Targeting Mitochondrial Dysfunction in Neurodegenerative Diseases: Expanding the Therapeutic Approaches by Plant-Derived Natural Products
Abstract
1. Introduction
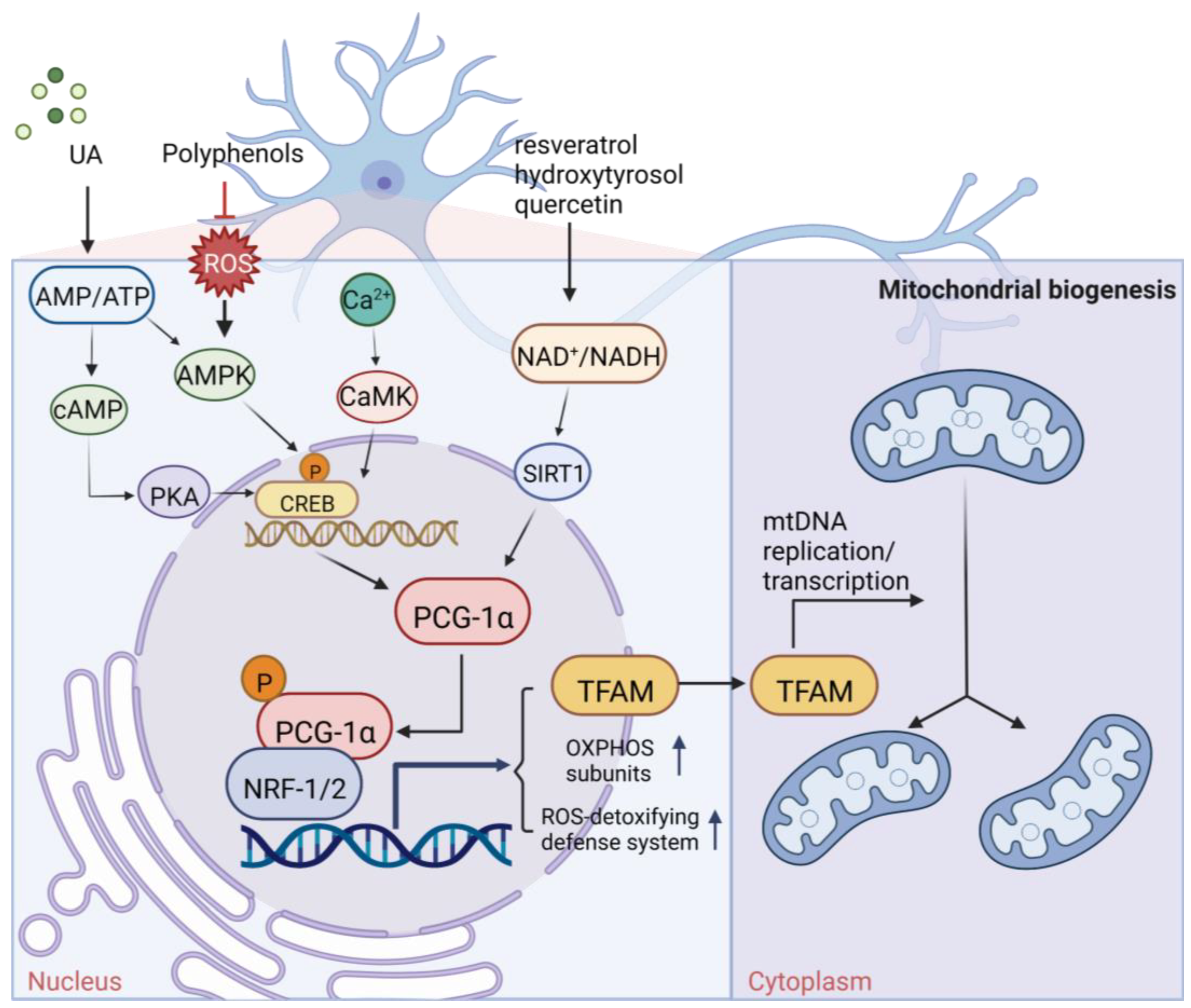
2. Plant-Derived Natural Products Stimulating Mitochondrial Biogenesis
3. Plant-Derived Natural Products Regulating Mitochondrial Fusion and Fission
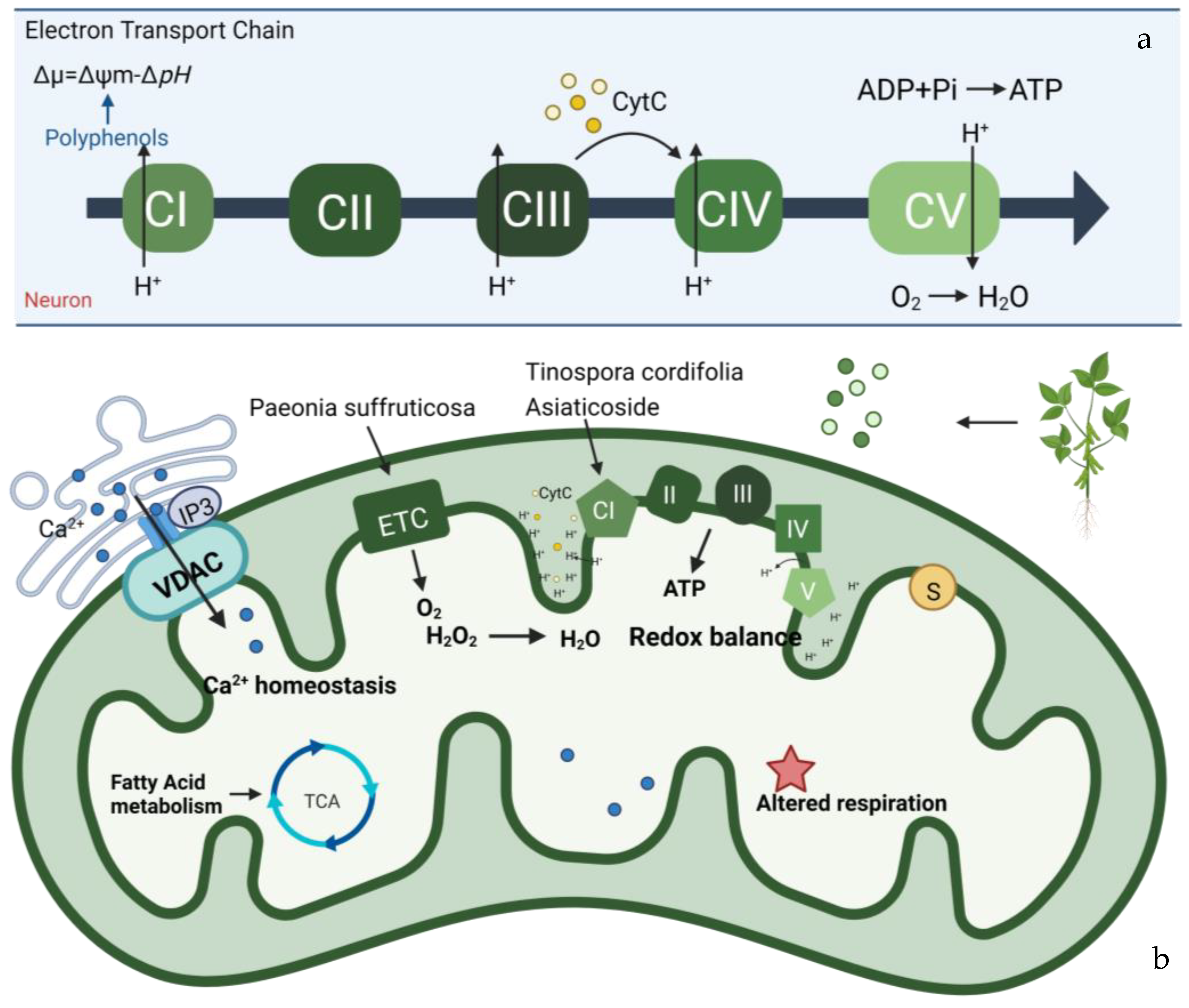
4. Plant-Derived Natural Products Improve Mitochondrial Bioenergetics
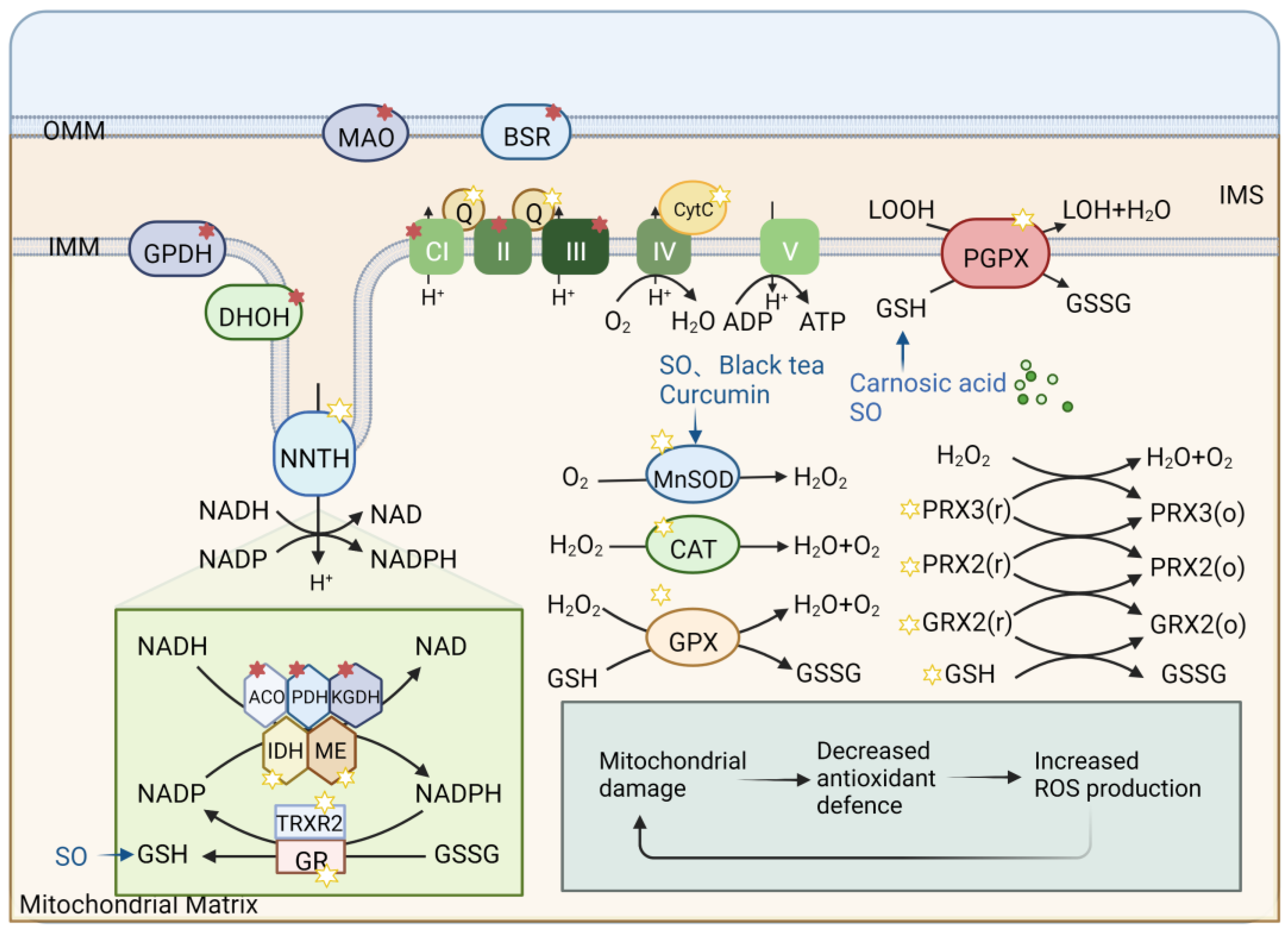
5. Plant-Derived Natural Products Preventing Mitochondrial Oxidative Stress
6. Plant-Derived Natural Products Modulate Mitochondrial Calcium (Ca2+) Homeostasis
7. Plant-Derived Natural Products Sustaining Mitochondrial Membrane Potential (∆Ψm)
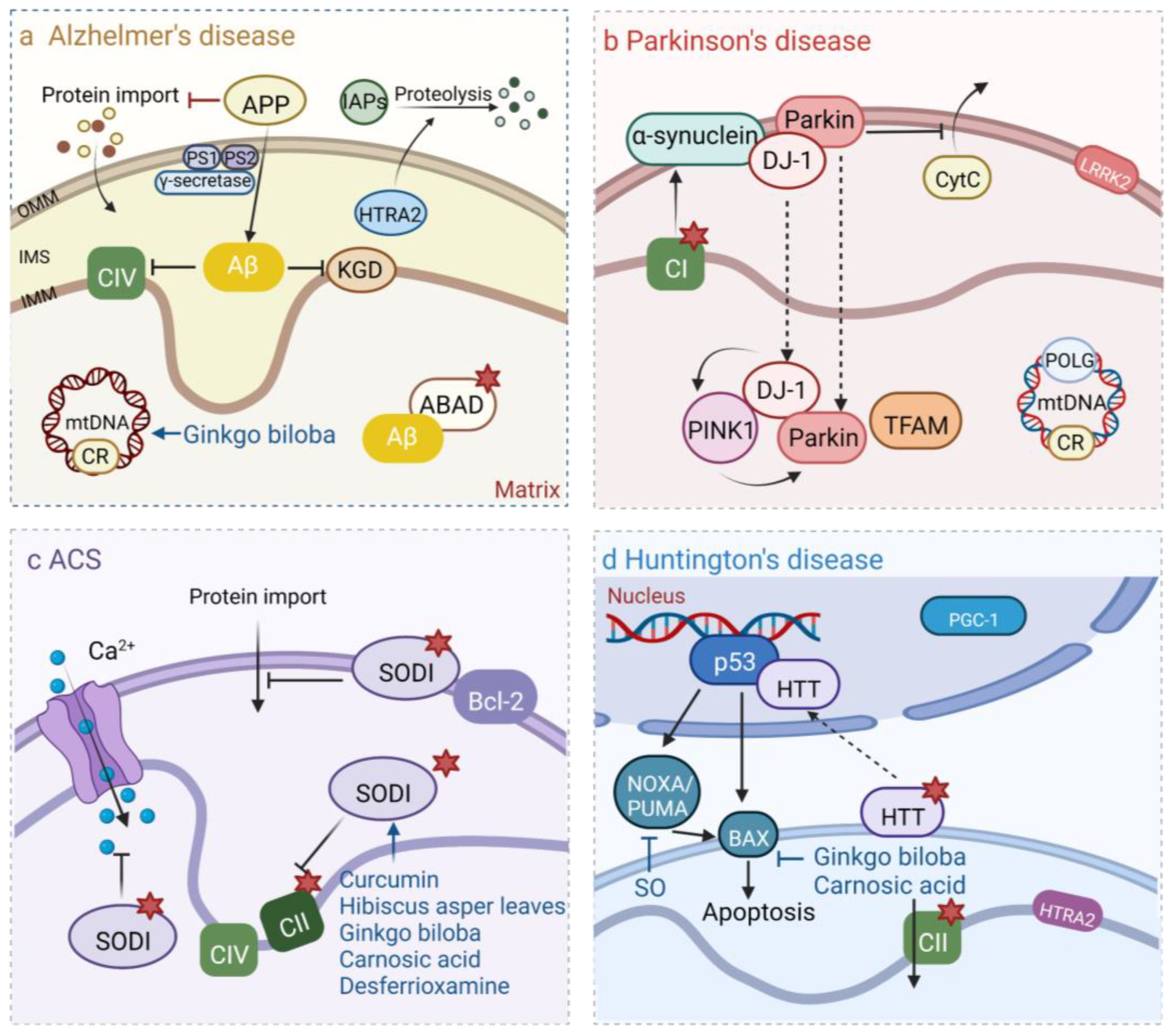
8. Plant-Derived Natural Products Maintain Mitochondrial DNA Stability
9. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Przedborski, S.; Vila, M.; Jackson-Lewis, V. Neurodegeneration: What is it and where are we? J. Clin. Investig. 2003, 111, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef]
- Hao, H.; Zheng, X.; Wang, G. Insights into drug discovery from natural medicines using reverse pharmacokinetics. Trends Pharmacol. Sci. 2014, 35, 168–177. [Google Scholar] [CrossRef]
- Surguchov, A.; Bernal, L.; Surguchev, A.A. Phytochemicals as Regulators of Genes Involved in Synucleinopathies. Biomolecules 2021, 11, 624. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, E.; Musich, P.R.; Lin, F. Mitochondrial dysfunction in neurodegenerative diseases and the potential countermeasure. CNS Neurosci. Ther. 2019, 25, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Devine, M.J.; Kittler, J.T. Mitochondria at the neuronal presynapse in health and disease. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Yun, U.W.; Yan, Z.; Amir, R.; Hong, S.; Jin, Y.W.; Lee, E.K.; Loake, G.J. Plant natural products: History, limitations and the potential of cambial meristematic cells. Biotechnol. Genet. Eng. Rev. 2012, 28, 47–59. [Google Scholar] [CrossRef]
- Maher, P. The Potential of Flavonoids for the Treatment of Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3056. [Google Scholar] [CrossRef]
- Golpich, M.; Amini, E.; Mohamed, Z.; Azman Ali, R.; Mohamed Ibrahim, N.; Ahmadiani, A. Mitochondrial Dysfunction and Biogenesis in Neurodegenerative diseases: Pathogenesis and Treatment. CNS Neurosci. Ther. 2017, 23, 5–22. [Google Scholar] [CrossRef]
- Ploumi, C.; Daskalaki, I.; Tavernarakis, N. Mitochondrial biogenesis and clearance: A balancing act. FEBS J. 2017, 284, 183–195. [Google Scholar] [CrossRef]
- Cameron, R.B.; Beeson, C.C.; Schnellmann, R.G. Development of Therapeutics That Induce Mitochondrial Biogenesis for the Treatment of Acute and Chronic Degenerative Diseases. J. Med. Chem. 2016, 59, 10411–10434. [Google Scholar] [CrossRef] [PubMed]
- Cameron, R.B.; Peterson, Y.K.; Beeson, C.C.; Schnellmann, R.G. Author Correction: Structural and pharmacological basis for the induction of mitochondrial biogenesis by formoterol but not clenbuterol. Sci. Rep. 2019, 9, 6790. [Google Scholar] [CrossRef] [PubMed]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Popov, L.D. Mitochondrial biogenesis: An update. J. Cell. Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, K.Z.; Chu, C.T. After the banquet: Mitochondrial biogenesis, mitophagy, and cell survival. Autophagy 2013, 9, 1663–1676. [Google Scholar] [CrossRef]
- Yamada, S.; Asa, S.L.; Kovacs, K. Oncocytomas and null cell adenomas of the human pituitary: Morphometric and in vitro functional comparison. Virchows Archiv. A Pathol. Anat. Histopathol. 1988, 413, 333–339. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, S.; Wu, J.; Sun, X.; Shen, Z.; Dong, J.; Huang, J. Promotion of Mitochondrial Biogenesis via Activation of AMPK-PGC1α Signaling Pathway by Ginger (Zingiber officinale Roscoe) Extract, and Its Major Active Component 6-Gingerol. J. Food Sci. 2019, 84, 2101–2111. [Google Scholar] [CrossRef]
- Chen, J.; Wong, H.S.; Leong, P.K.; Leung, H.Y.; Chan, W.M.; Ko, K.M. Ursolic acid induces mitochondrial biogenesis through the activation of AMPK and PGC-1 in C2C12 myotubes: A possible mechanism underlying its beneficial effect on exercise endurance. Food Funct. 2017, 8, 2425–2436. [Google Scholar] [CrossRef]
- Li, B.; Jiang, J.; Assaraf, Y.G.; Xiao, H.; Chen, Z.S.; Huang, C. Surmounting cancer drug resistance: New insights from the perspective of N(6)-methyladenosine RNA modification. Drug Resist. Updat. 2020, 53, 100720. [Google Scholar] [CrossRef]
- Renaud, J.; Martinoli, M.G. Considerations for the Use of Polyphenols as Therapies in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 1883. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.Z.; Jalili, F.; Farhadian, N.; Joshi, T.; Wang, M.; Zou, L.; Cao, H.; Farzaei, M.H.; Xiao, J. Polyphenols and neurodegenerative diseases: Focus on neuronal regeneration. Crit. Rev. Food Sci. Nutr. 2022, 62, 3421–3436. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, Z.; Feng, Z.; Hao, J.; Shen, W.; Li, X.; Sun, L.; Sharman, E.; Wang, Y.; Wertz, K.; et al. Hydroxytyrosol protects against oxidative damage by simultaneous activation of mitochondrial biogenesis and phase II detoxifying enzyme systems in retinal pigment epithelial cells. J. Nutr. Biochem. 2010, 21, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Williams, A.S.; Shanely, R.A.; Jin, F.; McAnulty, S.R.; Triplett, N.T.; Austin, M.D.; Henson, D.A. Quercetin’s influence on exercise performance and muscle mitochondrial biogenesis. Med. Sci. Sport. Exerc. 2010, 42, 338–345. [Google Scholar] [CrossRef]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Davis, B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiology. Regul. Integr. Comp. Physiol. 2009, 296, R1071–R1077. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef]
- Adebayo, M.; Singh, S.; Singh, A.P.; Dasgupta, S. Mitochondrial fusion and fission: The fine-tune balance for cellular homeostasis. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21620. [Google Scholar] [CrossRef]
- van der Bliek, A.M.; Shen, Q.; Kawajiri, S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb. Perspect. Biol. 2013, 5, e11072. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.N.; Leuthner, T.C.; Luz, A.L. Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology 2017, 391, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Pernas, L.; Scorrano, L. Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function. Annu. Rev. Physiol. 2016, 78, 505–531. [Google Scholar] [CrossRef]
- Sarzi, E.; Seveno, M.; Angebault, C.; Milea, D.; Rönnbäck, C.; Quilès, M.; Adrian, M.; Grenier, J.; Caignard, A.; Lacroux, A.; et al. Increased steroidogenesis promotes early-onset and severe vision loss in females with OPA1 dominant optic atrophy. Hum. Mol. Genet. 2016, 25, 2539–2551. [Google Scholar] [CrossRef]
- Praefcke, G.J.; McMahon, H.T. The dynamin superfamily: Universal membrane tubulation and fission molecules? Nat. Reviews. Mol. Cell Biol. 2004, 5, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C. Fusion and fission: Interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 2012, 46, 265–287. [Google Scholar] [CrossRef]
- Olichon, A.; Baricault, L.; Gas, N.; Guillou, E.; Valette, A.; Belenguer, P.; Lenaers, G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J. Biol. Chem. 2003, 278, 7743–7746. [Google Scholar] [CrossRef]
- Gomes, L.C.; Di Benedetto, G.; Scorrano, L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 2011, 13, 589–598. [Google Scholar] [CrossRef]
- Ehses, S.; Raschke, I.; Mancuso, G.; Bernacchia, A.; Geimer, S.; Tondera, D.; Martinou, J.C.; Westermann, B.; Rugarli, E.I.; Langer, T. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J. Cell Biol. 2009, 187, 1023–1036. [Google Scholar] [CrossRef]
- Chinta, S.J.; Andersen, J.K. Redox imbalance in Parkinson’s disease. Biochim. Biophys. Acta 2008, 1780, 1362–1367. [Google Scholar] [CrossRef]
- Bertholet, A.M.; Delerue, T.; Millet, A.M.; Moulis, M.F.; David, C.; Daloyau, M.; Arnauné-Pelloquin, L.; Davezac, N.; Mils, V.; Miquel, M.C.; et al. Mitochondrial fusion/fission dynamics in neurodegeneration and neuronal plasticity. Neurobiol. Dis. 2016, 90, 3–19. [Google Scholar] [CrossRef]
- Delettre, C.; Lenaers, G.; Griffoin, J.M.; Gigarel, N.; Lorenzo, C.; Belenguer, P.; Pelloquin, L.; Grosgeorge, J.; Turc-Carel, C.; Perret, E.; et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat. Genet. 2000, 26, 207–210. [Google Scholar] [CrossRef]
- Landes, T.; Emorine, L.J.; Courilleau, D.; Rojo, M.; Belenguer, P.; Arnauné-Pelloquin, L. The BH3-only Bnip3 binds to the dynamin Opa1 to promote mitochondrial fragmentation and apoptosis by distinct mechanisms. EMBO Rep. 2010, 11, 459–465. [Google Scholar] [CrossRef]
- Feely, S.M.; Laura, M.; Siskind, C.E.; Sottile, S.; Davis, M.; Gibbons, V.S.; Reilly, M.M.; Shy, M.E. MFN2 mutations cause severe phenotypes in most patients with CMT2A. Neurology 2011, 76, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.M.; Laura, M.; Fawcett, K.; Pandraud, A.; Liu, Y.T.; Davidson, G.L.; Rossor, A.M.; Polke, J.M.; Castleman, V.; Manji, H.; et al. Charcot-Marie-Tooth disease: Frequency of genetic subtypes and guidelines for genetic testing. J. Neurol. Neurosurg. Psychiatry 2012, 83, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Saporta, M.A.; Dang, V.; Volfson, D.; Zou, B.; Xie, X.S.; Adebola, A.; Liem, R.K.; Shy, M.; Dimos, J.T. Axonal Charcot-Marie-Tooth disease patient-derived motor neurons demonstrate disease-specific phenotypes including abnormal electrophysiological properties. Exp. Neurol. 2015, 263, 190–199. [Google Scholar] [CrossRef]
- Belenguer, P.; Pellegrini, L. The dynamin GTPase OPA1: More than mitochondria? Biochim. Biophys. Acta 2013, 1833, 176–183. [Google Scholar] [CrossRef]
- Cribbs, J.T.; Strack, S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007, 8, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Abrams, A.J.; Hufnagel, R.B.; Rebelo, A.; Zanna, C.; Patel, N.; Gonzalez, M.A.; Campeanu, I.J.; Griffin, L.B.; Groenewald, S.; Strickland, A.V.; et al. Mutations in SLC25A46, encoding a UGO1-like protein, cause an optic atrophy spectrum disorder. Nat. Genet. 2015, 47, 926–932. [Google Scholar] [CrossRef]
- Hasnat, M.; Yuan, Z.; Naveed, M.; Khan, A.; Raza, F.; Xu, D.; Ullah, A.; Sun, L.; Zhang, L.; Jiang, Z. Drp1-associated mitochondrial dysfunction and mitochondrial autophagy: A novel mechanism in triptolide-induced hepatotoxicity. Cell Biol. Toxicol. 2019, 35, 267–280. [Google Scholar] [CrossRef]
- Santín-Márquez, R.; Alarcón-Aguilar, A.; López-Diazguerrero, N.E.; Chondrogianni, N.; Königsberg, M. Sulforaphane–Role in aging and neurodegeneration. GeroScience 2019, 41, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Miao, X.; Zhong, Y.; Han, J.; Liu, Q.; Zhu, J.; Xia, X.; Peng, X. The renoprotective effect of diosgenin on aristolochic acid I-induced renal injury in rats: Impact on apoptosis, mitochondrial dynamics and autophagy. Food Funct. 2020, 11, 7456–7467. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.Z.; Zhou, W.; Yue, L.X.; Wang, Y.H.; Hao, F.R.; Li, P.Y.; Gao, Y. Repeated Aconitine Treatment Induced the Remodeling of Mitochondrial Function via AMPK-OPA1-ATP5A1 Pathway. Front. Pharmacol. 2021, 12, 646121. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, R.Y.; Zhou, J.H.; Xie, X.H.; Sun, G.B.; Sun, X.B. Calenduloside E Ameliorates Myocardial Ischemia-Reperfusion Injury through Regulation of AMPK and Mitochondrial OPA1. Oxidative Med. Cell. Longev. 2020, 2020, 2415269. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Herman, P.; Rothman, D.L.; Agarwal, D.; Hyder, F. Evaluating the gray and white matter energy budgets of human brain function. J. Cereb. Blood Flow Metab. 2018, 38, 1339–1353. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef] [PubMed]
- DiMauro, S.; Schon, E.A. Mitochondrial respiratory-chain diseases. N. Engl. J. Med. 2003, 348, 2656–2668. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Mitochondrial bioenergetics decay in aging: Beneficial effect of melatonin. Cell. Mol. Life Sci. 2017, 74, 3897–3911. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; de Bruijn, M.H.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial diseases. Nat. Rev. Dis. Prim. 2016, 2, 16080. [Google Scholar] [CrossRef]
- Wallace, D.C. Bioenergetics in human evolution and disease: Implications for the origins of biological complexity and the missing genetic variation of common diseases. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2013, 368, 20120267. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Petrosillo, G.; Paradies, V.; Ruggiero, F.M. Oxidative stress, mitochondrial bioenergetics, and cardiolipin in aging. Free Radic. Biol. Med. 2010, 48, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Petrosillo, G.; Paradies, V.; Reiter, R.J.; Ruggiero, F.M. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J. Pineal Res. 2010, 48, 297–310. [Google Scholar] [CrossRef]
- Guo, R.; Gu, J.; Zong, S.; Wu, M.; Yang, M. Structure and mechanism of mitochondrial electron transport chain. Biomed. J. 2018, 41, 9–20. [Google Scholar] [CrossRef]
- Papa, S.; Martino, P.L.; Capitanio, G.; Gaballo, A.; De Rasmo, D.; Signorile, A.; Petruzzella, V. The oxidative phosphorylation system in mammalian mitochondria. Adv. Exp. Med. Biol. 2012, 942, 3–37. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, S.; Lorenzi, I.; Rigoni, G.; Caicci, F.; Soriano, M.E. Regulation of Mitochondrial Electron Transport Chain Assembly. J. Mol. Biol. 2018, 430, 4849–4873. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Vizarra, E.; Zeviani, M. Mitochondrial disorders of the OXPHOS system. FEBS Lett. 2021, 595, 1062–1106. [Google Scholar] [CrossRef] [PubMed]
- Carelli, V.; Rugolo, M.; Sgarbi, G.; Ghelli, A.; Zanna, C.; Baracca, A.; Lenaz, G.; Napoli, E.; Martinuzzi, A.; Solaini, G. Bioenergetics shapes cellular death pathways in Leber’s hereditary optic neuropathy: A model of mitochondrial neurodegeneration. Biochim. Biophys. Acta 2004, 1658, 172–179. [Google Scholar] [CrossRef]
- Rak, M.; Bénit, P.; Chrétien, D.; Bouchereau, J.; Schiff, M.; El-Khoury, R.; Tzagoloff, A.; Rustin, P. Mitochondrial cytochrome c oxidase deficiency. Clin. Sci. 2016, 130, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Valnot, I.; von Kleist-Retzow, J.C.; Barrientos, A.; Gorbatyuk, M.; Taanman, J.W.; Mehaye, B.; Rustin, P.; Tzagoloff, A.; Munnich, A.; Rötig, A. A mutation in the human heme A:farnesyltransferase gene (COX10) causes cytochrome c oxidase deficiency. Hum. Mol. Genet. 2000, 9, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Antonicka, H.; Leary, S.C.; Guercin, G.H.; Agar, J.N.; Horvath, R.; Kennaway, N.G.; Harding, C.O.; Jaksch, M.; Shoubridge, E.A. Mutations in COX10 result in a defect in mitochondrial heme A biosynthesis and account for multiple, early-onset clinical phenotypes associated with isolated COX deficiency. Hum. Mol. Genet. 2003, 12, 2693–2702. [Google Scholar] [CrossRef] [PubMed]
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s Disease: Biomarkers, Treatment, and Risk Factors. Front. Neurosci. 2018, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, Z.; Solati, K.; Amini-Khoei, H. Phytotherapy in treatment of Parkinson’s disease: A review. Pharm. Biol. 2019, 57, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Gaki, G.S.; Papavassiliou, A.G. Oxidative stress-induced signaling pathways implicated in the pathogenesis of Parkinson’s disease. Neuromol. Med. 2014, 16, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Kosaraju, J.; Chinni, S.; Roy, P.D.; Kannan, E.; Antony, A.S.; Kumar, M.N. Neuroprotective effect of Tinospora cordifolia ethanol extract on 6-hydroxy dopamine induced Parkinsonism. Indian J. Pharmacol. 2014, 46, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Lieu, C.A.; Kunselman, A.R.; Manyam, B.V.; Venkiteswaran, K.; Subramanian, T. A water extract of Mucuna pruriens provides long-term amelioration of parkinsonism with reduced risk for dyskinesias. Park. Relat. Disord. 2010, 16, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Teerapattarakan, N.; Benya-Aphikul, H.; Tansawat, R.; Wanakhachornkrai, O.; Tantisira, M.H.; Rodsiri, R. Neuroprotective effect of a standardized extract of Centella asiatica ECa233 in rotenone-induced parkinsonism rats. Phytomed. Int. J. Phytother. Phytopharm. 2018, 44, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhou, L.; Wang, Y.; Nice, E.C.; Huang, C.; Zhang, H. A targeted nanomodulator capable of manipulating tumor microenvironment against metastasis. J. Control. Release 2022, 348, 590–600. [Google Scholar] [CrossRef]
- Praticò, D.; Uryu, K.; Leight, S.; Trojanoswki, J.Q.; Lee, V.M. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J. Neurosci. 2001, 21, 4183–4187. [Google Scholar] [CrossRef]
- Ohyagi, Y.; Yamada, T.; Nishioka, K.; Clarke, N.J.; Tomlinson, A.J.; Naylor, S.; Nakabeppu, Y.; Kira, J.; Younkin, S.G. Selective increase in cellular A beta 42 is related to apoptosis but not necrosis. Neuroreport 2000, 11, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Calingasan, N.Y.; Yu, F.; Mauck, W.M.; Toidze, M.; Almeida, C.G.; Takahashi, R.H.; Carlson, G.A.; Flint Beal, M.; Lin, M.T.; et al. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J. Neurochem. 2004, 89, 1308–1312. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Parola, M.; Bardini, P.; Piccini, A.; Borghi, R.; Guglielmotto, M.; Santoro, G.; Davit, A.; Danni, O.; Smith, M.A.; et al. Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J. Neurochem. 2005, 92, 628–636. [Google Scholar] [CrossRef]
- Lovell, M.A.; Xiong, S.; Xie, C.; Davies, P.; Markesbery, W.R. Induction of hyperphosphorylated tau in primary rat cortical neuron cultures mediated by oxidative stress and glycogen synthase kinase-3. J. Alzheimer’s Dis. 2004, 6, 659–671; discussion 673–681. [Google Scholar] [CrossRef] [PubMed]
- Morsy, A.; Trippier, P.C. Amyloid-Binding Alcohol Dehydrogenase (ABAD) Inhibitors for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2019, 62, 4252–4264. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, H.; Pavlov, P.F.; Wiehager, B.; Nishimura, T.; Winblad, B.; Ankarcrona, M. Association of Omi/HtrA2 with γ-secretase in mitochondria. Neurochem. Int. 2010, 57, 668–675. [Google Scholar] [CrossRef]
- Darios, F.; Corti, O.; Lücking, C.B.; Hampe, C.; Muriel, M.P.; Abbas, N.; Gu, W.J.; Hirsch, E.C.; Rooney, T.; Ruberg, M.; et al. Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum. Mol. Genet. 2003, 12, 517–526. [Google Scholar] [CrossRef]
- Chung, K.K.; Thomas, B.; Li, X.; Pletnikova, O.; Troncoso, J.C.; Marsh, L.; Dawson, V.L.; Dawson, T.M. S-nitrosylation of parkin regulates ubiquitination and compromises parkin’s protective function. Science 2004, 304, 1328–1331. [Google Scholar] [CrossRef]
- Canet-Avilés, R.M.; Wilson, M.A.; Miller, D.W.; Ahmad, R.; McLendon, C.; Bandyopadhyay, S.; Baptista, M.J.; Ringe, D.; Petsko, G.A.; Cookson, M.R. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. USA 2004, 101, 9103–9108. [Google Scholar] [CrossRef]
- Whitworth, A.J.; Theodore, D.A.; Greene, J.C.; Benes, H.; Wes, P.D.; Pallanck, L.J. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2005, 102, 8024–8029. [Google Scholar] [CrossRef]
- Hatziagapiou, K.; Kakouri, E.; Lambrou, G.I.; Bethanis, K.; Tarantilis, P.A. Antioxidant Properties of Crocus sativus L. and Its Constituents and Relevance to Neurodegenerative Diseases; Focus on Alzheimer’s and Parkinson’s Disease. Curr. Neuropharmacol. 2019, 17, 377–402. [Google Scholar] [CrossRef]
- Xu, C.L.; Qu, R.; Zhang, J.; Li, L.F.; Ma, S.P. Neuroprotective effects of madecassoside in early stage of Parkinson’s disease induced by MPTP in rats. Fitoterapia 2013, 90, 112–118. [Google Scholar] [CrossRef]
- Matthews, D.G.; Caruso, M.; Murchison, C.F.; Zhu, J.Y.; Wright, K.M.; Harris, C.J.; Gray, N.E.; Quinn, J.F.; Soumyanath, A. Centella Asiatica Improves Memory and Promotes Antioxidative Signaling in 5XFAD Mice. Antioxidants 2019, 8, 630. [Google Scholar] [CrossRef] [PubMed]
- Umka Welbat, J.; Sirichoat, A.; Chaijaroonkhanarak, W.; Prachaney, P.; Pannangrong, W.; Pakdeechote, P.; Sripanidkulchai, B.; Wigmore, P. Asiatic Acid Prevents the Deleterious Effects of Valproic Acid on Cognition and Hippocampal Cell Proliferation and Survival. Nutrients 2016, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Kerr, F.; Sofola-Adesakin, O.; Ivanov, D.K.; Gatliff, J.; Gomez Perez-Nievas, B.; Bertrand, H.C.; Martinez, P.; Callard, R.; Snoeren, I.; Cochemé, H.M.; et al. Direct Keap1-Nrf2 disruption as a potential therapeutic target for Alzheimer’s disease. PLoS Genet. 2017, 13, e1006593. [Google Scholar] [CrossRef] [PubMed]
- Brandes, M.S.; Gray, N.E. NRF2 as a Therapeutic Target in Neurodegenerative Diseases. ASN Neuro 2020, 12, 1759091419899782. [Google Scholar] [CrossRef]
- Wu, C.R.; Tsai, C.W.; Chang, S.W.; Lin, C.Y.; Huang, L.C.; Tsai, C.W. Carnosic acid protects against 6-hydroxydopamine-induced neurotoxicity in in vivo and in vitro model of Parkinson’s disease: Involvement of antioxidative enzymes induction. Chem.-Biol. Interact. 2015, 225, 40–46. [Google Scholar] [CrossRef]
- Guerrero, D. Community liaison: Working towards a partnership. Community Outlook 1990, 14, 18. [Google Scholar]
- Khuwaja, G.; Khan, M.M.; Ishrat, T.; Ahmad, A.; Raza, S.S.; Ashafaq, M.; Javed, H.; Khan, M.B.; Khan, A.; Vaibhav, K.; et al. Neuroprotective effects of curcumin on 6-hydroxydopamine-induced Parkinsonism in rats: Behavioral, neurochemical and immunohistochemical studies. Brain Res. 2011, 1368, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Haleagrahara, N.; Siew, C.J.; Ponnusamy, K. Effect of quercetin and desferrioxamine on 6-hydroxydopamine (6-OHDA) induced neurotoxicity in striatum of rats. J. Toxicol. Sci. 2013, 38, 25–33. [Google Scholar] [CrossRef]
- Ahmad, M.; Yousuf, S.; Khan, M.B.; Ahmad, A.S.; Saleem, S.; Hoda, M.N.; Islam, F. Protective effects of ethanolic extract of Delphinium denudatum in a rat model of Parkinson’s disease. Hum. Exp. Toxicol. 2006, 25, 361–368. [Google Scholar] [CrossRef]
- Shobana, C.; Kumar, R.R.; Sumathi, T. Alcoholic extract of Bacopa monniera Linn. protects against 6-hydroxydopamine-induced changes in behavioral and biochemical aspects: A pilot study. Cell. Mol. Neurobiol. 2012, 32, 1099–1112. [Google Scholar] [CrossRef]
- Chaturvedi, R.K.; Shukla, S.; Seth, K.; Chauhan, S.; Sinha, C.; Shukla, Y.; Agrawal, A.K. Neuroprotective and neurorescue effect of black tea extract in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Neurobiol. Dis. 2006, 22, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Beppe, G.J.; Dongmo, A.B.; Foyet, H.S.; Tsabang, N.; Olteanu, Z.; Cioanca, O.; Hancianu, M.; Dimo, T.; Hritcu, L. Memory-enhancing activities of the aqueous extract of Albizia adianthifolia leaves in the 6-hydroxydopamine-lesion rodent model of Parkinson’s disease. BMC Complement. Altern. Med. 2014, 14, 142. [Google Scholar] [CrossRef] [PubMed]
- Dar, N.J.; Hamid, A.; Ahmad, M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell. Mol. Life Sci. 2015, 72, 4445–4460. [Google Scholar] [CrossRef] [PubMed]
- Boyman, L.; Karbowski, M.; Lederer, W.J. Regulation of Mitochondrial ATP Production: Ca(2+) Signaling and Quality Control. Trends Mol. Med. 2020, 26, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Wu, Y.; Shamoto-Nagai, M.; Maruyama, W. Mitochondria in Neuroprotection by Phytochemicals: Bioactive Polyphenols Modulate Mitochondrial Apoptosis System, Function and Structure. Int. J. Mol. Sci. 2019, 20, 2451. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2007, 2, 257–273. [Google Scholar] [CrossRef]
- Kicinska, A.; Kampa, R.P.; Daniluk, J.; Sek, A.; Jarmuszkiewicz, W.; Szewczyk, A.; Bednarczyk, P. Regulation of the Mitochondrial BK(Ca) Channel by the Citrus Flavonoid Naringenin as a Potential Means of Preventing Cell Damage. Molecules 2020, 25, 3010. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P. Flavonoids and brain health: Multiple effects underpinned by common mechanisms. Genes Nutr. 2009, 4, 243–250. [Google Scholar] [CrossRef]
- Vashisht, A.; Trebak, M.; Motiani, R.K. STIM and Orai proteins as novel targets for cancer therapy. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am. J. Physiol. Cell Physiol. 2015, 309, C457–C469. [Google Scholar] [CrossRef]
- Ming, H.; Li, B.; Zhou, L.; Goel, A.; Huang, C. Long non-coding RNAs and cancer metastasis: Molecular basis and therapeutic implications. Biochim. Biophys. Acta. Rev. Cancer 2021, 1875, 188519. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, E.; Hermant, A.; Thevenet, J.; Bermont, F.; Kulkarni, S.S.; Ratajczak, J.; Santo-Domingo, J.; Dioum, E.H.; Canto, C.; Barron, D.; et al. The plant product quinic acid activates Ca(2+)-dependent mitochondrial function and promotes insulin secretion from pancreatic beta cells. Br. J. Pharmacol. 2019, 176, 3250–3263. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, L.; Marchesini, M.; Roti, G. Targeting oncogenic Notch signaling with SERCA inhibitors. J. Hematol. Oncol. 2021, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Madreiter-Sokolowski, C.T.; Sokolowski, A.A.; Graier, W.F. Dosis Facit Sanitatem-Concentration-Dependent Effects of Resveratrol on Mitochondria. Nutrients 2017, 9, 1117. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shamoto-Nagai, M.; Maruyama, W.; Osawa, T.; Naoi, M. Phytochemicals prevent mitochondrial membrane permeabilization and protect SH-SY5Y cells against apoptosis induced by PK11195, a ligand for outer membrane translocator protein. J. Neural Transm. 2017, 124, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Maruyama, W.; Yi, H. Rasagiline prevents apoptosis induced by PK11195, a ligand of the outer membrane translocator protein (18 kDa), in SH-SY5Y cells through suppression of cytochrome c release from mitochondria. J. Neural Transm. 2013, 120, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.M.; Thangthaeng, N.; Miller, M.G.; Shukitt-Hale, B. Effects of pterostilbene and resveratrol on brain and behavior. Neurochem. Int. 2015, 89, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Kogure, K.; Abe, K.; Kimata, Y.; Kitahama, K.; Yamashita, E.; Terada, H. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim. Biophys. Acta 2001, 1512, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Grougnet, R.; Magiatis, P.; Laborie, H.; Lazarou, D.; Papadopoulos, A.; Skaltsounis, A.L. Sesamolinol glucoside, disaminyl ether, and other lignans from sesame seeds. J. Agric. Food Chem. 2012, 60, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, A.; Zarb, C.; Caruana, M.; Ostermeier, U.; Ghio, S.; Högen, T.; Schmidt, F.; Giese, A.; Vassallo, N. Mitochondrial membrane permeabilisation by amyloid aggregates and protection by polyphenols. Biochim. Biophys. Acta 2013, 1828, 2532–2543. [Google Scholar] [CrossRef]
- Wang, D.M.; Li, S.Q.; Zhu, X.Y.; Wang, Y.; Wu, W.L.; Zhang, X.J. Protective effects of hesperidin against amyloid-β (Aβ) induced neurotoxicity through the voltage dependent anion channel 1 (VDAC1)-mediated mitochondrial apoptotic pathway in PC12 cells. Neurochem. Res. 2013, 38, 1034–1044. [Google Scholar] [CrossRef]
- Ghaffari, H.; Venkataramana, M.; Jalali Ghassam, B.; Chandra Nayaka, S.; Nataraju, A.; Geetha, N.P.; Prakash, H.S. Rosmarinic acid mediated neuroprotective effects against H2O2-induced neuronal cell damage in N2A cells. Life Sci. 2014, 113, 7–13. [Google Scholar] [CrossRef]
- Wang, X.J.; Chen, W.; Fu, X.T.; Ma, J.K.; Wang, M.H.; Hou, Y.J.; Tian, D.C.; Fu, X.Y.; Fan, C.D. Reversal of homocysteine-induced neurotoxicity in rat hippocampal neurons by astaxanthin: Evidences for mitochondrial dysfunction and signaling crosstalk. Cell Death Discov. 2018, 4, 50. [Google Scholar] [CrossRef]
- Lipsky, P.E.; Tao, X.L. A potential new treatment for rheumatoid arthritis: Thunder god vine. Semin. Arthritis Rheum. 1997, 26, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Kannaiyan, R.; Shanmugam, M.K.; Sethi, G. Molecular targets of celastrol derived from Thunder of God Vine: Potential role in the treatment of inflammatory disorders and cancer. Cancer Lett. 2011, 303, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lee, J.; Salazar Hernandez, M.A.; Mazitschek, R.; Ozcan, U. Treatment of obesity with celastrol. Cell 2015, 161, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, Q.; Luo, P.; Gu, L.; Shen, S.; Tang, H.; Zhang, Y.; Lyu, M.; Shi, Q.; Yang, C.; et al. Neuroprotective Effects of Celastrol in Neurodegenerative Diseases-Unscramble Its Major Mechanisms of Action and Targets. Aging Dis. 2022, 13, 815–836. [Google Scholar] [CrossRef]
- Li, J.; Hao, J. Treatment of Neurodegenerative Diseases with Bioactive Components of Tripterygium wilfordii. Am. J. Chin. Med. 2019, 47, 769–785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, M.; Wang, B.; Su, Z.; Guo, B.; Qin, L.; Zhang, W.; Zheng, R. The Nrf2-NLRP3-caspase-1 axis mediates the neuroprotective effects of Celastrol in Parkinson’s disease. Redox Biol. 2021, 47, 102134. [Google Scholar] [CrossRef]
- Geisberger, S.; Bartolomaeus, H.; Neubert, P.; Willebrand, R.; Zasada, C.; Bartolomaeus, T.; McParland, V.; Swinnen, D.; Geuzens, A.; Maifeld, A.; et al. Salt Transiently Inhibits Mitochondrial Energetics in Mononuclear Phagocytes. Circulation 2021, 144, 144–158. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, M.; Jin, G.; Jiang, Y.; Luan, Y. Cu-MOF chemodynamic nanoplatform via modulating glutathione and H(2)O(2) in tumor microenvironment for amplified cancer therapy. J. Colloid Interface Sci. 2021, 587, 358–366. [Google Scholar] [CrossRef]
- Boesch-Saadatmandi, C.; Loboda, A.; Wagner, A.E.; Stachurska, A.; Jozkowicz, A.; Dulak, J.; Döring, F.; Wolffram, S.; Rimbach, G. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: Role of miR-155. J. Nutr. Biochem. 2011, 22, 293–299. [Google Scholar] [CrossRef]
- Yu, X.; Xu, Y.; Zhang, S.; Sun, J.; Liu, P.; Xiao, L.; Tang, Y.; Liu, L.; Yao, P. Quercetin Attenuates Chronic Ethanol-Induced Hepatic Mitochondrial Damage through Enhanced Mitophagy. Nutrients 2016, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Corral-Debrinski, M.; Horton, T.; Lott, M.T.; Shoffner, J.M.; Beal, M.F.; Wallace, D.C. Mitochondrial DNA deletions in human brain: Regional variability and increase with advanced age. Nat. Genet. 1992, 2, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Trifunovic, A. Mitochondrial DNA and ageing. Biochim. Biophys. Acta 2006, 1757, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Lustbader, J.W.; Cirilli, M.; Lin, C.; Xu, H.W.; Takuma, K.; Wang, N.; Caspersen, C.; Chen, X.; Pollak, S.; Chaney, M.; et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science 2004, 304, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Casley, C.S.; Canevari, L.; Land, J.M.; Clark, J.B.; Sharpe, M.A. Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J. Neurochem. 2002, 80, 91–100. [Google Scholar] [CrossRef]
- Gibson, G.E.; Sheu, K.F.; Blass, J.P.; Baker, A.; Carlson, K.C.; Harding, B.; Perrino, P. Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer’s disease. Arch. Neurol. 1988, 45, 836–840. [Google Scholar] [CrossRef]
- Hansson, C.A.; Frykman, S.; Farmery, M.R.; Tjernberg, L.O.; Nilsberth, C.; Pursglove, S.E.; Ito, A.; Winblad, B.; Cowburn, R.F.; Thyberg, J.; et al. Nicastrin, presenilin, APH-1, and PEN-2 form active gamma-secretase complexes in mitochondria. J. Biol. Chem. 2004, 279, 51654–51660. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef]
- Shoffner, J.M.; Brown, M.D.; Torroni, A.; Lott, M.T.; Cabell, M.F.; Mirra, S.S.; Beal, M.F.; Yang, C.C.; Gearing, M.; Salvo, R.; et al. Mitochondrial DNA variants observed in Alzheimer disease and Parkinson disease patients. Genomics 1993, 17, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Pasinelli, P.; Belford, M.E.; Lennon, N.; Bacskai, B.J.; Hyman, B.T.; Trotti, D.; Brown, R.H., Jr. Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron 2004, 43, 19–30. [Google Scholar] [CrossRef]
- Liu, J.; Lillo, C.; Jonsson, P.A.; Vande Velde, C.; Ward, C.M.; Miller, T.M.; Subramaniam, J.R.; Rothstein, J.D.; Marklund, S.; Andersen, P.M.; et al. Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron 2004, 43, 5–17. [Google Scholar] [CrossRef]
- Gu, M.; Gash, M.T.; Mann, V.M.; Javoy-Agid, F.; Cooper, J.M.; Schapira, A.H. Mitochondrial defect in Huntington’s disease caudate nucleus. Ann. Neurol. 1996, 39, 385–389. [Google Scholar] [CrossRef]
- Ming, H.; Li, B.; Tian, H.; Zhou, L.; Jiang, J.; Zhang, T.; Qiao, L.; Wu, P.; Nice, E.C.; Zhang, W.; et al. A minimalist and robust chemo-photothermal nanoplatform capable of augmenting autophagy-modulated immune response against breast cancer. Mater. Today Bio 2022, 15, 100289. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.G.; Chen, W.F.; Xie, J.X.; Wong, M.S. Ginsenoside Rg1 protects against 6-OHDA-induced neurotoxicity in neuroblastoma SK-N-SH cells via IGF-I receptor and estrogen receptor pathways. J. Neurochem. 2009, 109, 1338–1347. [Google Scholar] [CrossRef]
- Kim, H.G.; Park, G.; Piao, Y.; Kang, M.S.; Pak, Y.K.; Hong, S.P.; Oh, M.S. Effects of the root bark of Paeonia suffruticosa on mitochondria-mediated neuroprotection in an MPTP-induced model of Parkinson’s disease. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2014, 65, 293–300. [Google Scholar] [CrossRef]
- Saha, S.; Ghosh, S. Tinospora cordifolia: One plant, many roles. Anc. Sci. Life 2012, 31, 151–159. [Google Scholar] [CrossRef]
- Sengupta, T.; Vinayagam, J.; Nagashayana, N.; Gowda, B.; Jaisankar, P.; Mohanakumar, K.P. Antiparkinsonian effects of aqueous methanolic extract of Hyoscyamus niger seeds result from its monoamine oxidase inhibitory and hydroxyl radical scavenging potency. Neurochem. Res. 2011, 36, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.N.; Luo, J.G.; Kong, L.Y. Phytotoxicity of lignanamides isolated from the seeds of Hyoscyamus niger. J. Agric. Food Chem. 2012, 60, 1682–1687. [Google Scholar] [CrossRef]
- Foyet, H.S.; Hritcu, L.; Ciobica, A.; Stefan, M.; Kamtchouing, P.; Cojocaru, D. Methanolic extract of Hibiscus asper leaves improves spatial memory deficits in the 6-hydroxydopamine-lesion rodent model of Parkinson’s disease. J. Ethnopharmacol. 2011, 133, 773–779. [Google Scholar] [CrossRef]
- Sierpina, V.S.; Wollschlaeger, B.; Blumenthal, M. Ginkgo biloba. Am. Fam. Physician 2003, 68, 923–926. [Google Scholar]
- Eisvand, F.; Razavi, B.M.; Hosseinzadeh, H. The effects of Ginkgo biloba on metabolic syndrome: A review. Phytother. Res. PTR 2020, 34, 1798–1811. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, S.; Borowski, T. Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation Res. 2013, 16, 313–326. [Google Scholar] [CrossRef]
- Li, S.; Lo, C.Y.; Pan, M.H.; Lai, C.S.; Ho, C.T. Black tea: Chemical analysis and stability. Food Funct. 2013, 4, 10–18. [Google Scholar] [CrossRef] [PubMed]
- de Pablos, R.M.; Espinosa-Oliva, A.M.; Hornedo-Ortega, R.; Cano, M.; Arguelles, S. Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases. Pharmacol. Res. 2019, 143, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Anand, U.; Ghosh, S.; Ray, D.; Ray, P.; Nandy, S.; Deshmukh, G.D.; Tripathi, V.; Dey, A. Bacosides from Bacopa monnieri extract: An overview of the effects on neurological disorders. Phytother. Res. 2021, 35, 5668–5679. [Google Scholar] [CrossRef]

| Name | Plant Origin | Function | Chemical Structures | References |
|---|---|---|---|---|
| Carnosic acid | Rosmarinus officinalis L. | 1. Improve mitochondrial activity 2. Reduced lipid peroxidation 3. GSH reduction 4. Increased protein expression of Gclc, SOD, and GR 5. Reduction of the Bcl-2/Bax ratio 6. Induction of caspase 3 cleavage 7. Induction of poly (ADP ribose) PARP cleavage | 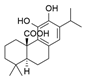 | [96] |
| Curcumin | Curcumalonga L. | 1. Decreased MDA 2. Increased GSH, GPx, GR, SOD, catalase TH and D2 receptor binding in brain tissue | 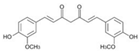 | [98] |
| Ginsenoside Rg1 | Ginseng | 1. Improve mitochondrial activity 2. Promote mitochondrial biogenesis 3. Expression of Bax and Bcl-2 mRNA and protein | 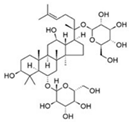 | [146] |
| Quercetin | Peas, potatoes, broad bean leaves, apple peels, etc | 1. Mitochondrial biogenesis is induced by SIRT1/PGC-1α pathway 2. Decreased protein carbonyl content | 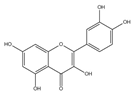 | [27,28] |
| Desferrioxamine | Some grasses, fruit trees, etc | 1. Decreased protein carbonyl content 2. Increased dopamine, GSH, and SOD levels | 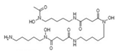 | [99] |
| Sulforaphane | Cruciferous plants, such as broccoli, Chinese kale, carrot, etc. | 1. Altering mitochondrial fusion and fission by inhibiting HDAC and DMT 2. Blocking DNA fragmentation and caspase-3 activation 3. Increased GSH levels | 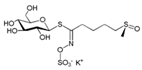 | [51] |
| 6-gingerol/6-chrysophanol | ginger | Promotion of OXPHOS subunit related proteins and activation of alpha signaling pathways promote mitochondrial biogenesis |  | [20] |
| Ursolic acid (UA) | Herbs, fruits, and vegetables | 1. Promote mitochondrial ATP production 2. The production of a small amount of ROS activates the AMPK-PGC1 pathway and further increases the expression of COX and uncoupled protein 3, thus inducing mitochondrial biogenesis | 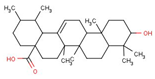 | [21] |
| Triptolide | Tripterygium wilfordii | 1. Acting on DRP1 leads to increased ROS generation, decreased mitochondrial depolarization, decreased mitochondrial number, and decreased ATP generation 2. Mitochondrial fission and mitochondrial autophagy 3. The specific pathway may be a new mechanism | 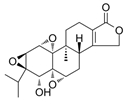 | [50] |
| Diosgenin | Widely used in a variety of plants. | Increased expression of mitochondrial fusion and fission-related proteins (including DRP1 and MFN2) | 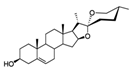 | [52] |
| Aconitine | Sichuan black, grass black, aconite, etc | Effect of OPA1 on mitochondrial function remodeling |  | [53] |
| Flavonoids | A plant widely occurring in nature | 1. Regulate mitochondrial large conductance calmodulating potassium channel and activate the regulation of Ca2+ signal 2. Attenuating the inhibition of thapsigargin on sarco/ER Ca2+- ATPase (SERCA) pumps 3. Inhibit neuronal apoptosis and promote neuronal survival and differentiation 4. Antioxidant activity | 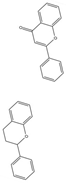 | [108,109,110,112] |
| Resveratrol | Grapes | 1. Induce mitochondrial biogenesis by inducing the SIRT1/PGC-1α pathway 2. Activate the mitochondrial ATP synthase-dependent respiration by increasing mitochondrial Ca2+ | 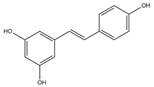 | [25] |
| Astaxanthin | Rhodiaceae, Chlorella, etc. | 1. Bcl2 and Bcl-cl were upregulated, while Bax and Bak were downregulated 2. Maintain Ca2+ homeostasis 3. Promote the anti-apoptosis of neuron cells | 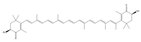 | [106,116,120,121] |
| Biapigenin | Cereals | 1. Activated related transcription factors enhanced Ca2+ efflux from mitochondria, which could reduce the Ca2+ burden of mitochondria 2. Protected cells against excitotoxicity |  | [106] |
| Mangiferin | Polyphenols | 1. Protected the MM potential Δψm 2. Prevent the activation of caspases in neurons 3. Inhibiting apoptosis |  | [106] |
| Saffron | Iris family of saffron | 1. Improve mitochondrial behavioral performances 2. Suppression of a-synuclein overexpression or aggregation 3. Promote the expression of the antioxidant system |  | [90] |
| Asiaticoside | Asiaticoside | 1. Mitochondria are protected by protecting the rate-limiting step of OXPHOS, CI activity 2. Effectively reduces neuronal death 3. The Nrf2/AREs pathway is activated to maintain mitochondrial Redox balance |  | [77,92,93] |
| Alkaloids | Tinospora cordifolia | 1. Increased mitochondrial complex I activity 2. Decreased MDA levels 3. Improve mitochondrial activity |  | [148] |
| Sesame seed oil (SO) | Sesame seed | 1. Increased GR, GST, GPx, CAT, GSH, and TBARS 2. Inhibit the activation of Nox2 and Cox2 3. Restored MnSOD expression | N.A. | [97] |
| Paeonia suffruticosa | Peony | 1. Increased total striatal dopamine 2. Reversed downregulation of Akt and the mitochondrial OXPHOS subunits | 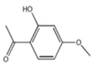 | [147] |
| Hyoscyamus niger seeds | Hyoscyamus niger seeds | 1. Attenuated motor disabilities 2. Increased level of GSH content and GPX, SOD, and CAT activities 3. Inhibiting MAO and scavenging hydroxyl free radicals | 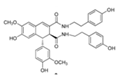 | [149,150] |
| Hibiscus asper leaves | Hibiscus asper leaves | 1. Increased SOD, GPX, and CAT activities, total GSH content 2. Reduced MDA level | N.A. | [151] |
| Bilobalid | Ginkgo biloba | 1. Increased GSH content 2. Decreased generation of TBARS 3. Increased SOD and CAT activities 4. Coding mitochondrial DNA increases the number of dopaminergic D2 receptors in the striatum. | 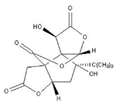 | [152,153] |
| Norditerpenoid alkaloids | Delphinium denudatum | 1. Decreased MDA levels 2. Increased GSH content 3. Increased SOD and CAT activities | 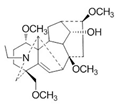 | [100] |
| Bacopa monnieri (Linn.) | Bacopa monnieri (L.) Wettst. | 1. Decreased MDA levels 2. Increased GSH content 3. Increased SOD and CAT activities | N.A. | [154] |
| Catechins | Black tea | 1. Recovery DA-D2 receptor binding 2. Decreased MDA levels 3. Increased GSH content 4. Increased SOD and CAT activities 5. Increased TH protein level and TH mRNA expression | 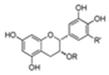 | [102,155] |
| Hydroxytyrosol | Olive | Induce mitochondrial biogenesis by inducing SIRT1/PGC-1α pathway | 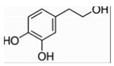 | [26,156] |
| Schumach | Leaf of Schumach | Promote the expression of antioxidant system | N.A. | [103] |
| Bacoside A3 | Withania somnifera | Reduces ROS in neurons, reduces the stress effect | 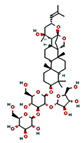 | [104,157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, L.; Li, B.; Shi, J.; Xu, J.; Yuan, M. Targeting Mitochondrial Dysfunction in Neurodegenerative Diseases: Expanding the Therapeutic Approaches by Plant-Derived Natural Products. Pharmaceuticals 2023, 16, 277. https://doi.org/10.3390/ph16020277
Zhang X, Wang L, Li B, Shi J, Xu J, Yuan M. Targeting Mitochondrial Dysfunction in Neurodegenerative Diseases: Expanding the Therapeutic Approaches by Plant-Derived Natural Products. Pharmaceuticals. 2023; 16(2):277. https://doi.org/10.3390/ph16020277
Chicago/Turabian StyleZhang, Xiaoyue, Longqin Wang, Bowen Li, Jiayan Shi, Jia Xu, and Minlan Yuan. 2023. "Targeting Mitochondrial Dysfunction in Neurodegenerative Diseases: Expanding the Therapeutic Approaches by Plant-Derived Natural Products" Pharmaceuticals 16, no. 2: 277. https://doi.org/10.3390/ph16020277
APA StyleZhang, X., Wang, L., Li, B., Shi, J., Xu, J., & Yuan, M. (2023). Targeting Mitochondrial Dysfunction in Neurodegenerative Diseases: Expanding the Therapeutic Approaches by Plant-Derived Natural Products. Pharmaceuticals, 16(2), 277. https://doi.org/10.3390/ph16020277






