Abstract
Oxidative stress is an imbalance between the increased production of reactive species and reduced antioxidant activity, which can cause a variety of disturbances including ocular diseases. Lycium barbarum polysaccharides (LBPs) are complex polysaccharides isolated from the fruit of L. barbarum, showing distinct roles in antioxidants. Moreover, it is relatively safe and non-toxic. In recent years, the antioxidant activities of LBPs have attracted remarkable attention. In order to illustrate its significance and underlying therapeutic value for vision, we comprehensively review the recent progress on the antioxidant mechanisms of LBP and its potential applications in ocular diseases, including diabetic retinopathy, hypertensive neuroretinopathy, age-related macular degeneration, retinitis pigmentosa, retinal ischemia/reperfusion injury, glaucoma, dry eye syndrome, and diabetic cataract.
1. Introduction
Reactive oxygen species (ROS) is a general term for oxygen-containing free and non-free radicals produced during the body’s metabolic process [1]. Although ROS is a byproduct of cell metabolism, cells need a certain amount of ROS to maintain body balance under normal physiological conditions [2]. Many studies have shown that ROS plays a vital role in biological processes such as cell proliferation and differentiation, gene expression, protein modification, cell signaling, and destruction of intracellular pathogens [3,4,5]. Excessive ROS production leads to oxidative stress, an imbalance between the increase in active species and the decrease in antioxidant activity [6]. These excess oxygen radicals can cause damage to macromolecules such as DNA, proteins and lipids, leading to a variety of oxidative stress-related diseases [7,8]. Studies have reported that more than 100 diseases are related to oxidative stress; they include neurodegenerative diseases, cancer, kidney disease, cardiovascular disease, diabetes, ocular degenerative diseases, autoimmune rheumatic diseases, and inflammatory diseases [9]. The eye is the primary light-sensitive organ exposed to various ultraviolet and visible light. Oxidative stress is exacerbated in the eye, damaging ocular tissues and functions, increasing vascular permeability, and eventually leading to microvascular abnormalities and neovascularization as well as various vision-threatening diseases such as age-related macular degeneration (AMD), cataract, dry eye syndrome (DES), retinitis pigmentosa (RP), and diabetic retinopathy (DR) [10,11].
Antioxidants are effective in preventing damage to the body from these free radicals [12]. However, many synthetic antioxidants are shown to be toxic and should not be used in large quantities for a long time [13]. Therefore, screening for natural and harmless antioxidants became an urgent demand. Many natural plant ingredients have been shown to possess antioxidant effects, particularly plant polysaccharides [14]. In recent years, natural plant polysaccharides have undertaken a significant role in the research of antioxidant drugs. They are proved to be reasonably effective in preventing oxidative stress caused by excess free radicals [15,16]. Lycium barbarum polysaccharides (LBPs), which are natural polysaccharides extracted from the fruits of L. barbarum, have been extensively studied [17]. LBPs nourish the eyes and liver in the traditional concept of Chinese medicine. Several studies explored the various functions of LBPs, such as immunity enhancement, anti-tumor activities, antioxidation, blood sugar and lipid regulation, and protection against radiation, and are promising as natural antioxidants [18]. In particular, the antioxidant effect of LBP has been extensively studied. Significant progress has been made in the study of the antioxidant mechanism of LBP and its application in ophthalmic diseases in recent decades. In this paper, we analyzed and reviewed the studies on the antioxidant effect of LBP in DR, hypertensive neuroretinopathy, AMD, RP, retinal ischemia/reperfusion injury, glaucoma, DES and diabetic cataract, by reviewing the recent studies on LBP at home and abroad, to provide new ideas and methods for the subsequent research on LBP. It also provides the theoretical basis for the early clinical application of LBP.
2. The Natural Existence and Acquisition of LBPs in Daily Life
2.1. Natural Existence of LBPs
L. barbarum L. (wolfberry or goji berry) (see Figure 1) is a perennial shrub belonging to the Solanaceae family, mainly distributed in East Asia, especially in South China, Korea, and Japan [19]. There are 80 species belonging to the genus Lycium globally, while only three of them (L. barbarum, L. Chinese, and L. ruthenicum) are used for medical purposes, known as goji berries in China. L. barbarum accounts for almost 90% of available goji berries in the market and has been widely cultivated in northwest China for more than 600 years [20]. As a nutritious and edible medicinal plant, goji berry has been used as a traditional Chinese medicine and food supplement in China for more than 2000 years [21]. L. barbarum is rich in polysaccharides, carotenoids, phenols, alkaloids, amines, betaine, essential oils, anthocyanins, trace elements, amino acids, and vitamins, among which LBPs are the main components accounting for 5–8% of the dried fruit [22,23].

Figure 1.
The dried fruit of Lycium barbarum.
2.2. Extraction and Biological Structure of LBPs
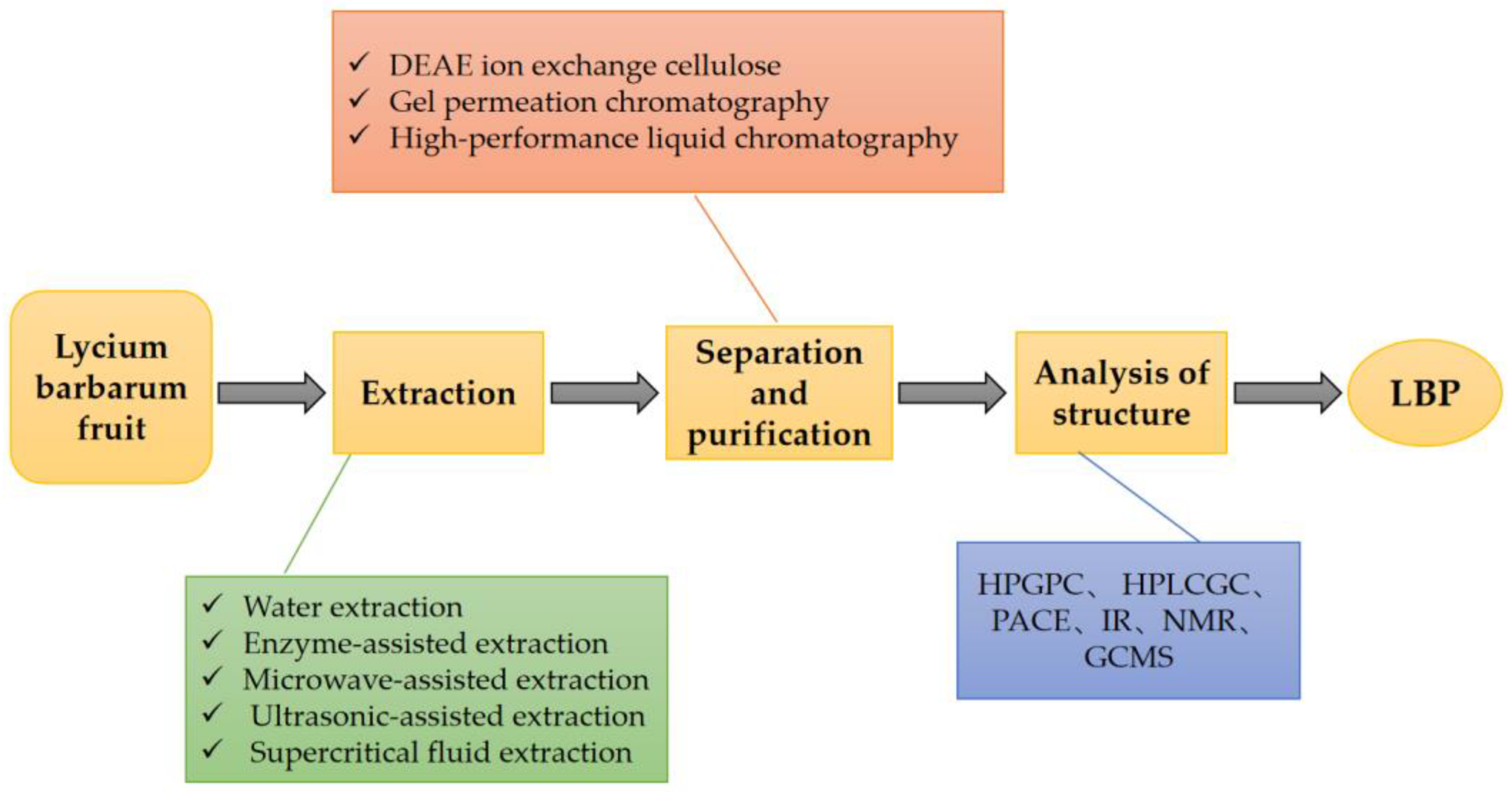
LBPs are derived from the extracts of the fruits of L. barbarum [18]. There are various methods for extracting LBPs, including the traditional water extraction method, enzyme-assisted method, microwave-assisted method, ultrasonic-assisted method, and supercritical fluid extraction method [24]. In all these extraction methods, the similar underlying principle is the degradation of the cell wall under certain conditions without affecting the polysaccharide activity [25]. Each extraction method has its pros and cons [26]. When selecting specific methods, it is essential to weigh the high extraction rate and biological activity of LBPs. There have been several reports of quantitative data on the polysaccharide content in Fructus lycii. A study determined the mass fraction of polysaccharides in dried L. barbarum as 23% [27]. Another study separated and purified LBPs and analyzed their structure [24]. The detailed process and method are shown in Figure 2.
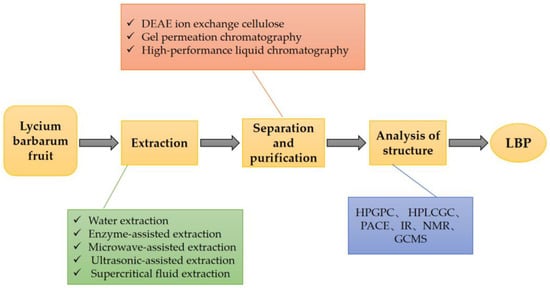
Figure 2.
The process of extraction, separation, and purification of LBPs. LBPs can be obtained by extraction, separation, purification, and structural analysis from L. barbarum fruit. DEAE: Diethylaminoethyl, HBGPC: high performance gel permeation chromatography, HPLC: high performance liquid chromatography, GC: gas chromatography, PACE: polyacrylamide co-electrophoresis, IR: infrared, NMR: nuclear magnetic resonance, GCMS: gas chromatographic mass spectrometry.
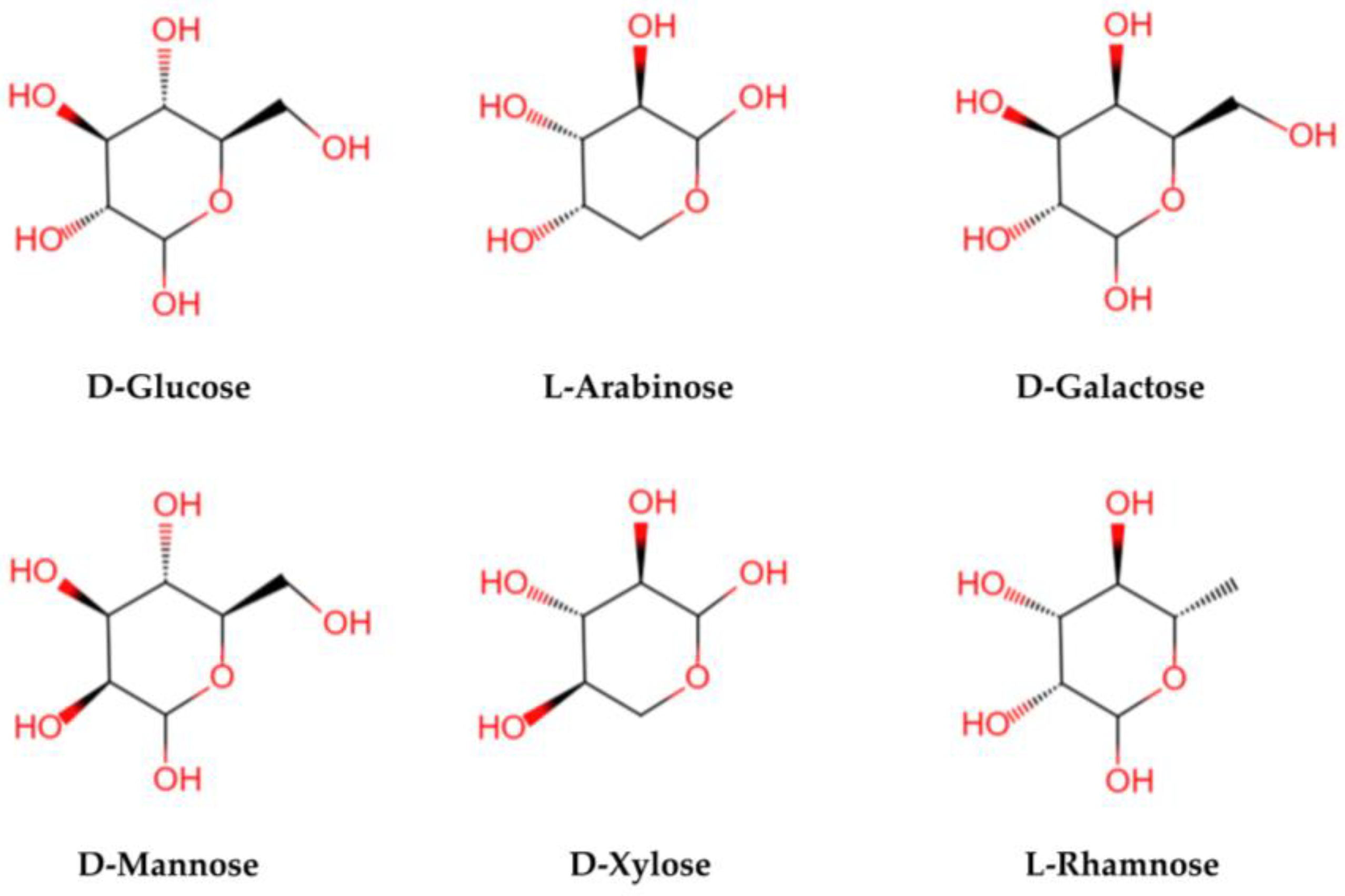
LBPs are glycopeptide chains consisting of acidic heteropolysaccharides and polypeptides or proteins. Polysaccharides are characterized by a complex structural hierarchy, with structure levels classified as primary, secondary, tertiary, and quaternary. Their advanced structures are based on their primary structures. The structure and composition of LBPs are different because of the different methods of separation and purification. The relative molecular mass is generally 10–2300 KD [18]. Structurally, LBPs contain six monosaccharides (arabinose, glucose, galactose, mannose, xylose, and rhamnose) (see Figure 3), galacturonic acid, and 18 amino acids. Monosaccharides are linked by glycosidic bonds that are mainly (1→3)-β-Galp, (1→4)-β-Galp, (1→4)-β-Galp, (1→6)-α-glucans, and (1→4)-α polygalacturonans with various branches and terminals [28,29]. The LBPs are highly branched, and the main chain is a (1→6) Galp-connected galactose [30]. It is the complex structure of a polysaccharide that determines its biological activity [26].
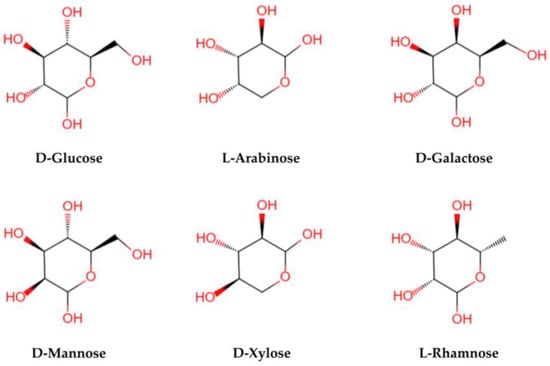
Figure 3.
The structural formula of main monosaccharides in LBPs. LBPs mainly contain six monosaccharides (glucose, arabinose, galactose, mannose, xylose, and rhamnose).
Polyphenols are also notable active ingredients in L. barbarum. At present, 53 kinds of polyphenols have been identified from L. barbarum, including 28 kinds of phenylpropane, 4 kinds of coumarins, 8 kinds of lignans, 5 kinds of flavonoids, 3 kinds of isoflavones, 2 kinds of chlorogenic acid derivatives, and 3 kinds of other components [31]. They showed strong oxygen radical absorption capacity and DPPH radical scavenging activity. There are many extraction methods for polyphenols. More and more green and sustainable extraction techniques have been discovered and applied [32,33]. At present, ultrasonic extraction, microwave extraction, enzyme-assisted extraction, and solvent extraction have been employed to extract polyphenols [34,35,36].
3. Biological Function of LBPs
3.1. Anti-Oxidative and Anti-Aging Effects
Stimulation by external factors induces excessive free radical production, leading to various diseases such as arthritis, atherosclerosis, cancer, chronic inflammation, diabetes, and senescence [37]. Several studies have validated that LBPs possess antioxidant and anti-aging properties attributed to the presence of carotenoids, ascorbic acid and their derivatives, flavonoids, and other phenolic compounds. The mechanisms include the direct or indirect scavenging of free radicals, lipid peroxidation inhibition, and the maintenance of cell membrane and macromolecular structure [18]. Zhang et al. [38] extracted L. barbarum seed dreg polysaccharides using four different solvents. They observed that concentrated alkaline preferred to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals, while hot buffer scavenged OH free radicals and the chelating agent (CHSS) chelated ferrous ions. They also found that polysaccharides had good antioxidant activities. Wang et al. [39] reported that LBPs extracted by different methods presented a solid ability to scavenge DPPH, hydroxyl free radicals, and superoxide anion radicals. In addition, LBPs could prolong the life span of nematodes under normal conditions, oxidative stress, and heat stress. The anti-aging role of LBPs by upregulating the expressions of daf-16, sod-3, and hsp-16.2 genes might be responsible for this. In addition, LBPs could reduce the expressions of age-related genes p21 and p53 in zebrafish embryos, thereby regulating the p53 signaling pathway to delay aging [40].
3.2. Immune Regulation
The immunomodulatory effect of LBPs has been widely studied. Research indicated that LBPs activated dendritic cells, macrophages, T and B lymphocytes, natural killer cells, and other immune cells to regulate the immune response in the body [41]. Zhang et al. [42] found that the Lycium barbarum polysaccharide LBPF4-OL might promote lymphocyte proliferation in wild-type (C3H/HeN) mice and induce the production of TNF-α and IL-1β in peritoneal macrophages isolated from the mice; LBPs functioned as immunomodulators. Bo et al. [43] showed that LBPs facilitated the maturation of dendritic cells; upregulated the expressions of MHCII, CD80, and CD86; and promoted antigen uptake. In addition, Su et al. found that LBPs upgraded the activation of follicular helper T cells and induced IL-21 secretion to enhance humoral immunity [44]. Sulfated LBPs can significantly enhance the immune activity of cultured chickens [45].
3.3. Neuroprotective Effects
LBPs were reported as neuroprotective. In ischemic nerve injury, LBPs inhibited the NR2B signaling pathway while activating the NR2A signaling pathway to play a neuroprotective role [46]. In a diabetic rat model, LBP treatment significantly increased the number of retinal ganglion cells (RGCs) and amacrine cells. The function of neuroprotection might be related to the activation of the Nrf2/HO-1 antioxidant pathway [47]. Yu et al. [48] showed that LBPs prevent oxygen-glucose deprivation/reperfusion-induced neuronal damage in primary hippocampal neurons by activating the PI3K/Akt/mTOR signaling pathway.
3.4. Anti-Tumor Effect
The antitumor effects of LBPs are mainly achieved by inhibiting the growth of tumor cells, promoting the apoptosis of neoplastic cells, enhancing the immune function of host cells, and reducing toxicity in combination with chemotherapy drugs [49]. Gong et al. [50] found that the component of LBPs, LBGP-I-3 (arabinogalactan fraction), possesses the most substantial inhibitive effect on the growth of hepatoma cells (SMMC-7721), cervical cancer cells (HeLa), and human breast cancer cells (MCF-7 cells). LBGP-I-3 facilitated the arrest of the MCF-7 cell cycle during the G0/G1 phase, which reduced the apoptosis-associated protein Bcl2/Bax ratio and increased the expressions of Caspase-3, 8, and 9 and ROS production. Additionally, mitochondrial membrane potential decreased with regulated expressions of phosphorylated Erk, JNK, and p38 proteins. Further, Deng et al. [51] observed that LBP-3 had the highest inhibitory efficiency, which could induce cell apoptosis, mitochondrial membrane potential destruction, and the arrest of mouse liver cancer cells, H22 cells in S-phase in vitro, which was confirmed in H22 tumor-bearing mice [52]. Furthermore, Zhang et al. [53] studied the effects of LBPs on the proliferation and apoptosis of the QGY7703 human hepatocellular carcinoma cells. It was found that the apoptosis of the cells treated by LBPs increased, accompanied by an increase in Ca2+ ions. It indicated that LBPs increased the concentration of calcium ions in cells, which was closely related to the signal transduction pathway of apoptosis, and affected the chemical sensitivity of tumor cells to anticancer agents. In addition, the combination of LBP with doxorubicin enhanced the antitumor activity of doxorubicin, increased the peripheral blood lymphocyte count, and promoted the cell cycle recovery of bone marrow cells [54].
3.5. Anti-Inflammatory Effect
LBPs have satisfactory anti-inflammatory effects and have been used to treat alcoholic liver disease, hepatitis, diabetes, kidney disease, and other disorders. Liao et al. [55] induced a renal injury model through a high-fat diet and treated the mice with oral administration of LBPs. It showed that the administration of LBPs restored the concentrations of lipid, blood urea nitrogen, serum creatinine, and urine protein. Additionally, the concentrations of SREGBP-1, TNF-α, and IL-6 were downregulated. In contrast, the concentrations of adiponectin and AMPK were upregulated in the kidney, which indicated that LBPs could enhance the anti-inflammatory responses and alleviate renal injury caused by lipid metabolism disorder. Xiao et al. [56] found that LBPs treatment of non-alcoholic steatohepatitis mice reduced liver inflammation and cell apoptosis, which might be achieved through the regulation of autophagy and the MAPK pathway. Du et al. [57] found that LBPs significantly inhibited albuminuria and reduced the blood urea nitrogen concentrations, as well as the expressions of serum inflammatory factors, including IL-2, IL-6, TNF-α, IFN-α, MCP-1, and ICAM-1 in diabetic rats, which indicated the anti-inflammatory properties of LBPs in diabetes mellitus.
3.6. Radiation Protection
Prolonged exposure to all types of radiation could cause severe damage to the skin, eyes, and nervous system, as well as accelerated aging. Plant polysaccharides have potential anti-radiation effects through antioxidation and cell protection. On the one hand, LBPs assume antioxidant roles in reducing oxidative stress. On the other, they reduce apoptosis and improve the survival of damaged cells [37]. Liang et al. [58] found that LBPs could improve UV-induced cell damage by boosting nuclear Nrf2 phosphorylation in HSF cells. Li et al. [59] found that LBPs pretreatment significantly attenuated the UVB-induced decrease in cell viability, ROS production, and DNA damage with immortalized human keratinocytes (HaCaT cells). LBPs have also been reported to protect cells from UV damage by increasing the concentrations of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) [60].
3.7. Hypolipidemic and Hypoglycemic Effects
Several studies have shown that LBPs could reduce blood sugar and dyslipidemia. This may be achieved by improving islet cell function and promoting their regeneration, improving insulin sensitivity, and increasing the expression of antioxidant enzyme genes [61]. Zhu et al. [62] found that LBPs promoted the proliferation of pancreatic cells and the release of insulin by RIN-m5f, a pancreatic cell line, which facilitated hypoglycemia. In a high-fat diet and streptozotocin-induced diabetic rat model [63], treatment with LBPs for 30 days significantly reduced fasting blood glucose and increased the concentration SOD. In addition, Li et al. [64] found that LBPs significantly reduced the blood glucose concentration of mice induced by a high-fat diet and triglyceride (TG) and DAG concentrations in serum and liver by regulating AMPK activity in hepatocytes and inhibiting the expression of SREBP-1c. Yang et al. [26] reported that LBPs reduced the concentration of glucose in the blood and alleviated insulin resistance in obese mice, which, in turn, reduced the serum concentrations of TG, total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) and increased that of high-density lipoprotein cholesterol (HDL-C). Meanwhile, the expressions of SOD and GSH increased while that of malonic dialdehyde (MDA) decreased. These results indicate that LBPs reduce blood glucose and lipid concentrations.
3.8. Regulation of Intestinal Flora
The human gut is home to more than 500 microorganisms that are involved in the body’s absorption and replacement, as well as the development of various diseases such as bowel cancer, obesity, type 2 diabetes, and autoimmune diseases [65,66]. Several studies showed that LBPs regulated intestinal flora and improved various functions of the body. Zhu et al. [67] found that LBPs treatment increased the growth of the probiotics Lactobacillus acidophilus and bifidobacterium longum in Man Rogosa Sharpe broth in vitro. In vivo, the administration of LBPs in mice increased the abundance of Proteobacteria and Firmicutes at the phylum level and promoted the emergence of probiotics, such as Akkermansia, Lactobacillus, and Prevotellaceae, at the genus level [67]. In short, LBPs affected the intestinal microbial community of mice and promoted the growth of beneficial bacteria. Zhao et al. [68] also proved that LBPs treatment increased the intestinal microbial diversity of rats with chronic prenatal stress and attenuated the emotional sadness of their offspring. In a high-fat diet/streptozotocin-induced diabetic mouse model, oral administration of LBPs for 6 weeks increased the number of microbes in the taxa of the genus Allobaculum involved in glycemic control, indicating that LBPs played an anti-diabetic role by altering the gut microbiome [69]. Cao et al. [70] found that the administration of LBP-3 (Arabingalactan) might alleviate DSS-induced chronic colitis by enriching potential probiotics (Rumenococci) and inhibiting the proliferation of harmful bacteria (Enterobacteriaceae).
3.9. Anti-Viral Activity
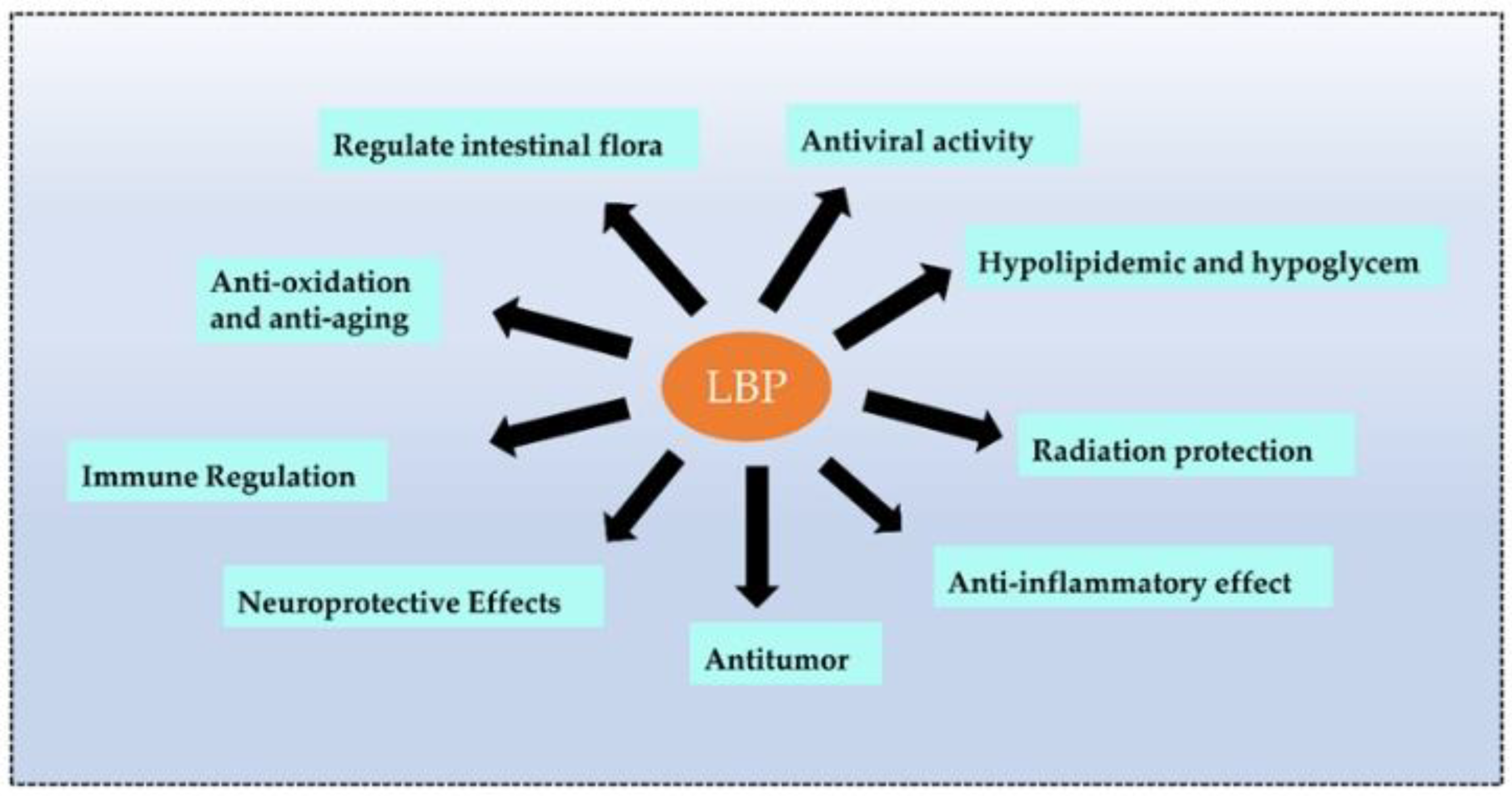
Recent studies revealed that plant polysaccharides could inhibit viruses and were more environmentally friendly and safer than antibiotics. Polysaccharides are biological macromolecules that effectively encapsulate viruses, preventing them from entering and attacking cells. LBPs also enhanced immune function and antiviral effects [71]. Wang et al. [72] carried out four types of sulfated modifications on extracted LBPs and found that LBPs prevented infections of the Newcastle virus in chicken embryo fibroblasts (CEF). The sulfated LBPs could exhibit higher inhibitory efficiency. Bo et al. [73] wrapped LBPs in liposomes and observed the promotion of splenic cell proliferation in vitro, a significant increase in the ratio of CD4+ T to CD8+ T cells, and the promotion of the secretion of macrophage cytokines. Moreover, LBPs significantly enhanced the immune response to the PCV2 vaccine in vivo [73] (the biological function of LBPs is shown in Figure 4).
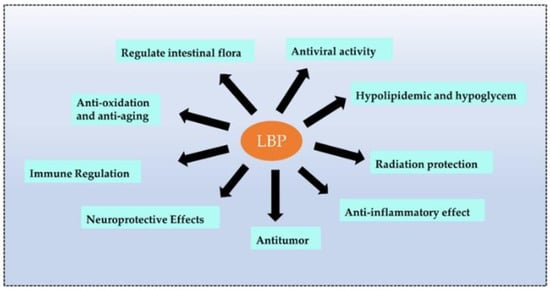
Figure 4.
The main biological functions of LBPs. LBPs have many functions, and the main functions are listed above.
4. Molecular Mechanisms Related to the Antioxidant Effect of LBPs
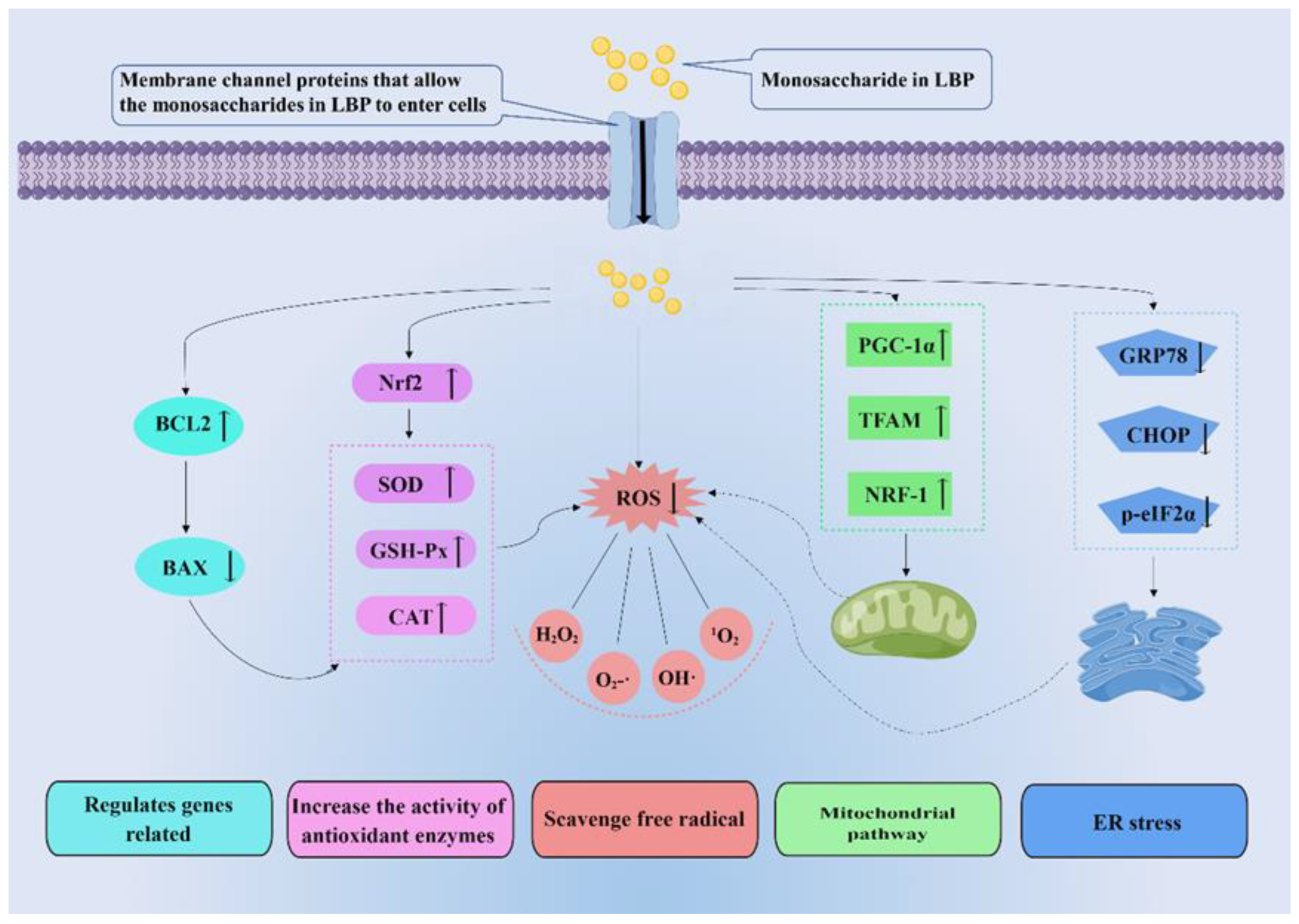
Anti-oxidation mitigates free radicals. The body has a redox system to balance the production of ROS. Antioxidants, substances that inhibit oxidation, are thought to protect against oxidative stress [74]. Antioxidants mainly consist of the following natural enzymes: SOD, catalase (CAT), and GSH-Px [75]. SOD is an antioxidant metal enzyme that can decompose superoxide into oxygen and hydrogen peroxide and reduce the peroxide effect of superoxide on lipids [76]. In addition, SOD is essential for activating CAT and GSH-Px. GSH-Px is a ubiquitous antioxidant enzyme that converts peroxides into non-toxic oxygen and water. It can also individually trap free radicals and convert them to non-toxic forms [77]. CAT further converts H2O2 to water and prevents the formation of hydroxyl radicals [76]. LBPs can improve the activity of these enzymes to achieve antioxidant effects. Therefore, research on antioxidants provides insights of great import into the prevention and treatment of various redox-related diseases [78]. In recent years, the antioxidant effects of plant polysaccharides have been widely studied. LBPs, the natural compounds, have been shown to exhibit antioxidant activity in several studies. LBPs may perform antioxidant roles by eliminating free radicals directly or indirectly (Figure 5).
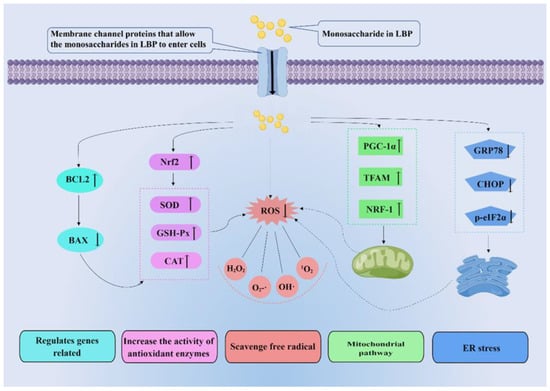
Figure 5.
Molecular mechanisms related to the antioxidant effect of LBPs. LBPs play antioxidant roles by directly or indirectly eliminating free radicals. Indirect ways of scavenging free radicals include regulating related genes, increasing the activity of antioxidant enzymes, mitochondrial pathway, and endoplasmic reticulum (ER) stress. Nrf2: nuclear respiratory factor 2; GSH-Px: glutathione peroxidase; CAT: catalase glutathione; ROS: reactive oxide species; PGC-1α: peroxisome proliferators-activated receptor γcoactivator-l alpha; TFAM: transcription factor A, mitochondrial; NRF-1: nuclear respiratory factor 1; GRP78: glucose regulated protein 78; CHOP: C/EBP homology protein; p-eIF2α: phosphorylated eukaryotic initiation factor-2 alpha.
4.1. LBPs Scavenge Free Radicals
There are four types of free radicals, including carbon, nitrogen, oxygen, and sulfur. Oxygen-containing free radicals are the most common [79]. The principal free radicals include hydrogen peroxide (H2O2), superoxide radical (O2•−), hydroxyl radical (OH•), singlet molecular oxygen (1O2), and lipid peroxide [80]. Polysaccharides may be antioxidative in the following ways. (1) Polysaccharides may break down free radicals into non-toxic substances by directly binding to them. (2) Polysaccharides can bind with metal ions, weaken lipid peroxidation, and inhibit free radical formation [81]. Sulfated polysaccharides seem to have stronger effects on free radical scavenging [81]. The direct scavenging of free radicals by LBP to achieve antioxidant effects has been widely reported in experimental studies. Liu et al. [82] extracted polysaccharides from Lycium ruthenicum by optimizing dynamic microwave-assisted extraction technology and tested their antioxidant activity in vitro. They found that polysaccharides were capable of scavenging DPPH, hydrogen peroxide, and superoxide anion radicals, and their activity of DPPH scavenging increased with increasing concentration. Skenderidis et al. found that the water extract of L. barbarum demonstrated free radical scavenging activity and inhibited DNA damage induced by peroxyl radicals in their study [83].
In addition, phenolic components are important secondary metabolites of natural products, and their antioxidant activities have been thoroughly studied [84]. In vitro, free radical scavenging and cell damage tests are commonly used to evaluate their antioxidant activity. Polyphenols have been found to alleviate oxidative stress-induced diseases such as cardiotoxicity and acute lung injury [85]. Catechins and other phenolic compounds can normalize lipid peroxidation and alleviate adriamycin-induced oxidative stress in rats [86]. Studies have shown that lycium berry polyphenols can increase the expression of antioxidant enzymes such as catalase, heme oxygenase-1, glutathione peroxidase, and SOD, as well as reduce the oxidative stress of lipopolysaccharide (LPS) stimulated acute lung injury mice [87].
4.2. LBPs Increase the Activity of Antioxidant Enzymes
Free radicals are atomic groups with unpaired electrons, which attack macromolecules in the human body and cause non-negligible damage to organisms [88]. ROS mainly include hydrogen peroxide (H2O2), superoxide anion (O2•−), singlet oxygen (1O2), hydroxyl radical (OH•), and hydroxyl ion (OH−) [88]. The body scavenges free radicals through oxidation-reduction by antioxidant enzymes, converting oxides into less toxic or harmless substances [89]. SOD, CAT, and GSH-Px can quickly eliminate oxygen free radicals and block the free radical chain reaction through interaction [89]. LBPs can regulate and enhance the activity of antioxidant enzymes to effectively remove free radicals [90]. Nuclear factor E2-related factor 2 (Nrf2) is a key regulator of antioxidant defense pathways [91]. LBPs can prevent oxidative stress by activating the Nrf2/ARE signaling pathway (see Figure 5) and activating the expression of genes encoding detoxification enzymes and antioxidant enzymes (such as heme oxygenase 1(HO-1), SOD2, CAT, and GSH) [92]. In the study of cyclophosphamide (CTX)-induced ovarian injury, LBPs upregulated the protein expressions of Nrf2, HO-1, and quinone oxidoreductase 1 and increased the activity of antioxidant enzymes in reducing oxidative stress damage [93]. In short, LBPs may improve the activity of antioxidant enzymes to protect tissues from oxidative damage.
4.3. LBPs Regulate Genes Related to Apoptosis, Ferroptosis, and Autophagy
Apoptosis is programmed cell death controlled by genes. The release of cytochrome c is a crucial step in apoptosis and is regulated by the B-cell lymphoma gene 2 (BCL2) family [94,95]. BCL2 is anti-apoptotic, while BAX is pro-apoptotic. The ratio of BCL2/BAX determines whether the cell initiates apoptosis [96]. LBPs can reduce apoptosis induced by oxidative stress by upregulating BCL2, downregulating BAX, and upregulating the activity of peroxidase to reduce the production of reactive oxygen species (see Figure 5). A study reported that [96] LBPs treatment reduced the apoptosis of and inhibited BAX in retinal pigment epithelium (RPE) cells and activated Bcl-2 in the hydrogen peroxidation-induced RPE oxidative damage model in turn protected RPE from oxidative stress damage. Alcohol causes excessive oxidative stress through processes such as increased malondialdehyde concentrations, generation of reactive oxygen species, and reduced antioxidant enzyme activity. LBPs could reverse it by regulating the balance between BAX and BCL2 [97]. In ischemia/reperfusion myocardial injury, LBPs therapy increased the rate of apoptosis and the concentration for SOD and P62 by activating the NRF2 signal to induce autophagy [98]. In addition, LBPs were associated with ferroptosis and autophagy. LBPs were considered as novel anticancer properties by triggering ferroptosis and might represent a therapeutic option for breast cancer. In this study, LBPs inhibited the activity and proliferation of human breast cancer cells, which were associated with high levels of ferroptosis [99]. Additionally, LBPs were protective against disease progression by regulating autophagy [98,100,101,102]. It was recently reported that LBPs played a direct role in suppressing pyroptosis. They inhibited the NLRP3 inflammasome in hyperoxia-induced acute lung injury and Aβ1-40 oligomer-induced RPE damage [103,104].
4.4. LBPs Can Regulate Mitochondrial Function
Mitochondria are continuously dynamic organelles that assume an indispensable role in cell homeostasis. Mitochondrial dysfunction leads to impaired cell survival and excessive production of ROS [105]. Excessive production of ROS exacerbates mitochondrial dysfunction and further deteriorates cell survival. PGC-1α, TFAM, and NRF-1 are three vital regulatory proteins in mitochondrial biogenesis [106,107]. LBPs can play antioxidant roles by regulating mitochondrial function. In the model of non-alcoholic fatty liver disease, LBPs treatment significantly reduced the intracellular lipid accumulation and concentrations of TG, alanine aminotransferase (ALT), aspartate transaminase (AST), and malondialdehyde, while increasing the concentrations of SOD, phospholipid hydroperoxides, GSH-Px, and CAT [108]. LBPs treatment up-regulated PGC-1 and further promoted the expression of TFRAM and NRF1, enhancing mitochondrial biogenesis and improving oxidative stress [108] (see Figure 5). Zhu et al. found that PM2.5 aggravated mitochondrial damage and induced cell apoptosis by upregulating the expression of pro-apoptotic BAX and downregulating the expression of anti-apoptotic BCL2, while LBPs could reverse this process to protect HaCaT cells [100]. In addition, LBPs inhibited the upregulation of GRK2, reduced the apoptosis of cardiomyocytes, and partially restored the imbalance of ischemia/reperfusion-induced mitochondrial division/fusion by regulating the expressions of Drp1, Opa1, and Mfn2 in AKT/eNOS signaling pathway in the ischemia/reperfusion injury model. A reduction in ROS production in cardiomyocytes was detected in vitro, which indicated the downregulation of cell apoptosis [109].
4.5. LBPs Alleviate Endoplasmic Reticulum Stress
The endoplasmic reticulum (ER) is the site of intracellular protein synthesis, folding, post-translational modification, and maintaining Ca2+ homeostasis [110]. The accumulation of misfolded proteins in organelles caused by external pathological factors is called endoplasmic reticulum stress (ERS) [111]. The occurrence of ERS is closely related to oxidative stress. ERS and oxidative stress can increase dysregulation of homeostasis, interfere with normal cell function, and activate pro-apoptotic signals through mutual positive feedback [112]. Glucose-regulated protein 78 (GRP78) and C/EBP homology protein (CHOP) expression were two notable markers of ERS [113]. Phosphorylated eukaryotic initiation factor-2 alpha (p-eIF2α) can reduce the transcription and translation of cells, thereby reducing protein production and regulating endoplasmic reticulum homeostasis [114]. LBPs have been shown in many studies to reduce oxidative damage by regulating endoplasmic reticulum stress (ERS). Yang et al. [115] found that LBPs intervention can improve ERS and oxidative stress, as well as increase the expression of antioxidase-related indexes in the testis of obese mice induced by the high-fat diet. Moreover, LBPs can ERS signaling by inhibiting the activation of p-eIF2α and GRP78-CHOP, reducing oxidative stress [115] (see Figure 5). In the oxidative stress injury of HaCaT cells induced by PM2.5 [100], the pretreatment of LBPs inhibited the expressions of intracellular GRP78 and CHOP, reduced the accumulation of apoptotic transcription factor CHOP in the nucleus, and regulated intracellular calcium ion homeostasis. These results suggested that LBPs reduced oxidative damage by inhibiting ER stress.
4.6. LBPs Can Promote Neuronal Regeneration in Oxidative Stress Damage
Some studies have shown that LBPs can counteract oxidative stress damage by promoting nerve regeneration. Oxidative stress shows an inhibitory effect in cavernosal nerve regeneration. A model of cavernosal nerve injury was developed by squeezing the cavernosal body, and continuous gavage of LBPs was conducted for 1 day, 7 days, and 14 days after surgery. The SOD and GSH-PX activities increased, and the serum MDA concentrations decreased in the treatment group. The number of myelinated axons in the cavernous nerve increased significantly in the treatment group 1 day after the injury, which reflected the nerve regeneration histologically. It suggested that LBPs effectively promoted nerve regeneration within two weeks before nerve injury to resist oxidative stress-induced neuronal damage [116]. In the study by Au et al., LBPs promoted intrinsic growth capacity and functional recovery of damaged neurons after severe peripheral nerve injury, as well as RGCs survival and axonal regeneration after optic nerve crush, which promoted the partial recovery of visual function [117].
5. Protective Role of LBPs in Oxidative Stress-Related Ocular Diseases
It is undeniable that LBPs have significant antioxidant effects. In ophthalmic diseases, the occurrence and development of many diseases are closely related to ROS. These diseases are called oxidative stress-related ocular diseases, including DR, hypertensive neuroretinopathy, AMD, RP, retinal ischemia/reperfusion injury, glaucoma, dry eye syndrome, and diabetic cataract. In the following, we will elaborate on the antioxidant effect of LBPs on improving oxidative stress-related ocular diseases (Table 1).

Table 1.
Reported studies evaluated LBP with oxidative stress-related ocular diseases.
5.1. Diabetic Retinopathy
DR is one of the most severe microvascular complications in diabetic patients, and it is also a common cause of clinical blindness [146,147]. The pathological features of DR include the thickening of the retinal basement membrane, vessel occlusion, pathological neovascularization, ischemia of local tissue, and hypoxia, which cause visual impairment through oxidative stress injury, inflammatory injury, and other pathways [148,149]. Oxidative stress has been shown to stimulate the expression of pro-apoptotic molecules leading to apoptosis, which has been identified as one of the key mechanisms underlying cell damage in DR [150]. The antioxidant properties of LBPs facilitate their protective effects in animal models and cell lines of DR. However, no study has evaluated the effects of LBPs in patients with DR. In animal models of DR, LBPs administration ameliorated the structural and functional changes in the retina caused by diabetes [118,119,120]. Recovery of the thicknesses of the entire retina and each layer, reduction of structural disorders in the inner and outer segments of the photoreceptors, and decrease of cavitation in the RPE cell layer were observed after LBPs intervention. At the same time, a decrease in basement membrane thickness with the increase in the vessel thickness and morphologically normal capillaries was observed, suggesting a decrease in abnormal vascular clusters and tortuous capillaries with vascular proliferation. At a functional level, LBPs administration was associated with a decrease in the amplitude of a-waves, b-waves, and oscillatory potentials on electroretinography [121]. At the cellular level, the possible mechanism of LBPs in DR is possibly related to critical organelle: LBPs reduce oxidative stress in mitochondria and ER, which are two primary outcomes of diabetes-induced oxidative damage. Continuous exposure to ROS damages mitochondria and impairs the electron transport system, which ultimately leads to mitochondrial DNA damage and subsequent transcriptional impairment [151]. DNA damage and apoptosis of mitochondria are associated with changes in retinal blood flow and disruption of the blood-retinal barrier (BRB) during the late stages of DR [152]. Experimental studies have shown that LBPs may promote mitochondrial biogenesis by upregulating certain metabolic genes [122], which protects DR. Similarly, LBPs can attenuate ER stress, as hyperglycemia-associated oxidative stress disrupts protein synthesis and protein folding within the ER, ultimately leading to ER stress and subsequent apoptosis [118].
5.2. Hypertensive Neuroretinopathy
Hypertension increases systemic arterial pressure and peripheral vascular resistance and is a major risk factor for systemic vascular diseases. The fundus changes it causes include arteriolar stenosis, cotton patches, retinal hemorrhage, papilloma, and other microvascular and optic nerve abnormalities [153]. Microglia are the principal immunoreactive cells in the neurovascular system. Their overactivation induces harmful factors, including ROS and proinflammatory cytokines, and plays a vital role in the pathogenesis of hypertensive retinopathy [154]. LBPs were found to have protective effects on neurons and blood vessels in animal models of acute and chronic ocular hypertension. Some studies have found that LBPs can protect nerve function by moderately activating microglia or inhibiting the activation of the NLRP3 inflammasome, thereby regulating autophagy and MAPK pathways to improve microglia damage [123,124].
5.3. Age-Related Macular Degeneration
AMD is a leading cause of central visual impairment globally [155]. A recent population-based study showed that goji berries might help prevent or delay the development of AMD [125,126,127]. The main pathological changes associated with AMD are the damage of RPE cells, retinal warts and atrophy, and the obstruction of the choroidal capillary. The risk factors for these pathological changes include oxidative stress, inflammation, aging, and hypertension [156,157,158]. In in vitro studies, LBPs had anti-oxidative and anti-apoptotic effects on RPE cells [131]. In addition, other studies [96,130] on human RPE cell lines consistently concluded that the protective effect of LBPs on RPE cells was mainly due to their antioxidant effects, leading to the reduction of endogenous ROS concentrations. Similarly, a study reported that LBPs effectively protected photoreceptor cells from oxidative damage through antioxidant activities, thereby improving photoreceptor function in animal models [128]. LBPs intervention downregulated oxidative stress markers and upregulated the antioxidant genes Nrf2 and TrxR1 in an AMD animal model [129].
5.4. Retinitis Pigmentosa
RP refers to a group of different types of hereditary bilateral retinal pigment dystrophy characterized by the progressive loss of rod and cone photoreceptor cells, which eventually leads to total blindness [159]. Previous studies reported several mechanisms related to the pathogenesis of RP, including oxidative stress, ER stress, cyclic guanosine monophosphate signaling dysregulation, calcium accumulation, and inflammatory responses [160]. Among them, oxidative stress might be a crucial pathway for the retina to be highly susceptible to oxidative stress [161]. In a double-blind placebo-controlled trial involving 42 patients with RP [132], both visual acuity and mean macular thickness of RP patients after oral administration of LBPs were better than those of the control group, and the authors speculated that LBPs might help delay or reduce cone degeneration in RP. In studies involving a mouse model of RP [131,134], LBPs demonstrated a protective effect on the structure and function of retinal nerve cells through their antioxidant properties. The morphologies of photoreceptors and bipolar cells were restored, and the arrangement of photoreceptor cell layers was improved after LBPs supplementation. Functionally, photopic b-wave changes were detected by electroretinography, manifesting as shortened latency, increased amplitude, and increased scotopic a-and b-waves. Further investigation into the specific mechanism of photoreceptor protection revealed that increased antioxidant activity was observed in the animal model after LBPs administration, with a higher glutathione redox/antioxidant ratio, which was commonly used to determine the status of oxidative stress [133].
5.5. Retinal Ischemia/Reperfusion Injury
Retinal ischemia/reperfusion injury involves various pathological changes, including BRB destruction, glial cell activation, oxidative stress, and neuronal death [162]. There is limited research on LBPs and retinal ischemia/reperfusion injury. However, it was confirmed that LBPs improved retinal injury in the phenomenon/reperfusion injury animal model [135]. Animal models of middle cerebral artery occlusion were commonly used to study focal cerebral ischemia in the brain and evaluate the effect of LBPs in the retina with ischemia/reperfusion injury. In the present study, LBPs administered 1 week before ischemia protected the retina from nerve cell death, glial cell activation, oxidative stress, retinal swelling, and BRB disruption 48 h after reperfusion [135,137].
5.6. Glaucoma
Glaucoma is a group of ocular diseases that result in vision loss and blindness by damaging retinal ganglion cells [163]. Based on previous studies on the pathogenesis of glaucoma, increased oxidative stress is thought to undertake an essential role in the pathogenesis of glaucoma [164,165,166]. The concentration of free radicals is known to increase with cell aging, which impairs mitochondrial energy production and normal neuronal function [167]. In treating glaucoma, neuroprotective strategies, in addition to lowering intraocular pressure, are necessary to protect healthy neurons and rescue damaged neurons [168]. The neuroprotective effects [169] of LBPs and their ability to modulate oxidative stress [170] have been demonstrated to protect RGCs in both ocular hypertension-dependent [138,171] and -independent [139,140,172] optic neuropathy models. Chan et al. [142] first demonstrated the dose-dependent preservation of RGCs with LBPs pretreatment in an in vivo rat model of ocular hypertension induced by the protective effects of LBPs pretreatment on RGCs in injury models, including those of chronic ocular hypertension [123,138,143,171], acute ocular hypertension [136,139], partial optic nerve transection [140,173], and ischemia-reperfusion injury. Therefore, the benefits of LBPs pretreatment in experimental glaucoma models are evident.
5.7. Dry Eye Syndrome
DES is a disease of the eye surface that causes discomfort and visual impairment, affecting life quality [174]. DES is characterized by an abnormal tear film, eye discomfort, and potential damage to the ocular surface between the eyelids [175]. The treatment options for DES are limited to palliative care with artificial tears and tear preservation techniques. However, specific treatments may worsen endophthalmitis, despite the maximum use of palliative care, for the most severe cases of DES associated with corneal ulcers [176]. In a population meta-analysis of patients with DES, a Chinese herbal preparation containing wolfberry showed excellent potential for improving symptoms [177]. There are few studies on LBPs and dry eye disease. However, a study reported that LBPs administration played a noteworthy role in alleviating DES caused by oxidative stress and inflammation [144].
5.8. Diabetic Cataract
Diabetic cataract is also a complication secondary to diabetes, which is characterized by the gradual accumulation of glucose on the lens of the eye. Its pathogenesis is a complex process of multiple factors [21,57,178]. Sirtuin1 (SIRT1) is nicotinamide adenine dinucleotide (NAD) tower-dependent deacetylase that affects cell aging, differentiation, apoptosis, and the regulation of fat and glucose metabolism [179]. SIRT1 has been identified as a key regulator in cataract prevention. Previous studies showed that SIRT1 executed a role in regulating oxidative stress response and apoptosis in lens epithelial cells [180,181]. A recent cellular study in vitro explored the mechanism underlying the improvement of diabetic cataracts facilitated by LBPs. It showed that LBPs hindered the development of cataracts in the lens and improved retinal function by upregulating Sirt1 [145]. In previous studies, we found that SIRT1, which was highly sensitive to cellular redox states, counteracts the effects of ROS by deacetylation of multiple cellular targets. The redox function of cells was affected by SIRT1 directly or indirectly, and the activity and expression of SIRT1 were affected by the redox state of cells through post-translational modifications [182]. Thus, it is believed that SIRT1 is closely related to cellular redox.
6. Conclusions and Perspectives
In conclusion, LBP can directly or indirectly eliminate free radicals, including improving the activity of antioxidant enzymes, regulating apoptosis-related protein Bcl2/Bax, and enhancing mitochondrial function to eliminate free radicals, thus reducing the damage of oxidative stress on the body. Its mechanisms mainly involve Nrf2/ARE, Bcl2/Bax, PGC-1α/TFAM/NRF-1, and GRP78/CHOP/p-eif2α-related signaling pathway. Furthermore, its antioxidant effects show great potential in AMD, DR, hypertensive neuroretinopathy, RP, and other eye diseases. Moreover, as natural components, LBPs have shown potential for oxidative stress-related eye diseases. LBPs have the advantages of high production with low price and high safety. Notably, their efficacy would not be affected by the quality of wolfberry; they may be ideal candidates for the development of healthcare products and drugs.
However, the relationship between their structure and activity is complex, and their mechanisms of action in cells remain to be investigated. For instance, the mechanisms underlying their antioxidant role via the regulation of the enzyme-like SOD, CAT, and GSH-Px and non-enzyme-like lipoic acid, glutathione, L-arginine, and coenzyme Q10 remain to be further explored. Furthermore, it has not been established that LBPs can prevent oxidative stress by blocking epigenetic changes in DNA. The possible effects of LBPs in the post-genomic era are intriguing. The exploration of LBPs concerning genomics, transcriptomics, proteomics, and metabolomics may provide opportunities for drug engineering. In addition, the molecular structure and function of polysaccharides obtained by different extraction methods are quite different. Further exploration of the relationship between its structure and function, as well as its extraction process, will provide more insights into the applications of LBPs. With the development of analytical techniques, the separation and structural analysis of polysaccharides will provide insights into their underlying mechanisms. Further research also should focus on the specific protective mechanisms of LBPs in ocular cells. In addition, most LBPs are absorbed through the gastrointestinal tract by oral administration rather than directly acting on the eyes, which may influence the efficiency of LBPs on the eye. The extraction of active ingredients of L. barbarum and their direct action on the eyes, for example, by using modified nanomaterials, may present future research directions [183,184,185]. Currently, most studies on the effects of LBP on eye diseases are at the empirical stage, and some are in clinical trials. The results of the research are one-sided, leaving room for further exploration. In the future, the clinical application of traditional Chinese medicine compounds based on LBPs should be considered for specific ocular diseases with multicenter and large-sample clinical studies. Due to the prominent effects of LBPs on redox-related diseases, their dietary addition should be considered to improve the diet structure to facilitate disease treatment.
Author Contributions
Conceptualization, G.D. and S.H.; formal analysis, Y.N., G.Z. and X.S.; investigation, Y.N., G.Z. and X.S.; writing—original draft preparation, Y.N., G.Z. and X.S.; writing—review and editing, S.H. and G.D.; funding acquisition, G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Guorui Dou, the National Natural Science Foundation of China (grant number: 81970814, 81670863) and Clinical AFFMU Foundation (grant number: 2021JSTS28).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
We thank Ziyi Zhou, who won the top prize in the National English Writing Contest, for thoroughly checking and modifying the language of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Seen, S.; Tong, L. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018, 96, e412–e420. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Holmgren, A.; Larsson, N.G.; Halliwell, B.; Chang, C.J.; Kalyanaraman, B.; Rhee, S.G.; Thornalley, P.J.; Partridge, L.; Gems, D.; et al. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011, 13, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Landry, W.D.; Cotter, T.G. ROS signalling, NADPH oxidases and cancer. Biochem. Soc. Trans. 2014, 42, 934–938. [Google Scholar] [CrossRef]
- Nüsse, O. Biochemistry of the phagosome: The challenge to study a transient organelle. Sci. World J. 2011, 11, 2364–2381. [Google Scholar] [CrossRef]
- Poli, G.; Leonarduzzi, G.; Biasi, F.; Chiarpotto, E. Oxidative stress and cell signalling. Curr. Med. Chem. 2004, 11, 1163–1182. [Google Scholar] [CrossRef]
- Ung, L.; Pattamatta, U.; Carnt, N.; Wilkinson-Berka, J.L.; Liew, G.; White, A.J.R. Oxidative stress and reactive oxygen species: A review of their role in ocular disease. Clin. Sci. 2017, 131, 2865–2883. [Google Scholar] [CrossRef] [PubMed]
- Tangvarasittichai, O.; Tangvarasittichai, S. Oxidative Stress, Ocular Disease and Diabetes Retinopathy. Curr. Pharm. Des. 2018, 24, 4726–4741. [Google Scholar] [CrossRef]
- Kabra, A.; Sharma, R.; Hano, C.; Kabra, R.; Martins, N.; Baghel, U.S. Phytochemical Composition, Antioxidant, and Antimicrobial Attributes of Different Solvent Extracts from Myrica esculenta Buch.-Ham.ex. D. Don Leaves. Biomolecules 2019, 9, 357. [Google Scholar] [CrossRef]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Saccà, S.C.; Roszkowska, A.M.; Izzotti, A. Environmental light and endogenous antioxidants as the main determinants of non-cancer ocular diseases. Mutat. Res. 2013, 752, 153–171. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid Med. Cell Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed]
- Nandhakumar, E.; Indumathi, P. In Vitro antioxidant activities of methanol and aqueous extract of Annona squamosa (L.) fruit pulp. J. Acupunct. Meridian Stud. 2013, 6, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Fejes, S.; Blázovics, A.; Lugasi, A.; Lemberkovics, E.; Petri, G.; Kéry, A. In vitro antioxidant activity of Anthriscus cerefolium L.(Hoffm.) extracts. J. Ethnopharmacol. 2000, 69, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Mei, X.; Hu, J. The Antioxidant Activities of Natural Polysaccharides. Curr. Drug Targets 2017, 18, 1296–1300. [Google Scholar] [CrossRef]
- Kruk, J.; Kubasik-Kladna, K.; Aboul-Enein, H.Y. The Role Oxidative Stress in the Pathogenesis of Eye Diseases: Current Status and a Dual Role of Physical Activity. Mini Rev. Med. Chem. 2015, 16, 241–257. [Google Scholar] [CrossRef]
- Guo, T.; Akan, O.D.; Luo, F.; Lin, Q. Dietary polysaccharides exert biological functions via epigenetic regulations: Advance and prospectives. Crit. Rev. Food Sci. Nutr. 2023, 63, 114–124. [Google Scholar] [CrossRef]
- Jiao, R.; Liu, Y.; Gao, H.; Xiao, J.; So, K.F. The Anti-Oxidant and Antitumor Properties of Plant Polysaccharides. Am. J. Chin. Med. 2016, 44, 463–488. [Google Scholar] [CrossRef]
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, Structural Characterization, and Biological Functions of Lycium Barbarum Polysaccharides: A Review. Biomolecules 2019, 9, 389. [Google Scholar] [CrossRef]
- Yang, S.; Chen, X.; Sun, J.; Qu, C.; Chen, X. Polysaccharides from traditional Asian food source and their antitumor activity. J. Food Biochem. 2022, 46, e13927. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Tao, W.; Zhang, X.; Gao, X.; Yong, J.; Zhao, J.; Zhang, L.; Li, Y.; Duan, J.A. Lycium ruthenicum studies: Molecular biology, Phytochemistry and pharmacology. Food Chem. 2018, 240, 759–766. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium Barbarum: A Traditional Chinese Herb and A Promising Anti-Aging Agent. Aging Dis. 2017, 8, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Potterat, O. Goji (Lycium barbarum and L.chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M. Protective effect of Lycium barbarum polysaccharides on streptozotocin-induced oxidative stress in rats. Int. J. Biol. Macromol. 2007, 40, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Masci, A.; Carradori, S.; Casadei, M.A.; Paolicelli, P.; Petralito, S.; Ragno, R.; Cesa, S. Lycium barbarum polysaccharides: Extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem. 2018, 254, 377–389. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, F.; Ming, K.; Wang, D.; Hu, Y.; Liu, J. Polysaccharides from Traditional Chinese Medicines: Extraction, Purification, Modification, and Biological Activity. Molecules 2016, 21, 1705. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, Q.; Li, S.; Pu, L.; Yu, L.; Liu, Y.; Lai, X. Effects of Lycium barbarum L. Polysaccharides on Vascular Retinopathy: An Insight Review. Molecules 2022, 27, 5628. [Google Scholar] [CrossRef]
- Yin, G.; Dang, Y. Optimization of extraction technology of the Lycium barbarum polysaccharides by Box–Behnken statistical design. Carbohydr. Polym. 2008, 74, 603–610. [Google Scholar] [CrossRef]
- Fakhfakh, J.; Athmouni, K.; Mallek-Fakhfakh, H.; Ayedi, H.; Allouche, N. Polysaccharide from Lycium arabicum: Structural Features, in Vitro Antioxidant Activities and Protective Effect against Oxidative Damage in Human Erythrocytes. Chem. Biodivers. 2020, 17, e2000614. [Google Scholar] [CrossRef]
- Ma, Q.; Zhai, R.; Xie, X.; Chen, T.; Zhang, Z.; Liu, H.; Nie, C.; Yuan, X.; Tu, A.; Tian, B.; et al. Hypoglycemic Effects of Lycium barbarum Polysaccharide in Type 2 Diabetes Mellitus Mice via Modulating Gut Microbiota. Front. Nutr. 2022, 9, 916271. [Google Scholar] [CrossRef]
- Zeng, P.; Li, J.; Chen, Y.; Zhang, L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar] [CrossRef]
- Zhou, Z.Q.; Xiao, J.; Fan, H.X.; Yu, Y.; He, R.R.; Feng, X.L.; Kurihara, H.; So, K.F.; Yao, X.S.; Gao, H. Polyphenols from wolfberry and their bioactivities. Food Chem. 2017, 214, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Chanioti, S.; Katsouli, M.; Tzia, C. Novel Processes for the Extraction of Phenolic Compounds from Olive Pomace and Their Protection by Encapsulation. Molecules 2021, 26, 1781. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhang, J.R.; Li, H.B.; Wu, D.T.; Geng, F.; Corke, H.; Wei, X.L.; Gan, R.Y. Green Extraction of Antioxidant Polyphenols from Green Tea (Camellia sinensis). Antioxidants 2020, 9, 785. [Google Scholar] [CrossRef]
- Pollini, L.; Rocchi, R.; Cossignani, L.; Mañes, J.; Compagnone, D.; Blasi, F. Phenol Profiling and Nutraceutical Potential of Lycium spp. Leaf Extracts Obtained with Ultrasound and Microwave Assisted Techniques. Antioxidants 2019, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Sang, J.; Sang, J.; Ma, Q.; Hou, X.F.; Li, C.Q. Extraction optimization and identification of anthocyanins from Nitraria tangutorun Bobr.seed meal and establishment of a green analytical method of anthocyanins. Food Chem. 2017, 218, 386–395. [Google Scholar] [CrossRef]
- Skenderidis, P.; Petrotos, K.; Giavasis, I.; Hadjichristodoulou, C.; Tsakalof, A. Optimization of ultrasound assisted extraction of of goji berry (Lycium barbarum) fruits and evaluation of extracts’ bioactivity. J. Food Process Eng. 2017, 40, e12522. [Google Scholar] [CrossRef]
- Yun, D.; Yan, Y.; Liu, J. Isolation, structure and biological activity of polysaccharides from the fruits of Lycium ruthenicum Murr: A review. Carbohydr. Polym. 2022, 291, 119618. [Google Scholar] [CrossRef]
- Zhang, X.X.; Ni, Z.J.; Zhang, F.; Thakur, K.; Zhang, J.G.; Khan, M.R.; Busquets, R.; Wei, Z.J. Physicochemical and antioxidant properties of Lycium barbarum seed dreg polysaccharides prepared by continuous extraction. Food Chem. X 2022, 14, 100282. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Q.; Fang, J.; Wang, C.; Wang, D.; Li, M. The anti-aging activity of Lycium barbarum polysaccharide extracted by yeast fermentation: In vivo and in vitro studies. Int. J. Biol. Macromol. 2022, 209, 2032–2041. [Google Scholar] [CrossRef]
- Xia, G.; Xin, N.; Liu, W.; Yao, H.; Hou, Y.; Qi, J. Inhibitory effect of Lycium barbarum polysaccharides on cell apoptosis and senescence is potentially mediated by the p53 signaling pathway. Mol. Med. Rep. 2014, 9, 1237–1241. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, W.; Zhang, Y. Immunoregulation and Lycium Barbarum. In Lycium Barbarum and Human Health; Chang, R.C., So, K.F., Eds.; Springer: Amsterdam, The Netherlands, 2015; pp. 27–44. [Google Scholar] [CrossRef]
- Zhang, X.R.; Qi, C.H.; Cheng, J.P.; Liu, G.; Huang, L.J.; Wang, Z.F.; Zhou, W.X.; Zhang, Y.X. Lycium barbarum polysaccharide LBPF4-OL may be a new Toll-like receptor 4/MD2-MAPK signaling pathway activator and inducer. Int. Immunopharmacol. 2014, 19, 132–141. [Google Scholar] [CrossRef]
- Bo, R.; Liu, Z.; Zhang, J.; Gu, P.; Ou, N.; Sun, Y.; Hu, Y.; Liu, J.; Wang, D. Mechanism of Lycium barbarum polysaccharides liposomes on activating murine dendritic cells. Carbohydr. Polym. 2019, 205, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Su, C.X.; Duan, X.G.; Liang, L.J.; Feng, W.; Zheng, J.; Fu, X.Y.; Yan, Y.M.; Ling, H.; Wang, N.P. Lycium barbarum polysaccharides as an adjuvant for recombinant vaccine through enhancement of humoral immunity by activating Tfh cells. Vet. Immunol. Immunopathol. 2014, 158, 98–104. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Wang, D.; Liu, J.; Zhang, J.; Abula, S.; Zhao, B.; Ruan, S. Sulfated modification can enhance the immune-enhancing activity of lycium barbarum polysaccharides. Cell Immunol. 2010, 263, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhu, L.; Li, T.; Tang, X.; Xiang, Y.; Han, X.; Xia, L.; Zeng, L.; Nie, J.; Huang, Y.; et al. Neuroprotective Mechanisms of Lycium barbarum Polysaccharides Against Ischemic Insults by Regulating NR2B and NR2A Containing NMDA Receptor Signaling Pathways. Front. Cell Neurosci. 2017, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Shi, Z.; Yang, T.G.; Yu, L.M.; Xu, A.L. The protective effects of lycium barbarum polysaccharides on retinal neurons in diabetic rats and its mechanism. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2019, 35, 55–59. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, X.; Pu, J.; Luo, P.; Ma, W.; Wang, J.; Wei, J.; Wang, Y.; Fei, Z. Lycium barbarum polysaccharide protects against oxygen glucose deprivation/reoxygenation-induced apoptosis and autophagic cell death via the PI3K/Akt/mTOR signaling pathway in primary cultured hippocampal neurons. Biochem. Biophys. Res. Commun. 2018, 495, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Huang, Q.; Zhao, K.; Shang, P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int. J. Biol. Macromol. 2013, 54, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Liu, Q.; Deng, Y.; Dang, T.; Dai, W.; Liu, T.; Liu, Y.; Sun, J.; Wang, L.; Liu, Y.; et al. Arabinogalactan derived from Lycium barbarum fruit inhibits cancer cell growth via cell cycle arrest and apoptosis. Int. J. Biol. Macromol. 2020, 149, 639–650. [Google Scholar] [CrossRef]
- Deng, X.; Li, X.; Luo, S.; Zheng, Y.; Luo, X.; Zhou, L. Antitumor activity of Lycium barbarum polysaccharides with different molecular weights: An in vitro and in vivo study. Food Nutr. Res. 2017, 61, 1399770. [Google Scholar] [CrossRef]
- Tang, W.M.; Chan, E.; Kwok, C.Y.; Lee, Y.K.; Wu, J.H.; Wan, C.W.; Chan, R.Y.; Yu, P.H.; Chan, S.W. A review of the anticancer and immunomodulatory effects of Lycium barbarum fruit. Inflammopharmacology 2012, 20, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, H.; Huang, J.; Li, Z.; Zhu, C.; Zhang, S. Effect of lycium barbarum polysaccharide on human hepatoma QGY7703 cells: Inhibition of proliferation and induction of apoptosis. Life Sci. 2005, 76, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Luo, S.; Luo, X.; Hu, M.; Ma, F.; Wang, Y.; Zhou, L.; Huang, R. Fraction From Lycium barbarum Polysaccharides Reduces Immunotoxicity and Enhances Antitumor Activity of Doxorubicin in Mice. Integr. Cancer Ther. 2018, 17, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Liu, B.; Zhong, W.; Wang, G.D.; Xu, Y.L.; Chen, X. Protective effect of Lycium barbarum polysaccharides against high-fat diet-induced renal injury and lipid deposition in rat kidneys. J. Biol. Regul. Homeost. Agents 2019, 33, 7–17. [Google Scholar]
- Xiao, J.; Xing, F.; Huo, J.; Fung, M.L.; Liong, E.C.; Ching, Y.P.; Xu, A.; Chang, R.C.; So, K.F.; Tipoe, G.L. Lycium barbarum polysaccharides therapeutically improve hepatic functions in non-alcoholic steatohepatitis rats and cellular steatosis model. Sci. Rep. 2014, 4, 5587. [Google Scholar] [CrossRef]
- Du, M.; Hu, X.; Kou, L.; Zhang, B.; Zhang, C. Lycium barbarum Polysaccharide Mediated the Antidiabetic and Antinephritic Effects in Diet-Streptozotocin-Induced Diabetic Sprague Dawley Rats via Regulation of NF-κB. BioMed Res. Int. 2016, 2016, 3140290. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Peng, L.; Li, R.; Li, H.; Mo, Z.; Dai, X.; Jiang, N.; Liu, Q.; Zhang, E.; Deng, H.; et al. Lycium barbarum polysaccharide protects HSF cells against ultraviolet-induced damage through the activation of Nrf2. Cell Mol. Biol. Lett. 2018, 23, 18. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Peng, L.; Jiang, N.; Liu, Q.; Zhang, E.; Liang, B.; Li, R.; Zhu, H. Lycium barbarum polysaccharide protects human keratinocytes against UVB-induced photo-damage. Free Radic. Res. 2017, 51, 200–210. [Google Scholar] [CrossRef]
- Guo, L.; Du, Q.Q.; Cheng, P.Q.; Yang, T.T.; Xing, C.Q.; Luo, X.Z.; Peng, X.C.; Qian, F.; Huang, J.R.; Tang, F.R. Neuroprotective Effects of Lycium barbarum Berry on Neurobehavioral Changes and Neuronal Loss in the Hippocampus of Mice Exposed to Acute Ionizing Radiation. Dose Response 2021, 19, 15593258211057768. [Google Scholar] [CrossRef]
- Yuan, D. Influence of LBP on Blood Glucose and Blood Lipid in Type 2 Diabetic Rats. J. Liaoning Med. Univ. 2015, 36, 12–14. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, W.; Yu, J.; Zou, S.; Wang, J.; Yao, W.; Gao, X. Characterization and hypoglycemic effect of a polysaccharide extracted from the fruit of Lycium barbarum L. Carbohydr. Polym. 2013, 98, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Tang, H.; Wang, F.; Yang, X.; Wang, Z.; Liu, H.; Pan, D.; Yang, C.; Wang, S.; Sun, G. An untargeted metabolomics approach reveals further insights of Lycium barbarum polysaccharides in high fat diet and streptozotocin-induced diabetic rats. Food Res. Int. 2019, 116, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Wang, Q.; Yang, Y. Crude extracts from Lycium barbarum suppress SREBP-1c expression and prevent diet-induced fatty liver through AMPK activation. BioMed Res. Int. 2014, 2014, 196198. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Zeng, M.Y.; Núñez, G. The interplay between host immune cells and gut microbiota in chronic inflammatory diseases. Exp. Mol. Med. 2017, 49, e339. [Google Scholar] [CrossRef]
- Kamiya, T.; Ohtani, N. The role of immune cells in the liver tumor microenvironment: An involvement of gut microbiota-derived factors. Int. Immunol. 2022, 34, 467–474. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, S.; Liu, J.; McLean, R.J.C.; Chu, W. Prebiotic, immuno-stimulating and gut microbiota-modulating effects of Lycium barbarum polysaccharide. Biomed. Pharmacother. 2020, 121, 109591. [Google Scholar] [CrossRef]
- Zhao, F.; Guan, S.; Fu, Y.; Wang, K.; Liu, Z.; Ng, T.B. Lycium barbarum polysaccharide attenuates emotional injury of offspring elicited by prenatal chronic stress in rats via regulation of gut microbiota. Biomed. Pharmacother. 2021, 143, 112087. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, T.; Xu, W.; Huang, Y.; Ran, L.; Yan, Y.; Mi, J.; Lu, L.; Sun, Y.; Zeng, X.; et al. The polysaccharides from the fruits of Lycium barbarum L. confer anti-diabetic effect by regulating gut microbiota and intestinal barrier. Carbohydr. Polym. 2022, 291, 119626. [Google Scholar] [CrossRef]
- Cao, C.; Zhu, B.; Liu, Z.; Wang, X.; Ai, C.; Gong, G.; Hu, M.; Huang, L.; Song, S. An arabinogalactan from Lycium barbarum attenuates DSS-induced chronic colitis in C57BL/6J. mice associated with the modulation of intestinal barrier function and gut microbiota. Food Funct. 2021, 12, 9829–9843. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. The antiviral activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 115, 77–82. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Wang, D.; Zhang, F.; Zhao, X.; Abula, S.; Fan, Y.; Guo, L. Lycium barbarum polysaccharide inhibits the infectivity of Newcastle disease virus to chicken embryo fibroblast. Int. J. Biol. Macromol. 2010, 46, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Bo, R.; Zheng, S.; Xing, J.; Luo, L.; Niu, Y.; Huang, Y.; Liu, Z.; Hu, Y.; Liu, J.; Wu, Y.; et al. The immunological activity of Lycium barbarum polysaccharides liposome in vitro and adjuvanticity against PCV2 in vivo. Int. J. Biol. Macromol. 2016, 85, 294–301. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Lee, R.; Lee, E.; Choi, T.G.; Lee, A.S.; Yoon, Y.I.; Park, G.C.; Namgoong, J.M.; Lee, S.G.; et al. Therapeutic strategies for liver diseases based on redox control systems. Biomed. Pharmacother. 2022, 156, 113764. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Duszewska, A.M.; Kucharski, M.; Tyczyński, P.; Smolarczyk, R. Oxidative Stress and Reproductive Function: Oxidative stress in polycystic ovary syndrome. Reproduction 2022, 164, F145–F154. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Wu, J.Q.; Kosten, T.R.; Zhang, X.Y. Free radicals, antioxidant defense systems, and schizophrenia. Progr. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 200–206. [Google Scholar] [CrossRef]
- Davinelli, S.; Medoro, A.; Intrieri, M.; Saso, L.; Scapagnini, G.; Kang, J.X. Targeting NRF2-KEAP1 axis by Omega-3 fatty acids and their derivatives: Emerging opportunities against aging and diseases. Free Radic. Biol. Med. 2022, 193, 736–750. [Google Scholar] [CrossRef]
- Zaric, B.L.; Macvanin, M.T.; Isenovic, E.R. Free radicals: Relationship to Human Diseases and Potential Therapeutic applications. Int. J. Biochem. Cell Biol. 2023, 154, 106346. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Ellagic acid: Insight into its protective effects in age-associated disorders. 3 Biotech. 2022, 12, 340. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxid. Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef]
- Liu, Z.; Dang, J.; Wang, Q.; Yu, M.; Jiang, L.; Mei, L.; Shao, Y.; Tao, Y. Optimization of polysaccharides from Lycium ruthenicum fruit using RSM and its anti-oxidant activity. Int. J. Biol. Macromol. 2013, 61, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Assessment of the antioxidant and antimutagenic activity of extracts from goji berry of Greek cultivation. Toxicol. Rep. 2018, 5, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Na, H.; Yourui, S.; Chenxu, D. Process optimization of lycium ruthenicum murr. powder preparation by airflow ultrafine pulverization at low temperature and characterization of its antioxidant activity. J. Chem. Soc. Pak. 2019, 41, 741–749. [Google Scholar]
- He, Q.; Du, B.; Xu, B. Extraction Optimization of Phenolics and Antioxidants from Black Goji Berry by Accelerated Solvent Extractor Using Response Surface Methodology. Appl. Sci. 2018, 8, 1905. [Google Scholar] [CrossRef]
- Tang, J.; Yan, Y.; Ran, L.; Mi, J.; Sun, Y.; Lu, L.; Gao, Y.; Zeng, X.; Cao, Y. Isolation, antioxidant property and protective effect on PC12 cell of the main anthocyanin in fruit of Lycium ruthenicum Murray. J. Funct. Foods 2017, 30, 97–107. [Google Scholar] [CrossRef]
- Yossa Nzeuwa, I.B.; Xia, H.; Shi, Y.; Yang, C.; Shah, M.W.; Guo, B.; Wang, L.; Sun, G. Fatty acid and mineral contents of Lycium ruthenicum Murr.and antioxidant activity against isoproterenol-induced acute myocardial ischemia in mice. Food Sci. Nutr. 2020, 8, 1075–1081. [Google Scholar] [CrossRef]
- Qi, Y.; Duan, G.; Fan, G.; Peng, N. Effect of Lycium barbarum polysaccharides on cell signal transduction pathways. Biomed. Pharmacother. 2022, 147, 112620. [Google Scholar] [CrossRef]
- Mu, S.; Yang, W.; Huang, G. Antioxidant activities and mechanisms of polysaccharides. Chem. Biol. Drug Des. 2021, 97, 628–632. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G.; Yang, Z.; Hou, Y. Antioxidant activity of Momordica charantia polysaccharide and its derivatives. Int. J. Biol. Macromol. 2019, 138, 673–680. [Google Scholar] [CrossRef]
- Schneider, K.S.; Chan, J.Y. Emerging role of Nrf2 in adipocytes and adipose biology. Adv Nutr. 2013, 4, 62–66. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Li, Y.; Wang, Q.; Gao, L.; Zhao, J. Dietary Lycium barbarum polysaccharide induces Nrf2/ARE pathway and ameliorates insulin resistance induced by high-fat via activation of PI3K/AKT signaling. Oxid. Med. Cell. Longev. 2014, 2014, 145641. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.M.; Zhang, J.Q.; Fei, Y.F. Lycium barbarum polysaccharide attenuates chemotherapy-induced ovarian injury by reducing oxidative stress. J. Obstet. Gynaecol. Res. 2017, 43, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Flores-Romero, H.; Hohorst, L.; John, M.; Albert, M.C.; King, L.E.; Beckmann, L.; Szabo, T.; Hertlein, V.; Luo, X.; Villunger, A.; et al. BCL-2-family protein tBID can act as a BAX-like effector of apoptosis. EMBO J. 2022, 41, e108690. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, T.; Czabotar, P.E. BAX, BAK, and BOK: A Coming of Age for the BCL-2 Family Effector Proteins. Cold Spring Harb. Perspect. Biol. 2020, 12, a036319. [Google Scholar] [CrossRef]
- Liu, L.; Lao, W.; Ji, Q.S.; Yang, Z.H.; Yu, G.C.; Zhong, J.X. Lycium barbarum polysaccharides protected human retinal pigment epithelial cells against oxidative stress-induced apoptosis. Int. J. Ophthalmol. 2015, 8, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Liu, J.; Di, D.; Liu, Y.; Wei, J. Hepatoprotective effect of crude polysaccharide isolated from Lycium barbarum L.against alcohol-induced oxidative damage involves Nrf2 signaling. Food Sci. Nutr. 2020, 8, 6528–6538. [Google Scholar] [CrossRef]
- Pan, H.; Niu, L.; Wu, Y.; Chen, L.; Zhou, X.; Zhao, Y. Lycium barbarum polysaccharide protects rats and cardiomyocytes against ischemia/reperfusion injury via Nrf2 activation through autophagy inhibition. Mol. Med. Rep. 2021, 24, 778. [Google Scholar] [CrossRef]
- Du, X.; Zhang, J.; Liu, L.; Xu, B.; Han, H.; Dai, W.; Pei, X.; Fu, X.; Hou, S. A novel anticancer property of Lycium barbarum polysaccharide in triggering ferroptosis of breast cancer cells. J. Zhejiang Univ Sci. B 2022, 23, 286–299. [Google Scholar] [CrossRef]
- Zhu, S.; Li, X.; Dang, B.; Wu, F.; Wang, C.; Lin, C. Lycium Barbarum polysaccharide protects HaCaT cells from PM2.5-induced apoptosis via inhibiting oxidative stress, ER stress and autophagy. Redox Rep. 2022, 27, 32–44. [Google Scholar] [CrossRef]
- Li, H.Y.; Huang, M.; Luo, Q.Y.; Hong, X.; Ramakrishna, S.; So, K.F. Lycium barbarum (Wolfberry) Increases Retinal Ganglion Cell Survival and Affects both Microglia/Macrophage Polarization and Autophagy after Rat Partial Optic Nerve Transection. Cell Transplant. 2019, 28, 607–618. [Google Scholar] [CrossRef]
- Liu, S.Y.; Chen, L.; Li, X.C.; Hu, Q.K.; He, L.J. Lycium barbarum polysaccharide protects diabetic peripheral neuropathy by enhancing autophagy via mTOR/p70S6K inhibition in Streptozotocin-induced diabetic rats. J. Chem. Neuroanat. 2018, 89, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.Y.; Zhang, H.D.; Liu, X.Y.; Xu, Y. Attenuation of hyperoxic acute lung injury by Lycium barbarum polysaccharide via inhibiting NLRP3 inflammasome. Arch. Pharm. Res. 2019, 42, 902–908. [Google Scholar] [CrossRef]
- Yang, M.; So, K.F.; Lo, A.C.Y.; Lam, W.C. The Effect of Lycium barbarum Polysaccharides on Pyroptosis-Associated Amyloid β(1-40) Oligomers-Induced Adult Retinal Pigment Epithelium 19 Cell Damage. Int. J. Mol. Sci. 2020, 21, 4658. [Google Scholar] [CrossRef] [PubMed]
- Chella Krishnan, K.; Kurt, Z.; Barrere-Cain, R.; Sabir, S.; Das, A.; Floyd, R.; Vergnes, L.; Zhao, Y.; Che, N.; Charugundla, S.; et al. Integration of Multi-omics Data from Mouse Diversity Panel Highlights Mitochondrial Dysfunction in Non-alcoholic Fatty Liver Disease. Cell Syst. 2018, 6, 103–115.e7. [Google Scholar] [CrossRef] [PubMed]
- Yenki, P.; Khodagholi, F.; Shaerzadeh, F. Inhibition of phosphorylation of JNK suppresses Aβ-induced ER stress and upregulates prosurvival mitochondrial proteins in rat hippocampus. J. Mol. Neurosci. 2013, 49, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, W.; Wang, L.; Li, Y.; Tan, B.; Lu, X.; Deng, Y.; Zhang, Y.; Guo, X.; Mu, J.; et al. Curcumin prevents cerebral ischemia reperfusion injury via increase of mitochondrial biogenesis. Neurochem. Res. 2014, 39, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Guo, Y.Q.; Fan, Y.N.; Tao, X.J.; Gao, Q.H.; Yang, J.J. Lycium barbarum Polysaccharides Promotes Mitochondrial Biogenesis and Energy Balance in a NAFLD Cell Model. Chin. J. Integr. Med. 2022, 28, 975–982. [Google Scholar] [CrossRef]
- Li, Y.; Yang, B.; Zhang, X.; Shen, X.; Ma, Y.; Jing, L. Lycium barbarum polysaccharide antagonizes cardiomyocyte apoptosis by inhibiting the upregulation of GRK2 induced by I/R injury, and salvage mitochondrial fission/fusion imbalance and AKT/eNOS signaling. Cell Signal. 2022, 92, 110252. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Ren, L.; Li, B.; Fang, J.; Zhai, Y.; He, X.; Du, E.; Miao, Y.; Hua, J.; Peng, S. Melatonin Relieves Busulfan-Induced Spermatogonial Stem Cell Apoptosis of Mouse Testis by Inhibiting Endoplasmic Reticulum Stress. Cell. Physiol. Biochem. 2017, 44, 2407–2421. [Google Scholar] [CrossRef]
- Oakes, S.A.; Papa, F.R. The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. 2015, 10, 173–194. [Google Scholar] [CrossRef]
- Karna, K.K.; Choi, B.R.; You, J.H.; Shin, Y.S.; Soni, K.K.; Cui, W.S.; Lee, S.W.; Kim, C.Y.; Kim, H.K.; Park, J.K. Cross-talk between ER stress and mitochondrial pathway mediated adriamycin-induced testicular toxicity and DA-9401 modulate adriamycin-induced apoptosis in Sprague-Dawley rats. Cancer Cell Int. 2019, 19, 85. [Google Scholar] [CrossRef]
- Kratochvílová, K.; Moráň, L.; Paďourová, S.; Stejskal, S.; Tesařová, L.; Šimara, P.; Hampl, A.; Koutná, I.; Vaňhara, P. The role of the endoplasmic reticulum stress in stemness, pluripotency and development. Eur. J. Cell Biol. 2016, 95, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, W.; Tsai, P.J.; Chen, P.H.; Ye, M.; Guo, J.; Su, Z. Mutual interaction between endoplasmic reticulum and mitochondria in nonalcoholic fatty liver disease. Lipids Health Dis. 2020, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.L.; Wei, Y.X.; Liao, B.Y.; Wei, G.J.; Qin, H.M.; Pang, X.X.; Wang, J.L. Effects of Lycium barbarum Polysaccharide on Endoplasmic Reticulum Stress and Oxidative Stress in Obese Mice. Front. Pharmacol. 2020, 11, 742. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.K.; Yu, H.L.; Liu, B.; Wang, H.; Luo, Q.; Ding, X.G. Antioxidative mechanism of Lycium barbarum polysaccharides promotes repair and regeneration following cavernous nerve injury. Neural Regen. Res. 2016, 11, 1312–1321. [Google Scholar] [CrossRef]
- Au, N.P.B.; Kumar, G.; Asthana, P.; Gao, F.; Kawaguchi, R.; Chang, R.C.C.; So, K.F.; Hu, Y.; Geschwind, D.H.; Coppola, G.; et al. Clinically relevant small-molecule promotes nerve repair and visual function recovery. npj Regen. Med. 2022, 7, 50. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, Y.; Jiang, Y.; Willard, L.; Ortiz, E.; Wark, L.; Medeiros, D.; Lin, D. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp. Biol. Med. 2011, 236, 1051–1063. [Google Scholar] [CrossRef]
- Hu, C.K.; Lee, Y.J.; Colitz, C.M.; Chang, C.J.; Lin, C.T. The protective effects of Lycium barbarum and Chrysanthemum morifolum on diabetic retinopathies in rats. Vet. Ophthalmol. 2012, 15 (Suppl. 2), 65–71. [Google Scholar] [CrossRef]
- Guo, J.; Xu, G.X.; Hou, Z.J.; Xu, J.B.; Huang, L.Y. Effect of Lycium barbarum polysaccharides on the retinal ultrastructure of streptozocin-induced diabetic rats. Zhongguo Zhong Xi Yi Jie He Za Zhi 2013, 33, 1404–1407. [Google Scholar]
- Yao, Q.; Yang, Y.; Lu, X.; Zhang, Q.; Luo, M.; Li, P.A.; Pan, Y. Lycium Barbarum Polysaccharides Improve Retinopathy in Diabetic Sprague-Dawley Rats. Evid. Based Complement. Altern. Med. 2018, 2018, 7943212. [Google Scholar] [CrossRef]
- Yu, H.; Wark, L.; Ji, H.; Willard, L.; Jaing, Y.; Han, J.; He, H.; Ortiz, E.; Zhang, Y.; Medeiros, D.M.; et al. Dietary wolfberry upregulates carotenoid metabolic genes and enhances mitochondrial biogenesis in the retina of db/db diabetic mice. Mol. Nutr. Food Res. 2013, 57, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.; Chan, H.C.; Yeung, S.C.; Yuen, W.H.; Zee, S.Y.; Chang, R.C.; So, K.F. Modulation of microglia by Wolfberry on the survival of retinal ganglion cells in a rat ocular hypertension model. J. Ocul. Biol. Dis. Inform. 2009, 2, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.S.; Feng, Q.; Lo, A.C.Y.; Chang, R.C.; Chung, S.K.; So, K.F. Lycium barbarum polysaccharides related RAGE and Aβ levels in the retina of mice with acute ocular hypertension and promote maintenance of blood retinal barrier. Neural Regen. Res. 2020, 15, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Bucheli, P.; Vidal, K.; Shen, L.; Gu, Z.; Zhang, C.; Miller, L.E.; Wang, J. Goji berry effects on macular characteristics and plasma antioxidant levels. Optom. Vis. Sci. 2011, 88, 257–262. [Google Scholar] [CrossRef]
- Li, S.; Liu, N.; Lin, L.; Sun, E.D.; Li, J.D.; Li, P.K. Macular pigment and serum zeaxanthin levels with Goji berry supplement in early age-related macular degeneration. Int. J. Ophthalmol. 2018, 11, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Holt, R.R.; Keen, C.L.; Morse, L.S.; Yiu, G.; Hackman, R.M. Goji Berry Intake Increases Macular Pigment Optical Density in Healthy Adults: A Randomized Pilot Trial. Nutrients 2021, 13, 4409. [Google Scholar] [CrossRef]
- Cheng, Y.P.; Ke, C.Y.; Kuo, C.C.; Lee, Y.J. Effect of a complex lutein formula in an animal model for light-induced retinal degeneration. Chin. J. Physiol. 2016, 59, 202–209. [Google Scholar] [CrossRef]
- Tang, L.; Bao, S.; Du, Y.; Jiang, Z.; Wuliji, A.O.; Ren, X.; Zhang, C.; Chu, H.; Kong, L.; Ma, H. Antioxidant effects of Lycium barbarum polysaccharides on photoreceptor degeneration in the light-exposed mouse retina. Biomed. Pharmacother. 2018, 103, 829–837. [Google Scholar] [CrossRef]
- Hsieh, F.C.; Hung, C.T.; Cheng, K.C.; Wu, C.Y.; Chen, Y.C.; Wu, Y.J.; Liu, W.; Chiu, C.C. Protective Effects of Lycium barbarum Extracts on UVB-Induced Damage in Human Retinal Pigment Epithelial Cells Accompanied by Attenuating ROS and DNA Damage. Oxid. Med. Cell. Longev. 2018, 2018, 4814928. [Google Scholar] [CrossRef]
- Liang, R.; Zhao, Q.; Zhu, Q.; He, X.; Gao, M.; Wang, Y. Lycium barbarum polysaccharide protects ARPE-19 cells against H(2)O(2)-induced oxidative stress via the Nrf2/HO-1 pathway. Mol. Med. Rep. 2021, 24, 769. [Google Scholar] [CrossRef]
- Chan, H.H.; Lam, H.I.; Choi, K.Y.; Li, S.Z.; Lakshmanan, Y.; Yu, W.Y.; Chang, R.C.; Lai, J.S.; So, K.F. Delay of cone degeneration in retinitis pigmentosa using a 12-month treatment with Lycium barbarum supplement. J. Ethnopharmacol. 2019, 236, 336–344. [Google Scholar] [CrossRef]
- Wang, K.; Xiao, J.; Peng, B.; Xing, F.; So, K.F.; Tipoe, G.L.; Lin, B. Retinal structure and function preservation by polysaccharides of wolfberry in a mouse model of retinal degeneration. Sci. Rep. 2014, 4, 7601. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, J.; Xiang, Z.; Xu, D.; So, K.F.; Vardi, N.; Xu, Y. Lycium Barbarum Polysaccharides Protect Retina in rd1 Mice During Photoreceptor Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Yang, D.; Yeung, C.M.; Yu, W.Y.; Chang, R.C.; So, K.F.; Wong, D.; Lo, A.C. Lycium barbarum polysaccharides reduce neuronal damage, blood-retinal barrier disruption and oxidative stress in retinal ischemia/reperfusion injury. PLoS ONE 2011, 6, e16380. [Google Scholar] [CrossRef]
- He, M.; Pan, H.; Chang, R.C.; So, K.F.; Brecha, N.C.; Pu, M. Activation of the Nrf2/HO-1 antioxidant pathway contributes to the protective effects of Lycium barbarum polysaccharides in the rodent retina after ischemia-reperfusion-induced damage. PLoS ONE 2014, 9, e84800. [Google Scholar] [CrossRef]
- Yang, D.; So, K.F.; Lo, A.C. Lycium barbarum polysaccharide extracts preserve retinal function and attenuate inner retinal neuronal damage in a mouse model of transient retinal ischaemia. Clin. Exp. Ophthalmol. 2017, 45, 717–729. [Google Scholar] [CrossRef]
- Mi, X.S.; Chiu, K.; Van, G.; Leung, J.W.; Lo, A.C.; Chung, S.K.; Chang, R.C.; So, K.F. Effect of Lycium barbarum Polysaccharides on the expression of endothelin-1 and its receptors in an ocular hypertension model of rat glaucoma. Neural Regen. Res. 2012, 7, 645–651. [Google Scholar] [CrossRef]
- Mi, X.S.; Feng, Q.; Lo, A.C.; Chang, R.C.; Lin, B.; Chung, S.K.; So, K.F. Protection of retinal ganglion cells and retinal vasculature by Lycium barbarum polysaccharides in a mouse model of acute ocular hypertension. PLoS ONE 2012, 7, e45469. [Google Scholar] [CrossRef]
- Chu, P.H.; Li, H.Y.; Chin, M.P.; So, K.F.; Chan, H.H. Effect of lycium barbarum (wolfberry) polysaccharides on preserving retinal function after partial optic nerve transection. PLoS ONE 2013, 8, e81339. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Chiu, K.; Yuan, Q.; Lin, B.; Chang, R.C.; So, K.F. Lycium barbarum (wolfberry) reduces secondary degeneration and oxidative stress, and inhibits JNK pathway in retina after partial optic nerve transection. PLoS ONE 2013, 8, e68881. [Google Scholar] [CrossRef]
- Chan, H.C.; Chang, R.C.; Koon-Ching Ip, A.; Chiu, K.; Yuen, W.H.; Zee, S.Y.; So, K.F. Neuroprotective effects of Lycium barbarum Lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Exp. Neurol. 2007, 203, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, Y.; Wong, F.S.Y.; Zuo, B.; So, K.F.; Bui, B.V.; Chan, H.H. Posttreatment Intervention With Lycium Barbarum Polysaccharides is Neuroprotective in a Rat Model of Chronic Ocular Hypertension. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4606–4618. [Google Scholar] [CrossRef]
- Chien, K.J.; Horng, C.T.; Huang, Y.S.; Hsieh, Y.H.; Wang, C.J.; Yang, J.S.; Lu, C.C.; Chen, F.A. Effects of Lycium barbarum (goji berry) on dry eye disease in rats. Mol. Med. Rep. 2018, 17, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Zhou, Y.; Yang, Y.; Cai, L.; Xu, L.; Han, X.; Guo, Y.; Li, P.A. Activation of Sirtuin1 by lyceum barbarum polysaccharides in protection against diabetic cataract. J. Ethnopharmacol. 2020, 261, 113165. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simó, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Vujosevic, S.; Aldington, S.J.; Silva, P.; Hernández, C.; Scanlon, P.; Peto, T.; Simó, R. Screening for diabetic retinopathy: New perspectives and challenges. Lancet Diabetes Endocrinol. 2020, 8, 337–347. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Silva, P.S.; Stitt, A.W. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat. Rev. Endocrinol. 2021, 17, 195–206. [Google Scholar] [CrossRef]
- Yildirim, Z.; Uçgun, N.I.; Kiliç, N.; Gürsel, E.; Sepici-Dinçel, A. Antioxidant enzymes and diabetic retinopathy. Ann. N. Y. Acad. Sci. 2007, 1100, 199–206. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Mishra, M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim. Biophys. Acta 2015, 1852, 2474–2483. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Mohammad, G.; dos Santos, J.M.; Zhong, Q. Abrogation of MMP-9 gene protects against the development of retinopathy in diabetic mice by preventing mitochondrial damage. Diabetes 2011, 60, 3023–3033. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Park, J.H.; Won, Y.; Lee, M.W.; Shin, Y.I.; Jo, Y.J.; Kim, J.Y. Retinal Microvascular Change in Hypertension as measured by Optical Coherence Tomography Angiography. Sci. Rep. 2019, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Milner, R. The GFAP Monoclonal Antibody GA-5 Identifies Astrocyte Remodeling and Glio-Vascular Uncoupling During the Evolution of EAE. Cell. Mol. Neurobiol. 2022, 42, 1615–1622. [Google Scholar] [CrossRef]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef]
- Chen, M.; Luo, C.; Zhao, J.; Devarajan, G.; Xu, H. Immune regulation in the aging retina. Prog. Retin. Eye Res. 2019, 69, 159–172. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Newton, F.; Megaw, R. Mechanisms of Photoreceptor Death in Retinitis Pigmentosa. Genes 2020, 11, 1120. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, M.; Wang, Z.J. (Z)-7, 4’-Dimethoxy-6-hydroxy-aurone-4-O-β-glucopyranoside mitigates retinal degeneration in Rd10 mouse model through inhibiting oxidative stress and inflammatory responses. Cutan. Ocul. Toxicol. 2020, 39, 36–42. [Google Scholar] [CrossRef]
- Verkman, A.S.; Ruiz-Ederra, J.; Levin, M.H. Functions of aquaporins in the eye. Prog. Retin. Eye Res. 2008, 27, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Calkins, D.J. Adaptive responses to neurodegenerative stress in glaucoma. Prog. Retin. Eye Res. 2021, 84, 100953. [Google Scholar] [CrossRef]
- Tezel, G. Oxidative stress in glaucomatous neurodegeneration: Mechanisms and consequences. Prog. Retin. Eye Res. 2006, 25, 490–513. [Google Scholar] [CrossRef]
- McMonnies, C. Reactive oxygen species, oxidative stress, glaucoma and hyperbaric oxygen therapy. J. Optom. 2018, 11, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Srivastava, A.; Sihota, R.; Kaur, J. Evaluation of oxidative stress markers in aqueous humor of primary open angle glaucoma and primary angle closure glaucoma patients. Curr. Eye Res. 2014, 39, 823–829. [Google Scholar] [CrossRef]
- Harman, D. Free radical theory of aging: An update: Increasing the functional life span. Ann. N. Y. Acad. Sci. 2006, 1067, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.A.; Crowe, M.E.; Quigley, H.A. Neuroprotection for glaucoma: Requirements for clinical translation. Exp. Eye Res. 2017, 157, 34–37. [Google Scholar] [CrossRef]
- Yu, M.S.; Ho, Y.S.; So, K.F.; Yuen, W.H.; Chang, R.C. Cytoprotective effects of Lycium barbarum against reducing stress on endoplasmic reticulum. Int. J. Mol. Med. 2006, 17, 1157–1161. [Google Scholar] [CrossRef]
- Chang, J.S.; Lee, Y.J.; Wilkie, D.A.; Lin, C.T. The Neuroprotective and antioxidative effects of submicron and blended Lycium barbarum in experimental retinal degeneration in rats. J. Vet. Med. Sci. 2018, 80, 1108–1115. [Google Scholar] [CrossRef]
- Chiu, K.; Zhou, Y.; Yeung, S.C.; Lok, C.K.; Chan, O.O.; Chang, R.C.; So, K.F.; Chiu, J.F. Up-regulation of crystallins is involved in the neuroprotective effect of wolfberry on survival of retinal ganglion cells in rat ocular hypertension model. J. Cell. Biochem. 2010, 110, 311–320. [Google Scholar] [CrossRef]
- Li, H.Y.; Ruan, Y.W.; Kau, P.W.; Chiu, K.; Chang, R.C.; Chan, H.H.; So, K.F. Effect of Lycium barbarum (Wolfberry) on alleviating axonal degeneration after partial optic nerve transection. Cell Transplant. 2015, 24, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Ju, R.H.; Zhang, J.Q.; Ke, X.Y.; Lu, X.H.; Liang, L.F.; Wang, W.J. Spontaneous regression of retinopathy of prematurity: Incidence and predictive factors. Int. J. Ophthalmol. 2013, 6, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Dogru, M.; Kawashima, M.; Nakamura, S.; Tsubota, K. Advances in the diagnosis and treatment of dry eye. Prog. Retin. Eye Res. 2020, 78, 100842. [Google Scholar] [CrossRef] [PubMed]
- Walter, K. What Is Dry Eye Disease? JAMA 2022, 328, 84. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.A.; Penn, C.C.; Negvesky, G.J.; Butrus, S.I. Fungal and parasitic infections of the eye. Clin. Microbiol. Rev. 2000, 13, 662–685. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Fu, H.; Liu, H. The clinical efficiency of lycium-rehmannia pills in treating dry eye symptom: A meta-analysis. Medicine 2020, 99, e20887. [Google Scholar] [CrossRef]
- Congdon, N.G.; Friedman, D.S.; Lietman, T. Important causes of visual impairment in the world today. JAMA 2003, 290, 2057–2060. [Google Scholar] [CrossRef]
- Ding, R.B.; Bao, J.; Deng, C.X. Emerging roles of SIRT1 in fatty liver diseases. Int. J. Biol. Sci. 2017, 13, 852–867. [Google Scholar] [CrossRef]
- Burgering, B.M.; Kops, G.J. Cell cycle and death control: Long live Forkheads. Trends Biochem. Sci. 2002, 27, 352–360. [Google Scholar] [CrossRef]
- Mimura, T.; Kaji, Y.; Noma, H.; Funatsu, H.; Okamoto, S. The role of SIRT1 in ocular aging. Exp. Eye Res. 2013, 116, 17–26. [Google Scholar] [CrossRef]
- Hwang, J.W.; Yao, H.; Caito, S.; Sundar, I.K.; Rahman, I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic. Biol. Med. 2013, 61, 95–110. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; He, L.; Chen, N.; Ramakrishna, S.; So, K.F.; Mo, X. Lycium barbarum polysaccharide encapsulated Poly lactic-co-glycolic acid Nanofibers: Cost effective herbal medicine for potential application in peripheral nerve tissue engineering. Sci. Rep. 2018, 8, 8669. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, X.; Zhang, J.; Liang, L.; Miao, F.; Ji, T.; Ye, Z.; Chu, M.; Ren, J.; Xu, X. Synthesis, stability and anti-fatigue activity of selenium nanoparticles stabilized by Lycium barbarum polysaccharides. Int. J. Biol. Macromol. 2021, 179, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Li, W.; Sun, J.; Jia, L.; Guan, Q.; Guo, Y.; Wang, Y. A review on plant polysaccharide based on drug delivery system for construction and application, with emphasis on traditional Chinese medicine polysaccharide. Int. J. Biol. Macromol. 2022, 211, 711–728. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).