Pharmacological Treatments and Natural Biocompounds in Weight Management
Abstract
1. Introduction
2. Drugs-Induced Obesity
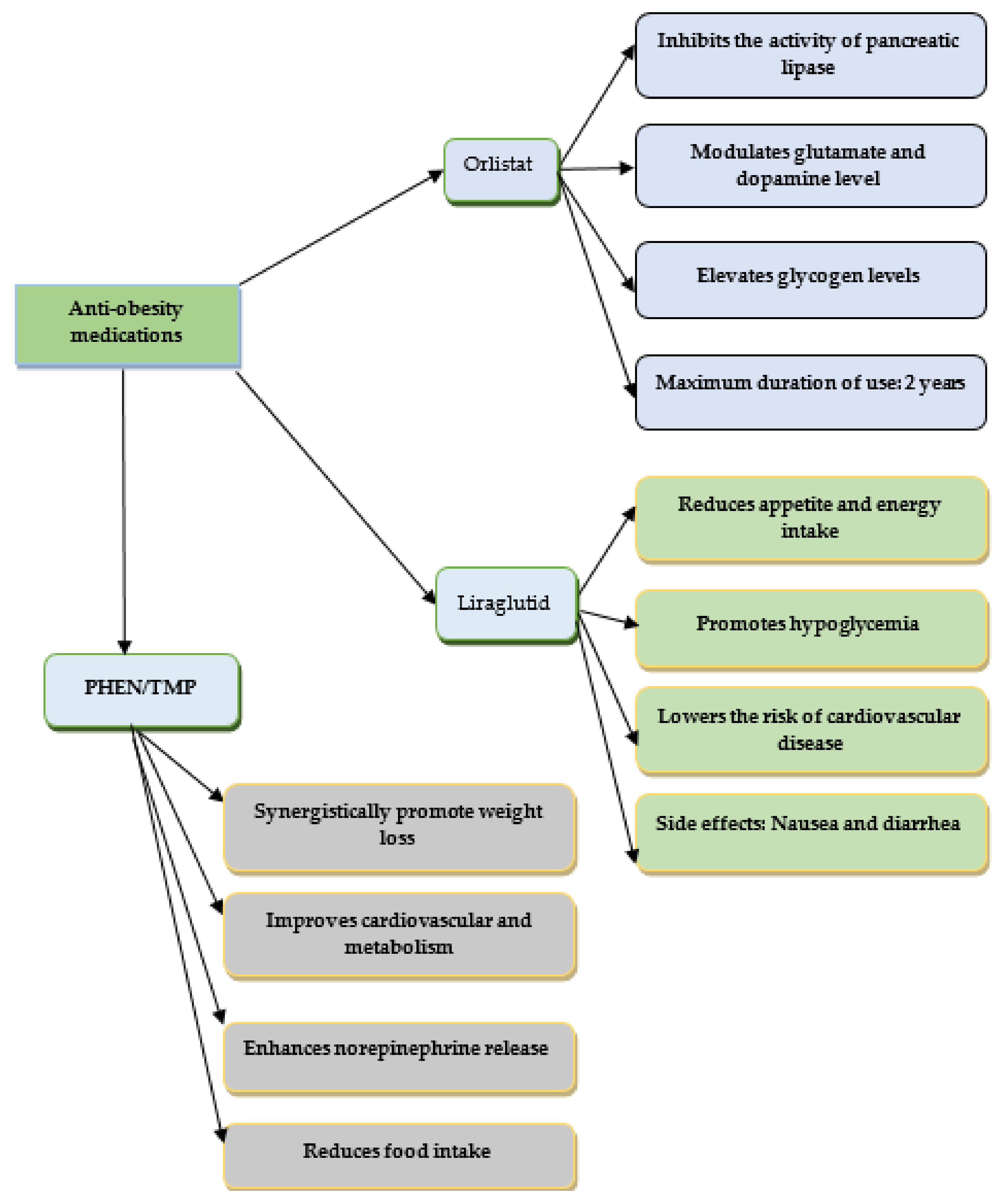
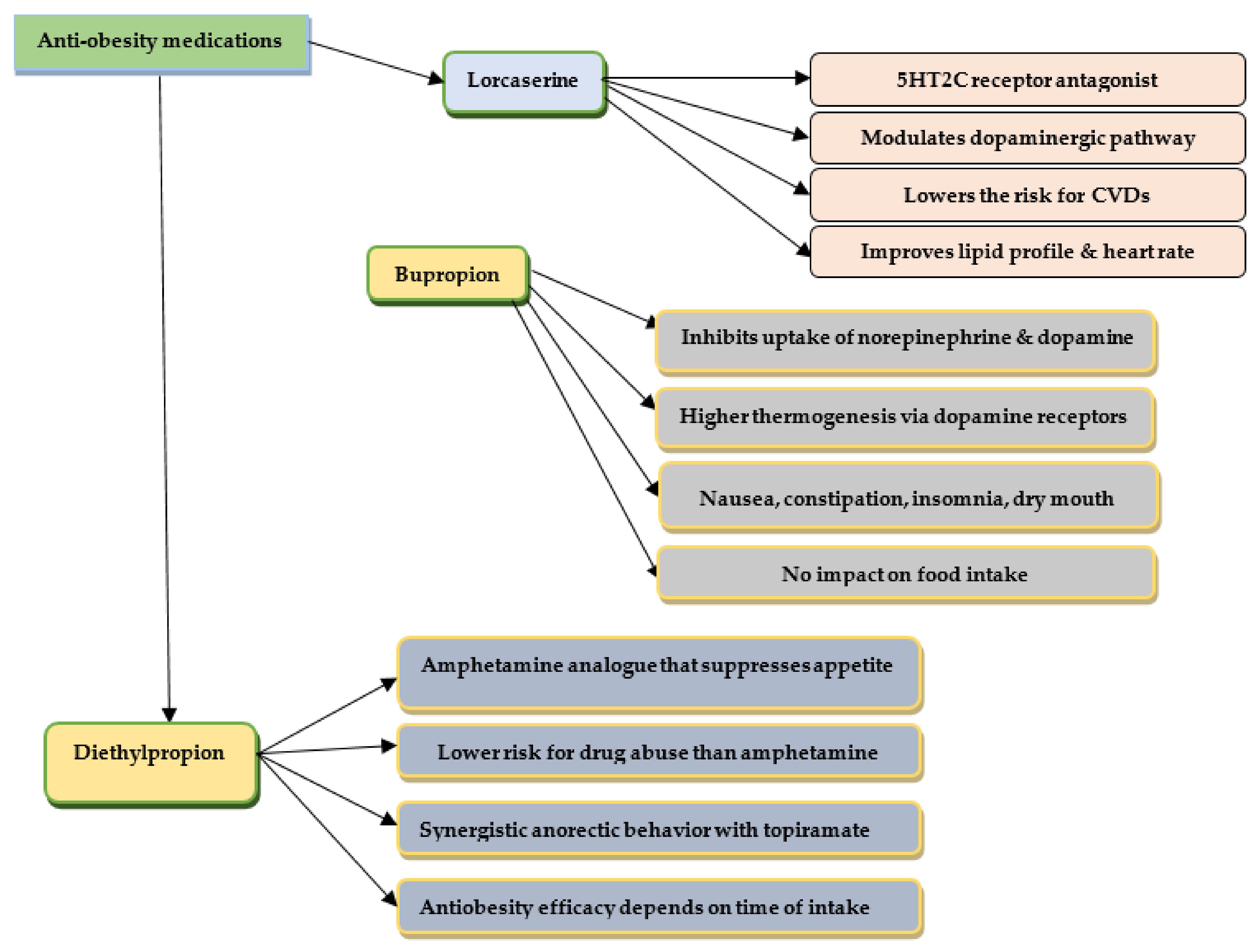
3. Novel Drugs in Obesity Treatment
3.1. Orlistat
3.2. Liraglutide 3.0
3.3. Phentermine/Topiramate
3.4. Phentermine/Diethylpropion
3.5. Lorcaserin
3.6. Bupropion
3.7. The Use of Antidiabetic Drugs and Natural Constituents in the Prevention and Treatment of Obesity
4. Limits in the Pharmacological Treatment of Obesity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, X.; Jin, Y.; Li, D.; Zhang, J.; Han, J.; Li, Y. Multidisciplinary Progress in Obesity Research. Genes 2022, 13, 1772. [Google Scholar] [CrossRef] [PubMed]
- Coulter, A.A.; Rebello, C.J.; Greenway, F.L. Centrally Acting Agents for Obesity: Past, Present, and Future. Drugs 2018, 78, 1113–1132. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, C.C.; Li, J.; Tian, Q.; Du, Y.J. Mechanism of Action of Acupuncture in Obesity: A Perspective from the Hypothalamus. Front. Endocrinol. 2021, 12, 632324. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef] [PubMed]
- Swiatkiewicz, I.; Wozniak, A.; Taub, P.R. Time-Restricted Eating and Metabolic Syndrome: Current Status and Future Perspectives. Nutrients 2021, 13, 221. [Google Scholar] [CrossRef]
- Thomas-Valdés, S.; Vaz-Tostes, M.; Anunciação, P.; Silva, B.; Pinheiro-Sant’Ana, H. Association between Vitamin Deficiency and Metabolic Disorders Related to Obesity. Crit. Rev. Food Sci. Nutr. 2016, 57, 3332–3343. [Google Scholar] [CrossRef]
- Kushner, R.F. Weight Loss Strategies for Treatment of Obesity: Lifestyle Management and Pharmacotherapy. Prog. Cardiovasc. Dis. 2018, 61, 246–252. [Google Scholar] [CrossRef]
- Kang, J.; Park, C.-Y. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef]
- Kim, G.W.; Lin, J.E.; Blomain, E.S.; Waldman, S.A. Antiobesity pharmacotherapy: New drugs and emerging targets. Clin. Pharm. Ther. 2014, 95, 53–66. [Google Scholar] [CrossRef]
- Moreira, F.A.; Crippa, J.A. The psychiatric side-effects of rimonabant. Braz. J. Psychiatry 2009, 31, 145–153. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Tschop, M.H.; Wilding, J.P. Anti-obesity drugs: Past, present, and future. Dis. Model. Mech. 2012, 5, 621–626. [Google Scholar] [CrossRef]
- Verhaegen, A.A.; Van Gaal, L.F. Drug-induced obesity and its metabolic consequences: A review focusing on mechanisms and possible therapeutic options. J. Endocrinol. Investig. 2017, 40, 1165–1174. [Google Scholar] [CrossRef]
- Mangge, H.; Bengesser, S.; Dalkner, N.; Birner, A.; Fellendorf, F.; Platzer, M.; Queissner, R.; Pilz, R.; Maget, A.; Reininghaus, B.; et al. Weight Gain During Treatment of Bipolar Disorder (BD)-Facts and Therapeutic Options. Front. Nutr. 2019, 6, 76. [Google Scholar] [CrossRef]
- Gafoor, R.; Booth, H.P.; Gulliford, M.C. Antidepressant utilisation and incidence of weight gain during ten years’ follow-up: Population based cohort study. BMJ 2018, 361, k1951. [Google Scholar] [CrossRef]
- Cunningham, J.I.; Eyerman, D.J.; Todtenkopf, M.S.; Dean, R.L.; Deaver, D.R.; Sanchez, C.; Namchuk, M. Samidorphan mitigates olanzapine-induced weight gain and metabolic dysfunction in rats and non-human primates. J. Psychopharmacol. 2019, 33, 1303–1316. [Google Scholar] [CrossRef]
- Ellul, P.; Delorme, R.; Cortese, S. Metformin for Weight Gain Associated with Second-Generation Antipsychotics in Children and Adolescents: A Systematic Review and Meta-Analysis. CNS Drugs 2018, 32, 1103–1112. [Google Scholar] [CrossRef]
- Pisano, S.; Coppola, G.; Catone, G.; Carotenuto, M.; Iuliano, R.; D’Esposito, V.; Cabaro, S.; Giudice, E.; Bravaccio, C.; Formisano, P. Differences in Metabolic Factors Between Antipsychotic-Induced Weight Gain and Non-pharmacological Obesity in Youths. Clin. Drug Investig. 2018, 38, 457–462. [Google Scholar] [CrossRef]
- Ko, K.; Kim, K.-K.; Lee, K.R. Does Weight Gain Associated with Thiazolidinedione Use Negatively Affect Cardiometabolic Health? J. Obes. Metab. Syndr. 2017, 26, 102–106. [Google Scholar] [CrossRef]
- Horowitz, M.; Wilcock, M. Newer generation antidepressants and withdrawal effects: Reconsidering the role of antidepressants and helping patients to stop. Drug. Ther. Bull. 2022, 60, 7–12. [Google Scholar] [CrossRef]
- Ricardo, L.; Singh, S.; Cifuentes, L.; Decker, P.; Gonzalez Izundegui, D.; Moyer, A.; Hurtado, M.; Camilleri, M.; Bielinski, S.; Acosta, A. Association between CYP metabolizer phenotypes and selective serotonin reuptake inhibitors induced weight gain: A retrospective cohort study. BMC Med. 2022, 20, 261. [Google Scholar] [CrossRef]
- Hunt, H.; Donaldson, K.; Strem, M.; Tudor, I.; Sweet-Smith, S.; Sidhu, S. Effect of Miricorilant, a Selective Glucocorticoid Receptor Modulator, on Olanzapine-Associated Weight Gain in Healthy Subjects: A Proof-of-Concept Study. J. Clin. Psychopharmacol. 2021, 6, 632. [Google Scholar] [CrossRef] [PubMed]
- Galati, A.; Brown, E.S.; Bove, R.; Vaidya, A.; Gelfand, J. Glucocorticoids for therapeutic immunosuppression: Clinical pearls for the practicing neurologist. J. Neurol. Sci. 2021, 430, 120004. [Google Scholar] [CrossRef] [PubMed]
- Lexington, R.; Iyer, A.M.; van der Valk, E.S.; Hoogeveen, E.K.; Meijer, O.C.; van der Voorn, B.; van Rossum, E.F.C. Variation in glucocorticoid sensitivity and the relation with obesity. Obes. Rev. 2022, 23, e13401. [Google Scholar]
- Segula, D. Complications of obesity in adults: A short review of the literature. Malawi Med. J. 2014, 26, 20–24. [Google Scholar]
- Ruban, A.; Stoenchev, K.; Ashrafian, H.; Teare, J. Current treatments for obesity. Clin. Med. 2019, 19, 205–212. [Google Scholar] [CrossRef]
- Muller, T.D.; Bluher, M.; Tschop, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef]
- Ryan, D.H. Drugs for Treating Obesity. Handb Exp. Pharm. 2022, 274, 387–414. [Google Scholar]
- Shi, Q.; Wang, Y.; Hao, Q.; Vandvik, P.O.; Guyatt, G.; Li, J.; Chen, Z.; Xu, S.; Shen, Y.; Ge, L.; et al. Pharmacotherapy for adults with overweight and obesity: A systematic review and network meta-analysis of randomised controlled trials. Lancet 2022, 399, 259–269. [Google Scholar] [CrossRef]
- Ahmad, N.N.; Robinson, S.; Kennedy-Martin, T.; Poon, J.L.; Kan, H. Clinical outcomes associated with anti-obesity medications in real-world practice: A systematic literature review. Obes. Rev. 2021, 22, e13326. [Google Scholar] [CrossRef]
- Qi, X. Review of the Clinical Effect of Orlistat. IOP Conf. Ser. Mater. Sci. Eng. 2018, 301, 012063. [Google Scholar] [CrossRef]
- O’meara, S.; Riemsma, R.; Shirran, L.; Stirk, L.; Riet, G. A systematic review of the clinical effectiveness of orlistat used for the management of obesity. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2004, 5, 51–68. [Google Scholar] [CrossRef]
- Hollywood, A.; Ogden, J. Taking Orlistat: Predicting Weight Loss over 6 Months. J. Obes. 2011, 2011, 806896. [Google Scholar] [CrossRef]
- Gorgojo-Martinez, J.J.; Basagoiti-Carreno, B.; Sanz-Velasco, A.; Serrano-Moreno, C.; Almodovar-Ruiz, F. Effectiveness and tolerability of orlistat and liraglutide in patients with obesity in a real-world setting: The XENSOR Study. Int. J. Clin. Pract. 2019, 73, e13399. [Google Scholar] [CrossRef]
- Khedr, N.; Ebeid, A.; Khalil, R. New insights into weight management by orlistat in comparison with cinnamon as a natural lipase inhibitor. Endocrine 2020, 67, 109–116. [Google Scholar] [CrossRef]
- Hauner, H. Adipositastherapie—Legale und illegale Arzneimittel und die ZukunftObesity treatment—Legal and illegal drugs and the future. Der Internist 2021, 62. [Google Scholar]
- Bansal, A.B.; Al Khalili, Y. Orlistat. BTI-StatPearls 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542202/ (accessed on 29 December 2022).
- Grudén, S.; Forslund, A.; Alderborn, G.; Söderhäll, A.; Hellström, P.; Holmbäck, U. Safety of a Novel Weight Loss Combination Product Containing Orlistat and Acarbose. Clin. Pharmacol. Drug Dev. 2021, 10, 1242–1247. [Google Scholar] [CrossRef]
- Noori, S.; Mirzababaei, A.; Amini, M.R.; Clark, C.C.T.; Mirzaei, K. Effect of orlistat on serum uric acid level in adults: A systematic review and meta-analysis of randomised controlled trials. Int. J. Clin. Pr. 2021, 75, e14674. [Google Scholar] [CrossRef]
- Mehta, A.; Marso, S.P.; Neeland, I.J. Liraglutide for weight management: A critical review of the evidence. Obes. Sci. Pr. 2017, 3, 3–14. [Google Scholar] [CrossRef]
- Garvey, W.; Birkenfeld, A.; Dicker, D.; Mingrone, G.; Pedersen, S.; Satylganova, A.; Skovgaard, D.; Sugimoto, D.; Jensen, C.; Mosenzon, O. Efficacy and Safety of Liraglutide 3.0 mg in Individuals with Overweight or Obesity and Type 2 Diabetes Treated With Basal Insulin: The SCALE Insulin Randomized Controlled Trial. Diabetes Care 2020, 43, dc191745. [Google Scholar] [CrossRef]
- Metz, J.A.; Stern, J.S.; Kris-Etherton, P.; Reusser, M.E.; Morris, C.D.; Hatton, D.C.; Oparil, S.; Haynes, R.B.; Resnick, L.M.; Pi-Sunyer, F.X.; et al. A randomized trial of improved weight loss with a prepared meal plan in overweight and obese patients: Impact on cardiovascular risk reduction. Arch. Intern. Med. 2000, 160, 2150–2158. [Google Scholar] [CrossRef]
- Kelly, A.S.; Auerbach, P.; Barrientos-Perez, M.; Gies, I.; Hale, P.M.; Marcus, C.; Mastrandrea, L.D.; Prabhu, N.; Arslanian, S.; Investigators, N.N.T. A Randomized, Controlled Trial of Liraglutide for Adolescents with Obesity. N. Engl. J. Med. 2020, 382, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Shao, L.; Zhang, Y.M.; Tu, Y.J.; Zhang, Y.; Tomlinson, B.; Chan, P.; Liu, Z. An evaluation of liraglutide including its efficacy and safety for the treatment of obesity. Expert Opin. Pharm. 2020, 21, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Muratori, F.; Vignati, F.; Di Sacco, G.; Gavazzi, L.; Pellegrino, D.; Del Prete, M. Efficacy of liraglutide 3.0 mg treatment on weight loss in patients with weight regain after bariatric surgery. Eat. Weight Disord. 2022, 27, 2775–2781. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Smith, A. Patient initiation and maintenance of GLP-1 RAs for treatment of obesity. Expert Rev. Clin. Pharm. 2021, 14, 1193–1204. [Google Scholar] [CrossRef]
- Konwar, M.; Bose, D.; Jaiswal, S.K.; Maurya, M.; Ravi, R. Efficacy and Safety of Liraglutide 3.0 mg in Patients with Overweight and Obese with or without Diabetes: A Systematic Review and Meta-Analysis. Int. J. Clin. Pract. 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Trenson, L.; Trenson, S.; van Nes, F.; Moyson, C.; Lannoo, M.; Deleus, E.; Meulemans, A.; Matthys, C.; Mertens, A.; Van der Schueren, B.; et al. Liraglutide for Weight Management in the Real World: Significant Weight Loss Even if the Maximal Daily Dose Is Not Achieved. Obes. Facts. 2022, 15, 83–89. [Google Scholar] [CrossRef]
- Cosentino, G.; Conrad, A.O.; Uwaifo, G.I. Phentermine and topiramate for the management of obesity: A review. Drug Des. Devel Ther. 2013, 7, 267–278. [Google Scholar]
- Aronne, L.J.; Wadden, T.A.; Peterson, C.; Winslow, D.; Odeh, S.; Gadde, K.M. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity 2013, 21, 2163–2171. [Google Scholar] [CrossRef]
- Hsia, D.S.; Gosselin, N.H.; Williams, J.; Farhat, N.; Marier, J.F.; Shih, W.; Peterson, C.; Siegel, R. A randomized, double-blind, placebo-controlled, pharmacokinetic and pharmacodynamic study of a fixed-dose combination of phentermine/topiramate in adolescents with obesity. Diabetes Obes. Metab. 2020, 22, 480–491. [Google Scholar] [CrossRef]
- Garvey, W.T.; Ryan, D.H.; Look, M.; Gadde, K.M.; Allison, D.B.; Peterson, C.A.; Schwiers, M.; Day, W.W.; Bowden, C.H. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): A randomized, placebo-controlled, phase 3 extension study. Am. J. Clin. Nutr. 2012, 95, 297–308. [Google Scholar] [CrossRef]
- Dhillon, S. Phentermine/Topiramate: Pediatric First Approval. Pediatr. Drugs 2022, 24, 715–720. [Google Scholar] [CrossRef]
- In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Disease: Bethesda, MD, USA, 2012.
- Valle-Jones, J.C.; Brodie, N.H.; O’Hara, H.; O’Hara, J.; McGhie, R.L. A comparative study of phentermine and diethylpropion in the treatment of obese patients in general practice. Pharmatherapeutica 1983, 3, 300–304. [Google Scholar] [PubMed]
- Cercato, C.; Roizenblatt, V.A.; Leanca, C.C.; Segal, A.; Lopes Filho, A.P.; Mancini, M.C.; Halpern, A. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of diethylpropion in the treatment of obese subjects. Int. J. Obes. 2009, 33, 857–865. [Google Scholar] [CrossRef]
- Cortes-Moreno, G.Y.; Roa-Coria, J.E.; Zuniga-Romero, A.; Huerta-Cruz, J.C.; Lara-Padilla, E.; Del Valle-Laisequilla, C.F.; Rocha-Gonzalez, H.I.; Reyes-Garcia, J.G. Anorectic efficacy and safety of the diethylpropion-topiramate combination in rats. Drug Dev. Res. 2018, 79, 225–233. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Solorio, J.; Perez, C.; Hoyo-Vadillo, C.; Simon, S.; Gutierrez, R. The efficacy of the appetite suppressant, diethylpropion, is dependent on both when it is given (day vs. night) and under conditions of high fat dietary restriction. Appetite 2016, 100, 152–161. [Google Scholar]
- Soto, M.H.; Diaz, M.J.; Escobar, J.Y.; Fernandez del Valle, C. Cost-Effectiveness Analysis of Amfepramone (Diethylpropion) for the Obesity Treatment in Mexico. Value Health 2014, 17, A531–A532. [Google Scholar] [CrossRef]
- Poyatos, L.; Torres, A.; Papaseit, E.; Perez-Mana, C.; Hladun, O.; Nunez-Montero, M.; de la Rosa, G.; Torrens, M.; Fuster, D.; Muga, R.; et al. Abuse Potential of Cathinones in Humans: A Systematic Review. J. Clin. Med. 2022, 11, 1004. [Google Scholar] [CrossRef]
- Tchang, B.G.; Abel, B.; Zecca, C.; Saunders, K.H.; Shukla, A.P. An up-to-date evaluation of lorcaserin hydrochloride for the treatment of obesity. Expert Opin. Pharm. 2020, 21, 21–28. [Google Scholar] [CrossRef]
- Bohula, E.A.; Wiviott, S.D.; McGuire, D.K.; Inzucchi, S.E.; Kuder, J.; Im, K.; Fanola, C.L.; Qamar, A.; Brown, C.; Budaj, A.; et al. Investigators, Cardiovascular Safety of Lorcaserin in Overweight or Obese Patients. N. Engl. J. Med. 2018, 379, 1107–1117. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Zhou, S.; Shanahan, W.; Fain, R. Lorcaserin in Obese and Overweight Patients Taking Prohibited Serotonergic Agents: A Retrospective Analysis. Clin. Ther. 2016, 38, 1498–1509. [Google Scholar] [CrossRef]
- Jing, M.; Wang, S.; Li, D.; Wang, Z.; Li, Z.; Lu, Y.; Sun, T.; Qiu, C.; Chen, F.; Yu, H.; et al. Lorcaserin Inhibit Glucose-Stimulated Insulin Secretion and Calcium Influx in Murine Pancreatic Islets. Front. Pharm. 2021, 12, 761966. [Google Scholar] [CrossRef] [PubMed]
- Dela Pena, I.C.; Figueroa, J.D.; Shi, W.X. Hypothesis: Amelioration of obesity-induced cognitive dysfunction via a lorcaserin-betahistine combination treatment. Pharm. Res. Perspect. 2022, 10, e00947. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Goulis, D.G.; Giouleme, O.; Germanidis, G.S.; Goulas, A. Anti-obesity Medications for the Management of Nonalcoholic Fatty Liver Disease. Curr. Obes. Rep. 2022, 11, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Billes, S.K.; Sinnayah, P.; Cowley, M.A. Naltrexone/bupropion for obesity: An investigational combination pharmacotherapy for weight loss. Pharm. Res. 2014, 84, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fava, M.; Rush, A.J.; Thase, M.E.; Clayton, A.; Stahl, S.M.; Pradko, J.F.; Johnston, J.A. 15 years of clinical experience with bupropion HCl: From bupropion to bupropion SR to bupropion XL. Prim. Care Companion. J. Clin Psychiatry 2005, 7, 106–113. [Google Scholar] [CrossRef]
- Gadde, K.M.; Parker, C.B.; Maner, L.G.; Wagner, H.R., 2nd; Logue, E.J.; Drezner, M.K.; Krishnan, K.R. Bupropion for weight loss: An investigation of efficacy and tolerability in overweight and obese women. Obes. Res. 2001, 9, 544–551. [Google Scholar] [CrossRef]
- Liu, Y.L.; Connoley, I.P.; Heal, D.J.; Stock, M.J. Pharmacological characterisation of the thermogenic effect of bupropion. Eur. J. Pharm. 2004, 498, 219–225. [Google Scholar] [CrossRef]
- Sherman, M.M.; Ungureanu, S.; Rey, J.A. Naltrexone/Bupropion ER (Contrave): Newly Approved Treatment Option for Chronic Weight Management in Obese Adults. Pharm. Ther. 2016, 41, 164–172. [Google Scholar]
- Tek, C. Naltrexone HCI/bupropion HCI for chronic weight management in obese adults: Patient selection and perspectives. Patient Prefer. Adherence 2016, 10, 751–759. [Google Scholar]
- Pi-Sunyer, X.; Apovian, C.M.; McElroy, S.L.; Dunayevich, E.; Acevedo, L.M.; Greenway, F.L. Psychiatric adverse events and effects on mood with prolonged-release naltrexone/bupropion combination therapy: A pooled analysis. Int. J. Obes. 2019, 43, 2085–2094. [Google Scholar] [CrossRef]
- Roberto da Silva, G.; Carneiro, M.G.; Barbosa, M.P.; Costa, J.A.; de Souza, I.A.; Dos Santos Oliveira, L.; de Vasconcelos, D.A.A.; do Nascimento, E.; Matos, R.J.B.; Lopes de Souza, S.; et al. Naltrexone/bupropion modifies weight, food intake, and Drd2 gene expression in rats. J. Endocrinol. 2022, 253, 85–96. [Google Scholar] [CrossRef]
- Salari, N.; Jafari, S.; Darvishi, N.; Valipour, E.; Mohammadi, M.; Mansouri, K.; Shohaimi, S. The best drug supplement for obesity treatment: A systematic review and network meta-analysis. Diabetol. Metab. Syndr. 2021, 13, 110. [Google Scholar] [CrossRef]
- Aras, M.; Tchang, B.G.; Pape, J. Obesity and Diabetes. Nurs. Clin. North. Am. 2021, 56, 527–541. [Google Scholar] [CrossRef]
- Yerevanian, A.; Soukas, A.A. Metformin: Mechanisms in Human Obesity and Weight Loss. Curr. Obes. Rep. 2019, 8, 156–164. [Google Scholar] [CrossRef]
- Loi, H.; Kramar, S.; Laborde, C.; Marsal, D.; Pizzinat, N.; Cussac, D.; Roncalli, J.; Boal, F.; Tronchere, H.; Oleshchuk, O.; et al. Metformin Attenuates Postinfarction Myocardial Fibrosis and Inflammation in Mice. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Seifarth, C.; Schehler, B.; Schneider, H.J. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp. Clin. Endocrinol. Diabetes 2013, 121, 27–31. [Google Scholar] [CrossRef]
- American Diabetes, A. Standards of Medical Care in Diabetes-2019 Abridged for Primary Care Providers. Clin. Diabetes 2019, 37, 11–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, C.; Li, X.; Yang, M.; Tao, L.; Zheng, X.; Jia, Y. Beinaglutide showed significant weight loss benefit and effective glycemic control for the treatment of type 2 diabetes in a real-world setting: A 3-month, multicenter, observational, retrospective, open-label study. Obes. Sci. Pract. 2019, 5, 366–375. [Google Scholar] [CrossRef]
- Gao, L.; Huang, H.; Zhang, L.; Zhang, N.; Fu, Y.; Zhu, D.; Bi, Y.; Feng, W. Comparison of Beinaglutide Versus Metformin for Weight Loss in Overweight and Obese Non-diabetic Patients. Exp. Clin. Endocrinol. Diabetes 2022, 130, 358–367. [Google Scholar] [CrossRef]
- Wang, G.-Y.; Wang, H.-Q.; Zhang, F.-J.; Jiao, A.-F.; Li, Y.-Y.; Zhang, J.-M.; Huang, Z.-L.; Gao, Y.-H.; Chi, Y.-J.; Ma, C.-M.; et al. The effect of beinaglutide on visceral fat and bodyweight in obese type 2 diabetic patients. Arch. Med. Sci. 2020, 7. [Google Scholar] [CrossRef]
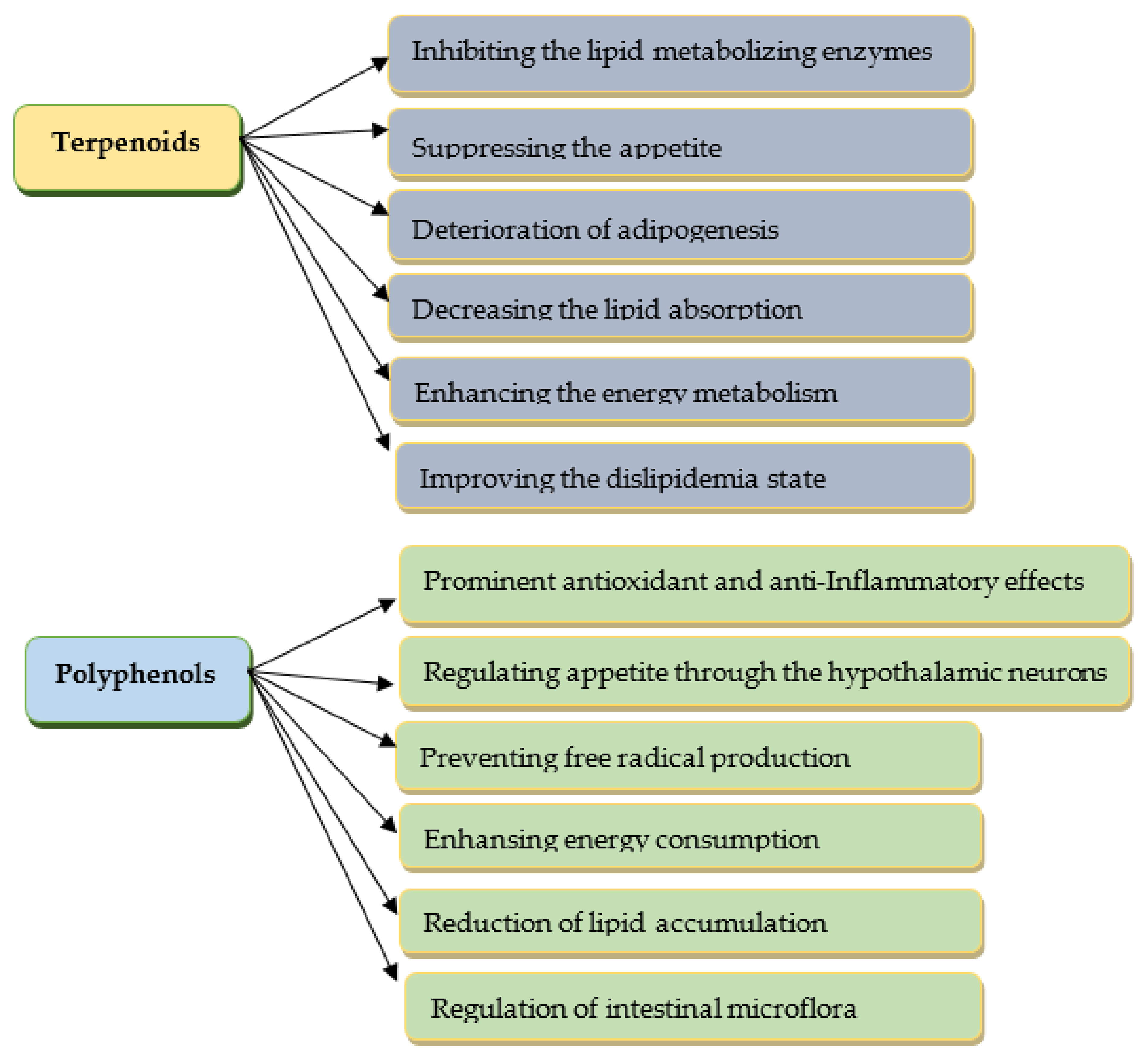
- Rahman, M.M.; Islam, M.R.; Shohag, S.; Hossain, M.E.; Rahaman, M.S.; Islam, F.; Ahmed, M.; Mitra, S.; Khandaker, M.U.; Idris, A.M.; et al. The Multifunctional Role of Herbal Products in the Management of Diabetes and Obesity: A Comprehensive Review. Molecules 2022, 27, 1713. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Yadav, P.; Vashishth, D.; Sharma, K.A.K.; Chahal, J.; Dalal, S.; Kataria, S. A Review on Obesity Management through Natural Compounds and a Green Nanomedicine-Based Approach. Molecules 2021, 26, 3278. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-D.; Ge, X.-C.; Jiang, S.-Y.; Yang, Y.-Y. Potential lipolytic regulators derived from natural products as effective approaches to treat obesity. Front. Endocrinol. 2022, 13. [Google Scholar] [CrossRef]
- Chu, W.-L.; Phang, S.-M. Marine Algae as a Potential Source for Anti-Obesity Agents. Mar. Drugs 2016, 14, 222. [Google Scholar]
- Sahib, N.; Saari, N.A.I.; Khatib, A.; Mahomoodally, F.; Abdul Hamid, A. Plants’ Metabolites as Potential Antiobesity Agents. Sci. World J. 2012, 2012, 436039. [Google Scholar]
- Moorthy, M.; Sundralingam, U.; Palanisamy, U. Polyphenols as Prebiotics in the Management of High-Fat Diet-Induced Obesity: A Systematic Review of Animal Studies. Foods 2021, 10, 299. [Google Scholar] [CrossRef]
- Perez-Torres, I.; Castrejon-Tellez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Farhat, G.; Drummond, S.; Al-Dujaili, E. Polyphenols and Their Role in Obesity Management: A Systematic Review of Randomized Clinical Trials. Phytother. Res. PTR 2017, 31, 1005–1018. [Google Scholar] [CrossRef]
- Quispe, C.; Cruz-Martins, N.; Manca, M.L.; Manconi, M.; Sytar, O.; Hudz, N.; Shanaida, M.; Kumar, M.; Taheri, Y.; Martorell, M.; et al. Nano-Derived Therapeutic Formulations with Curcumin in Inflammation-Related Diseases. Oxid Med. Cell Longev. 2021, 2021, 3149223. [Google Scholar] [CrossRef]
- Koshovyi, O.; Granica, S.; Piwowarski, J.; Stremoukhov, O.; Kostenko, Y.; Kravchenko, G.; Krasilnikova, O.; Zagayko, A. Highbush Blueberry (Vaccinium corymbosum L.) Leaves Extract and Its Modified Arginine Preparation for the Management of Metabolic Syndrome-Chemical Analysis and Bioactivity in Rat Model. Nutrients 2021, 13, 2870. [Google Scholar] [CrossRef]
- Suzuki, T.; Pervin, M.; Goto, S.; Isemura, M.; Nakamura, Y. Beneficial Effects of Tea and the Green Tea Catechin Epigallocatechin-3-gallate on Obesity. Molecules 2016, 21, 1305. [Google Scholar] [CrossRef]
- Gouveia, H.; Urquiza-Martinez, M.V.; Manhaes-de-Castro, R.; Costa-de-Santana, B.J.R.; Villarreal, J.P.; Mercado-Camargo, R.; Torner, L.; de Souza Aquino, J.; Toscano, A.E.; Guzman-Quevedo, O. Effects of the Treatment with Flavonoids on Metabolic Syndrome Components in Humans: A Systematic Review Focusing on Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 8344. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef]
- Skakun, N.; Stepanova, N. Comparative evaluation of the hepatoprotective, antioxidant and choleretic activity of flavonoid drugs. Vrachebnoe Delo 1989, 52–54. [Google Scholar]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Chen, L.; Pu, Y.; Xu, Y.; He, X.; Cao, J.; Ma, Y.; Jiang, W. Anti-diabetic and anti-obesity: Efficacy evaluation and exploitation of polyphenols in fruits and vegetables. Food Res. Int. 2022, 157, 111202. [Google Scholar] [CrossRef]
- Molinari, R.; Merendino, N.; Costantini, L. Polyphenols as modulators of pre-established gut microbiota dysbiosis: State-of-the-art. Biofactors 2022, 48, 255–273. [Google Scholar] [CrossRef]
- Basu, A.; Feng, D.; Planinic, P.; Ebersole, J.L.; Lyons, T.J.; Alexander, J.M. Dietary Blueberry and Soluble Fiber Supplementation Reduces Risk of Gestational Diabetes in Women with Obesity in a Randomized Controlled Trial. J. Nutr. 2021, 151, 1128–1138. [Google Scholar] [CrossRef]
- Zagayko, A.L.; Kolisnyk, T.Y.; Chumak, O.I.; Ruban, O.A.; Koshovyi, O.M. Evaluation of anti-obesity and lipid-lowering properties of Vaccinium myrtillus leaves powder extract in a hamster model. J. Basic Clin Physiol. Pharm. 2018, 29, 697–703. [Google Scholar] [CrossRef]
- Erpel, F.; Mateos, R.; Pérez-Jiménez, J.; Pérez-Correa, J.R. Phlorotannins: From isolation and structural characterization, to the evaluation of their antidiabetic and anticancer potential. Food Res. Int. 2020, 137, 109589. [Google Scholar] [CrossRef] [PubMed]
- Hirata, B.K.S.; Cruz, M.M.; de Sa, R.; Farias, T.S.M.; Machado, M.M.F.; Bueno, A.A.; Alonso-Vale, M.I.C.; Telles, M.M. Potential Anti-obesogenic Effects of Ginkgo biloba Observed in Epididymal White Adipose Tissue of Obese Rats. Front. Endocrinol. 2019, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Thomaz, F.M.; de Jesus Simao, J.; da Silva, V.S.; Machado, M.M.F.; Oyama, L.M.; Ribeiro, E.B.; Alonso Vale, M.I.C.; Telles, M.M. Ginkgo biloba Extract Stimulates Adipogenesis in 3T3-L1 Preadipocytes. Pharmaceuticals 2022, 15, 1294. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lee, J.; Salazar Hernandez, M.A.; Mazitschek, R.; Ozcan, U. Treatment of obesity with celastrol. Cell 2015, 161, 999–1011. [Google Scholar] [CrossRef]
- Xu, S.; Feng, Y.; He, W.; Xu, W.; Xu, W.; Yang, H.; Li, X. Celastrol in metabolic diseases: Progress and application prospects. Pharm. Res. 2021, 167, 105572. [Google Scholar] [CrossRef]
- Marrelli, M.; Conforti, F.; Araniti, F.; Statti, G.A. Effects of Saponins on Lipid Metabolism: A Review of Potential Health Benefits in the Treatment of Obesity. Molecules 2016, 21, 1404. [Google Scholar] [CrossRef]
- Zheng, J.; Zheng, S.; Feng, Q.; Zhang, Q.; Xiao, X. Dietary capsaicin and its anti-obesity potency: From mechanism to clinical implications. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef]
- Mounien, L.; Tourniaire, F.; Landrier, J.F. Anti-Obesity Effect of Carotenoids: Direct Impact on Adipose Tissue and Adipose Tissue-Driven Indirect Effects. Nutrients 2019, 11, 1562. [Google Scholar] [CrossRef]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, B.; Bai, Y.; Miao, T.; Rui, L.; Zhang, H.; Xia, B.; Li, Y.; Gao, S.; Wang, X.D.; et al. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharm. Res 2020, 159, 104966. [Google Scholar] [CrossRef]
- Bjørklund, G.; Gasmi, A.; Lenchyk, L.; Shanaida, M.; Zafar, S.; Mujawdiya, P.; Lysiuk, R.; Antonyak, H.; Noor, S.; Akram, M.; et al. The Role of Astaxanthin as a Nutraceutical in Health and Age-Related Conditions. Molecules 2022, 27, 7167. [Google Scholar] [CrossRef]
- Radice, R.P.; Limongi, A.R.; Viviano, E.; Padula, M.C.; Martelli, G.; Bermano, G. Effects of astaxanthin in animal models of obesity-associated diseases: A systematic review and meta-analysis. Free Radic. Biol Med. 2021, 171, 156–168. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Shanaida, M.; Ongenae, A.; Lysiuk, R.; Dosa, M.D.; Tsal, O.; Piscopo, S.; Chirumbolo, S.; Bjørklund, G. Calanus oil in the treatment of obesity-related low-grade inflammation, insulin resistance, and atherosclerosis. Appl. Microbiol. Biotechnol. 2020, 104, 967–979. [Google Scholar] [CrossRef]
- Bielawiec, P.; Harasim-Symbor, E.; Chabowski, A. Phytocannabinoids: Useful Drugs for the Treatment of Obesity? Special Focus on Cannabidiol. Front. Endocrinol. 2020, 11, 114. [Google Scholar] [CrossRef]
- Rossi, F.; Punzo, F.; Umano, G.R.; Argenziano, M.; Miraglia Del Giudice, E. Role of Cannabinoids in Obesity. Int. J. Mol. Sci. 2018, 19, 5875. [Google Scholar] [CrossRef]
- De Blasio, A.; D’Anneo, A.; Lauricella, M.; Emanuele, S.; Giuliano, M.; Pratelli, G.; Calvaruso, G.; Carlisi, D. The Beneficial Effects of Essential Oils in Anti-Obesity Treatment. Int. J. Mol. Sci. 2021, 22, 11832. [Google Scholar] [CrossRef]
- Shen, S.; Liao, Q.; Lyu, M.; Wong, Y.-K.; Zhang, X.; Zhou, J.; Ma, N.; Wang, J. The potential of artemisinins as anti-obesity agents via modulating the immune system. Pharmacol. Ther. 2020, 216, 107696. [Google Scholar] [CrossRef]
- Islam, M.; Ali, E.; Mubarak, M. Anti-obesity effect of plant diterpenes and their derivatives: A review. Phytother. Res. 2020, 34. [Google Scholar] [CrossRef]
- El-Shazly, S.A.; Ahmed, M.M.; Al-Harbi, M.S.; Alkafafy, M.E.; El-Sawy, H.B.; Amer, S.A.M. Physiological and molecular study on the anti-obesity effects of pineapple (Ananas comosus) juice in male Wistar rat. Food Sci. Biotechnol. 2018, 27, 1429–1438. [Google Scholar] [CrossRef]
- Kang, Y.M.; Kang, H.A.; Cominguez, D.C.; Kim, S.H.; An, H.J. Papain Ameliorates Lipid Accumulation and Inflammation in High-Fat Diet-Induced Obesity Mice and 3T3-L1 Adipocytes via AMPK Activation. Int. J. Mol. Sci. 2021, 22, 9983. [Google Scholar] [CrossRef] [PubMed]
- Ranaweera, S.S.; Natraj, P.; Rajan, P.; Dayarathne, L.A.; Mihindukulasooriya, S.P.; Dinh, D.T.T.; Jee, Y.; Han, C.H. Anti-obesity effect of sulforaphane in broccoli leaf extract on 3T3-L1 adipocytes and ob/ob mice. J. Nutr. Biochem. 2022, 100, 108885. [Google Scholar] [CrossRef] [PubMed]
- Mashmoul, M.; Azlan, A.; Khaza’ai, H.; Mohd Yusof, B.N.; Noor, M.S. Saffron: A Natural Potent Antioxidant as a Promising Anti-Obesity Drug. Antioxidants 2013, 2, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Mykhailenko, O.; Petrikaite, V.; Korinek, M.; El-Shazly, M.; Chen, B.H.; Yen, C.H.; Hsieh, C.F.; Bezruk, I.; Dabrisiute, A.; Ivanauskas, L.; et al. Bio-guided bioactive profiling and HPLC-DAD fingerprinting of Ukrainian saffron (Crocus sativus stigmas): Moving from correlation toward causation. BMC Complement. Med. Ther. 2021, 21, 203. [Google Scholar] [CrossRef]
- Gasmi Benahmed, A.; Gasmi, A.; Arshad, M.; Shanaida, M.; Lysiuk, R.; Peana, M.; Pshyk-Titko, I.; Adamiv, S.; Shanaida, Y.; Bjørklund, G. Health benefits of xylitol. Appl. Microbiol. Biotechnol. 2020, 104, 7225–7237. [Google Scholar] [CrossRef]
- Pearlman, M.; Obert, J.; Casey, L. The Association Between Artificial Sweeteners and Obesity. Curr. Gastroenterol. Rep. 2017, 19, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Xu, M.; Li, Y. Adipose Tissue Aging and Metabolic Disorder, and the Impact of Nutritional Interventions. Nutrients 2022, 14, 3134. [Google Scholar] [CrossRef]
- Gons’kyĭ, I.; Korda, M.; Klishch, I. Status of the free radical oxidation and antioxidant system in rats with toxic liver damage; effect of tocopherol and dimethylsulfoxide. Ukr. Biokhim. Zh. 1991, 63, 112–116. [Google Scholar]
- Dietrich, M.O.; Horvath, T.L. Limitations in anti-obesity drug development: The critical role of hunger-promoting neurons. Nat Rev. Drug. Discov. 2012, 11, 675–691. [Google Scholar] [CrossRef]
- Onakpoya, I.; Heneghan, C.; Aronson, J. Post-marketing withdrawal of anti-obesity medicinal products because of adverse drug reactions: A systematic review. BMC Med. 2016, 14, 1. [Google Scholar] [CrossRef]
- Yanovski, S.Z.; Yanovski, J.A. Long-term drug treatment for obesity: A systematic and clinical review. JAMA 2014, 311, 74–86. [Google Scholar] [CrossRef]
- Rosa-Goncalves, P.; Majerowicz, D. Pharmacotherapy of Obesity: Limits and Perspectives. Am. J. Cardiovasc. Drugs 2019, 19, 349–364. [Google Scholar] [CrossRef]
- Gadde, K.M.; Atkins, K.D. The limits and challenges of antiobesity pharmacotherapy. Expert Opin. Pharm. 2020, 21, 1319–1328. [Google Scholar] [CrossRef]
- May, M.; Schindler, C.; Engeli, S. Modern pharmacological treatment of obese patients. Ther. Adv Endocrinol. Metab. 2020, 11, 2042018819897527. [Google Scholar] [CrossRef]
- Li, J.; Duan, H.; Liu, Y.; Wang, L.; Zhou, X. Biomaterial-Based Therapeutic Strategies for Obesity and Its Comorbidities. Pharmaceutics 2022, 14, 144. [Google Scholar] [CrossRef]
- Van Nuland, M.; Ververs, T.F.; Lam, M. Dosing Therapeutic Radiopharmaceuticals in Obese Patients. Int. J. Mol. Sci. 2022, 23, 818. [Google Scholar] [CrossRef]
- Kang, J.; Kim, K.W.; Seo, Y.; Song, M.Y.; Chung, W.S. Effects of electroacupuncture for obesity: A protocol for systematic review and meta-analysis. Medicine 2022, 101, e29018. [Google Scholar] [CrossRef]
- Elpasty, S.S.A.; Helal, E.G.E.; Algendy, A.M.M.; Yousef, H.N. Effects of the Antiobesity Drugs Aplex and Venera on Certain Biochemical and Physiological Indices in Obese Adult Male Albino Rats. Adv Pharm. Pharm. Sci. 2022, 2022, 3776676. [Google Scholar] [CrossRef]
- Nicolucci, A.; Maffeis, C. The adolescent with obesity: What perspectives for treatment? Ital. J. Pediatr. 2022, 48, 1–9. [Google Scholar] [CrossRef]
- Angelidi, A.M.; Belanger, M.J.; Kokkinos, A.; Koliaki, C.C.; Mantzoros, C.S. Novel Noninvasive Approaches to the Treatment of Obesity: From Pharmacotherapy to Gene Therapy. Endocr. Rev. 2022, 43, 507–557. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasmi, A.; Mujawdiya, P.K.; Nehaoua, A.; Shanaida, M.; Semenova, Y.; Piscopo, S.; Menzel, A.; Voloshyn, V.; Voloshyn, O.; Shanaida, V.; et al. Pharmacological Treatments and Natural Biocompounds in Weight Management. Pharmaceuticals 2023, 16, 212. https://doi.org/10.3390/ph16020212
Gasmi A, Mujawdiya PK, Nehaoua A, Shanaida M, Semenova Y, Piscopo S, Menzel A, Voloshyn V, Voloshyn O, Shanaida V, et al. Pharmacological Treatments and Natural Biocompounds in Weight Management. Pharmaceuticals. 2023; 16(2):212. https://doi.org/10.3390/ph16020212
Chicago/Turabian StyleGasmi, Amin, Pavan Kumar Mujawdiya, Amine Nehaoua, Mariia Shanaida, Yuliya Semenova, Salva Piscopo, Alain Menzel, Volodymyr Voloshyn, Olena Voloshyn, Volodymyr Shanaida, and et al. 2023. "Pharmacological Treatments and Natural Biocompounds in Weight Management" Pharmaceuticals 16, no. 2: 212. https://doi.org/10.3390/ph16020212
APA StyleGasmi, A., Mujawdiya, P. K., Nehaoua, A., Shanaida, M., Semenova, Y., Piscopo, S., Menzel, A., Voloshyn, V., Voloshyn, O., Shanaida, V., & Bjørklund, G. (2023). Pharmacological Treatments and Natural Biocompounds in Weight Management. Pharmaceuticals, 16(2), 212. https://doi.org/10.3390/ph16020212








