Efficacy of Selective Internal Radiation Therapy for Hepatocellular Carcinoma Post-Incomplete Response to Chemoembolization
Abstract
:1. Introduction
2. Results
2.1. Patients’ Characteristics
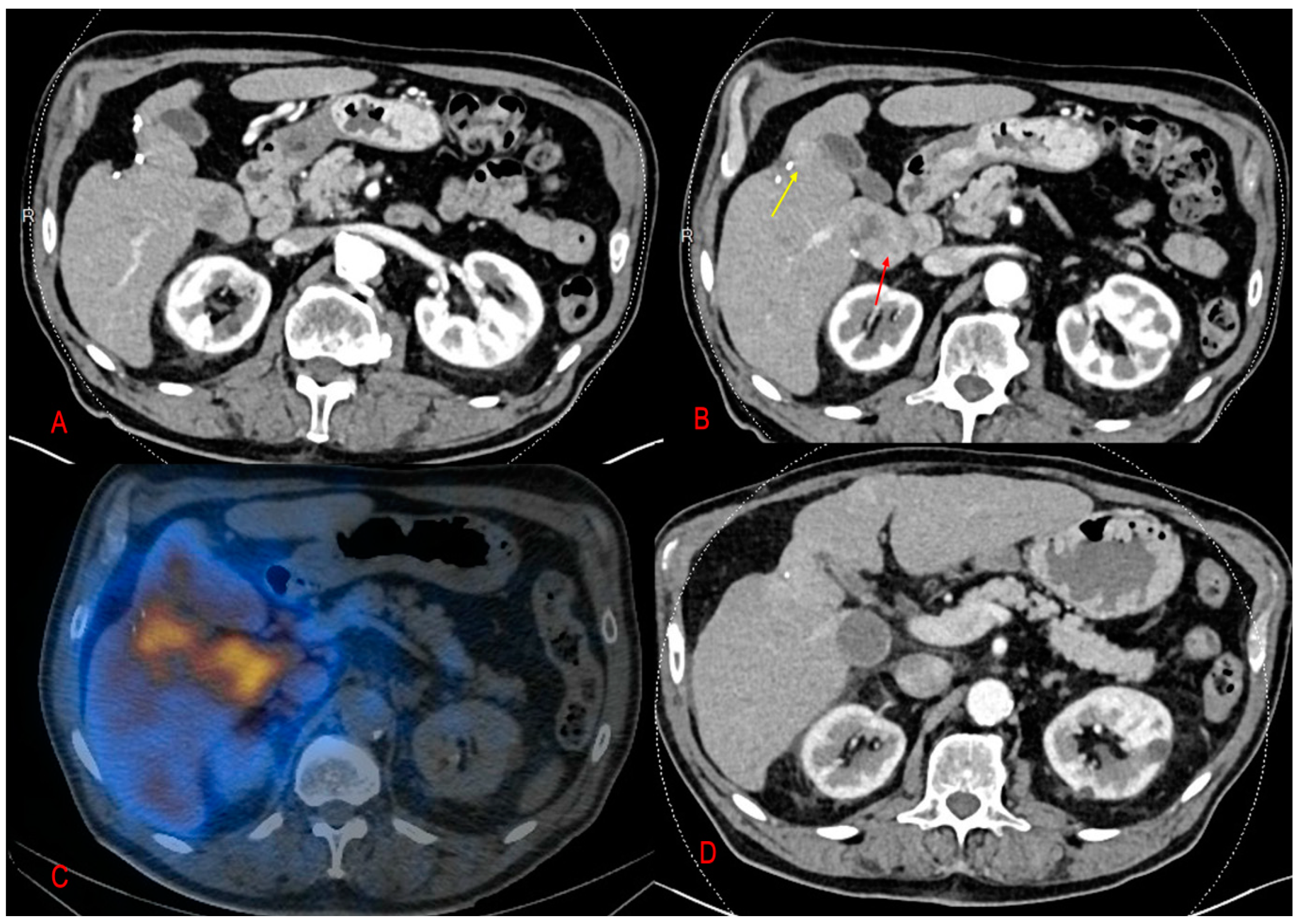
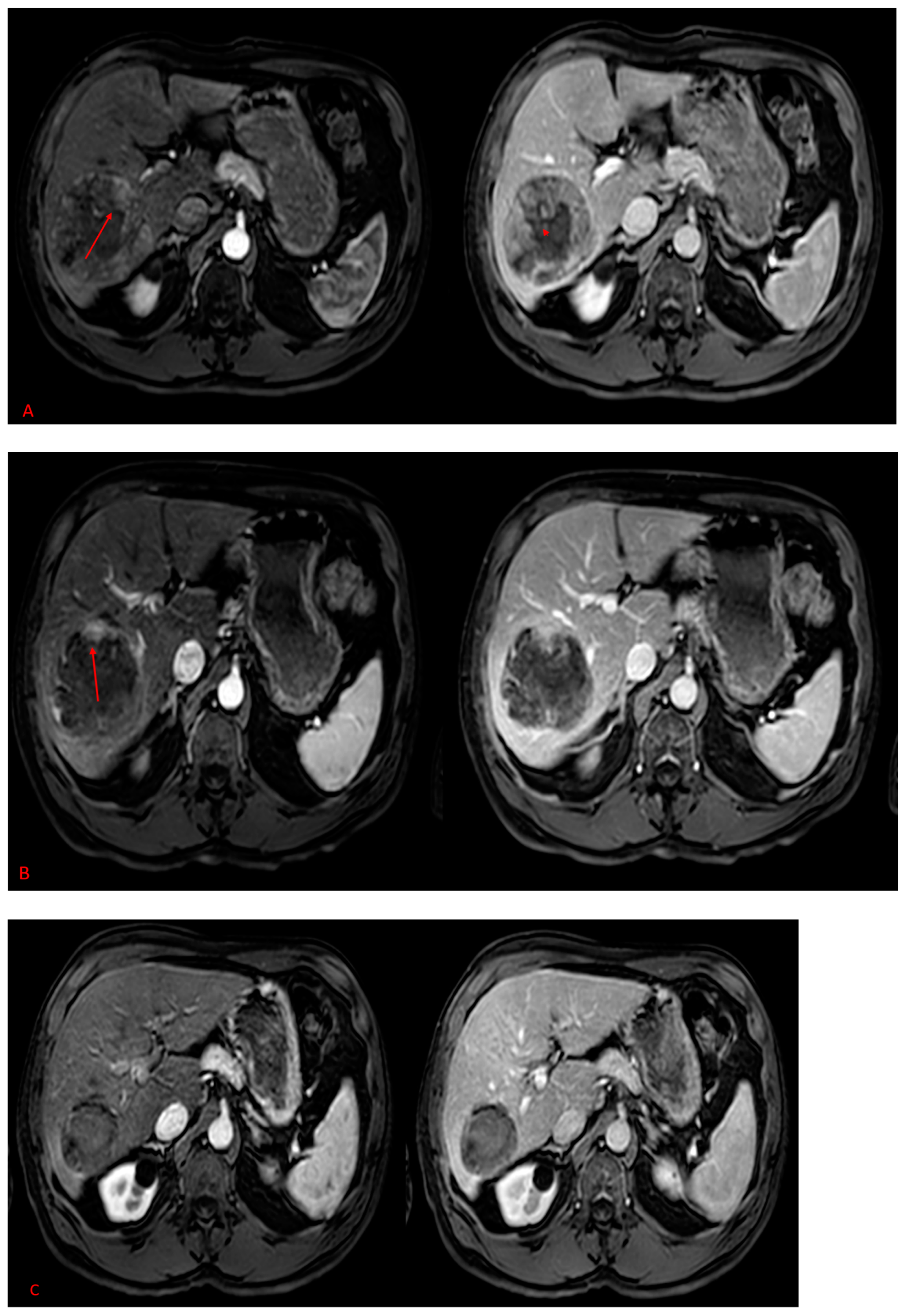
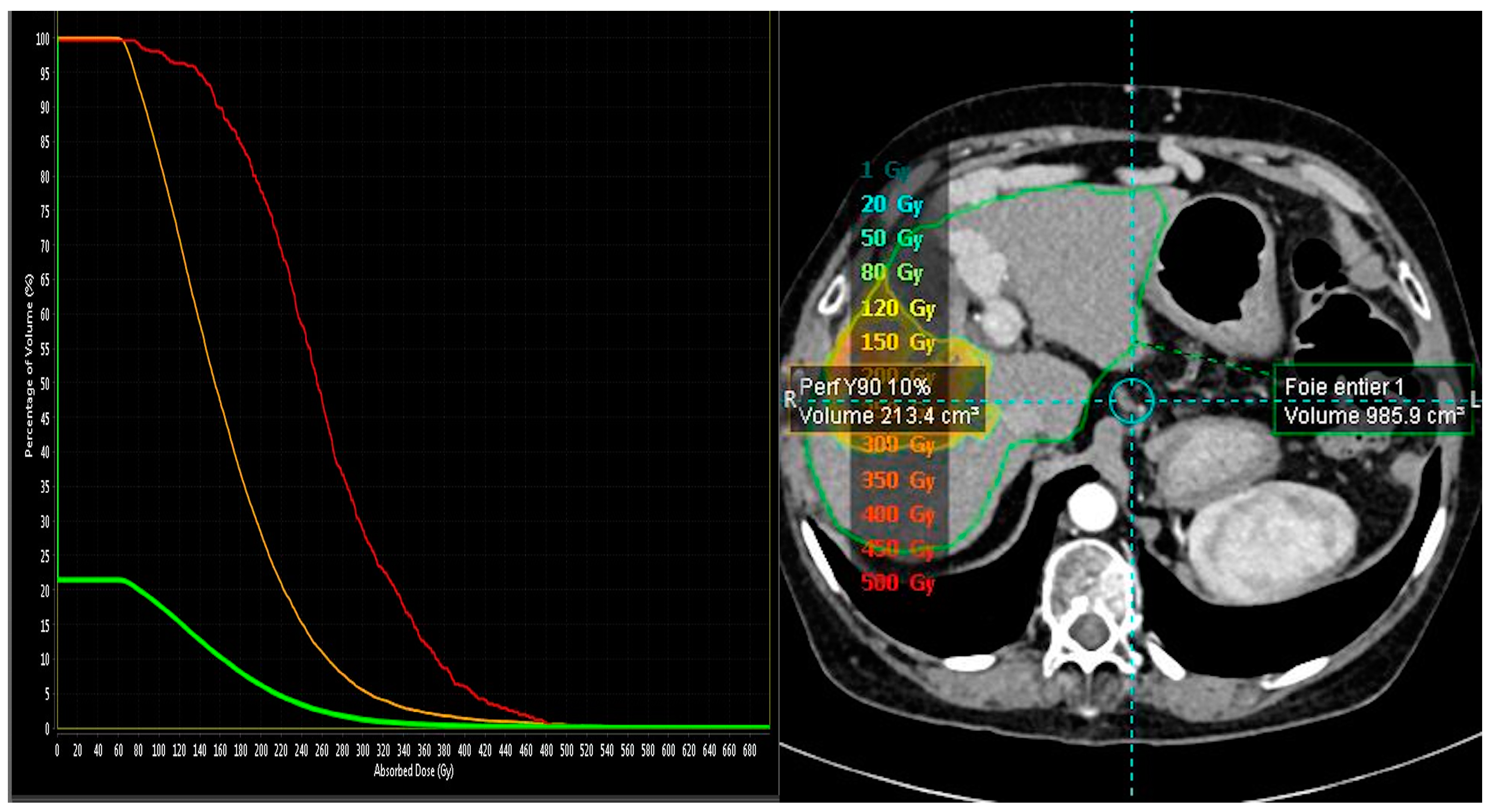
2.2. MAA-Based Dosimetry Analysis
2.3. 90Y-Based Dosimetry Analysis
3. Discussion
4. Methods and Materials
4.1. Study Design
4.2. Treatment Protocol
4.3. Data Analysis and Response Categorization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, Z.J.; Tsilimigras, D.I.; Ruff, S.M.; Mohseni, A.; Kamel, I.R.; Cloyd, J.M.; Pawlik, T.M. Management of Hepatocellular Carcinoma: A Review. JAMA Surg. 2023, 158, 410–420. [Google Scholar] [CrossRef]
- Wege, H.; Li, J.; Ittrich, H. Treatment Lines in Hepatocellular Carcinoma. Visc. Med. 2019, 35, 266–272. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, J.M.; Lee, J.S.; Lee, H.Y.; Park, B.H.; Kim, Y.H.; Han, J.K.; Choi, B.I. Hepatocellular carcinoma: Diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology 2015, 275, 97–109. [Google Scholar] [CrossRef]
- Kadalayil, L.; Benini, R.; Pallan, L.; O’Beirne, J.; Marelli, L.; Yu, D.; Hackshaw, A.; Fox, R.; Johnson, P.; Burroughs, A.K.; et al. A Simple Prognostic Scoring System for Patients Receiving Transarterial Embolisation for Hepatocellular Cancer. Ann. Oncol. 2013, 24, 2565–2570. [Google Scholar] [CrossRef]
- Kim, S.P.; Cohalan, C.; Kopek, N.; Enger, S.A. A guide to 90Y radioembolization and its dosimetry. Phys. Medica 2019, 68, 132–145. [Google Scholar] [CrossRef]
- Knesaurek, K.; Tuli, A.; Kim, E.; Kostakoglu, L. Comparison of Y-90 dosimetry derived from post-therapy PET/CT and bremsstrahlung SPECT imaging. J. Nucl. Med. 2016, 57 (Suppl. S2), 1893. [Google Scholar]
- Lewandowski, R.J.; Kulik, L.M.; Riaz, A.; Senthilnathan, S.; Mulcahy, M.F.; Ryu, R.K.; Ibrahim, S.M.; Sato, K.T.; Baker, T.; Miller, F.H.; et al. A Comparative Analysis of Transarterial Downstaging for Hepatocellular Carcinoma: Chemoembolization versus Radioembolization. Am. J. Transplant. 2009, 9, 1920–1928. [Google Scholar] [CrossRef]
- Clark, T.W.I. Complications of Hepatic Chemoembolization. Semin Interv. Radiol. 2006, 23, 119–125. [Google Scholar] [CrossRef]
- Commission de la Transparence. Available online: https://www.has-sante.fr/upload/docs/evamed/CT-19001_TECENTRIQ_PIC_EI_AvisDef_CT19001.pdf (accessed on 27 November 2023).
- Gomaa, A.I.; Khan, S.A.; Leen, E.L.; Waked, I.; Taylor-Robinson, S.D. Diagnosis of hepatocellular carcinoma. World J. Gastroenterol. WJG 2009, 15, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Kloeckner, R.; Ruckes, C.; Kronfeld, K.; Wörns, M.A.; Weinmann, A.; Galle, P.R.; Lang, H.; Otto, G.; Eichhorn, W.; Schreckenberger, M.; et al. Selective internal radiotherapy (SIRT) versus transarterial chemoembolization (TACE) for the treatment of intrahepatic cholangiocellular carcinoma (CCC): Study protocol for a randomized controlled trial. Trials 2014, 15, 311. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Osuga, K.; Mikami, K.; Higashihara, H.; Onishi, H.; Nakaya, Y.; Tatsumi, M.; Hori, M.; Kim, T.; Tomoda, K.; et al. Angiographic evaluation of hepatic arterial damage after transarterial chemoembolization for hepatocellular carcinoma. Radiat. Med. 2008, 26, 206–212. [Google Scholar] [CrossRef]
- Choi, J.; Lee, D.; Shim, J.H.; Kim, K.M.; Lim, Y.-S.; Lee, Y.S.; Lee, H.C. Evaluation of transarterial chemoembolization refractoriness in patients with hepatocellular carcinoma. PLoS ONE 2020, 15, e0229696. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Randomised, multicentre prospective trial of transarterial chemoembolization (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020, 69, 1492–1501. [Google Scholar] [CrossRef]
- Vente, M.A.D.; Wondergem, M.; Van Der Tweel, I.; Van Den Bosch, M.A.A.J.; Zonnenberg, B.A.; Lam, M.G.E.H.; Nijsen, J.F.W. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: A structured meta-analysis. Eur. Radiol. 2009, 19, 951–959. [Google Scholar] [CrossRef]
- Lau, W.Y.; Ho, S.; Leung, T.W.T.; Chan, M.; Ho, R.; Johnson, P.J.; Li, A.K.C. Selective internal radiation therapy for nonresectable hepatocellular carcinoma with intraarterial infusion of 90yttrium microspheres. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 583–592. [Google Scholar] [CrossRef]
- Van Thai, N.; Thinh, N.T.; Ky, T.D.; Bang, M.H.; Giang, D.T.; Ha, L.N.; Son, M.H.; Tien, D.D.; Lee, H.W. Efficacy and safety of selective internal radiation therapy with yttrium-90 for the treatment of unresectable hepatocellular carcinoma. BMC Gastroenterol. 2021, 21, 216. [Google Scholar] [CrossRef]
- Moctezuma-Velazquez, C.; Montano-Loza, A.J.; Meza-Junco, J.; Burak, K.; Ma, M.; Bain, V.G.; Kneteman, N.; Sarlieve, P.; Owen, R.J. Selective Internal Radiation Therapy for Hepatocellular Carcinoma Across the Barcelona Clinic Liver Cancer Stages. Dig. Dis. Sci. 2021, 66, 899–911. [Google Scholar] [CrossRef]
- Levillain, H.; Bagni, O.; Deroose, C.M.; Dieudonné, A.; Gnesin, S.; Grosser, O.S.; Kappadath, S.C.; Kennedy, A.; Kokabi, N.; Liu, D.M.; et al. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1570–1584. [Google Scholar] [CrossRef]
- Lam, M.; Garin, E.; Maccauro, M.; Kappadath, S.C.; Sze, D.Y.; Cantasdemir, M.; Turkmen, C.; Haste, P.; Herrmann, K.; Alsuhaibani, H.S.; et al. A global evaluation of advanced dosimetry in transarterial radioembolization of hepatocellular carcinoma with Yttrium-90: The TARGET study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3340–3352. [Google Scholar] [CrossRef]
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; de Baere, T.; Milliner, M. Personalized versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): A randomized, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 17–29. [Google Scholar] [CrossRef]
- Hermann, A.-L.; Dieudonné, A.; Ronot, M.; Sanchez, M.; Pereira, H.; Chatellier, G.; Garin, E.; Castera, L.; Lebtahi, R.; Vilgrain, V. Relationship of Tumor Radiation-absorbed Dose to Survival and Response in Hepatocellular Carcinoma Treated with Transarterial Radioembolization with 90Y in the SARAH Study. Radiology 2020, 296, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Lam, M.; Chiesa, C.; Konijnenberg, M.; Cremonesi, M.; Flamen, P.; Gnesin, S.; Bodei, L.; Kracmerova, T.; Luster, M.; et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1682–1699. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.; Rose, S.C.; Al Jammal, O.; Hsieh, L.J.; Rockwell, H.D.; Duncan, D.P.; Minocha, J.; Berman, Z.T. Boosted-Dose Yttrium-90 Radiation Segmentectomy or Lobectomy for Hepatocellular Carcinoma Refractory to Prior Transarterial Embolization or Chemoembolization: A Single Institution Retrospective Case Series. Cardiovasc. Interv. Radiol. 2023, 46, 460–469. [Google Scholar] [CrossRef]
- Ricke, J.; Klümpen, H.J.; Amthauer, H.; Bargellini, I.; Bartenstein, P.; de Toni, E.N.; Gasbarrini, A.; Pech, M.; Peck-Radosavljevic, M.; Popovič, P.; et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J. Hepatol. 2019, 71, 1164–1174. [Google Scholar] [CrossRef]
- TheraSphereTM Y-90 Glass Microspheres—Brief Summary. 2023. Available online: https://www.bostonscientific.com/en-US/products/cancer-therapies/therasphere-y90-glass-microspheres/therasphere-y90-microspheres-brief-summary.html (accessed on 27 November 2023).
- Sirtex—SIR-Spheres® Y-90 Resin Microspheres. 2023. Available online: https://www.sirtex.com/ap/products/sir-spheres-y-90-resin-microspheres/ (accessed on 27 November 2023).
| Age | 69 | (58–78) |
| Female | 4 | 17% |
| Male | 19 | 83% |
| Liver function status | ||
| Child–Pugh A | 21 | 9% |
| Child–Pugh B | 2 | 9% |
| Liver cirrhosis | 17 | 73.9 |
| Alcohol abuse | 14 | 58% |
| Hepatitis B virus | 4 | 17% |
| NASH | 2 | 8% |
| Tumor number | ||
| Unifocal | 15 | 65% |
| Multifocal | 8 | 33% |
| Tumor size | 30 mm | (12–70) |
| Portal veinous invasion | ||
| Tumoral PVT | 13 | 54% |
| Absence of tumoral PVT | 5 | 21% |
| AFP ng/ml | 84.4 | (6–51,470) |
| BCLC | ||
| Stage A | 2 | 8% |
| Stage B | 4 | 17% |
| Stage C | 17 | 74% |
| Microsphere Type/Absorbed Tumor Dose | n/(Median and IQR) |
|---|---|
| SIRT Microsphere | 23 |
| SIR-Sphere | 15 |
| TheraSphere | 8 |
| Absorbed Tumor Dose | |
| SIR-Sphere | 268 Gy (107.1–243.2) |
| For complete and partial responses n = 11 | 167.4 Gy (127.7–243.2) |
| For stable or progression responses n = 4 | 79.6 Gy (50.7–183.7) |
| Thera-Sphere | 445 Gy (349.5–563.5) |
| For complete and partial responses n = 6 | 520 Gy (422.5–587.5) |
| For stable or progression responses n = 2 | 319 Gy (288.5–349.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binzaqr, S.; Debordeaux, F.; Blanc, J.-F.; Papadopoulos, P.; Hindie, E.; Lapouyade, B.; Pinaquy, J.-B. Efficacy of Selective Internal Radiation Therapy for Hepatocellular Carcinoma Post-Incomplete Response to Chemoembolization. Pharmaceuticals 2023, 16, 1676. https://doi.org/10.3390/ph16121676
Binzaqr S, Debordeaux F, Blanc J-F, Papadopoulos P, Hindie E, Lapouyade B, Pinaquy J-B. Efficacy of Selective Internal Radiation Therapy for Hepatocellular Carcinoma Post-Incomplete Response to Chemoembolization. Pharmaceuticals. 2023; 16(12):1676. https://doi.org/10.3390/ph16121676
Chicago/Turabian StyleBinzaqr, Salma, Frederic Debordeaux, Jean-Frédéric Blanc, Panteleimon Papadopoulos, Elif Hindie, Bruno Lapouyade, and Jean-Baptiste Pinaquy. 2023. "Efficacy of Selective Internal Radiation Therapy for Hepatocellular Carcinoma Post-Incomplete Response to Chemoembolization" Pharmaceuticals 16, no. 12: 1676. https://doi.org/10.3390/ph16121676
APA StyleBinzaqr, S., Debordeaux, F., Blanc, J.-F., Papadopoulos, P., Hindie, E., Lapouyade, B., & Pinaquy, J.-B. (2023). Efficacy of Selective Internal Radiation Therapy for Hepatocellular Carcinoma Post-Incomplete Response to Chemoembolization. Pharmaceuticals, 16(12), 1676. https://doi.org/10.3390/ph16121676







