effects due to their broad targets. This suggests a higher chance of success for isoform-specific HDACi or the necessity to inject such radioactive agents via CED directly into the GB tumor or its vicinity [164]. Another option is the use of nanovectors with theranostic properties to optimize the tumor delivery of potent HDACi, which could improve their anti-GB properties in vivo [165]. Other criteria to consider for the development of GB TRT agents were recently published by our group [166].
HDAC brain PET has been studied for the potential detection of various neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease, and limited studies have investigated their potential for glioma imaging [160,163,164]. Most HDAC radiopharmaceuticals are structurally related to SAHA and include 6-([18F]fluoroacetamido)-1-hexanoicanilide ([18F]FAHA), 6-(di-[18F]fluoroacetamido)-1-hexanoicanilide ([18F]DFAHA), 6-(tri-[18F]fluoroacetamido)-1-hexanoicanilide ([18F]TFAHA), [18F]F-SAHA (Figure 3A), N1-(4-(2-fluoroethyl)phenyl)-N8-hydroxyoctanediamide ([18F]FE-SAHA), [18F]fluoro-ethyltriazolesuberohydroxamine acid ([18F]FET-SAHA) (Figure 3B), [125/131I]-iodo-SAHA and two 11C-labeled SAHA-based ligands [162,167,168,169,170,171,172,173].
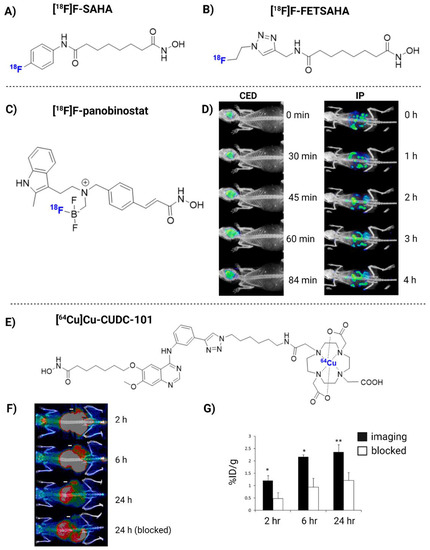
Figure 3.
The chemical structure of [18F]F-SAHA (A), [18F]FET-SAHA (B), [18F]F-panobinostat (C) and [64Cu]Cu-CUDC-101 (E). [18F]F-panobinostat PET imaging when delivered via convection-enhanced delivery (CED) or intraperitoneal (IP) (D). In vivo PET imaging of [64Cu]Cu-CUDC-101 in MDA-MB-231-bearing tumor mice, with or without co-injection of cold CUDC-101 (F,G). Images reproduced with permission from [180,184]. Copyright 2013 and 2018 American Chemical Society.
In 2006, the first 18F-labeled SAHA analogue ([18F]FAHA) was radiosynthesized by Mukhopadhyay et al. [168]. Soon thereafter, Nishii et al. confirmed PET in vivo brain uptake in rats of up to 0.44%ID/g between 5 and 60 min [169]. Moreover, blocking studies revealed a specificity similar to that of SAHA, suggesting that [18F]FAHA is a clinically relevant PET tracer capable of targeting HDAC IIa expression [170]. [18F]FAHA has also shown potential to monitor alterations in HDAC activity/expression in a rat model of chemotherapy-induced brain neurotoxicity [174]. Concerns were raised about [18F]fluoroacetate ([18F]FACE), a radiometabolite of the rapidly metabolized [18F]FAHA, that also crosses the BBB and therefore complicates [18F]FAHA quantification [171]. Fortunately, in non-human primates (NHP), the contribution of [18F]FACE to the 18F activity signal was minimal in the first 30 min post-administration [175]. A [18F]FAHA-like substrate developed by Seo et al. displayed an insufficient BBB permeability and HDAC specificity [167]. Bonomi et al. modified the structure of [18F]FAHA to add two or three fluorine groups ([18F]DFAHA or [18F]TFAHA, respectively), which increased the lipophilicity and thus BBB permeability [172]. In 2019, [18F]TFAHA was finally studied in GB rat models that confirmed tumor uptake 20 min post-radiotracer administration, which significantly reduced after administration of HDACi MC1568. [18F]TFAHA accumulation was also observed in normal brain structures known to overexpress HDAC class IIa: the hippocampus, nucleus accumbens, periaqueductal gray matter and cerebellum [163].
Brain uptake was reported of another radiolabeled SAHA-analogue, [18F]FE-SAHA, but its metabolic instability remains a substantial obstacle (high uptake in the kidneys, liver, bone and small intestines) [176]. Kim et al. developed [18F]FET-SAHA, which showed improved metabolic stability over [18F]FE-SAHA and accumulation in sarcoma tumors [173]. [125/131I]-iodo-SAHA maintained a comparable profile (e.g., similar toxicity and pharmacokinetics) to SAHA. However, in tumor-bearing mice, it showed no preferential tumor accumulation, rapid efflux and unspecific washout. Moreover, accumulation in the liver and kidneys was high [177]. Thus, none of the proposed SAHA-based radiopharmaceuticals have reached a clinical phase.
Another group of HDACi-based radiopharmaceuticals, including [11C] trichostatin A, [11C]MS-275, [11C]KB631, [11C]4-phenylbutyric acid, [11C]valproate, [11C]butyric acid, [11C]CN89, [11C]CN107, [18F]F-panobinostat and [11C]PCI34051 demonstrated inadequate BBB penetration, which discourages their application for the HDAC-based imaging of GB, despite possible application in other tumor types [161,167,172,178,179,180,181]. [18F]F-panobinostat has bioactivity similar to that of unmodified panobinostat against diffuse intrinsic pontine glioma and U87MG glioma cells (nM efficacy), with low toxicity to healthy astrocytes [180]. [18F]F-panobinostat has also shown potential for PET-guided CED in order to achieve high brain concentrations in healthy mice and a pediatric diffuse midline glioma model, which could be translated to high-grade glioma (Figure 3C,D) [182].
Recently, radiolabeling of trifluoromethyloxadiazole (TFMO)-bearing class-IIa HDACi were explored, and NT160 was identified as a potent inhibitor of class-IIa HDAC4. [18F]F-NT160 was capable of BBB crossing, and binding to class-IIa HDACs was confirmed in mouse brain tissue [183].
In addition, radiometal-nuclide-labeled ligands have also been developed, such as a 64Cu-labeled hydroxamic acid-based radioligand 7-(4-(3-ethynylphenylamino)-7-methoxyquinazolin-6-yloxy)-N-hydroxyheptanamide (CUDC-101). CUDC-101 entered into Phase I clinical trial testing in multiple tumor types (but excluding glioma). [64Cu]Cu-CUDC-101 exhibits the capability to image HDAC expression in triple-negative breast cancer (Figure 3E–G). However, most radioligands conjugated to metal chelators fail to cross an intact BBB [184]. To this end, [64Cu]Cu-CUDC-101 may be a good candidate to explore CED-based administration for GB therapy.
As the development of highly brain-penetrant HDACi has been a persistent challenge, research is now shifting from radiolabeling existing HDACi to the development of novel brain-penetrant radiotracers; in particular, adamantane-conjugated radioligands seem promising [164]. The most advanced candidate is (E)-3-(4-((((3r,5r,7r)-adamantan-1-ylmethyl)([11C]methyl)amino)methyl)phenyl)-N-hydroxyacrylamide ([11C]martinostat), an adamantane-based hydroxamic acid with selective binding to HDAC 1, 2, 3 and 6 with subnanomolar potency and fast-binding kinetics [185]. In vivo, [11C]C-martinostat has shown a selective, reversible and dose-dependent binding, excellent signal-to-noise ratio and desirable safety profiles in rodents, pigs and humans [185,186,187]. Next, an 18F-labeled derivative of martinostat, [18F]F-MGS3, was developed by Strebl et al. [188]. [18F]F-MGS3 exhibited HDAC-specific binding, as well as comparable brain uptake and regional distribution compared to [11C]martinostat. However, [18F]F-MGS3 warrants more efficient radiosynthesis as poor yields and manual synthesis only allowed for low doses to be administered [188]. Lastly, [18F]F-bavarostat ([18F]F-EKZ-001) appears to be useful for HDAC6 quantification. In NHPs, [18F]F-EKZ-001 displayed rapid and high brain tissue uptake and excellent specific binding which was subsequently confirmed in healthy human adults [189,190].

Table 2.
Preclinical development of HDAC radiopharmaceuticals.
Table 2.
Preclinical development of HDAC radiopharmaceuticals.
| Radio- Pharmaceutical | (Pre)clinical Model | Year | Main Outcome and Findings | Ref |
|---|---|---|---|---|
| [18F]FAHA | Healthy rats | 2007 | (+) Uptake increased rapidly up to 0.44%ID/g (5–60 min). Target blocking (SAHA) decreased uptake | [169] |
| Healthy NHP | 2009 | (−) Rapidly metabolized to [18F]FACE, which enters the brain | [171] | |
| Healthy NHP | 2013 | (-) Lack of BBB permeability and specificity | [167] | |
| Healthy NHP | 2013 | (+) BBB crossing. Limited influence of [18F]FACE to brain uptake (first 30 min) | [175] | |
| NNK-treated A/J mice | 2014 | (+) Midbrain, cerebellum and brainstem uptake was displaced by SAHA with <10% remaining | [170] | |
| Healthy mice | 2018 | (+) Specific uptake consistent with increased HDAC levels | [174] | |
| [18F]DFAHA | Healthy rats | 2015 | (+) Selectivity for HDAC Class IIa > [18F]FAHA, favorably low unspecific brain accumulation | [172] |
| [18F]TFAHA | Healthy rats | 2015 | (+) Selectivity for HDAC class IIa > [18F]DFAHA and [18F]FAHA | [172] |
| Intracerebral 9L and U87-MG rat xenografts | 2019 | (+) Increased accumulation at 20 min post-radiotracer administration (+) Target specificity, i.e., significant reduction uptake in 9L tumors after administration of HDACi MC1568 but not the SIRT1 specific inhibitor EX-527 | [163] | |
| AD mouse model | 2021 | (+) Potential as an epigenetic radiotracer for AD | [191] | |
| [18F]F-SAHA | A2780 OC mice | 2011 | (+) Exhibits nM potency. Target binding efficacy can be quantitated within 24 h | [162] |
| [125/131I]-iodo-SAHA | Thyroid, hepatoma, colon carcinoma- bearing mice | 2008 | (−) Equally toxic as SAHA. Rapid efflux and rapid washout and no preferential tumor accumulation. High (unwanted) accumulation in liver and kidneys | [177] |
| [18F]FE-SAHA | Mice LNCaP xenografts | 2011 | (+) Tumor uptake (−) High (unwanted) uptake in small intestines, kidneys, liver and bone (suspected defluorination) | [176] |
| [18F]FET-SAHA | RR1022 sarcoma rat | 2018 | (+) Significant accumulation in tumors with rapid blood clearance (gastrointestinal/renal excretion). Tracer accumulation was receptor-specific | [173] |
| [11C]TSA | Healthy NHP | 2013 | (−) Lack of BBB permeability and HDAC-specificity | [167] |
| [11C]MS-275 | Healthy mice, rats, NHP | 2010 | (−) Poor brain penetration and lack of tracer specificity | [178] |
| [11C]KB631 | B16.F10 murine melanoma-bearing mice | 2019 | (+) Showed HDAC6-selective binding (−) Lack of brain penetrance in rats, possibly due to the hydroxamate moiety | [181] |
| [11C]CN89 | Healthy rats, NHP | 2013 | (−) Poor BBB penetration | [179] |
| [11C]CN107 | ||||
| [18F]F-panobinostat | DIPG IV and XIII + U87MG glioma cells | 2018 | (+) Retains nM efficacy in glioma cells in vitro. Highly selective to glioma, with low toxicity to healthy astrocyte controls. Successful delivery to the murine central nervous system via CED (Figure 3D) | [180] |
| [11C]-4-phenylbutyric acid | Healthy NHP | 2013 | (−) Low brain uptake. Showed 15% metabolization after 30 min. High (unwanted) uptake in liver and heart | [161] |
| [11C]valproic acid | Healthy NHP | 2013 | (−) Low brain uptake. Showed 2% metabolization after 30 min. Exceptionally high (unwanted) heart uptake possibly due to its involvement in lipid metabolism | [161] |
| [11C]butyric Acid | Healthy NHP | 2013 | (−) Low brain uptake. Rapid metabolization (plasma: 80% metabolized after 5 min). Relatively high (unwanted) uptake in spleen and pancreas | [161] |
| [11C]PCI34051 | Healthy rats/NHP | 2013 | (−) Poor BBB penetration. Low uptake in the brain within 80 min. Pretreatment with 2 mg/kg standard did not improve retention or permeability | [179] |
| [64Cu]Cu-CUDC-101 | MDA-MB-231 xenograft mice | 2013 | (+) Specific binding to HDACs in vitro (nM). High TBR in vivo (Figure 3F) | [184] |
| [11C]martinostat | Healthy rats | 2014 | (+) Can quantify target engagement of structurally distinct, brain-penetrant hydroxamate HDACi in living rat brain | [192] |
| Healthy NHP | 2014 | (+) Highly selective and specific. Testing in humans is warranted | [185] | |
| Healthy NHP | 2015 | (+) Allows quantification of brain HDAC expression. Reversible and dose-dependent binding. Slow washout kinetics observed | [193] | |
| Healthy humans | 2016 | (+) Selectively binds HDAC1, 2 and 3 | [186] | |
| Healthy pigs | 2020 | (+) Allowed for accurate in vivo measurement of cerebral HDAC1—3 protein levels. Excellent signal-to-noise ratio | [187] | |
| [18F]F-MGS3 | Healthy rats, NHP | 2016 | (+) Exhibits specific binding/comparable brain uptake and regional distribution to [11C]martinostat | [188] |
| [18F]F-bavarostat | Healthy rats, NHP | 2017 | (+) Selective HDAC6 inhibitor. Excellent brain penetrance. Low amount of nonspecific binding observed after pre-treatment with 1 mg/kg unlabeled bavarostat | [194] |
| Healthy NHP | 2020 | (+) Excellent brain penetrance. Good HDAC6 selectivity, enabling quantification | [189] | |
| Healthy humans | 2021 | (+) Safe to administer and accurate quantification of HDAC6 expression in the human brain | [190] | |
| [18F]F-NT160 | Healthy mice | 2022 | (+) Can cross the BBB and bind to class-IIa HDACs in vivo in mice brain tissue | [183] |
AD = Alzheimer’s disease; BBB = blood brain barrier; CED = convection enhanced delivery, CNS = central nervous system; DIPG = diffuse intrinsic pontine glioma; HDAC = histone deacetylase; i.v. = intravenous; LNCaP = lymph node carcinoma of the prostate; NHP = non-human primate; NNK = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; OC = ovarian cancer; PET = positron emission tomography; TBR = tumor-to-background ratio.
5. Challenges and Future Outlook
Although extensive research has been performed on HDACi in glioma with clear radio- and/or chemosensitizing effects, the potential of radiolabeled HDACi has only been confirmed in the field of neurodegenerative diseases and been primarily diagnostic, with the goal of quantifying HDAC expression and/or monitoring treatment response. Their potential for GB imaging and TRT is underexplored. Whilst furthering this field of research should be recommended, one of the major issues that slowed down recent translation to clinics was poor BBB penetration, poor specificity and diverse target locations. Interestingly, adamantane-conjugated radioligands seem promising to increase brain penetrance [164]. Only two radiotracers have been investigated in healthy adults: [11C]martinostat and [18F]F-bavarostat, confirming the ability to quantify HDAC expression [186,190]. Both should be recommended for GB HDAC imaging as they have shown target specificity and reported brain penetrance. [18F]TFAHA is the only radiopharmaceutical that has been evaluated in GB models, with uptake in GB tumors but also in normal brain structures known to overexpress HDAC class IIa [163]. Another recommendable radiopharmaceutical is [18F]F-NT160 featuring potent binding to class-IIa HDACs and BBB crossing in mice [183]. However, future studies are needed to increase its tumor specific uptake while preventing damage to healthy tissues.
The potential for HDACi-based radiopharmaceuticals in GB can currently be formulated as (1) biomarkers for HDAC expression, (2) elucidate the roles of HDAC class enzymes and (3) dose optimization of cold HDACi [163]. Cancer resistance and the toxic effects of HDACi are currently an issue to translate radiolabeled HDACi for potential application in TRT. HDACi are often pan-specific towards a specific HDAC class. As their substrates are present all over the human brain, targeting HDACi in GB may cause unwanted effects on healthy tissues too. However, CED could be considered to mitigate any adverse effects and circumvent the BBB. Targeting multiple HDAC proteins could also be advantageous due to the heterogeneous nature of GB. Research should be initiated to confirm this, including optimal combinatorial strategies for HDACi that permit efficacy as well as safety in GB.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph16020227/s1, Table S1: Representative summary of the preclinical development for candidate HDACi-derived glioma therapy.
Author Contributions
Conceptualization, J.B.; writing—original draft preparation, L.E., J.B. and E.N.S.; writing—review and editing, E.N.S., T.E., I.G. and J.B.; final approval of the version published, L.E., E.N.S., T.E., I.G. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This review includes data from the thesis of Liesbeth Everix (MSc in Biomedical sciences, Ghent University 2021). BioRender was used for creating the images.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. A Critical Overview of Targeted Therapies for Glioblastoma. Front. Oncol. 2018, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- LoPresti, P. HDAC6 in Diseases of Cognition and of Neurons. Cells 2020, 10, 12. [Google Scholar] [CrossRef]
- Mottamal, M.; Zheng, S.; Huang, T.L.; Wang, G. Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules 2015, 20, 3898–3941. [Google Scholar] [CrossRef]
- McClure, J.J.; Li, X.; Chou, C.J. Advances and Challenges of HDAC Inhibitors in Cancer Therapeutics. Adv. Cancer Res. 2018, 138, 183–211. [Google Scholar] [CrossRef]
- Parbin, S.; Kar, S.; Shilpi, A.; Sengupta, D.; Deb, M.; Rath, S.K.; Patra, S.K. Histone deacetylases: A saga of perturbed acetylation homeostasis in cancer. J. Histochem. Cytochem. 2014, 62, 11–33. [Google Scholar] [CrossRef]
- Was, H.; Krol, S.K.; Rotili, D.; Mai, A.; Wojtas, B.; Kaminska, B.; Maleszewska, M. Histone deacetylase inhibitors exert anti-tumor effects on human adherent and stem-like glioma cells. Clin. Epigenetics 2019, 11, 11. [Google Scholar] [CrossRef]
- Bezecny, P. Histone deacetylase inhibitors in glioblastoma: Pre-clinical and clinical experience. Med. Oncol. 2014, 31, 985. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, M.; Zhou, Y.; Guo, W.; Yi, M.; Zhang, Z.; Ding, Y.; Wang, Y. The application of histone deacetylases inhibitors in glioblastoma. J. Exp. Clin. Cancer Res. 2020, 39, 138. [Google Scholar] [CrossRef]
- Singh, A.K.; Bishayee, A.; Pandey, A.K. Targeting Histone Deacetylases with Natural and Synthetic Agents: An Emerging Anticancer Strategy. Nutrients 2018, 10, 731. [Google Scholar] [CrossRef]
- Yelton, C.J.; Ray, S.K. Histone deacetylase enzymes and selective histone deacetylase inhibitors for antitumor effects and enhancement of antitumor immunity in glioblastoma. Neuroimmunol. Neuroinflamm. 2018, 5, 46. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Chen, J.; Tan, Q.; Xie, C.; Li, C.; Zhan, W.; Wang, M. Silencing of histone deacetylase 2 suppresses malignancy for proliferation, migration, and invasion of glioblastoma cells and enhances temozolomide sensitivity. Cancer Chemother Pharmacol. 2016, 78, 1289–1296. [Google Scholar] [CrossRef]
- Mottet, D.; Pirotte, S.; Lamour, V.; Hagedorn, M.; Javerzat, S.; Bikfalvi, A.; Bellahcène, A.; Verdin, E.; Castronovo, V. HDAC4 represses p21(WAF1/Cip1) expression in human cancer cells through a Sp1-dependent, p53-independent mechanism. Oncogene 2009, 28, 243–256. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, J.M.; Chen, J.K.; Yan, X.L.; Chen, H.M.; Nong, W.X.; Huang, H.Q. Histone deacetylase 5 promotes the proliferation of glioma cells by upregulation of Notch 1. Mol. Med. Rep. 2014, 10, 2045–2050. [Google Scholar] [CrossRef]
- Shi, P.; Hoang-Minh, L.B.; Tian, J.; Cheng, A.; Basrai, R.; Kalaria, N.; Lebowitz, J.J.; Khoshbouei, H.; Deleyrolle, L.P.; Sarkisian, M.R. HDAC6 Signaling at Primary Cilia Promotes Proliferation and Restricts Differentiation of Glioma Cells. Cancers 2021, 13, 1644. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Chen, X.; Zhang, L.; Wang, X. Histone deacetylase 6 promotes growth of glioblastoma through inhibition of SMAD2 signaling. Tumour. Biol. 2015, 36, 9661–9665. [Google Scholar] [CrossRef]
- Huang, Z.; Xia, Y.; Hu, K.; Zeng, S.; Wu, L.; Liu, S.; Zhi, C.; Lai, M.; Chen, D.; Xie, L.; et al. Histone deacetylase 6 promotes growth of glioblastoma through the MKK7/JNK/c-Jun signaling pathway. J. Neurochem. 2020, 152, 221–234. [Google Scholar] [CrossRef]
- Kim, G.W.; Lee, D.H.; Yeon, S.K.; Jeon, Y.H.; Yoo, J.; Lee, S.W.; Kwon, S.H. Temozolomide-resistant Glioblastoma Depends on HDAC6 Activity Through Regulation of DNA Mismatch Repair. Anticancer Res. 2019, 39, 6731–6741. [Google Scholar] [CrossRef]
- Chueh, A.C.; Tse, J.W.T.; Dickinson, M.; Ioannidis, P.; Jenkins, L.; Togel, L.; Tan, B.; Luk, I.; Davalos-Salas, M.; Nightingale, R.; et al. ATF3 Repression of BCL-XL Determines Apoptotic Sensitivity to HDAC Inhibitors across Tumor Types. Clin. Cancer Res. 2017, 23, 5573–5584. [Google Scholar] [CrossRef] [PubMed]
- Bondarev, A.D.; Attwood, M.M.; Jonsson, J.; Chubarev, V.N.; Tarasov, V.V.; Schiöth, H.B. Recent developments of HDAC inhibitors: Emerging indications and novel molecules. Br. J. Clin. Pharmacol. 2021, 87, 4577–4597. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, G.; Mercatelli, D.; Di Muzio, G.; Triboli, L.; De Rosa, P.; Perini, G.; Giorgi, F.M. Histone Deacetylases (HDACs): Evolution, Specificity, Role in Transcriptional Complexes, and Pharmacological Actionability. Genes 2020, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Jenke, R.; Reßing, N.; Hansen, F.K.; Aigner, A.; Büch, T. Anticancer Therapy with HDAC Inhibitors: Mechanism-Based Combination Strategies and Future Perspectives. Cancers 2021, 13, 634. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Lee, I.N.; Huang, C.; Wu, Y.P.; Chung, C.Y.; Lee, M.H.; Lin, M.H.; Yang, J.T. Valproic acid-induced amphiregulin secretion confers resistance to temozolomide treatment in human glioma cells. BMC Cancer 2019, 19, 756. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, P.; Tang, F.; Lian, H.; Chen, X.; Zhang, Y.; He, X.; Liu, W.; Xie, C. HDAC6 promotes cell proliferation and confers resistance to temozolomide in glioblastoma. Cancer Lett. 2016, 379, 134–142. [Google Scholar] [CrossRef]
- Yang, W.B.; Wu, A.C.; Hsu, T.I.; Liou, J.P.; Lo, W.L.; Chang, K.Y.; Chen, P.Y.; Kikkawa, U.; Yang, S.T.; Kao, T.J.; et al. Histone deacetylase 6 acts upstream of DNA damage response activation to support the survival of glioblastoma cells. Cell Death Dis. 2021, 12, 884. [Google Scholar] [CrossRef]
- Groselj, B.; Sharma, N.L.; Hamdy, F.C.; Kerr, M.; Kiltie, A.E. Histone deacetylase inhibitors as radiosensitisers: Effects on DNA damage signalling and repair. Br. J. Cancer 2013, 108, 748–754. [Google Scholar] [CrossRef]
- Shabason, J.E.; Tofilon, P.J.; Camphausen, K. Grand rounds at the National Institutes of Health: HDAC inhibitors as radiation modifiers, from bench to clinic. J. Cell Mol. Med. 2011, 15, 2735–2744. [Google Scholar] [CrossRef]
- Camphausen, K.; Tofilon, P.J. Inhibition of histone deacetylation: A strategy for tumor radiosensitization. J. Clin. Oncol. 2007, 25, 4051–4056. [Google Scholar] [CrossRef]
- Camphausen, K.; Cerna, D.; Scott, T.; Sproull, M.; Burgan, W.E.; Cerra, M.A.; Fine, H.; Tofilon, P.J. Enhancement of in vitro and in vivo tumor cell radiosensitivity by valproic acid. Int. J. Cancer 2005, 114, 380–386. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, J.H.; Kim, I.H. Susceptibility and radiosensitization of human glioblastoma cells to trichostatin A, a histone deacetylase inhibitor. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 1174–1180. [Google Scholar] [CrossRef]
- Camphausen, K.; Burgan, W.; Cerra, M.; Oswald, K.A.; Trepel, J.B.; Lee, M.J.; Tofilon, P.J. Enhanced radiation-induced cell killing and prolongation of gammaH2AX foci expression by the histone deacetylase inhibitor MS-275. Cancer Res. 2004, 64, 316–321. [Google Scholar] [CrossRef]
- Diss, E.; Nalabothula, N.; Nguyen, D.; Chang, E.; Kwok, Y.; Carrier, F. Vorinostat(SAHA) Promotes Hyper-Radiosensitivity in Wild Type p53 Human Glioblastoma Cells. J. Clin. Oncol. Res. 2014, 2, 1. [Google Scholar]
- Barazzuol, L.; Jeynes, J.C.; Merchant, M.J.; Wéra, A.C.; Barry, M.A.; Kirkby, K.J.; Suzuki, M. Radiosensitization of glioblastoma cells using a histone deacetylase inhibitor (SAHA) comparing carbon ions with X-rays. Int. J. Radiat. Biol. 2015, 91, 90–98. [Google Scholar] [CrossRef]
- Conti, C.; Leo, E.; Eichler, G.S.; Sordet, O.; Martin, M.M.; Fan, A.; Aladjem, M.I.; Pommier, Y. Inhibition of histone deacetylase in cancer cells slows down replication forks, activates dormant origins, and induces DNA damage. Cancer Res. 2010, 70, 4470–4480. [Google Scholar] [CrossRef]
- Namdar, M.; Perez, G.; Ngo, L.; Marks, P.A. Selective inhibition of histone deacetylase 6 (HDAC6) induces DNA damage and sensitizes transformed cells to anticancer agents. Proc. Natl. Acad. Sci. USA 2010, 107, 20003–20008. [Google Scholar] [CrossRef]
- Lee, P.; Murphy, B.; Miller, R.; Menon, V.; Banik, N.L.; Giglio, P.; Lindhorst, S.M.; Varma, A.K.; Vandergrift, W.A., 3rd; Patel, S.J.; et al. Mechanisms and clinical significance of histone deacetylase inhibitors: Epigenetic glioblastoma therapy. Anticancer Res. 2015, 35, 615–625. [Google Scholar]
- Chiao, M.T.; Cheng, W.Y.; Yang, Y.C.; Shen, C.C.; Ko, J.L. Suberoylanilide hydroxamic acid (SAHA) causes tumor growth slowdown and triggers autophagy in glioblastoma stem cells. Autophagy 2013, 9, 1509–1526. [Google Scholar] [CrossRef]
- Galanis, E.; Jaeckle, K.A.; Maurer, M.J.; Reid, J.M.; Ames, M.M.; Hardwick, J.S.; Reilly, J.F.; Loboda, A.; Nebozhyn, M.; Fantin, V.R.; et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: A north central cancer treatment group study. J. Clin. Oncol. 2009, 27, 2052–2058. [Google Scholar] [CrossRef]
- Lee, E.Q.; Puduvalli, V.K.; Reid, J.M.; Kuhn, J.G.; Lamborn, K.R.; Cloughesy, T.F.; Chang, S.M.; Drappatz, J.; Yung, W.K.; Gilbert, M.R.; et al. Phase I study of vorinostat in combination with temozolomide in patients with high-grade gliomas: North American Brain Tumor Consortium Study 04-03. Clin. Cancer Res. 2012, 18, 6032–6039. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 5 September 2022).
- Puduvalli, V.K.; Wu, J.; Yuan, Y.; Armstrong, T.S.; Groves, M.D.; Raizer, J.J.; Giglio, P.; Colman, H.; Peereboom, D.M.; Walbert, T.; et al. Brain Tumor Trials Collaborative Bayesian Adaptive Randomized Phase II trial of bevacizumab plus vorinostat versus bevacizumab alone in adults with recurrent glioblastoma (BTTC-1102). J. Clin. Oncol. 2015, 33, 2012. [Google Scholar] [CrossRef]
- Peters, K.B.; Lipp, E.S.; Miller, E.; Herndon, J.E., 2nd; McSherry, F.; Desjardins, A.; Reardon, D.A.; Friedman, H.S. Phase I/II trial of vorinostat, bevacizumab, and daily temozolomide for recurrent malignant gliomas. J. Neurooncol. 2018, 137, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Ghiaseddin, A.; Reardon, D.; Massey, W.; Mannerino, A.; Lipp, E.S.; Herndon, J.E., 2nd; McSherry, F.; Desjardins, A.; Randazzo, D.; Friedman, H.S.; et al. Phase II Study of Bevacizumab and Vorinostat for Patients with Recurrent World Health Organization Grade 4 Malignant Glioma. Oncologist 2018, 23, 157-e21. [Google Scholar] [CrossRef]
- Kang, D.W.; Hwang, W.C.; Noh, Y.N.; Kang, Y.; Jang, Y.; Kim, J.A.; Min, D.S. Phospholipase D1 is upregulated by vorinostat and confers resistance to vorinostat in glioblastoma. J. Cell. Physiol. 2021, 236, 549–560. [Google Scholar] [CrossRef]
- Gurbani, S.S.; Yoon, Y.; Weinberg, B.D.; Salgado, E.; Press, R.H.; Cordova, J.S.; Ramesh, K.K.; Liang, Z.; Velazquez Vega, J.; Voloschin, A.; et al. Assessing Treatment Response of Glioblastoma to an HDAC Inhibitor Using Whole-Brain Spectroscopic MRI. Tomography 2019, 5, 53–60. [Google Scholar] [CrossRef]
- Xu, K.; Ramesh, K.; Huang, V.; Gurbani, S.S.; Cordova, J.S.; Schreibmann, E.; Weinberg, B.D.; Sengupta, S.; Voloschin, A.D.; Holdhoff, M.; et al. Final Report on Clinical Outcomes and Tumor Recurrence Patterns of a Pilot Study Assessing Efficacy of Belinostat (PXD-101) with Chemoradiation for Newly Diagnosed Glioblastoma. Tomography 2022, 8, 688–700. [Google Scholar] [CrossRef]
- Iwamoto, F.M.; Lamborn, K.R.; Kuhn, J.G.; Wen, P.Y.; Yung, W.K.; Gilbert, M.R.; Chang, S.M.; Lieberman, F.S.; Prados, M.D.; Fine, H.A. A phase I/II trial of the histone deacetylase inhibitor romidepsin for adults with recurrent malignant glioma: North American Brain Tumor Consortium Study 03-03. Neuro-Oncology 2011, 13, 509–516. [Google Scholar] [CrossRef]
- Furumai, R.; Matsuyama, A.; Kobashi, N.; Lee, K.H.; Nishiyama, M.; Nakajima, H.; Tanaka, A.; Komatsu, Y.; Nishino, N.; Yoshida, M.; et al. FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases. Cancer Res. 2002, 62, 4916–4921. [Google Scholar]
- Van Veggel, M.; Westerman, E.; Hamberg, P. Clinical Pharmacokinetics and Pharmacodynamics of Panobinostat. Clin. pharmacokinet. 2018, 57, 21–29. [Google Scholar] [CrossRef]
- Lee, E.Q.; Reardon, D.A.; Schiff, D.; Drappatz, J.; Muzikansky, A.; Grimm, S.A.; Norden, A.D.; Nayak, L.; Beroukhim, R.; Rinne, M.L.; et al. Phase II study of panobinostat in combination with bevacizumab for recurrent glioblastoma and anaplastic glioma. Neuro-Oncology 2015, 17, 862–867. [Google Scholar] [CrossRef]
- Shi, W.; Palmer, J.D.; Werner-Wasik, M.; Andrews, D.W.; Evans, J.J.; Glass, J.; Kim, L.; Bar-Ad, V.; Judy, K.; Farrell, C.; et al. Phase I trial of panobinostat and fractionated stereotactic re-irradiation therapy for recurrent high grade gliomas. J. Neuro-Oncol. 2016, 127, 535–539. [Google Scholar] [CrossRef]
- Singleton, W.G.B.; Bienemann, A.S.; Woolley, M.; Johnson, D.; Lewis, O.; Wyatt, M.J.; Damment, S.J.P.; Boulter, L.J.; Killick-Cole, C.L.; Asby, D.J.; et al. The distribution, clearance, and brainstem toxicity of panobinostat administered by convection-enhanced delivery. J. Neurosurg. Pediatr. 2018, 22, 288–296. [Google Scholar] [CrossRef]
- Han, W.; Guan, W. Valproic Acid: A Promising Therapeutic Agent in Glioma Treatment. Front. Oncol. 2021, 11, 687362. [Google Scholar] [CrossRef]
- Krauze, A.V.; Myrehaug, S.D.; Chang, M.G.; Holdford, D.J.; Smith, S.; Shih, J.; Tofilon, P.J.; Fine, H.A.; Camphausen, K. A Phase 2 Study of Concurrent Radiation Therapy, Temozolomide, and the Histone Deacetylase Inhibitor Valproic Acid for Patients With Glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 986–992. [Google Scholar] [CrossRef]
- Tsai, H.C.; Wei, K.C.; Chen, P.Y.; Huang, C.Y.; Chen, K.T.; Lin, Y.J.; Cheng, H.W.; Chen, Y.R.; Wang, H.T. Valproic Acid Enhanced Temozolomide-Induced Anticancer Activity in Human Glioma Through the p53-PUMA Apoptosis Pathway. Front. Oncol. 2021, 11, 722754. [Google Scholar] [CrossRef]
- Krauze, A.V.; Megan, M.; Theresa, C.Z.; Peter, M.; Shih, J.H.; Tofilon, P.J.; Rowe, L.; Gilbert, M.; Camphausen, K. The addition of Valproic acid to concurrent radiation therapy and temozolomide improves patient outcome: A Correlative analysis of RTOG 0525, SEER and a Phase II NCI trial. Cancer Stud. Ther. 2020, 5, 722754. [Google Scholar] [CrossRef]
- Shim, H.; Wei, L.; Holder, C.A.; Guo, Y.; Hu, X.P.; Miller, A.H.; Olson, J.J. Use of high-resolution volumetric MR spectroscopic imaging in assessing treatment response of glioblastoma to an HDAC inhibitor. Am. J. Roentgenol. 2014, 203, W158–W165. [Google Scholar] [CrossRef]
- Chinnaiyan, P.; Chowdhary, S.; Potthast, L.; Prabhu, A.; Tsai, Y.Y.; Sarcar, B.; Kahali, S.; Brem, S.; Yu, H.M.; Rojiani, A.; et al. Phase I trial of vorinostat combined with bevacizumab and CPT-11 in recurrent glioblastoma. Neuro-oncology 2012, 14, 93–100. [Google Scholar] [CrossRef]
- Friday, B.B.; Anderson, S.K.; Buckner, J.; Yu, C.; Giannini, C.; Geoffroy, F.; Schwerkoske, J.; Mazurczak, M.; Gross, H.; Pajon, E.; et al. Phase II trial of vorinostat in combination with bortezomib in recurrent glioblastoma: A north central cancer treatment group study. Neuro-oncology 2012, 14, 215–221. [Google Scholar] [CrossRef]
- Galanis, E.; Anderson, S.K.; Miller, C.R.; Sarkaria, J.N.; Jaeckle, K.; Buckner, J.C.; Ligon, K.L.; Ballman, K.V.; Moore, D.F., Jr.; Nebozhyn, M.; et al. Phase I/II trial of vorinostat combined with temozolomide and radiation therapy for newly diagnosed glioblastoma: Results of Alliance N0874/ABTC 02. Neuro-oncology 2018, 20, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Kuwabara, Y.; Suehiro, S.; Yamashita, D.; Tanaka, M.; Tanaka, A.; Ohue, S.; Araki, H. Valproic acid reduces hair loss and improves survival in patients receiving temozolomide-based radiation therapy for high-grade glioma. Eur. J. Clin. Pharmacol. 2017, 73, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, H.; Tan, Q.; Xie, C.; Zhan, W.; Sharma, A.; Sharma, H.S.; Zhang, Z. The therapeutic and neuroprotective effects of an antiepileptic drug valproic acid in glioma patients. Prog. Brain Res. 2020, 258, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, M.; Dielemans, J.C.; van Breemen, M.S.; Zwinkels, H.; Walchenbach, R.; Taphoorn, M.J.; Vecht, C.J. Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro-oncology 2013, 15, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Valiyaveettil, D.; Malik, M.; Joseph, D.M.; Ahmed, S.F.; Kothwal, S.A.; Vijayasaradhi, M. Effect of valproic acid on survival in glioblastoma: A prospective single-arm study. S. Asian J. Cancer 2018, 7, 159–162. [Google Scholar] [CrossRef]
- Redjal, N.; Reinshagen, C.; Le, A.; Walcott, B.P.; McDonnell, E.; Dietrich, J.; Nahed, B.V. Valproic acid, compared to other antiepileptic drugs, is associated with improved overall and progression-free survival in glioblastoma but worse outcome in grade II/III gliomas treated with temozolomide. J. Neuro-Oncol. 2016, 127, 505–514. [Google Scholar] [CrossRef]
- Ugur, H.C.; Ramakrishna, N.; Bello, L.; Menon, L.G.; Kim, S.K.; Black, P.M.; Carroll, R.S. Continuous intracranial administration of suberoylanilide hydroxamic acid (SAHA) inhibits tumor growth in an orthotopic glioma model. J. Neuro-Oncol. 2007, 83, 267–275. [Google Scholar] [CrossRef]
- Jane, E.P.; Premkumar, D.R.; Addo-Yobo, S.O.; Pollack, I.F. Abrogation of mitogen-activated protein kinase and Akt signaling by vandetanib synergistically potentiates histone deacetylase inhibitor-induced apoptosis in human glioma cells. J. Pharmacol. Exp. Ther. 2009, 331, 327–337. [Google Scholar] [CrossRef]
- Orzan, F.; Pellegatta, S.; Poliani, P.L.; Pisati, F.; Caldera, V.; Menghi, F.; Kapetis, D.; Marras, C.; Schiffer, D.; Finocchiaro, G. Enhancer of Zeste 2 (EZH2) is up-regulated in malignant gliomas and in glioma stem-like cells. Neuropathol. Appl. Neurobiol. 2011, 37, 381–394. [Google Scholar] [CrossRef]
- Singh, M.M.; Manton, C.A.; Bhat, K.P.; Tsai, W.W.; Aldape, K.; Barton, M.C.; Chandra, J. Inhibition of LSD1 sensitizes glioblastoma cells to histone deacetylase inhibitors. Neuro-Oncology 2011, 13, 894–903. [Google Scholar] [CrossRef]
- Berghauser Pont, L.M.; Spoor, J.K.; Venkatesan, S.; Swagemakers, S.; Kloezeman, J.J.; Dirven, C.M.; van der Spek, P.J.; Lamfers, M.L.; Leenstra, S. The Bcl-2 inhibitor Obatoclax overcomes resistance to histone deacetylase inhibitors SAHA and LBH589 as radiosensitizers in patient-derived glioblastoma stem-like cells. Genes Cancer 2014, 5, 445–459. [Google Scholar] [CrossRef]
- Cornago, M.; Garcia-Alberich, C.; Blasco-Angulo, N.; Vall-Llaura, N.; Nager, M.; Herreros, J.; Comella, J.X.; Sanchis, D.; Llovera, M. Histone deacetylase inhibitors promote glioma cell death by G2 checkpoint abrogation leading to mitotic catastrophe. Cell Death Dis. 2014, 5, e1435. [Google Scholar] [CrossRef]
- Rasmussen, R.D.; Gajjar, M.K.; Jensen, K.E.; Hamerlik, P. Enhanced efficacy of combined HDAC and PARP targeting in glioblastoma. Mol. Oncol. 2016, 10, 751–763. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, C.; Feldman, M.J.; Wang, H.; Pang, Y.; Maggio, D.M.; Zhu, D.; Nesvick, C.L.; Dmitriev, P.; Bullova, P.; et al. Vorinostat suppresses hypoxia signaling by modulating nuclear translocation of hypoxia inducible factor 1 alpha. Oncotarget 2017, 8, 56110–56125. [Google Scholar] [CrossRef]
- Lohitesh, K.; Saini, H.; Srivastava, A.; Mukherjee, S.; Roy, A.; Chowdhury, R. Autophagy inhibition potentiates SAHA-mediated apoptosis in glioblastoma cells by accumulation of damaged mitochondria. Oncol. Rep. 2018, 39, 2787–2796. [Google Scholar] [CrossRef]
- Gonçalves, R.M.; Agnes, J.P.; Delgobo, M.; de Souza, P.O.; Thomé, M.P.; Heimfarth, L.; Lenz, G.; Moreira, J.C.F.; Zanotto-Filho, A. Late autophagy inhibitor chloroquine improves efficacy of the histone deacetylase inhibitor SAHA and temozolomide in gliomas. Biochem. Pharmacol. 2019, 163, 440–450. [Google Scholar] [CrossRef]
- Khathayer, F.; Taylor, M.A.; Ray, S.K. Synergism of 4HPR and SAHA increases anti-tumor actions in glioblastoma cells. Apoptosis 2020, 25, 217–232. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, Z.; Copland, J.A.; Mehrling, T.; Tun, H.W. Combined alkylation and histone deacetylase inhibition with EDO-S101 has significant therapeutic activity against brain tumors in preclinical models. Oncotarget 2018, 9, 28155–28164. [Google Scholar] [CrossRef]
- Kusaczuk, M.; Krętowski, R.; Stypułkowska, A.; Cechowska-Pasko, M. Molecular and cellular effects of a novel hydroxamate-based HDAC inhibitor - belinostat - in glioblastoma cell lines: A preliminary report. Investig. New Drugs 2016, 34, 552–564. [Google Scholar] [CrossRef]
- Berghauser Pont, L.M.; Kleijn, A.; Kloezeman, J.J.; van den Bossche, W.; Kaufmann, J.K.; de Vrij, J.; Leenstra, S.; Dirven, C.M.; Lamfers, M.L. The HDAC Inhibitors Scriptaid and LBH589 Combined with the Oncolytic Virus Delta24-RGD Exert Enhanced Anti-Tumor Efficacy in Patient-Derived Glioblastoma Cells. PLoS ONE 2015, 10, e0127058. [Google Scholar] [CrossRef]
- Meng, W.; Wang, B.; Mao, W.; Wang, J.; Zhao, Y.; Li, Q.; Zhang, C.; Tang, Y.; Ma, J. Enhanced efficacy of histone deacetylase inhibitor combined with bromodomain inhibitor in glioblastoma. J. Exp. Clin. Cancer Res. 2018, 37, 241. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Zhang, Y.; Shang, E.; Shu, C.; Torrini, C.; Zhao, J.; Bianchetti, E.; Mela, A.; Humala, N.; Mahajan, A.; et al. HDAC inhibitors elicit metabolic reprogramming by targeting super-enhancers in glioblastoma models. J. Clin. Investig. 2020, 130, 3699–3716. [Google Scholar] [CrossRef] [PubMed]
- De La Rosa, J.; Urdiciain, A.; Zazpe, I.; Zelaya, M.V.; Meléndez, B.; Rey, J.A.; Idoate, M.A.; Castresana, J.S. The synergistic effect of DZ-NEP, panobinostat and temozolomide reduces clonogenicity and induces apoptosis in glioblastoma cells. Int. J. Oncol. 2020, 56, 283–300. [Google Scholar] [CrossRef] [PubMed]
- De La Rosa, J.; Urdiciain, A.; Zelaya, M.V.; Zazpe, I.; Meléndez, B.; Rey, J.A.; Idoate, M.A.; Castresana, J.S. APR-246 combined with 3-deazaneplanocin A, panobinostat or temozolomide reduces clonogenicity and induces apoptosis in glioblastoma cells. Int. J. Oncol. 2021, 58, 312–330. [Google Scholar] [CrossRef]
- Pratap, U.P.; Sareddy, G.R.; Liu, Z.; Venkata, P.P.; Liu, J.; Tang, W.; Altwegg, K.A.; Ebrahimi, B.; Li, X.; Tekmal, R.R.; et al. Histone deacetylase inhibitors enhance estrogen receptor beta expression and augment agonist-mediated tumor suppression in glioblastoma. Neurooncol. Adv. 2021, 3, vdab099. [Google Scholar] [CrossRef]
- Knüpfer, M.M.; Hernáiz-Driever, P.; Poppenborg, H.; Wolff, J.E.; Cinatl, J. Valproic acid inhibits proliferation and changes expression of CD44 and CD56 of malignant glioma cells in vitro. Anticancer Res. 1998, 18, 3585–3589. [Google Scholar]
- Chavez-Blanco, A.; Perez-Plasencia, C.; Perez-Cardenas, E.; Carrasco-Legleu, C.; Rangel-Lopez, E.; Segura-Pacheco, B.; Taja-Chayeb, L.; Trejo-Becerril, C.; Gonzalez-Fierro, A.; Candelaria, M.; et al. Antineoplastic effects of the DNA methylation inhibitor hydralazine and the histone deacetylase inhibitor valproic acid in cancer cell lines. Cancer Cell Int. 2006, 6, 2. [Google Scholar] [CrossRef]
- Das, C.M.; Aguilera, D.; Vasquez, H.; Prasad, P.; Zhang, M.; Wolff, J.E.; Gopalakrishnan, V. Valproic acid induces p21 and topoisomerase-II (alpha/beta) expression and synergistically enhances etoposide cytotoxicity in human glioblastoma cell lines. J. Neurooncol. 2007, 85, 159–170. [Google Scholar] [CrossRef]
- Papi, A.; Ferreri, A.M.; Rocchi, P.; Guerra, F.; Orlandi, M. Epigenetic modifiers as anticancer drugs: Effectiveness of valproic acid in neural crest-derived tumor cells. Anticancer Res. 2010, 30, 535–540. [Google Scholar]
- Alvarez, A.A.; Field, M.; Bushnev, S.; Longo, M.S.; Sugaya, K. The effects of histone deacetylase inhibitors on glioblastoma-derived stem cells. J. Mol. Neurosci. 2015, 55, 7–20. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Yuan, X.; Hu, Z.; Li, H.; Wu, M.; Yuan, J.; Zhao, Z.; Su, J.; Wang, X.; et al. Valproic Acid Promotes Human Glioma U87 Cells Apoptosis and Inhibits Glycogen Synthase Kinase-3β Through ERK/Akt Signaling. Cell Physiol. Biochem. 2016, 39, 2173–2185. [Google Scholar] [CrossRef]
- Chang, Y.L.; Huang, L.C.; Chen, Y.C.; Wang, Y.W.; Hueng, D.Y.; Huang, S.M. The synergistic effects of valproic acid and fluvastatin on apoptosis induction in glioblastoma multiforme cell lines. Int. J. Biochem. Cell Biol. 2017, 92, 155–163. [Google Scholar] [CrossRef]
- Tseng, J.H.; Chen, C.Y.; Chen, P.C.; Hsiao, S.H.; Fan, C.C.; Liang, Y.C.; Chen, C.P. Valproic acid inhibits glioblastoma multiforme cell growth via paraoxonase 2 expression. Oncotarget 2017, 8, 14666–14679. [Google Scholar] [CrossRef]
- Garcia, C.G.; Kahn, S.A.; Geraldo, L.H.M.; Romano, I.; Domith, I.; Silva, D.; Dos Santos Assunção, F.; Ferreira, M.J.; Portugal, C.C.; de Souza, J.M.; et al. Combination Therapy with Sulfasalazine and Valproic Acid Promotes Human Glioblastoma Cell Death Through Imbalance of the Intracellular Oxidative Response. Mol. Neurobiol. 2018, 55, 6816–6833. [Google Scholar] [CrossRef]
- Riva, G.; Cilibrasi, C.; Bazzoni, R.; Cadamuro, M.; Negroni, C.; Butta, V.; Strazzabosco, M.; Dalprà, L.; Lavitrano, M.; Bentivegna, A. Valproic Acid Inhibits Proliferation and Reduces Invasiveness in Glioma Stem Cells Through Wnt/β Catenin Signalling Activation. Genes 2018, 9, 522. [Google Scholar] [CrossRef]
- Berendsen, S.; Frijlink, E.; Kroonen, J.; Spliet, W.G.M.; van Hecke, W.; Seute, T.; Snijders, T.J.; Robe, P.A. Effects of valproic acid on histone deacetylase inhibition in vitro and in glioblastoma patient samples. Neurooncol. Adv. 2019, 1, vdz025. [Google Scholar] [CrossRef]
- Sanaei, M.; Kavoosi, F. The effect of valproic acid on intrinsic, extrinsic, and JAK/STAT pathways in neuroblastoma and glioblastoma cell lines. Res. Pharm. Sci. 2022, 17, 392–409. [Google Scholar] [CrossRef]
- Tarasenko, N.; Chekroun-Setti, H.; Nudelman, A.; Rephaeli, A. Comparison of the anticancer properties of a novel valproic acid prodrug to leading histone deacetylase inhibitors. J. Cell Biochem. 2018, 119, 3417–3428. [Google Scholar] [CrossRef]
- Wetzel, M.; Premkumar, D.R.; Arnold, B.; Pollack, I.F. Effect of trichostatin A, a histone deacetylase inhibitor, on glioma proliferation in vitro by inducing cell cycle arrest and apoptosis. J. Neurosurg. 2005, 103, 549–556. [Google Scholar] [CrossRef]
- Svechnikova, I.; Almqvist, P.M.; Ekström, T.J. HDAC inhibitors effectively induce cell type-specific differentiation in human glioblastoma cell lines of different origin. Int. J. Oncol. 2008, 32, 821–827. [Google Scholar]
- Gao, J.; Chen, T.; Liu, J.; Liu, W.; Hu, G.; Guo, X.; Yin, B.; Gong, Y.; Zhao, J.; Qiang, B.; et al. Loss of NECL1, a novel tumor suppressor, can be restored in glioma by HDAC inhibitor-Trichostatin A through Sp1 binding site. Glia 2009, 57, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Foltz, G.; Yoon, J.G.; Lee, H.; Ma, L.; Tian, Q.; Hood, L.; Madan, A. Epigenetic regulation of wnt pathway antagonists in human glioblastoma multiforme. Genes Cancer 2010, 1, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Höring, E.; Podlech, O.; Silkenstedt, B.; Rota, I.A.; Adamopoulou, E.; Naumann, U. The histone deacetylase inhibitor trichostatin a promotes apoptosis and antitumor immunity in glioblastoma cells. Anticancer Res. 2013, 33, 1351–1360. [Google Scholar] [PubMed]
- Sassi Fde, A.; Caesar, L.; Jaeger, M.; Nör, C.; Abujamra, A.L.; Schwartsmann, G.; de Farias, C.B.; Brunetto, A.L.; Lopez, P.L.; Roesler, R. Inhibitory activities of trichostatin a in U87 glioblastoma cells and tumorsphere-derived cells. J. Mol. Neurosci. 2014, 54, 27–40. [Google Scholar] [CrossRef]
- Sun, P.; Xia, S.; Lal, B.; Eberhart, C.G.; Quinones-Hinojosa, A.; Maciaczyk, J.; Matsui, W.; Dimeco, F.; Piccirillo, S.M.; Vescovi, A.L.; et al. DNER, an epigenetically modulated gene, regulates glioblastoma-derived neurosphere cell differentiation and tumor propagation. Stem Cells 2009, 27, 1473–1486. [Google Scholar] [CrossRef]
- Carol, H.; Gorlick, R.; Kolb, E.A.; Morton, C.L.; Manesh, D.M.; Keir, S.T.; Reynolds, C.P.; Kang, M.H.; Maris, J.M.; Wozniak, A.; et al. Initial testing (stage 1) of the histone deacetylase inhibitor, quisinostat (JNJ-26481585), by the Pediatric Preclinical Testing Program. Pediatr. Blood Cancer 2014, 61, 245–252. [Google Scholar] [CrossRef]
- Bouché, M.; Dong, Y.C.; Sheikh, S.; Taing, K.; Saxena, D.; Hsu, J.C.; Chen, M.H.; Salinas, R.D.; Song, H.; Burdick, J.A.; et al. Novel Treatment for Glioblastoma Delivered by a Radiation Responsive and Radiopaque Hydrogel. ACS Biomater. Sci. Eng. 2021, 7, 3209–3220. [Google Scholar] [CrossRef]
- Zhang, W.; Lv, S.; Liu, J.; Zang, Z.; Yin, J.; An, N.; Yang, H.; Song, Y. PCI-24781 down-regulates EZH2 expression and then promotes glioma apoptosis by suppressing the PIK3K/Akt/mTOR pathway. Genet. Mol. Biol. 2014, 37, 716–724. [Google Scholar] [CrossRef]
- Asklund, T.; Appelskog, I.B.; Ammerpohl, O.; Ekström, T.J.; Almqvist, P.M. Histone deacetylase inhibitor 4-phenylbutyrate modulates glial fibrillary acidic protein and connexin 43 expression, and enhances gap-junction communication, in human glioblastoma cells. Eur. J. Cancer 2004, 40, 1073–1081. [Google Scholar] [CrossRef]
- Kusaczuk, M.; Krętowski, R.; Bartoszewicz, M.; Cechowska-Pasko, M. Phenylbutyrate-a pan-HDAC inhibitor-suppresses proliferation of glioblastoma LN-229 cell line. Tumour. Biol. 2016, 37, 931–942. [Google Scholar] [CrossRef]
- Engelhard, H.H.; Duncan, H.A.; Kim, S.; Criswell, P.S.; Van Eldik, L. Therapeutic effects of sodium butyrate on glioma cells in vitro and in the rat C6 glioma model. Neurosurgery 2001, 48, 616–624. [Google Scholar] [CrossRef]
- Nakagawa, H.; Sasagawa, S.; Itoh, K. Sodium butyrate induces senescence and inhibits the invasiveness of glioblastoma cells. Oncol. Lett. 2018, 15, 1495–1502. [Google Scholar] [CrossRef]
- Taylor, M.A.; Khathayer, F.; Ray, S.K. Quercetin and Sodium Butyrate Synergistically Increase Apoptosis in Rat C6 and Human T98G Glioblastoma Cells Through Inhibition of Autophagy. Neurochem. Res. 2019, 44, 1715–1725. [Google Scholar] [CrossRef]
- Majchrzak-Celińska, A.; Kleszcz, R.; Stasiłowicz-Krzemień, A.; Cielecka-Piontek, J. Sodium Butyrate Enhances Curcuminoids Permeability through the Blood-Brain Barrier, Restores Wnt/β-Catenin Pathway Antagonists Gene Expression and Reduces the Viability of Glioblastoma Cells. Int. J. Mol. Sci. 2021, 22, 11285. [Google Scholar] [CrossRef]
- Pająk, B.; Siwiak-Niedbalska, E.; Jaśkiewicz, A.; Sołtyka, M.; Zieliński, R.; Domoradzki, T.; Fokt, I.; Skóra, S.; Priebe, W. Synergistic Anticancer Effect of Glycolysis and Histone Deacetylases Inhibitors in a Glioblastoma Model. Biomedicines 2021, 9, 1749. [Google Scholar] [CrossRef]
- Zhang, G.; Gan, Y.H. Synergistic antitumor effects of the combined treatment with an HDAC6 inhibitor and a COX-2 inhibitor through activation of PTEN. Oncol. Rep. 2017, 38, 2657–2666. [Google Scholar] [CrossRef]
- Urdiciain, A.; Erausquin, E.; Meléndez, B.; Rey, J.A.; Idoate, M.A.; Castresana, J.S. Tubastatin A, an inhibitor of HDAC6, enhances temozolomide-induced apoptosis and reverses the malignant phenotype of glioblastoma cells. Int. J. Oncol. 2019, 54, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Auzmendi-Iriarte, J.; Saenz-Antoñanzas, A.; Mikelez-Alonso, I.; Carrasco-Garcia, E.; Tellaetxe-Abete, M.; Lawrie, C.H.; Sampron, N.; Cortajarena, A.L.; Matheu, A. Characterization of a new small-molecule inhibitor of HDAC6 in glioblastoma. Cell Death Dis. 2020, 11, 417. [Google Scholar] [CrossRef]
- Yin, C.; Li, P. Growth Suppression of Glioma Cells Using HDAC6 Inhibitor, Tubacin. Open Med. 2018, 13, 221–226. [Google Scholar] [CrossRef]
- Liffers, K.; Kolbe, K.; Westphal, M.; Lamszus, K.; Schulte, A. Histone Deacetylase Inhibitors Resensitize EGFR/EGFRvIII-Overexpressing, Erlotinib-Resistant Glioblastoma Cells to Tyrosine Kinase Inhibition. Target Oncol. 2016, 11, 29–40. [Google Scholar] [CrossRef]
- Was, H.; Krol, S.K.; Rotili, D.; Mai, A.; Wojtas, B.; Kaminska, B.; Maleszewska, M. Histone deacetylase inhibitors exert anti-tumor effects on human adherent and stem-like glioma cells. Clin. Epigenetics 2019, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Koul, N.; Joseph, C.; Dixit, D.; Ghosh, S.; Sen, E. HDAC inhibitor, scriptaid, induces glioma cell apoptosis through JNK activation and inhibits telomerase activity. J. Cell. Mol. Med. 2010, 14, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Ramos, J.; Luo, W.; Sirisawad, M.; Verner, E.; Buggy, J.J. A novel histone deacetylase 8 (HDAC8)-specific inhibitor PCI-34051 induces apoptosis in T-cell lymphomas. Leukemia 2008, 22, 1026–1034. [Google Scholar] [CrossRef]
- Angeletti, F.; Fossati, G.; Pattarozzi, A.; Würth, R.; Solari, A.; Daga, A.; Masiello, I.; Barbieri, F.; Florio, T.; Comincini, S. Inhibition of the Autophagy Pathway Synergistically Potentiates the Cytotoxic Activity of Givinostat (ITF2357) on Human Glioblastoma Cancer Stem Cells. Front. Mol. Neurosci. 2016, 9, 107. [Google Scholar] [CrossRef]
- Taiarol, L.; Bigogno, C.; Sesana, S.; Kravicz, M.; Viale, F.; Pozzi, E.; Monza, L.; Carozzi, V.A.; Meregalli, C.; Valtorta, S.; et al. Givinostat-Liposomes: Anti-Tumor Effect on 2D and 3D Glioblastoma Models and Pharmacokinetics. Cancers 2022, 14, 2978. [Google Scholar] [CrossRef]
- Marampon, F.; Leoni, F.; Mancini, A.; Pietrantoni, I.; Codenotti, S.; Ferella, L.; Megiorni, F.; Porro, G.; Galbiati, E.; Pozzi, P.; et al. Correction to: Histone deacetylase inhibitor ITF2357 (givinostat) reverts transformed phenotype and counteracts stemness in in vitro and in vivo models of human glioblastoma. J. Cancer Res. Clin. Oncol. 2019, 145, 2411. [Google Scholar] [CrossRef]
- Pont, L.M.; Naipal, K.; Kloezeman, J.J.; Venkatesan, S.; van den Bent, M.; van Gent, D.C.; Dirven, C.M.; Kanaar, R.; Lamfers, M.L.; Leenstra, S. DNA damage response and anti-apoptotic proteins predict radiosensitization efficacy of HDAC inhibitors SAHA and LBH589 in patient-derived glioblastoma cells. Cancer Lett. 2015, 356, 525–535. [Google Scholar] [CrossRef]
- Eyupoglu, I.Y.; Hahnen, E.; Trankle, C.; Savaskan, N.E.; Siebzehnrubl, F.A.; Buslei, R.; Lemke, D.; Wick, W.; Fahlbusch, R.; Blumcke, I. Experimental therapy of malignant gliomas using the inhibitor of histone deacetylase MS-275. Mol. Cancer Ther. 2006, 5, 1248–1255. [Google Scholar] [CrossRef]
- Buyandelger, B.; Bar, E.E.; Hung, K.S.; Chen, R.M.; Chiang, Y.H.; Liou, J.P.; Huang, H.M.; Wang, J.Y. Histone deacetylase inhibitor MPT0B291 suppresses Glioma Growth in vitro and in vivo partially through acetylation of p53. Int. J. Biol. Sci. 2020, 16, 3184–3199. [Google Scholar] [CrossRef]
- Choi, S.A.; Kwak, P.A.; Park, C.K.; Wang, K.C.; Phi, J.H.; Lee, J.Y.; Lee, C.S.; Lee, J.H.; Kim, S.K. A novel histone deacetylase inhibitor, CKD5, has potent anti-cancer effects in glioblastoma. Oncotarget 2017, 8, 9123–9133. [Google Scholar] [CrossRef]
- Bacon, C.L.; O’Driscoll, E.; Regan, C.M. Valproic acid suppresses G1 phase-dependent sialylation of a 65 kDa glycoprotein in the C6glioma cell cycle. Int. J. Dev. Neurosci. 1998, 15, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sampath, D.; Lang, F.F.; Prabhu, S.; Rao, G.; Fuller, G.N.; Liu, Y.; Puduvalli, V.K. Vorinostat modulates cell cycle regulatory proteins in glioma cells and human glioma slice cultures. J. Neurooncol. 2011, 105, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Sawa, H.; Murakami, H.; Kumagai, M.; Nakasato, M.; Yamauchi, S.; Matsuyama, N.; Tamura, Y.; Satone, A.; Ide, W.; Hashimoto, I.; et al. Histone deacetylase inhibitor, FK228, induces apoptosis and suppresses cell proliferation of human glioblastoma cells in vitro and in vivo. Acta Neuropathol. 2004, 107, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Franco-Molina, M.A.; Santana-Krímskaya, S.E.; Madrigal-de-León, L.M.; Coronado-Cerda, E.E.; Zárate-Triviño, D.G.; Hernández-Martínez, S.P.; García-Coronado, P.L.; Rodríguez-Padilla, C. Evaluation of the cytotoxic and immunogenic potential of temozolamide, panobinostat, and Lophophora williamsii extract against C6 glioma cells. Excli. J. 2021, 20, 614–624. [Google Scholar] [CrossRef]
- Hsu, Y.F.; Sheu, J.R.; Hsiao, G.; Lin, C.H.; Chang, T.H.; Chiu, P.T.; Wang, C.Y.; Hsu, M.J. p53 in trichostatin A induced C6 glioma cell death. Biochim. Biophys. Acta 2011, 1810, 504–513. [Google Scholar] [CrossRef]
- Staberg, M.; Michaelsen, S.R.; Rasmussen, R.D.; Villingshoj, M.; Poulsen, H.S.; Hamerlik, P. Inhibition of histone deacetylases sensitizes glioblastoma cells to lomustine. Cell. Oncol. 2017, 40, 21–32. [Google Scholar] [CrossRef]
- Egler, V.; Korur, S.; Failly, M.; Boulay, J.L.; Imber, R.; Lino, M.M.; Merlo, A. Histone deacetylase inhibition and blockade of the glycolytic pathway synergistically induce glioblastoma cell death. Clin. Cancer Res. 2008, 14, 3132–3140. [Google Scholar] [CrossRef]
- Yu, C.; Friday, B.B.; Yang, L.; Atadja, P.; Wigle, D.; Sarkaria, J.; Adjei, A.A. Mitochondrial Bax translocation partially mediates synergistic cytotoxicity between histone deacetylase inhibitors and proteasome inhibitors in glioma cells. Neuro-Oncology 2008, 10, 309–319. [Google Scholar] [CrossRef]
- Bangert, A.; Hacker, S.; Cristofanon, S.; Debatin, K.M.; Fulda, S. Chemosensitization of glioblastoma cells by the histone deacetylase inhibitor MS275. Anticancer Drugs 2011, 22, 494–499. [Google Scholar] [CrossRef]
- Vengoji, R.; Atri, P.; Macha, M.A.; Seshacharyulu, P.; Perumal, N.; Mallya, K.; Liu, Y.; Smith, L.M.; Rachagani, S.; Mahapatra, S.; et al. Differential gene expression-based connectivity mapping identified novel drug candidate and improved Temozolomide efficacy for Glioblastoma. J. Exp. Clin. Cancer Res. 2021, 40, 335. [Google Scholar] [CrossRef]
- Li, Z.Y.; Li, Q.Z.; Chen, L.; Chen, B.D.; Wang, B.; Zhang, X.J.; Li, W.P. Histone Deacetylase Inhibitor RGFP109 Overcomes Temozolomide Resistance by Blocking NF-κB-Dependent Transcription in Glioblastoma Cell Lines. Neurochem. Res. 2016, 41, 3192–3205. [Google Scholar] [CrossRef]
- Zhang, I.; Beus, M.; Stochaj, U.; Le, P.U.; Zorc, B.; Rajic, Z.; Petrecca, K.; Maysinger, D. Inhibition of glioblastoma cell proliferation, invasion, and mechanism of action of a novel hydroxamic acid hybrid molecule. Cell Death Discov. 2018, 4, 41. [Google Scholar] [CrossRef]
- Kitange, G.J.; Mladek, A.C.; Carlson, B.L.; Schroeder, M.A.; Pokorny, J.L.; Cen, L.; Decker, P.A.; Wu, W.; Lomberk, G.A.; Gupta, S.K.; et al. Inhibition of histone deacetylation potentiates the evolution of acquired temozolomide resistance linked to MGMT upregulation in glioblastoma xenografts. Clin. Cancer Res. 2012, 18, 4070–4079. [Google Scholar] [CrossRef]
- Pastorino, O.; Gentile, M.T.; Mancini, A.; Del Gaudio, N.; Di Costanzo, A.; Bajetto, A.; Franco, P.; Altucci, L.; Florio, T.; Stoppelli, M.P.; et al. Histone Deacetylase Inhibitors Impair Vasculogenic Mimicry from Glioblastoma Cells. Cancers 2019, 11, 747. [Google Scholar] [CrossRef]
- Yao, Z.G.; Li, W.H.; Hua, F.; Cheng, H.X.; Zhao, M.Q.; Sun, X.C.; Qin, Y.J.; Li, J.M. LBH589 Inhibits Glioblastoma Growth and Angiogenesis Through Suppression of HIF-1α Expression. J. Neuropathol. Exp. Neurol. 2017, 76, 1000–1007. [Google Scholar] [CrossRef]
- An, Z.; Gluck, C.B.; Choy, M.L.; Kaufman, L.J. Suberoylanilide hydroxamic acid limits migration and invasion of glioma cells in two and three dimensional culture. Cancer Lett. 2010, 292, 215–227. [Google Scholar] [CrossRef]
- Perez, T.; Bergès, R.; Maccario, H.; Oddoux, S.; Honoré, S. Low concentrations of vorinostat decrease EB1 expression in GBM cells and affect microtubule dynamics, cell survival and migration. Oncotarget 2021, 12, 304–315. [Google Scholar] [CrossRef]
- Rampazzo, E.; Manfreda, L.; Bresolin, S.; Cani, A.; Mariotto, E.; Bortolozzi, R.; Della Puppa, A.; Viola, G.; Persano, L. Histone Deacetylase Inhibitors Impair Glioblastoma Cell Motility and Proliferation. Cancers 2022, 14, 1897. [Google Scholar] [CrossRef]
- Eyupoglu, I.Y.; Hahnen, E.; Buslei, R.; Siebzehnrubl, F.A.; Savaskan, N.E.; Luders, M.; Trankle, C.; Wick, W.; Weller, M.; Fahlbusch, R.; et al. Suberoylanilide hydroxamic acid (SAHA) has potent anti-glioma properties in vitro, ex vivo and in vivo. J. Neurochem. 2005, 93, 992–999. [Google Scholar] [CrossRef]
- Yin, D.; Ong, J.M.; Hu, J.; Desmond, J.C.; Kawamata, N.; Konda, B.M.; Black, K.L.; Koeffler, H.P. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: Effects on gene expression and growth of glioma cells in vitro and in vivo. Clin. Cancer Res. 2007, 13, 1045–1052. [Google Scholar] [CrossRef]
- Alexanian, A.R.; Brannon, A. Unique combinations of epigenetic modifiers synergistically impair the viability of the U87 glioblastoma cell line while exhibiting minor or moderate effects on normal stem cell growth. Med. Oncol. 2022, 39, 86. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Shang, E.; Schiffgens, S.; Torrini, C.; Shu, C.; Akman, H.O.; Prabhu, V.V.; Allen, J.E.; Westhoff, M.A.; Karpel-Massler, G.; et al. Induction of Synthetic Lethality by Activation of Mitochondrial ClpP and Inhibition of HDAC1/2 in Glioblastoma. Clin. Cancer Res. 2022, 28, 1881–1895. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, D.R.; Jane, E.P.; Agostino, N.R.; DiDomenico, J.D.; Pollack, I.F. Bortezomib-induced sensitization of malignant human glioma cells to vorinostat-induced apoptosis depends on reactive oxygen species production, mitochondrial dysfunction, Noxa upregulation, Mcl-1 cleavage, and DNA damage. Mol. Carcinog. 2013, 52, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Wang, B.; Mao, W.; Wang, J.; Zhao, Y.; Li, Q.; Zhang, C.; Ma, J. Enhanced efficacy of histone deacetylase inhibitor panobinostat combined with dual PI3K/mTOR inhibitor BEZ235 against glioblastoma. Nagoya J. Med. Sci. 2019, 81, 93–102. [Google Scholar] [CrossRef]
- Essien, E.I.; Hofer, T.P.; Atkinson, M.J.; Anastasov, N. Combining HDAC and MEK Inhibitors with Radiation against Glioblastoma-Derived Spheres. Cells 2022, 11, 775. [Google Scholar] [CrossRef]
- Marino, A.M.; Sofiadis, A.; Baryawno, N.; Johnsen, J.I.; Larsson, C.; Vukojevic, V.; Ekstrom, T.J. Enhanced effects by 4-phenylbutyrate in combination with RTK inhibitors on proliferation in brain tumor cell models. Biochem. Biophys. Res. Commun. 2011, 411, 208–212. [Google Scholar] [CrossRef]
- Zhang, Y.; Ishida, C.T.; Ishida, W.; Lo, S.L.; Zhao, J.; Shu, C.; Bianchetti, E.; Kleiner, G.; Sanchez-Quintero, M.J.; Quinzii, C.M.; et al. Combined HDAC and Bromodomain Protein Inhibition Reprograms Tumor Cell Metabolism and Elicits Synthetic Lethality in Glioblastoma. Clin. Cancer Res. 2018, 24, 3941–3954. [Google Scholar] [CrossRef]
- Kim, G.H.; Choi, S.Y.; Oh, T.I.; Kan, S.Y.; Kang, H.; Lee, S.; Oh, T.; Ko, H.M.; Lim, J.H. IDH1(R132H) Causes Resistance to HDAC Inhibitors by Increasing NANOG in Glioblastoma Cells. Int. J. Mol. Sci. 2019, 20, 2679. [Google Scholar] [CrossRef]
- Wang, C.; Schroeder, F.A.; Hooker, J.M. Visualizing epigenetics: Current advances and advantages in HDAC PET imaging techniques. Neuroscience 2014, 264, 186–197. [Google Scholar] [CrossRef]
- Kim, S.W.; Hooker, J.M.; Otto, N.; Win, K.; Muench, L.; Shea, C.; Carter, P.; King, P.; Reid, A.E.; Volkow, N.D.; et al. Whole-body pharmacokinetics of HDAC inhibitor drugs, butyric acid, valproic acid and 4-phenylbutyric acid measured with carbon-11 labeled analogs by PET. J. Nucl. Med. 2013, 40, 912–918. [Google Scholar] [CrossRef]
- Hendricks, J.A.; Keliher, E.J.; Marinelli, B.; Reiner, T.; Weissleder, R.; Mazitschek, R. In vivo PET imaging of histone deacetylases by 18F-suberoylanilide hydroxamic acid (18F-SAHA). J. Med. Chem. 2011, 54, 5576–5582. [Google Scholar] [CrossRef]
- Laws, M.T.; Bonomi, R.E.; Kamal, S.; Gelovani, D.J.; Llaniguez, J.; Potukutchi, S.; Lu, X.; Mangner, T.; Gelovani, J.G. Molecular imaging HDACs class IIa expression-activity and pharmacologic inhibition in intracerebral glioma models in rats using PET/CT/(MRI) with [(18)F]TFAHA. Sci. Rep. 2019, 9, 3595. [Google Scholar] [CrossRef]
- Tago, T.; Toyohara, J. Advances in the Development of PET Ligands Targeting Histone Deacetylases for the Assessment of Neurodegenerative Diseases. Molecules 2018, 23, 300. [Google Scholar] [CrossRef]
- El Bahhaj, F.; Denis, I.; Pichavant, L.; Delatouche, R.; Collette, F.; Linot, C.; Pouliquen, D.; Grégoire, M.; Héroguez, V.; Blanquart, C.; et al. Histone Deacetylase Inhibitors Delivery using Nanoparticles with Intrinsic Passive Tumor Targeting Properties for Tumor Therapy. Theranostics 2016, 6, 795–807. [Google Scholar] [CrossRef]
- Bolcaen, J.; Kleynhans, J.; Nair, S.; Verhoeven, J.; Goethals, I.; Sathekge, M.; Vandevoorde, C.; Ebenhan, T. A perspective on the radiopharmaceutical requirements for imaging and therapy of glioblastoma. Theranostics 2021, 11, 7911–7947. [Google Scholar] [CrossRef]
- Seo, Y.J.; Muench, L.; Reid, A.; Chen, J.; Kang, Y.; Hooker, J.M.; Volkow, N.D.; Fowler, J.S.; Kim, S.W. Radionuclide labeling and evaluation of candidate radioligands for PET imaging of histone deacetylase in the brain. Bioorg. Med. Chem. Lett. 2013, 23, 6700–6705. [Google Scholar] [CrossRef]
- Mukhopadhyay, U.; Tong, W.P.; Gelovani, J.G.; Alauddin, M.M. Radiosynthesis of 6-([18F]fluoroacetamido)-1-hexanoicanilide ([18F]FAHA) for PET imaging of histone deacetylase (HDAC). J. Label Compd. Radiopharm. 2006, 49, 997–1006. [Google Scholar] [CrossRef]
- Nishii, R.; Mukhopadhyay, U.; Yeh, H.; Soghomonyan, S.; Volgin, A.; Alauddin, M.; Tong, W.; Gelovani, J. PET imaging of histone deacetylase activity in a rat brain using 6-([18F]-fluoroacetamide)-1-hexanoicanilide ([18F]-FAHA). J. Nucl. Med. 2007, 48, 336P. [Google Scholar]
- Tang, W.; Kuruvilla, S.A.; Galitovskiy, V.; Pan, M.L.; Grando, S.A.; Mukherjee, J. Targeting histone deacetylase in lung cancer for early diagnosis: (18)F-FAHA PET/CT imaging of NNK-treated A/J mice model. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 324–332. [Google Scholar]
- Reid, A.E.; Hooker, J.; Shumay, E.; Logan, J.; Shea, C.; Kim, S.W.; Collins, S.; Xu, Y.; Volkow, N.; Fowler, J.S. Evaluation of 6-([(18)F]fluoroacetamido)-1-hexanoicanilide for PET imaging of histone deacetylase in the baboon brain. J. Nucl. Med. 2009, 36, 247–258. [Google Scholar] [CrossRef]
- Bonomi, R.; Mukhopadhyay, U.; Shavrin, A.; Yeh, H.H.; Majhi, A.; Dewage, S.W.; Najjar, A.; Lu, X.; Cisneros, G.A.; Tong, W.P.; et al. Novel Histone Deacetylase Class IIa Selective Substrate Radiotracers for PET Imaging of Epigenetic Regulation in the Brain. PLoS ONE 2015, 10, e0133512. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Kim, H.S.; Kim, M.; Kwon, J.; Kim, E.M.; Hwang, H.; Oh, P.S.; Lim, S.T.; Sohn, M.H.; Kim, D.H.; et al. Synthesis and Evaluation of 2-[(18)F]Fluoroethyltriazolesuberohydroxamine Acid for Histone Deacetylase in a Tumor Model as a Positron Emission Tomography Radiotracer. Cancer Biother Radiopharm 2018, 33, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Fukumitsu, N.; Yeh, S.H.; Flores Ii, L.G.; Mukhopadhyay, U.; Young, D.; Ogawa, K.; Jeong, H.J.; Tong, W.; Gelovani, J.G. In Vivo 6-([(18)F]Fluoroacetamido)-1-hexanoicanilide PET Imaging of Altered Histone Deacetylase Activity in Chemotherapy-Induced Neurotoxicity. Contrast Media Mol. Imaging 2018, 2018, 3612027. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.H.; Tian, M.; Hinz, R.; Young, D.; Shavrin, A.; Mukhapadhyay, U.; Flores, L.G.; Balatoni, J.; Soghomonyan, S.; Jeong, H.J.; et al. Imaging epigenetic regulation by histone deacetylases in the brain using PET/MRI with (1)(8)F-FAHA. Neuroimage 2013, 64, 630–639. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Pillarsetty, N.; Divilov, V.; Blasberg, R.A.; Lewis, J.S. The synthesis and evaluation of N1-(4-(2-[18F]-fluoroethyl)phenyl)-N8-hydroxyoctanediamide ([18F]-FESAHA), a PET radiotracer designed for the delineation of histone deacetylase expression in cancer. J. Nucl. Med. 2011, 38, 683–696. [Google Scholar] [CrossRef]
- Haberkorn, U.; Beijer, B.; Altmann, A.; Gelovani, J.; Strauss, L.; Dimitrakopoulou-Strauss, A.; Eisenhut, M.; Mier, W. Uptake and biodistribution of the histone deacetylase inhibitor SAHA in tumor bearing animals. J. Nucl. Med. 2008, 49, 332P. [Google Scholar]
- Hooker, J.M.; Kim, S.W.; Alexoff, D.; Xu, Y.; Shea, C.; Reid, A.; Volkow, N.; Fowler, J.S. Histone deacetylase inhibitor, MS-275, exhibits poor brain penetration: PK studies of [C]MS-275 using Positron Emission Tomography. ACS Chem. Neurosci. 2010, 1, 65–73. [Google Scholar] [CrossRef]
- Wang, W.J.; Long, L.M.; Yang, N.; Zhang, Q.Q.; Ji, W.J.; Zhao, J.H.; Qin, Z.H.; Wang, Z.; Chen, G.; Liang, Z.Q. NVP-BEZ235, a novel dual PI3K/mTOR inhibitor, enhances the radiosensitivity of human glioma stem cells in vitro. Acta Pharmacol. Sin. 2013, 34, 681–690. [Google Scholar] [CrossRef]
- Kommidi, H.; Tosi, U.; Maachani, U.B.; Guo, H.; Marnell, C.S.; Law, B.; Souweidane, M.M.; Ting, R. (18)F-Radiolabeled Panobinostat Allows for Positron Emission Tomography Guided Delivery of a Histone Deacetylase Inhibitor. ACS Med. Chem. Lett. 2018, 9, 114–119. [Google Scholar] [CrossRef]
- Vermeulen, K.; Ahamed, M.; Luyten, K.; Bormans, G. Evaluation of [(11)C]KB631 as a PET tracer for in vivo visualisation of HDAC6 in B16.F10 melanoma. J. Nucl. Med. 2019, 74-75, 1–11. [Google Scholar] [CrossRef]
- Tosi, U.; Kommidi, H.; Adeuyan, O.; Guo, H.; Maachani, U.B.; Chen, N.; Su, T.; Zhang, G.; Pisapia, D.J.; Dahmane, N.; et al. PET, image-guided HDAC inhibition of pediatric diffuse midline glioma improves survival in murine models. Sci. Adv. 2020, 6, eabb4105. [Google Scholar] [CrossRef]
- Turkman, N.; Liu, D.; Pirola, I. Design, synthesis, biochemical evaluation, radiolabeling and in vivo imaging with high affinity class-IIa histone deacetylase inhibitor for molecular imaging and targeted therapy. Eur. J. Med. Chem. 2022, 228, 114011. [Google Scholar] [CrossRef]
- Meng, Q.; Li, F.; Jiang, S.; Li, Z. Novel (64)Cu-Labeled CUDC-101 for in Vivo PET Imaging of Histone Deacetylases. ACS Med. Chem. Lett. 2013, 4, 858–862. [Google Scholar] [CrossRef]
- Wang, C.; Schroeder, F.A.; Wey, H.Y.; Borra, R.; Wagner, F.F.; Reis, S.; Kim, S.W.; Holson, E.B.; Haggarty, S.J.; Hooker, J.M. In vivo imaging of histone deacetylases (HDACs) in the central nervous system and major peripheral organs. J. Med. Chem. 2014, 57, 7999–8009. [Google Scholar] [CrossRef]
- Wey, H.Y.; Gilbert, T.M.; Zurcher, N.R.; She, A.; Bhanot, A.; Taillon, B.D.; Schroeder, F.A.; Wang, C.; Haggarty, S.J.; Hooker, J.M. Insights into neuroepigenetics through human histone deacetylase PET imaging. Sci. Transl. Med. 2016, 8, 351ra106. [Google Scholar] [CrossRef]
- Donovan, L.L.; Magnussen, J.H.; Dyssegaard, A.; Lehel, S.; Hooker, J.M.; Knudsen, G.M.; Hansen, H.D. Imaging HDACs In Vivo: Cross-Validation of the [(11)C]Martinostat Radioligand in the Pig Brain. Mol. Imaging Biol. 2020, 22, 569–577. [Google Scholar] [CrossRef]
- Strebl, M.G.; Wang, C.; Schroeder, F.A.; Placzek, M.S.; Wey, H.Y.; Van de Bittner, G.C.; Neelamegam, R.; Hooker, J.M. Development of a Fluorinated Class-I HDAC Radiotracer Reveals Key Chemical Determinants of Brain Penetrance. ACS Chem. Neurosci. 2016, 7, 528–533. [Google Scholar] [CrossRef]
- Fang, X.T.; Zheng, M.Q.; Holden, D.; Fowles, K.; Tamagnan, G.; Hooker, J.; Huang, Y.Y.; Carson, R. Assessment of HDAC6 PET radiotracer F-18-Bavarostat. J. Nucl. Med. 2020, 61, 1021. [Google Scholar]
- Koole, M.; Van Weehaeghe, D.; Serdons, K.; Herbots, M.; Cawthorne, C.; Celen, S.; Schroeder, F.A.; Hooker, J.M.; Bormans, G.; de Hoon, J.; et al. Clinical validation of the novel HDAC6 radiotracer [(18)F]EKZ-001 in the human brain. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 596–611. [Google Scholar] [CrossRef]
- Chen, Y.A.; Lu, C.H.; Ke, C.C.; Chiu, S.J.; Chang, C.W.; Yang, B.H.; Gelovani, J.G.; Liu, R.S. Evaluation of Class IIa Histone Deacetylases Expression and In Vivo Epigenetic Imaging in a Transgenic Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 8633. [Google Scholar] [CrossRef]
- Schroeder, F.A.; Wang, C.; Van de Bittner, G.C.; Neelamegam, R.; Takakura, W.R.; Karunakaran, A.; Wey, H.Y.; Reis, S.A.; Gale, J.; Zhang, Y.L.; et al. PET imaging demonstrates histone deacetylase target engagement and clarifies brain penetrance of known and novel small molecule inhibitors in rat. ACS Chem. Neurosci. 2014, 5, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Wey, H.Y.; Wang, C.; Schroeder, F.A.; Logan, J.; Price, J.C.; Hooker, J.M. Kinetic Analysis and Quantification of [(1)(1)C]Martinostat for in Vivo HDAC Imaging of the Brain. ACS Chem. Neurosci. 2015, 6, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Strebl, M.G.; Campbell, A.J.; Zhao, W.N.; Schroeder, F.A.; Riley, M.M.; Chindavong, P.S.; Morin, T.M.; Haggarty, S.J.; Wagner, F.F.; Ritter, T.; et al. HDAC6 Brain Mapping with [(18)F]Bavarostat Enabled by a Ru-Mediated Deoxyfluorination. ACS Cent. Sci. 2017, 3, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).