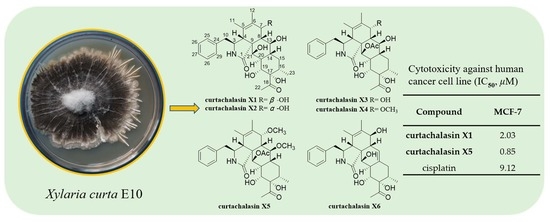
Six Unprecedented Cytochalasin Derivatives from the Potato Endophytic Fungus Xylaria curta E10 and Their Cytotoxicity
Abstract
1. Introduction
2. Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Culture and Fermentation of Fungal Material
3.3. Extraction and Isolation
3.4. Quantum Chemical Calculations
3.5. Cytotoxicity Assay against MCF-7
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bianconi, E.; Tassinari, R.; Alessandrini, A.; Ragazzini, G.; Cavallini, C.; Abruzzo, P.M.; Petrocelli, G.; Pampanella, L.; Casadei, R.; Maioli, M.; et al. Cytochalasin B modulates nanomechanical patterning and fate in human adipose-derived stem. Cells 2022, 11, 1629. [Google Scholar] [CrossRef] [PubMed]
- Qiao, K.J.; Chooi, Y.H.; Tang, Y. Identification and engineering of the cytochalasin gene cluster from Aspergillus clavatus NRRL 1. Metab. Eng. 2011, 13, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.X.; Ye, Y.; Zhang, Y.H. Large-scale culture as a complementary and practical method for discovering natural products with novel skeletons. Nat. Prod. Rep. 2021, 38, 1775–1793. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.D.; Tong, Q.Y.; Zhang, X.T.; Zhou, P.; Dai, C.; Wang, J.P.; Chen, C.M.; Zhu, H.C.; Zhang, Y.H. Amichalasines A-C: Three cytochalasan heterotrimers from aspergillus micronesiensis PG-1. Org. Lett. 2019, 21, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Li, G.; Qiao, Y.N.; Sun, Y.; Peng, X.P.; Lou, H.X. Chamiside A, a cytochalasan with a tricyclic core skeleton from the endophytic fungus Chaetomium nigricolor F5. Org. Lett. 2019, 21, 3319–3322. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Wu, S.H.; Zhai, Y.Z.; Xuan, Q.C.; Wang, T. Secondary metabolites from the genus xylaria and their bioactivities. Chem. Biodivers. 2014, 11, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.L.; Dong, S.H.; Li, H.Y.; Wei, W.J.; Tu, Y.Q.; Gao, K. Cytochalasins from Xylaria sp. CFL5, an Endophytic Fungus of Cephalotaxus fortune. Nat. Prod. Bioprospect. 2020, 11, 87–98. [Google Scholar] [CrossRef]
- Helaly, S.E.; Thongbai, B.; Stadler, M. Diversity of biologically active secondary metabolites from endophytic and saprotrophic fungi of the ascomycete order Xylariales. Nat. Prod. Rep. 2018, 35, 992–1014. [Google Scholar] [CrossRef]
- Guo, C.J.; Wu, P.; Xue, J.H.; Li, H.X.; Wei, X.Y. Xylaropyrones B and C, new γ-pyrones from the endophytic fungus Xylaria sp. SC1440. Nat. Prod. Res. 2018, 32, 1525–1531. [Google Scholar] [CrossRef]
- Xu, K.; Li, R.J.; Zhu, R.X.; Li, X.B.; Xu, Y.L.; He, Q.B.; Xie, F.; Qiao, Y.N.; Luan, X.Y.; Lou, H.X. Xylarins A-D, two pairs of diastereoisomeric isoindoline alkaloids from the endolichenic fungus Xylaria sp. Org. Lett. 2021, 23, 7751–7754. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, J.H.; Zhao, J.C.; Wang, Y.L.; Dong, P.P.; Liu, X.G.; Zhang, T.Y.; Wu, Y.Y.; Shang, D.J.; Zhang, Y.X.; et al. Xylarianins A-D from the endophytic fungus Xylaria sp. SYPF 8246 as natural inhibitors of human carboxylesterase 2. Bioorg. Chem. 2018, 81, 350–355. [Google Scholar] [CrossRef]
- Song, J.T.; Xu, K.; Liu, C.Y.; Wang, T.; Luan, X.Y.; Zhu, L.H.; Chu, Z.J.; Fu, X.J.; Chang, W.Q.; Wang, X.N.; et al. Bioactive specialised metabolites from the endophytic fungus Xylaria sp. of Cudrania tricuspidata. Phytochemistry 2022, 196, 113079. [Google Scholar] [CrossRef] [PubMed]
- Deyrup, S.T.; Gloer, J.B.; O’Donnell, K.; Wicklow, D.T. Kolokosides A-D: Triterpenoid glycosides from a Hawaiian isolate of Xylaria sp. J. Nat. Prod. 2007, 70, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.H.; Sun, Z.C.; Peng, J.X.; Zhu, M.L.; Che, Q.; Zhang, G.J.; Zhu, T.J.; Gu, Q.Q.; Li, D.H. Secondary Metabolites Produced by Combined Culture of Penicillium crustosum and a Xylaria sp. J. Nat. Prod. 2019, 82, 2013–2017. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.N.; Chang, S.S.; Li, Y.H.; Xi, X.M.; Chen, M.H.; He, N.; Wang, M.Y.; Zhao, W.L.; Xie, Y.Y. Molecular networking-based screening led to the discovery of a cyclic heptadepsipeptide from an endolichenic Xylaria sp. J. Nat. Prod. 2022, 85, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sang, Y.N.; Tang, S.Q.; Zhang, P. New curtachalasin from the Xylaria sp. DO 1801. Nat. Prod. Res. 2021, 35, 3701–3706. [Google Scholar] [CrossRef]
- Tchoukoua, A.; Ota, T.; Akanuma, R.; Ju, Y.M.; Supratman, U.; Murayama, T.; Koseki, T.; Shiono, Y. A phytotoxic bicyclic lactone and other compounds from endophyte Xylaria curta. Nat. Prod. Res. 2017, 31, 2113–2118. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Li, Z.H.; Feng, T.; Li, J.; Sun, H.; Huang, R.; Yuan, Q.X.; Ai, H.L.; Liu, J.K. Curtachalasins A and B, two cytochalasans with a tetracyclic skeleton from the endophytic fungus Xylaria curta E10. Org. Lett. 2018, 20, 7758–7761. [Google Scholar] [CrossRef]
- Wang, W.X.; Lei, X.X.; Ai, H.L.; Bai, X.; Li, J.; He, J.; Li, Z.H.; Zheng, Y.S.; Feng, T.; Liu, J.K. Cytochalasans from the endophytic fungus Xylaria cf. curta with resistance reversal activity against fluconazole-resistant Candida albicans. Org. Lett. 2019, 21, 1108–1111. [Google Scholar] [CrossRef]
- Wang, W.X.; Cheng, G.G.; Li, Z.H.; Ai, H.L.; He, J.; Li, J.; Feng, T.; Liu, J.K. Curtachalasins, immunosuppressive agents from the endophytic fungus Xylaria cf. curta. Org. Biomol. Chem. 2019, 17, 7985–7994. [Google Scholar] [CrossRef]
- Becker, K.; Stadler, M. Recent progress in biodiversity research on the Xylariales and their secondary metabolism. J. Antibiot. 2021, 74, 1–23. [Google Scholar] [CrossRef] [PubMed]
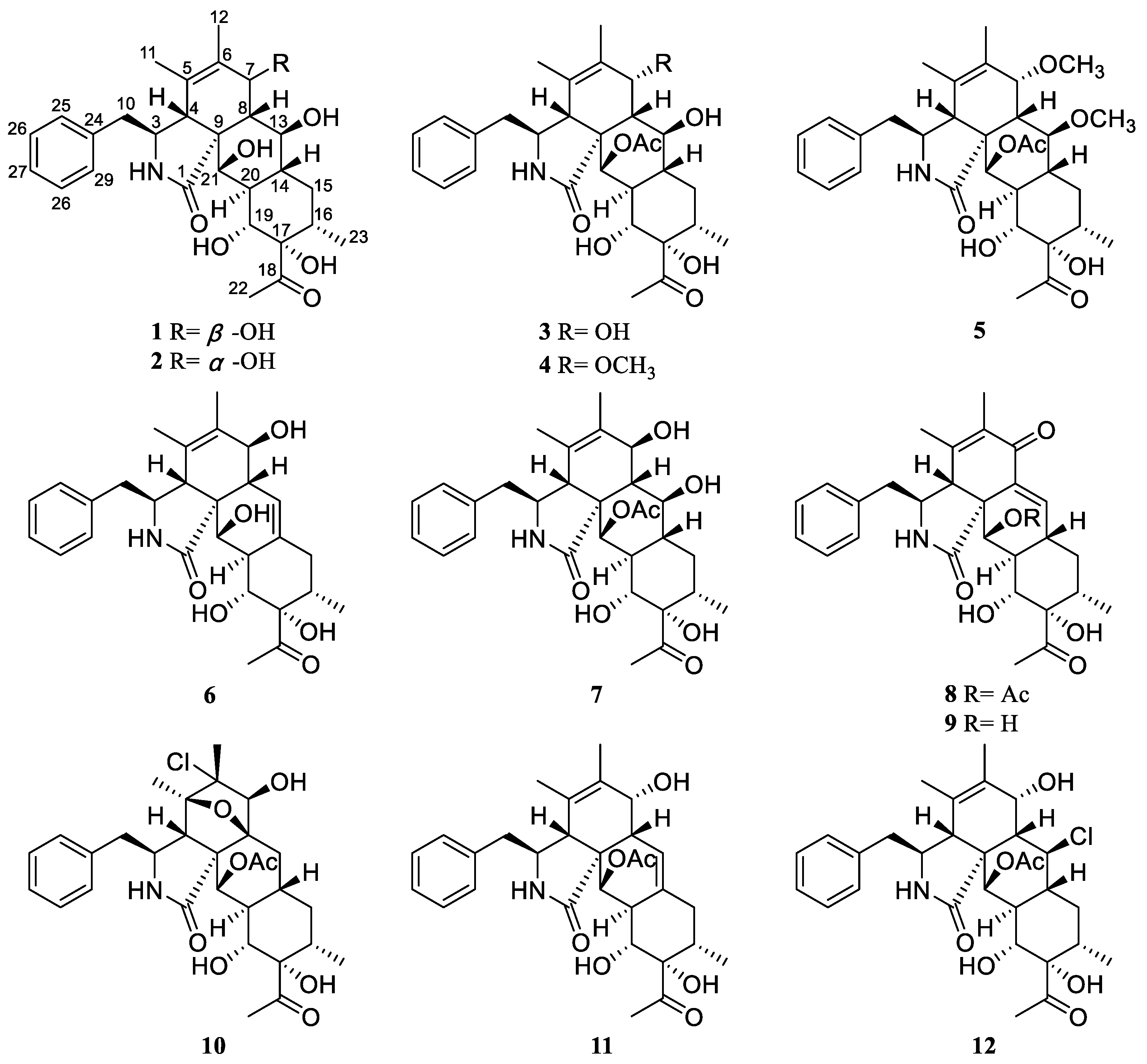

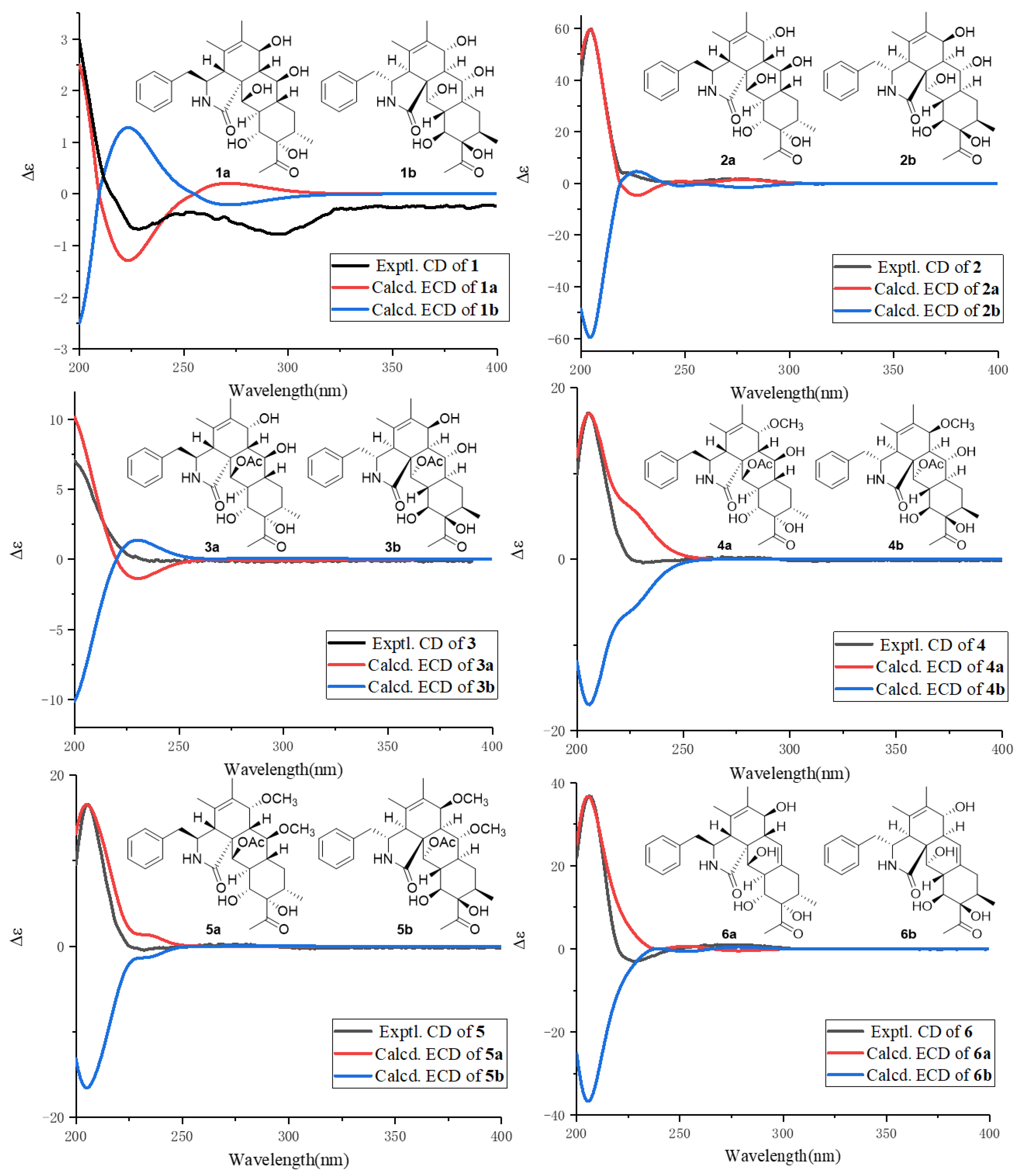
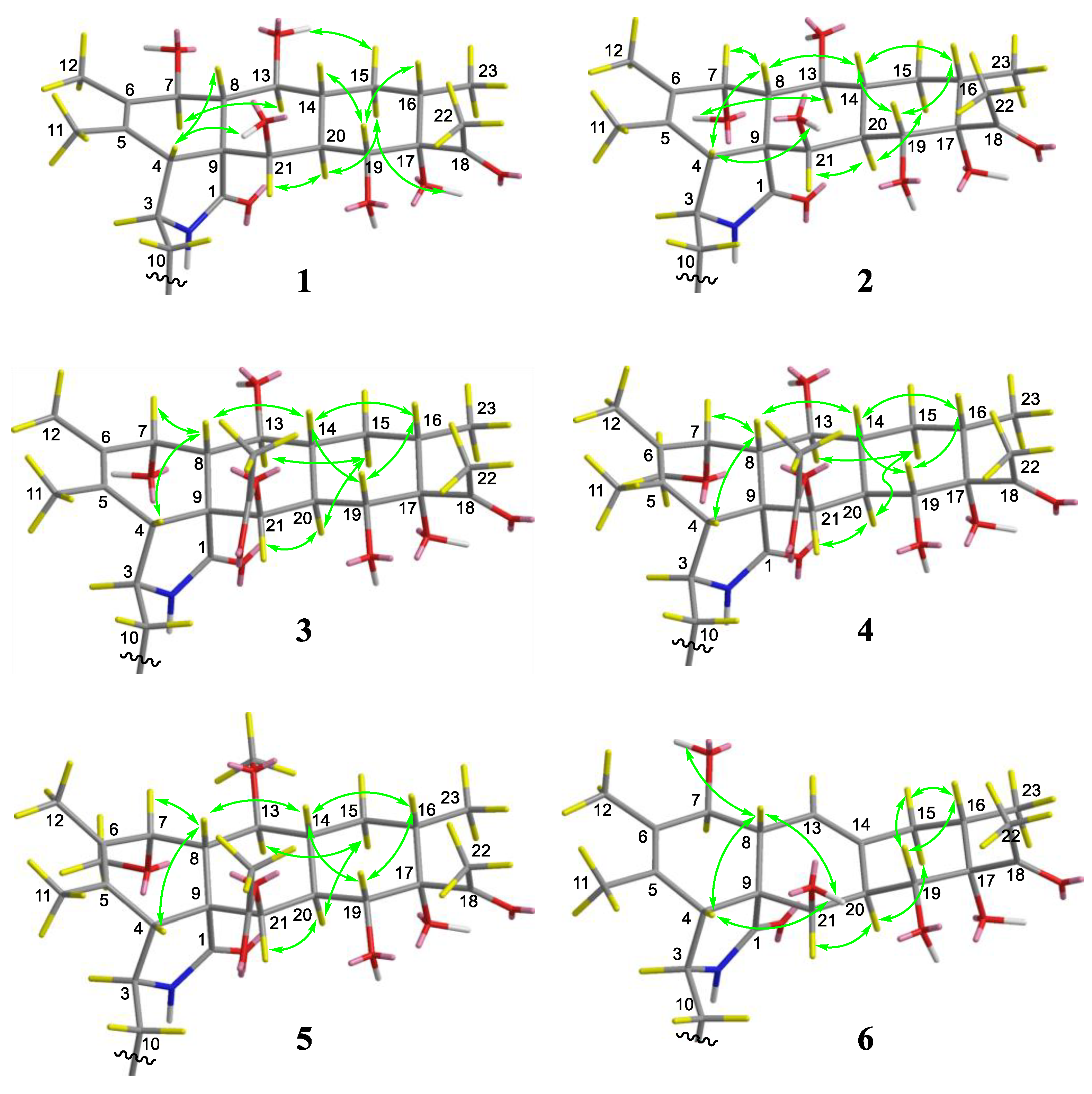
| No. | 1 a | 2 a | 3 b | 4 a | 5 b | 6 a |
|---|---|---|---|---|---|---|
| 1 | 177.1, C | 178.4, C | 178.7, C | 174.7, C | 178.7, C | 176.1, C |
| 3 | 59.4, CH | 58.5, CH | 61.2, CH | 59.9, CH | 61.5, CH | 58.5, CH |
| 4 | 47.2, CH | 47.2, CH | 49.5, CH | 47.9, CH | 49.3, CH | 47.3, CH |
| 5 | 126.2, C | 128.3, C | 130.4, C | 128.6, C | 129.6, C | 126.8, C |
| 6 | 132.8, C | 130.7, C | 131.4, C | 130.4, C | 133.0, C | 134.7, C |
| 7 | 71.8, CH | 66.7, CH | 68.0, CH | 78.1, CH | 77.5, CH | 68.1, CH |
| 8 | 43.4, CH | 44.0, CH | 46.6, CH | 46.9, CH | 46.6, CH | 39.2, CH |
| 9 | 52.1, C | 50.4, C | 50.6, C | 50.0, C | 50.8, C | 50.5, C |
| 10 | 43.9, CH2 | 44.5, CH2 | 44.8, CH2 | 44.2, CH2 | 45.3, CH2 | 44.1, CH2 |
| 11 | 17.0, CH3 | 18.5, CH3 | 17.1, CH3 | 16.8, CH3 | 17.3, CH3 | 17.3, CH3 |
| 12 | 14.2, CH3 | 16.8, CH3 | 18.4, CH3 | 20.1, CH3 | 21.1, CH3 | 15.1, CH3 |
| 13 | 73.3, CH | 67.8, CH | 69.3, CH | 66.1, CH | 80.4, CH | 119.9, CH |
| 14 | 39.1, CH | 39.7, CH | 42.1, CH | 42.4, CH | 41.4, CH | 132.6, C |
| 15 | 31.7, CH2 | 32.4, CH2 | 33.0, CH2 | 33.4, CH2 | 32.9, CH2 | 37.4, CH2 |
| 16 | 35.9, CH | 36.1, CH | 37.3, CH | 35.5, CH | 37.3, CH | 35.7, CH |
| 17 | 84.6, C | 84.7, C | 85.7, C | 84.3, C | 85.6, C | 84.8, C |
| 18 | 214.8, C | 215.8, C | 215.7, C | 214.9, C | 215.4, C | 214.8, C |
| 19 | 72.9, CH | 73.2, CH | 74.1, CH | 72.9, CH | 74.0, CH | 72.3, CH |
| 20 | 41.3, CH | 41.7, CH | 42.1, CH | 41.5, CH | 42.2, CH | 43.1, CH |
| 21 | 67.0, CH | 67.8, CH | 73.3, CH | 71.5, CH | 73.2, CH | 67.4, CH |
| 22 | 26.7, CH3 | 26.7, CH3 | 26.3, CH3 | 26.1, CH3 | 26.2, CH3 | 26.6, CH3 |
| 23 | 15.7, CH3 | 15.7, CH3 | 15.3, CH3 | 15.4, CH3 | 15.4, CH3 | 15.3, CH3 |
| 24 | 138.7, C | 138.7, C | 138.7, C | 138.5, C | 139.2, C | 138.8, C |
| 25, 29 | 130.0, CH | 130.1, CH | 130.6, CH | 129.8, CH | 130.7, CH | 130.0, CH |
| 26, 28 | 128.8, CH | 128.8, CH | 129.6, CH | 128.9, CH | 130.3, CH | 128.8, CH |
| 27 | 126.8, CH | 126.8, CH | 127.7, CH | 126.9, CH | 127.7, CH | 126.8, CH |
| 21-OAc | 172.5, C | 170.4, C | 172.5, C | |||
| 20.9, CH3 | 21.2, CH3 | 20.8, CH3 | ||||
| 7-OCH3 | 59.3 | 59.8 | ||||
| 13-OCH3 | 57.5 |
| No. | 1 a | 2 a | 3 b |
|---|---|---|---|
| 3 | 3.12, m | 3.15, m | 3.09, m |
| 4 | 2.91, br s | 2.87, br s | 2.39, br s |
| 7 | 4.01, d, (10.0) | 3.93, dd, (5.0, 3.0) | 4.19, d, (2.4) |
| 8 | 1.59, dd, (10.0, 10.0) | 1.56, d, (3.0) | 1.63, d, (11.0, 2.4) |
| 10 | 2.86, dd, (12.8, 5.3) | 2.86, dd, (12.8, 4.8) | 2.98, dd, (13.1, 5.2) |
| 2.74, dd, (12.8, 9.6) | 2.67, dd, (12.8, 10.0) | 2.90, dd, (13.1, 9.2) | |
| 11 | 1.01, s | 1.56, s | 1.04, s |
| 12 | 1.48, s | 0.93, s | 1.69, s |
| 13 | 4.38, dd, (10.0, 3.7) | 4.18, t, 10.4 | 4.26, dd, (11.0, 10.0) |
| 14 | 1.52, m | 1.41, ddd, (12.0, 9.2, 2.8) | 1.55, m |
| 15 | 1.73, m | 1.80, m | 1.95, dt, (12.8, 3.7) |
| 1.01, m | 1.05, dd, (12.0, 12.0) | 1.30, m | |
| 16 | 1.77, m | 1.77, m | 1.89, m |
| 19 | 3.76, dd, (10.2, 8.8) | 3.75, dd, (10.6, 8.8) | 3.50, d, (10.6) |
| 20 | 1.99, ddd, (10.2, 10.2,1.8) | 2.21, dd, (10.6, 2.0) | 2.69, ddd, (12.0, 10.6, 2.2) |
| 21 | 4.03, d, (6.0, 1.8) | 3.90, dd, (5.8, 2.0) | 5.50, d, (2.2) |
| 22 | 2.19, s | 2.19, s | 2.25, s |
| 23 | 0.66, d, (6.6) | 0.66, d, (6.4) | 0.77, d, (6.8) |
| 25, 29 | 7.32, d, (6.8) | 7.32, d, (7.1) | 7.26, d, (7.2) |
| 26, 28 | 7.33, t, (6.8) | 7.32, t, (7.1) | 7.30, t, (7.2) |
| 27 | 7.24, t, (6.8) | 7.23, t, (7.1) | 7.20, t, (7.2) |
| 21-OAc | 2.20, s | ||
| 7-OH | 5.95, d, (2.9) | 3.51, d, (5.0) | |
| 13-OH | 5.56, d, (3.7) | 4.10, d, (6.8) | |
| 17-OH | 4.40, s | 4.34, s | |
| 19-OH | 4.54, d, (8.8) | 4.54, d, (8.8) | |
| 21-OH | 5.14, d, (6.0) | 5.13, d, (5.8) |
| No. | 4 a | 5 b | 6 a |
|---|---|---|---|
| 3 | 3.15, m | 3.32, m | 3.11, m |
| 4 | 2.27, br s | 2.39, br s | 2.80, br s |
| 7 | 3.91, d, (2.0) | 3.75, d, (1.8) | 3.61, dd, (9.8, 7.7) |
| 8 | 1.98, dd, (11.2, 2.0) | 1.82, dd, (10.8, 1.8) | 2.20, m |
| 10 | 2.87, dd, (13.0, 4.2) | 2.97, dd, (13.1, 5.2) | 2.88, dd, (12.8, 5.3) |
| 2.78, dd, (13.0, 9.8) | 2.84, dd, (13.1, 9.2) | 2.76, dd, (12.8, 9.7) | |
| 11 | 0.85, s | 1.03, s | 1.03, s |
| 12 | 1.66, s | 1.78, s | 1.52, s |
| 13 | 5.06, dd, (11.0, 11.0) | 4.24, dd, (10.8, 10.8) | 5.57, d, (2.1) |
| 14 | 1.76, dd, (11.0, 2.3) | 1.57, m | |
| 15 | 1.91, m | 1.83, m | 2.07, m |
| 1.21, d, (11.7) | 1.36, dd, (12.2, 12.2) | 1.86, m | |
| 16 | 1.88, m | 1.88, m | 1.82, m |
| 19 | 3.37, m | 3.48, d, (9.7) | 3.94, dd, (10.6, 8.6) |
| 20 | 2.75, m | 2.77, ddd, (10.0, 10.0, 2.3) | 2.45, m |
| 21 | 5.41, d, (2.0) | 5.42, d, (2.3) | 4.18, dd, (6.4, 3.7) |
| 22 | 2.18, s | 2.25, s | 2.20, s |
| 23 | 0.68, d, (6.5) | 0.77, d, (6.6) | 0.66, d, (6.6) |
| 25, 29 | 7.25, d, (7.2) | 7.29, d, (7.0) | 7.32, d, (7.2) |
| 26, 28 | 7.32, t, (7.2) | 7.28, t, (7.0) | 7.33, t, (7.2) |
| 27 | 7.20, t, (7.2) | 7.20, t, (7.0) | 7.24, t, (7.2) |
| 21-OAc | 2.14, s | 2.16, s | |
| 7-OCH3 | 3.29, s | 3.48, s | |
| 13-OCH3 | 3.27, s | ||
| 7-OH | 4.74, d, (7.7) | ||
| 17-OH | 4.50, s | ||
| 19-OH | 4.70, d, (8.6) | ||
| 21-OH | 5.20, d, (6.4) |
| Compound | MCF-7 | Compound | MCF-7 |
|---|---|---|---|
| 1 | 2.03 ± 0.63 | 8 | >40 |
| 2 | >40 | 9 | >40 |
| 3 | 13.86 ± 1.62 | 10 | >40 |
| 4 | >40 | 11 | >40 |
| 5 | 0.85 ± 0.13 | 12 | >40 |
| 6 | >40 | cisplatin | 9.12 ± 0.41 |
| 7 | >40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Fan, Y.; Ye, K.; Pan, X.; Ma, X.; Ai, H.; Shi, B.; Liu, J. Six Unprecedented Cytochalasin Derivatives from the Potato Endophytic Fungus Xylaria curta E10 and Their Cytotoxicity. Pharmaceuticals 2023, 16, 193. https://doi.org/10.3390/ph16020193
Zhang X, Fan Y, Ye K, Pan X, Ma X, Ai H, Shi B, Liu J. Six Unprecedented Cytochalasin Derivatives from the Potato Endophytic Fungus Xylaria curta E10 and Their Cytotoxicity. Pharmaceuticals. 2023; 16(2):193. https://doi.org/10.3390/ph16020193
Chicago/Turabian StyleZhang, Xian, Yinzhong Fan, Ke Ye, Xiaoyan Pan, Xujun Ma, Honglian Ai, Baobao Shi, and Jikai Liu. 2023. "Six Unprecedented Cytochalasin Derivatives from the Potato Endophytic Fungus Xylaria curta E10 and Their Cytotoxicity" Pharmaceuticals 16, no. 2: 193. https://doi.org/10.3390/ph16020193
APA StyleZhang, X., Fan, Y., Ye, K., Pan, X., Ma, X., Ai, H., Shi, B., & Liu, J. (2023). Six Unprecedented Cytochalasin Derivatives from the Potato Endophytic Fungus Xylaria curta E10 and Their Cytotoxicity. Pharmaceuticals, 16(2), 193. https://doi.org/10.3390/ph16020193