Combination Therapy for the Treatment of Shingles with an Immunostimulatory Vaccine Virus and Acyclovir
Abstract
:1. Introduction
2. Repurposing Is a Drug Development Strategy
3. The Rationale of Using Repurposed IBDV Veterinary Vaccine to Control Unrelated Viral Infections
3.1. The IBDV Superinfection Therapy (SIT) Is an Intentional Viral Coinfection Strategy
3.2. The Safety of the Veterinary IBDV Vaccine Drug Candidate
4. IBDV R903/78 Drug Candidate Is Simple to Manufacture and Will Be Affordable in Resource-Limited Countries
5. Phase I/II Combination Clinical Trial of ACV Plus IBDV-R903/78 Drug Candidate for the Treatment of Facial HZ in Elderly Patients
5.1. Design Overview
5.2. Study Objectives
5.2.1. Primary
5.2.2. Secondary
5.3. Participants
5.3.1. Inclusion Criteria
5.3.2. Exclusion Criteria
5.4. Randomization and Intervention
- (A)
- Lead-in dose escalation study section
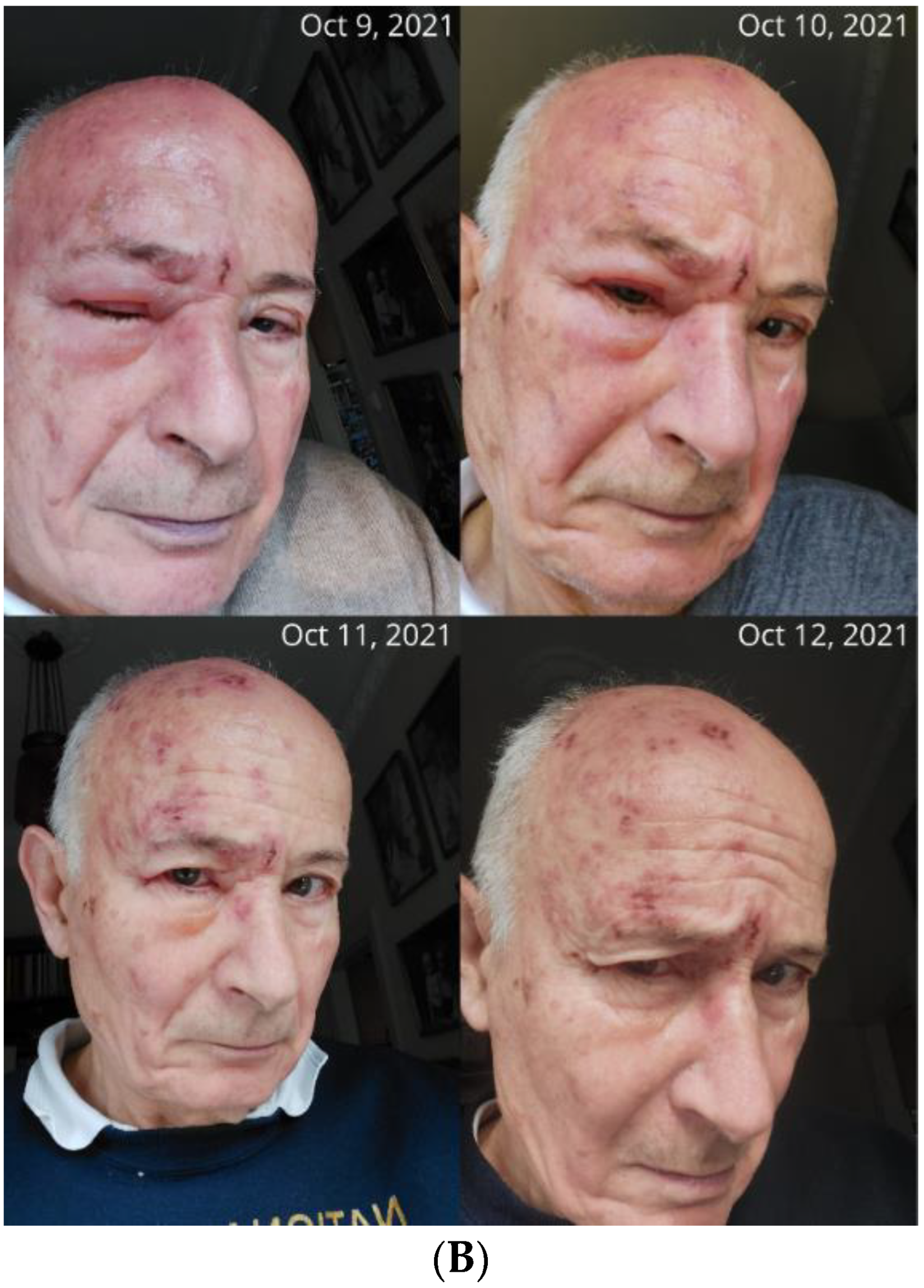
- (B)
- Comparative study section
5.5. Study Assessments
5.5.1. MTD and Systemic Toxicity
5.5.2. Efficacy
5.5.3. Safety
5.5.4. Statistical Methods
6. Discussion
7. Conclusions
8. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, J.I. Herpesvirus latency. J. Clin. Investig. 2020, 130, 3361–3369. [Google Scholar] [CrossRef] [PubMed]
- Gilden, D.; Mahalingam, R.; Nagel, M.A.; Pugazhenthi, S.; Cohrs, R.J. Review: The neurobiology of varicella zoster virus infection. Neuropathol. Appl. Neurobiol. 2011, 37, 441–463. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.X.; Lee, M.S.; Nambudiri, V.E. Global herpes zoster incidence, burden of disease, and vaccine availability: A narrative review. Ther. Adv. Vaccines Immunother. 2022, 10, 25151355221084535. [Google Scholar] [CrossRef] [PubMed]
- Janniger, C.K.; Eastern, J.S.; Hospenthal, D.R.; Moon, J.E. Herpes Zoster Clinical Presentation. Medscape J. Med. 2021, 2, 2–4. [Google Scholar]
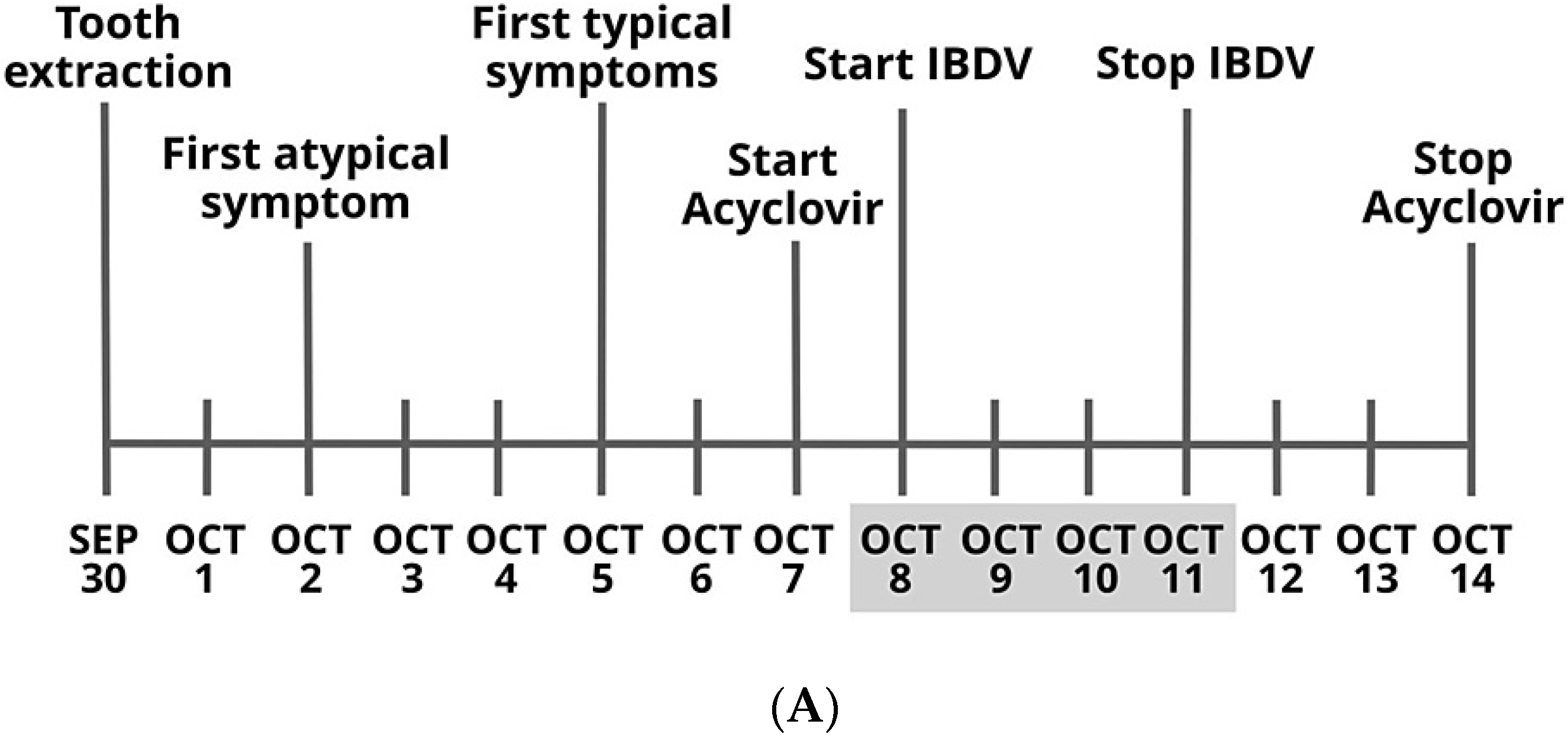
- Bakacs, T. Healing of Severe Herpes Zoster Ophthalmicus Within a Few Days: An Autobiographical Case Report. Cureus 2021, 13, e20303. [Google Scholar] [CrossRef]
- Bakacs, T.; Chumakov, K.; Safadi, R.; Kovesdi, I. Editorial: Fighting fire with fire: Using non-pathogenic viruses to control unrelated infections. Front. Immunol. 2022, 13, 1046851. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Grimshaw, A.A.; Axson, S.A.; Choe, S.H.; Miller, J.E. Drug repurposing: A systematic review on root causes, barriers and facilitators. BMC Health Serv. Res. 2022, 22, 970. [Google Scholar] [CrossRef]
- Pantziarka, P.; Verbaanderd, C.; Huys, I.; Bouche, G.; Meheus, L. Repurposing drugs in oncology: From candidate selection to clinical adoption. Semin. Cancer Biol. 2021, 68, 186–191. [Google Scholar] [CrossRef]
- Vandeborne, L.; Pantziarka, P.; Van Nuffel, A.M.T.; Bouche, G. Repurposing Infectious Diseases Vaccines Against Cancer. Front. Oncol. 2021, 11, 688755. [Google Scholar] [CrossRef]
- Seo, S.-U.; Seong, B.-L. Prospects on Repurposing a Live Attenuated Vaccine for the Control of Unrelated Infections. Front. Immunol. 2022, 13, 1767. [Google Scholar] [CrossRef]
- Yagovkina, N.V.; Zheleznov, L.M.; Subbotina, K.A.; Tsaan, A.A.; Kozlovskaya, L.I.; Gordeychuk, I.V.; Korduban, A.K.; Ivin, Y.Y.; Kovpak, A.A.; Piniaeva, A.N.; et al. Vaccination With Oral Polio Vaccine Reduces COVID-19 Incidence. Front. Immunol. 2022, 13, 907341. [Google Scholar] [CrossRef] [PubMed]
- Bakacs, T.; Volker, S.; Kovesdi, I. An Orally Administered Nonpathogenic Attenuated Vaccine Virus Can Be Used to Control SARSCoV-2 Infection: A Complementary Plan B to COVID-19 Vaccination. Cureus 2022, 14, e28467. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- Katze, M.G.; He, Y.; Gale, M. Viruses and interferon: A fight for supremacy. Nat. Rev. Immunol. 2002, 2, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Chumakov, M.P.; Voroshilova, M.K.; Antsupova, A.S.; Boiko, V.M.; Blinova, M.I.; Priimiagi, L.S.; Rodin, V.I.; Seibil, V.B.; Siniak, K.M.; Smorodintsev, A.A.; et al. Live enteroviral vaccines for the emergency nonspecific prevention of mass respiratory diseases during fall-winter epidemics of influenza and acute respiratory diseases. Zh. Mikrobiol. Epidemiol. Immunobiol. 1992, 11–12, 37–40. [Google Scholar]
- Kovesdi, I.; Bakacs, T. Therapeutic exploitation of viral interference. Infect. Disord. Drug Targets 2019, 19, 1. [Google Scholar] [CrossRef]
- Qin, Y.; Zheng, S.J. Infectious Bursal Disease Virus-Host Interactions: Multifunctional Viral Proteins that Perform Multiple and Differing Jobs. Int. J. Mol. Sci. 2017, 18, 161. [Google Scholar] [CrossRef]
- Holmes, E.C. The comparative genomics of viral emergence. Proc. Natl. Acad. Sci. USA 2010, 107, 1742–1746. [Google Scholar] [CrossRef]
- Hornyak, A.; Lipinski, K.S.; Bakonyi, T.; Forgach, P.; Horvath, E.; Farsang, A.; Hedley, S.J.; Palya, V.; Bakacs, T.; Kovesdi, I. Effective multiple oral administration of reverse genetics engineered infectious bursal disease virus in mice in the presence of neutralizing antibodies. J. Gene Med. 2015, 17, 116–131. [Google Scholar] [CrossRef]
- Csatary, L.K.; Telegdy, L.; Gergely, P.; Bodey, B.; Bakacs, T. Preliminary report of a controlled trial of MTH-68/B virus vaccine treatment in acute B and C hepatitis: A phase II study. Anticancer Res. 1998, 18, 1279–1282. [Google Scholar]
- Csatary, L.K.; Schnabel, R.; Bakacs, T. Successful treatment of decompensated chronic viral hepatitis by bursal disease virus vaccine. Anticancer Res. 1999, 19, 629–633. [Google Scholar] [PubMed]
- Bakacs, T.; Mehrishi, J.N. Examination of the value of treatment of decompensated viral hepatitis patients by intentionally coinfecting them with an apathogenic IBDV and using the lessons learnt to seriously consider treating patients infected with HIV using the apathogenic hepatitis G virus. Vaccine 2004, 23, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Bakacs, T.; Safadi, R.; Puskas, L.G.; Feher, L.Z.; Kovesdi, I. Sequential Combination of a Strong Interferon Inducer Viral Vector With Low Doses of Nivolumab Plus Ipilimumab Could Provide Functional Cure in Chronic Hepatitis B Virus infections: Technical Report Proposing a New Modality. Cureus 2022, 14, e22750. [Google Scholar] [CrossRef] [PubMed]
- Jordan, I.; John, K.; Howing, K.; Lohr, V.; Penzes, Z.; Gubucz-Sombor, E.; Fu, Y.; Gao, P.; Harder, T.; Zadori, Z.; et al. Continuous cell lines from the Muscovy duck as potential replacement for primary cells in the production of avian vaccines. Avian Pathol. 2016, 45, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Vohra, S.; Shamseer, L.; Sampson, M.; Bukutu, C.; Schmid, C.H.; Tate, R.; Nikles, J.; Zucker, D.R.; Kravitz, R.; Guyatt, G.; et al. CONSORT extension for reporting N-of-1 trials (CENT) 2015 Statement. J. Clin. Epidemiol. 2016, 76, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Margolis, A.; Giuliano, C. Making the switch: From case studies to N-of-1 trials. Epilepsy Behav. Rep. 2019, 12, 100336. [Google Scholar] [CrossRef]
- Pott, H., Jr.; de Oliveira, M.F.B.; Gambero, S.; Amazonas, R.B. Randomized clinical trial of famciclovir or acyclovir for the treatment of herpes zoster in adults. Int. J. Infect. Dis. 2018, 72, 11–15. [Google Scholar] [CrossRef]
- Gerada, C.; Campbell, T.M.; Kennedy, J.J.; McSharry, B.P.; Steain, M.; Slobedman, B.; Abendroth, A. Manipulation of the Innate Immune Response by Varicella Zoster Virus. Front. Immunol. 2020, 11, 1. [Google Scholar] [CrossRef]
- Virgin, H.W. The virome in mammalian physiology and disease. Cell 2014, 157, 142–150. [Google Scholar] [CrossRef]
- Majewska, A.; Mlynarczyk-Bonikowska, B. 40 Years after the Registration of Acyclovir: Do We Need New Anti-Herpetic Drugs? Int. J. Mol. Sci. 2022, 23, 3431. [Google Scholar] [CrossRef]
- Rison, R.A.; Shepphird, J.K.; Kidd, M.R. How to choose the best journal for your case report. J. Med. Case Rep. 2017, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, E.; Rocha, V. History of the clinical use of umbilical cord blood hematopoietic cells. Cytotherapy 2005, 7, 219–227. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakacs, T.; Sandig, V.; Kovesdi, I. Combination Therapy for the Treatment of Shingles with an Immunostimulatory Vaccine Virus and Acyclovir. Pharmaceuticals 2023, 16, 226. https://doi.org/10.3390/ph16020226
Bakacs T, Sandig V, Kovesdi I. Combination Therapy for the Treatment of Shingles with an Immunostimulatory Vaccine Virus and Acyclovir. Pharmaceuticals. 2023; 16(2):226. https://doi.org/10.3390/ph16020226
Chicago/Turabian StyleBakacs, Tibor, Volker Sandig, and Imre Kovesdi. 2023. "Combination Therapy for the Treatment of Shingles with an Immunostimulatory Vaccine Virus and Acyclovir" Pharmaceuticals 16, no. 2: 226. https://doi.org/10.3390/ph16020226
APA StyleBakacs, T., Sandig, V., & Kovesdi, I. (2023). Combination Therapy for the Treatment of Shingles with an Immunostimulatory Vaccine Virus and Acyclovir. Pharmaceuticals, 16(2), 226. https://doi.org/10.3390/ph16020226





